Plant roots play a crucial role in plant growth, vegetation dynamics, and ecosystem functioning (e.g., net primary productivity, nutrient cycling) as well as plant responses to environmental changes (Carmona et al., 2021; Laughlin et al., 2021; Chandregowda et al., 2022; Encinas-Valero et al., 2022; Hong et al., 2022). Although extensive research has examined both the form and function of stems and leaves (Wright et al., 2004; Diaz et al., 2016; Joswig et al., 2022), our understanding of plant roots is greatly limited. The core function of plant roots is to acquire and transport water and nutrients, which is undertaken by only a few terminal root branch orders with primary root structures, i.e., absorptive roots (Guo et al., 2008b).
Root absorption is facilitated by microbial symbiosis (e.g., with mycorrhizal fungi) and a range of root traits, including root morphology (e.g., root diameter), physiology (e.g., root respiration), anatomy (e.g., root cortex and stele), chemistry (e.g., root nitrogen content), mechanics (e.g., tensile strength) and microbial symbiosis (e.g., with mycorrhizal fungi) (McCormack et al., 2017; Wambsganss et al., 2021; Wen et al., 2022; Yan et al., 2022; Betekhtina et al., 2023; Weemstra et al., 2023). Among these root functional traits, root diameter is considered the most important, given that it usually has the largest inter-specific variation (Kong et al., 2014; Valverde-Barrantes et al., 2017; Weigelt et al., 2021). Such great variation in root diameter controls a range of root and mycorrhizal traits (Eissenstat et al., 2015; Li et al., 2018; Bergmann et al., 2020; Wen et al., 2022). Importantly, absorptive root diameter is the most phylogenetically conserved trait, suggesting that the inter-specific variation of root diameter is closely related to plant evolution (Comas et al., 2012; Chen et al., 2013; Pineiro et al., 2020; Lugli et al., 2021).
Roots are mainly composed of two cylindrical components, i.e., cortex and stele. This anatomy determines the absorptive diameter of roots. The cortex absorbs water and nutrients both directly and indirectly, through symbiotic mycorrhizal fungi (Brundrett, 2002; Ma et al., 2018; Rich et al., 2021). Root steles are responsible for the transportation of water and nutrients to stems and leaves, and of photosynthates to meet energy demands during nutrient absorption. Recent research has discovered that root growth is allometric, i.e., the thickness of the cortex increases linearly and at a higher rate than that of the stele (hereafter, “root allometry”) (Gu et al., 2014; Kong et al., 2014), root allometry has since been reported in over 200 plant species (Kong et al., 2019).
Thick root cortex is physiologically disadvantageous for resource absorption (water and nutrients) because it increases the resistance resources encounter (Steudle and Carol, 1998). In contrast, a thick stele, which bears thick vascular conduits (e.g., vessels), usually has a high capacity to transport resources (Kong et al., 2014). Consequently, root allometry may increase the imbalance between resource absorption and transportation. Thick absorptive roots usually have low specific root length (root length per unit root biomass) and hence limited root surface area for resource absorption (Pregitzer et al., 2002). Root allometry, thus, does not enhance resource absorption by roots themselves; instead, the fast increase in nutrient absorption required to eventually balance nutrient absorption and transportation must be mediated by other agents, e.g., mycorrhizal fungi (Kong et al., 2017). Thicker steles usually have thicker vascular conduits (e.g., sieve tubes), which improve transportation of leaf photosynthates belowground (Jensen et al., 2016). This may, in turn, increase allometric root growth through the construction and maintenance of even thicker cortex (see Kong et al., 2021 for details). Thus, structural allometry in roots (cortex and stele) may reflect the functional coordination of the uptake, transportation and utilization of elements essential for plant life (water, nutrients and carbon). If so, root allometry paves a new way of understanding how root form and function are related to plant evolution and adaptation to environmental changes (McCormack et al., 2017; Kong et al., 2019; Bergmann et al., 2020; Zhou et al., 2022).
Although several studies have examined allometric growth in roots, various plant groups have been neglected, including Orchidaceae and vine plants. Furthermore, little is known about whether and how root allometry varies among different mycorrhizal types, e.g., arbuscular mycorrhiza (AM), ectomycorrhiza (EM), and Orchid mycorrhiza (OM) (Brundrett, 2002; Martin et al., 2017). AM fungi and EM fungi are known to differ structurally and functionally in ways that may be affected by root allometry. For instance, AM fungi penetrate root cortical cells and form arbuscules to facilitate nutrient transfer, whereas EM fungi create a sheathing mantle on the exterior of the root epidermis. Thus, increased root cortical space would provide a greater habitat for AM fungi, whereas EM fungi habitat is proportional to the root epidermal surface area (Martin et al., 2008; Terrer et al., 2016, 2017, 2018). It is important to learn whether the structural and functional differences between AM and EM affect their host root structures, i.e., the allometric assembly of root cortex and stele.
Absorptive root diameter shows a strong phylogenetic signal (Kong et al., 2014; Valverde-barrantes et al., 2017; Wang et al., 2018), indicating that root anatomical traits are more influenced by ancestry than by environmental conditions. This is important for our understanding of how roots and even entire plants respond to climate change. However, we lack empirical evidence that indicates whether and/or how environmental changes (e.g., nitrogen deposition, elevation of atmospheric CO2 concentration) influence root allometry. It is necessary to test the generality of root allometry using a large species pool that includes neglected plant groups and that considers environmental changes.
Here, we aim to (1) test the generality of root allometry in different plant growth forms, mycorrhizal types, and in response to different environmental change scenarios; (2) summarize current knowledge on how root allometry is formed and on the ecological and evolutionary implications of the root allometry; and (3) propose promising directions that should be pursued in future studies on root allometry.
2. MethodsWe screened data on cortex thickness and stele radius for absorptive roots in Web of Science, Google Scholar, FRED 3.0 (http://roots.ornl.gov/) and CNKI (China's national knowledge infrastructure, https://www.cnki.net/) using the following keywords: “cortex”, “stele”, “anatomic structure”, “allometric relationship” or “root diameter”. Our search yielded 498,754 papers and reports. We then refined these results according to additional criteria: (1) Studies included in our analysis must be empirical rather than a review or perspective; (2) Data on root diameter and stele radius must be accessible. We also included some unpublished data (Supplementary data 2) on root anatomical traits that were measured at the same sites and following the same sampling procedures as in our previous study (Kong et al., 2014).
In total, our data set included 32 empirical studies (Supplementary data 1) with 698 observations of 512 species at 41 sites globally (Fig. 1). Specifically, there were 272 woody species and 240 non-woody species (78 grass, 92 herb, 37 fern, 28 Orchidaceae and 5 non-woody liana species). In addition, 13 liana species were included in the data set. For the same species sampled in different studies, we used the mean value of the root traits across studies as the trait value of this species. For some studies with only data on stele radius and root diameter, we used the difference between root diameter and stele radius, namely the stele tissue and the tissue outside the stele (ToS), including the epidermis, exodermis and cortex. The thickness of the cortex, i.e., tToS, has been used as a proxy approximate to the cortex thickness (Kong et al., 2019). For studies with root trait data displayed in figures, or only photos of root anatomical structures, we digitalized root trait data using the software “SigmaScan Pro software (v.5.0, SPSS Inc., Chicago, USA)” and the software “IMAGE J (NIH Image, Bethesda, MD, USA)”, respectively. All 204 plant species in Kong’s study were included in our study.
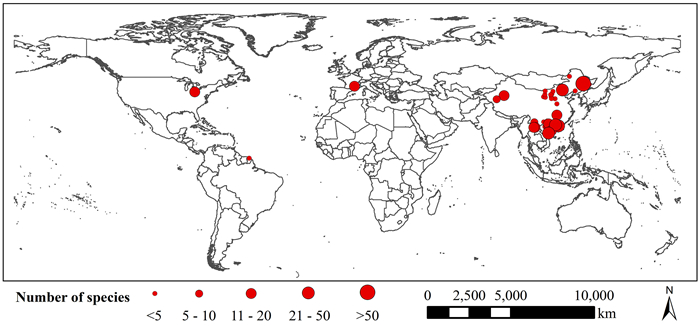
|
| Fig. 1 A global map of 32 studies reporting data on the cortex thickness and stele radius in absorptive roots. Each study is represented by a red circle, and the size of the circle is proportional to the number of species in the study. |
The existence of root allometry was tested by the following steps. First, we calculated the slope of regression of the stele radius with root diameter (Slope(RD-SR)) and the slope of the regression of the cortex thickness with root diameter (Slope(RD-CT)). Then, a slope comparison was conducted between Slope(RD-SR) and Slope(RD-CT) using the method of standardized major axis (Falster et al., 2006). A significant difference in the slope comparison indicates the existence of the root allometry. Finally, we tested whether root allometry exists across the total observations and total species, among different plant growth forms (woody vs. non-woody), mycorrhizal types (AM, EM and OM), and environmental change scenarios (e.g., nitrogen deposition, elevation of atmospheric CO2 concentration).
3. Generality of root allometry in absorptive rootsRoot allometry was observed in the root anatomical structures of 512 species (Table 1; Fig. 2a and b). Root allometry was observed in 22 of 26 studies in which root anatomical structures were examined in at least three species (Table 2; Fig. S1; Supplementary data 1).
| Species class | Number of species | Slope(RD-CT) | Slope(RD-SR) | Slope(RD-CT)/Slope(RD-SR) | Slope comparison | ||
| Species level | All species | 512 | 0.40*** | 0.12*** | 3.33 | *** | |
| Woody | 272 | 0.53*** | 0.07*** | 7.57 | *** | ||
| Non-woody | Fern + grass + herb + Orchidaceae + liana | 240 | 0.39*** | 0.12*** | 3.25 | *** | |
| Fern | 37 | 0.31*** | 0.17*** | 1.82 | *** | ||
| Grass | 78 | 0.39*** | 0.19*** | 2.05 | *** | ||
| Herb | 92 | 0.38*** | 0.11*** | 3.45 | *** | ||
| Orchidaceae | 28 | 0.41*** | 0.08*** | 5.12 | *** | ||
| Mycorrhiza type | AM | Woody + non-woody | 429 | 0.43*** | 0.13*** | 3.31 | *** |
| Woody | 197 | 0.53*** | 0.07*** | 7.57 | *** | ||
| Non-woody | 212 | 0.35*** | 0.18*** | 1.94 | *** | ||
| EM | Coniferous + broadleaf | 20 | 0.19ns | 0.21*** | – | – | |
| Coniferous | 6 | 0.11ns | 0.22ns | – | – | ||
| Broadleaf | 14 | 0.58*** | 0.10*** | 5.80 | *** | ||
| AM&EM | 13 | 0.33** | 0.13** | 2.54 | ** | ||
| OM | 28 | 0.41*** | 0.08*** | 5.13 | *** | ||
| Different treatments | Control + CO2 increase | 17 | 0.27*** | 0.18*** | 1.50 | ** | |
| Control | 17 | 0.26*** | 0.18*** | 1.44 | – | ||
| CO2 increase | 17 | 0.28*** | 0.18*** | 1.56 | * | ||
| Control + N increase | 14 | 0.43*** | 0.13*** | 3.31 | *** | ||
| Control | 12 | 0.40*** | 0.19** | 2.11 | *** | ||
| N increase | 14 | 0.43*** | 0.11*** | 3.91 | *** | ||
| Control + P increase | 12 | 0.41*** | 0.17** | 2.41 | *** | ||
| Control | 12 | 0.40*** | 0.19** | 2.11 | * | ||
| P increase | 12 | 0.43*** | 0.15** | 2.87 | ** | ||
| Dry + rainy season | 8 | 0.25*** | 0.20*** | 1.25 | – | ||
| Dry season | 8 | 0.42*** | 0.12* | 3.50 | * | ||
| Rainy season | 8 | 0.24*** | 0.21* | 1.14 | – | ||
| “Slope comparison”: statistical test of the difference between the two slopes is performed using standardized major axis. ***, ** and * indicate significance levels at p < 0.001, p < 0.01, and p < 0.05, respectively, ns indicates no significance (p > 0.05). Slope(RD-CT): Slope(RD-SR) and the slope comparison are denoted by “-” when either of the slopes is statistically significant. |
|||||||
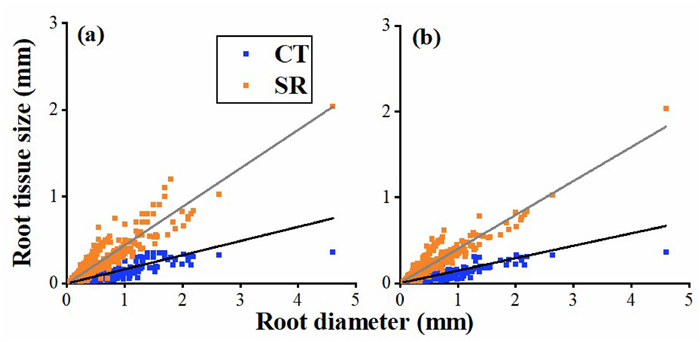
|
| Fig. 2 The pattern of root allometry across 698 observations (a) and 512 species (b). CT: cortex thickness; SR: stele radius. |
| Study | Number of species | Slope(RD-CT) | Slope(RD-SR) | Slope(RD-CT): Slope(RD-SR) | Slope comparison |
| Wahl and Ryser, 2000 | 19 | 0.27*** | 0.23*** | 1.17 | – |
| Hummel et al., 2007 | 14 | 0.37*** | 0.13* | 2.85 | * |
| Hou, 2007 | 20 | 0.39*** | 0.12*** | 3.25 | *** |
| Guo et al., 2008b | 23 | 0.37*** | 0.14*** | 2.64 | ** |
| Xu, 2011 | 27 | 0.40*** | 0.10*** | 4.00 | *** |
| Long, 2013 | 5 | 0.39** | 0.07* | 5.57 | ** |
| Kong et al., 2014 | 96 | 0.75*** | 0.10*** | 7.50 | *** |
| Dong et al., 2015 | 9 | 0.47*** | 0.03ns | – | – |
| Liu et al., 2016 | 12 | 0.39*** | 0.11*** | 3.55 | *** |
| Kong et al., 2016 | 7 | 0.32*** | 0.08** | 4.00 | ** |
| Valverde-Barrantes et al., 2016 | 34 | 0.47*** | 0.03*** | 15.67 | *** |
| Wang et al., 2016 | 6 | 0.40*** | 0.13* | 3.08 | ** |
| Wang et al., 2018 | 12 | 0.06ns | 0.25*** | – | – |
| Zhou et al., 2018 | 15 | 0.70*** | 0.004ns | – | – |
| Liu et al., 2019 | 16 | 0.89*** | 0.08* | 11.13 | *** |
| Wang et al., 2019 | 91 | 0.35*** | 0.11*** | 3.18 | *** |
| Wang, 2020 | 34 | 0.27*** | 0.18*** | 1.50 | ** |
| Wang et al., 2020 | 3 | 0.52ns | 0.14ns | – | – |
| Yamauchi et al., 2021 | 18 | 0.37*** | 0.13*** | 2.85 | *** |
| Xu, 2021 | 42 | 0.48*** | 0.10*** | 4.80 | *** |
| Zhou et al., 2022 | 32 | 0.39*** | 0.04*** | 9.75 | *** |
| Li et al., 2022 | 8 | 0.09ns | 0.05ns | – | – |
| Xiang et al., 2022 | 26 | 0.33*** | 0.15*** | 2.20 | ** |
| Yuan et al., 2022 | 16 | 0.25*** | 0.20*** | 1.25 | – |
| Xu et al., 2022 | 10 | 0.39*** | 0.11*** | 3.55 | *** |
| Cao et al., 2022 | 17 | 0.42*** | 0.08*** | 5.25 | *** |
| “Study”: see supplementary data 1 for the details of these studies. “Slope comparison”: statistical test of the difference between the two slopes is performed using standardized major axis. ***, ** and * indicate significance levels at p < 0.001, p < 0.01, and p < 0.05, respectively; ns indicates no significance (p > 0.05). Slope(RD-CT): Slope(RD-SR) and slope comparison are denoted by “-” when either of the slopes is statistically significant. |
|||||
Root allometry occurs in different plant growth forms. For example, in woody species, Slope(RD-CT): Slope(RD-SR) was 7.6, and in non-woody species the Slope(RD-CT) was 3.3 (Table 1; Fig. S3). The presence of root allometry in woody and non-woody species is very similar to that reported by Kong et al. (2019), which used less than half the plant species as in this current study. Interestingly, we also observed that the Slope(RD-CT): Slope(RD-SR) ratio was higher in woody vines than in non-woody vines (4.2 vs. 2.5, Table S1; Fig. S5a-c). The difference in root allometry between woody and non-woody species arise mainly from higher Slope(RD-CT) of the woody species than that of the non-woody species (Table 1). This could be because woody plants rely more on mycorrhizal fungi than do non-woody species (Ma et al., 2018); hence, thicker cortex (i.e., higher Slope(RD-CT)) is needed to accommodate more mycorrhizal fungi.
Root allometry was observed in ferns, grasses, herbs, and even the Orchidaceae bearing the thickest absorptive roots (up to 4.6 mm) (Zhu et al., 2016) among terrestrial plants (Table 1; Fig. S4d). Interestingly, we observed a slope ratio of 5.1 for Slope(RD-CT): Slope(RD-SR) in Orchidaceae species, higher than the slope ratio in ferns (1.8), grasses (2.1) and herbs (3.5) (Table 1). The difference in root allometry (indicated by the above slope ratios) in these non-woody species is mainly owed to the lower Slope(RD-SR) of the Orchidaceae species than that of other non-woody species (Table 1). This could be because Orchidaceae species, as the most evolutionarily advanced angiosperms, could have vascular conduits with higher transport efficiency than other non-woody species; this may ensure that Orchidaceae species have a higher increase of matter transport efficiency by a smaller increase in conduit and stele diameter, i.e., low Slope(RD-SR). Another possibility may be that Orchidaceae species usually grow in shady habitats, which have low transpiration demand, resulting in a low increase in conduit and stele diameter with increasing root diameter, i.e., low Slope(RD-SR).
Among dominant mycorrhizal types, root allometry is observed in AM (Fig. S6a), in both woody and non-woody plants (Fig. S7a and 7b), and in the dual mycorrhizas of AM and EM plants (i.e., roots are colonized by both AM and EM) but not in EM plants (Table 1; Fig. S5b and S5c). We observed root allometry in broadleaf EM trees but not the coniferous EM trees (Table 2; Fig. S8). Thus, the inclusion of the coniferous EM trees greatly influenced our statistical analysis of root allometry across the EM plants. However, only six coniferous EM plant species were included in our data set, and we cannot rule out the existence of the root allometry in other coniferous EM trees. If root allometry is absent in all coniferous EM trees, it might be interesting to explore the underlying mechanisms, given the distinct anatomical structures of coniferous and broadleaf tree roots (Guo et al., 2008b; Chen et al., 2016). For example, coniferous EM trees usually have vascular conduits (tracheid and sieve cells) with lower matter (water and photosynthates) transport efficiency relative to those of broadleaf EM plants, which have conduits with higher transport efficiency (vessels and sieve tubes) (Guo et al., 2008b). However, coniferous species usually have a special type of pit on the side walls of conduits, i.e., bordered pits, which confer the conduits with higher transport efficiency than that expected only by the conduit diameter. It is possible that bordered pits rather than conduit and stele radius vary in tandem with root diameter. This would decouple stele radius (and cortex thickness) from root diameter, and hence result in no root allometry.
Root anatomical structures have been measured sparsely under different environmental change scenarios (e.g., soil nitrogen or phosphorus fertilization, elevation of atmospheric CO2 concentration, and seasonality in precipitation) (Table 1; Fig. S9), which provides an opportunity to test the consistency of root allometry. Although root allometry in some scenarios does not hold statistically (Fig. S9), this is apparently due to the inclusion of a few species with “exceptionally” sized root cortex and stele or the inclusion of some species with “exceptional” responses to environmental changes (e. g., Fig. S9a2, a3, b2, b3, d2, d3). Here, we found that root allometry does occur after exclusion of these exceptional species (Fig. S9). These results suggest that root allometry is relatively insensitive to environmental changes. Unexpectedly, we note that cortex thickness increases slower and stele radius increases faster with increasing root diameter in the rainy season than in the dry season, causing an equal increase rate of the cortex thickness and stele radius in the rainy season (Fig. S8d2, d3). It would be interesting to test whether seasonality impacts root allometry in other plant species.
Together, the widespread existence of root allometry between root cortex and stele across different climatic zones (tropical, sub-tropical and temperate), ecosystem types (forests, grasslands, deserts and mangroves), mycorrhizal types (AM, some EM, AM&EM, OM), phylogenetic gradients (from ferns to Orchidaceae), and some environmental change scenarios supports the general existence of the root allometry in terrestrial plants.
4. Mechanisms of the root allometric relationshipCurrently, two mechanisms have been proposed to explain why root allometry is formed. One is the nutrient absorption-transportation balance theory (Kong et al., 2017) and the other is the carbon supply-consumption balance theory (Kong et al., 2021; Colombi et al., 2022) (Fig. 3). Both theories run according to the principle of functional balance of matter (nutrients, photosynthates) transport within the root stele as well as the physical law of fluid transport in the conduits, namely the “Hagen–Poiseuille law” (Jensen et al., 2016). Here, we briefly explain the two mechanisms.

|
| Fig. 3 Mechanisms for root allometry in absorptive roots. For simplicity, we illustrate two plant species with thin and thick absorptive roots, respectively, followed by the root cross-sectional area (a). Symbols in (a): cortex, the area between the two green circles; stele, inner green circle; vessels: blue circles; sieves: orange circles. Root allometry is shown in (b) where the cortex thickness increases much faster than the stele radius. Functional change of the root cortex (mycorrhizal association, nutrient acquisition, carbon consumption) and the stele (nutrient transportation, carbon supply) with increasing root diameter is shown in (c) with root longitudinal section area. Symbols in (c): cortex, the yellow cells; stele, the blue and orange columns; mycorrhizal fungi, the intermingled lines within the cortex cells. Thickness of the arrowed lines in (c) represents the size of nutrients and carbon flux through the roots. More mycorrhizal fungi in the cortex indicates more nutrient acquisition through the mycorrhizal fungi. Functional balance of nutrients and carbon with increasing root diameter is shown in (d). In the functional balance framework, the root allometry is formed to meet the balance between nutrient absorption (via mycorrhizal fungi) and nutrient transportation (via the stele), i.e., the nutrient balance theory (d1) and between carbon supply (via the stele) and carbon consumption (via the cortex), i.e., the carbon balance theory (d2). The conceptual models in (d1) and (d2) are redrawn from Kong et al. (2017) and Kong et al. (2021). |
There are two paralleled vascular systems in root steles, i.e., vessels responsible for transporting water and nutrients upward to stems and leaves, and sieves responsible for transporting photosynthates downward to meet the carbon demands of absorptive roots. According to the Hagen–Poiseuille law, both volumetric flow rates in the conduits (i.e., water and nutrient transportation via vessels and photosynthate transportation via sieves) scale to the fourth power of the root radius (∝r4, r is radius of the absorptive roots); in contrast, even the maximum nutrient absorption (via mycorrhizal fungi in the cortex) and carbon consumption (in the cortex) scales to less than the second power of root radius (∝r2). In this case, only a much faster increase in cortex thickness than in stele radius (i.e., the allometric relationship) can lead to a functional balance between nutrient absorption (via mycorrhizal fungi in the cortex) and nutrient transportation (via vessels in the stele) and a balance between the carbon supply (via sieves in the stele) and carbon consumption (via the cortex and mycorrhizal fungi).
Although the above two mechanisms have been widely accepted, we should keep in mind that there are important limitations to these theories. First, the nutrient absorption-transportation balance theory is based on a universal mycorrhizal association in terrestrial plant roots, although many plant species have no mycorrhizal associations (Van der Heijden et al., 2015; Brundrett and Tedersoo, 2018; Correia et al., 2018). Second, the two theories seem to be independent of one another, although they are both based on two interconnected vascular conduits, i.e., vessels and sieves in roots. It is also interesting to learn about how the two theories are linked with leaves. This is because leaves function as an important nutrient sink and the source of carbon, both of which are functionally linked to absorptive roots. Thirdly, empirical evidence is still needed to test the predictions of the functional balance that underlies the formation of root allometry. In testing the predictions, we should also consider the temporal dynamics of carbon supply from leaves as well as the carbon cost in root exudates, mycorrhizal symbiosis and root turnover.
5. Implications of root allometry 5.1. The “root economics spectrum"Similar to the well-known leaf economics spectrum (Wright et al., 2004), absorptive roots have long been assumed to vary following the root economics spectrum, a leading trait dimension in roots conveying a trade-off between nutrient acquisition and conservation (Freschet et al., 2010; Reich, 2014; Kramer-Walter et al., 2016; Bergmann et al., 2020). If the nutrient acquisition-conservation tradeoff (i.e., root economics spectrum) exists in absorptive roots, there should be a positive correlation between root diameter and root tissue density, given that thicker absorptive roots have longer lifespan (i.e., longer conservation of the nutrients in the roots) (Guo et al., 2008a; Gu et al., 2017; Liese et al., 2019), and roots with higher root tissue density usually have lower activity in nutrient acquisition (Zadworny et al., 2017; Stock et al., 2021). Given the higher tissue density of the stele than the cortex (Valverde-barrantes et al., 2021), root allometry theoretically leads to a negative relationship between root tissue density and root diameter (Han and Zhu, 2021, Kong et al., 2016, Weemstra et al., 2016). Such a prediction has been confirmed using global root trait data, which runs against the prediction from the root economics spectrum (Kong et al., 2019). This in turn suggests that root allometry may constitute indirect evidence against the existence of the traditionally recognized root economics spectrum (Kong et al., 2019).
5.2. Plant evolutionThe evolution of angiosperms is closely related to the decline of atmospheric CO2 concentrations since the mid-Cretaceous (Beerling and Berner, 2005; Gerhart and Ward, 2010). For example, the reduction of atmospheric CO2 concentrations often lowers leaf photosynthesis, consequently causing a “carbon starvation” in plants. To survive in this carbon limitation condition, plants tend to increase stomatal conductance to compensatively improve leaf CO2 fixation (Zhou et al., 2022; Holtta et al., 2017). However, large stomatal conductance enhances transpiration water loss, causing a physiological drought stress to plants (Khan et al., 2007; Wang et al., 2018). The surge of leaf vein density in angiosperms since the mid-Cretaceous, and hence more efficient water supply to the mesophyll cell for photosynthesis, has been considered evidence for plant adaptation to physiological drought (Baraloto et al., 2010; Feild et al., 2011; Baird et al., 2021; Yan et al., 2022).
Coordinated with the evolutionary change in leaves, thinning of the absorptive roots is regarded as an adaptation to physiological drought stress caused by the decline of atmospheric CO2 concentrations (Fig. 4a) (Comas et al., 2012; Chen et al., 2013; Ma et al., 2018). Root allometry may also be an adaptation to drought stress, as it effectively reduces the resistance of water and nutrients entering the root tissues and reduces carbon consumption by the root cortex (Kong et al., 2017, 2021) (Fig. 4b). Therefore, even though root allometry is present in non-angiosperms, e.g., ferns, whose origin is much earlier than the Cretaceous, this adaptation has likely been instrumental in helping angiosperms survive in carbon- and water-limited environments for millions of years. From this point of view, root allometry provides insights into how roots, whole plants and even ecosystems respond and adapt to on-going geological and environmental changes. Further, the Orchidaceae plants, as the most advanced angiosperms, have the thickest absorptive roots. Therefore, the formation of root allometry in these plants may not be linked with plant adaptation to the decline of atmospheric CO2. It would be interesting to discover what root allometry implies for the plant ecology and evolution of these plants.
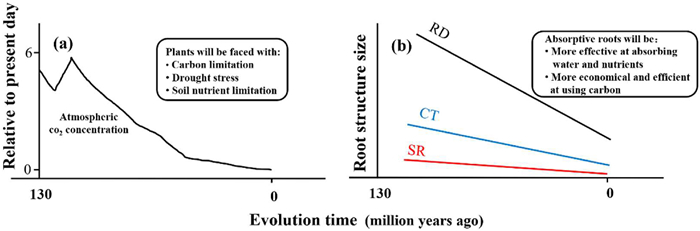
|
| Fig. 4 The co-variation of atmospheric CO2 concentration (a) and anatomical structures of absorptive roots in angiosperms (b) since the Cretaceous. The pattern for the change of atmospheric CO2 concentration is redrawn from Beerling and Franks (2010). The resultant stress by the change in atmospheric CO2 concentration (Comas et al., 2012) and the adaptive responses of the absorptive roots are indicated as inlets (Kong et al., 2017, 2021) (see the text for the details). RD: absorptive root diameter; CT: cortex thickness, SR: stele radius. |
To date, studies have concentrated on the general pattern of root allometry, i.e., higher Slope(RD-CT) than Slope(RD-SR). However, our analysis has shown a great variation in the Slope(RD-CT): Slope(RD-SR) ratio from the minimum of 1.2 to the maximum of 15.7 (Table 2). We are still unclear why there is so large a difference in root allometry among studies, across species or even within species. This is a fascinating area that could stimulate new insights into our understanding of the root form and function.
6.2. More plant species need to be examinedRoot allometry has been observed in over 500 plant species, much smaller than the total number of the vascular plant species (about 390,000) on Earth (Cantwell-Jones et al., 2022). Even in global root trait data sets, such as FRED 3.0 (Iversen et al., 2021), measurements of root anatomical traits are far less common than those of other root traits such as root diameter and root tissue density. Therefore, it is necessary to examine root anatomical structures in more plant species, especially the families with a large number of species like Orchidaceae (> 20,000 species).
6.3. Environmental changes and root allometryFew studies have examined whether environmental changes alter root allometry in absorptive roots. Moreover, these studies have only examined a few environmental changes, failing to examine interactions among these environmental factors. In addition to studies that examine how environmental changes relate to root allometry, we need to pay more attention to plants growing in naturally stressed conditions such as alpine forests, deserts, and coast environments in high salinity (Cao et al., 2023). Comparison of the root allometry among these conditions could be instructive for our understanding and prediction of vegetation dynamics under global climate change.
6.4. Plant above-ground linkage and root allometryPlant growth and evolution depends on the functional coordination between plant above- and below-ground organs (Aritsara et al., 2022; Weigelt et al., 2021; Zhou et al., 2022). Root allometry may be accompanied by a faster supply of leaf photosynthates to the roots. However, we know little about how plants with thick absorptive roots shape their leaf structures to meet the functional balance of water, nutrients and carbon between roots and leaves. Another interesting question is whether and how root allometry is coordinated with plant reproductive organs like flowers, fruits and seeds given that the reproductive organs can compete with roots for photosynthates and with leaves for water and nutrients. Therefore, linking root allometry with plant above-ground organs is an intriguing area to deepen our understanding of the co-evolution among plant organs and the co-evolution between plants and animals for pollination or seed dispersal.
AcknowledgementsWe are grateful for Dr. Meng Zhou, Oscar J. Valverde-Barrantes and WenHua Xiang for their assistance in data collection. Thanks are also given to Dr. J. Aaron Hogan (Department of Biology, University of Florida) and the other anonymous reviewer for their insightful comments and inputs for improvement of this review paper. This study was funded by the National Natural Science Foundation of China (32171746, 42077450, 31870522 and 31670550), Funding for Characteristic and Backbone Forestry Discipline Group of Henan Province, and the Scientific Research Foundation of Henan Agricultural University (30500854), Research Funds for overseas returnee in Henan Province, China.
Author contributions
Y.Z. and D.K. conceived the ideas of this review paper, D.K. and Y.Z. performed the data analysis, Y.Z. wrote the first draft of this manuscript and Y.Z., D.K., J.C., Q.Y., M.W., and Y.Z. all contributed to the editing and revision of the final version of the manuscript.
Declaration of competing interest
We declare that we have no conflict interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.05.002.
Aritsara, A.N.A., Wang, S., Li, B.N., et al., 2022. Divergent leaf and fine root “pressure-volume relationships” across habitats with varying water availability. Plant Physiol., 190: 2246-2259. DOI:10.1093/plphys/kiac403 |
Baird, A.S., Taylor, S.H., Pasquet-Kok, J., et al., 2021. Developmental and biophysical determinants of grass leaf size worldwide. Nature, 592: 242-257. DOI:10.1038/s41586-021-03370-0 |
Baraloto, C., Paine, C.E.T., Poorter, L., et al., 2010. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett., 13: 1338-1347. DOI:10.1111/j.1461–0248.2010.01517.x |
Beerling, D.J., Berner, R.A., 2005. Feedbacks and the coevolution of plants and atmospheric CO2. Proc. Natl. Acad. Sci. U.S.A., 102: 1302-1305. DOI:10.1073/pnas.0408724102 |
Beerling, D.J., Franks, P.J., 2010. The hidden cost of transpiration. Nature, 464: 495-496. DOI:10.1038/464495a |
Bergmann, J., Weigelt, A., van Der Plas, F., et al., 2020. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv., 6: 1193-1208. DOI:10.1126/sciadv.aba1193 |
Betekhtina, A.A., Tukova, D.E., Veselkin, D.V., Root structure syndromes of four families of monocots in the Middle Urals. Plant Divers., 45: 722-731. DOI:10.1016/j.pld.2023.01.007 |
Brundrett, M.C., 2002. Coevolution of roots and mycorrhizas of land plants. New Phytol., 154: 275-304. DOI:10.1046/j.1469–8137.2002.00397.x |
Brundrett, M.C., Tedersoo, L., 2018. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol., 220: 1108-1115. DOI:10.1111/nph.14976 |
Cantwell-Jones, A., Ball, J., Collar, D., et al., 2022. Global plant diversity as a reservoir of micronutrients for humanity. Nat. Plants, 8: 225-232. DOI:10.1038/s41477-022-01100-6 |
Cao, J.J., Chen, J., Yang, Q.P., et al., 2023. Leaf hydraulics coordinated with leaf economics and leaf size in mangrove species along a salinity gradient. Plant Divers., 45: 309-314. DOI:10.1016/j.pld.2022.01.002 |
Cao, J.J., Yang, Q.P., Chen, J., et al. 2022. Novel leaf-root coordination driven by leaf water storage tissues in mangroves, BioRxiv 501578 [Preprint]. https://doi.org/10.1101/2022.07.26.501578.
|
Carmona, C.P., Bueno, C.G., Toussaint, A., et al., 2021. Fine-root traits in the global spectrum of plant form and function. Nature, 597: 683-687. DOI:10.1038/s41586-021-03871-y |
Chandregowda, M.H., Tjoelker, M.G., Pendall, E., et al., 2022. Root trait shifts towards an avoidance strategy promote productivity and recovery in C-3 and C-4 pasture grasses under drought. Funct. Ecol., 36: 1754-1771. DOI:10.1111/1365–2435.14085 |
Chen, W.L., Zeng, H., Eissenstat, D.M., et al., 2013. Variation of first-order root traits across climatic gradients and evolutionary trends in geological time. Global Ecol. Biogeogr., 22: 846-856. DOI:10.1111/geb.12048 |
Chen, W.L., Koide, R.T., Adams, T.S., et al., 2016. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. U.S.A., 113: 8741-8746. DOI:10.1073/pnas.1601006113 |
Colombi, T., Chakrawal, A., Herrmann, A.M., 2022. Carbon supply-consumption balance in plant roots: effects of carbon use efficiency and root anatomical plasticity. New Phytol., 233: 1542-1547. DOI:10.1111/nph.17598 |
Comas, L.H., Mueller, K.E., Taylor, L.L., et al., 2012. Evolutionary patterns and biogeochemical significance of angiosperm toot traits. Int. J. Plant Sci., 173: 584-595. DOI:10.1086/665823 |
Correia, M., Heleno, R., Vargas, P., et al., 2018. Should I stay or should I go? Mycorrhizal plants are more likely to invest in long-distance seed dispersal than non-mycorrhizal plants. Ecol. Lett., 21: 683-691. DOI:10.1111/ele.12936 |
Diaz, S., Kattge, J., Cornelissen, J.H.C., et al., 2016. The global spectrum of plant form and function. Nature, 529: 167-171. DOI:10.1038/nature16489 |
Dong, X.Y., Wang, H.F., Gu, J.C., et al., 2015. Root morphology, histology and chemistry of nine fern species (Pteridophyta) in a temperate forest. Plant Soil, 393: 215-227. DOI:10.1007/s11104-015-2484-7 |
Eissenstat, D.M., Kucharski, J.M., Zadworny, M., et al., 2015. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol., 208: 114-124. DOI:10.1111/nph.13451 |
Encinas-Valero, M., Esteban, R., Hereş, A.M., et al., 2022. Holm oak decline is determined by shifts in fine root phenotypic plasticity in response to belowground stress. New Phytol., 235: 2237-2251. DOI:10.1111/nph.18182 |
Falster, D.S., Warton, D.I., Wright, I.J., et al., 2006. User's guide to SMATR: Standardised MajorAxis Tests & Routines Version 2.0, Copyright 2006.
|
Feild, T.S., Brodribb, T.J., Iglesias, A., et al., 2011. Fossil evidence for Cretaceous escalation in angiosperm leaf vein evolution. Proc. Natl. Acad. Sci. U.S.A., 108: 8363-8366. DOI:10.1073/pnas.1014456108 |
Freschet, G.T., Cornelissen, J.H.C., Van Logtestijn, R.S.P., et al., 2010. Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol., 98: 362-373. DOI:10.1111/j.1365–2745.2009.01615.x |
Gerhart, L.M., Ward, J.K., 2010. Plant responses to low CO2 of the past. New Phytol., 188: 674-695. DOI:10.1111/j.1469–8137.2010.03441.x |
Gu, J.C., Xu, Y., Dong, X.Y., et al., 2014. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiol., 34: 415-425. DOI:10.1093/treephys/tpu019 |
Gu, J.C., Wang, Y., Fahey, T.J., et al., 2017. Effects of root diameter, branch order, soil depth and season of birth on fine root life span in five temperate tree species. Eur. J. For. Res., 136: 727-738. DOI:10.1007/s10342-017-1068-x |
Guo, D.L., Mitchell, R.J., Withington, J.M., et al., 2008a. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J. Ecol., 96: 737-745. DOI:10.1111/j.1365–2745.2008.01385.x |
Guo, D.L., Xia, M.X., Wei, X., et al., 2008b. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol., 180: 673-683. DOI:10.1111/j.1469–8137.2008.02573.x |
Han, M.G., Zhu, B., 2021. Linking root respiration to chemistry and morphology across species. Global Change Biol., 27: 190-201. DOI:10.1111/gcb.15391 |
Holtta, T., Lintunen, A., Chan, T., et al., 2017. A steady-state stomatal model of balanced leaf gas exchange, hydraulics and maximal source-sink flux. Tree Physiol., 37: 851-868. DOI:10.1093/treephys/tpx011 |
Hong, Y., Zhou, Q., Hao, Y., et al., 2022. Crafting the plant root metabolome for improved microbe-assisted stress resilience. New Phytol., 234: 1945-1950. DOI:10.1111/nph.17908 |
Hou, X.Q., 2007. Studies on mycorrhizal biology of Dendrobium. Peking Union Medical College and Chinese Academy of Medical Sciences.
|
Hummel, I., Violle, C., Devaux, J., et al., 2007. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytol., 173: 313-321. DOI:10.1111/j.1469–8137.2006.01912.x |
Iversen, C.M., McCormack, M.L., Baer, J.K., et al., 2021. Fine-root Ecology Database (FRED): A Global Collection of Root Trait Data with Coincident Site, Vegetation, Edaphic, and Climatic Data. Version 3.
|
Jensen, K.H., Berg-Sørensen, K., Bruus, H., et al., 2016. Sap flow and sugar transport in plants. Rev. Mod. Phys., 88: 035007. DOI:10.1103/RevModPhys.88.035007 |
Joswig, J.S., Wirth, C., Schuman, M.C., et al., 2022. Climatic and soil factors explain the two-dimensional spectrum of global plant trait variation. Nat. Ecol. Evol., 6: 36-50. |
Khan, H.U.R., Link, W., Hocking, T.J., et al., 2007. Evaluation of physiological traits for improving drought tolerance in faba bean (Vicia faba L.). Plant Soil, 292: 205-217. DOI:10.1007/s11104-007-9217-5 |
Kong, D.L., Wang, J., Zeng, H., et al., 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol., 203: 863-872. DOI:10.1111/nph.12842 |
Kong, D.L., Wang, J., Zeng, H., et al., 2017. The nutrient absorption–transportation hypothesis: optimizing structural traits in absorptive roots. New Phytol., 213: 1569-1572. DOI:10.1111/nph.14344 |
Kong, D.L., Wang, J., Wu, H., et al., 2019. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun., 10: 2203. DOI:10.1038/s41467-019-10245-6 |
Kong, D.L., Wang, J.J., Kardol, P, et al., 2016. Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences, 13: 415-424. DOI:10.5194/bg-13-415-2016 |
Kong, D.L., Wang, J.J., Valverde-Barrantes, O.J., et al., 2021. A framework to assess the carbon supply-consumption balance in plant roots. New Phytol., 229: 659-664. DOI:10.1111/nph.16807 |
Kramer-Walter, K.R., Bellingham, P.J., Millar, T.R., et al., 2016. Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol., 104: 1299-1310. DOI:10.1111/1365–2745.12562 |
Laughlin, D.C., Mommer, L., Sabatini, F.M., et al., 2021. Root traits explain plant species distributions along climatic gradients yet challenge the nature of ecological trade-offs. Nat. Ecol. Evol., 5: 1123-1134. DOI:10.1038/s41559-021-01471-7 |
Li, X., Dong, J.L., Chu, W.Y., et al., 2018. The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant Soil, 425: 415-432. DOI:10.1007/s11104-017-3555-8 |
Li, Z.Y., Wang, Y., Mu, L.Q., 2022. How does deforestation affect the growth of Cypripedium (Orchidaceae) species? A simulation experiment in Northeast China. Forests, 13: 166. DOI:10.3390/f13020166 |
Liese, R., Leuschner, C., Meier, I.C., 2019. The effect of drought and season on root life span in temperate arbuscular mycorrhizal and ectomycorrhizal tree species. J. Ecol., 107: 2226-2239. DOI:10.1111/1365–2745.13181 |
Liu, B., He, J., Zeng, F., et al., 2016. Life span and structure of ephemeral root modules of different functional groups from a desert system. New Phytol, 211: 103-112. DOI:10.1111/nph.13880 |
Liu, C., Xiang, W.H., Zou, L.M., et al., 2019. Variation in the functional traits of fine roots is linked to phylogenetics in the common tree species of Chinese subtropical forests. Plant Soil, 436: 347-364. DOI:10.1007/s11104-019-03934-0 |
Long, Y.Q., 2013. The Research of the Linkage of Root Function with Root Branch Order. Peking University.
|
Lugli, L.F., Rosa, J.S., Andersen, K.M., et al., 2021. Rapid responses of root traits and productivity to phosphorus and cation additions in a tropical lowland forest in Amazonia. New Phytol., 230: 116-128. DOI:10.1111/nph.17154 |
Ma, Z.Q., Guo, D.L., Xu, X., et al., 2018. Evolutionary history resolves global organization of root functional traits. Nature, 555: 94-97. DOI:10.1038/nature25783 |
Martin, F., Aerts, A., Ahrén, D., et al., 2008. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature, 452: 88-92. DOI:10.1038/nature06556 |
Martin, F.M., Uroz, S., Barker, D.G., 2017. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science, 356: eaad4501. DOI:10.1126/science.aad4501 |
McCormack, M.L., Guo, D.L., Iversen, C.M., et al., 2017. Building a better foundation: improving root-trait measurements to understand and model plant and ecosystem processes. New Phytol., 215: 27-37. DOI:10.1111/nph.14459 |
Pineiro, J., Ochoa-Hueso, R., Drake, J.E., et al., 2020. Water availability drives fine root dynamics in a Eucalyptus woodland under elevated atmospheric CO2 concentration. Funct. Ecol., 34: 2389-2402. DOI:10.1111/1365–2435.13660 |
Pregitzer, K.S., DeForest, J.L., Burton, A.J., et al., 2002. Fine root architecture of nine north American trees. Ecol. Monogr., 72: 293-309. DOI:10.1890/0012-9615(2002)072[0293:FRAONN]2.0.CO;2 |
Reich, P.B., 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol., 102: 275-301. DOI:10.1111/1365–2745.12211 |
Rich, M.K., Vigneron, N., Libourel, C., et al., 2021. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science, 372: 864-868. DOI:10.1126/science.abg0929 |
Steudle, E., Carol, A.P., 1998. How does water get through roots?. J. Exp. Bot., 322: 775-788. |
Stock, S.C., Koester, M., Boy, J., et al., 2021. Plant carbon investment in fine roots and arbuscular mycorrhizal fungi: a cross-biome study on nutrient acquisition strategies. Sci. Total Environ., 781: 146748. DOI:10.1016/j.scitotenv.2021.146748 |
Terrer, C., Vicca, S., Stocker, B.D., et al., 2016. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science, 353: 72-74. DOI:10.1126/science.aaf4610 |
Terrer, C., Vicca, S., Stocker, B.D., et al., 2017. Response to Comment on “Mycorrhizal association as a primary control of the CO2 fertilization effect”. Science, 355: 358. DOI:10.1126/science.aai8242 |
Terrer, C., Vicca, S., Stocker, B.D., et al., 2018. Ecosystem responses to elevated CO2 governed by plant–soil interactions and the cost of nitrogen acquisition. New Phytol., 217: 507-522. DOI:10.1111/nph.14872 |
Valverde-Barrantes, O.J., Freschet, G.T., Roumet, C., et al., 2017. A worldview of root traits: the influence of ancestry, growth form, climate and mycorrhizal association on the functional trait variation of fine-root tissues in seed plants. New Phytol., 215: 1562-1573. DOI:10.1111/nph.14571 |
Valverde-Barrantes, O.J., Authier, L., Schimann, H., et al., 2021. Root anatomy helps to reconcile observed root trait syndromes in tropical tree species. Am. J. Bot., 108: 744-755. DOI:10.1002/ajb2.1659 |
Valverde-Barrantes, O.J., Horning, A.L., Smemo, K.A., et al., 2016. Phylogenetically structured traits in root systems influence arbuscular mycorrhizal colonization in woody angiosperms. Plant Soil, 404: 1-12. DOI:10.1007/s11104-016-2820-6 |
Van der Heijden, M.G.A., Martin, F.M., Selosse, M.A., et al., 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol., 205: 1406-1423. DOI:10.1111/nph.13288 |
Wahl, S., Ryser, P., 2000. Root tissue structure is linked to ecological strategies of grasses. New Phytol., 148: 459-471. DOI:10.1046/j.1469–8137.2000.00775.x |
Wambsganss, J., Freschet, G.T., Beyer, F., et al., 2021. Tree species mixing causes a shift in fine-root soil exploitation strategies across European forests. Funct. Ecol., 35: 1886-1902. DOI:10.1111/1365–2435.13856 |
Wang, N., 2020. Effects of elevated atmospheric CO2 concentration on leaf and absorptive root and functional traits. In: Seedlings of 17 temperate woody and herbaceous. Northeast Forestry University.
|
Wang, Y., Dong, X.Y., Wang, H.F., et al., 2016. Root tip morphology, anatomy, chemistry and potential hydraulic conductivity vary with soil depth in three temperate hardwood species. Tree Physiol., 361: 99-108. DOI:10.1093/treephys/tpv094 |
Wang, X.X., Du, T.T., Huang, J.L., et al., 2018. Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. J. Exp. Bot., 69: 4033-4045. DOI:10.1093/jxb/ery188 |
Weemstra, M., Mommer, L., Visser, E.J., et al., 2016. Towards a multidimensional root trait framework: a tree root review. New Phytol., 211: 1159-1169. DOI:10.1111/nph.14003 |
Wang, H.F., Wang, Z.Q., Dong, X.Y., 2019. Anatomical structures of fine roots of 91 vascular plant species from four groups in a temperate forest in Northeast China. PLoS One, 14: e0215126. DOI:10.1371/journal.pone.0215126 |
Wang, Y.M., Wang, Y., Wang, S.Y., et al., 2020. Fine root anatomical and morphological traits of three temperate liana species in northeastern China. J. Beijing For. Univ., 42: 42-49. |
Weemstra, M., Kuyper, T.W., Sterck, F.J., Umaña, M.N., 2023. Incorporating belowground traits: avenues towards a whole-tree perspective on performance. Oikos, 2023: e08827. DOI:10.1111/oik.08827 |
Weigelt, A., Mommer, L., Andraczek, K., et al., 2021. An integrated framework of plant form and function: the belowground perspective. New Phytol., 232: 42-59. DOI:10.1111/nph.17590 |
Wen, Z.H., White, P.J., Shen, J.B., et al., 2022. Linking root exudation to belowground economic traits for resource acquisition. New Phytol., 233: 1620-1635. DOI:10.1111/nph.17854 |
Wright, I.J., Reich, P.B., Westoby, M., et al., 2004. The worldwide leaf economics spectrum. Nature, 428: 821-827. DOI:10.1038/nature02403 |
Xiang, W., Huang, D.L., Zhu, S.D., 2022. Absorptive root anatomical traits of 26 tropical and subtropical fern species. J. Plant Ecol., 46: 593-601. DOI:10.17521/cjpe.2021.0328 |
Xu, Y., 2011. Fine root morphology, anatomy and tissue nitrogen and carbon of the first five order roots in twenty seven Chinese tropical hardwood tree species. Northeast Forestry University.
|
Xu, L.Y., 2021. Effects of nitrogen and phosphorus on leaf and root functional traits. In: Seedlings of Species. Northeast Forestry University.
|
Xu, H.W., Ren, Y., Liu, X.J., et al., 2022. Relationship between root tip diameter and anatomical traits among ten species of climbing plants in tropical forest. Mol. Plant Breed., 20: 987-995. |
Yamauchi, T., Pedersen, O., Nakazono, M., et al., 2021. Key root traits of Poaceae for adaptation to soil water gradients. New Phytol., 229: 3133-3140. DOI:10.1111/nph.17093 |
Yan, H., Freschet, G.T., Wang, H.M., et al., 2022. Mycorrhizal symbiosis pathway and edaphic fertility frame root economics space among tree species. New Phytol., 234: 1639-1653. DOI:10.1111/nph.18066 |
Yuan, Y.M., Liu, J.Y., Gao, X.L., et al., 2022. Root traits of seven Stipa species and their relations with environmental factors in temperature grasslands. Acta Ecologica Sinica, 21: 1-11. |
Zadworny, M., McCormack, M.L., Zytkowiak, R., et al., 2017. Patterns of structural and defense investments in fine roots of Scots pine (Pinus sylvestris L.) across a strong temperature and latitudinal gradient in Europe. Global Change Biol., 23: 1218-1231. DOI:10.1111/gcb.13514 |
Zhou, M., Bai, W.M., Zhang, Y.S., et al., 2018. Multi-dimensional patterns of variation in root traits among coexisting herbaceous species in temperate steppes. J. Ecol., 106: 2320-2331. DOI:10.1111/1365–2745.12977 |
Zhou, M., Guo, Y.M., Sheng, J., et al., 2022. Using anatomical traits to understand root functions across root orders of herbaceous species in a temperate steppe. New Phytol., 234: 422-434. DOI:10.1111/nph.17978 |
Zhu, L.Q., Xu, Y.X., Zhao, L.J., et al., 2016. Anatomical structure and environmental adaptability of Cymbidium cyperifolium in karst area. Guihaia, 36: 1179–1185+1164. |



