b. Department of Environment Studies, Panjab University, Chandigarh 160014, India
An alien plant species is acknowledged as 'invasive' when it begins to expand significantly over a broad geographical range by overcoming the physiological constraints and environmental barriers (Richardson et al., 2000; Shackleton et al., 2019). During its range expansion, a species adjusts itself in the novel habitats either via phenotypic plasticity and/or adaptive evolution (Davidson et al., 2011; Oduor et al., 2016; Fox et al., 2019). Phenotypic plasticity is a phenomenon by virtue of which the individuals of a species with a common genotype express variable phenotypes in response to their surroundings (Agrawal, 2001; Richards et al., 2006). On the contrary, adaptive evolution is a genetically determined alteration in the natural behavior of a species (Hairston et al., 2005). Ecologists suggest that both plasticity and genetic adaptations are equally dominant in the field of invasion biology and these events give rise to spatial or temporal intraspecific variations in the population of a species (Richards et al., 2006; Prentis et al., 2008; Davidson et al., 2011; Oduor et al., 2016; Liao et al., 2020).
Intraspecific variations maximize the fitness of a species under heterogeneous environmental conditions (Agrawal, 2001; Richards et al., 2006; Zhao et al., 2012; Nolting et al., 2021). Such disparities, induced stochastically, environmentally, or genetically, amongst the con-specific individuals often provide raw material for the natural selection of traits (Bolnik et al., 2011). Invasive species can afford benefits of such variations to evolve into more adaptable and sustainable ecophenes, ecotypes, and ecospecies (Bradshaw, 1965; Anderson and Treshow, 1980; Schlichting, 1986; Prentis et al., 2008). Therefore, research pertaining to intraspecific variations holds a great importance in the invasion ecology. But, despite that, the number of studies evaluating ecological and evolutionary significance of intraspecific adaptations in invasive species are scarce (van Kleunen and Fischer, 2005; Oduor et al., 2016).
Intraspecific variations are represented by the altered phenotypic expressions of individuals of a species and usually rooted in the underlying metabolic and/or metabolomic changes. For instance, polymorphism in plant secondary metabolites is induced by the diversity in biochemical interactions between plants and their natural enemies (Moore et al., 2014). Genetically and environmentally actuated variations in accumulation, breakdown, and remobilization of primary metabolites can influence plant physiology and biomass allocation, which in turn affect the stress tolerance ability of a species (Aspinwall et al., 2015). For example, thermotolerance in coffee genotypes stems from the genetic variability that affects physiological and biochemical pathways (de Oliveira et al., 2020); heteroblasty in Pinus pinaster Aiton genotypes, resulting from the altered gene expressions and levels of specific metabolite accumulation, is responsible for their plasticity to carbon dioxide (de Simón et al., 2018). Examining the linkages between intraspecific variations occurring at the molecular, biochemical, and phenotypic levels is crucial for interpreting the mechanisms behind the plant ecological responses (Aspinwall et al., 2015).
Parthenium hysterophorus L. (ragweed parthenium; Asteraceae) is an obnoxious invasive weed of the tropical and subtropical regions that has occupied the non-native biomes across five continents (Kaur et al., 2021). The plant is previously known to reflect intraspecific variations in the morpho-functional traits (Kaur et al., 2019), physiological and biochemical attributes (Bajwa et al., 2017), reproductive biology (Hanif et al., 2012), phenology (Kaur et al., 2017), and phytotoxicity (Kaur et al., 2022). Prominent intraspecific variations have been noticed in the Australian individuals of P. hysterophorus, where these are classified into two different biotypes, i.e., Toogoolawah and Clermont, depending upon their history of introduction, invasion potential, distribution, and morpho-physiological traits (Bajwa et al., 2017; 2018). Diversity in the populations of P. hysterophorus has also been reported in South America, which is presumed to be an outcome of polyploidy and hybridization (Picman and Towers, 1982).
In India, phenotypic variations have been noticed in the population of P. hysterophorus in Chandigarh. Two morphotypes (tagged as PA and PB) were identified during the field surveys based on their distinct morpho-functional characteristics (Kaur et al., 2019). PA had larger leaf area, greater leaf biomass and higher content of chlorophyll as compared with PB. In contrast, PB had a larger stem circumference, greater stem specific density, higher twig dry matter content, profuse branching, bigger canopy, and better reproductive output as compared with PA (Kaur et al., 2019). Apart from that, chemical characterization of the morphotypes revealed that the allelochemical composition also varied between PA and PB (Kaur et al., 2022). It also led to the variable impact of these morphotypes on the associated vegetation (Kaur et al., 2019, 2022). Although these studies highlighted the presence of intraspecific variations in P. hysterophorus, they could not provide an appropriate explanation regarding the origin and basis of these variations.
In the present study, we propagated the morphotypes of P. hysterophorus (PA and PB) in an experimental screenhouse in two different seasons (winter and summer). Apart from the key morpho-functional traits, protein and carbohydrate metabolism was also studied in the leaves and roots of the morphotypes. The major objectives of the present study were a) to substantiate the persistence of intraspecific variations in the population of P. hysterophorus and b) to understand if the genesis of these variations is driven by hereditary factors and/or phenotypic plasticity.
2. Materials and methods 2.1. Experimental designA total of five study sites (Site Ⅰ–Ⅴ) were established in the peri-urban regions of Chandigarh, India (within 30°42′N‒30°45′N and 76°44′E−76°50′E) based on heavy infestations of P. hysterophorus and unevenness in the appearance of its individuals (Fig. 1). Sixty-four plots (10 × 10 m size) were set up in the five study sites (11–14 plots per site) at a minimum distance of 500 m from each other (Fig. 1). These were used for field investigations during our previous study, and it was established during that study that the individuals of P. hysterophorus in different plots differ in terms of their morpho-functional characteristics (Kaur et al., 2019). Based on morpho-functional variations observed under field conditions, the plots were categorized into two groups (PA and PB) using UPGMA hierarchical clustering, representing the two morphotypes of P. hysterophorus (Kaur et al., 2019; Fig. 1). Each site constituted a variable number of both PA and PB inhabiting plots (Fig. 1).

|
| Fig. 1 Graphical representation of the experimental design applied in the study. |
Fully-ripened seeds of the two morphotypes of P. hysterophorus were collected from the plots categorized as PA (n = 5–7 plots per site) and PB (n = 6–7 plots per site) as per Kaur et al. (2019) during July‒September 2017, when the plants were at their peak flowering stage. The collection was done randomly within a plot, without counting the number of individuals and number of seeds per individual; however, a Ziplock bag of size 8 × 6 cm was filled with the seeds from each plot. Since the site of collection, season of collection, and the phenological stage were kept the same for both the morphotypes, the influence of any physiological or genotypic factor on the seed biology was assumed to be negligible. However, soil samples collected from different study sites showed slight variations in the soil chemistry and nutrient composition (Table 1). Therefore, study sites were considered as random factors to account for the environmental variations at the time of seed collection. The seeds, collected from the plots inhabiting morphotype PA and PB, were pooled separately for each study site. These were air-dried for three days, followed by manual thrashing to separate out clean and healthy seed-sets of PA and PB. The seeds were subjected to dry storage for a period of 5–10 months as suggested by Tamado et al. (2002).
| pH | Phenolics (μg/mg) | OM (%) | N (kg/h) | P (kg/h) | K (kg/h) | Mg (%) | Ca (%) | |
| Plots invaded by morphotype PA | ||||||||
| Site Ⅰ | 7.82 ± 0.11a | 1.11 ± 0.06a | 3.69 ± 0.17a | 132.5 ± 2.26ab | 32.07 ± 2.07a | 203.00 ± 1.73a | 2.65 ± 0.03a | 1.73 ± 0.03a |
| Site Ⅱ | 7.91 ± 0.09a | 1.31 ± 0.02ab | 3.84 ± 0.24a | 94.08 ± 0.91c | 16.93 ± 1.68b | 55.50 ± 6.06b | 2.30 ± 0.40a | 2.63 ± 0.14b |
| Site Ⅲ | 7.66 ± 0.02a | 1.28 ± 0.04ab | 3.30 ± 0.01a | 159.15 ± 2.26b | 10.35 ± 0.78c | 75.50 ± 0.29b | 2.60 ± 0.12a | 2.53 ± 0.03b |
| Site Ⅳ | 7.66 ± 0.10a | 1.21 ± 0.06ab | 3.81 ± 0.08a | 116.81 ± 9.51ac | 8.60 ± 1.27c | 78.00 ± 0.01b | 2.40 ± 0.25a | 2.33 ± 0.03b |
| Site Ⅴ | 7.64 ± 0.08a | 1.48 ± 0.09b | 3.80 ± 0.19a | 94.86 ± 8.60c | 32.6 ± 0.52a | 55.50 ± 9.53b | 2.40 ± 0.17a | 2.63 ± 0.03b |
| Plots invaded by morphotype PB | ||||||||
| Site Ⅰ | 7.83 ± 0.08a | 1.22 ± 0.02a | 3.95 ± 0.07a | 137.21 ± 0.34a | 31.07 ± 1.05a | 226.50 ± 4.33a | 1.30 ± 1.53a | 1.33 ± 0.12a |
| Site Ⅱ | 8.11 ± 0.05b | 1.47 ± 0.13ab | 4.09 ± 0.05a | 108.97 ± 4.07a | 15.3 ± 2.61b | 81.00 ± 5.20b | 1.80 ± 0.21ab | 1.70 ± 0.15ab |
| Site Ⅲ | 7.72 ± 0.05a | 1.46 ± 0.02ab | 3.46 ± 0.02b | 282.24 ± 16.29b | 12.55 ± 0.43b | 160.50 ± 4.30c | 2.23 ± 0.15b | 1.50 ± 0.06ab |
| Site Ⅳ | 7.83 ± 0.07a | 1.47 ± 0.02ab | 3.93 ± 0.01a | 118.48 ± 5.94a | 7.03 ± 1.51b | 87.00 ± 1.73b | 2.27 ± 0.03b | 1.83 ± 0.15ab |
| Site Ⅴ | 7.82 ± 0.03a | 1.68 ± 0.07b | 3.98 ± 0.04a | 108.19 ± 0.91a | 30.73 ± 4.74a | 147.00 ± 5.20c | 1.57 ± 0.09a | 2.00 ± 0.17b |
A screenhouse experiment was set up twice in two contrasting growth seasons, i.e., winter (date of transplantation: 2nd Jan 2018) and summer (date of transplantation: 2nd May 2018) (Fig. 1). In each season, seeds were sown in 10 plastic trays (five trays per morphotype with each tray representing one study site) and after the emergence of two true leaves, the seedlings were transplanted in earthenware pots (diameter: 25 cm, depth: 22 cm) in a screenhouse established at Department of Botany, Panjab University, Chandigarh, India. Initially, in each season, a total of 100 pots (50 pots per morphotype with 10 pots representing one study site) were maintained. Up to five plants per pot were maintained during the initial 30 days, and later reduced to one plant per pot to provide proper space to the growing plants. However, a few plants could not survive until the flowering stage and therefore, a maximum of 42 (9, 8, 9, 7, and 9 pots for site Ⅰ, Ⅱ, Ⅲ, Ⅳ, and Ⅴ) and 48 pots (9, 10, 10, 9, and 10 pots for site Ⅰ, Ⅱ, Ⅲ, Ⅳ, and Ⅴ) per morphotype were available at the end of the study in winter and summer, respectively. The 1st harvest was carried out 30 DAT (days after transplantation) in both the seasons (n = 50) (Fig. 1), whereas the 2nd harvest was carried out when the plants attained a stage of maximum flowering (BBCH stage 65/605; Kaur et al., 2017), which took 180 DAT in winter (n = 42) and 80 DAT in summer (n = 48) (Fig. 1). Morpho-functional traits were studied only during the 2nd harvest, whereas biochemical traits were studied during both 1st and 2nd harvest. The meteorological data pertaining to temperature (T; ℃) and relative humidity (RH; %) during the study period was obtained from India Meteorological Department, Chandigarh, India.
2.2. Morpho-functional traitsSelected morpho-functional traits, i.e., stem circumference (SC; cm), number of higher order branches (HOB), number of capitula (Ncapitula), canopy cover (Ccover; cm2), stem specific density (SSD; mg mm−3), twig dry matter content (TDMC; mg g−1), leaf area (LA; cm2), total chlorophyll content (Tchl; μg mg−1 d. wt.), and biomass allocated to leaves (Bleaf; g), stem (Bstem; g), and capitula (Bcapitula; g) were measured in the individuals of PA and PB at the time of 2nd harvest during both the seasons (n = 42 in winter and n = 48 in summer). The traits were selected based on phenotypic differences recorded between the two morphotypes during the field studies (Kaur et al., 2019).
SC was determined by measuring the length of thread used to encompass circumference of the plant stem at 0.5 cm above the ground level. Total number of branches originating from the main stem and total number of capitula produced by the plants were counted. Ccover was estimated as the area of the ground covered by vertical projections of the outermost boundary of plant foliage. SSD was calculated by dividing the oven dried mass of the stem section from its volume which was measured when the stem section was fresh (Cornelissen et al., 2003). TDMC was measured as the ratio of biomass of the terminal twig of a plant to its fresh mass (Cornelissen et al., 2003). LA was measured by scanning the leaf and analyzing the image using ImageJ software version k 1.45 (Cornelissen et al., 2003).
For the estimation of Tchl, methodology provided by Hiscox and Israelstam (1979) was used. Twenty-five milligrams of fresh leaf sample were incubated in 4 ml of dimethyl sulphoxide (DMSO) at 60 ℃ for 1 h. Thereafter, the absorbance of the solution was read on Shimadzu UV-1800 double beam spectrophotometer at 645 nm and 663 nm taking DMSO as blank. Tchl was calculated as per following equation (Arnon, 1949):
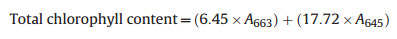
|
where A663 and A645 are the absorbance at 663 nm and 645 nm, respectively. The final values were expressed on a dry weight basis (μg mg−1 d. wt.) using dry weight equivalents of each sample as suggested by Rani and Kohli (1991). For obtaining the dry weight/biomass, samples were oven dried at 60 ℃ for 72 h.
2.3. Protein and carbohydrate metabolismTo measure differences at the biochemical levels, protein and carbohydrate metabolism were studied in the leaves and roots of two morphotypes of P. hysterophorus at the time of 1st (n = 50 for both winter and summer) and 2nd harvest (n = 42 in winter and n = 48 in summer) in both the seasons.
2.3.1. Total water-soluble protein content (TPC)TPC was estimated as per the method given by Bradford (1976). The plant extract was prepared by homogenizing 100 mg of the fresh plant tissue (leaf/root) in 10 ml of distilled water followed by centrifugation at 15, 000×g for 20 min at 4 ℃ in a cold centrifuge (Sigma Inc., USA). The reagent mixture was prepared by mixing 0.1 g of Coomassie brilliant blue dye, 50 ml of ethanol, 100 ml of ortho-phosphoric acid, and 50 ml of distilled water. The solution, when properly mixed, was further diluted four times using distilled water and filtered through Whatman filter paper #1. For protein estimation, 5 ml of the reagent were added to 1 ml of the plant extract. The absorbance of the reaction mixture was read at 595 nm against a standard of bovine serum albumin, and the content of protein was expressed as μg mg−1 f. wt.
2.3.2. ProteasesThe activity of proteases was determined based on the protocol given by Basha and Beavers (1975). For the extraction of proteolytic enzymes, 100 mg of the fresh plant tissue (leaf/root) were homogenized in 10 ml of 0.1 M sodium phosphate buffer (pH = 7) and the homogenate was centrifuged in a cold centrifuge at 15, 000×g for 25 min at 4 ℃. Reagent A was prepared by dissolving 1% casein into 0.1 M phosphate buffer (pH = 6) and reagent B was prepared by mixing solutions (ⅰ) 2% sodium carbonate in 0.1 N sodium hydroxide and (ⅱ) 0.5% of copper sulphate (CuSO4.5H2O) in 1% sodium citrate in the ratio of 50:1 (v/v). For the determination of proteases, 1 ml of reagent A was added to 1 ml of the plant extract, and the reaction mixture was incubated at 37 ℃ for an hour. Then, 2 ml of 15% trichloroacetic acid were added to precipitate the proteins. The reaction mixture was centrifuged at 10, 000×g for 5 min to release amino acids in the supernatant. Thereafter, 1 ml of this supernatant was assayed as per Bradford (1976). The activity of proteases was calculated and expressed as μg h−1 mg−1 protein.
2.3.3. Total water-soluble carbohydrate content (TCC)TCC was determined using the method described by Loewus (1952). One hundred milligrams of the fresh plant tissue (leaf/root) were macerated in 10 ml of distilled water and the homogenate was centrifuged in a cold centrifuge at 20, 000×g for 15 min at 4 ℃. A reagent was prepared by dissolving 0.2% anthrone in concentrated sulphuric acid (w/v). For determining carbohydrate content, 4 ml of the reagent were added to 1 ml of the plant extract with continuous shaking. Thereafter, the reaction mixture was boiled for 20 min until turned brownish yellow in color. It was allowed to cool down for a while, and then its absorbance was read at 620 nm against glucose as standard. The content of carbohydrates was expressed as μg mg−1 f. wt.
2.3.4. α- and β-AmylasesThe activities of α- and β-amylases were determined using the method given by Mahajan et al. (2013). One hundred milligrams of the fresh plant tissue (leaf/root) were homogenized in 10 ml of 0.1 M sodium phosphate buffer (pH = 7). The homogenate was centrifuged in a cold centrifuge at 15, 000×g for 25 min at 4 ℃.
For assaying a-amylases activity, Reagent A was prepared by boiling 150 mg of soluble starch, 600 mg of potassium di-hydrogen phosphate, and 20 mg of anhydrous calcium chloride in 100 ml of distilled water for 1 min. The solution was cooled, filtered, and stored in a refrigerator at 4 ℃. Reagent B was prepared by adding 25.4 mg iodine and 0.4 g of potassium iodide in 100 ml of distilled water. The reaction mixture contained 0.5 ml of extract, 1 ml of reagent A, and incubated for 30 min at room temperature. To this, 1 ml of 0.1 M EDTA was added. Thereafter, 0.2 ml of this reaction mixture was added with 3 ml of reagent B with vigorous shaking. The absorbance of the solution was read at 630 nm against starch as standard. The activity of α-amylases was calculated in terms of leftover starch and expressed as μg min−1 mg−1 protein.
For assaying β-amylases activity, Reagent A was prepared by dissolving 0.2 g of soluble starch in 0.067 M phosphate buffer (pH = 6) and Reagent B was prepared by adding 2.5 g dinitrosalicylic acid in 50 ml of distilled water. Once dissolved completely, it was added with 4 g of sodium hydroxide and 75 g of sodium potassium tartrate and the final volume was made 250 ml with distilled water. The reaction mixture consisted of 0.5 ml of extract, 0.7 ml of reagent A and 0.1 ml of 0.1 M EDTA, and incubated at 30 ℃ for 30 min. Then, 1 ml of reagent B was added to terminate the reaction and the reaction mixture was boiled for 20 min in a water bath. After letting it cool down, 3 ml of distilled water were added. The yellow-orange color of the solution was read at 560 nm against maltose as standard. The activity of β-amylases was calculated in terms of maltose units and expressed as μg min−1 mg−1 protein.
2.4. Statistical analysesGeneralized Linear Mixed Model (GLMM) was applied to test the effects of fixed factors, i.e., parent morphotypes, growth season, and their interaction (morphotype × season) and random factor, i.e., study sites on all the studied parameters. Tukey's post hoc analysis was used to determine the significance levels of differences observed in the four sets of observations (Winter PA, Winter PB, Summer PA, and Summer PB). Shapiro–Wilk and Levene's test were used to determine the normal distribution and equal variances in the data, respectively. The analyses were carried out in SPSS ver. 16.0 and Minitab ver. 21.1.1 and the significance of results was checked at p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001. A standardized principal component analysis (PCA) was also performed using factoextra and ggbiplot package in R v.4.0.3 to validate trait responses under different sets of observations.
3. Results 3.1. Growth conditions during the study periodThe two seasons depicted remarkable differences in growth conditions at the time of transplantation and early growth (Fig. 1). The average T and RH recorded at the time of transplantation during winter were 14.6 ℃ and 88% and during summer were 32.1 ℃ and 21% (Fig. 1). At the time of 1st harvest, the average T was around 13.8 ℃ and 32.2 ℃ during winter and summer, respectively, whereas average RH was recorded to be nearly 92% in winter and 48% in summer (Fig. 1). However, for the period of 2nd harvest the growth conditions were nearly similar and a minor difference of 5.2 ℃ and 6% was recorded between the average T and RH observed during winter and summer, respectively, at the time of 2nd harvest (Fig. 1).
3.2. Morpho-functional and biochemical differences in the morphotypes of Parthenium hysterophorusGLMM tested the effects of fixed factors, i.e., parent morphotypes (P [PA, PB]), growth seasons (S [winter, summer]) and their interaction (P × S) and random factor, i.e., study sites on all the parameters. Morpho-functional parameters were significantly affected by the parent morphotypes (P) except in case of TDMC, LA, Bleaf, and Bstem (Table 2). On the contrary, the effect of growth seasons (S) was highly significant on all the parameters (Table 2). Further, the interaction of parent morphotype and season (P × S) significantly affected several growth parameters, i.e., Ncapitula, SSD, TDMC, LA, Tchl, and Bcapitula (Table 2). In contrast, study sites did not affect any of the parameters significantly. SC and Ncapitula varied significantly between PA and PB in winter; however, no variation was noticed in any parameter between PA and PB in summer (Table 2). HOB, Ccover, LA, Tchl, and Beaf were found to differ significantly between the two seasons, irrespective of the parent morphotype (Table 2). SC and Ncapitula differed between winter PA and summer PA and PB, whereas these parameters in the individuals of winter PB differed only from summer PB (Table 2). Similarly, in case of SSD, winter PA and PB differed from summer PA but not from summer PB (Table 2), whereas in Bstem winter PA and PB differed from summer PB only. On the other hand, TDMC varied only between winter PB and summer PA and PB and Bcapitula was dissimilar only between winter PA and summer PA and PB (Table 2).
| Winter | Summer | Effect | ||||||
| PA | PB | PA | PB | Fixed factors | Random factor | |||
| Stem circumference (SC; cm) | 1.53 ± 0.15a | 1.68 ± 0.05b | 2.05 ± 0.06bc | 2.15 ± 0.12c | P** | Sitesns | ||
| S*** | ||||||||
| P × Sns | ||||||||
| Number of higher order branches (HOB) | 27.0 ± 3.65a | 21.0 ± 3.18a | 37.2 ± 2.4b | 33.8 ± 3.12b | P** | Sitesns | ||
| S*** | ||||||||
| P × Sns | ||||||||
| Number of capitula (Ncapitula) | 2101.8 ± 198.6a | 3449.0 ± 140.6b | 4078.6 ± 396.1bc | 4768.6 ± 291.3c | P*** | Sitesns | ||
| S*** | ||||||||
| P × S** | ||||||||
| Canopy cover (Ccover; cm2) | 37.16 ± 2.05a | 33.52 ± 2.46a | 77.88 ± 5.37b | 71.04 ± 4.32b | P*** | Sitesns | ||
| S*** | ||||||||
| P × Sns | ||||||||
| Stem specific density (SSD; mg mm−3) | 0.07 ± 0.01a | 0.07 ± 0.01a | 0.15 ± 0.01b | 0.11 ± 0.01ab | P*** | Sitesns | ||
| S*** | ||||||||
| P × S*** | ||||||||
| Twig dry matter content (TDMC; mg g−1) | 254.32 ± 6.60ab | 246.52 ± 8.49a | 279.84 ± 7.13b | 286.46 ± 3.30b | Pns | Sitesns | ||
| S*** | ||||||||
| P × S* | ||||||||
| Leaf area (LA; cm2) | 70.34 ± 4.88a | 63.76 ± 4.69a | 23.36 ± 2.04b | 28.26 ± 2.74b | Pns | Sitesns | ||
| S*** | ||||||||
| P × S*** | ||||||||
| Total chlorophyll content (Tchl; μg mg−1 d. wt.) | 8.52 ± 0.17a | 8.02 ± 0.10a | 7.37 ± 0.16b | 7.31 ± 0.19b | P*** | Sitesns | ||
| S*** | ||||||||
| P × S** | ||||||||
| Biomass allocated to leaves (Bleaf; g) | 6.78 ± 0.21a | 7.01 ± 0.25a | 4.36 ± 0.11b | 5.16 ± 0.18b | Pns | Sitesns | ||
| S*** | ||||||||
| P × Sns | ||||||||
| Biomass allocated to stem (Bstem; g) | 18.71 ± 0.56a | 22.54 ± 0.73a | 28.91 ± 1.05ab | 31.11 ± 1.45b | Pns | Sitesns | ||
| S*** | ||||||||
| P × Sns | ||||||||
| Biomass allocated to capitula (Bcapitula; g) | 6.89 ± 0.26a | 7.32 ± 0.31ab | 8.67 ± 1.23b | 8.23 ± 0.98b | P* | Sitesns | ||
| S** | ||||||||
| P × S* | ||||||||
Total water-soluble protein content and the activity of the proteolytic enzyme in the leaves and roots of P. hysterophorus were affected by the parent morphotype (P), seasons (S), and interaction between morphotypes and seasons (P × S) during the 1st harvest. However, during the 2nd harvest, only the effect of seasons (S) and interaction between morphotypes and seasons (P × S) was evident in case of the total protein content in roots (Fig. 2). The effect of study sites was insignificant on the protein metabolism of P. hysterophorus. A significant difference in the total protein content and activity of proteases was observed between winter (PA and PB) and summer (PA and PB) at the time of 1st harvest in both leaves and roots (Fig. 2). This difference was eliminated in the leaves during 2nd harvest, whereas in roots the total protein content still varied between winter PB and summer (PA and PB) (Fig. 2). However, no significant variation in the protein metabolism was noted between individuals of PA and PB within a season (Fig. 2).
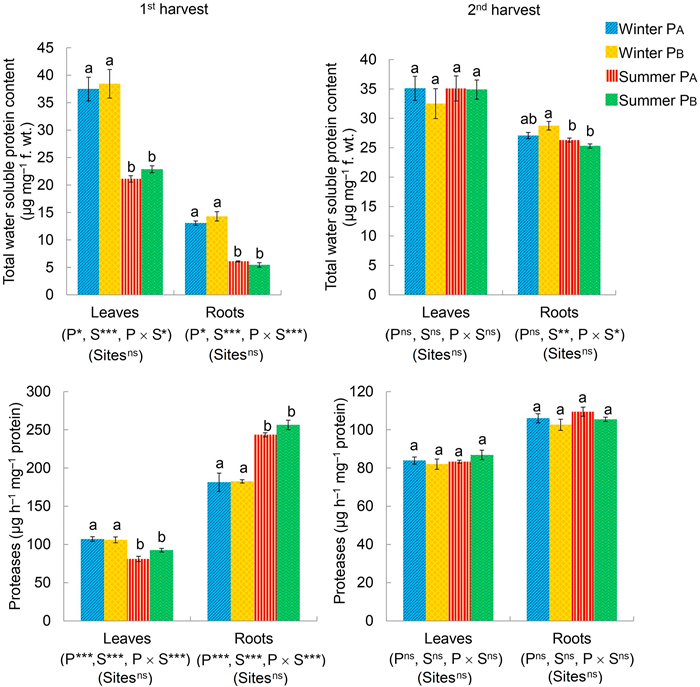
|
| Fig. 2 Total water-soluble protein content and the activity of proteases in Parthenium hysterophorus, measured at 1st (30 DAT [days after transplantation]) and 2nd (180 DAT in winter; 80 DAT in summer) harvest, as a function of fixed factors, i.e., parent morphotypes (P [PA, PB]), growth seasons (S [winter, summer]), and their interactions (P × S), and random factor, i.e., study sites (Sites) as determined by Generalized Linear Mixed Model (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; nsnon-significant). Dissimilar alphabets represent significant differences within the four sets of observations (Winter PA, Winter PB, Summer PA, and Summer PB) at p ≤ 0.05 using Tukey's post-hoc analysis. |
During the 1st harvest, the parent morphotypes (P) significantly affected total water-soluble carbohydrate content in the leaves, the activity of α-amylases in the roots, and the activity of β-amylases in both leaves and roots of P. hysterophorus (Fig. 3). Season of transplantation (S) affected the carbohydrate metabolism in leaves as well as roots during the 1st harvest (Fig. 3). Further, the interactions between morphotypes and seasons (P × S) affected the carbohydrate metabolism except for the activity of α-amylases in the roots of the plant at the time of 1st harvest (Fig. 3). During 2nd harvest, only the interaction (P × S) affected total carbohydrate content in the leaves of the plant. However, the study sites did not influence any of the parameters significantly. In the 1st harvest, carbohydrate metabolism varied between the two seasons in the leaves of P. hysterophorus, but in case of roots, a similarity was noted in total carbohydrate content between winter PA and summer PA and the activity of α-amylases between winter PA and summer PB (Fig. 3). No significant variation was, however, observed between the individuals of PA and PB within a season at the time of 1st harvest. During the 2nd harvest, total carbohydrate content in the leaves of individuals of winter PA varied from winter PB and summer PA, but no such variation was recorded in the sugar metabolizing enzymes (Fig. 3).
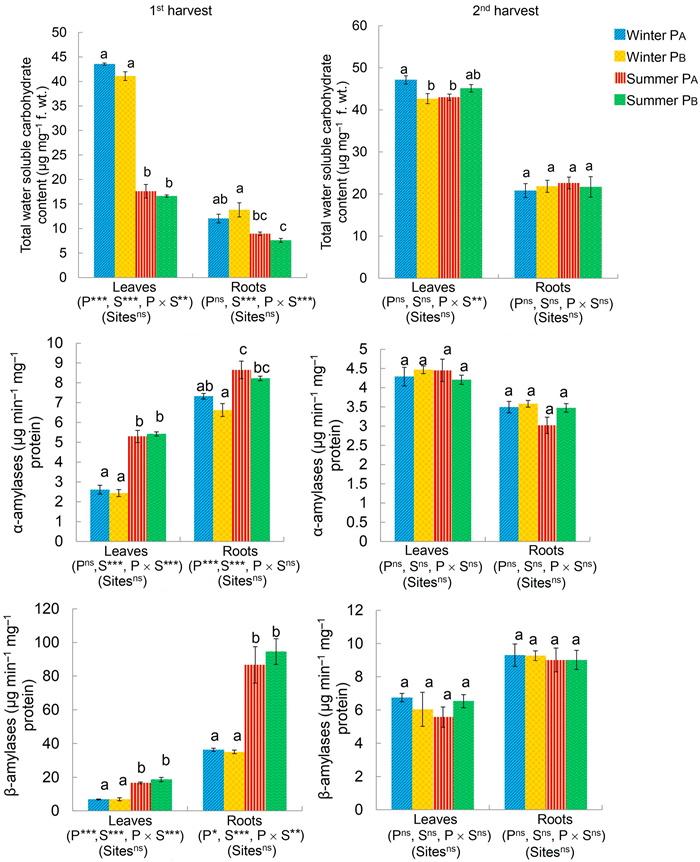
|
| Fig. 3 Total water-soluble carbohydrate content and the activity of α- and β-amylases in Parthenium hysterophorus, measured at 1st (30 DAT [days after transplantation]) and 2nd (180 DAT in winter; 80 DAT in summer) harvest, as a function of fixed factors, i.e., parent morphotypes (P [PA, PB]), growth seasons (S [winter, summer]), and their interactions (P × S), and random factor, i.e., study sites (Sites) as determined by Generalized Linear Mixed Model (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; nsnon-significant). Dissimilar alphabets represent significant differences within the four sets of observations (Winter PA, Winter PB, Summer PA, and Summer PB) at p ≤ 0.05 using Tukey's post-hoc analysis. |
Biochemical traits at 1st harvest, and biochemical and morpho-functional traits at 2nd harvest were individually analyzed via standardized PCA (Fig. 4). In both cases, the first two principal components (PC1 and PC2) explained the maximum variance in the dataset, i.e., 89.9%, and 46.6%, and, therefore, the results of PCA are presented as biplots (Fig. 4). The analysis deduced the interrelationship among morpho-functional and biochemical parameters and their response towards different combinations of parent morphotypes and seasons (Winter PA, Winter PB, Summer PA, and Summer PB) in a multi-trait space (Fig. 4). In agreement with the results of GLMM and Tukey's test, the confidence ellipses indicated a significant difference between winter (PA and PB) and summer (PA and PB), which were prominently separated across the biplot. Minor overlapping of the ellipses was observed within the seasons during both the harvests (Fig. 4). At the first harvest, total water-soluble protein and carbohydrate content were the maximum in winter-propagated seedlings, whereas the metabolizing enzymes were higher in the summer-propagated seedlings (Fig. 4a). Except for the Tchl, Bleaf, and LA, all the morpho-functional parameters favored the individuals propagated in summer season. On the contrary, biochemical traits at 2nd harvest were scattered across the biplot (Fig. 4b).

|
| Fig. 4 Biplots of standardized Principal Component Analysis (PCA) representing (a) biochemical trait responses at the 1st harvest, and (b) biochemical and morpho-functional trait responses at the 2nd harvest within the four sets of observations (Winter PA, Winter PB, Summer PA, and Summer PB). SC: stem circumference; HOB: number of higher order branches; Ncapitula: number of capitula; Ccover: canopy cover; SSD: stem specific density; TDMC: twig dry matter content; LA: leaf area; Tchl: total chlorophyll content; Bleaf: biomass allocated to leaves; Bstem: biomass allocated to stem; Bcapitula: biomass allocated to capitula; L_PC and R_PC: total water-soluble protein content in leaves and roots; L_P and R_P: activity of proteases in leaves and roots; L_CC and R_CC: total water-soluble carbohydrate content in leaves and roots; L_AA and R_AA: activity of α-amylases in leaves and roots; L_BA and R_BA: activity of β -amylases in leaves and roots. |
The present study was conducted to validate the presence of phenotypic disparities, in terms of morpho-functional and biochemical (protein and carbohydrate metabolism) traits, in the population of P. hysterophorus. The morphotypes of P. hysterophorus (PA and PB), identified under field conditions, were propagated in two contrasting growth seasons (winter and summer). Summer represented high temperature conditions and considered a favorable season for the growth and development of P. hysterophorus (since it is a tropical weed); whereas winter representing low temperature conditions, is considered an unfavorable growth period for the plant (Williams and Groves, 1980; Kohli and Rani, 1994). Low temperature conditions do not inhibit germination of P. hysterophorus (Tamado et al., 2002; Kaur et al., 2017); although, unlike summer, the plant undergoes a state of dormancy in rosette form (Williams and Groves, 1980; Batish et al., 2012; Kaur et al., 2017). Growth conditions at the time of transplantation and early growth strikingly varied between the two seasons (winter and summer), whereas these became stable at the time of plant maturation in both the seasons due to delayed flowering in winter transplanted seedlings.
Although significant differences were observed in the morpho-functional parameters in the individuals of P. hysterophorus, interestingly, most of these differences were observed between the individuals propagated in different seasons rather than between the individuals propagated from different parent morphotypes. Based on the observed differences, it can be speculated that the individuals propagated in winter were like morphotype PA (as seen from better leaf traits such as LA, Tchl, and Bleaf). However, those propagated in summer were analogous to morphotype PB (with greater SC, HOB, Ncapitula, Ccover, SSD, TDMC, and Bstem and Bcapitula). It was irrespective of the parent morphotype from which these were propagated. Parallel to the morpho-functional traits, biochemical traits also varied among the individuals harvested from different seasons. Total water-soluble protein and carbohydrate content were found to be the maximum in the seedlings harvested during winter. In contrast, the activity of protein and sugar metabolizing enzymes were the highest in the individuals propagated during summer. External factors and biotic/abiotic stresses regulate the protein and carbohydrate metabolism in a plant (Lloyd et al., 2005; Streb and Zeeman, 2012; Ghosh and Xu, 2014; Yue et al., 2019), which also include seasonal shifts and temperature fluctuations (Koch, 1996; Schaberg et al., 2000; Anderson et al., 2005; He et al., 2005; Impa et al., 2020).
The most notable observation was that the biochemical variations were evident only during early growth of the plant, whereas morpho-functional variations persisted in the mature individuals of P. hysterophorus. T/RH based fluctuations at the time of early growth resulted in metabolic changes in P. hysterophorus. These physiological and biochemical alterations may have regulated the growth and development of a plant species in the long run (Caruso et al., 2005; Roche et al., 2019). It can, therefore, be concluded that the environmental conditions at the time of germination/transplantation and early development regulate growth related traits and phenotype of P. hysterophorus. Previously, it has been reported that the germination season can have a strong effect on life history traits, and the seasonal changes at the time of germination/early growth affect growth cycle, morphology, resource allocation patterns, and seed biology in a plant species (Lu et al., 2016; Domingos and Bilsborrow, 2021). In Lactuca serriola L., individuals germinating in the winter season produced more seeds compared with plants germinated in other seasons (Marks and Prince, 1981). By varying the seasons of germination, not only the phenotypic expression of important life-history characteristics was modulated in Arabidopsis thaliana (L.) Heynh., but the mode of natural selection was also altered (Donohue, 2002). Similar variations have also been noticed in characteristics of several other plant species in response to season of germination; for example, in Dimorphotheca sinuate DC., Ursinia calenduliflora (DC.) N.E.Br., Heliophila pendula Willd. (van Rooyen et al., 1992), Diplotaxis erucoides (L.) DC. (Sans and Masalles, 1994), Amaranthus retroflexus L., Chenopodium glaucum L. (Zhou et al., 2005), Thlaspi arvense L. (Saarinen et al., 2011), Isatis violascens Bunge (Lu et al., 2016), and Fagopyrum esculentum Moench (Domingos and Bilsborrow, 2021). The trait differentiation in some of these species was found to be associated with the duration of rosette stage (Marks and Prince, 1981; Donohue, 2002; Lu et al., 2016). In the present study, prolonged duration of rosette stage in winter might have influenced the growth and development patterns of P. hysterophorus.
4.2. Factors regulating intraspecific variations in Parthenium hysterophorusAn attempt has also been made to assess a sound basis of the observed intraspecific variations in P. hysterophorus through this study. Both inherent, i.e., parent morphotypes (P [PA, PB]) and environmental factors, i.e., seasons (S [winter, summer]) were taken into consideration. The independent effects of these factors as well as the effect of their interaction (P × S) were studied on the morpho-functional traits and biochemical aspects (protein and carbohydrate metabolism) of the plant. In addition, the effect of study sites used for the collection of seeds of P. hysterophorus was also assessed. It can be summarized from the results of GLMM and PCA that environmental factor (S) had the most pronounced effect on all the studied parameters. In contrast, parent morphotypes (P) and interactions between the fixed factors (P × S) significantly affected some of the morpho-functional traits and biochemical parameters. This suggests that a combination of inherent and environmental factors may regulate phenotypes of P. hysterophorus. On the contrary, study sites used for the seed collection did not impart a significant effect on any of the morpho-functional and biochemical parameter.
Phenotypic traits usually change plastically in response to the environment, but very often these changes are underlain by alteration of gene expression (Moore et al., 2014; Chevin et al., 2022). This results in a genotype × environment (G × E) interaction, which can be viewed as genetic variation in reaction norms and can sometimes cause genetic variance to differ among environments (Moore et al., 2014). Relating gene expression plasticity to phenotypic plasticity may provide useful information about which genes influence phenotypic characteristics, even in the absence of genetic variation (Chevin et al., 2022). In addition, the phenomena of plasticity and genetic differentiation are not mutually exclusive and in certain species, both are proposed to act synergistically for promoting invasiveness. Composite interactions between adaptive and plastic responses drive the persistence and spread of certain aggressive invasive species such as Acer sp. (Lamarque et al., 2015), Bromus tectorum L. (Hufft and Zelikova, 2016), Alliaria petiolata (M.Bieb.) Cavara & Grande (Blossey et al., 2017), and Chromolaena odorata (L.) R.M.King and H. Rob (Liao et al., 2020). Spartina alterniflora Loisel., an invasive perennial grass found in marshy ecosystems, has developed ecotypes through interplay of genetic and plastic factors in response to inconsistent edaphic environment (Anderson and Treshow, 1980).
The role of phenotypic plasticity in imparting invasive character to P. hysterophorus has already been established via several studies (Navie et al., 1996; Annapurna and Singh, 2003; Kadam et al., 2009; Tanveer et al., 2015; Rathee et al., 2021). Comparison drawn between its biotypes identified in Australia (Clermont and Toogoolawah) also showed variations in its invasion potential, germination ecology, and stress-tolerance ability (Bajwa et al., 2018). Plasticity in P. hysterophorus helps overcoming environmental constraints, consequently facilitating its expansion towards previously uninhabited ranges. Enhanced reproductive fitness aids the elevational range expansion of the weed (Rathee et al., 2021). Despite studies reporting biochemical and proteomic alterations in P. hysterophorus in response to abiotic stress factors (Ahmad et al., 2017; Bajwa et al., 2017), marker-based metabolomic analyses that could reveal the variations at genetic level are lacking. The present study infers that a combination of inherent and environmental factors might be responsible for producing phenotypic variability in the population of P. hysterophorus. Nevertheless, further studies are required to validate the involvement of genetic factors and to assess the evolutionary importance of these intraspecific variations. Phenotypic variability in P. hysterophorus is often studied under limited study systems with limited sources of genotypic and environmental variations, and therefore, plant populations collected over a broad geographical area and those including large sample sizes might provide better insights into the complex ecological responses of P. hysterophorus.
5. ConclusionsThe analyses revealed that the contrasting growth conditions at the time of transplantation and early growth may regulate the phenotype of P. hysterophorus. The pattern of intraspecific variations observed during the study is justified to consider morphotype PA as winter biotype and morphotype PB as summer biotype of P. hysterophorus. The study points towards the role of plasticity or a combination of genetic and environmental (G × E) factors in producing the phenotypic variability observed in the population of P. hysterophorus.
A significant implication of high intraspecific variation is the ability of a species to evolve rapidly. Earlier, environmental variance was assumed to be a steady feature; however, of late, research has explored the possibilities of both natural and artificial selection of plastic responses. Trait variance linked to environmental changes can evolve and may respond more strongly to selection than the mean trait values (Moore et al., 2014). In fact, it is becoming increasingly clear that phenotypic plasticity can provide fitness and act as a buffer against environmental changes (Aspinwall et al., 2015). Moreover, within-species variations in ecological responses allow species coexistence in a variety of plant communities, which is a prerequisite for finding niche space (Moore et al., 2014). As climate change is affecting both the biotic and abiotic environments, interactions between genes and the environment could determine threshold of a species' response to key environmental drivers and availability of the genetic variance needed to adapt to new environments (Moore et al., 2014; Aspinwall et al., 2015).
These implications are even crucial in case of invasive species, which tend to respond more strongly to the heterogeneous environmental conditions. Invasiveness of a species is specifically augmented if intraspecific variability conferred a fitness advantage only to an invader and not to the associated native or naturalized species (Ferrero et al., 2022). Understanding the origin and magnitude of trait variations in invasive species, particularly under diverse environmental and geographical regimes, is very critical. The series of information in this direction may help in drawing important conclusions about invasive strategies of a species, devising better management approaches, and supplementing the efficacy of the ongoing management policies. At the same time, insights provided by such studies will upgrade the fundamental knowledge about ecological and evolutionary processes in invasive species.
AcknowledgementsAK is thankful to University Grants Commission (UGC), New Delhi, India, for the research fellowship.
Author contributions
DRB and SK conceived the idea for this study. DRB and AK designed the study. AK conducted the experiments, analyzed the data. SK, HPS, and DRB edited the earlier versions of the manuscript. All authors interpreted results and contributed to the final draft of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Agrawal, A.A., 2001. Phenotypic plasticity in the interactions and evolution of species. Science, 294: 321-326. DOI:10.1126/science.1060701 |
Ahmad, J., Bashir, H., Bagheri, R., et al., 2017. Drought and salinity induced changes in ecophysiology and proteomic profile of Parthenium hysterophorus. PLoS One, 12: e0185118. DOI:10.1371/journal.pone.0185118 |
Anderson, C.M., Treshow, M., 1980. A review of environmental and genetic factors that affect height in Spartina alterniflora Loisel. (Salt marsh cord grass). Estuaries, 3: 168-176. DOI:10.2307/1352066 |
Anderson, J.V., Gesch, R.W., Jia, Y., et al., 2005. Seasonal shifts in dormancy status, carbohydrate metabolism, and related gene expression in crown buds of leafy spurge. Plant Cell Environ., 28: 1567-1578. DOI:10.1111/j.1365–3040.2005.01393.x |
Annapurna, C., Singh, J.S., 2003. Variation of Parthenium hysterophorus in response to soil quality: implications for invasiveness. Weed Res., 43: 190-198. DOI:10.1046/j.1365-3180.2003.00332.x |
Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol., 24: 1-15. DOI:10.1104/pp.24.1.1 |
Aspinwall, M.J., Loik, M.E., Resco de Dios, V., et al., 2015. Utilizing intraspecific variation in phenotypic plasticity to bolster agricultural and forest productivity under climate change. Plant Cell Environ., 38: 1752-1764. DOI:10.1111/pce.12424 |
Bajwa, A.A., Chauhan, B.S., Adkins, S., 2017. Morphological, physiological and biochemical responses of two Australian biotypes of Parthenium hysterophorus to different soil moisture regimes. Environ. Sci. Pollut. Res., 24: 16186-16194. DOI:10.1007/s11356-017-9176-1 |
Bajwa, A.A., Chauhan, B.S., Adkins, S.W., 2018. Germination ecology of two Australian biotypes of ragweed parthenium (Parthenium hysterophorus) relates to their invasiveness. Weed Sci., 66: 62-70. DOI:10.1017/wsc.2017.61 |
Basha, S.M.M., Beevers, L., 1975. The development of proteolytic activity and protein degradation during the germination of Pisum sativum L. Planta, 124: 77-87. DOI:10.1007/BF00390070 |
Batish, D.R., Kohli, R.K., Singh, H.P., et al., 2012. Biology, ecology and spread of the invasive weed Parthenium hysterophorus in India. In: Bhatt, J.R., Singh, J.S., Singh, S.P., Tripathi, R.S., Kohli, R.K. (Eds.), Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent. CAB International, UK, pp. 10–18.
|
Blossey, B., Nuzzo, V., Dávalos, A., 2017. Climate and rapid local adaptation as drivers of germination and seed bank dynamics of Alliaria petiolata (garlic mustard) in North America. J. Ecol., 105: 1485-1495. DOI:10.1111/1365–2745.12854 |
Bolnik, D.I., Amarasekare, P., Araújo, M.S., et al., 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol., 26: 183-192. DOI:10.1016/j.tree.2011.01.009 |
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248-254. DOI:10.1016/0003-2697(76)90527-3 |
Bradshaw, A.D., 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet., 13: 115-155. |
Caruso, C.M., Maherali, H., Mikulyuk, A., et al., 2005. Genetic variance and covariance for physiological traits in Lobelia: are there constraints on adaptive evolution?. Evolution, 59: 826-837. |
Cornelissen, J.H.C., Lavorel, S., Garnier, E., et al., 2003. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot., 51: 335-380. DOI:10.1071/BT02124 |
Davidson, A.M., Jennions, M., Nicotra, A.B., 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett., 14: 419-431. DOI:10.1111/j.1461–0248.2011.01596.x |
de Oliveira, R.R., Ribeiro, T.H.C., Cardon, C.H., et al., 2020. Elevated temperatures impose transcriptional constraints and elicit intraspecific differences between coffee genotypes. Front. Plant Sci., 11: 1113. DOI:10.3389/fpls.2020.01113 |
de Simón, B.F., Cadahía, E., Aranda, I., 2018. Metabolic response to elevated CO2 levels in Pinus pinaster Aiton needles in an ontogenetic and genotypic-dependent way. Plant Physiol. Biochem., 132: 202-212. DOI:10.1016/j.plaphy.2018.09.006 |
Domingos, I.F.N., Bilsborrow, P.E., 2021. The effect of variety and sowing date on the growth, development, yield and quality of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Agron., 126: 126264. DOI:10.1016/j.eja.2021.126264 |
Donohue, K., 2002. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology, 83: 1006-1016. DOI:10.1890/0012-9658(2002)083[1006:GTINSO]2.0.CO;2 |
Ferrero, M.C., Tecco, P.A., Gurvich, D.E., 2022. Is intraspecific variability an advantage in mountain invasions? Comparing functional trait variation in an invasive and a native woody species along multiple environmental gradients. Biol. Invasions, 24: 1393-1412. DOI:10.1007/s10530-021-02722-1 |
Fox, R.J., Donelson, J.M., Schunter, C., et al., 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R. Soc. B, 374: 20180174. DOI:10.1098/rstb.2018.0174 |
Ghosh, D., Xu, J., 2014. Abiotic stress responses in plant roots: a proteomics perspective. Front. Plant Sci., 5: 6. |
, Ellner, S.P., Geber, M.A., et al., 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett., 8: 1114-1127. DOI:10.1111/j.1461–0248.2005.00812.x |
Hanif, Z., Adkins, S.W., Prentis, P.J., et al., 2012. Characterization of the reproductive behavior and invasive potential of parthenium weed in Australia. Pak. J. Weed Sci. Res., 18: 767-774. |
He, Y., Liu, X., Huang, B., 2005. Changes in protein content, protease activity, and amino acid content associated with heat injury in creeping bentgrass. J. Am. Soc. Hortic. Sci., 130: 842-847. DOI:10.21273/jashs.130.6.842 |
Hiscox, J.D., Israelstam, G.F., 1979. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot., 57: 1332-1334. DOI:10.1139/b79-163 |
Hufft, R.A., Zelikova, T.J., 2016. Ecological genetics, local adaptation, and phenotypic plasticity in Bromus tectorum in the context of a changing climate. In: Germino, M.J., Chambers, J.C., Brown, C.S. (Eds.), Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US: Causes, Consequences and Management Implications. Springer International publishing, Switzerland, pp. 133–154.
|
Impa, S.M., Vennapusa, A.R., Bheemanahalli, R., et al., 2020. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ., 43: 431-447. DOI:10.1111/pce.13671 |
Kadam, R.M., Dhavle, S.D., Allapure, R.B., et al., 2009. Evolution of phenological plasticity in Parthenium hysterophorus in response to air pollution stress and unordered environmental variation. Asian J. Environ. Sci., 3: 131-133. |
Kaur, A., Batish, D.R., Kaur, S., et al., 2017. Phenological behaviour of Parthenium hysterophorus in response to climatic variations according to the extended BBCH scale. Ann. Appl. Biol., 171: 316-326. DOI:10.1111/aab.12374 |
Kaur, A., Kaur, S., Singh, H.P., et al., 2019. Phenotypic variations alter the ecological impact of invasive alien species: lessons from Parthenium hysterophorus. J. Environ. Manag., 241: 187-197. DOI:10.1016/j.jenvman.2019.03.129 |
Kaur, A., Batish, D.R., Chauhan, B.S., et al., 2021. Parthenium hysterophorus. In: Chauhan, B.S. (Ed.), Biology and Management of Problematic Crop Weed Species. Academic Press, USA, pp. 311–333.
|
Kaur, A., Kaur, S., Singh, H.P., et al., 2022. Alterations in phytotoxicity and allelochemistry in response to intraspecific variation in Parthenium hysterophorus. Ecol. Complex., 50: 100999. DOI:10.1016/j.ecocom.2022.100999 |
Koch, K.E., 1996. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 47: 509-540. DOI:10.1146/annurev.arplant.47.1.509 |
Kohli, R.K., Rani, D., 1994. Parthenium hysterophorus- a review. Res. Bull. Panjab Univ. Sci., 44: 105-149. |
Lamarque, L.J., Lortie, C.J., Porté, A.J., et al., 2015. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees. Biol. Invasions, 17: 1109-1122. DOI:10.1007/s10530-014-0781-3 |
Liao, Z.Y., Scheepens, J.F., Li, Q.M., et al., 2020. Founder effects, post-introduction evolution and phenotypic plasticity contribute to invasion success of a genetically impoverished invader. Oecologia, 192: 105-118. DOI:10.1007/s00442-019-04566-y |
Lloyd, J.R., Kossmann, J., Ritte, G., 2005. Leaf starch degradation comes out of the shadows. Trends Plant Sci., 10: 130-137. DOI:10.1016/j.tplants.2005.01.001 |
Loewus, F.A., 1952. Improvement in anthrone method for determination of carbohydrates. Anal. Chem., 24: 219. DOI:10.1021/ac60061a050 |
Lu, J.J., Tan, D.Y., Baskin, C.C., et al., 2016. Effects of germination season on life history traits and on transgenerational plasticity in seed dormancy in a cold desert annual. Sci. Rep., 6: 25076. DOI:10.1038/srep25076 |
Mahajan, P., Singh, H.P., Batish, D.R., et al., 2013. Cr(Ⅵ) imposed toxicity in maize seedlings assessed in terms of disruption in carbohydrate metabolism.. Biol. Trace Elem. Res., 156: 316-322. DOI:10.1007/s12011-013-9806-5 |
Marks, M., Prince, S., 1981. Influence of germination date on survival and fecundity in wild lettuce Lactuca serriola. Oikos, 36: 326-330. DOI:10.2307/3544630 |
Moore, B.D., Andrew, R.L., Külheim, C., et al., 2014. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol., 201: 733-750. DOI:10.1111/nph.12526 |
Navie, S.C., McFadyen, R.E., Panetta, F.D., et al., 1996. A comparison of the growth and phenology of two introduced biotypes of Parthenium hysterophorus. In: Shepherd, R.H.C. (Ed.), Eleventh Australian Weeds Conference Proceedings. Weed Science Society of Victoria, Australia, pp. 313–316.
|
Nolting, K.M., Prunier, R., Midgley, G.F., et al., 2021. Intraspecific trait variation influences physiological performance and fitness in the South Africa shrub genus Protea (Proteaceae). Ann. Bot., 127: 519-531. DOI:10.1093/aob/mcaa060 |
Oduor, A.M., Leimu, R., van Kleunen, M., 2016. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J. Ecol., 104: 957-968. DOI:10.1111/1365–2745.12578 |
Picman, A.K., Towers, G.H.N., 1982. Sesquiterpene lactones in various populations of Parthenium hysterophorus. Biochem. Systemat. Ecol., 10: 145-153. DOI:10.1016/0305-1978(82)90021-7 |
Prentis, P.J., Wilson, J.R.U., Dormontt, E.E., et al., 2008. Adaptive evolution in invasive species. Trends Plant Sci., 13: 288-294. DOI:10.1016/j.tplants.2008.03.004 |
Rani, D., Kohli, R.K., 1991. Fresh matter is not an appropriate relation unit for chlorophyll content: experience from experiments on effects of herbicide and allelopathic substance. Photosynthetica, 25: 655-658. |
Rathee, S., Ahmad, M., Sharma, P., et al., 2021. Biomass allocation and phenotypic plasticity are key elements of successful invasion of Parthenium hysterophorus at high elevation. Environ. Exp. Bot., 184: 104392. DOI:10.1016/j.envexpbot.2021.104392 |
Richards, C.L., Bossdorf, O., Muth, N.Z., et al., 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett., 9: 981-993. DOI:10.1111/j.1461–0248.2006.00950.x |
Richardson, D.M., Pyšek, P., Rejmánek, M., et al., 2000. Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib., 6: 93-107. DOI:10.1046/j.1472-4642.2000.00083.x |
Roche, J., Mouloungui, Z., Cerny, M., et al., 2019. Effect of sowing dates on fatty acids and phytosterols patterns of Carthamus tinctorius L. Appl. Sci., 9: 2839. DOI:10.3390/app9142839 |
Saarinen, T., Lundell, R., Åström, H., et al., 2011. Parental overwintering history affects the responses of Thlaspi arvense to warming winters in the North. Environ. Exp. Bot., 72: 409-414. DOI:10.1016/j.envexpbot.2011.02.012 |
Sans, F.X., Masalles, R.M., 1994. Life-history variation in the annual arable weed Diplotaxis erucoides (Cruciferae). Can. J. Bot., 72: 10-19. DOI:10.1139/b94-003 |
Schaberg, P.G., Snyder, M.C., Shane, J.B., et al., 2000. Seasonal patterns of carbohydrate reserves in red spruce seedlings. Tree Physiol., 20: 549-555. DOI:10.1093/treephys/20.8.549 |
Schlichting, C.D., 1986. The evolution of phenotypic plasticity in plants. Annu. Rev. Ecol. Evol. Syst., 17: 667-693. DOI:10.1146/annurev.es.17.110186.003315 |
Shackleton, R.T., Shackleton, C.M., Kull, C.A., 2019. The role of invasive alien species in shaping local livelihoods and human well-being: a review. J. Environ. Manag., 229: 145-157. DOI:10.1016/j.jenvman.2018.05.007 |
Chevin, L.M., Leung, C., Le Rouzic, A., et al., 2022. Using phenotypic plasticity to understand the structure and evolution of the genotype–phenotype map. Genetica, 150: 209-221. DOI:10.1007/s10709-021-00135-5 |
Streb, S., Zeeman, S.C., 2012. Starch metabolism in Arabidopsis. Arabidopsis Book, 10: e0160. DOI:10.1199/tab.0160 |
Tamado, T., Schutz, W., Milberg, P., 2002. Germination ecology of the weed Parthenium hysterophorus in eastern Ethiopia. Ann. Appl. Biol., 140: 263-270. DOI:10.1111/j.1744–7348.2002.tb00180.x |
Tanveer, A., Khaliq, A., Ali, H.H., et al., 2015. Interference and management of parthenium: the world's most important invasive weed. Crop Protect., 68: 49-59. DOI:10.1016/j.cropro.2014.11.005 |
van Kleunen, M., Fischer, M., 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol., 166: 49-60. DOI:10.1111/j.1469–8137.2004.01296.x |
van Rooyen, M.W., Grobbelaar, N., Theron, G.K., et al., 1992. The ephemerals of Namaqualand: effect of germination date on development of three species. J. Arid Environ., 22: 51-66. DOI:10.1016/S0140-1963(18)30656-6 |
Williams, J.D., Groves, R.H., 1980. The influence of temperature and photoperiod on growth and development of Parthenium hysterophorus L. Weed Res., 20: 47-52. DOI:10.1111/j.1365–3180.1980.tb00040.x |
Yue, C., Cao, H., Lin, H., et al., 2019. Expression patterns of alpha-amylase and beta-amylase genes provide insights into the molecular mechanisms underlying the responses of tea plants (Camellia sinensis) to stress and postharvest processing treatments. Planta, 250: 281-298. DOI:10.1007/s00425-019-03171-w |
Zhao, Y., Yang, X., Xi, X., et al., 2012. Phenotypic plasticity in the invasion of crofton weed (Eupatorium adenophorum) in China. Weed Sci., 60: 431-439. DOI:10.1614/WS-D-11-00198.1 |
Zhou, D., Wang, T., Valentine, I., 2005. Phenotypic plasticity of life-history characters in response to different germination timing in two annual weeds. Can. J. Bot., 83: 28-36. DOI:10.1139/b04-148 |



