b. Geological Studies Unit, Indian Statistical Institute, 203, B.T. Road, Kolkata 700108, West Bengal, India;
c. Centre of Advanced Study, Department of Botany, University of Calcutta, 35, B.C. Road, Kolkata 700019, India
Horsetails, the unique survivors of a very ancient group of vascular plants, the Sphenophyta, have a history reaching back to the Upper Devonian and may represent the world's oldest fossil genus of living vascular plants (Stewart and Rothwell, 1993). Equisetum L. is the only living, free-sporing morphologically distinct genus of sphenophyte (PPG I, 2016). As the sole living representative of the ancient group of Sphenopsida (Hauke, 1963; Wu and Qin, 1991; Taylor and Taylor 1993; Taylor et al., 2009), fossil evidence of Equisetum has long been sought after by botanists and palaeontologists, as it may be a key to understanding the paleoenvironment.
Extant species of Equisetum are herbaceous in nature (small annual herbs) (Wu and Qin 1991; Zhang et al., 2007; Aung et al., 2020). These plants have unique morphological features, including articulated stems, with longitudinal ridges or furrows, enclosed within leaf sheaths (Taylor and Taylor, 1993). Some species, such as E. giganteum L. and E. myriochaetum Schltdl. & Cham., grow up to 5 and 8 m in height respectively (Hauke, 1963). They primarily grow in wet areas such as moist woods, ditches, wetlands, and road fill where sufficient groundwater is available (Hauke, 1990). Equisetum has a sub-cosmopolitan distribution, ranging from southern portions of South America and Africa to above the Arctic Circle, but no modern representative is found in Australia, New Zealand, or Antarctica (Scagel et al., 1984; Hauke, 1990). Present-day Equisetum species are also notably absent from the islands of the central Pacific, Indian, and South Atlantic oceans (Schaffner, 1930). The greatest concentration of Equisetum species is found between 40° and 60° North latitude.
Molecular studies have traditionally divided Equisetum into two sub-genera: Equisetum and Hippochaete (Hauke, 1963; Des Marais et al., 2003; Guillon, 2004; Zhang, 2004; Li, 2007; Zhang et al., 2007; Elgorriaga et al., 2018). The sub-genus Equisetum is characterised by wide, superficial, scattered, or two-more banded stomata; non-apiculate cones, and annual regularly branched stems, whereas sub-genus Hippochaete is characterised by sunken stomata arranged in single lines; apiculate cones and perennial unbranched stems (Hauke, 1990; Cullen and Rudall, 2016). Christenhusz et al. (2019) proposed a new sub-genus, Paramochaete Christenh. and Husby, based on phylogenetic studies of four plastid DNA regions.
Fossil Equisetales were herbaceous and had the same basic body plan as present-day horsetails (Behrensmeyer, 1992). Equisetopsida first appeared in the late Devonian and diversified during the Carboniferous period. However, during the Triassic Equisetum became less diverse and limited its herbaceous growth, likely due to the increasingly dry, arid to semi-arid conditions (Behrensmeyer, 1992). In the Jurassic, Equisetales habitats were decimated and plants became smaller (Schaffner, 1930). During the Cenozoic period, Equisetales again diversified, although the rapid rise of angiosperms and the success of ferns, cycads, and conifers in dry sites may have led to the decrease in Equisetum stem size and an absence of secondary growth (Schaffner, 1930; Koske et al., 1985; Stewart and Rothwell, 1993). The oldest fossil of the sub-genus Equisetum is E. bryanii Gould, which has well-preserved stems and leaf sheaths, from the Jurassic of south-eastern Queensland (Gould, 1968). Some fossilized tuberous rhizome parts of Equisetum have also been reported from Asia (Guo, 2000; Zhou et al., 2003; Sun et al., 2013; Yang et al., 2016; Deng et al., 2020), Europe (Denk et al., 2005), and North America (Skog and Dilcher, 1994).
To date, one fossil species of Equisetum (Equisetitis sahnii Borkar et al.) has been reported from the Decan Intertrappean Beds (Late Cretaceous to Early Palaeocene) of Madhya Pradesh, Central India (Borkar et al., 2017). Unfortunately, no reliable fossil evidence has been reported from the Cenozoic sediments of India. Mehrotra et al. (2009) have reported a fragmentary stem impression from the Oligocene sediments of Assam and compared them with the stem of modern Equisetum; but proper identification was not definitive. We have discovered Equisetum fossils in appreciable number (thirteen well-preserved impressed and compressed fossilized parts of the jointed stem with nodes and internodes and leaf sheaths) from the middle part of Siwalik (Late Miocene) sediments of Himachal Pradesh, the western Himalayas. Here, we describe a new fossil species of Equisetum based on morphological and epidermal characters, compare our fossil specimens with modern as well as earlier reported fossil species of Equisetum, review the Cenozoic fossil history of Equisetum in detail, and discuss the palaeoecological implications of our discovery. Our fossil finding constitutes the first recognition of Equisetum from Indian Cenozoic. Despite the rich paleobotanical heritage of the Siwalik period, our fossils are the only record of equisetaleans in Siwalik. This discovery also constitutes the youngest known Equisetum fossils from India.
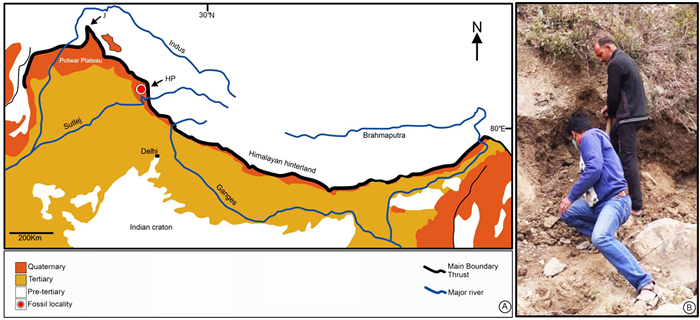
2. Materials and methods 2.1. Materials and localityThe fossil specimens (impression-compression of stems with nodes and internodes enclosed with leaf sheaths with parts and counterparts and an impression of strobilus) similar to Equisetum described in the present study were collected from the middle Siwalik beds exposed in a northeast roadside road cutting section (31°44′26″N, 76°43′33″E) near Sarkaghat town, which is located about 60 km from the district headquarters at Mandi District of Himachal Pradesh, western India (Fig. 1).
|
| Fig. 1 General location of Siwalik formation and fossiliferous exposure. (A) map showing the present fossil locality (after Brozovick and Burbank, 2000; HP—Himachal Pradesh reentrant, J—Jhelum reentrant); (B) a fossiliferous exposure from where fossil Equisetum specimens were recovered. |
The middle Siwalik succession has been dated to the Late Miocene (Brozovic and Burbank, 2000). About 8–10 km from our locality is the Nalad Khad section (31°46′N, 76°43′E), which is located on the western limb of the Sarkaghat anticline and in the Jawalamukhi thrust sheet. Brozovic and Burbank (2000) palaeomagnetically dated (~12–8 Ma) the Nalad Khad section.
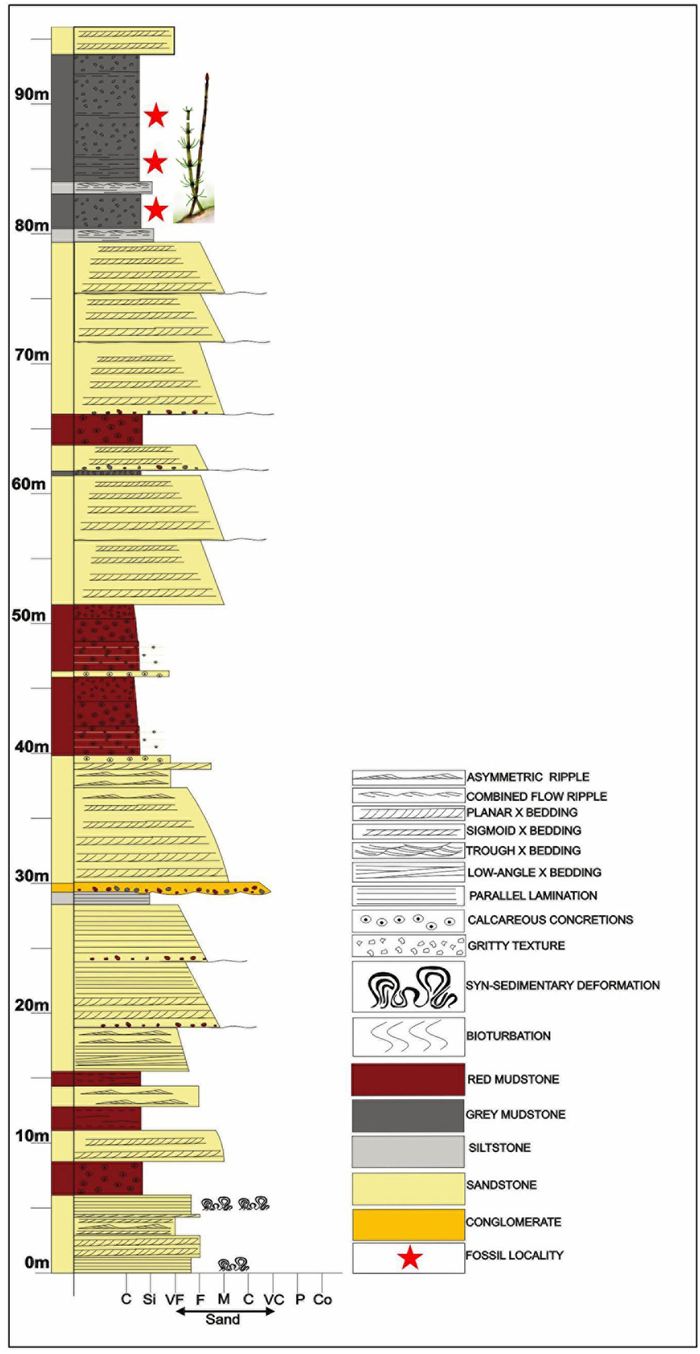
2.3. Sedimentary contextThe middle Siwalik succession essentially consists of thick medium to fine-grained sandstone bodies separated by red or grey mudstone beds (Fig. 2). A sharp erosive base of the fining-upward sandstone units, often marked by intraformational conglomerate layers and capped by 4–12 m thick red mudstone with calcareous paleosols indicates the fluvial origin of the middle Siwalik succession. The sandstones are 5–11 m thick, amalgamated to form sandstone bodies up to 40 m thick. The sandstone-red mudstone succession is interlayered with a few grey mudstone layers (14–22 m thick). The well-preserved fossil specimens of Equisetum are mostly recovered from these grey mudstones. About 300 m of the middle Siwalik succession were logged for details of the lithologic characters and to show the position of the fossil locality (Fig. 2).
|
| Fig. 2 The detailed sedimentological lithology of the middle Siwalik rocks exposed in the road-cutting section near Sarkaghat, Himachal Pradesh. |
Fossil specimens were recovered by manual laceration of large fossiliferous sedimentary rock with the aid of heavy sledgehammers and chisels. We cleaned specimens carefully with a wet paintbrush and extra flakes of sediments were scratched by a fine sharp needle. Fossil specimens were first observed with a hand lens and photographed by a digital camera (Canon EOS 1500D) (Figs. 3–9 and S1). We identified fossil specimens based on a detailed morphological study using an optical light microscope and epidermal characters using inverted fluorescence microscopy. The characteristic morphological details (e.g., ridges, furrows, leaf sheath, sheath leaf teeth) were observed on a stereo zoom microscope (Olympus SZX7; Maycam DC5 camera). Morphological characters were measured by ImageJ software (v.1.44p; National Institutes of Health, Bethesda, Maryland, USA). The ridges of the fossil stems bearing fine longitudinal striae, whorled leaf sheaths, the shape of teeth, and details were drawn in CorelDraw v.2021 and edited with Adobe Photoshop software (Figs. 3–9). The terminology for the morphological descriptions of fossil specimens follows Pole and McLoughlin (2017) and Chen et al. (2021). For descriptions of epidermal anatomy, we follow the terms of Thomasson (1980) and Brown (1977). We compared current fossils with modern species of Equisetum using digital herbarium catalogues, viz., (Kew herbarium catalogue. https://apps.kew.org/herbcat/gotoCiteUs.do. accessed on 28-08-2022), United States National Herbarium (PteridoPortal. 2022. https://www.pteridoportal.org. accessed on 30-08-2022; STRI Research Portal. https://panamabiota.org/stri/taxa/index. accessed on 02-09-2022), and the Florida Plant Atlas (http://florida.plantatlas.usf.edu. accessed on 04-09-2022) (Table S1). Author citation of a new plant fossil species is mainly after Punt (1994). The recovered fossil specimens (SKBU/PPL/HP/P/E1–21) are kept in the Department of Botany, Palaeobotany and Palynology Laboratory, Sidho-Kanho-Birsha University, Purulia, India.
|
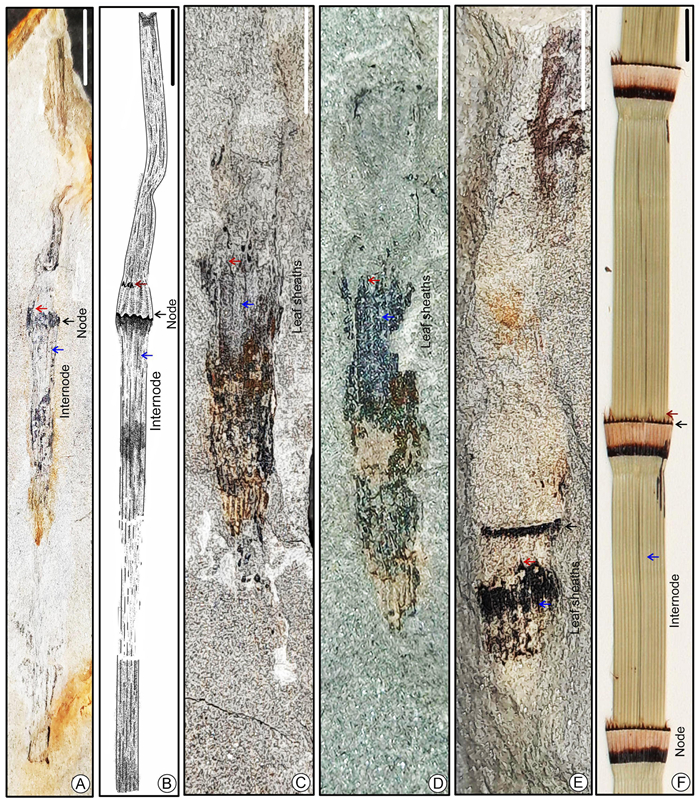
| Fig. 3 (A) A fossil stem species Equisetum siwalikum sp. nov. with distinct nodes and internodes (Holotype: SKBUH/PPL/HP/P/E1) (scale bar = 5 mm); (B) line drawing of holotype specimen; (C–E) another fossil specimens showing stem axis with a whorl of basely fused sheath leaves above a node (specimen numbers SKBUH/PPL/HP/P/E2, E3, E4); (F) Modern stem of Equisetum having similar nature of node, internode and leaf sheaths (scale bar = 5 mm) (ridges marked by blue arrows, leaf sheaths marked by red arrows and bulbous nodes marked by black arrows). |

|
| Fig. 4 (A) Enlarged view of a fossil stem of specimen SKBUH/PPL/HP/P/E4; (B) Line drawing of (A) showing acute triangle-shaped leaf teeth; (C) modern stem of Equisetum enclosed by similar nature of leaf sheaths (scale bar (A–C) = 5 mm); (D, E) enlarged view of specimen no. SKBUH/PPL/HP/P/E3; (F) line drawing of specimen SKBUH/PPL/HP/P/E2 (scale bar = 5 mm); (G, H) enlarged view of specimen no. SKBUH/PPL/HP/P/E4 and SKBUH/PPL/HP/P/E1 respectively (scale bar = 2 mm) (ridges marked by blue arrows, leaf sheaths marked by red arrows, and bulbous stem marked by black arrows). |

|
| Fig. 5 (A) Two fossil stem specimens of Equisetum consisting of characteristic ridges and furrows (SKBUH/PPL/HP/P/E5-6); (B, C) enlarged view of SKBUH/PPL/HP/P/E6; (D) line drawing of image C; (E) enlarged view of modern Equisetum showing jointed stem enclosed with leaf sheaths; (F) enlarged view of a portion of image C; (G) enlarged view a portion of modern Equisetum stem; (H) enlarged view of the fossilized bulbous node of specimen SKBUH/PPL/HP/P/E5; (I) line drawing of image H; (J) a portion of modern stem showing characteristic triangular leaf sheaths with acute tips; (scale bar = 10 mm) (ridges marked by yellow arrows and furrows marked by pink arrows, leaf sheaths marked by red arrows). |

|
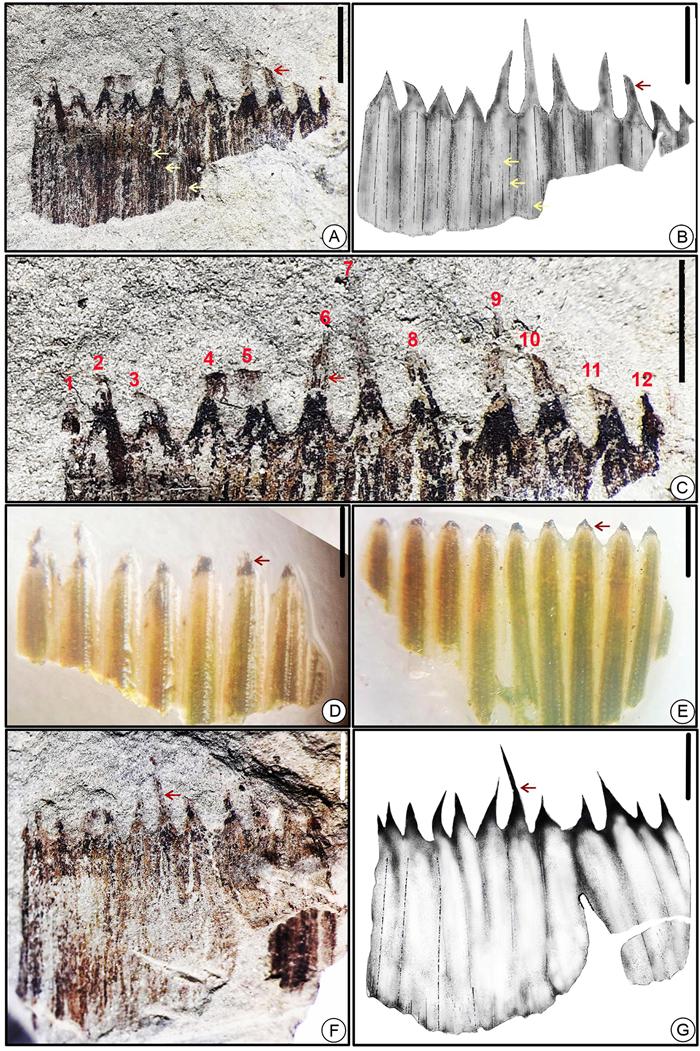
| Fig. 6 (A–E) Isolated leaf sheaths of fossil Equisetum stem specimens showing pointed acute-shaped teeth (SKBUH/PPL/HP/P/E7-11); (F) an impression of elongate-shaped strobilus showing hexagonal sporangiophores marked by black arrows (SKBUH/PPL/HP/P/E12); (G) strobilus of modern Equisetum showing sporangiophores marked by arrows; (H) seemingly compressed whorl of fertile stem sheath (SKBUH/PPL/HP/P/E13); (I) fertile stem sheath of modern horsetail; (scale bar = 2 mm) (leaf sheaths marked by red arrows). |
|
| Fig. 7 (A) Enlarged view of the leaf sheath of fossil Equisetum specimen SKBUH/PPL/HP/P/E10 showing apically pointed teeth and basally fused lamina; (B) line drawing of image A; (C) enlarged view of teeth of leaf sheath of image A; (D, E) enlarged view of leaf sheaths of modern Equisetum; (F) enlarged view of leaf sheath of fossil Equisetum (SKBUH/PPL/HP/P/E11); (G) line drawing of (F); (scale bar = 2 mm) (longitudinal striae marked by yellow arrows, leaf sheaths marked by red arrows). |
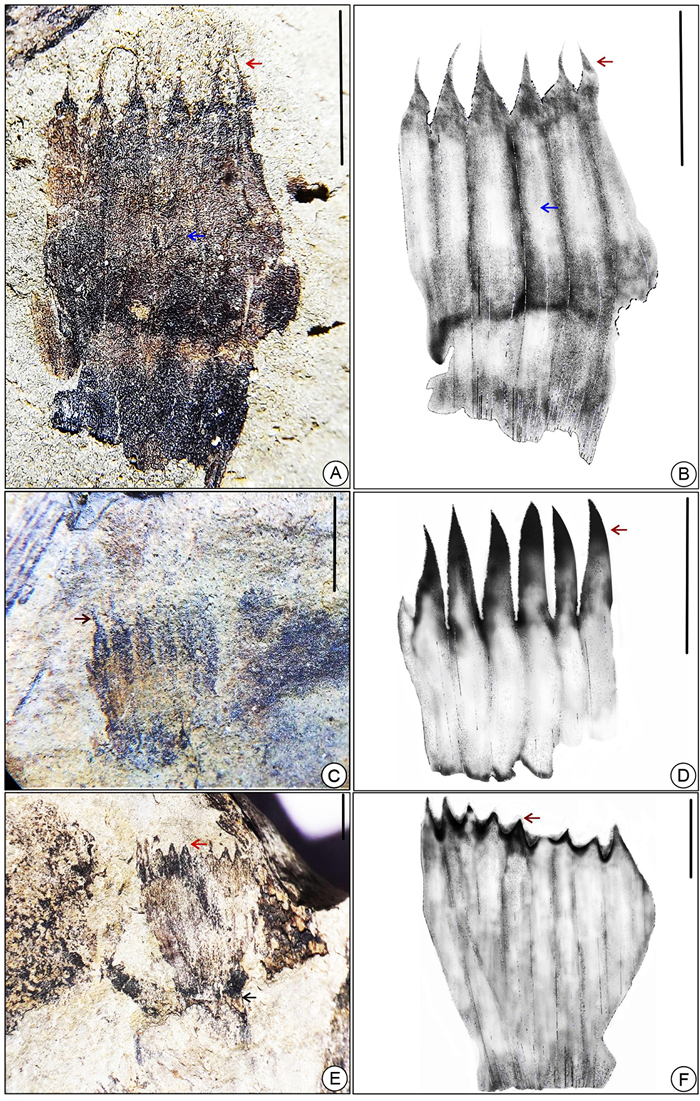
|
| Fig. 8 (A–F) Leaf sheaths of fossil Equisetum, (A) enlarged view of fossil specimen SKBUH/PPL/HP/P/E8; (B) line drawing of (A); (C) enlarged view of fossil specimen SKBUH/PPL/HP/P/E7; (D) line drawing of (C); (E) enlarged view of fossil specimen SKBUH/PPL/HP/P/E13, (F) line drawing of (E); (scale bar = 2 mm) (ridges mark by blue arrows, leaf sheaths mark by red arrows). |
|
| Fig. 9 (A) Fossil stems specimen SKBUH/PPL/HP/P/E9 showing whorl of basely fused leaf sheaths with pointed teeth (marked by red arrow) at the node; (B) enlarged view of (A); (C) line drawing of (A); (D) carbonaceous compression a portion of stem showing leaf sheaths enclosed at nodal region (SKBUH/PPL/HP/P/E14) (scale bar = 2 mm). |
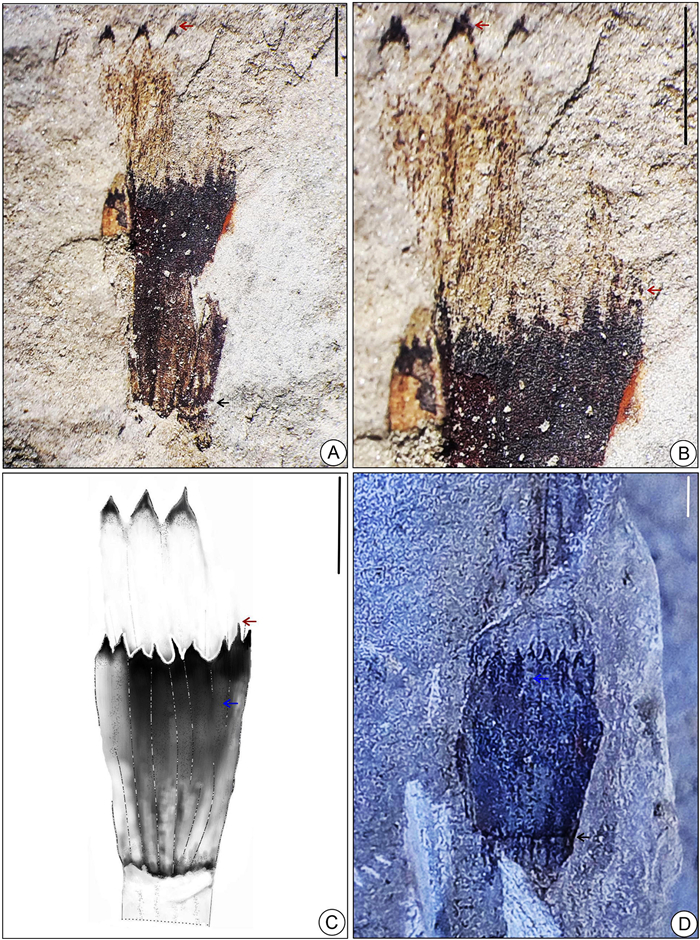
The fluorescence technique identifies the cuticular characters for the taxonomic identification of plant macrofossils (Hu et al., 2016) and has an advantage for fossil research because the fossil material does not need to be chemically treated and will not be damaged (Kerp, 1990). In our study, inverted fluorescence microscopy was used to investigate the epidermal features (epidermal cells and stomata) of compressed leaf sheath specimens (SKBU/PPL/HP/P/E12) (Fig. 10). We used a Carl Zeiss, Axio Vert microscope with transmitted light: halogen lamp, LED (wavelength 400–700 nm, peak at 460 nm); reflected light: HBO 50, HBO 100, HXP 120 C, LED modules (wavelength, nm): 365, 385, 420, 445, 455, 470, 505, 530, 590, 615, 625 or neutral white: 540–580 nm.

|
| Fig. 10 Inverted fluorescence microscopic image of leaf sheath cuticle of Equisetum siwalikum sp. nov.; (A–C) leaf sheath teeth (SKBUH/PPL/HP/P/E10); (D, E) row of rectangular epidermal cells (SKBUH/PPL/HP/P/E9); (F) ridges and furrows of stem (G) epidermal cell of leaf sheath; (H) well-preserved epidermal cells and elongated superficial stomata (scale bar = 20 μm). |
XRD analysis was used to study the mineral composition of Equisetum compressed stems (Table S2). The stem fossil sample (SKBU/PPL/HP/P/E5) was sectioned to approximate thickness with a jeweler's saw. After that sectioned fossilized stem cuticle samples were chipped with a small hammer. Small chipping particles were washed with distilled water and dried for 4 h in a hot air oven at 60 ℃. After that the sample was crushed and powdered using a mortar and pestle. A 2–3 mg powdered sample was taken for XRD analysis. X-ray diffraction was carried out on a Panalytical X'Pert Pro diffractometer. The instrument was equipped with a Cu Kα source, capable of high-resolution and lower-resolution measurements. The divergence slit size was 1.9076° and phase identification was made by X'pert High Score plus software.
3. Results 3.1. SystematicsDivision: Pteridophyta Schimp. in K.A. Zittel, 1879–1890.
Class: Polypodiopsida Cronquist, Takht. and W. Zimm., 1966
Order: Equisetales DC. ex Bercht. and J. Presl, 1820
Family: Equisetaceae Michx. ex DC, 1804
Genus: Equisetum L., 1753
Subgenus: Equisetum L., 1753
Species: Equisetum siwalikum Kundu, Hazra et Khan sp. nov.
Holotype: SKBUH/PPL/HP/E1 (Fig. 3A)
Additional specimens: SKBUH/PPL/HP/E2–22 (Figs. 3C–E; 4D, E; 5A; 6A–D, F, G; 8C; 9D; Figs. S1A–G)
Repository: Palaeobotany and Palynology Laboratory, Department of Botany, Sidho-Kanho-Birsha University, Purulia.
Type locality: Road cutting section (31°44′26″N, 76°43′33″E) along the Sarkaghat-Dharmpur road near Sarkaghat area in Mandi district, Himachal Pradesh, western India.
Type horizon: Middle part of the Siwalik succession of sediments (Late Miocene)
Etymology: The Latin specific epithet 'siwalikum' is chosen for the Siwalik deposits from where fossil specimens were recovered.
Specific diagnosis
Preserved articulated stem 3–4 mm in wide, composed of nodes and internodes, with longitudinal ridges and furrows; nodes enclosed with distinct leaf sheaths, 5–7 mm in length and 3–5 mm in width; leaf sheath teeth triangular, acute and joined towards the base; strobilus bilaterally symmetrical, elliptical to elongate in shape; in leaf sheaths of stem epidermal cells well-developed, stomata wide and superficial.
Description
External morphological features (Figs. 3–9 and S1)
Stems
Some fossil specimens preserved as external impressions (Fig. 3A and B) and some as siliceous compressions with well-preserved cuticles (Fig. 3C, D, E and S1C); stem well-preserved, narrow, preserved length 10–11 cm, jointed, composed of distinct nodes and internodes (Figs. 3A and 5B) with longitudinal ridges and furrows (Figs. 3C and D, 10F and S1C), ridges slightly pronounced in stem exteriors bearing fine longitudinal striae; nodes bulbous and enclosed with distinct leaf sheaths (Figs. 3A–E and 5H), 5–7 mm in length and 3–5 mm in width; internodes 10–15 mm in length and 3–4 mm in width; leaf sheath dark in colour, spreading, attached to ring-like meristem, consisting of ~ 5–8 basally fused linear leaves (Fig. 3A, C, D, E), maximum preserved leaf sheath length ~ 6–7 mm, distally free with triangular acute leaf teeth, joined towards the base (Figs. 3C, D and S1B, C).
Isolated leaf-sheaths
Well-preserved as external impressions and carbonaceous compressions (Fig. 6A–E and G); 1 cm in width and 6–7 mm in length; 11–12 longitudinal ridges present, 0.5–0.9 cm apart; ridges slightly more pronounced and bearing fine longitudinal striae; remnants of each leaf sheath linear to narrowly triangular, apically pointed (Figs. 6A–E, G; 7A, C, F; 8A, C, E; 9A, B, D and 10A–C) with longitudinally straight and basally fused lamina segments.
Strobilus
Poorly preserved; bilaterally symmetrical, elliptical to elongate in shape (Fig. 6F); preserved strobilus about 6 mm in length and 2 mm in width, seemingly consists of whorls of the hexagonal sporangiophores; two sporangiophores seen (Fig. 6F).
Epidermal characters (Fig. 10)
Epidermal cells of the compressed leaf sheath specimen ~45 μm in length and ~30 μm in width; regularly arranged, generally rectangular (Fig. 10D and E) with oblique end walls; anticlinal walls more or less straight and periclinal walls smooth; stomata elongated, irregularly distributed, and randomly oriented, 35–40 μm in length and 28–34 μm in width, stomata superficial, not sunken, one lateral subsidiary cell per guard cell (Fig. 10H); guard cell superficial, elongated, 9–12 μm long and 4–6 μm wide..
3.2. XRD analysis (Table S2 and Fig. 11)The principal components of fossilized Equisetum stem are quartz (SiO2) and muscovite. Our XRD analysis reveals that 67.79% quartz, 63.86% muscovite, and 14.78% calcite were deposited in 2–3 mg powdered Equisetum compressed stem. The total value of the relative intensity of all known and unknown minerals is 219.65 and the total value of the relative intensity of quartz is 148.91, muscovite 140.273, and calcite 32.48.
3.3. Floristic components in the middle Siwalik sedimentsBesides Equisetum, the sediments yielded plant megafossils of many other angiosperm taxa such as Gynocardia (Achriaceae), Millettia, Albizia, Cynometra (Fabaceae), Ventilago (Rhamnaceae), Terminalia (Combretaceae), Daemonorops (Arecaceae) as well as diverse monocots (Prasad et al., 2013; Kundu et al., 2021) and pteridophytic ferns such as Microsorum Link of Polypodiaceae, and Adiantum L., Ampelopteris (Retz.) Copel., Pteris L., Christella H. Lev. attributable to the Thelypteridaceae. In addition, some fossil forms are assignable to modern genera (e.g., Quercus, Populus, Betula, Carpinus, Viburnum) typical of cool temperate deciduous woodlands found today at high elevations in the Himalayas.
4. Discussion 4.1. Comparison with modern species of EquisetumThe significant morphological characteristics for the identification of the extant Equisetum are jointed stems with longitudinal ridges and furrows and that are composed of distinct nodes and internodes enclosed with leaf sheaths (Fig. 3F) (Zhang, 2004; Zhang and Nicholas, 2012). Because our Siwalik Late Miocene specimens possess the above-mentioned morphological features, we regard modern Equisetum as the most closely related genus to the recovered fossil specimens. Additionally, the present fossil specimens characterised by superficial stomata (Fig. 10H) exhibit characteristics of the sub-genus Equisetum (Hauke, 1963, 1990). However, the other sub-genus of Equisetum, Hippochaete, is characterised by sunken stomata arranged in single lines. Seven modern species, such as E. variegatum Schleich, E. giganteum L., E. hyemale L., E. laevigatum A. Braun and Engelm, E. myriochaetum Schltdl. and Cham., E. ramosissimum Desf., E. scirpoides Michx., belong to the sub-genus Hippochaete (Hauke, 1963). Only two extant species, E. diffusum D. Don and E. fluviatile L., placed under subgenus Equisetum, are more or less similar to our Siwalik fossil specimens in the length of internodes, as well as the length and width of leaf sheaths (Table S3). However, the width of the stem of the above-mentioned two modern species is comparatively narrower than that of the current fossil specimens. In addition, the number of ridges was greater in the Siwalik specimens than in modern species. Thus, our fossil specimens are ascribed to the genus Equisetum (subgenus Equisetum) based on morphological and epidermal characters.
4.2. Comparisons with Cenozoic fossil species of EquisetumEquisetum-like fossils, commonly known as Equisetostachys, Equisetites, and Equisetum, have been reported from the Mesozoic and Cenozoic sediments of the Northern Hemisphere by stems fragments, leaf sheaths, rhizomes, or tubers (Becker, 1969; Brown, 1975; McIver and Basinger, 1989; Sun et al., 2013). The Mesozoic sphenophyte fossils were assigned to the organ genus Equisetostachys and the fossil genus Equisetites, sharing some morphological similarities with extant Equisetum (Kelber and van Konijnenburg-van Cittert, 1998; DiMichele et al., 2005; Villar de Seoane, 2005; Weber 2005). However, most Cenozoic sphenophyte fossils, especially the vegetative parts, have been assigned to the genus Equisetum (Table 1; Si and Li, 1963; Mei and Tian, 1988; Taylor and Taylor, 1993; Kelber and van Konijnenburg-van Cittert, 1998; Silva–Pineda et al., 2009).
| Age | Fossil species | Locality | Organ | Reference |
| Pleistocene | Equisetum fluviatile | Canada | – | Penhallow (1896) |
| E. limossum | Canada | – | Penhallow (1896) | |
| E. scirpoides | Ontario | – | Penhallow (1896) | |
| E. sylvaticum | Canada | – | Dowson and Penhallow (1890) | |
| Equisetum sp. | California | – | LaMotte (1944) | |
| Equisetum sp. | Canada | – | LaMotte (1944) | |
| Pliocene | E. yongpingense | China | Rhizome with nodes and internodes | Aung et al. (2020) |
| Equisetum sp. | America | Leaf-sheaths | Thomasson (1980) | |
| E. oregonense | America | Stem and leaf sheaths | Knowlton (1902) | |
| Equisetum sp. | America | Stem | Knowlton (1902) | |
| E. aravense | United States | – | Knowlton et al. (1921) | |
| E. myriochaetum | Central Mexico | Fragmented stem | Silva–Pineda et al. (2009) | |
| Equisetum sp. | California | – | Lesquereux (1883); Axelrod (1985) | |
| Equisetum sp. | California | – | Lesquereux (1883) | |
| Miocene | Equisetum sp. | Japan | Stem | Huzioka (1963, 1964); Ina (1992); Yabe (2008) |
| Equisetum sp. | South Korea | Rhizome tuber with nodes and internodes | Jeong et al. (2017) | |
| E. miocenicum | America | Stem | Graham (1963) | |
| Equisetum sp. | New Zealand | Stem | Pole and Mcloughlin (2017) | |
| E. alexanderi | America | – | Brown (1937) | |
| Equisetum sp. | Oregon | – | Arnold (1937) | |
| E. winkleri | Switzerland | – | Heer (1868) | |
| Equisetum sp. | Iceland | Rhizome tuber | Denk et al. (2005) | |
| E. octangulatum | Canada | Stem with node | Smith (1938) | |
| Equisetum sp. | United States | – | Knowlton (1898) | |
| E. yenbaiense | Vietnam | Rhizome with nodes and internodes | Aung et al. (2020) | |
| E. cf. pratense | China | One branch of tuber; Incomplete stem, isolated leaf sheaths | Zhang et al. (2007) | |
| E. oppositum | China | Rhizome tuber | Ma et al. (2012) | |
| Equisetum sp. | China | Rhizome tuber | Tao and Du (1987) | |
| Equisetum sp. | Iceland | Rhizome with nodules | Denk et al. (2011) | |
| Equisetum sp. | Spain | Stem with nodes and internodes | Barrón and Postigo-Mijarra (2011) | |
| Equisetum sp. nov. (Current fossil) | India | Compressed and impressed stem with nodes and internodes, leaf sheaths, an impression of strobilus | This study | |
| Oligocene | E. cf. arcticum | America | Rhizome tuber | Becker (1969) |
| E. aquatile | America | Stem | Becker (1973) | |
| E. insculptum | America | Stem | Jennings (1920) | |
| E. florissantense | America | – | MacGinitie (1955) | |
| United States | – | Cockerell (1915) | ||
| Equisetum sp. | Germany | Shoot fragment | Uhl et al. (2002) | |
| Equisetum sp. | Oregon | – | Arnold (1937) | |
| Equisetum sp. | India | Stem? | Mehrotra et al. (2009) | |
| E. arcticum | Japan | – | Yamamoto and Yonesaka (1999); Momohara (2005) | |
| E. kitamurae | Japan | – | Matsuo (1971) | |
| Equisetum sp. | Japan | – | Uemura et al. (1999) | |
| E. oppositum | China | Tuber | Deng et al. (2020) | |
| E. cf. fluviatoides | China | Stem | Chen et al. (2021) | |
| Equisetum sp. | Ethiopian plateau | Stem | García Massini et al. (2010) | |
| Late Eocene- Early Oligocene | Equisetum sp. | Australia | Stem | Rozefelds et al. (2019) |
| E. similkamense | Columbia | – | Dawson (1879) | |
| Eocene | E. clarnoi | America | Stem | Brown (1975) |
| E. haydenii | America | – | Lesquereux (1878); Lamotte (1952) | |
| E. oregoense | – | Newberry (1898); Knowlton (1902) | ||
| E. hornii | – | Lesquereux (1888) | ||
| E. perlaevigatum | Knowlton (1930) | |||
| E. wyomingense | United States | – | Cockerell (1925) | |
| E. winchesteri | United States | – | Brown (1929) | |
| E. tipperarense | Ireland | – | Berry (1930) | |
| Equisetum sp. | Texas | – | Berry (1930) | |
| Equisetum sp. | United States | – | Berry (1929) | |
| Equisetum | America | – | MacGinitie (1969) | |
| Equisetum | America | – | Buchheim and Surdam (1981) | |
| Equisetum | America | – | Myers (1991) | |
| E. arcticum | Russia | Stem | Pavlyutkin et al. (2020) | |
| Equisetum sp. | Russia | Stem | Pavlyutkin et al. (2020) | |
| Equisetum | Canada | – | Lowe et al. (2018) | |
| Equisetum | Germany | – | Wilde (1995) | |
| Equisetum sp. | China | – | Guo (1979) | |
| E. hunchunense | China | Rhizome tuber | Guo (2000) | |
| E. ezoense | Japan | – | Endo (1968) | |
| Equisetum sp. | Japan | – | Matsuo (1967) | |
| Equisetum sp. | Alberta | – | Berry (1930) | |
| Palaeocene-Eocene | E. magnum | United States | Rhizome and stem | Hickey (1977) |
| E. newberryi | North America | – | Knowlton (1919) | |
| E. robustum | Washington state | – | Newberry (1898) | |
| E. oppositum | China | Rhizome tuber | Yang et al. (2016) | |
| Palaeocene | E. alexoensis | Canada | Stem | Bell (1949) |
| E. arcticum | Norway | – | Heer (1868) | |
| E. parlatorii | Alberta | – | Penhallow (1908) | |
| Equisetum sp. | Alberta and Canada | – | Dawson (1875) | |
| E. boreale | Greenland | – | Heer (1868); Bell (1949) | |
| E. canaliculatum | United States | – | Knowlton (1898) | |
| E. deciduum | United States | – | Knowlton (1898) | |
| E. coloradense | Colorado | – | Knowlton (1919; 1930) | |
| Equisetum sp. | Colorado | – | Knowlton (1919; 1930) | |
| E. fluviatoides | Canada | Vegetative and fertile remains | Mciver and Basinger (1989) | |
| Equisetum sp. | California | – | LaMotte (1944) | |
| E. haguel | United States | – | Knowlton (1898) |
Here, we compare our Siwalik specimens with earlier reported stem fossil species of Equisetum (E. oregonense Knowlton, E. insculptum Jennings; E. octangulatum Smith, E. alexoensis Bell, E. miocenicum Graham, E. aquatile Becker, E. clarnoi Brown, E. magnum Hickey, E. fluviatoides Mciver and Basinger, Equisetum sp. Uhl et al., E. myriochaetum Silva–Pineda et al., Equisetum sp. Pole and McLoughlin, Equisetum cf. fluviatoides Chen et al., E. arcticum Pavlyutkin et al.) from the Cenozoic Period of Asia, North America, Europe, and Africa (Table 2; Knowlton, 1902; Jennings 1920; Smith, 1938; Bell, 1949; Graham, 1963; Becker, 1973; Brown, 1975; Hickey, 1977; Mciver and Basinger, 1989; Verdcour, 1999; Uhl et al., 2002; Silva–Pineda et al., 2009; Pole and McLoughlin, 2017; Chen et al., 2021; Pavlyutkin et al., 2020). The current Siwalik specimens are characterized by the narrow-jointed stem, 10–15 mm long internodes, 10–12 teeth present in deep brown leaf sheath, basal fused lamina, and apically pointed tip with longitudinal striae, key characters that differentiate our specimens from earlier reported stem fossil species of Equisetum. The width of the stem of five fossil species (e.g., E. oregonense, E. miocenicum, Equisetum sp., E. magnum, E. octangulatum, and E. alexoensis) is much wider (9–45 mm) than those of our specimens (2–5 mm). However, the width of the stem of two fossil species (Equisetum sp. and E. aquatile) is much narrower (0.5–2 mm) than our specimens. In E. myriochaetum, E. cf. fluviatoides, E. clarnoi, and E. fluviatoides, the length of the internode ranges from 13 to 20 mm, whereas the length of internode in our fossil specimens is 10–15 mm. In addition, our specimens differ from Equisetum cf. fluviatoides, and E. clarnoi in having prominent leaf sheaths with pointed tips. Among the rest, Equisetum sp., E. insculptum, and E. arcticum are almost similar in widths of the stem (less than 5 mm), but the number of ridges preserved in those two fossil species is > 14 (Table 2).
| Fossil species of Equisetum | External morphological features | Epidermal anatomy | Reference | |||||||
| Stem width (mm) | Internode length (mm) | No. of ridges | Leaf sheath length (mm) | Leaf teeth number in the leaf sheath | Leaf sheath shape | Size of teeth | ||||
| Length (mm) | Width (mm) | |||||||||
| Equisetum sp. (Subgenus: Hippochaete) | – | – | – | – | Fragmentary (2–4) | – | 2.4–3.5 mm | 0.5– 0.6 mm | Bar-shaped thickenings surrounding stomatal openings; sunken stomata arranged in two regular rows on the flanks of each segment and by the massive development of sheath ornamentation | Thomasson (1980) |
| E. oregonense | 30 mm | – | – | – | – | Obtuse dentation | – | – | – | Knowlton (1902) |
| Equisetum sp. | 5 mm | – | 16 | 8 mm | 16 | Sharp teeth | – | – | – | Knowlton (1902) |
| E. myriochaetum | 4–20 mm | 13–82 mm | 4–20 | – | – | Sharp teeth | 3–5 mm | 0.5 mm | – | Silva–Pineda et al. (2009) |
| E. miocenicum | 14 mm | 10–45 mm | – | – | Nodal sheath narrow, inconspicuous | – | – | – | Graham (1963) | |
| E. octangulatum | 45 mm | 33 mm | 4–5 | – | – | – | – | – | – | Smith (1938) |
| Equisetum sp. | 9–19 mm | 62–63 | Visible | – | – | Apically pointed teeth | – | – | – | Pole and Mcloughlin (2017) |
| Equisetum sp. | 0.5–2 mm | 2 mm | – | – | – | Needle-like leaves | – | – | – | Barrón and Postigo-Mijarra (2011) |
| E. aquatile | 0.3–0.7 mm | Visible | 10–12 | – | – | – | – | – | – | Becker (1973) |
| E. insculptum | 4 mm | Visible | 18 | – | – | – | – | – | – | Jennings (1920) |
| Equisetum sp. | – | 12–13 mm | – | – | – | – | – | Uhl et al. (2002) | ||
| E. cf. fluviatoides | 3–19 mm | 10–30 mm | 12–30 | – | – | – | – | – | – | Chen et al. (2021) |
| E. clarnoi | < 8 mm | 25 mm | Visible | – | – | – | – | – | Sunken stomata arranged vertically in a single line flanking each of the external biangulate stem ridges | Brown (1975) |
| E. arcticum | 5 | Visible | 14–16 | – | – | – | – | – | – | Pavlyutkin et al. (2020) |
| E. magnum | 10–18 | > 43 | 30–60 | – | – | Acute to acuminate | 1–3 mm | 3 mm | – | Hickey (1977) |
| E. alexoensis | < 10 mm | 50 mm | > 24 | – | – | – | – | – | – | Bell (1949) |
| E. fluviatoides | 3–19 mm | 30 mm | 12–30 | 9–23 mm | 12–30 | Attenuate | – | – | – | Mciver and Basinger (1989) |
| E. siwalikum sp. nov. | 2–5 mm | 10–15 mm | 10–12 | 2–5.5 mm | 10–12 | Pointed acute apex | 2–2.5 mm | 0.5–1 mm | Epidermal cells | This study |
| Irregularly rectangular oblique end walls; anticlinal walls more or less straight and periclinal walls smooth; stomata elongated, irregularly distributed and randomly oriented, stomata superficial, one lateral subsidiary cell per guard cell | ||||||||||
Our use of fluorescence microscopy allows us to compare the epidermal features of our specimens to those of previously discovered Equisetum fossils, although to date epidermal anatomy has only been characterized in two fossil species of Equisetum (Brown, 1975; Thomasson, 1980). One species, Equisetum sp., was reported by Thomasson (1980) from the Ash Hollow Formation (Late Miocene to Pliocene) and the other species, E. clarnoi, from Clarno Chert Formation, Oregon (Middle Eocene) of North America (Brown, 1975). Our specimens represent the first authentic record of Equisetum specimens with epidermal anatomy from Asia. Our specimen differs from the North American fossils in having superficial stomata. However, both North American fossil species of Equisetum species exhibited sunken stomata.
Taken together, macro and micromorphological analyses indicate that the Late Miocene Himachal specimens differ from earlier reported fossil species of Equisetum. We have, thus, described these specimens under a new specific name. Because the fossil specimens resemble Equisetum and are recorded from the Siwalik sediments for the first time, they are described here as a new species, E. siwalikum Kundu, Hazra et Khan sp. nov.
4.3. Silica on Equisetum fossil stemModern Equisetum tissue is often rich in phosphorus, potassium, calcium, magnesium, and silicon (Marsh et al., 2000). Among all terrestrial plants, only the horsetails require silicon as an essential mineral nutrient (Epstein, 1999). The silicon concentrations in Equisetum stems are the highest among vascular plants (Hodson et al., 2005). Silica accumulates on the epidermis of the Equisetum plants and is also incorporated into the cell walls, increasing their rigidity and stability (Currie and Perry, 2009). Kaufman et al. (1971) suggest that the silica on Equisetum stems protects horsetails against insect feeding or fungal diseases and also reduces water loss through the epidermis.
Our XRD analysis of Siwalik compressed stems reveals that 67.79% of mineral content in Equisetum compressed stems consisted of quartz. This finding confirms the preservation potential of silica in the fossil stem of horsetails (Fig. 11) and corroborates the mineral data on modern Equisetum stems. The present study represents the first report on the mineral composition of the fossil stem of Equisetum. Silica studied from the fossilized stem may be an indicator of defense mechanisms of past Equisetum against herbivores or fungi and provides insights into how horsetails interacted with their environment. A thorough further study of the mineral composition in plants will offer useful insights into the evolution of minerals over geological time.
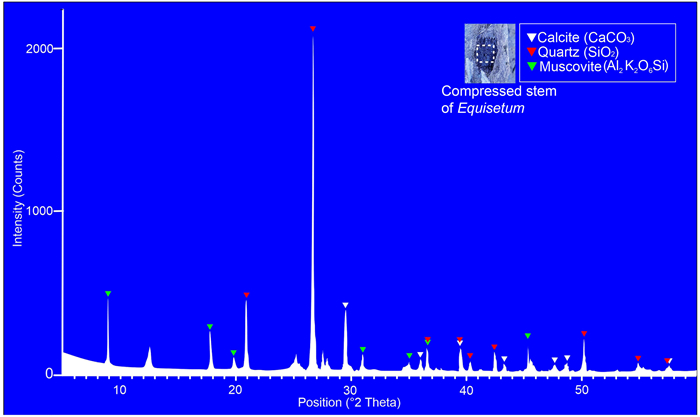
|
| Fig. 11 X-ray diffraction analysis of a powdered sample of compressed Equisetum stem (SKBUH/PPL/HP/P/E5). |
The Cenozoic Equisetum fossils have been recorded mainly from Asia, North America, and Europe, with a few fossil records from Australia, New Zealand, and South America (Table 1; Fig. 12). Ample fossil evidence indicates that this sphenopsid was widespread during the Cenozoic period. Fossil evidence indicates that the genus Equisetum flourished without hindrance from the Eocene to Pleistocene in North America (Table 1). Two fossil species of Equisetum, E. arcticum and Equisetum sp., have been reported from the Early Eocene Uglovka Formation of Russia (Pavlyutkin et al., 2020). Another two Eocene fossil species (E. tipperarense Berry and Equisetum sp.) were reported by Berry (1930) and Wilde (1995) from Ireland and Germany respectively. A fossilised shoot fragment of Equisetum with nodes and internodes was also reported from the Oligocene flora of Hochstetten-Dhaun of Germany (Uhl et al., 2002). Four Miocene fossil species of Equisetum have been reported from the European continental region (Heer, 1868; Denk et al., 2005; 2011; Barrón and Postigo-Mijarra, 2011). A fossil species E. oppositum was recovered at different epochs (Palaeocene-Eocene, Oligocene, and Miocene) of the Cenozoic Era from China (Ma et al., 2012; Yang et al., 2016; Deng et al., 2020), which indicates that this Equisetum species used to grow in abundance during that era. Aung et al. (2020) described two fossil species, E. yongpingense Aung et al., recovered from the middle Miocene sediments of Yunnan Province, China, and E. yenbaiense Aung et al., collected from the Late Miocene sediments of Yenbai Province, Vietnam. Additionally, some Equisetum fossils have also been reported from the Cenozoic sediments of China (Tao and Du, 1987; Zhang et al., 2007; Chen et al., 2021). Two Miocene fossil species of Equisetum have been reported from the eastern part of Asia (Matsuo, 1971; Uemura et al., 1999; Yamamoto and Yonesaka, 1999; Momohara, 2005; Yabe, 2008; Jeong et al., 2017). Massini et al. (2010) reported a fossilized stem of this sphenophyte from the northwestern Ethiopian Plateau of Africa. However, only two Equisetum fossils have been recorded from Australia and New Zealand (Pole and McLoughlin, 2017; Rozefelds et al., 2019). Horsetails have frequently been recorded from Cenozoic deposits in China, USA, Europe, Australia, New Zealand, and South America collectively, indicating that the genus was widely distributed during the Cenozoic (Table 1). The decline of equisetaleans from Australia and New Zealand at the present day is linked to the substantial climatic and environmental changes that occurred as a result of Australia's rapid northward drift from Antarctica during the Cenozoic (Macphail et al., 1994; Pole and McLoughlin, 2017).
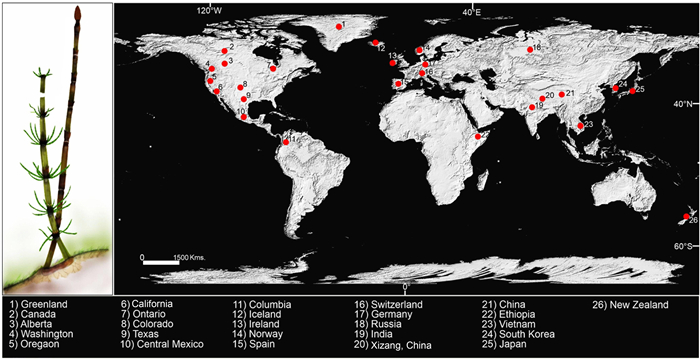
|
| Fig. 12 Cenozoic macrofossil records of Equisetum in the World. |
Equisetum is an antique genus and comprises the only surviving representatives of the class Sphenopsida (Scagel et al., 1984), which first appeared in the fossil record during the Late Devonian, according to macrofossil evidence of the earliest unambiguous sphenopsid Pseudobornia ursina Scagel et al. (Scagel et al., 1984; Stewart and Rothwell, 1993). After that sphenopsids diversified greatly during the Early Carboniferous (Stewart and Rothwell, 1993). The Carboniferous sphenopsids are presently divided into two orders: Sphenophyllales and Equisetales (Stewart and Rothwell, 1993). The common components of the palaeoflora are represented by Equisetales, including Archaeocalamitaceae, Calamitaceae, and Equisetaceae (Stewart and Rothwell, 1993). The family Equisetaceae consists of the living genus Equisetum as well as other extinct herbaceous sphenopsids resembling Equisetum.
The Carboniferous period represented the peak of diversity and abundance of pteridophytes and coal swamps dominated by giant arborescent lycopods that indicated a warm and humid climate with wet tropical low-lying areas (Lesquereux, 1879–1884; Pearson, 1995; Rothwell, 1996). During this period, two land masses (Laurasia and Gondwana) collided and began the formation of the supercontinent Pangea (Parrish, 1993). However, climate changes in the Early Permian began the demise of the great coal swamps. During this time, the equatorial regions of Pangea became drier and rainfall became more seasonal (Parrish, 1993). In addition, the climate became cooler with extensive glaciation in the southern hemisphere. This scenario continued through the Triassic (Kelber and van Konijnenburg-van Cittert, 1998), when arid to semiarid climates prevailed (Stewart and Rothwell, 1993). This led to a universal change from hydric to mesic conditions, which are less favorable to the growth of sphenopsids. Additionally, sphenopsids reduced their ability to compete with the increasingly successful growth of ferns, cycads, and conifers (Koske et al., 1985). These changes probably led to the extinction of both Calamites (during the Early Permian) and Sphenophyllales (by the end of the Permian).
The above-mentioned major extinctions left the remaining members of the Equisetales as the only representatives of the Sphenopsida (Kidston, 1892; Stewart and Rothwell, 1993) and Equisetum as the sole living representative of the ancient group of Sphenopsida (Hauke, 1963; Wu and Qin, 1991; Taylor and Taylor 1993; Taylor et al., 2009). Thus, botanists and palaeontologists have searched for the fossil evidence of Equisetum because it is considered a key element in understanding the paleoenvironment. Hickey (1977) reported fossil species E. magnum from the Golden Valley Formation of North Dakota of Eocene sediments, which indicate an emergent aquatic paleovegetation with poorly drained low land and low pH and Eh favorable for the development of aquatic and marsh plants. Based on fossil evidence of Equisetum, Chen et al. (2021) concluded that the Qaidam Basin of the northeastern Tibetan Plateau in China was humid and covered by a water drainage system during the Oligocene. In addition, some fossiliferous parts of Equisetum (stem with node and internodes, rhizome tuber, strobilus) have been preserved in situ in association with ferns (Collinson, 1988; Denk et al., 2005; Yabe, 2008; Yang et al., 2016; Jeong et al., 2017) that indicate swampy to marshy floodplains. The evidence of in-situ Equisetum stems in association with well-preserved ferns (Marsilea sp., Acrostichum sp.) and abundant fossil fishes (Cyprinidae) in the same layer of the fossil locality indicate that Equisetum grew in wet conditions (river side or lake side) (Zhang et al., 2007; García Massini et al., 2010; Ma et al., 2012; Yang et al., 2018; Aung et al., 2020). Modern-day Equisetum primarily grows on open, sunny riverside banks, lake margins, marshes, and in other wet places such as moist woods, ditches, and wetlands (Hauke, 1963, Husby, 2013). Some species grow in open, standing water and few grow on roadsides and railroad embankments, where sufficient underground water is available. According to Hauke (1963; 1978), Equisetum is a rapid colonizer of disturbed habitats that directly indicates wetland habitats.
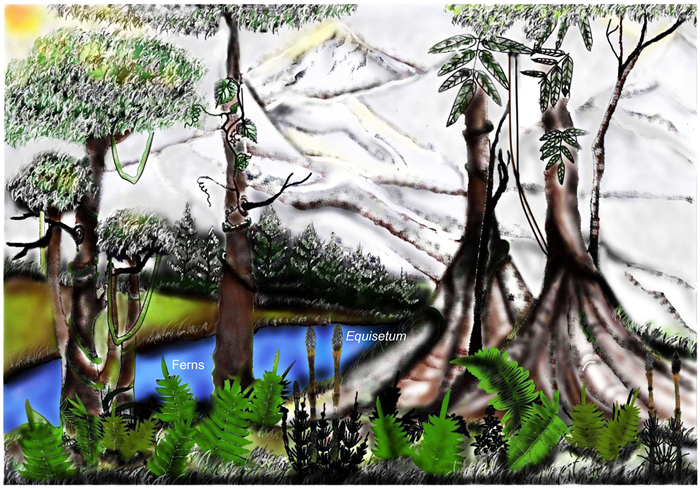
Ample megafossil evidence of Equisetum recovered from the middle Siwalik (Late Miocene) strata of Himachal Pradesh indicates that this sphenopsid once existed in the western Himalayas during the Siwalik period. Thus, we can conclude that our fossil locality was humid, and surrounded by swamp and lowland regions during the time of deposition. Generally, Equisetum grows in wet conditions around water reservoir sides. The occurrence of Equisetum in association with ferns generally suggests an impression of flooded terranean or marshy land conditions (Collinson, 1988; Falder et al., 1999; Falcon-Lang and Cantrill, 2002; Cantrill and Hunter, 2005). This observation can be extended back through the period of Siwalik sedimentation because Equisetum co-occurs with ferns found in Siwalik strata (Late Miocene) of the Himachal Pradesh (Fig. 13). Interestingly, besides Equisetum, we recovered a large number (approx. one hundred) of ferns similar to modern Cyclosorus Link, Ampelopteris (Retz.) Copel., Christella H. Lev., Pteris L., Adiantum L. of Thelypteridaceae and Microsorum Link of Polypodiaceae from the same fossil locality (Fig. 13), suggesting that the same ecological conditions prevailed in the Siwalik area during the time of deposition. The fossil locality now has a drier tropical climate (Srikantia and Bhargava, 2021). This clearly shows that there has been a substantial change in the climate in this region since the Late Miocene. Two main factors such as Himalayan uplift and recession of the Tethys Sea are responsible for this change. We reconstructed the palaeoecology of the ancient Siwalik forests that showed floristic components, including Equisetum and ferns, during the Late Miocene on the fossil locality of Himachal Pradesh (Fig. 14).

|
| Fig. 13 Recovered fern fossils from the same fossil locality in association with Equisetum; (A) cf. Christella; (B–D) cf. Cyclosorus; (F) cf. Ampelopteris (scale bar = 1 cm). |
|
| Fig. 14 Palaeoecological reconstruction of the ancient forest during the late Miocene in Himachal Pradesh, western India. |
Presently, Equisetum is a rare element in the flora of Himachal Pradesh (Chowdhery and Wadhwa, 1984) from where the fossil Equisetum and ferns were recovered. This sphenophyte now grows in some regions of this state of western India. We have collected a modern species, E. ramosissimum Desf., from forests adjacent to the fossil locality (Fig. S2). However, the width of the stem of the above-mentioned modern species is comparatively narrower (1–2 mm) than the fossil specimens (2–5 mm). Thus, we suggest that during Siwalik time Equisetum was comparatively larger than present-day Equisetum. It is presumed that significant changes in the climate due to the Himalayan Orogeny during the Late Miocene might be a possible reason for the disappearance of this sphenophyte from the present-day vegetation as well as the decrease in size of the present-day Equisetum. The rise of opportunistic angiosperms may be another cause of vulnerability of Equisetum growth (Pole and McLoughlin, 2017). Therefore, the decline of equisetaleans, otherwise unknown from the Siwalik period, was possibly a consequence of severe environmental changes experienced in the Siwalik, coupled with competition from opportunistic angiosperms. These factors may explain why our fossil evidence is the only record of equisetaleans in Siwalik despite the rich angiosperm heritage of the Siwalik period (Prasad, 2008).
AcknowledgementsThe authors are grateful to DST-SERB, GOI, New Delhi, India (File no. CRG/2020/001303) for their financial assistance. SK, TH, and MAK thankfully acknowledge the Department of Botany, Sidho-Kanho-Birsha University, Department of Geological Science, Jadavpur University, and S. N. Bose Innovation Centre, Kalyani University for providing facilities to accomplish this work. SB acknowledges the Centre of Advanced Study (Phase-Ⅶ), the Department of Botany, the University of Calcutta for providing the necessary facilities. TC gratefully acknowledges the Indian Statistical Institute, Kolkata for providing the necessary facilities.
Author contributions
All authors contributed to the study conception and design. Preparation of materials, data collection and analysis were performed by SK, TH and MAK. The first draft of the manuscript was written by SK, MAK and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.01.004.
Arnold, C.A., 1937. Observations on the Fossil Flora of Eastern and Southeastern Oregon, vol. 8. University of Michigan Press and Arbor, pp. 79–102.
|
Aung, A.T., Huang, J., Van Do, T., et al., 2020. Three new fossil records of Equisetum (Equisetaceae) from the Neogene of south-western China and northern Vietnam. PhytoKeys, 138: 3-15. DOI:10.3897/phytokeys.138.38674 |
Axelrod, D.I., 1985. Miocene Floras from the Middlegate Basin, West-Central Nevada, 129. University of California Press.
|
Barrón, E., Postigo-Mijarra, J.M., 2011. Early Miocene fluvial-lacustrine and swamp vegetation of La Rinconada mine (Ribesalbes-Alcora basin, Eastern Spain). Rev. Palaeobot. Palynol., 165: 11-26. DOI:10.1016/j.revpalbo.2011.02.001 |
Becker, H.F., 1969. Fossil plants of the tertiary beaverhead basins in southwestern Montana. Palaeontograph. Abteilung B, 127: 1-142. |
Becker, H.F., 1973. The York Ranch flora of the upper Ruby river basin, southwestern Montana. Palaeontograph. Abteilung B, 143: 18-93. DOI:10.1007/978-3-663-19797-3_5 |
Behrensmeyer, A.K., 1992. Paleoenvironments and taphonomy. Terrestrial Ecosystem through Time, Evolutionary Paleoecology of Terrestrial Plants and Animals. The University of Chicago Press, pp. 15–136.
|
Bell, W.A., 1949. Uppermost cretaceous and Paleocene floras of western Alberta. Bull. Can. Petrol. Geol., 13: 1-231. DOI:10.2307/2305808 |
Berry, E.W., 1929. A Revision of the flora of the Latah Formation. U.S. Government Printing Office.
|
Berry, E.W., 1930. Revision of the Lower Eocene Wilcox Flora of the Southeastern States: With Descriptions of New Species, Chiefly from Tennessee and Kentucky, vol. 156. US Government Printing Office.
|
Borkar, S.U., Nagrale, V.D., Korpenwar, A.N., et al., 2017. Report of Equisetitis sahnii gen. et sp. nov. A Pteridophytic strobilus from the Late Cretaceous Beds of Singpur Madhya Pradesh, India. Int. J. Appl. Res., 3: 272-275. |
Brown, J.T., 1975. Equisetum clarnoi, a new species based on petrifactions from the Eocene of Oregon. Am. J. Bot., 62: 410-415. DOI:10.1002/j.1537-2197.1975.tb14064.x |
Brown, J.T., 1977. Evidence for epidogenetic growth in Paracalamites from South West Africa. S. Afr. J. Sci., 73: 235-237. |
Brown, R.W., 1929. Additions to the Flora of the Green River Formation: U.S.. Geological Survey Professional Paper, 154: 279-299. |
Brown, R.W., 1937. Additions to Some Fossil Floras of the Western United States. U. S, 186. Geological Survey publication, pp. 163–206.
|
Brozovic, N., Burbank, D.W., 2000. Dynamic fluvial systems and gravel progradation in the Himalayan foreland. Geol. Soc. Am. Bull., 112: 394-412. DOI:10.1130/0016-7606(2000)112<394:DFSAGP>2.0.CO;2 |
Buchheim, H.P., Surdam, R.R., 1981. Paleoenvironments and fossil fishes of the Laney member. In: Gray, J., Boucot, A.J., Berry, W.B.N. (Eds.), Green River Formation, Wyoming. Hutchinson Ross publication, Stroudsburg (PA), pp. 415–452.
|
Cantrill, D.J., Hunter, M.A., 2005. Macrofossil floras of the Latady basin, Antarctic Peninsula. N. Z. J. Geol. Geophys., 48: 537-553. DOI:10.1080/00288306.2005.9515132 |
Chen, H.Y., Yang, T., Han, L., et al., 2021. The Oligocene Equisetum from Qaidam Basin, northeastern Tibetan Plateau in China and its implications. Hist. Biol., 33: 2845-2853. DOI:10.1080/08912963.2020.1830280 |
Chowdhery, H.J., Wadhwa, B.M., 1984. Flora of India. In: Series 2, Flora of Himachal Pradesh Analysis, 1. Botanical Survey of India.
|
Christenhusz, M.J., Bangiolo, L., Chase, M.W., et al., 2019. Phylogenetics, classification and typification of extant horsetails (Equisetum, Equisetaceae). Bot. J. Linn. Soc., 189: 311-352. DOI:10.1093/botlinnean/boz002 |
Cockerell, T.D.A., 1915. Shorter notes. Torreya, 15: 265-267. |
Cockerell, T.D.A., 1925. Plant and insect fossils from the Green River Eocene of Colorado. Proc. U. S. Natl. Mus.. |
Collinson, M.E., 1988. Freshwater macrophytes in palaeolimnology. Palaeogeogr. Palaeoclimatol. Palaeoecol., 62: 317-342. DOI:10.1016/0031-0182(88)90060-0 |
Cullen, E., Rudall, P.J., 2016. The remarkable stomata of horsetails (Equisetum): patterning, ultrastructure and development. Ann. Bot., 118: 207-218. DOI:10.1093/aob/mcw094 |
Currie, H.A., Perry, C.C., 2009. Chemical evidence for intrinsic 'Si' within Equisetum cell walls. Phytochemistry, 70: 2089-2095. DOI:10.1016/j.phytochem.2009.07.039 |
Dawson, J.W., 1875. Note on the plants, collected by G.M. Dawson, from the lignite tertiary deposits, near the forty-ninth parallel. In: Dawson, G.M. (Ed.), British North American Boundary Commission; Report on the Geology and Resources in the Vicinity of the Forty-Ninth Parallel. Dawson Brothers, Montreal, pp. 237–331.
|
Dawson, J.W., 1879. List of Tertiary fossil plants from localities in the southern part of British Columbia, with the description of a new species of Equisetum. Geol. Surv. Can., Report of Progress, 1877-1878, Government Printing Bureau 186B-187B.
|
Dawson, S.W., Penhallow, D.P., 1890. On the Pleistocene flora of Canada. Geol. Soc. Am. Bull., 1: 311-334. DOI:10.1130/GSAB-1-311 |
Deng, W., Su, T., Wappler, T., et al., 2020. Sharp changes in plant diversity and plant-herbivore interactions during the Eocene–Oligocene transition on the southeastern Qinghai-Tibetan Plateau. Global Planet. Change, 194: 103293. DOI:10.1016/j.gloplacha.2020.103293 |
Denk, T., Grímsson, F., Kvaček, Z., 2005. The Miocene floras of Iceland and their significance for late Cainozoic North Atlantic biogeography. Bot. J. Linn. Soc., 149: 369-417. DOI:10.1111/j.1095–8339.2005.00441.x |
Denk, T., Grímsson, F., Zetter, R., et al., 2011. Climate evolution in the northern North Atlantic – 15 Ma to present. In: Late Cainozoic Floras of Iceland. Topics in Geobiology. Springer, Dordrecht, pp. 669–721.
|
Des Marais, D.L., Smith, A.R., Britton, D.M., et al., 2003. Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcL and trnL–F). Int. J. Plant Sci., 164: 737-751. DOI:10.1086/376817 |
DiMichele, W.A., Cittert, K.V., Looy, C.V., et al., 2005. Equisetites from the early Permian of North-central Texas. Nonmarine Permian, 30: 56-59. |
Elgorriaga, A., Escapa, I.H., Rothwell, G.W., et al., 2018. Origin of Equisetum: evolution of horsetails (Equisetales) within the major euphyllophyte clade Sphenopsida. Am. J. Bot., 105: 1286-1303. DOI:10.1002/ajb2.1125 |
Endo, S., 1968. The flora from the Eocene Woodwardia formation, Ishikari coal field, Hokkaido, Japan. Bull. Natl. Sci. Mus. (Tokyo), 11: 411-449. |
Epstein, E., 1999. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50: 641-664. DOI:10.1146/annurev.arplant.50.1.641 |
Falcon-Lang, H.J., Cantrill, D.J., 2002. Terrestrial paleoecology of the cretaceous (early Aptian) cerro Negro formation, south Shetland islands, Antarctica: a record of polar vegetation in a volcanic arc environment. Palaios, 17: 491-506. DOI:10.1669/0883-1351(2002)017<0491:TPOTCE>2.0.CO;2 |
Falder, A.B., Stockey, R.A., Rothwell, G.W., 1999. In situ fossil seedlings of a Metasequoia-like taxodiaceous conifer from Paleocene River floodplain deposits of central Alberta, Canada. Am. J. Bot., 86: 900-902. DOI:10.2307/2656710 |
García Massini, J.L., Jacobs, B.F., Tabor, N.J., 2010. Paleobotany and sedimentology of Late Oligocene terrestrial strata from the northwestern Ethiopian Plateau. Palaeontol. Electron., 13: 1-51. |
Gould, R.E., 1968. Morphology of Equisetum laterale Phillips, 1829, and E. bryanii sp. nov. from the Mesozoic of South-eastern Queensland. Aust. J. Bot., 16: 153-176. DOI:10.1071/BT9680153 |
Graham, A., 1963. Systematic revision of the Sucker creek and trout creek Miocene floras of southeastern Oregon. Am. J. Bot., 50: 921-936. DOI:10.1002/j.1537-2197.1963.tb06572.x |
Guillon, J.M., 2004. Phylogeny of horsetails (Equisetum) based on the chloroplast rsp4 gene and adjacent noncoding sequences. Syst. Bot., 29: 251-259. DOI:10.1600/036364404774195467 |
Guo, S.X., 1979. Late Cretaceous and early Tertiary palaeovegetation and its stratigraphical significance on Guangdong and Guangxi provinces. In: Institute of Vertebrate Paleontology and Paleoanthropology and Nanjing Institute of Geology and Palaeontology (Eds.), Mesozoic and Cenozoic Red Beds in South China. Science Press, Beijing, pp. 223–230.
|
Guo, S.X., 2000. New material of the late cretaceous flora from Hunchun of Jilin, northeast China. Acta Palaeontol. Sin., 39: 226-250. |
Hauke, R.L., 1963. A taxonomical monograph of the genus Equisetum subgenus Hippochaete. Nova Hedwigia, 8: 1-123. |
Hauke, R.L., 1978. A taxonomic monograph of Equisetum subgenus Equisetum. Nova Hedwigia, 30: 385-455. |
Hauke, R.L., 1990. Equisetaceae. In: Pteridophytes and Gymnosperms. Springer, Berlin, Heidelberg, pp. 46–48.
|
Heer, O., 1868. Flora fossilis arctica – Die fossile Flora der Polarläander enthaltend die in Nordgrönland, auf der Melville-Insel, im Banksland, am Mackenzie, in Island und in Spitzbergen entdeckten fossilen pflanze. Friedrich Schulthess, Zürich.
|
Hickey, L.J., 1977. Stratigraphy and paleobotany of the Golden Valley Formation (early Tertiary) of western North Dakota. Geol. Soc. Am. Mem.?, 150. |
Hodson, M.J., White, P.J., Mead, A., et al., 2005. Phylogenetic variation in the silicon composition of plants. Ann. Bot., 96: 1027-1046. DOI:10.1093/aob/mci255 |
Hu, Y.Q., Mingram, J., Stebich, M., et al., 2016. A key for the identification of conifer stomata from N.E. China based on fluorescence microscopy. Rev. Palaeobot. Palynol., 233: 12-21. DOI:10.1016/j.revpalbo.2016.06.005 |
Husby, C., 2013. Biology and functional ecology of Equisetum with emphasis on the giant horsetails. Bot. Rev., 79: 147-177. DOI:10.1007/s12229-012-9113-4 |
Huzioka, K., 1963. The Utto flora of Northern Honshu. In: Participants in the Tertiary Paleobotany Project (Ed.), Tertiary Floras of Japan–Miocene Floras. The Collaborating Association to Commemorate the 80th Anniversary of the Geological Survey of Japan. Kawasaki (Japan), Geol. Surv. Jpn, pp. 153–216.
|
Huzioka, K., 1964. The Aniai Flora of Akita Prefecture, and the Aniai-type Floras in Honshu, Japan. J. Mining Coll. Akita Univ. A Ⅲ: 41-105. |
Ina, H., 1992. Miocene vegetational and climatic history of the eastern part of the Setouchi Geologic Province, Japan. Earth Planet Sci. Lett., 39: 47-82. |
Jennings, O.E., 1920. Fossil Plants from the Beds of Volcanic Ash Near Missoula, Western Montana, vol. 8. Board of Trustees of the Carnegie Institute, pp. 385–450.
|
Jeong, E.K., Kim, H.J., Uemura, K., et al., 2017. Miocene fossil plants from the Eoil basin (Gampo area), Gyeongju, Korea. Geosci. J., 21: 483-494. DOI:10.1007/s12303-017-0004-x |
Kaufman, P.B., Bigelow, W.C., Schmid, R., et al., 1971. Electron microscope analysis of silica in epidermal cells of Equisetum. Am. J. Bot., 58: 309-316. DOI:10.1002/j.1537-2197.1971.tb09978.x |
Kelber, K.P., van Konijnenburg-van Cittert, J.H., 1998. Equisetites arenaceus from the Upper Triassic of Germany with evidence for reproductive strategies. Rev. Palaeobot. Palynol., 100: 1-26. DOI:10.1016/S0034-6667(97)00061-4 |
Kerp, H., 1990. The study of fossil gymnosperms by means of cuticular analysis. Palaios, 5: 548-569. DOI:10.2307/3514861 |
Kidston, R., 1892. On the occurrence of the genus Equisetum (E. hemingwayi, Kidston) in the Yorkshire coal-measures. Ann. Mag. Nat. Hist., 9: 138-141. DOI:10.1080/00222939208677287 |
Knowlton, C.H., Ripley, W.S., Weatherby, C.A., 1921. Third report of the committee on floral areas. Rhodora, 23: 209-220. |
Knowlton, F.H., 1898. The Fossil Plants of the Payette Formation. The Mining Districts of the Idaho Basin and the Boise Ridge, Idaho, 18. US Geological Survey Annual Report, pp. 721–44.
|
Knowlton, F.H., 1902. Fossil Flora of the John Day Basin, Oregon, 204. Government Printing Office, US.
|
Knowlton, F.H., 1919. A Catalogue of the Mesozoic and Cenozoic Plants of North America. US Government Printing Office, p. 696.
|
Knowlton, F.H., 1930. The Flora of the Denver and Associated Formations of Colorado, 155–157. US Government Printing Office.
|
Koske, R.E., Friese, C.F., Olexia, P.D., et al., 1985. Vesicular-arbuscular mycorrhizas in Equisetum. Trans. Br. Mycol. Soc., 85: 350-353. DOI:10.1016/S0007-1536(85)80202-3 |
Kundu, S., Hazra, T., Bera, S., et al., 2021. Occurrence of monocot leaf remains from the Siwalik (late Miocene) sediments of Himachal Pradesh, western Himalaya. J. Bot. Soc. Bengal, 75: 151-155. |
LaMotte, R.S., 1944. Supplement to Catalogue of Mesozoic and Cenozoic Plants of North America. US Government Printing Office, pp. 1919–1937.
|
LaMotte, R.S., 1952. Catalogue of the cenozoic plants of North America through 1950. Geol. Soc. Am, 51: 1-381. DOI:10.1130/MEM51-p1 |
Lesquereux, L., 1878. Contributions to the fossil flora of the western territories. Ⅱ. The Tertiary flora. In: Hayden, F.V. (Ed.), Report of the US Geological Survey. Government Printing Office, Washington (DC), pp. 67–69.
|
Lesquereux, L., 1879–1884. Description of the coal flora of the Carboniferous Formation in Pennsylvania and throughout the United States: Second Pennsylvania Geological Survey, 3 vols. Atlas (1879), pp. 1–18, 85 pls; v. 1-2 (1880), pp. 1–694. v. 3 (1884), pp. 695–975.
|
Lesquereux, L., 1883. The Cretaceous and Tertiary Flora: U.S. Geological Survey Territories, Report, 183.
|
Lesquereux, L., 1888. List of fossil plants collected by Mr. I. C. Russell, at Black Creek, near Gadsden, Ala., with descriptions of several new species. Proc. U. S. Natl. Mus., 11: 83-87. DOI:10.5479/si.00963801.11–688.83 |
Li, C.S., 2007. Advances in plant sciences on Equisetum L. (Equisetaceae). Beijing: Higher Education Press.
|
Lowe, A.J., Greenwood, D.R., West, C.K., et al., 2018. Plant community ecology and climate on an upland volcanic landscape during the early Eocene climatic optimum: McAbee fossil beds, British columbia, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol., 511: 433-448. DOI:10.1016/j.palaeo.2018.09.010 |
Ma, H.J., Zhang, S.T., Su, T., et al., 2012. New materials of Equisetum from the upper Miocene of Mangkang, eastern Xizang and its ecological implications. J. Jilin Univ. (Sci. Ed.), 42: 189-195. |
MacGinitie, H.D., 1955. Fossil Plants of the Florissant Beds, 559. Carnegie Institution of Washington Pub, Colorado, pp. 1–198.
|
MacGinitie, H.D., 1969. The Eocene green River flora of northwestern Colorado and northeastern Utah. Univ. Calif. Publ. Geol. Sci., 83: 1-40. |
Macphail, M.K., Alley, N.F., Truswell, E.M., et al., 1994. Early Tertiary vegetation: evidence from spores and pollen. In: Hill, R.S. (Ed.), History of the Australian Vegetation: Cretaceous to Recent. Cambridge University Press, Cambridge, pp. 189–261.
|
Marsh, A.S., Arnone, J.A., Bormann, B.T., et al., 2000. The role of Equisetum in nutrient cycling in an Alaskan shrub wetland. J. Ecol., 88: 999-1011. DOI:10.1046/j.1365-2745.2000.00520.x |
Matsuo, H., 1967. Palaeogene Floras of Northwestern Kyushu. Part 1. The Takashima flora, Kanazawa, pp. 15–90.
|
Matsuo, H., 1971. Palaeogene mega-plant remains of the Tsushima islands, Japan. Bull. Natl. Mus. Nat. Sci. Ser. B, Bot, 14: 671-710. |
McIver, E.E., Basinger, J.F., 1989. The morphology and relationships of Equisetum fluviatoides sp. nov. From the Paleocene Ravenscrag Formation of Saskatchewan, Canada. Canad. J. Bot., 67: 2937-2943. DOI:10.1139/b89-376 |
Mehrotra, R.C., Dilcher, D.L., Lott, T.A., 2009. Notes on elements of the Oligocene flora from the Makum coalfield, Assam, India. Palaeobotanist, 58: 1-9. DOI:10.54991/jop.2009.76 |
Mei, M.T., Tian, B.L., 1988. Floras of Coal-bearing Strata from China. Xuzhou (JS). China Mining and Technology University Publishing House. (In Chinese).
|
Momohara, A., 2005. Paleoecology and history of Metasequoia in Japan, with reference to its extinction and survival in East Asia. In: LePage, B.A., Williams, C.J., Yang, H. (Eds.), The Geobiology and ecology of Metasequoia. Topics in Geobiology. Springer, Dordrecht, pp. 115–136.
|
Myers, J.A., 1991. The early middle Eocene torrey flora, Del mar, California. In: Abbott, P.L., May, L.A. (Eds.), Eocene Geologic History San Diego Region, 201. Pacific section SEPM, Los Angeles (CA).
|
Newberry, J.S., 1898. The Later Extinct Floras of North America, 35. US Government Printing Office, Washington.
|
Parrish, J.T., 1993. Climate of the supercontinent Pangea. J. Geol., 101: 215-233. DOI:10.1086/648217 |
Pavlyutkin, B.I., Petrenko, T.I., Chekryzhov, I.Y., et al., 2020. The plant biostratigraphy of the Cenozoic coal-bearing formations in Primorye, Russian Far East. Int. J. Coal Geol., 220: 103414. DOI:10.1016/j.coal.2020.103414 |
Pearson, L.C., 1995. The Diversity and Evolution of plants. CRC Press.
|
Penhallow, D.P., 1896. Contributions to the Pleistocene Flora of Canada, 2. John Durie and Son, London, England.
|
Penhallow, D.P., 1908. A Report on Tertiary Plants of British Columbia. Government Printing Bureau, Canada, p. 1013 collected by Lawrence M. Lambe in 1906: together with a discussion of previously recorded Tertiary floras.
|
Pole, M., McLoughlin, S., 2017. The first Cenozoic Equisetum from New Zealand. Geobios, 50: 259-265. DOI:10.1016/j.geobios.2017.04.001 |
PPG I, 2016. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol., 54: 563-603. DOI:10.1111/jse.12229 |
Prasad, M., 2008. Angiospermous fossil leaves from the Siwalik foreland and their paleoclimatic implication. Palaeobotanist, 57: 177-215. DOI:10.54991/jop.2008.238 |
Prasad, M., Mohan, L., Singh, S.K., 2013. First record of fossil leaves from Siwalik (Upper Miocene) sediments of Mandi District, Himachal Pradesh, India: palaeoclimatic and phytogeographical implications. Palaeobotanist, 62: 165-180. DOI:10.54991/jop.2013.342 |
Punt, W., 1994. Format of descriptions of new taxa of fossil plants (genera, species). Rev. Palaeobot. Palynol., 80: 7-8. |
Rothwell, G.W., 1996. Pteridophytic evolution: an often-underappreciated phytological success story. Rev. Palaeobot. Palynol., 90: 209-222. DOI:10.1016/0034-6667(95)00084-4 |
Rozefelds, A.C., Dettmann, M.E., Milroy, A.K., et al., 2019. The unexpected, recent history of horsetails in Australia. Aust. Syst. Bot., 32: 255-268. |
Scagel, R.F., Bondini, R.J., Maze, J.R., et al., 1984. Plants, an Evolutionary Survey. Wadsworth Publishing Company, Belmont.
|
Schaffner, J.H., 1930. Geographic distribution of the species of Equisetum in relation to their phylogeny. Am. Fern J., 20: 89-106. DOI:10.2307/1543867 |
Si, X. J., Li, X. X., 1963. Mesozoic fossil plants in China. Beijing: Science Press.
|
Silva–Pineda, A., Leon, M.P.V., Aguilar, F.J., et al., 2009. An upper Pliocene Equisetum (Equisetales) from the Atotonilco el Grande formation in Central Mexico. Paleontol. J., 43: 216-225. DOI:10.1134/S0031030109020142 |
Skog, J.E., Dilcher, D.L., 1994. Lower vascular plants of the Dakota formation in Kansas and Nebraska, USA. Rev. Palaeobot. Palynol., 80: 1-18. DOI:10.1016/0034-6667(94)90089-2 |
Smith, H.V., 1938. Some new and interesting late Tertiary plants from Sucker creek, Idaho-Oregon boundary. J. Torrey Bot. Soc., 65: 557-564. DOI:10.2307/2480794 |
Srikantia, S.V., Bhargava, O.N., 2021. Geology of Himachal Pradesh. J. Geol. Soc. India, 1: 402. |
Stewart, W.N., Rothwell, G.W., 1993. Paleobotany and the Evolution of Plants. second ed. Cambridge: Cambridge University Press.
|
Sun, B.N., Du, B.X., Ferguson, D.K., et al., 2013. Fossil Equisetum from the lower cretaceous in Jiuquan basin, Gansu, northwest China and its paleoclimatic significance. Palaeogeogr. Palaeoclimatol. Palaeoecol., 385: 202-212. DOI:10.1016/j.palaeo.2013.06.005 |
Tao, J.R., Du, N.Q., 1987. The Miocene plants and Betulaceae distribution history in Mangkang. Acta Bot. Sin., 29: 649-655. |
Taylor, T.N., Taylor, E.L., 1993. The Biology and Evolution of Fossil Plants. PrenticeHall, Englewood Cliffs (NJ).
|
Taylor, E.L., Taylor, T.N., Krings, M., 2009. Paleobotany: the Biology and Evolution of Fossil Plants. second ed. USA: Academic Press.
|
Thomasson, J.R., 1980. A fossil Equisetum sp. (family Equisetaceae, subgenus Hippochaetae) from the late tertiary Ash Hollow Formation of Nebraska. Am. J. Bot., 67: 125-127. DOI:10.1002/j.1537-2197.1980.tb07631.x |
Uemura, K., Doi, E., Takahashi, F., 1999. Plant megafossil assemblage from the Kiwado formation (Oligocene) from Ouchiyama-Kami in Yamaguchi Prefectre, western Honshu, Japan. Bull. Mine City Mus., 15: 1-51. |
Uhl, D., Walther, H., Krings, M., 2002. The Palaeogene flora of Hochstetten-Dhaun (Nahe-area, Rhineland-Palatinate, SW-Germany). Feddes Repert., 113: 477-491. DOI:10.1002/fedr.200290000 |
Verdcour, B., 1999. Flora of Tropical East Africa. Equisetaceae. Royal Botanical Gardens, Kew.
|
Villar de Seoane, L., 2005. Equisetites pusillus sp. nov. from the Aptian of Patagonia, Argentina, vol. 7. Revista del Museo Argentino de Ciencias Naturales, pp. 43–49.
|
Weber, R., 2005. Equisetites aequecaliginosus sp. nov., ein Riesenschachtelhalm aus der sp ä ttriassischen Formation Santa Clara, Sonora, Mexiko. Revue De Pal É Obiologie, Gen È Ve, 24: 331-364. |
Wilde, V., 1995. Die Makroflora aus dem Mitteleozän des Geiseltalgebietes, kurze Übersicht und Vergleiche. Hallesches Jahrbuch für Geowissenschaften – Beihefte, 17: 121-138. |
Wu, Z.H., Qin, R.C., 1991. Fern Families and Genera of China. Beijing: Science Press.
|
Yabe, A., 2008. Plant megafossil assemblage from the lower Miocene Ito-o Formation, Fukui Prefecture, Central Japan. Mem. Fukui Prefectural Dinosaur Mus., 7: 1-24. |
Yamamoto, J., Yonesaka, M., 1999. Plant fossils from the upper part of the Shirakawa Formation in Kobe, Hyogo Prefecture, Japan, with reference to their mode of occurrence and inferred Paleoenvironments. Chigakukenkyu, 48: 65-88. |
Yang, G.L., Wangm, Z.X., Chen, J.W., et al., 2016. Equisetum cf. oppositum (Equisetaceae) from the Paleocene-Eocene of Tibet in southwestern China and its paleoenvironmental implications. Arabian J. Geosci., 9: 1-10. DOI:10.1007/978-3-031-02301-9_1 |
Yang, T., Zhang, L., Li, W.J., et al., 2018. New schizothoracine from Oligocene of Qaidam Basin, northern Tibetan Plateau, China, and its significance. J. Vertebr. Paleontol., 38: 2. DOI:10.1007/s12200-017-0753-1 |
Zhang, L.B., 2004. Flora Reipublicae Popularis Sinicae. Beijing: Science Press.
|
Zhang, L.B., Nicholas, J.T., 2012. Flora of China. Beijing: Science Press.
|
Zhang, Y.L., Ferguson, D.K., Ablaev, A.G., et al., 2007. Equisetum cf. pratense (Equisetaceae) from the Miocene of Yunnan in southwestern China and its palaeoecological implications. Int. J. Plant Sci., 168: 351-359. DOI:10.1086/510411 |
Zhou, Z.H., Barrett, P.M., Hilton, J., 2003. An exceptionally preserved lower Cretaceous ecosystem. Nature, 421: 807-814. DOI:10.1038/nature01420 |



