b. College of Life Sciences, Shaanxi Normal University, Xi'an 710119, China;
c. Yunnan Forestry Technological College, Kunming 650224, China
Biotic interactions often vary in predictable ways along environmental gradients, and this variation in turn shapes concomitant clines in ecosystem structure and function, and traits that mediate mutualistic and antagonistic interactions (Schemske et al., 2009; Moeller et al., 2017; Chen et al., 2019). Thus, studying the geographic variation in biotic interactions is often considered central to the explanation of geographic diversity patterns and the prediction for the responses of biodiversity to global change (Moles et al., 2020; Song et al., 2022).
One of the most important biotic interactions is that between plants and pollinators. Most flowering plants (ca. 87.5%) in the world depend on animal pollinators for sexual reproduction (Ollerton et al., 2011). These plants, in turn, play a significant role in maintaining the functional integrity of terrestrial ecosystems. The strength of plant–pollinator interactions has been documented to be particularly important for plant reproductive success and the evolution of floral traits and mating systems (Knight et al., 2005). Mating systems often vary within or among plant lineages, and include complete outcrossers, complete selfers and intermediate selfers (Goodwillie et al., 2005; Raduski et al., 2012; Moeller et al., 2017). Although selfed progeny may often suffer reduced fitness relative to outcrossed progeny due to inbreeding depression, selfing provides the advantage of reproductive assurance in environments disadvantageous to outcrossing (Schoen and Brown, 1991; Morgan and Wilson, 2005). One such environment is the globally distributed high-latitude or alpine life-zone, which is characterized by cold temperatures, variable weather, and high thermal variation (Scherrer and Körner, 2011). These environmental conditions have been shown to decrease pollinator diversity, richness and activity, i.e., pollinator visitation (Arroyo et al., 1982, 1983). One of the most common plant adaptations to low or unpredictable pollinator visitation is autonomous self-pollination (Arroyo et al., 2006; Duan et al., 2007a; Zhang and Li, 2008; Xiong et al., 2013). As a means for assuring seed production, autonomous self-pollination has been hypothesized to be greater in temperate regions or high elevations than in tropical regions or low elevations (Olesen and Jordano, 2002; García-Camacho and Totland, 2009; Sakai et al., 2016). Given that the degree of pollinator dependence decreases with the increase in auto-fertility (mainly through autonomous selfing) (Melathopoulos et al., 2015; Rodger et al., 2021), plants at higher latitudes or higher elevations should be less dependent on pollinators for seed production.
Studies of elevational gradients are excellent proxies for examining clinal variation in biotic interactions, with few confounding effects (Körner, 2007; Galmán et al., 2018). However, ecologists have paid less attention to how pollinator dependence varies along elevational gradients than to how it changes along latitudinal gradients (e.g., Ollerton et al., 2011; Ratto et al., 2018; Rodger et al., 2021). Furthermore, the few studies available have found that pollinator dependence is negatively related (Eriksen et al., 1993; Abdusalam and Li, 2019), positively related (Wirth et al., 2010) or unrelated to elevation (Bingham and Ranker, 2000; Arroyo et al., 2006). Thus, despite widespread interest, there remains no consensus on whether variation in pollinator dependence along elevational gradients is generalizable.
Several factors may account for inconsistencies in the relationship between elevation and pollinator dependence. First, few studies have spanned a large enough elevational gradient, and contrasting sites in two or three regions rather than thoroughly sampling gradients may cloud the evidence (e.g., Song et al., 2020). Second, some studies suggested that intraspecific comparison or studying only a relatively small number of species may result in completely opposite geographical patterns (e.g., Moles et al., 2011a). Third, elevational patterns of pollinator dependence may be diluted by using inconsistent methods (Moles et al., 2011b); for example, for a specific plant species, high self-compatibility does not necessarily reflect the realized ability of autonomous selfing under natural conditions (Wirth et al., 2010).
The Qinghai-Tibet Plateau (QTP), the largest and highest plateau on Earth, varies in elevation from several hundred meters to more than 6000 m, spanning an unusually large elevational range that can be found in only a few regions in the world (Zhang et al., 2016a). Furthermore, due to its high plant diversity and the complexity of local environments (Sun et al., 2017), this area has attracted many evolutionary biologists to probe the mechanisms of pollination adaptation, having a large number of studies with data on reproductive success both when pollinators are experimentally excluded and natural pollinated (Peng et al., 2012, 2014; Sun et al., 2014; Tong et al., 2021). Thus, QTP is an ideal model to quantify the elevational pattern of pollinator dependence using a large-scale study that samples a range of plant species along a wide elevational range.
In addition to elevation, plant reproductive traits might also determine to what extent plants depend on pollinators. Previous research has indicated that autonomous self-fertilization is favored when plant reproductive success is limited by a lack of pollinators or inefficient pollen transfer (Kalisz et al., 2004). These findings suggest that the degree of pollinator dependence decreases with an increase in pollen limitation. Self-incompatible plants depend on pollinators almost completely for seed production. Thus, pollinator dependence is predicted to be higher in self-incompatible plants than in self-compatible plants. Another reproductive trait that may influence pollinator dependence is flower size. Previous studies have shown that flower size is often positively correlated with the ability of plants to autonomously self-fertilize (Randle et al., 2009; Toräng et al., 2017), indicating that pollinator dependence may increase with flower size. Because pollinator diversity, abundance and activity are higher during the middle of the flower growing season (Körner, 2003), plants are also predicted to be more dependent on pollinators when they flower during the middle of the growing season than when they flower at the beginning or the end of the growing season. Finally, flower longevity and the presence or absence of nectar may influence pollinator dependence. Studies have shown that pollinators are more likely to visit flowers with longer longevity or nectar (Primack, 1985; Larson and Barrett, 2000). Thus, plants are more likely to depend on pollinators if they have long-lived flowers or are nectariferous than if they have short-lived flowers or are nectariless.
In this study, to test the above hypotheses, we compiled a data set of fruit or seed production in pollination experiments for animal-pollinated flowering plants in QTP region. Specifically, we aimed to answer the following questions: (1) What is the degree of pollinator dependence for the flowering plants growing in the QTP region? (2) Is there an overall elevational gradient in pollinator dependence? (3) Which reproductive traits of plants are significantly associated with the degree of pollinator dependence? By addressing these questions, this study delivers the first large-scale assessment of elevational gradient in pollinator dependence in the QTP region. It sheds light on the possible effects of pollinator declines on plant reproduction under global change and species distribution.
2. Materials and methods 2.1. Study areaThe study covered the geographical range of the QTP, extending from the southern edge of the Himalayan Range to the northern edge of the Kunlun Mountains, and from the western boundary of the Pamir Mountains to the eastern edge of the Hengduan Mountains. This area covered ca. 2, 600, 000 km2, and most of it lies at an elevation of more than 4000 m a.s.l (Fig. 1a). The study area consists of three major regions: the Hengduan Mountains to the East, the Himalayas in the South and Tibetan Plateau (Zhang et al., 2002; Liu et al., 2022). The main topographic features include very high mountains, lower mountains, hills, plains and tablelands (Pan et al., 2004). Due to the complicated geographical features and environmental heterogeneity, the QTP contains various climates and vegetation types, including tropical season forests to subtropical forests and alpine scree, and harbors extremely high plant diversity and numerous endemic species (Zhang et al., 2016a; Sun et al., 2017). Thus, the Hengduan Mountains and the eastern Himalayas have been regarded as two of the most important biodiversity hotspots in the world (Myers et al., 2000; Sun et al., 2017).

|
| Fig. 1 Data set of pollinator dependence in populations of flowering plants in the Qinghai-Tibet Plateau. (a) Geographical distribution, (b) frequency distribution of pollinator dependence index among the total sample of species investigated, and (c) phylogenetic distribution of species level. Yellow bars indicate the degree of pollinator dependence in each species. |
Our database was created by searching the Web of Science, Google Scholar, a Chinese publications database, CNKI (Chinese National Knowledge Infrastructure) for the following keywords: "Tibet" or "Xizang" or "Yunnan" or "Sichuan" or "Gansu" or "Qinghai" or "Xinjiang" or "Himalaya" or "Hengduan" or "Qinghai-Tibetan" in combination with the terms "pollination" or "reproduct" or "mating system" or "fruit set" or "seed set" We also checked reference lists in papers through a database search and paper collections of authors to include as many studies as possible from the study area. The search was completed 10 July 2022. Our initial search yielded 1730 articles. After screening the titles and abstracts of these articles, 90 articles were deemed relevant to our study.
2.3. Data collectionWe collected data according to several criteria: 1) studies of animal-pollinated plants were included, whereas studies of abiotic-pollinated plants were excluded (e.g., wind-pollinated), following Rodger et al. (2021); 2) only studies conducted in the QTP region were considered; 3) studies were included that involved an experiment where pollinators were excluded using a physical barrier such as a paper bag, and plant reproductive success was measured in the presence and absence of pollinators; 4) only studies made under field conditions in wild populations were considered; those conducted in glasshouses were not included because, under such experimental conditions, pollinator dependence may differ from those occurring in wild populations.
For fruit set, seed set and seed number data, means, SEs, or SDs and sample sizes of both natural pollination and bagged treatments were extracted from the text, tables, or figures. For data expressed in figures, we obtained the values using the WEBPLOTDIGITIZER (v.4.1) image tool. For species with diclinous flowers, we defined their reproductive output under bagged treatments as 0 when it was not reported. For each study, we collected the latitude, longitude, and elevation of the study population when reported, or used Google Earth to obtain the coordinate values according to the location description. Studies were excluded if they contained no clear information on the study site location. The study sites we included spanned more than 3600 m of elevation (from 990 m a.s.l. to 4620 m a.s.l.).
To understand the associations of pollinator dependence with various reproductive traits, data on several key traits were collected, including on pollen limitation, compatibility status, flower size, flowering time, floral longevity and reward type. Following the method of Larson and Barrett (2000), the pollen limitation index was calculated as 1- (percentage fruit set or seed set in natural pollination/percentage fruit set or seed set in supplemental cross pollination). Compatibility status was classified into self-compatible and self-incompatible. When the information on compatibility status was not directly reported in the collected papers, we calculated the index of self-incompatibility (ISI) with the reproductive success of self-pollinated treatments and cross-pollinated treatments from the collected papers. Self-incompatible species were defined by the criterion of having ISI value ≥ 0.8, and species with an index < 0.8 were considered self-compatible (Grossenbacher et al., 2017). Conventionally, species that are dioecious are classified as self-incompatible (Raduski et al., 2012). Flower size was recorded from the collected papers or Flora of China, based on the maximum length or width of the corolla (Stratton, 1989; Song et al., 2022). Floral longevity was defined as the length of time a flower remained open (i.e., from anthesis to flower senescence) under natural pollination conditions following Ashman (2004) and Song et al. (2022). Pollination rewards of a plant were divided into two types, nectariferous or nectariless, according to descriptions from the collected papers or other studies on the same species. Following the method of Jiang and Xie (2020), flowering time was classified into three categories: early spring (before May), summer (May to August), and autumn (after August), given the seasonal patterns of diversity, abundance and activity capacity of pollinators in this region.
2.4. Data analysesGiven that many data points collected in our sample reported no clear standard deviation or error, use of weighted regression would have excluded too many data points. We used the unweighted mean pollinator dependence for each data point in our analyses, which is a commonly used method (e.g., Turcotte et al., 2014; Zhang et al., 2016b; Song et al., 2022). We used the pollinator dependence index to quantify the degree of pollinator dependence. Following the methods of Melathopoulos et al. (2015) and Rodger et al. (2021), the pollinator dependence index, the proportional contribution of pollinators to plant reproduction for each record, was calculated as (reproductive output in natural pollination treatments - reproductive output in pollinator exclusion treatments)/reproductive output in natural pollination treatments. When reproductive output in natural treatments was zero (1 record) or reproductive output in pollinator exclusion treatments was higher than that in natural pollination treatments (4 records), the pollinator dependence index was treated as zero (Rodger et al., 2021). To maintain the independence of data from individual studies, the pollinator dependence index for a given species conducted at the same site within a reference was averaged (e.g., Zhang et al., 2016b; Song et al., 2022). Before analyses, data on pollinator dependence indexes was log10-transformed for normality (Moles et al., 2009).
To determine whether the pollinator dependence index was constrained by phylogeny, we conducted phylogenetic analyses. First, we determined the taxonomical status of each species using the 'plantlist' package (Zhang et al., 2022) and then generated a species-level phylogenetic tree for the plant species included in our data set using the package U.PhyloMaker (Jin and Qian, 2023). Specifically, we used the megatree GBOTB.extended TPL.tre (Jin and Qian, 2022), which was derived from the megatrees reported in Smith and Brown (2018) and Zanne et al. (2014), as a phylogenetic backbone, and the functions build.nodes.1 and Scenario 3 (Jin and Qian, 2022; Qian et al., 2023) to generate the phylogenetic tree. Subsequently, we quantified the phylogenetic signal (K statistic) of the pollinator dependence index using the phylosignal function in the PICANTE package (Kembel et al., 2010). Given the significant phylogenetic signal for the pollinator dependence index among species (see the Results section), we constructed a variance-covariance matrix by transforming the phylogenetic tree using the vcv.phylo function in the package PHYTOOLS (Revell, 2012). This matrix was included in subsequent analyses.
To test the relationships between pollinator dependence index and elevation, we performed Bayesian phylogenetic mixed models using the brm function in the BRMS package (Bürkner, 2017), with elevation as a fixed effect and species as random effects. Species was set as a random-effect factor in the model to account for interspecific variation in pollinator dependence. Phylogenetic variance-covariance matrix was also included in the models to account for the phylogenetic relationships among species.
Next, we tested the associations of pollinator dependence index with six reproductive traits (pollen limitation index, compatibility status, flower size, flowering time, floral longevity, and reward type) using Bayesian phylogenetic mixed models, described above. In these models, each of the potential predictor variables was considered separately as fixed-effect terms.
All statistical analyses were performed in R (v.4.2.2; R Core Team, 2022).
3. ResultsOur literature survey extracted information from 90 studies of 121 locations, spanning almost all vegetation types in the QTP (Fig. 1a). Our data set consists of 200 data points comprising 161 species–site combinations, and 112 angiosperm species from 30 families (Appendix S1). Of the studies examined, 58 were from the Hengduan Mountains, 7 from the Himalayas, and 22 from the Tibetan Plateau. Two studies were conducted in two subregions simultaneously. Species from the following families were represented most: Gentianaceae (15.5%), Primulaceae (10.5%), Orchidaceae (10.0%), Orobanchaceae (9.5%), Asteraceae (7.0%) and Lamiaceae (5.5%).
3.1. Pollinator dependence of the flowering plants in the QTP regionOverall, the pollinator dependence index was strongly and negatively correlated with the ability of autonomous selfing (R2 = 0.67; Fig. S1). For the 112 species sampled, pollinators contributed 76.2% of seed production [95% confidence interval = 69.8%–82.6%] (Fig. 1b), with a median of 98.2% (95% confidence interval = 93.6%–100%). Pollinators made at least some contribution to seed production in 97.3% of species (i.e., pollinator dependence index > 0), and only 2.7% of species were able to produce seeds in the absence of pollinators (pollination dependence index = 0). A total of 67.9% of species needed animal pollinators for at least 80% of their seed production (i.e., pollinator dependence index ≥ 0.8). Of these species, 44.6% depended completely on pollinators for seed production (i.e., pollinator dependence index = 1) (Fig. 1b), indicating that these plant species would produce no seed without pollinator visitation. Pollinator dependence index exhibited a significant phylogenetic signal (K = 0.15, P < 0.01; Fig. 1c), indicating that related species within our data set have a similar reliance on pollinators for seed production.
3.2. Associations of pollinator dependence with elevation and subregionContrary to our prediction, there was no significant elevational gradient in pollinator dependence (Fig. 2a), and this was maintained in analyses after accounting for latitude (Table 1) or in analyses that included only self-compatible species (slope = 0.0000096, 95% credible intervals: −0.0001 to 0.0001). Similarly, no significant quadratic association between pollinator dependence and elevation was detected (Table S1). Given that vegetation types vary from forests at low elevations to alpine grasslands and the subnival belt at high elevations (Fig. S2), we pooled these data to determine the effect of vegetation type on pollinator dependence, but no significant effect was found (Fig. 2b). When we divided the QTP region into three subregions, there was no significant difference in pollinator dependence among subregions; in addition, pollinator dependence did not change along an elevational gradient within any subregion (Fig. S3). Taken together, these findings indicate that pollinator dependence does not decrease along elevational gradients in the QTP.
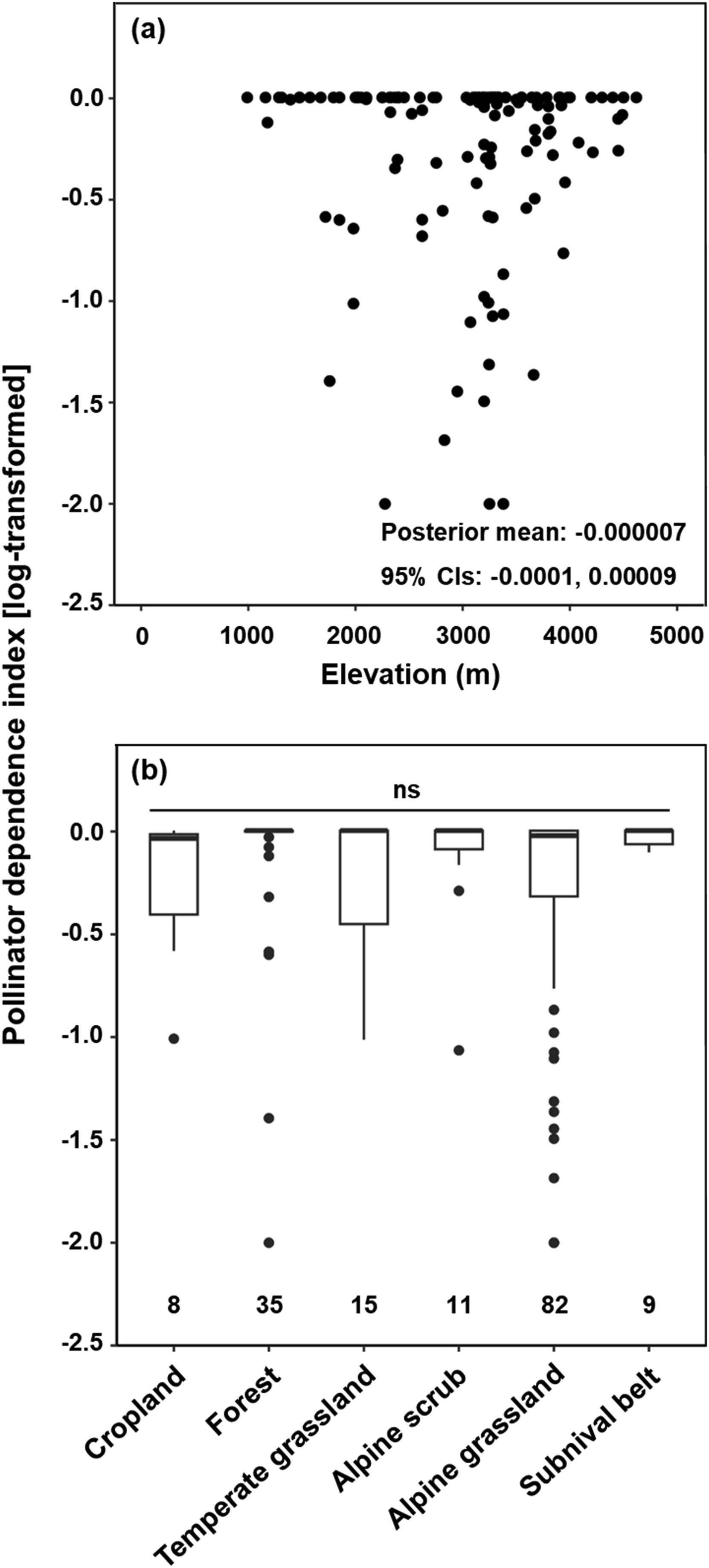
|
| Fig. 2 Relationships between pollinator dependence index and elevation (a) and vegetation type (b) for a sample of 183 species–site combinations (122 angiosperm species). Analyses were based on Bayesian phylogenetic mixed models with study species as random effect. In panel (a), each point represents the pollinator dependence of one species at one study site. There is no statistically significant relationship if the 95% credible intervals (CIs) of the slope includes zero. In panel (b), the boxes indicate the first and third quartiles, the horizontal lines the median, vertical lines the range, and points represent outliers. ns denotes that the 95% credible intervals (CIs) of the slope include zero (meaning that this is not significant). |
| Model | Fixed effect | Posterior mean | 0.025 quantile | 0.975 quantile |
| 1 | Elevation | −0.000068 | −0.0001 | 0.00009 |
| 2 | Elevation + Latitude | |||
| Elevation | 0.0000062 | −0.000098 | 0.00011 | |
| Latitude | 0.017 | −0.057 | 0.041 | |
The pollinator dependence index was not significantly correlated with pollen limitation index, flower size, floral longevity, or reward type. However, we found that the pollinator dependence index was significantly correlated with compatibility status and flowering time (Fig. 3), although these reproductive traits explained little variance (R2 = 0.064 and 0.042, respectively). Specifically, self-incompatible species were more dependent on pollinators than were self-compatible species (Fig. 3b). In addition, the pollinator dependence index was lower in late-flowering plants than in early- or mid-flowering plants (Fig. 3d).
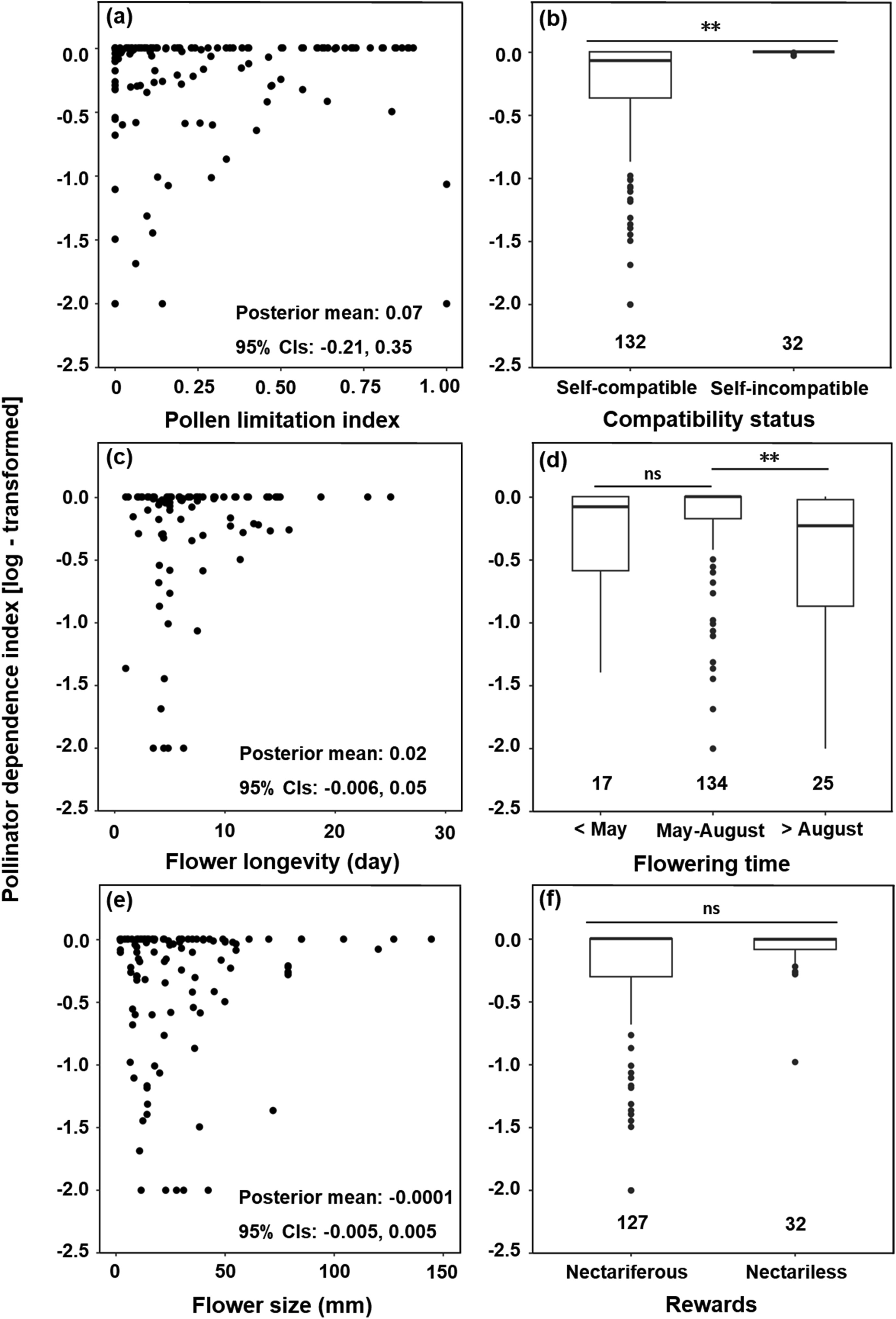
|
| Fig. 3 Relationships between pollinator dependence index and six reproductive traits: (a) pollen limitation, (b) compatibility status, (c) floral longevity, (d) flowering time, (e) flower size, and (f) reward type. Analyses were based on Bayesian phylogenetic mixed models with study species as random effect. In (a, c, e), each point represents the pollinator dependence index of one species at one study site. There is no statistically significant relationship if the 95% credible intervals (CIs) of the slope includes zero. In panel (b, d, f), the boxes indicate the first and third quartiles, the horizontal lines the median, vertical lines the range, and black points represent outliers. ∗∗ denotes that the 95% CIs do not include zero (meaning that this is significant). |
Study of large-scale geographic variation in plant–pollinator interactions is important for both basic and applied questions in community structure and function, the evolution of floral traits, and the development of optimal conservation strategies (Burkle and Alarcón, 2011). Several studies have investigated the patterns of elevational variation in reproductive traits and reproductive processes associated with pollinators at global or regional scales, such as pollen limitation (García-Camacho and Totland, 2009; Jiang and Xie, 2020), breeding system (Vamosi and Queenborough, 2010), flower size (Kuriya et al., 2015), floral longevity (Song et al., 2022), and pollination specialization (Lara-Romero et al., 2019). We provide the first regional-scale assessment of elevational gradients in key reproductive traits – pollinator dependence. Our study reveals that flowering plants depend substantially on pollinators for sexual reproduction in the QTP region. Contrary to our expectation, there was no significant elevational variation in pollinator dependence, indicating that flowering plants at higher elevations are not less dependent on pollinators for seed production, at least in our study region. We also found that two reproductive traits, including compatibility status and flowering time, had a significant effect on the degree of pollinator dependence.
4.1. Pollinator dependence of the flowering plants in the QTP regionAlthough autonomous self-fertilization is common in flowering plants (ca. 20% of plants use self-fertilization as their predominant mating strategy) (Barrett, 2002), our results showed that 97.3% of animal-pollinated flowering plants depended to some extent on pollinators for seed production in the QTP region. Overall, pollinators contributed to 76.2% of seed production for animal-pollinated flowering plants, which is 16.2% higher than the global average level of 60% (Rodger et al., 2021). Meanwhile, compared with other important mountains (several global biodiversity hotspots) around the world, our results also showed a much higher proportion of plant species that rely on pollinators for all of their seed production in QTP region (45% of species depend completely on pollinators; the corresponding values are 21%, 24% and 27% for Alps in Europe, Rocky Mountains in North America, and Andes Mountains in South America, respectively; Rodger et al., 2021). Together, these results suggest that flowering plants experience a much higher degree of pollinator dependence in the QTP region, such that seed production in most plant species may be reduced due to reductions in pollinator visitation.
Several factors may explain why flowering plants are highly dependent on pollinators for seed production in the QTP region. Firstly, the harsh environmental conditions in the QTP region may drive plants to outcross as the deleterious effects of inbreeding depression are expected to be more pronounced under stressful rather than benign conditions (Armbruster and Reed, 2005). Secondly, the QTP region has a high pollinator diversity, with bees, which are effective pollinators, performing the majority of pollinator services (Tong et al., 2021). Thirdly, plants in the QTP region possess diverse reproductive strategies that may intensify their interactions with pollinators. For example, "glasshouse plants" (Song et al., 2013, 2014, 2015), "cushion plants" (Chen et al., 2021), "woolly plants" (Peng et al., 2015), and plants with sun-tracking flowers (Zhang et al., 2010) provide brood-sites or play nurse roles for pollinators. Lastly, the QTP is one of the cleanest regions on earth. In contrast to much of the world, where pollinator services have been negatively affected by agricultural intensification, habitat fragmentation, and urbanization (Bennett et al., 2020), the ecological conservation redline established over the last decades by the Chinese government has prevented land use changes that would decrease pollination services in the QTP region (The State Council Information Office of the People's Republic of China, 2018).
4.2. Association of pollinator dependence with elevationWe found no evidence for an elevational gradient in pollinator dependence index, regardless of whether we examined the whole QTP or subregions of the QTP (i.e., Hengduan Mountains, Himalaya, and Tibetan Plateau; Fig. S3). Nor did pollinator dependence index vary significantly along an elevational gradient when we analyzed only self-compatible species. Furthermore, the pollinator dependence index did not vary with vegetation type, which generally varies with elevation. In other words, plants in higher elevations (e.g., subnival belt, the highest vegetation belt) did not show a lower pollinator dependence compared to plants at lower elevations (e.g., forests or temperate grasslands). These data, like data for many other reproductive traits (e.g., García-Camacho and Totland, 2009; Maglianesi et al., 2015), are not consistent with the traditional idea that plants at high elevations show lower levels of pollinator dependence (Arroyo et al., 2006; Körner and Paulsen, 2009). The prediction of lower levels of pollinator dependence at high elevations is based on the assumption that low levels of pollinator diversity, abundance, and activity may limit the pollen transfer of plants at high elevations (Arroyo et al., 1982, 2006; Bingham and Orthner, 1998). However, empirical studies and meta-analyses have tended to not support the hypothesis that pollination efficiency is lower at high elevations (e.g., Bingham and Orthner, 1998; Duan et al., 2007b; García-Camacho and Totland, 2009; Trunschke and Stöcklin, 2017; Ma et al., 2023; but Jiang and Xie, 2020). For example, a study conducted in the Rocky Mountains showed that although pollinator diversity and visitation rate were lower in high-elevation populations of Campanula rotundifolia (Campanulaceae), pollination efficiency was higher as primary pollinators have shifted from less efficient solitary bees to highly efficient bumblebees (Bingham and Orthner, 1998). Another study conducted at the community level in the Andes Mountains found that the pollinator number per plant species did not differ between high and low elevations (Medan et al., 2002). Furthermore, even if pollinator availability is really lower at high elevations, plants growing in these areas have often evolved a series of adaptive traits. For example, in the subnival belt of the Hengduan Mountains, showy flowers (attracting pollinators) and generalized floral traits (suited to a wide range of pollinators) are common (Peng et al., 2012), which can to some extent compensate for the scarcity of pollinators in high alpine areas (Arroyo et al., 2006). In addition, low pollinator availability does not necessarily translate to strong pollen limitation because reproductive output of plants is commonly constrained by both pollen availability and provisioning resources (Haig and Westoby, 1988). Thus, harsher environments (e.g., cold temperature and low resource availability) experienced in high elevations may constrain plant reproduction more than pollen limitation by constraining photosynthetic activity (Totland, 2001) and carbon transfer to the ovary from leaves and storage structures (Devlin and Witham 1983; Song et al., 2013). Under such circumstances, selection for autonomous self-fertilization as a mode of reproductive assurance due to pollination deficit may be partly counteracted by resource limitation in harsh alpine environments.
4.3. Association of pollinator dependence with various reproductive traitsThe reproductive assurance hypothesis predicts that low pollinator dependence should be selected for in environments where a lack of mates or pollinators limits outcross reproduction (Kalisz et al., 2004; Eckert et al., 2006). However, our data showed that the pollen limitation index had no significant effect on the pollinator dependence index (Fig. 3a). Indeed, a large number of empirical studies that quantified the relationships between pollinator dependence and pollen limitation or pollination environments have not supported the reproductive assurance hypothesis (e.g., Herlihy and Eckert, 2005; Koski et al., 2017). One possible explanation for why pollinator dependence and pollen limitation are not correlated in this study, similar to previous studies, is that plant adaptations of pollinators (e.g., the degree of pollinator dependence and ability to autonomously self-fertilize) are driven by historical pollination conditions, but the rates at which these traits evolve is much slower than the rates at which environmental conditions change (Koski et al., 2017). The congruence of evidence from our comprehensive synthesis of data from the literature and a series of intra-specific studies (e.g., Herlihy and Eckert, 2005; Koski et al., 2017) strongly suggests that the widely accepted reproductive assurance hypothesis needs to be reconsidered (Busch and Delph, 2012).
Our results confirmed our prediction that seed production was more dependent on pollinators in self-incompatible than in self-compatible plants (Fig. 3b). However, the association between pollinator dependence index and compatibility status was unexpectedly weak, explaining only 6.4% of the variation. This may be explained by the high outcrossing rates in many self-compatible plants (e.g., Duan et al., 2005, 2007a; Zhang et al., 2011; Peng et al., 2016). In fact, among the 82 self-compatible species in our data set, 41.5% (34 species) did not have the ability to self autonomously at all (Fig. S4). Although selfing can provide reproductive assurance when lack of pollinators or inefficient pollen transfer limit reproductive success, it also has detrimental effects on reproductive fitness, such as inbreeding depression, gamete discounting, and seed discounting (Goodwillie et al., 2005; Eckert et al., 2006). Indeed, genetic self-incompatibility is only one way in which plants can diminish selfing. Plants have evolved several mechanisms to avoid selfing, such as heterostyly, physiological self-incompatibility, and spatial or temporal separation of male and female functions (herkogamy and dichogamy, respectively) (reviewed by Li et al., 2022).
We predicted that plant dependence on pollinators would increase in long-lived or nectariferous flowers (Ashman, 2004; Pyke, 2016). However, the pollinator dependence index was not significantly affected by either floral longevity or the presence or absence of nectar (Fig. 3c, f). Indeed, studies of the relationships between floral longevity and pollination efficiency or pollination environments have provided mixed results with little concordance among comparative studies (e.g., Trunschke and Stöcklin, 2017; Song et al., 2022). For example, Ishii and Sakai (2000) found that extended floral longevity did not increase fruit set in Erythronium japonicum (Liliaceae), whereas Foster and Caruso (2022) showed that increased floral longevity even resulted in a reduction in seed production by 34% in Lobelia siphilitica (Campanulaceae). The lack of a significant difference in the degree of pollinator dependence between nectariferous and nectariless plants may be the result of the artificial dichotomy between them, as previous studies have shown that nectar amount and secreting dynamics have significant effects on pollinator visitation (Guerra et al., 2014; Li et al., 2021). One additional explanation is that nectar is not the only reward for pollinators (Cook et al., 2002).
Previous studies have suggested that pollinators are scarce at the beginning and the end of the growing season (Körner, 2003). However, we found that in the QTP region, pollinator dependence was only lower in late-flowering plants (Fig. 3d). This finding is consistent with those obtained from other regions (Molau, 1993; Gugerli, 1998). For example, in northern Sweden, early-flowering species have been shown to outbreed at high rates, and a great fraction of late-flowering plants can self or are apomictic (Molau, 1993). One possible explanation for the high outcrossing and pollinator dependence in early-flowering plants is that pollinator presence may have been underestimated (Körner, 2003).
Since small flowers are often associated with self-compatible plants and large flowers are often associated with self-incompatible plants (Thomann et al., 2013), we expected that the dependence on pollinators would decrease with reduced flower size. However, no significant relationship between pollinator dependence index and flower size was found in our study. One possible reason for this finding is that the fitness cost of autonomous selfing can offset the reproductive assurance, resulting in a mixed mating system, even a high outcrossing rate, in many self-compatible plants as discussed above (Kalisz et al., 2004; Song et al., 2022).
4.4. Potential effects of pollinator decline on plant pollination under global changeThe persistence of populations and/or their migration to new sites demands sustained seed production. Mounting evidence indicates a rapid decline in pollinator richness and density at a global scale due to global change, including habitat loss and fragmentation, agrochemicals, pathogens, alien species and climate warming (Potts et al., 2010; Millard et al., 2021). The high reliance on pollinators for seed production observed in our study leads us to speculate that if plant reproductive strategies cannot adapt to pollinator declines by decreasing reliance on pollinators, seed production in these plants will decrease severely in response to climate change (Thomann et al., 2013). Indeed, the rates at which adaptive traits evolve do not always match the rate at which pollination environments change (Thomann et al., 2013). Furthermore, several studies have found no relationship between pollinator dependence and pollination environment or pollen limitation (e.g., Koski et al., 2017; 2019), including the findings of our investigation (Fig. 3a). Pollinator declines are likely to affect plants with specific traits and plants that grow in specific habitats. For example, pollinator declines will likely strongly affect plants that are self-incompatible and early-flowering plants because these plants are more dependent on pollinators than self-compatible plants or mid-flowering plants. Habitats such as the subnival belt are more likely to suffer pollinator declines, greatly affecting plants that depend on pollinators in these habitats (Post et al., 2009; Miller-Struttmann et al., 2022).
5. ConclusionsOur synthesis provides the first large-scale assessment of elevational gradients in pollinator dependence. We found that flowering plants growing in QTP region, which encompasses several important global biodiversity hotspots, are highly dependent on pollinators. We also found that pollinator dependence did not decrease with increasing elevation in the QTP. Our study suggests that plants in QTP region, especially self-incompatible or early-flowering plants growing at high elevations (e.g., the subnival belt), may be particularly sensitive to pollinator declines caused by global change.
AcknowledgementsThis work was supported by the Second Tibetan Plateau Scientific Expedition and Research program (2019QZKK0502), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20050203), the National Natural Science Foundation of China (31770249 and 32071669), the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2017HB062), the Ten-thousand Talents Program of Yunnan Province (YNWR-QNBJ-2018-208), and the Yunnan Innovation Team Project (202305AS350004).
Author contributions
B.S. and H.S. conceived and designed the study. Y.W.X., R.M., and Y.Q.G. collected the data. L.S. and Y.W.X. analyzed the data. B.S. and Y.W.X wrote the draft of the manuscript. All authors read, commented and approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendice A and B. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.03.006
Abdusalam, A., Li, Q.-J., 2019. Elevation-related variation in the population characteristics of distylous Primula nivalis affects female fitness and inbreeding depression. Plant Divers., 41: 250-257. DOI:10.1016/j.pld.2019.06.004 |
Armbruster, P., Reed, D.H., 2005. Inbreeding depression in benign and stressful environments. Heredity, 95: 235-242. DOI:10.1038/sj.hdy.6800721 |
Arroyo, M.T.K., Armesto, J.J., Primack, R., 1983. Tendencias altitudinales y latitudinales en mecanismos de polinización en la zona andina de los Andes templados de Sudamérica. Rev. Chil. Hist. Nat., 56: 159-180. |
Arroyo, M.T.K., Muñoz, M.S., Henríquez, C., et al., 2006. Erratic pollination, high selfing levels and their correlates and consequences in an altitudinally widespread above-tree-line species in the high Andes of Chile. Acta Oecol., 30: 248-257. DOI:10.1016/j.actao.2006.05.006 |
Arroyo, M.T.K., Primack, R., Armesto, J., 1982. Community studies in pollination ecology in the high temperate Andes of central Chile. I. Pollination mechanisms and altitudinal variation. Am. J. Bot., 69: 82-97. DOI:10.1002/j.1537-2197.1982.tb13237.x |
Ashman, T.L., 2004. Flower longevity. In: Nooden, L.D. (Ed.), Plant Cell Death Process. Elsevier, London, U. K, pp. 349–362.
|
Barrett, S.C., 2002. The evolution of plant sexual diversity. Nat. Rev. Genet., 3: 274-284. DOI:10.1038/nrg776 |
Bennett, J.M., Steets, J.A., Burns, J.H., et al., 2020. Land use and pollinator dependency drives global patterns of pollen limitation in the Anthropocene. Nat. Commun., 11: 1-6. DOI:10.1038/s41467-019-13993-7 |
Bingham, R.A., Orthner, A.R., 1998. Efficient pollination of alpine plants. Nature, 391: 238-239. DOI:10.1038/34564 |
Bingham, R.A., Ranker, T.A., 2000. Genetic diversity in alpine and foothill populations of Campanula rotundifolia (Campanulaceae). Int. J. Plant Sci., 161: 403-411. DOI:10.1086/314272 |
Burkle, L.A., Alarcón, R., 2011. The future of plant-pollinator diversity: understanding interaction networks across time, space, and global change. Am. J. Bot., 98: 528-538. DOI:10.3732/ajb.1000391 |
Bürkner, P.C., 2017. brms: an R package for Bayesian multilevel models using Stan. J. Stat. Software, 80: 1-28. |
Busch, J.W., Delph, L.F., 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Ann. Bot., 109: 553-562. DOI:10.1093/aob/mcr219 |
Chen, J.-G., Zhang, Y.-Z., Zhang, H.-R., et al., 2021. The positive effects of the alpine cushion plant Arenaria polytrichoides on insect dynamics are determined by both physical and biotic factors. Sci. Total Environ., 762: 143091. DOI:10.1016/j.scitotenv.2020.143091 |
Chen, S.-C., Tamme, R., Thomson, F.J., et al., 2019. Seeds tend to disperse further in the tropics. Ecol. Lett., 22: 954-961. DOI:10.1111/ele.13255 |
Cook, S.M., Bartlet, E., Murray, D.A., et al., 2002. The role of pollen odour in the attraction of pollen beetles to oilseed rape flowers. Entomol. Exp. Appl., 104: 43-50. DOI:10.1046/j.1570-7458.2002.00989.x |
Devlin, R.M., Witham, F.H., 1983. Plant Physiology, 4th edn. Willard Grant, Boston.
|
Duan, Y.-W., He, Y.-P., Liu, J.-Q., 2005. Reproductive ecology of the Qinghai-Tibet Plateau endemic Gentiana straminea (Gentianaceae), a hermaphrodite perennial characterized by herkogamy and dichogamy. Acta Oecol., 27: 225-232. DOI:10.1016/j.actao.2005.01.003 |
Duan, Y.-W., He, Y.-P., Zhang, T.-F., et al., 2007a. Delayed selfing in an alpine species Gentianopsis barbata. Chin. J. Plant Ecol., 31: 110. DOI:10.17521/cjpe.2007.0014 |
Duan, Y.-W., Zhang, T.-F., Liu, J.-Q., 2007b. Pollination biology of Anisodus tanguticus (Solanaceae). Biodivers. Sci., 15: 584-591. DOI:10.1360/biodiv.070108 |
Eckert, C.G., Samis, K.E., Dart, S., 2006. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder, L.D., Barrett, S.C.H. (Eds.), Ecology and evolution of flowers. Oxford University Press, U.K., pp. 183-203.
|
Eriksen, B., Molau, U., Svensson, M., 1993. Reproductive strategies in two arctic Pedicularis species (Scrophulariaceae). Ecography, 16: 154-166. DOI:10.1111/j.1600-0587.1993.tb00067.x |
Foster, O., Caruso, C.M., 2022. Evidence for a cost of increased floral longevity in female and hermaphrodite Lobelia siphilitica (Campanulaceae). Int. J. Plant Sci., 183: 186-192. DOI:10.1086/718386 |
Galmán, A., Abdala-Roberts, L., Zhang, S., et al., 2018. A global analysis of elevational gradients in leaf herbivory and its underlying drivers: effects of plant growth form, leaf habit and climatic correlates. J. Ecol., 106: 413-421. DOI:10.1111/1365-2745.12866 |
García-Camacho, R., Totland, Ø., 2009. Pollen limitation in the alpine: a meta-analysis. Arctic Antarct. Alpine Res., 41: 103-111. DOI:10.1657/1523-0430-41.1.103 |
Goodwillie, C., Kalisz, S., Eckert, C.G., 2005. The evolutionary enigma of mixed mating system in plants: occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst., 36: 47-79. DOI:10.1146/annurev.ecolsys.36.091704.175539 |
Grossenbacher, D.L., Brandvain, Y., Auld, J.R., et al., 2017. Self-compatibility is over-represented on islands. New Phytol., 215: 469-478. DOI:10.1111/nph.14534 |
Guerra, T.J., Galetto, L., Silva, W.R., 2014. Nectar secretion dynamic links pollinator behavior to consequences for plant reproductive success in the ornithophilous mistletoe Psittacanthus robustus. Plant Biol., 16: 956-966. DOI:10.1111/plb.12146 |
Gugerli, F., 1998. Effect of elevation on sexual reproduction in alpine populations of Saxifraga oppositifolia (Saxifragaceae). Oecologia, 114: 60-66. DOI:10.1007/s004420050420 |
Haig, D., Westoby, M., 1988. On limits to seed production. Am. Nat., 131: 757-759. DOI:10.1086/284817 |
Herlihy, C.R., Eckert, C.G., 2005. Evolution of self-fertilization at geographical range margins? A comparison of demographic, floral, and mating system variables in central vs. peripheral populations of Aquilegia canadensis (Ranunculaceae). Am. J. Bot., 92: 744-751. DOI:10.3732/ajb.92.4.744 |
Ishii, H.S., Sakai, S., 2000. Optimal timing of corolla abscission: experimental study on Erythronium japonicum (Liliaceae). Funct. Ecol., 14: 122-128. DOI:10.1046/j.1365-2435.2000.00401.x |
Jiang, X.-F., Xie, Y.-P., 2020. Meta-analysis reveals severe pollen limitation for the flowering plants growing in East Himalaya-Hengduan Mountains region. BMC Ecology, 20: 1-9. DOI:10.1186/s12898-019-0268-2 |
Jin, Y., Qian, H., 2022. V.PhyloMaker2: an updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers., 44: 335-339. DOI:10.1016/j.pld.2022.05.005 |
Jin, Y., Qian, H., 2023. U.PhyloMaker: an R package that can generate large phylogenetic trees for plants and animals. Plant Divers., 45: 347-352. DOI:10.1016/j.pld.2022.12.007 |
Kalisz, S., Vogler, D.W., Hanley, K.M., 2004. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature, 430: 884-887. DOI:10.1038/nature02776 |
Kembel, S.W., Cowan, P.D., Helmus, M.R., et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26: 1463-1464. DOI:10.1093/bioinformatics/btq166 |
Knight, T.M., Steets, J.A., Vamosi, J.C., et al., 2005. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst., 36: 467-497. DOI:10.1146/annurev.ecolsys.36.102403.115320 |
Körner, C., 2003. Alpine Plant Life. Springer Verlag, Berlin.
|
Körner, C., 2007. The use of 'altitude' in ecological research. Trends Ecol. Evol., 22: 569-574. DOI:10.1016/j.tree.2007.09.006 |
Körner, C., Paulsen, J., 2009. Exploring and explaining mountain biodiversity. In: Spehn, E.M., Körner, C. (Eds.), Data mining for global trends in mountain biodiversity. CRC Press, Boca Raton, Florida, U.S.A.
|
Koski, M.H., Galloway, L.F., Busch, J.W., 2019. Pollen limitation and autonomous selfing ability interact to shape variation in outcrossing rate across a species range. Am. J. Bot., 106: 1240-1247. DOI:10.1002/ajb2.1342 |
Koski, M.H., Grossenbacher, D.L., Busch, J.W., et al., 2017. A geographic cline in the ability to self-fertilize is unrelated to the pollination environment. Ecology, 98: 2930-2939. DOI:10.1002/ecy.2001 |
Kuriya, S., Hattori, M., Nagano, Y., et al., 2015. Altitudinal flower size variation correlates with local pollinator size in a bumblebee-pollinated herb, Prunella vulgaris L. (Lamiaceae). J. Evol. Biol., 28: 1761-1769. DOI:10.1111/jeb.12693 |
Lara-Romero, C., Seguí, J., Pérez-Delgado, A., et al., 2019. Beta diversity and specialization in plant-pollinator networks along an elevational gradient. J. Biogeogr., 46: 1598-1610. |
Larson, B.M., Barrett, S.C., 2000. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc., 69: 503-520. DOI:10.1111/j.1095-8312.2000.tb01221.x |
Li, D.-F., Yan, X.-C., Lin, Y., et al., 2021. Do flowers removed of either nectar or pollen attract fewer bumblebee pollinators? An experimental test in Impatiens oxyanthera. AoB Plants, 13: plab029. DOI:10.1093/aobpla/plab029 |
Li, M.-R., Sui, Y., Wang, X.-N., et al., 2022. High outcrossing rates in a self-compatible and highly aggregated host-generalist mistletoe. Mol. Ecol., 31: 6489-6504. DOI:10.1111/mec.16720 |
Liu, J., Milne, R.I., Zhu, G.-F., et al., 2022. Name and scale matter: clarifying the geography of Tibetan Plateau and adjacent mountain regions. Global Planet. Change, 215: 103893. DOI:10.1016/j.gloplacha.2022.103893 |
Ma, Y.-M., Cha, Y.-P., Tong, Z.-L., et al., 2023. The nonlinear change in pollinator assemblages and self-mating syndromes of Primula atrodentata along elevation gradients. J. Plant Ecol., 16: rtac109. DOI:10.1093/jpe/rtac109 |
Maglianesi, M.A., Blüthgen, N., Böhning-Gaese, K., et al., 2015. Functional structure and specialization in three tropical plant-hummingbird interaction networks across an elevational gradient in Costa Rica. Ecography, 38: 1119-1128. DOI:10.1111/ecog.01538 |
Medan, D., Montaldo, N.H., Devoto, M., et al., 2002. Plant-pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arctic Antarct. Alpine Res., 34: 233-241. DOI:10.2307/1552480 |
Melathopoulos, A.P., Cutler, G.C., Tyedmers, P., 2015. Where is the value in valuing pollination ecosystem services to agriculture?. Ecol. Econ., 109: 59-70. DOI:10.1016/j.ecolecon.2014.11.007 |
Millard, J., Outhwaite, C.L., Kinnersley, R., et al., 2021. Global effects of land-use intensity on local pollinator biodiversity. Nat. Commun., 12: 1-11. DOI:10.1038/s41467-020-20314-w |
Miller-Struttmann, N., Miller, Z., Galen, C., 2022. Climate driven disruption of transitional alpine bumble bee communities. Global Change Biol., 28: 6165-6179. DOI:10.1111/gcb.16348 |
Moeller, D.A., Briscoe Runquist, R.D., Moe, A.M., et al., 2017. Global biogeography of mating system variation in seed plants. Ecol. Lett., 20: 375-384. DOI:10.1111/ele.12738 |
Molau, U., 1993. Relationships between flowering phenology and life history strategies in tundra plants. Arct. Alp. Res., 25: 391-402. DOI:10.2307/1551922 |
Moles, A.T., Bonser, S.P., Poore, A.G., et al., 2011a. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Funct. Ecol., 25: 380-388. DOI:10.1111/j.1365-2435.2010.01814.x |
Moles, A.T., Laffan, S.W., Keighery, M., et al., 2020. A hairy situation: plant species in warm, sunny places are more likely to have pubescent leaves. J. Biogeogr., 47: 1934-1944. DOI:10.1111/jbi.13870 |
Moles, A.T., Wallis, I.R., Foley, W.J., et al., 2011b. Putting plant resistance traits on the map: a test of the idea that plants are better defended at lower latitudes. New Phytol., 191: 777-788. DOI:10.1111/j.1469-8137.2011.03732.x |
Moles, A.T., Warton, D.I., Warman, L., et al., 2009. Global patterns in plant height. J. Ecol., 97: 923-932. DOI:10.1111/j.1365-2745.2009.01526.x |
Morgan, M.T., Wilson, W.G., 2005. Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution, 59: 1143-1148. |
Myers, N., Mittermeier, R.A., Mittermeier, C.G., et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Olesen, J.M., Jordano, P., 2002. Geographic patterns in plant-pollinator mutualistic networks. Ecology, 83: 2416-2424. |
Ollerton, J., Winfree, R., Tarrant, S., 2011. How many flowering plants are pollinated by animals?. Oikos, 120: 321-326. DOI:10.1111/j.1600-0706.2010.18644.x |
Pan, B.-T., Gao, H.-S., Li, B.-Y., et al., 2004. Step-like landforms and uplift of the Qinghai-Xizang plateau. Quat. Sci., 24: 50-57. DOI:10.1186/1471-2202-5-50 |
Peng, D.-L., Niu, Y., Song, B., et al., 2015. Woolly and overlapping leaves dampen temperature fluctuations in reproductive organ of an alpine Himalayan forb. J. Plant Ecol., 8: 159-165. DOI:10.1093/jpe/rtv014 |
Peng, D.-L., Ou, X.-K., Xu, B., et al., 2014. Plant sexual systems correlated with morphological traits: reflecting reproductive strategies of alpine plants. J. Syst. Evol., 52: 368-377. DOI:10.1111/jse.12046 |
Peng, D.-L., Song, B., Yang, Y., et al., 2016. Overlapping leaves covering flowers in the alpine species Eriophyton wallichii (Lamiaceae): key driving factors and their potential impact on pollination. PLoS One, 11: e0164177. DOI:10.1371/journal.pone.0164177 |
Peng, D.-L., Zhang, Z.-Q., Niu, Y., et al., 2012. Advances in the studies of reproductive strategies of alpine plants. Biodivers. Sci., 20: 286-299. DOI:10.3166/ejc.18.286-300 |
Post, E., Forchhammer, M.C., Bret-Harte, M.S., et al., 2009. Ecological dynamics across the Arctic associated with recent climate change. Science, 325: 1355-1358. DOI:10.1126/science.1173113 |
Potts, S.G., Biesmeijer, J.C., Kremen, C., et al., 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol., 25: 345-353. DOI:10.1016/j.tree.2010.01.007 |
Primack, R.B., 1985. Longevity of individual flowers. Annu. Rev. Ecol. Syst., 16: 15-37. DOI:10.1146/annurev.es.16.110185.000311 |
Pyke, G.H., 2016. Floral nectar: pollinator attraction or manipulation?. Trends Ecol. Evol., 31: 339-341. DOI:10.1016/j.tree.2016.02.013 |
Qian, H., Zhang, J., Jiang, M., 2023. Global patterns of taxonomic and phylogenetic diversity of flowering plants: biodiversity hotspots and coldspots. Plant Divers., 45: 265-271. DOI:10.1016/j.pld.2023.01.009 |
R Core Team, 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
|
Raduski, A.R., Haney, E.B., Igić, B., 2012. The expression of self-incompatibility in angiosperms is bimodal. Evolution, 66: 1275-1283. DOI:10.1111/j.1558-5646.2011.01505.x |
Randle, A.M., Slyder, J.B., Kalisz, S., 2009. Can differences in autonomous selfing ability explain differences in range size among sister-taxa pairs of Collinsia (Plantaginaceae)? An extension of Baker's Law. New Phytol., 183: 618-629. DOI:10.1111/j.1469-8137.2009.02946.x |
Ratto, F., Simmons, B.I., Spake, R., et al., 2018. Global importance of vertebrate pollinators for plant reproductive success: a meta-analysis. Front. Ecol. Environ., 16: 82-90. DOI:10.1002/fee.1763 |
Revell, L.J., 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol., 3: 217-223. DOI:10.1111/j.2041-210X.2011.00169.x |
Rodger, J.G., Bennett, J.M., Razanajatovo, M., et al., 2021. Widespread vulnerability of flowering plant seed production to pollinator declines. Sci. Adv., 7: eabd3524. DOI:10.1126/sciadv.abd3524 |
Sakai, S., Metelmann, S., Toquenaga, Y., et al., 2016. Geographical variation in the heterogeneity of mutualistic networks. R. Soc. Open Sci., 3: 150630. DOI:10.1098/rsos.150630 |
Schemske, D.W., Mittelbach, G.G., Cornell, H.V., et al., 2009. Is there a latitudinal gradient in the importance of biotic interactions?. Annu. Rev. Ecol. Evol. Syst., 40: 245. DOI:10.1146/annurev.ecolsys.39.110707.173430 |
Scherrer, D., Körner, C., 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J. Biogeogr., 38: 406-416. DOI:10.1111/j.1365-2699.2010.02407.x |
Schoen, D.J., Brown, A.H., 1991. Whole-and part-flower self-pollination in Glycine clandestina and G. argyrea and the evolution of autogamy. Evolution, 45: 1651-1664. DOI:10.2307/2409786 |
Smith, S.A., Brown, J.W., 2018. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot., 105: 302-314. DOI:10.1002/ajb2.1019 |
Song, B., Chen, G., Stöcklin, J., et al., 2014. A new pollinating seed-consuming mutualism between Rheum nobile and a fly fungus gnat, Bradysia sp., involving pollinator attraction by a specific floral compound. New Phytol., 203: 1109-1118. DOI:10.1111/nph.12856 |
Song, B., Stöcklin, J., Peng, D.-L., et al., 2015. The bracts of the alpine 'glasshouse' plant Rheum alexandrae (Polygonaceae) enhance reproductive fitness of its pollinating seed-consuming mutualist. Bot. J. Linn. Soc., 179: 349-359. DOI:10.1111/boj.12312 |
Song, B., Sun, L., Barrett, S.C., et al., 2022. Global analysis of floral longevity reveals latitudinal gradients and biotic and abiotic correlates. New Phytol., 235: 2054-2065. DOI:10.1111/nph.18271 |
Song, B., Sun, L., Lev-Yadun, S., et al., 2020. Plants are more likely to be spiny at mid-elevations in the Qinghai-Tibetan Plateau, south-western China. J. Biogeogr., 47: 250-260. DOI:10.1111/jbi.13724 |
Song, B., Zhang, Z.-Q., Stöcklin, J., et al., 2013. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas. Oecologia, 172: 359-370. DOI:10.1007/s00442-012-2518-2 |
Stratton, D.A., 1989. Longevity of individual flowers in a Costa Rican cloud forest: ecological correlates and phylogenetic constraints. Biotropica, 21: 308-318. DOI:10.2307/2388281 |
Sun, H., Niu, Y., Chen, Y.-S., et al., 2014. Survival and reproduction of plant species in the Qinghai-Tibet Plateau. J. Syst. Evol., 52: 378-396. DOI:10.1111/jse.12092 |
Sun, H., Zhang, J.-W., Deng, T., et al., 2017. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers., 39: 161-166. DOI:10.1016/j.pld.2017.09.004 |
The State Council Information Office of the People's Republic of China, 2018. Ecological Progress on the Qinghai-Tibet Plateau. http://english.www.gov.cn/archive/white_paper/2018/07/18/content_281476227186598.htm.
|
Thomann, M., Imbert, E., Devaux, C., et al., 2013. Flowering plants under global pollinator decline. Trends Plant Sci., 18: 353-359. DOI:10.1016/j.tplants.2013.04.002 |
Tong, Z.-Y., Wu, L.-Y., Huang, S.-Q., 2021. Reproductive strategies of animal-pollinated plants on high mountains: a review of studies from the "Third Pole". J. Syst. Evol., 59: 1159-1169. DOI:10.1111/jse.12680 |
Toräng, P., Vikström, L., Wunder, J., et al., 2017. Evolution of the selfing syndrome: anther orientation and herkogamy together determine reproductive assurance in a self-compatible plant. Evolution, 71: 2206-2218. DOI:10.1111/evo.13308 |
Totland, Ø., 2001. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology, 82: 2233-2244. DOI:10.1890/0012-9658(2001)082[2233:EDPLAS]2.0.CO;2 |
Trunschke, J., Stöcklin, J., 2017. Plasticity of flower longevity in alpine plants is increased in populations from high elevation compared to low elevation populations. Alpine Bot., 127: 41-51. DOI:10.1007/s00035-016-0176-4 |
Turcotte, M.M., Thomsen, C.J., Broadhead, G.T., et al., 2014. Percentage leaf herbivory across vascular plant species. Ecology, 95: 788. DOI:10.1890/13-1741.1 |
Vamosi, S.M., Queenborough, S.A., 2010. Breeding systems and phylogenetic diversity of seed plants along a large-scale elevational gradient. J. Biogeogr., 37: 465-476. DOI:10.1111/j.1365-2699.2009.02214.x |
Wirth, L.R., Graf, R., Gugerli, F., et al., 2010. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (Boraginaceae). Am. J. Bot., 97: 899-901. DOI:10.3732/ajb.0900297 |
Xiong, Y.-Z., Fang, Q., Huang, S.-Q., 2013. Pollinator scarcity drives the shift to delayed selfing in Himalayan mayapple Podophyllum hexandrum (Berberidaceae). AoB Plants, 5: plt037. |
Zanne, A.E., Tank, D.C., Cornwell, et al., 2014. Three keys to the radiation of angiosperms into freezing environments. Nature, 506: 89-92. DOI:10.1038/nature12872 |
Zhang, D.-C., Ye, J.-X., Sun, H., 2016a. Quantitative approaches to identify floristic units and centres of species endemism in the Qinghai-Tibetan Plateau, south-western China. J. Biogeogr., 43: 2465-2476. DOI:10.1111/jbi.12819 |
Zhang, J. -L., Liu, B., Liu, S., et al., 2022. Helixcn/plantlist: looking up the status of plant Scientific names based on the plant list database. Searching the Chinese Names and Making checklists of plants R package version 0.8.0. Available from https://github.com/helixcn/plantlist/.
|
Zhang, S., Ai, H.-L., Yu, W.-B., et al., 2010. Flower heliotropism of Anemone rivularis (Ranunculaceae) in the Himalayas: effects on floral temperature and reproductive fitness. Plant Ecol., 209: 301-312. DOI:10.1007/s11258-010-9739-4 |
Zhang, S., Zhang, Y.-X., Ma, K.-M., 2016b. Latitudinal variation in herbivory: hemispheric asymmetries and the role of climatic drivers. J. Ecol., 104: 1089-1095. DOI:10.1111/1365-2745.12588 |
Zhang, Y., Li, B., Zheng, D., 2002. A discussion on the boundary and area of the Tibetan Plateau in China. Geogr. Res., 21: 1-8. |
Zhang, Z.-Q., Kress, W.J., Xie, W.-J., et al., 2011. Reproductive biology of two Himalayan alpine gingers (Roscoea spp., Zingiberaceae) in China: pollination syndrome and compensatory floral mechanisms. Plant Biol., 13: 582-589. DOI:10.1111/j.1438-8677.2010.00423.x |
Zhang, Z.-Q., Li, Q.-J., 2008. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae). Ann. Bot., 102: 531-538. DOI:10.1093/aob/mcn136 |



