b. College of Life Sciences, Yunnan University, Shengming Kexueyuan Building #2, Chenggong Campus, Dongwaihuan South Road, University Town, Chenggong New District, Kunming, Yunnan 650504, China;
c. Institute of Highland Forest Science, Chinese Academy of Forestry, Kunming, Yunnan 650224, China;
d. Forestry and Forest Products Research Institute, Forest Research and Management Organization, Matsunosato 1, Tsukuba-shi, Ibaraki-ken, 305-8687, Japan;
e. Faculty of Life & Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba, Ibaraki, 305-8572, Japan;
f. College of Natural Resources, Yunnan University, Chenggong Campus, Dongwaihuan South Road, University Town, Chenggong New District, Kunming, Yunnan 650504, China;
g. Forestry and Grassland Bureau of Diqing Tibetan Autonomous Prefecture, 1st Floor of Government Building, Shengping Town, Deqing County, Yunnan 674500, China;
h. Forestry and Grassland Bureau of Weixi Lisu Autonomous County, Nanjian Dao #7, Baohe Town, Weixi Lisu Autonomous County, Yunnan 674600, China
Current patterns of biodiversity are the outcome of both historical and recent ecological conditions. Relict plants, especially relict trees, which have been able to survive impressive environmental changes over millions of years, are thus important sources of valuable information about both past and recent biogeographical and evolutionary processes (Kozlowski and Gratzfeld, 2013). Relict species also provide insight into the effects of anthropogenic activities, as these species usually suffer more severely from human activities than do non-relict populations (Habel and Assmann, 2010). China has multiple refugia for many relict species (Tang, 2015; Tang et al., 2018). However, anthropogenic impacts in the past have reduced the range of many relict plants to small and fragmented habitats. Rare and relict plants are the most vulnerable component of the flora, as the loss of any of them means an irreparable loss for biodiversity as a whole. Therefore, studies on current status of its forests and populations are an important task for effective conservation of plant diversity.
Historically, the genus Pseudotsuga was widespread in the Northern Hemisphere, dating to the Eocene in the USA (Schorn, 1994), the Oligocene in the USA and Germany (Lakhanpal, 1958; Denk et al., 2005), the Miocene and Pliocene in North America, Europe and East Asia (e.g. Tanai and Suzuki, 1963; Ozaki, 1991; Denk et al., 2005; Palamarev et al., 2005; Gugger et al., 2010; Yabe, 2011; Kunzmann, 2014). Present genus Pseudotsuga has a disjunct distribution, and is confined to western North America, Mexico, and eastern Asia (China and Japan) (Lavender and Hermann, 2014). The number of extant species has long been debated, but two in western North America (P. menziesii, P. macrocarpa) and four in eastern Asia (P. sinensis, P. forrestii, and P. brevifolia in China, P. japonica in Japan) are generally recognized. A species previously known as P. wilsoniana is now treated as P. sinensis var. wilsoniana (FOC, 2021).
Pseudotsuga forrestii is now narrowly distributed in small forest patches and isolated stands in southwestern China. The timber is used for construction, bridge building, vehicles, coffins, and furniture, as well as landscaping (Sun et al., 2003; Kong et al., 2018). Excessive deforestation, utilization and habitat degradation have greatly reduced the number of P. forrestii (Fu, 1992; Sun et al., 2003), which is a vulnerable species according the IUCN Criteria in China (Qin et al., 2017) and is listed as a second-grade protected plant of China.
Preliminary studies described Pseudotsuga forrestii distribution (Kong et al., 2018) and distributional suitability in Dayao County, Yunnan (Jin et al., 2021), as well as population characteristics in Deqin County, Yunnan (Li et al., 2022). To date, the forests containing P. forrestii as the 1st dominant have not been systematically studied. In this study, we clarify (1) P. forrestii forest types and characteristics; (2) population structure in terms of DBH, age, and seedling recruitment; and (3) growth trends across its entire distribution range in China.
2. Material and methods 2.1. Study areasWe made efforts to search forests containing Pseudotsuga forrestii as the 1st dominant in its distribution range in China. We investigated 49 forest plots in Deqin, Weixi and Lanping Counties in northwestern Yunnan, and Chayu County in southeastern Tibet (Fig. 1). Deqin and Weixi counties belong to Diqing Tibetan Autonomous Prefecture and are located in the three parallel rivers (Nu River, Lancang River and Jinsha River) region. The Yunling Mountains are the main mountain ranges, in which the Jinsha and Lancang Rivers (Mekong River) are located. The Yunling Mountains extend from north to south and are strongly cut. Lanping County is situated in Nujiang Lisu Autonomous Prefecture, located in the middle of the Hengduan Mountains; the terrain is a mountain plain canyon. The main mountain ranges in the county are the two major mountain systems bounded by the Lancang River (Mekong River), the Yunling Mountains in the east and the Nujiang Mountains in the west, with the main body of each mountain range running north-south and the mountains gradually decreasing from north to south. Chayu County is located in the western section of the Hengduan Mountains in southeastern Tibet. The typical landforms are high mountains and mountain valleys.
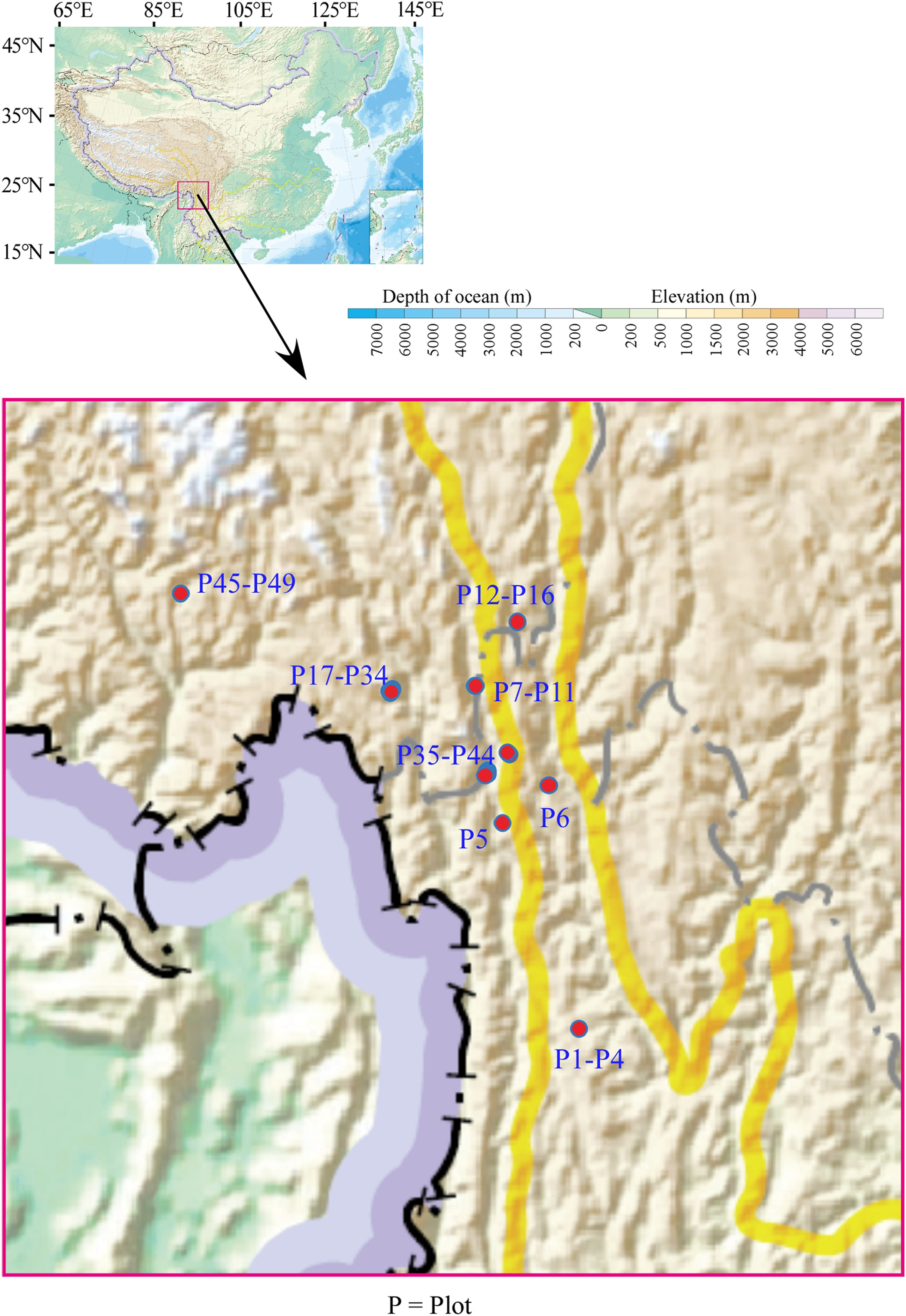
|
| Fig. 1 The distribution of plots of forest stands containing Pseudotsuga forrestii as the 1st dominant. |
Elevations of the study plots ranged from 2400 to 3300 m a.s.l. Information on environmental characteristics of plots is shown in Table S1.
The climate of study plots is largely controlled in summer by the Indian Ocean monsoon. The annual mean temperature is -2–12.4 ℃. The mean temperature of the warmest month, July, is 3.7–19.7 ℃ and of the coldest month, January, is -7.8–4.3 ℃. The annual mean precipitation is 527–1100 mm, and the evapotranspiration is 329–682 mm. The moisture index is 1.
2.2. SpeciesPseudotsuga forrestii has been treated as P. sinensis var. forrestii (Silba, 1990), or P. sinensis subsp. forrestii (Silba, 2008). In addition, both P. sinensis var. forrestii and P. sinensis subsp. forrestii have been treated as a synonym of P. sinensis var. sinensis (Farjon, 2010; Govaerts, 2011). However, in the most updated English version Flora of China (FOC, 2021) it is treated as a species Pseudotsuga forrestii. We follow the Flora of China (FOC, 2021), treating it as an independent species P. forrestii. It is monoecious, evergreen coniferous canopy tree species. Its bark is dark brown-gray, rough, and deeply fissured longitudinally. Leaves are pectinately arranged and subsessile. Seed cones are ovoid. Seed scales at the middle of the cones are suborbicular or rhombic-orbicular, glabrous abaxially, and the base is cuneate-orbicular. Bracts are obviously longer than seed scales, reflexed, and the cusp is lanceolate (FOC, 2021). P. forrestii is distributed in the Hengduan Mountains, including the mountain areas at elevations of 2400–3300 m in northwestern Yunnan, western Sichuan, and southeastern Tibet. Forest communities of P. forrestii are only found in northwestern Yunnan and southeastern Tibet. Trees of P. forrestii are scattered in western Sichuan, however, no forest communities containing P. forrestii as the 1st dominant are found.
2.3. Data collection and analysesWe established 49 plots that consisted of various types of Pseudotsuga forrestii-dominated plant communities in southwestern China, including in Yunnan and Tibet. The plot size was from 20 m × 20 m–20 m × 40 m based on the smallest area for the maximum number of species. We divided each plot into 10 m × 10 m subplots. For the species in each plot, all individuals at least 1.3 m tall were identified to the species level, numbered and tagged, and their diameter at breast height (DBH) and height were recorded. In addition, general information about each plot was noted, such as slope position, elevation, slope exposure, slope inclination, as well as human disturbance history. Woody stems (height ≥1.3 m) in the overstory were classified into two categories based on their vertical position and height: arborous layer (height ≥ 5 m) and shrub layer (1.3 m ≤ height < 5 m tall). According to the general canopy heights of the investigated forests, the arborous layer included emergent (height > 25 m), canopy (10 m ≤ height ≤ 25 m), and subcanopy (5 m ≤ height < 10 m) sublayers. For all woody species less than 1.3 m tall in the understory, each individual was identified to species level, counted, and measured for height and percent cover. In each plot, we set up five 1 m × 1 m squares to investigate the herbaceous taxa in the understory. Five 1 m × 1 m squares were respectively located in the four corners and the center of each subplot. Herb taxa in the understory were identified and the coverage and number of individuals of each species were recorded.
We obtained 44 increment cores from Pseudotsuga forrestii trees of varying DBHs in the study area. For each tree trunk, a single increment core was taken from 1.3 m above ground level. The length of time from the position at 1.3 m in height to ground level was estimated to be eight years based on the tree rings in the stem base of saplings with a height ≤ 1.3 m. Thus, eight years was added to ages we obtained from each increment core. Tree age was determined using the software WinDENRO tree ring analysis system. From this analysis, we were also able to determine ring widths and to calculate basal area increments (BAI). The following formula was used to calculate BAI: T-Y, where T is the basal area at year X (last year of growth) and Y is the basal area of the tree measured up to the year previous to X. BAI is used in forest growth studies because it accurately quantifies wood production based on the ever-increasing diameter of a growing tree (Rubino and McCarthy, 2000).
For all woody individuals ≥ 1.3 m tall, DBH was used to calculate basal area and then basal area (BA) for each species found in a plot. To measure the abundance of species, we used relative importance value (RIV) = (Relative density + Relative basal area)/2 for species in the overstory, and RIV = (Relative density + Relative coverage)/2 for species in the understory. Plant communities were classified using a floristic similarity dendrogram with Correlation and Flexible Beta Method [PCORD software (McCune and Mefford, 1999)]. The communities were named by dominant/indicator species of the overstory. Diversity was calculated for each forest stand using species richness (number of species), Pielou's evenness index E, Shannon–Wiener's diversity index H′ (Pielou, 1969) and Simpson's diversity index D (Lande, 1996). Differences in species richness and diversity indices among habitats were analyzed by the nonparametric Kruskal–Wallis all-pairwise comparisons test, using Analyze-it software (United Kingdom).
3. Results 3.1. Forest types and stratificationFloristic similarity cluster analysis (at a 38% floristic similarity threshold) classified 49 vegetation plots into four distinct forest communities (Fig. 2A).

|
| Fig. 2 (A) Dendrogram of floristic similarity for 49 plots; (B) The frequency distribution in height-classes of woody species (height ≥ 1.3 m) of each forest type. |
Forest community Type 1 is Pseudotsuga forrestii - Quercus guyavifolia - Acer davidii evergreen coniferous and broad-leaved mixed forest. Type 1 forest communities occur at elevations of 2613–3020 m in Hexi Xiang, Lanping County and Yunling Xiang, Deqin County, northwestern Yunnan, Chawa Xiang, Chayu County, southeastern Tibet. These forests are mainly distributed on slopes nearby villages.
Forest community Type 2 is Pseudotsuga forrestii - Pinus yunnanensis - Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest. Type 2 forest communities occur at elevations of 2670–2990 m in Yunling Xiang, Deqin County, northwestern Yunnan. In these forests, human activities such as road construction and logging have been common.
Forest community Type 3 is Pseudotsuga forrestii evergreen coniferous forest. Type 3 forest communities occur at elevations of 2420–3280 m in Badi Xiang, Weixi County and Xiaruo Xiang, Deqin County, northwestern Yunnan and Chayu County, southeastern Tibet. These forests are mainly found on steep slopes along rivers in gorges, locations in which natural disturbances are common.
Forest community Type 4 is Pseudotsuga forrestii - Abies georgei var. smithii evergreen coniferous forest. This forest community type occurs at elevations of 2660–3040 m in Walong Xiang, Chayu County, southeastern Tibet. This forest type is located on the slopes of valleys that have a river running through them.
Each forest type had multiple layers of stratification, including an arborous layer, shrub layer and understory. The vertical stratification of woody species ≥ 1.3 m tall is shown in Fig. 2B.
In Pseudotsuga forrestii - Quercus guyavifolia - Acer davidii evergreen coniferous and broad-leaved mixed forest (Type 1), P. forrestii reached 28 m tall in the emergent sublayer, and absolutely dominated the canopy (10–25 m). Evergreen sclerophyllous Q. guyavifolia trees co-dominated the canopy. A. davidii dominated the subcanopy. Accompanying species were Quercus variabilis, Pinus armandii, Pinus wallichiana, etc. In the shrub layer, besides the saplings of canopy species including Quercus semecarpifolia, A. davidii, Taxus wallichiana var. chinensis, etc., there were species of Rhododendron, Hippophae, Jasminum, Platycarya, etc. In the understory, there were Pteris cretica, Fragaria vesca, Dendrocalamus membranaceus, etc.
In Pseudotsuga forrestii - Pinus yunnanensis - Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest (Type 2), P. forrestii reached 28 m tall, and dominated the canopy (10–25 m). P. yunnanensis reached 27 m in the emergent sublayer, and with P. forrestii and Q. guyavifolia co-dominated the subcanopy. It was accompanied by trees of Acer oliverianum, P. wallichiana, Abies delavayi, etc. The shrub layer was dominated by saplings of Q. guyavifolia and shrub Rhododendron decorum, accompanied by saplings of P. forrestii, and Platycarya strobilacea. The bamboo Dendrocalamus membranaceus predominated the understory. Few herbaceous species were present.
In Pseudotsuga forrestii evergreen coniferous forest (Type 3), P. reached 42 m tall and occupied the emergent sublayer (25–42 m). P. forrestii dominated the canopy and subcanopy. Quercus guyavifolia and Tsuga dumosa, etc. were companions. The shrub layer was dominated by saplings of P. forrestii and Pinus armandii, and the shrub Rhododendron rubiginosum. In the understory, the bamboo D. membranaceus and two herbaceous species, Fragaria vesca and Eriophorum comosum, were dominants.
In Pseudotsuga forrestii - Abies georgei var. smithii evergreen coniferous forest (Type 4), a few P. forrestii trees reached 31 m tall in the emergent sublayer, while A. georgei var. smithii reached 35 m tall. P. forrestii and A. georgei var. smithii co-dominated the canopy, and P. forrestii dominated the subcanopy, which included accompanying species Pinus armandii, Picea asperata, etc. In the shrub layer, there were mainly saplings of P. forrestii, Tsuga dumosa, and the shrubs Rhododendron decorum, Rhamnus davurica, etc. In the understory, Fragaria vesca and bamboo D. membranaceus were dominants. Few herbaceous species were present.
The representative Pseudotsuga forrestii forest stands and their habitats are shown in Fig. 3A–F.

|
| Fig. 3 Pseudotsuga forrestii and its representative forest stands and habitats. (A) The seed corn of P. forrestii; (B) P. forrestii forest at ca. 2890 m a.s.l. along steep slopes by a river in Foshan Xiang, Deqin County, Yunnan; (C) P. forrestii forest at ca. 2970 m a.s.l. in Foshan Xiang, Deqin County, Yunnan; (D) Humid understory, with mosses, of a P. forrestii forest, where prayer flags were hung by local villagers at ca. 2900 m a.s.l. in Yunling Xiang, Deqin County; (E) P. forrestii forest at ca. 2890 m a.s.l. in Chawalong Xiang, Chayu County, Tibet; (F) P. forrestii forest at ca. 2880 m a.s.l. in Chawalong Xiang, Chayu County, Tibet. Photographs: Xin–Xin Zhu (A), Shi-Qian Yao (B), (C), (D) and (F); Zi Wang (E). |
We recorded a total of 166 plant species in 71 families and 127 genera in all the Pseudotsuga forrestii forest communities. Among all the taxa, 12 species in 2 families and 8 genera were gymnosperms, 143 species in 62 families and 109 genera were angiosperms, 11 species in 7 families and 10 genera were ferns. P. forrestii forests have both warm temperate and temperate affinities. Specifically, approximately 20% of families and 11% of the genera consisted of tropical elements, whereas 8% of the families and 28% of the genera consisted of temperate elements.
The species composition of the arborous layer, shrub layer and understory of each forest type is shown in Tables S2–S4, respectively.
Species richness and diversity indices of each forest type are shown in Fig. 4A–E. For each forest type, the number of woody species varied between 18–84. Pseudotsuga forrestii evergreen coniferous forest (Type 3) had the highest number (84) of woody species (Fig. 4A). The average number of woody species among plots of each forest type ranged from 8 to 11. Simpson diversity, Pielou evenness, Shannon–Wiener diversity indices ranged from 0.75 to 0.76, 0.74–0.81, and 1.62–1.93, respectively. The average number of woody species did not differ significantly between forest types, nor did diversity indices (Fig. 4B–E).

|
| Fig. 4 Woody species (height ≥ 1.3 m) richness and diversity of each forest type. Type 1: Pseudotsuga forrestii - Quercus guyavifolia - Acer davidii evergreen coniferous and broad-leaved mixed forest; Type 2: Pseudotsuga forrestii - Pinus yunnanensis - Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest; Type 3: Pseudotsuga forrestii evergreen coniferous forest; Type 4: Pseudotsuga forrestii - Abies georgei var. smithii evergreen coniferous forest. In (B)–(E), forest sharing the same letters do not differ significantly by the non-parametric Kruskal–Wallis all-pairwise comparisons test (p < 0.05). Bar: Standard deviation. |
The DBH-class frequency distribution of individuals of dominant species in each forest type is shown in Fig. 5. All the dominant species had multimodal distribution.
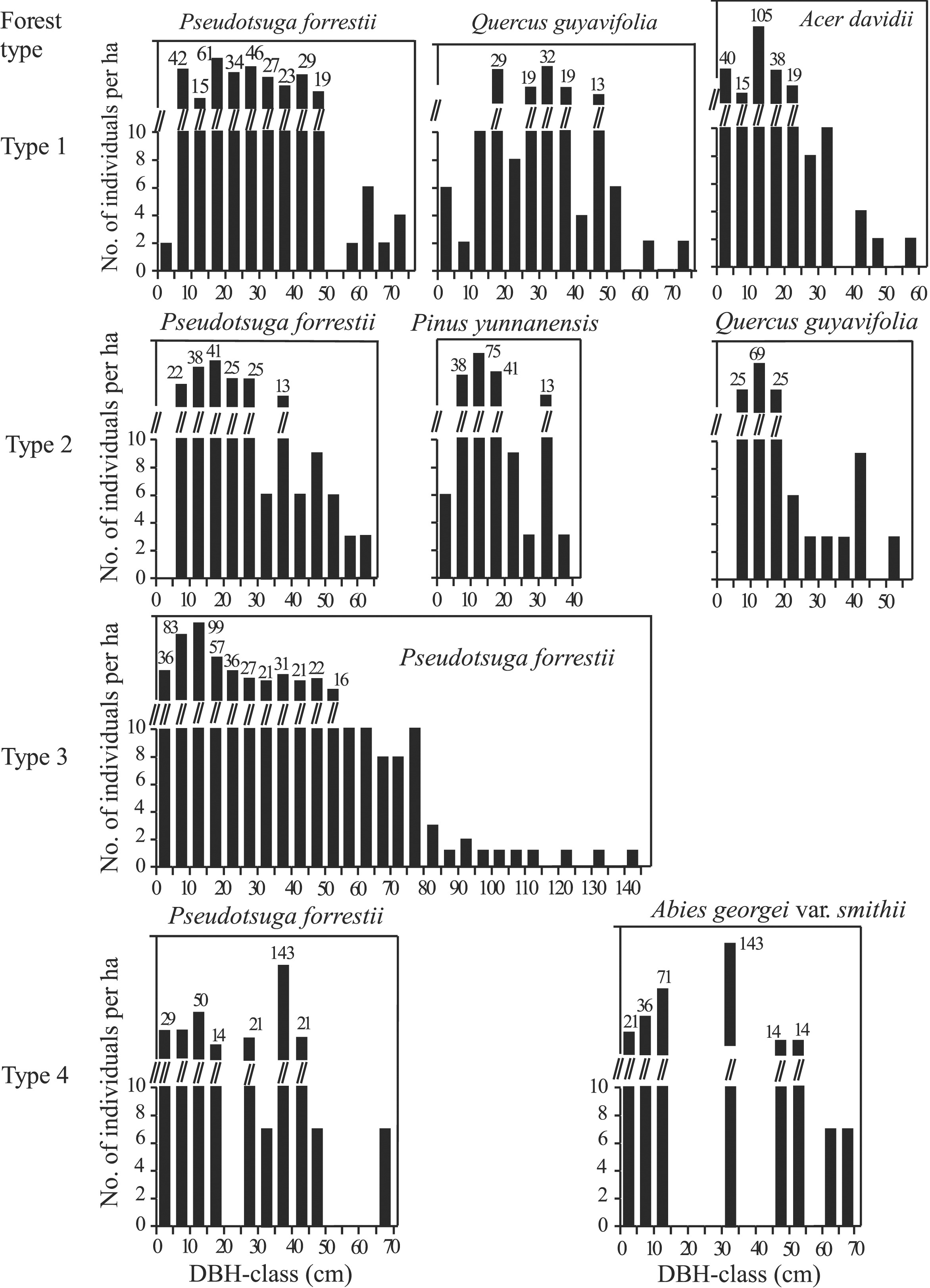
|
| Fig. 5 The frequency distribution in DBH-classes of dominant species in each forest type. Type 1: Pseudotsuga forrestii -Quercus guyavifolia -Acer davidii evergreen coniferous and broad-leaved mixed forest; Type 2: Pseudotsuga forrestii - Pinus yunnanensis - Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest; Type 3: Pseudotsuga forrestii evergreen coniferous forest; Type 4: Pseudotsuga forrestii - Abies georgei var. smithii evergreen coniferous forest. |
In Pesudosuga forrestii - Quercus guyavifolia - Acer davidii evergreen coniferous and broad-leaved mixed forest (Type 1), the number of P. forrestii trees had three peaks (61, 46, 42 trees respectively in the 15–20 cm, 25–30 cm, 5–10 cm DBH). Trees were absent in the 50–55 cm DBH class, while a few trees were found between 55 and 75 cm DBH. Only two trees were found smaller than 5 cm DBH. Q. guyavifolia and A. davidii were discontinuously distributed in 0–75 cm and 0–60 cm DBH, respectively. In Pseudotsuga forrestii - Pinus yunnanensis - Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest (Type 2), P. forrestii trees with DBH between 5 and 65 cm DBH were unevenly distributed. No trees with a DBH < 5 cm were found. P. yunnanensis had 75 trees with DBH between 10 and 15 cm, and a few individuals with DBH between 0 and 5 cm, 25–30 cm and 35–40 cm. Q. guyavifolia trees were discontinuously distributed in 0–55 cm DBH, and were mainly found in 5–20 cm DBH.
In Pseudotsuga forrestii evergreen coniferous forest (Type 3), the tallest P. forrestii individual reached 143 cm DBH. Most individuals had a DBH between 5 and 20 cm. The number of individuals generally decreased with increasing DBH from 50 to 80 cm. There were only 13 trees per hectare with a DBH between 80 and 143 cm.
In Pseudotsuga forrestii - Abies georgei var. smithii evergreen coniferous forest (Type 4), P. forrestii individuals were discontinuously distributed in 0–70 cm DBH. Most trees (143) had a DBH between 35 and 40 cm, while only 50 trees had a DBH between 10 and 15 cm DBH. No P. forrestii trees were found with a DBH between 20 and 25 cm or 50–65 cm. A. georgei var. smithii generally showed a similar distribution pattern in DBH-classes to that of P. forrestii.
3.3.2. Age structureIn Pseudotsuga forrestii, DBH and age were strongly positively correlated (p < 0.05; Fig. 6; y = 0.0125x2+2.2198x+3.1322, R2 = 0.8633, n = 61).

|
| Fig. 6 The relationship of DBH and age of Pseudotsuga forrestii. The increment core samples were taken in P. forrestii evergreen coniferous forest (Type 3). |
The age-class frequency distribution of individuals (height ≥ 1.3 m) of Pseudotsuga forrestii in each forest type is shown in Fig. 7. All the age-structures were multimodal. The observed maximum age of P. forrestii was 570 years in Pseudotsuga forrestii evergreen coniferous forest (Type 3), whereas it was 210 or 220 years in the other three forest types. P. forrestii in Pseudotsuga forrestii evergreen coniferous forest (Type 3) appeared to have relatively frequent episodes of regeneration compared to the other three forest types. The age class data for all the forest types showed three peaks over four hectares: 145 trees between the ages of 100–115, 113 trees between the ages of 35–40 years, and 101 trees between the ages of 20–25 years. There were also 128 trees in four hectares aged between 10 and 20 years. The number of trees greater than 200 years old was rather limited.
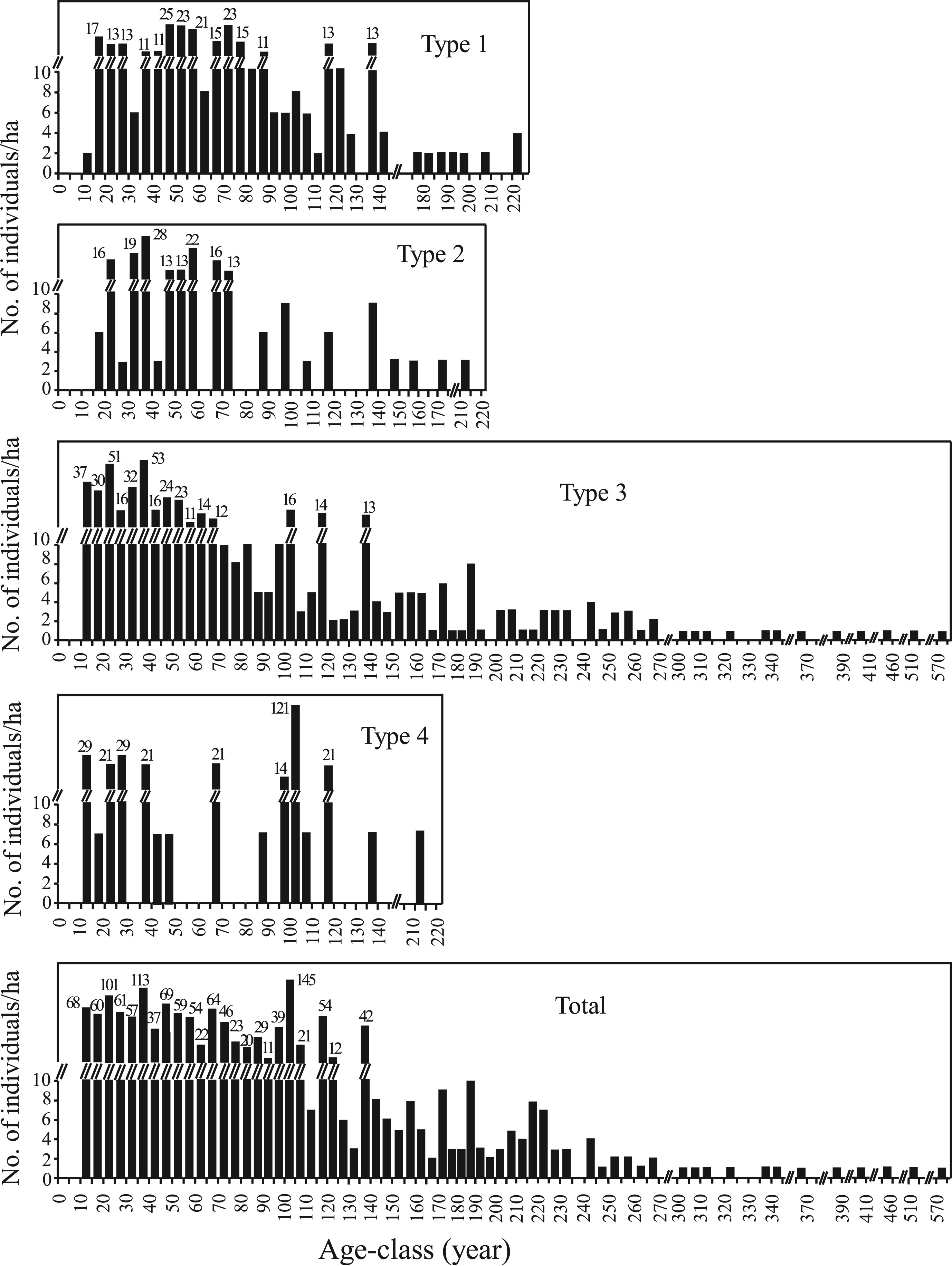
|
| Fig. 7 The age structure of Pseudotsuga forrestii (height ≥ 1.3 m). |
Seedlings of Pseudotsuga forrestii were found in diverse micro-habitats, e.g., nearby water sources, along roadsides, and under canopy gaps (Fig. 8). According to our field observations, seedlings were shade intolerant and no seedlings were found under closed canopy. The number of seedlings/saplings near water sources decreased dramatically from 83 to 15 individuals. Very limited number of seedlings/saplings were eventually found in more open micro-habitats such as canopy gaps and roadsides, where moderate disturbances frequently occurred (Fig. 8).
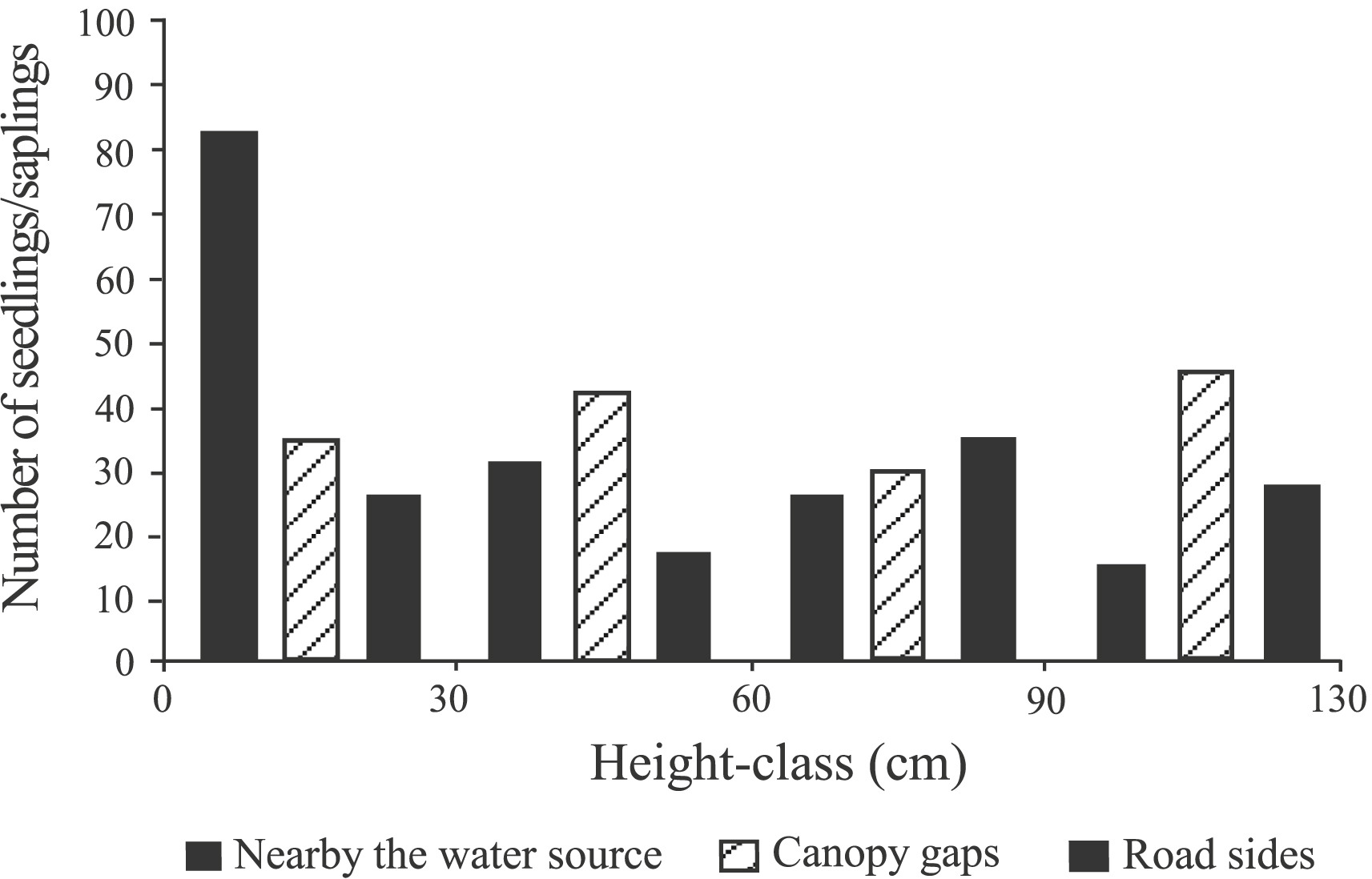
|
| Fig. 8 The frequency distribution in height-classes of Pseudotsuga forrestii seedlings/saplings in various habitats. |
The ring width and basal area increment among the 44 samples were highly variable. Overall, the average radial growth rate of Pseudotsuga forrestii trees decreased as the trees aged, from 4.07 to 0.05 mm/year. In general, when P. forrestii trees were less than 20 years old, the average growth rate of ring width was relatively high (2.93 mm/year) and ranged from 5.86 to 1.10 mm/year. Thereafter, the average radial growth rate fell to 1.77 mm/year between ages 20–50 years, 1.16 mm/year between 50 and 100 years, and 0.81 mm/year between 100 and 370 years. Trees in all the five age classes (8–80 years, 80–160 years, 160–240 years, 240–320 years, 320–372 years) had a similar pattern, with the radial growth rate starting high and decreasing in a rough L-shape (Fig. 9A). P. forrestii trees exhibited basal area increments (BAI) that increased (628.8 mm2/year on average) greatly with increasing age for the first 50 years (Fig. 9B). Thereafter, the growth rate of BAI also increased, but fluctuated greatly, especially in trees of the 240–320 age-class (Fig. 9B), which reached the maximum BAI of 3791 mm2/year between the ages of 275 and 310. Moreover, from the age of 10–100, P. forrestii trees grew faster in the 80–160 age-class than in the other age-classes (Fig. 9A and B).

|
| Fig. 9 Growth trends of Pseudotsuga forrestii. (A) The ring width for trees (height ≥ 1.3 m) in the five age classes (i.e., 8–80, 80–160, 160–240, 240–320, 320–372 years); (B) The basal area at the breast height (1.3 m tall) increment for trees in the five age classes. |
The distribution and forest characteristics of Pseudotsuga forrestii are compared with other species of Pseudotsuga in the world in Table 1. The elevational distribution range 2400–3300 m a.s.l. of P. forrestii falls within the warm-temperate coniferous and broad-leaved mixed forest zone and temperate coniferous forest zone in NW Yunnan, SE Tibet, W Sichuan, SW China. P. forrestii seedlings are shade intolerant. Regeneration requires moderate disturbances. Its habitats are generally moist on steep slopes along rivers in gorges; mosses are commonly present on the forest floor. The most important canopy trees associated with P. forrestii are Quercus guyavifolia, Acer. davidii, Pinus yunnanensis, Abies georgei var. smithii, Pinus armandii, and Tsuga dumosa. Shrubs associates are Eurya yunnanensis, Rhododendron decorum, Metapanax delavayi, etc.
| Species | Distribution Region | Height (m) | DBH (cm) | Age (year) | Major habitats & elevations | Shade tolerance | Major associated species | Source |
| P. forrestii | a | 15–28 (42) | 22–70 (143) | 53–220 (570) | Steep slopes along rivers in gorgers at ca. 2420 m–3280 m a.s.l | Intolerant | Quercus guyavifolia, Acer davidii, Pinus yunnanensis, Abies georgei var. smithii, Tsuga dumosa, etc. | This study |
| P. sinensis var. sinensis | b | 20–35 (Max.: NA) | 15–60 (Max.: NA) | NA | Slopes in hills and mountains or Karst limestone mountain slopes at 600–2800 m a.s.l. | Intolerant | Species of Pinus, Calocedrus, Castanopsis and Quercus, etc. | Zuo, 1995; Chen et al., 2001; Guo et al., 2007; Xu and Yu, 2010; Li and Xie, 2015; Xiong et al., 2017; Thomas and Yang, 2019 |
| P. sinensis var. wilsonianaa | c | 25 (50) | Rang: NA; Max.: 200 cm | NA | Exposed rocky slopes at (800) 1000–2000 (2700) m a.s.l. | NA | Species of Tsuga, Chamaecyparis, Pinus and Fagaceae, etc. | Su, 1980; ETEFN, 1993; Huang, 1994; TaiBIF, 2022 |
| P. brevifolia | d | NA | NA | NA | Calcareous and rocky soils in mountains at ca. 1300 m a.s.l. | NA | NA | FOC, 2021 |
| P. japonica | e | 15–30 (43) | 15–70 (143) | NA | Xeric sites, steep slopes, narrow ridges, rarely gentle slopes and valleys at 400–1100 m a.s.l. | Intolerant | Abies firma, Pinus densiflora, Pinus parviflora, Tsuga sieboldii, Quercus spp., Cleyera japonica, Pieris japonica, etc. | Yatoh, 1964; Hirata, 1975; Kobayashi and Yokoyama, 1981; Yamamoto, 1992; SFB and OYO, 2011 |
| P. menziesiis | f | 19–205 (127) | 10–61 (490) | 750 (1400) | A wide variety of soils and climates at ca. 240–3260 m a.s.l. | Intolerant | Acer circinatum, Gaultheria shallon, Rhododendron macrophyllum, Corylus cornuta var. californica, Holodiscus discolor, etc. | Franklin and Dyrness, 1973; Lavender and Hermann, 2014 |
| Common ranges of variables are presented with maximum values in parentheses. a. NW Yunnan, SE Tibet, W Sichuan; b. S Anhui, N Fujian, N Guizhou, W Hubei, NW Hunan, NE Jiangxi, Zhejiang, S Shaanxi, SE Sichuan, Central and NE Yunnan, N Vietnam; c. Central Mountain Range in Taiwan; d. SW Guangxi and Guizhou; e. Yanase district in southeastern Shikok to the Kii Peninsula in southern Honshu, Japan; f. Western North America. a In Flora of Taiwan, it is treated as Pseudotsuga wilsoniana. |
||||||||
Additional Pseudotsuga species are distributed in China, Japan, and western North America. P. sinensis var. sinensis, which has a wider distribution range than P. forrestii, is found in hills and mountains, also in karst limestone areas at 600–2800 m a.s.l. in S Anhui, N Fujian, N Guizhou, W Hubei, NW Hunan, NE Jiangxi, S Shaanxi, SE Sichuan, C and NE Yunnan, Zhejiang, China. P. sinensis var. sinensis is also scattered at ca. 400 m a.s.l. in karst limestone region, in northern Vietnam (Thomas and Yang, 2019). P. sinensis var. sinensis' associated genera are similar to associates of P. forrestii including Pinus and Quercus. However, associates Calocedrus and Castanopsis of P. sinensis var. sinensis are not associates of P. forrestii (Zuo, 1995; Chen et al., 2001; Guo et al., 2007; Xu and Yu, 2010; Li and Xie, 2015; Xiong et al., 2017; FOC, 2021). The variety P. sinensis var. wilsoniana is confined to Taiwan, and is associated with evergreen broad-leaved species or species of Tsuga, Chamaecyparis, Pinus and Fagaceae at (800) 1000–2000 (2700) m a.s.l. (Su, 1980; ETEFN, 1993; Huang, 1994; TaiBIF, 2022). An additional Chinese species, P. brevifolia, is very rare, and is scattered on S-facing slopes and mountain tops, on calcareous and rocky soils at ca. 1300 m a.s.l. in SW Guangxi, and Guizhou (FOC, 2021). Information on taxa associated with P. brevifolia is not available. Although P. forrestii and P. sinensis var. sinensis are shade intolerant; it is unclear whether P. sinensis var. wilsoniana and P. brevifolia share this trait.
The Japanese species, Pseudotsuga japonica, which is distributed from southeastern Shikok to southern Honshu, has a higher latitudinal range (34°22′N and 33°26′N) than does Pseudotsuga forestii; however, P. japonica is confined to a lower elevational range (400–1100 m a.s.l.) (Yatoh, 1964; Kobayashi and Yokoyama, 1981). Pure stands of P. japonica are rare; canopy trees are exclusively conifers such as Abies firma, Pinus densiflora, Pinus parviflora and Tsuga sieboldii, subcanopy trees are Quercus spp., including Quercus glauca, Quercus salicina and Quercus sessilifolia. Shrub associates are Cleyera japonica, Pieris japonica, etc. (Yamamoto, 1992). P. japonica is a ridge-specialist. On the most xeric sites, such as steep narrow ridge, its regeneration can be maintained (Yamamoto, 1992). P. japonica seedlings are shade intolerant (Hirata, 1975; Yamamoto, 1992), similar to those of P. forrestii.
The Douglas fir, Pseudotsuga menziesiis, is distributed in western North America between 240 and 3260 m a.s.l., and grows under a wide variety of soils and climatic conditions in its native range, which extends from latitude 19°N to 55°N, resembling an inverted V with uneven sides (Hermann and Lavender, 1990). The distribution of P. menziesiis is much wider than that of either P. forrestii in southwestern China or P. japonica in Japan. In addition, P. menziesiis in general has much larger appearance and form in the max. DBH (490 cm) and height (127 m) than other species of Pseudotsuga (max. 143 cm DBH, max. 42 m tall for P. forrestii; max. 200 cm DBH, max. 50 m tall for P. sinensis var. wilsoniana; max. 143 cm DBH, max. 43 m tall for P. japonica) (Table 1). Periodic recurrence of catastrophic wildfires has created vast, almost pure stands of coastal Douglas fir, Pseudotsuga menziesii var. menziesii, throughout its range north of the Umpqua River in Oregon. Although logging has mainly eliminated the original old-growth forest, clear-cutting combined with slash burning has helped maintain P. menziesii var. menziesii as the major component in second-growth stands. Where regeneration of P. menziesii var. menziesii was only partially successful or failed, Alnus rubra has become an associate of Douglas fir or has replaced it altogether. The shrubs associated with coastal Douglas fir through its central and northern ranges are Acer circinatum, Gaultheria shallon, Rhododendron macrophyllum, etc. Toward the drier southern end of its range, common shrub associates include Corylus cornuta var. californica, Holodiscus discolor, Symphoricarpos mollis, Arctostaphylos spp., etc. (Hermann and Lavender, 1990). P. menziesii var. menziesii has the nature of shade intolerance, similar to P. forrestii, P. sinensis var. sinensis and P. japonica.
4.2. Growth rate and regenerationOur results show that Pseudotsuga forrestii has a lower radial growth rate (0.05–4.06 mm/year) than the relict conifer Metasequoia glyptostroboides (0.34–7.81 mm/year, Tang et al., 2011) in its native habitats in Lichuan, south-central China. This is probably because M. glyptostroboides is a fast-growing deciduous conifer. M. glyptostroboides is light demanding, but able to adapt to a variety of habitats, including river ditches, hill sides with deep soils, roadsides, nearby houses and farmland (Tang et al., 2011). P. forrestii grows faster (radial growth rate 0.97 mm/year on average) than the relict evergreen conifer Thujia sutchuenensis [0.73 mm/year on average, recalculated from Tang et al., 2015] in its native habitats in Chengkou County (Chongqing Municipality), because T. sutchuenensis, which grows in extremely harsh cliffs and rocky habitats, has distinctive traits of stunted and morphologically deformed crowns, very slow growth and exceptional longevity (Tang et al., 2015). These different growth rates mainly result from differences in life histories and ecological traits of these species.
Pseudotsuga forrestii bears fruits in alternate years. P. forrestii seeds are dispersed by wind and water. Smaller numbers of older P. forrestii seedlings near water sources may indicate failed competition with other plants. However, P. forrestii is highly adaptive, and can grow in cruel habitats such as steep slopes, landslide-prone sites and roadsides where canopy gaps have been created that reduce competition from other species. Its regeneration requires moderate (intermediate) disturbances. This is similar to many relict coniferous species such as P. japonica (Yamamoto, 1992), Thuja sutchuenensis (Tang et al., 2015), Cathaya argyrophylla (Qian et al., 2016). Pseudotsuga forrestii evergreen coniferous forest (Type 3) appears to have relatively frequent episodes of regeneration. This may result from frequent natural disturbances on the steep slopes along Lancang River.
4.3. Conservation recommendationsIn the study area, trees of Pseudotsuga forrestii have long been used by Tibetans and Lisu villagers. Tibetans use large trees to construct houses and small trees to build fences. The Lisu people use P. forrestii wood for coffins and dig holes in the trunks of P. forrestii to make bee nests (Kong et al., 2018). Local villagers are unaware that P. forrestii trees are a protected plant. Thus, one conservation priority should be to inform local people of the importance of protecting this species. Conservation efforts should also prioritize the maintenance of P. forrestii population sizes. Furthermore, logging the vulnerable relict timber P. forrestii should be strictly prohibited. We recommend that P. forrestii forests near villages in Yunling Xiang, Deqin County be selectively marked and species other than P. forrestii (e.g., Quercus guyavifolia and Pinus yunnanensis) with DBHs > 30 cm be sustainably harvested. P. forrestii seedlings are light demanding; thus, sustainably harvesting timbers of other species will promote P. forrestii regeneration by opening space in the canopy and understory to more sunlight, water, and nutrients for seedlings establishment. Because P. forrestii stands and populations are highly fragmented, we also recommend that future studies on genetic structure and diversity of various population sizes of P. forrestii provide basic information on enhancing gene flow among the populations for conservation.
AcknowledgementsThis study received financial support from the Science and Technology Department of Yunnan University, China (2019YNU002), Major Program for Basic Research Project of Yunnan Province, China (202101BC070002), the Special Foundation for National Science and Technology Basic Resources Investigation of China (2019FY202300).
Author contributions
CQT designed the study, analyzed the data and wrote the manuscript. SQY organized and analyzed the data. SL identified the botanical specimens. S-QY, PBH, JRW, MCP, CYW, TM, YPL, SLu, YH conducted the fieldwork. All the authors contributed discussion to improve the manuscript.
Declaration of competing interest
None.
Appendice A and B. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.10.005
Chen, W.-H., Shui, Y.-M., Wang, W., 2001. Community investigation and conservation of Calocedrus macrolepsis and Pseudotsuga sinensis in Yimen County, Yunnan Province, China. Acta Bot. Yunnan., 23: 189-200. |
Denk, T., Grímsson, F., Kvaček, Z., 2005. The Miocene floras of Iceland and their significance for late Cainozoic North Atlantic biogeography. Bot. J. Linn. Soc., 149: 369-417. DOI:10.1111/j.1095-8339.2005.00441.x |
ETEFN, 1993. Edinburgh Taiwan Expedition Field Notes. Kew.
|
Farjon, A., 2010. A Handbook of the World's Conifers 2. BRILL, Leiden, Boston, pp. 533-1111.
|
FOC (Flora of China), 2021. Flora of China (English version). Available from: website. http://www.iplant.cn/info/Pseudotsuga?t=foc. (Accessed 5 May 2021).
|
Franklin, J.F., Dyrness, C.T., 1973. Natural Vegetation of Oregon and Washington. USDA Forest Service. General Technical Report PNW-8. Pacific Northwest Forest and Range Experiment Station, Portland, OR. 417 p. (Reprinted 1988, Oregon State University Press, Corvallis).
|
Fu, L.G., 1992. Rare and Endangered Plants, vol. 1. Science Press, Beijing, pp. 114-118 (in Chinese).
|
Govaerts, R.H.A., 2011. World Checklist of Selected Plant Families Published Update Facilitated by the Trustees of the. Royal Botanic Gardens, Kew.
|
Gugger, P.F., Sugita, S., Cavender-Bares, J., 2010. Phylogeography of Douglas-fir based on mitochondrial and chloroplast DNA sequences: testing hypotheses from the fossil record. Mol. Ecol., 19: 1877-1926. DOI:10.1111/j.1365-294X.2010.04622.x |
Guo, W, Shen, R.-J., Wu, J.-H., et al., 2007. Analysis on community composition and structure of Pseudotsuga gaussenii in Sanqing Mountain of Jiangxi Province. J. Plant Resour. Environ., 16: 46-52. |
Habel, J.C., Assmann, T., 2010. Relict Species: Phylogeography and Conservation Biology. Springer-Verlag Berlin Heidelberg, Germany.
|
Hermann, R.K., Lavender, D.P., 1990. Pseudotsuga menziesii (mirb.) Franco. Douglasfir. In: Burns, R.M., Honkala, B.H., tech (Eds.), Coords., Silvics of North America, vol. 654. USDA Agricultural Handbook, Washington, D.C., pp. 527-540 vol. 1, Conifers.
|
Hirata, Y., 1975. Primeval forests of Pseudotsuga japonica Beissner. In: Report for the Investigation of Natural Environments in the Upper District of Kinokawa River. Nara Prefecture, Nara, pp. 1-22 (in Japanese).
|
Huang, T. -C., senior (Eds.), 1994. Flora of Taiwan Pteridophyta Gymnospermae. Taipei: Editorial Commission of the Flora of Taiwan, second ed., vol. 1
|
Jin, Q., Jin, H., Xie, J., et al., 2021. Adaptive detection of Pseudotsuga forrestii Craib. Forest Investigation Design, 50: 17-23. |
Kobayashi, Y., Yokoyama, T., 1981. Pseudotsuga Carr. In: Asakawa, S., Katsuta, M., Yokoyama, T. (Eds.), Seeds of Woody Plants in Japan: Gymnospermae. Japan Forest Tree Breeding Association, Tokyo, pp. 34-36 (in Japanese).
|
Kong, W., Liu, P., Ma, F., et al., 2018. Distribution status and protection suggestions of Pseudotsuga forrestii in Deqen Tibetan autonomous prefecture. For. Invent. Plan., 43: 89-103. DOI:10.3969/j.issn.1671-3168.2018.04.020 |
Kozlowski, G., Gratzfeld, J, 2013. Zelkova—an ancient tree. Global status and conservation action. Natural History Museum Fribourg, Switzerland.
|
Kunzmann, L., 2014. On the fossil history of Pseudotsuga Carr. (Pinaceae) in Europe. Paleobiodivers. Paleoenviron., 94: 393-409. DOI:10.1007/s12549-014-0156-x |
Lakhanpal, R.N., 1958. The Rujada flora of west central Oregon. Univ. Calif. Publ. Geol. Sci., 35: 1-66. |
Lande, R., 1996. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos, 76: 5-13. DOI:10.2307/3545743 |
Lavender, D.P., Hermann, R.K., 2014. Douglas-fir. The Genus Pseudotsuga. Oregon Forest Research Laboratory, Oregon State University, Corvallis.
|
Li, M. -G., Xie, S. -X, 2015. Study on community and population Structure of Pseudotsuga sinensis forest in karst mountainous region of Guizhou Province. Tianjin Agricul. Sci., 21: 150-153. |
Li, M., Liu, P., Kong, W., et al., 2022. Population structure and dynamic characteristics of endangered Pseudotsuga forestii Craib. Acta Ecol. Sin., 42(13). https://kns.cnki.net/kcms/detail/11.2031.Q.20220316.1737.057.html. |
McCune, B., Mefford, M.J., 1999. PC-ORD: Multivariate Analysis of Ecological Data. MjMSoftware Design. Gleneden Beach, USA.
|
Ozaki, K., 1991. Late Miocene and Pliocene Floras in Central Honshu, Japan. Bulletin of Kanagawa Prefectural Museum. Natural Science Special Issue, Yokohama.
|
Palamarev, E., Bozukov, V., Uzunova, K., et al., 2005. Catalogue of the Cenozoic plants of Bulgaria (Eocene to Pliocene). Phytol. Balc., 11: 215-365. |
Pielou, E.C., 1969. An Introduction to Mathematical Ecology. Wiley, New York.
|
Qian, S.H., Yang, Y.C., Tang, C.Q., et al., 2016. Effective conservation measures are needed for wild Cathaya argyrophylla populations in China: insights from the population structure and regeneration characteristics. For. Ecol. Manag., 361: 358-367. DOI:10.1016/j.foreco.2015.11.041 |
Qin, H., Yang, Y., Dong, S., et al., 2017. Threatened species list of China's Higher Plants. Biodivers. Sci., 25: 696-744. DOI:10.17520/biods.2017144 |
Rubino, D.L., McCarthy, B.C., 2000. Dendroclimatological analysis of white oak (Quercus alba L., Fagaceae) from an old-growth forest of southeastern Ohio, USA. J. Torrey Bot. Soc., 127: 240-250. DOI:10.2307/3088761 |
Schorn, H.E., 1994. A preliminary discussion of fossil larches (Larix, Pinaceae) from the Arctic. Quat. Int., 22: 173-183. |
SFB (Shikoku Forestry Bureau), OYO (OYO Corporation), 2011. Report on the Current Status of Forest Genetic Resources Conservation Forests (Togasawara) in 2010. Shikoku Forestry Bureau (in Japanese).
|
Silba, J., 1990. A supplement to the international census of the coniferae, Ⅱ. Phytologia, 68: 7-78. |
Silba, J., 2008. Pseudotsuga sinensis subsp. forrestii (Craib) Silba. J. Int. Conifer Preserv. Soc., 15: 63. |
Su, H.J., 1980. Studies on rare and endangered forest plants. National Univ. Exp. For. Res. Rep., 125. |
Sun, W.-B., Kong, F.-C., Zhou, Y., et al., 2003. Current status of germplasm resources of genus Pseudotsuga in Yunnan and the strategies on conservation and utilization. Guihaia, 23: 15-18. |
TaiBIF, 2022. https://portal.taibif.tw/. (Accessed 12 May 2022).
|
Tanai, T., Suzuki, N., 1963. Miocene floras of southwestern Hokkaido, Japan. Tertiary floras of Japan, Miocene floras. Collab. Assoc. Commemorate 80th Anniversary Geological Survey Japan, 1: 9-149. |
Tang, C.Q., 2015. The subtropical vegetation of southwestern China: plant distribution, diversity and ecology. In: Plants and Vegetation, vol. 11. Springer, Dordrecht.
|
Tang, C.Q., Yang, Y., Ohsawa, M., et al., 2011. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv., 144: 279-289. |
Tang, C.Q., Yang, Y., Ohsawa, M., et al., 2015. Community structure and survival of Tertiary relict species Thuja sutchuenensis (Cupressaceae) in the subtropical Daba Mountains, southwestern China. PLoS One, 10: e0125307. DOI:10.1371/journal.pone.0125307 |
Tang, C.Q., Matsui, T., Ohashi, H., et al., 2018. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun., 9: 4488. DOI:10.1038/s41467-018-06837-3 |
Thomas, P., 2019. Pseudotsuga Sinensis Var. Sinensis, from the Website: 'Threatened Conifers of the World' (https://threatenedconifers.rbge.org.uk/conifers/pseudotsuga-sinensis-var.-sinensis. (Accessed 13 July 2022).
|
Yamamoto, S. -I., 1992. Preliminary studies on the species composition, stand structure and regeneration characteristics of an old-growth Pseudotsuga japonica forest at the Sannoko on Kii Peninsula, southwestern Japan. For. Habitats, 34: 50-58. |
Yatoh, K., 1964. Dendrology, Conifers. Asakura Shoten, Tokyo (in Japanese).
|
Xiong, B.-M., Wang, Z.-X., Tian, K., et al., 2017. Coenological characteristics of forests in Qizimei Mountains Nature Reserve. Guihaia, 37: 434-441. DOI:10.1002/jsfa.7742 |
Xu, Q.-H., Yu, X.-L., 2010. Preliminary study on the characteristics of community in Yangmingshan, Hunan. J. Fujian For. Sci. Tech., 37: 10-14. |
Yabe, A., 2011. Pseudotsuga tanaii Huzioka from the earliest Miocene Shichiku flora of northeast Japan: Systematics and ecological conditions. Paleontol. Res., 15: 1-11. DOI:10.2517/1342-8144-15.1.001 |
Zuo, J.B., 1995. Study on community characteristics and natural regeneration of Pseudotsuga sinensis in western Guizhou. Guizhou For. Sci. Tech., 23: 14-21. |



