b. Programa de Doctorado en Ciencias Mención Ecología y Evolución, Escuela de Graduados, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile;
c. Jardín Botánico de Bogotá José Celestino Mutis, Subdirección Científica, Línea de Investigación en Restauración Ecológica, 111071, Bogotá, Colombia;
d. Facultad del Medio Ambiente y Recursos Naturales, Universidad Distrital Francisco José de Caldas, Sede Vivero, 111711, Bogotá, Colombia
Wildfires represent the most significant disturbance in terrestrial ecosystems (Bond and Keeley, 2005; Pausas and Ribeiro, 2017), and their influence on plant structure, composition, and functional traits depends on the nature of the fire regime including frequency, intensity, type, and season (Keeley et al., 2011). For example, the natural fires in the High tropical Andes happened early Holocene (Di Pasquale et al., 2008), and the activity for the High Colombian Cordillera Oriental increased in the Middle and Late Holocene of the marked influence of ENSO (Espinoza et al., 2022); nevertheless, the fire regime changed since people arrived. The páramo ecosystem is an equatorial neotropical alpine treeless vegetation (Hofstede and Rossenaar, 1995), located above 3600 m altitude (Cortés-Duque and Sarmiento-Pinzón, 2013). It is burned periodically by anthropogenic fires (Hofstede, 1995; Keating, 2007; Horn and Kappelle, 2009; White, 2013) due to converting land (Premauer and Vargas-Ríos, 2004; Cleef, 2008). Furthermore, the dominant plants of the páramo burn easily because the architecture of their canopy is composed of dead biomass (e.g., branches, twigs, and leaves), which form a flammable fuel (Ramsay and Oxley, 1996). For this reason, the páramo ecosystem is considered a fire-dependent ecosystem (Horn and Kappelle, 2009; Zomer and Ramsay, 2020) because some species depend on wildfires to complete their life cycle. Therefore, changes in fire regimes could lead to new arrangements in the community structure. Consequently, the postfire environment provides a more significant amount of light and space due to low competition, promoting the resprout and recruitment of plants of some species (Laegaard, 1992; Ramsay and Oxley, 1996; Ramsay, 1999; Gutiérrez-Salazar and Ramsay, 2020). Furthermore, Vaccinium, Pernettia, Hypericum, and other species have responses stimulated by fire (Horn, 1989, 1990; Bader et al., 2007; Vargas-Ríos, 1997; Zomer and Ramsay, 2020).
In the case of subpáramo, the lowest subdivision of páramo, located in the montane belt between 3200 and 3600 m a.s.l., taking into account the climate, physiognomic and floristic attributes of the vegetation and stratified shrubs of the families Asteraceae, Hypericaceae, and Ericaceae are found below the grasses (Cuatrecasas, 1958). The subpáramo susceptibility is fire-related to the necromass accumulation in rosettes, and the alteration of the fire regime has affected the forest–páramo interface. However, it will depend on the traits of the species present in the ecotone and other factors like the low moisture content in the vegetation and soil during the dry season since, under extreme drought conditions, the high soil organic content can lead to ignition and sustaining fires (Bader et al., 2007; Armenteras et al., 2020). Furthermore, the frequency of burning depends on on-site accessibility (i.e., public access) and agricultural activity. In this last activity, the frequency of burning depends on the rate of vegetation recovery after the fire but is typically every 2–4 years (i.e., Ecuador páramo) (Ramsay and Oxley, 1996). Therefore, knowing the history and fire regime of the páramos is essential to estimate its ecological effects. However, it is difficult to build fire regimes because of the absence of cloud-free satellite images in the páramos, which makes remote monitoring of fires difficult (Borrelli et al., 2015); additionally, insufficient resources to study and the low or null amount of research on fires (Gutierrez-Salazar and Ramsay, 2020). However, some studies carried out in the Eastern Cordillera show the importance of fire in the history of high Andean landscapes and highlight its role in the configuration of vegetation (Espinoza et al., 2022). Therefore, the subpáramo represents an interesting target area to study vegetation's response to the impact of wildfires and its ability to recover.
The persistence and dispersal traits of the species found under a disturbance regime such as fire can be considered as an expression of several traits that, in combination, allow the plant to persist, avoiding danger in critical stages or tolerating and allowing resprout or recruitment (McIntyre et al., 1999; Cárdenas-Arévalo and Vargas-Ríos, 2008). Plant species with fire-tolerance traits are relatively common in fire-prone ecosystems favoring faster regeneration (Cavero and Ederra, 1999; Keeley and Fotheringham, 2009; Keeley et al., 2011; Pausas and Keeley, 2014; Accatino et al., 2016; Lawes et al., 2016). The most extensive response trait is resprouting, which has been identified as the primary regeneration mechanism in ecosystems frequently affected by wildfires (Clarke et al., 2013; Torres et al., 2014). On the other hand, recruitment is a mechanism that shows the resilience of an ecosystem to different disturbances, maintaining ecological stability through the fecundity and growth of new individuals of the species that make up the ecosystem, allowing natural regeneration by sexual means. The efficiency of species recruitment after a disturbance requires a series of functional characteristics, such as the capacity to germinate and establish seedlings in an environment usually modified in terms of its thermal, light, nutrient, and competition profiles (Knox and Clarke, 2006). Additionally, seed dispersal by wind or birds can be regarded as a fire-avoid trait, as it allows the rapid establishment of new individuals and species in post-disturbance conditions (Whelan, 1986).
This study investigates the degree to which plant communities change over time. We evaluated whether functional traits shifted predictably over time and differed between burnt and unburnt sites over 2.5 years postfire. Additionally, we documented how these traits changed during the second and third years after the disturbance (13th to 31st months) in woody and non-woody species. Most subpáramo species can survive harsh environmental conditions, such as low night temperatures, high solar radiation, and strong winds. The hypothesis was to identify several species capable of regeneration after a wildfire. Due to seed dispersal, resprouting was expected as the main regeneration trait, while it was thought that seed regeneration would rise over time. In contrast to compositional and structural studies, the functional trait perspective constitutes a novel approach to assessing natural regeneration after wildfire disturbances in páramo (Sturm and Rangel, 1985; Ramsay and Oxley, 1996; Vargas-Ríos, 1997; Keeley et al., 2011; Lamont et al., 2019).
2. Materials and methods 2.1. Study siteThe study was conducted in Eastern Andean Cordillera in Bogotá city, Colombia, in the subpáramo Cerro Aguanoso (4° 34′ 28.67″ N, 74° 02′ 8.48″ W) (Fig. 1a). This subpáramo is located in the Bosque Oriental Forest Reserve, part of the Estructura Ecologica Principal of Bogotá, a strategic ecosystem with a network of environmental corridors whose primary components are the system of protected areas, urban parks, ecological corridors (van der Hammen and Andrade, 2003). The annual mean temperature fluctuates between 8 and 12 ℃, while mean annual precipitation ranges between 1000 and 1500 mm with a bimodal distribution pattern. The wet season between December and March is characterized by less than 100 mm monthly precipitation. Relative humidity of 43.6% in the wet season. The elevation of the subpáramo in reserve ranges from 3000 to 3500 m a.s.l (IDEAM, 2007; CAR, 2016; Peyre et al., 2018). The area has a black humid soils with an acid pH (value < 5.5), as is typical for the high and humid regions of the páramos.
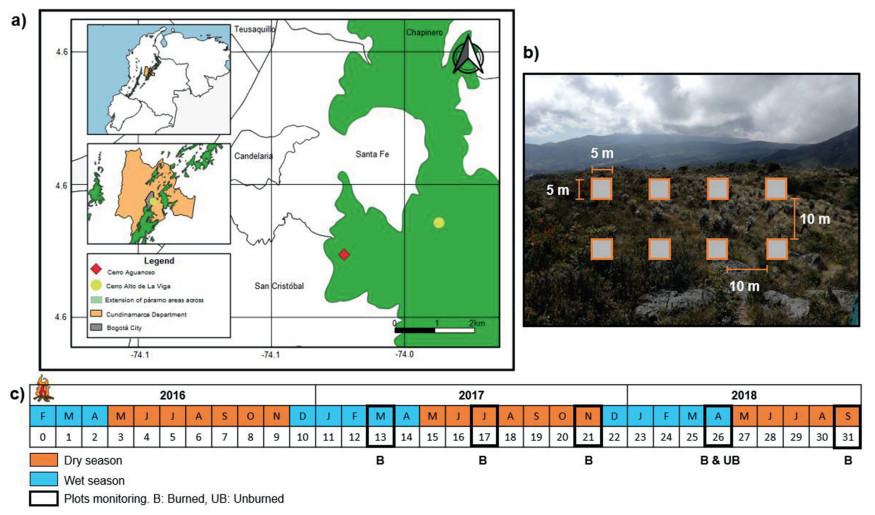
|
| Fig. 1 Location of the study area in the Bosque Oriental Forest Reserve, Colombia. (a) The geographic location of the burned site (Cerro Aguanoso, B) and unburned site (Cerro Alto de la Viga, UB). (b) Schematic distribution of plots along the sampling transects; orange rectangles grey with represent the position of plots. (c) In the sampling period, the black boxes represent the sampling months and season, blue is the wet season, and orange is the dry season. |
During the extremely dry conditions generated by the 2015–2016 El Niño climatic event (Huang et al., 2016), between January and February 2016. A forest fire broke out in the Bosque Oriental Forest Reserve, affecting around 128 ha (Rodríguez and Pinilla, 2022), of which 8.23 ha correspond to low vegetation associated with the Cerro Aguanoso subpáramo. The vegetation was affected, the fire affected the woody plants, and their stems were charred to a great extent. In addition, the fire affected the surface cover of the soil, consuming the litter, as well as mosses and herbaceous plants. Rodríguez and Pinilla (2022) determined the severity of burning where 0.92 ha presented low severity, 4.82 ha moderate severity, and 2.49 ha medium to high severity. For this, the authors used the normalized difference vegetation index (NDVI), which shows that the vigor of 2016 was low (0.4–0.54) and that, five years after the fire, 2021 increased to medium (0.54–0.68), which reflects a uniformity in the area affected by the fire and a decrease in patches.
2.2. Reference ecosystemWe consider the subpáramo Cerro Alto de la Viga (4°34′53.1″ N, 74°01′56.9" W, Fig. 1a) as a reference ecosystem, referring to an unburned site (Fig. 1b). The reference ecosystem was selected taking into account the framework proposed by Durbecq et al. (2020), who postulates the need to delimit a geographic area in which habitat types similar to restoration sites occur, taking into account that these sites are not degraded and that they have environmental conditions similar to those of the restoration sites. The subpáramo is located 2.4 km from the Cerro Aguanoso, which is also part of the Bosque Oriental de Bogotá Forest Reserve. The annual mean temperature fluctuates between 8 and 11.3 ℃, with a relative humidity of 69.6% in the wet season. The elevation of the subpáramo in reserve ranges from 3000 to 3549 m a.s.l (IDEAM, 2007; CAR, 2016). The pH values in the upper layers of the soil are between 3.5 and 5.2. Regarding the vegetation, this hill has two vegetation covers of shrub and frailejonal, with species of genera such as Diplostephium, Pentacalia, Gynoxys, Hypericum, Pernettya, and Gaultheria (Rangel, 2000). In relation to its richness, it has 22 families, 16 genera, and 42 species of plants (IAvH, 2015). Due to its ecological characteristics and state of conservation, Cerro Alto de la Viga is a good reference ecosystem (control) since it has ecologically stable landscapes (climate, soil altitude, and vegetation type) similar to the ecosystem disturbed by fire, such as the case of Cerro Aguanoso.
2.3. Vegetation samplingThe vegetation regeneration was monitored in Cerro Aguanoso (burned site at 3520 m a.s.l) and Alto de La Viga (unburned site at 3505 m a.s.l) with 16 plots of 5 × 5 m each site, in the same elevation and randomly distributed on two slopes (west and east) especially difficult due to the inherent heterogeneity of the environment (Fig. 1b). All plots were implemented in the burned site one year later and two years later in the unburned area, with a single sampling. In each plot of burned site, all seedlings were documented at intervals of four months, while in the unburned area, they made only one (Rodríguez and Vargas, 2002; Ramírez-Tixe, 2013; Castro-Bonilla, 2015) (Fig. 1c). Each seedling was identified at the species level and classified as resprouting (e.g., root or rhizome resprout; Pausas and Keeley, 2014) or seed emergence. In each plot (only burned site), the individual was tagged with metal flags for follow-up monitoring. Two samples per species were collected outside the sampling plots and stored in the Herbarium of the Bogotá Botanical Garden.
2.4. Functional dataThe traits used in the study are described in Table 1. We collected functional traits related to dispersal mode and fruit type data from online databases (i.e., Plant trait database (TRY), Seed information database (SID), and World Species Web) and compiled primary source literature (Santamaría and Rodríguez, 2018). The plants were classified according to their woodiness (i.e., woody and non-woody) to establish the relationship between tolerance or vulnerability to fire. Each individual was classified in agreement to the challenges plants in the study site face in two strategy groups: persistence and dispersal. The persistence traits included in this study were height and regeneration mechanism (resprout and seedling), plant height relates to competitive ability (particularly for light), and seedling and resprout correlated to the ability to occupy space over time. In contrast, germination is largely a matter of specific physiological tolerances which are not likely to be related to any simple trait (Weiher et al., 1999). The ability to resprout is the most general and fundamental trait for disturbance tolerance (Noble and Slatyer, 1980). Selected dispersal traits included dispersal mode and fruit type (eight categories). Fruit type and the dispersal mode is related to dispersal distance and could be estimated for dispersal efficiency (Weiher et al., 1999).
| Strategy group | Trait | Type | Description | Source |
| Persistence | Regeneration mechanism | Resprout | Species can resprout after a fire from any plant structure, such as rhizomes, roots, etc. | Pausas et al. (2004) |
| Seedling | Species that depend on some traits in the seed banks to recover after a fire. | Pausas et al. (2004) | ||
| Height | Height | Height is a crucial component of a plant species' ecological strategy because height is a major determinant of a plant's ability to compete for light. | Falster and Westoby (2005) | |
| Dispersal | Fruit type | Achene | Dry and indehiscent fruit from a single seed in which the seed coating is not part of the fruit layer. | Li (2012) |
| Berry | Fleshy, indehiscent fruit, usually containing several seeds. | Li (2012) | ||
| Capsule | Dry single fruit made of two or more carpels opens to release the seeds. | Li (2012) | ||
| Caryopsis | Dry and indehiscent fruit, like achene, but with the coat of the seed fused with the coat of the fruit. | Li (2012) | ||
| Drupe | Fleshy, indehiscent fruit with a hard stone containing a single seed. | Li (2012) | ||
| Pome | Fleshy fruit with a thin skin, not formed by the ovary but by another part of the plant. | Li (2012) | ||
| Polidrupe | Multiple fruits come from a pluricarp and coricarp gynoecium that originates from numerous drupes. | Li (2012) | ||
| Unfruitful | Species that have no fruit; dispersal by sporangia or sori. | Cárdenas-Arévalo and Vargas-Ríos (2008) | ||
| Dispersal mode | Anemochory | Transport of seeds by wind. The seeds exhibit features facilitating dispersal over long distances. | Frantzen and Bouman (1989); Schulze et al. (2005); Vargas-Ríos and Pérez-Martínez (2014) | |
| Autochory | The plant disperses the seeds over a short distance, including gravity (barochory). | Frantzen and Bouman (1989); Schulze et al. (2005); Vargas-Ríos and Pérez-Martínez (2014) | ||
| Zoochory | Transport and distribution of seeds with the help of animals, including endo and ectozoochory. | Frantzen and Bouman (1989); Schulze et al. (2005); Vargas-Ríos and Pérez-Martínez (2014) |
We calculated community-weighted means (CWM) for all functional traits through (eq. (1)): where s = total number of species, Wi = relative abundance of species i in plot j, and Xi = trait value for species i (Casanoves et al., 2011).

|
(1) |
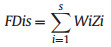
|
(2) |
We evaluated functional dispersion (FDis) to identify differences in the relative abundance of traits among communities (Laliberte and Legendre, 2010) (Eq. (2)). Where Wi is the relative abundance of species i and Zi is the distance of species to the weighted centroid. CWM and FDis were calculated using the FD package dbFD function in R (Laliberte and Legendre, 2010).
To determine the importance of the four big traits evaluated between CWM, FDis, and time, we used the t-Student test. In the case of the post-fire months, month 13 was used as a control. The t. test function of R was used. We used Spearman correlation to account for possible nonlinear relationships between traits and time. Overall, we tested for the significance of correlations for 14 CWM type of traits and four traits FDis variables (correlations were considered significant for p < 0.05). We also present graphs of changes in these variables over time traits. Spearman correlation was determined using the Stats package with corr function in R.
We used Permutational Analysis of Variance (PERMANOVA; Anderson, 2001) to test for differences in CWM and functional diversity community between burned and unburned sites, with the adonis 2 function in the vegan package with 9999 permutations (Oksanen et al., 2020). We used Bray Curtis distance and the metaMDS function to determine the stress. We tested the compositional differences between sites using Non-metric multidimensional scaling (NMDS) to illustrate the functional differences in two dimensions (Clarke, 1993). We performed all analyses under R statistical environment (R Development Core Team, 2022).
3. Results 3.1. Compositional shifts in the burned site over timeOur results show a functional change over time between woody and non-woody species richness and their functional diversity (Tables 1 and 2; Fig. 2). The CWM for woody species had differences between mechanism regeneration (p < 0.001) and height (p < 0.001) concerning their species richness. The CWM had a significant positive correlation with all the trait types except for dispersal mode such as zoochory (r = 0.18) and fruit-type such as berry (r = 0.05) and polydrupe (r = −0.02) which had a significant negative correlation (Table 2). Comparing the burned and unburned sites related to the richness, they do not have autochory in dispersal mode and pome in fruit type. Only in the unburned site, the unfruitful fruit type was not found. In both sites, there is a positive correlation between richness with height, seedlings, and fruit type, this latest, with capsule and drupe; in contrast, a negative correlation was found for achene. For non-woody species, CWM related to species richness was found to be significantly different in regeneration mechanism (p < 0.01), height (p < 0.001), and fruit type (p < 0.01). Regarding to correlation we found positive correlation in all traits, except to dispersal mode (zoochory: r = −0.19), fruit type (achene: r = −0.02; drupe: r = −0.41) and resprout (r = −0.01). Comparing the burned and unburned sites (Table 3), we no find autochory and polidrupe in the burn site, while in both sites, the unfruitful fruit was not found. In burned sites, there is only a positive correlation with the caryopsis fruit type, and there are differences in the correlation of the capsules.
| Strategy group | Trait | Type | Functional structure Woody (CWM) | Functional structure Non-woody (CWM) | |||||||||||
| Burned site | Unburned site | Burned site | Unburned site | ||||||||||||
| Mean ± SD | Spearman (r) | Mean ± SD | Spearman (r) | t | Mean ± SD | Spearman (r) | Mean ± SD | Spearman (r) | t | ||||||
| Persistence | Regeneration mechanism | Resprout | 1.28 ± 0.52 | 0.23 | – | – | −2.91 | 1.44 ± 0.18 | −0.01 | – | – | −2.45 | |||
| Seedling | 0.42 ± 0.21 | 0.53 | – | – | 0.44 ± 0.42 | 0.68 | – | – | |||||||
| Height | Height | 0.44 ± 0.09 | 0.36 | 1.72 ± 0.05 | −0.01 | 33.56 | 0.80 ± 0.11 | 0.36 | 1.53 ± 0.07 | 0.27 | 10.01 | ||||
| Dispersal | Dispersal mode | Anemochory | 0.25 ± 0.05 | −0.30 | 0.26 ± 0.07 | −0.17 | 0 | 0.11 ± 0.08 | 0.19 | 0.37 ± 0.05 | 0.37 | −0.26 | |||
| Autochory | – | – | – | – | – | – | 0.03 ± 0.05 | 0.11 | |||||||
| Zoochory | 0.74 ± 0.05 | 0.18 | 0.73 ± 0.07 | 0.17 | 0.88 ± 0.08 | −0.19 | 0.59 ± 0.08 | −0.38 | |||||||
| Fruit type | Achene | 0.43 ± 0.10 | −0.21 | 0.40 ± 0.12 | −0.60 | −0.53 | 0.43 ± 0.18 | −0.02 | 0.28 ± 0.06 | −0.01 | −2.54 | ||||
| Berry | 0.26 ± 0.08 | 0.05 | 0.17 ± 0.09 | −0.57 | 0.30 ± 0.12 | −0.22 | 0.08 ± 0.07 | 0.23 | |||||||
| Capsule | 0.10 ± 0.08 | 0.29 | 0.15 ± 0.11 | 0.77 | 0.02 ± 0.05 | 0.70 | 0.01 ± 0.01 | −0.51 | |||||||
| Caryopsis | 0.09 ± 0.07 | −0.30 | 0.17 ± 0.07 | −0.02 | 0.11 ± 0.15 | 0.23 | 0.22 ± 0.05 | 0.27 | |||||||
| Drupe | 0.01 ± 0.08 | 0.28 | 0.01 ± 0.01 | 0.53 | 0.03 ± 0.04 | −0.41 | 0.01 ± 0.01 | 0.64 | |||||||
| Pome | – | – | – | – | 0.07 ± 0.08 | −0.23 | 0.01 ± 0.04 | 0.35 | |||||||
| Polidrupe | 0.07 ± 0.03 | −0.02 | 0.08 ± 0.04 | 0.54 | – | – | 0.05 ± 0.05 | 0.15 | |||||||
| Unfruitful | 0.01 ± 0.01 | 0.30 | – | – | – | – | – | – | |||||||

|
| Fig. 2 Photographic representation of subpáramo regeneration post-fire process. (a) Year of the wildfire (2016). (b) First-year post-fire (2017). (c) Second-year post-fire (2018). |
| Strategy group | Trait | Type | Functional structure Woody (CWM) | Functional structure Non-woody (CWM) | |||||
| Mean ± SD | Spearman (r) | t | Mean ± SD | Spearman (r) | t | ||||
| Persistence | Regeneration mechanism | Resprout | 1.28 ± 0.52 | 0.52 | −2.91 | 1.44 ± 0.18 | 0.52 | −2.45 | |
| Seedling | 0.42 ± 0.21 | 0.72 | 0.44 ± 0.42 | 0.75 | |||||
| Height | Height | 0.44 ± 0.09 | 0.93 | −6.99 | 0.80 ± 0.11 | 0.75 | −5.68 | ||
| Dispersal | Dispersal mode | Anemochory | 0.25 ± 0.05 | −0.07 | 0.00 | 0.11 ± 0.08 | 0.26 | −0.09 | |
| Autochory | |||||||||
| Zoochory | 0.74 ± 0.05 | 0.07 | 0.88 ± 0.08 | −0.26 | |||||
| Fruit type | Achene | 0.43 ± 0.10 | −0.17 | −2.52 | 0.43 ± 0.18 | 0.06 | 0.06 | ||
| Berry | 0.26 ± 0.08 | −0.05 | 0.30 ± 0.12 | −0.01 | |||||
| Capsule | 0.10 ± 0.08 | 0.26 | 0.02 ± 0.05 | 0.38 | |||||
| Caryopsis | 0.09 ± 0.07 | 0.03 | 0.11 ± 0.15 | −0.26 | |||||
| Drupe | 0.01 ± 0.08 | −0.03 | 0.03 ± 0.04 | ||||||
| Pome | 0.07 ± 0.08 | −0.05 | |||||||
| Polidrupe | 0.07 ± 0.03 | 0.01 | |||||||
| Unfruitful | 0.01 ± 0.01 | −0.01 | |||||||
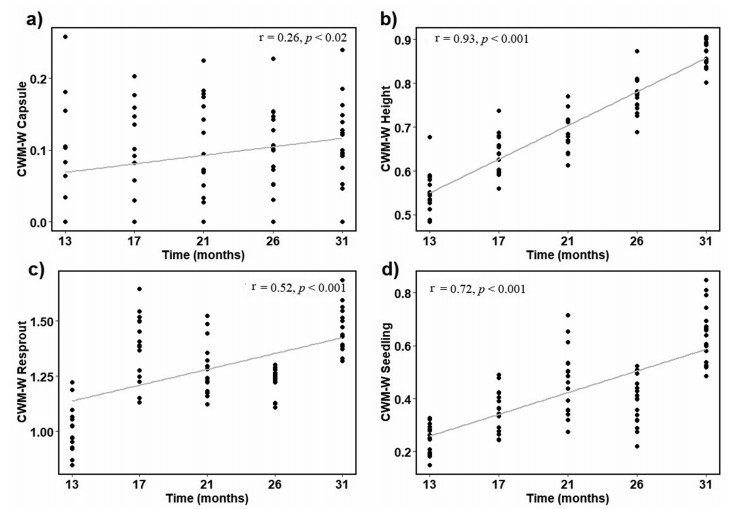
The CWM for woody species over post-fire time had significant differences only in the regeneration mechanism (p < 0.001) and height (p < 0.001). Regarding to correlation shows positive changes only in the fruit type (capsule: r = 0.26, Fig. 3a), height (r = 0.93, Fig. 3b), and regeneration mechanisms (resprout: r = 0.52, Fig. 3c; seedling: r = 0.72, Fig. 3d) (Table 4). For non-woody species was significant and positive correlation for the fruit type (capsule: r = 0.38, Fig. 4), for the dispersal mode (anemochory: r = 0.26, Fig. 4c), height (r = 0.75, Fig. 4e), and for regeneration mechanisms (resprout: r = 0.52, Fig. 4f; seedling: r = 0.75, Fig. 4g). Non-woody species was significant differences between regeneration mechanism (p < 0.01) and, height (p < 0.001). The CWM was negative correlation with caryopsis (r = −0.26, Fig. 4b) and zoochory (r = −0.26, Fig. 4d). Comparing woody and non-woody species between burned sites we found differences between dispersal mode, and fruit type (Table 5). Comparing woody and non-woody species in burned sites shows that the regeneration mechanisms correlates with species richness and post-fire time (Tables S1 and S2).
|
| Fig. 3 Spearman correlation results show changes in the CWM for woody species of persistence and dispersal traits over time in burned site communities along post-fire time. |
| Strategy group | Trait | Type | Functional dispersion Woody (FDis) | Functional dispersion Non-Woody (FDis) | |||||||||||||
| Burned site | Unburned site | Burned site | Unburned site | ||||||||||||||
| Mean ± SD | Spearman (r) | Mean ± SD | Spearman (r) | t | Mean ± SD | Spearman (r) | Mean ± SD | Spearman (r) | t | ||||||||
| Persistence | Regeneration mechanism | Resprout | 0.68 ± 0.13 | 0.23 | −2.94 | 0.52 ± 0.13 | 0.63 | 0.32 | |||||||||
| Seedling | 0.69 ± 0.18 | 0.53 | 0.66 ± 0.27 | 0.37 | |||||||||||||
| Height | 0.56 ± 0.18 | 0.06 | 0.63 ± 0.22 | 0.01 | 1.33 | 0.62 ± 0.20 | −0.22 | 0.69 ± 0.21 | −0.28 | 1.28 | |||||||
| Dispersal | Dispersal mode | 0.37 ± 0.05 | −0.19 | 0.37 ± 0.06 | −0.17 | 0.13 | 0.18 ± 0.13 | 0.19 | 0.38 ± 0.03 | 0.50 | 5.87 | ||||||
| Fruit type | 0.30 ± 0.02 | 0.17 | 0.33 ± 0.02 | 0.60 | 5.22 | 0.32 ± 0.05 | 0.02 | 0.31 ± 0.01 | 0.49 | −0.75 | |||||||

|
| Fig. 4 Spearman correlation show changes in the CWM for non-woody species of persistence and dispersal traits over time in burned sites community along post-fire time. |
| Strategy group | Trait | Type | Functional dispersion Woody (FDis) | Functional dispersion Non-Woody (FDis) | |||||
| Mean ± SD | Spearman (ρ) | T | Mean ± SD | Spearman (ρ) | t | ||||
| Persistence | Regeneration mechanism | Resprout | 0.68 ± 0.13 | 0.21 | −2.94 | 0.52 ± 0.13 | 0.22 | 0.32 | |
| Seedling | 0.69 ± 0.18 | 0.17 | 0.66 ± 0.27 | −0.04 | |||||
| Height | 0.56 ± 0.18 | −0.09 | 2.64 | 0.62 ± 0.20 | −0.41 | 2.01 | |||
| Dispersal | Dispersal mode | 0.37 ± 0.05 | −0.07 | 0.13 | 0.18 ± 0.13 | 0.30 | −0.36 | ||
| Fruit type | 0.30 ± 0.02 | 0.23 | −1.72 | 0.32 ± 0.05 | −0.15 | −0.12 | |||
The functional dispersion (FDis) for unburned sites, showed significant differences in the richness of woody plants with the regeneration mechanisms (p < 0.001) and fruit type (p < 0.001). Non-woody plants showed significant differences in the dispersal mode (p < 0.001), having been positively correlated with dispersal mode (r = 0.50) and fruit type (r = 0.49, Fig. 5a). Regarding functional dispersion (FDis) related to post-fire time, for woody species, differences were found between regeneration mechanism (p < 0.001) and height (p < 0.0001); and the positive correlation in the fruit type (r = 0.23, Fig. 5a) and resprout (r = 0.21, Fig. 5b) (Table 4). For non-woody species, we found significant differences only in the height (p < 0.0001). Positive relationship was found with the dispersal mode (r = 0.30; Fig. 6a) and resprout (r = 0.22, Fig. 6c), while was a negative correlation with height (r = −0.41, Fig. 6b) (Table 5). Comparing woody and non-woody species in burned sites shows that FDis does not correlate with species richness and post-fire time (Tables S3 and S4).

|
| Fig. 5 Spearman correlation show changes in the functional dispersal (FDis) for woody species of persistence and dispersal traits over time in burned sites community along post-fire time. |

|
| Fig. 6 Spearman correlation show changes in the functional dispersal (FDis) for non-woody species of persistence and dispersal traits over time in burned sites community along post-fire time. |
We identified 68 species in total across all the sites (Table S5, Fig. S1; see abundance per species in Fig. S2). Cerro Aguanoso (burned site) included 53 species (17 unique, Table S5), and Alto de la Viga (Unburned site) contained 51 species (15 unique, Table S5). We found differences in functional composition (CWM and FDis) between the burned and unburned site (CWM: r = 0.048, F = 4.706, p < 0.001; FDis: r = 0.051, F = 5.016, p < 0.01). NMDS analysis shows a clear superposition among burned and unburned sites (CWM stress = 0.13, FDis stress < 0.001; Fig. 7 and Table S6). High CWM trait values characterized functional composition in the reference site (unburned). FDis, the unburned site also showed higher values in the persistence and dispersion traits groups, considering that the regeneration traits were not evaluated.

|
| Fig. 7 Non-metric multidimensional scaling (NMDS) showing patterns of functional diversity (FD) based on (a) CWM and (b) FDis (Burned site: pink, unburned site: blue. Non-metric Fit R2 = 0.965. The ellipse depicts the 95% confidence ellipse around each sample group. |
We observed changes in functional community composition in the Cerro Aguanoso (burned site) postfire time. As expected, functional structure traits are most significant with richness. Thus, there are substantial changes in subpáramo functionality along succession for the areas under study. These changes in functional diversity were related to an increase in species richness. These results could be explained by the fact that the traits evaluated are related to stress-tolerant species. Furthermore, they confer success in the initial stages of succession due to their ability to grow without competition and occupy the available space to replace species (Vargas-Ríos, 1997). These results are relevant, considering the páramo ecosystem generally has few functional types and strategies; this trait convergence is driven by environmental filtering of high mountain features (Cruz and Lasso, 2021). The functional composition shows how wildfire is a triggering factor for persistence and dispersal strategy groups. Our results show high values in CWM typically occurring within local communities and between sites with similar abiotic conditions (burned and unburned areas). The changes in CWM trait values along abiotic gradients could involve the combined action of mechanisms that constrain and maintain local functional diversity where the abundance is relatively high and thus contribute more strongly to CWM (Muscarella and Uriarte, 2016).
Our results show a significant differences and correlation between CWM and post-fire time in regeneration mechanisms and height, and dispersal mode (only in non-woody plants). This patron could be explained because of their lifespan and quick regeneration (Sklenár and Ramsay, 2001) dominated by positive relation with CWM and anemochory, where plants with dry, woody, and dehiscent capsules that rely on wind for seed dispersal are prevalent in open habitats (Reginato et al., 2020), therefore, in the time after the fire, they colonized a greater number of sites. Furthermore, there is a clear positive correlation between patterns in species richness and functional composition, where the climate becomes less hospitable, and those plants that survive are restricted to favorable microsites (Ramsay and Oxley, 1996). Likewise, injury temperatures are lower in herbs, with cushion plants and grasses being the most resistant; this explains why these groups reacted positively to disturbance (Rada et al., 2019).
The correlation of traits could be seen between the regeneration mechanisms, in our case the sexual one, with the type of fruit and the form of dispersion. In non-woody species, there is a positive correlation between fruit types such as capsule (Fig. 4a) and caryopsis (Fig. 4b), which are dispersed by anemochory (Fig. 4c), allowing them to have a general colonization strategy like pioneer species (Sklenár and Ramsay, 2001). For the dispersal group, the dominance of anemochory showed that subpáramo plant communities have shorter dispersal ranges (Tamme et al., 2014) because the tropical Andean communities have higher thermal optima (i.e., cold-adapted species) than species with other dispersal modes. Many species exhibit structures such as wings, plums, or hairs that increase their resistance to air currents (Frantzen and Bouman, 1989). Anemochory has been reported as a relevant regeneration mechanism in high-montane ecosystems (Frantzen and Bouman, 1989; Melcher et al., 2000). There is a tendency for low plants due to vegetation type concerning height. This trait would also be closely related to the regeneration mechanism, where it is said that species that germinate are taller than those that resprout (Falster and Westoby, 2005). The differences could be due to the open spaces and low competition in the first months after the fire. Although the short dispersal mode may be less tolerant of drier conditions and less successful at migrating, these species could be vulnerable under warming scenarios (Tovar et al., 2020).
The most relevant results in the persistence group are the significant positive differences in seedling and resprout recruitment traits in the post-fire time for woody and non-woody plants. On the one hand, significant seedling differences in germination by seeds between postfire months due to the open gaps generated by the fire reduce the competition for light favoring the establishment of a variety of species by seedlings and the arrival of new propagules by seed dispersal, thus being a trait of fire avoidance (Laegaard, 1992; Ramsay and Oxley, 1996; Kappelle and Horn, 2016). On the other hand, we found that resprouting has differences; being a dominant regeneration mechanism, in the case of non-woody species, the resprouts are generated through rhizomes, stem tubers, and root tubers (Table 2). This characteristic is ancestral in most plant lineages (Pausas, 2012; Pausas et al., 2016, 2018; Lamont et al., 2019) and has been recognized as a typical trait of response to fire (Bond and Midgley, 2001; Lawes and Clarke, 2011; Lamont et al., 2019), especially during the early stages of regeneration (Horn, 1989; Bilbao et al., 2009; Horn and Kappelle, 2009). The importance of resprouting in this ecosystem coincides with studies in páramo and high-montane dry forests, where many species (particularly woody species) demonstrate the ability to resprout after a fire (Horn, 1989; 1990; Horn and Kappelle, 2009; Lippok et al., 2013). According to Kauffman (1991), resprouts might start to appear almost immediately after the fire, as soon as the conditions are favorable, while seed regeneration takes more time as it depends on seed dispersal, fruit production patterns, and the distance to seed sources (Laegaard, 1992; Ramsay, 1999; Ramsay and Oxley, 1996). Furthermore, seed regeneration could be essential for species that do not resprout (Otterstrom et al., 2006). Moreover, the importance of seedlings in this ecosystem is supported by the high survival rate of recruits documented 2.5 years after the fire.
Our study found few functional similarities between the traits evaluated in the sampled sites. Since functional dispersion reflects the regularity of the distribution of abundance in functional space, the similarities in regeneration mechanisms qualities among successional areas may explain the only similarities in sites (Mouchet et al., 2010). The spatiotemporal variation, especially in functional dispersion of resprouting (in woody and non-woody), would be the result of changing environmental conditions strong enough to influence species abundances and to produce temporal changes (Laliberte and Legendre, 2010).
The significant differences evidence the functional divergence in the burned area, specifically in woody species (Table 4, Table 5). These results are explained because the space occupied is not used homogeneously. The values of functional dispersion demonstrate the reorganization of the burned subpáramo. In this sense, the persistence traits have the higher FDis, whereby there is less competition between species since there is a more significant differentiation of functional traits between the functional niches of the species and, therefore, less redundancy (Mason et al., 2003), and they are changing over time postfire. Biotic interactions could also be an important driving force in the divergence of traits (Kraft and Ackerly, 2014).
Our research revealed significant changes in trait structure and dispersion functional during the post-fire period, indicating the growing importance of restricting similarity. In addition, the larger functional dispersion suggests that long-term changes in subpáramo communities will involve diversification of strategy groups, such as resprout and fruit type in woody plants and resprout and dispersal method in non-woody plants. Therefore, reproductive trait divergence is a major factor in assembly, which must be considered in restoration practices. Further studies should explore whether these interactions drive convergence in the páramo (Cruz and Lasso, 2021).
4.2. Changing composition functional between burned and unburned sites over timeWe found significant differences in functional composition between the burned site (Cerro Aguanoso) and the unburned site (Cerro Alto de la Viga). Moderate to high fire severity altered the functional structure significantly than the undisturbed site. This can partly be explained by an incomplete recovery after a fire. The páramo and subpáramo have low primary productivity, so 2.5 years are a short time for vegetation recovery and restoring a natural functional structure (Hofstede and Rossenaar, 1995). This recovery could be a second phase, as described by Zomer and Ramsay (2020), who says after the fire, a survival and recruitment phase occurs in more open conditions. After that, increases in herbaceous forms prevent other plants from establishing and reducing diversity.
The differences between these communities could also be due to differences in the species encountered. On the one hand, heterogeneity in vegetation structure leads to irregular fuel distribution and variable fire temperatures (residence time) (Zomer and Ramsay, 2020), resulting in differential plant mortality and postfire establishment and growth (Ramsay, 1999; Ramsay and Oxley, 1996). On the other hand, alternatively, this irregularity and variability in postfire recovery have been related to higher levels of biodiversity at the landscape scale due to the high degree of endemic species and significant differences in species composition between páramos (Cleef, 1981; van der Hammen and Cleef, 1986; Londoño et al., 2014). For this reason, we found variation in values for CWM and functional dispersion, generating divergent ecosystems.
These results could help to assume that species that do not show similarities in essential functional characteristics will respond differently to changes in climatic conditions (Díaz and Cabido, 1997). For example, the altitudinal conditions above 3000 m a.s.l., geographical location, incoming solar radiation, specific humidity, and precipitation conditions in both horizontal and vertical gradients (Rada, 2016), such as these two subpáramos, which are 2.4 km separated. Furthermore, the functional composition of unburned and burned sites in general show responses to water stress own of the páramo ecosystem, suggesting that they may be susceptible to the changing environmental conditions, which in turn result in allowing resilience and fragile functional stability of the high elevation ecosystems (Azócar et al., 2017).
Although this study may have some limitations, it is one of the few that focus on the functional analysis of the postfire of the Andean subpáramo in Colombia (Cruz and Lasso, 2021). It provides significant evidence for its characterization as a fire-prone and fire-tolerant ecosystem. The region is subjected to distinct seasonality, with pronounced dry seasons (CAR, 2016). The identified tolerance and avoided traits to fire, as well as the presence of certain stems types with heat- and cold-protective structures (Sturm and Rangel, 1985; Ramsay and Oxley, 1996; Vargas-Ríos, 1997; Horn and Kappelle, 2009), favor the overall positive response of the ecosystem to severe wildfires (Horn, 1989; 1990; Myers, 2006; Horn and Kappelle, 2009, 2016). Therefore, as suggested by Naccarella et al. (2020), the fire might be preventing the advance of the current high Andean ecosystems, as generated a response to postfire resprouting and seedling recruitment. However, the role of this disturbance in maintaining local dynamics will depend on the frequency. Thus, considering the slow growth rate in these high-altitude areas, recurrent fires might significantly affect the composition and structure of the vegetation on longer time scales (Oosterhoorn and Kappelle, 2000) and the regeneration opportunities of the variety of fire-tolerance traits.
5. ConclusionsOur study presents the functional differences found in an Andean subpáramo affected by the fire. Floristic, physiological, and physiognomic elements correlated with the survival and recovery of certain disturbances such as forest fires and the generation of ecosystem functions are shown. So much so that the seedlings have a high functional value, which shows how the regeneration of the subpáramo is directed; this correlates broadly with anemochory, a predominant mode of dispersal shown as early recovery indicating the ability to colonize new spaces created by fire rapidly. Likewise, resprout has previously been recognized as a typical feature of fire-prone ecosystems and appears to be, along with seedlings, the dominant regeneration mechanism in the study area. We also found a high functional divergence due to fire changes and the difference in species richness between the months evaluated after the fire and the reference site. Therefore, recognizing the study's limitations, future studies should investigate the composition and functional dispersion of páramo ecosystems to have more tools to determine if the heterogeneity is not only at the level of taxonomic diversity but also functional. All of this information can help to understand if the prevalence of resprouting is the result of repeated disturbances in the past, as an ancestral trait, or if the seedling could increase even more in the future, in the face of more frequent wildfire events that, beyond presenting a resilient ecosystem, it could reveal its vulnerability to extreme changes in environmental conditions and to change in short periods of fire return.
AcknowledgmentsThanks to the Scientific Sub-direction of the Bogotá Botanical Garden in Colombia, which funded this research [Investment project No. 1121, 2016]. In addition to ANID PhD fellowship, Chile [No. 21190817, 2019] and Vicerrectoría de Investigación, Desarrollo y Creación Artística (VIDCA) grant from the Universidad Austral de Chile [No. TD-2021-01, 2021] awarded to KOZ. Thanks to Enoc Sánchez-Londoño and the operational team assigned to the study in APIRE Cerro Aguanoso. We appreciate the insightful and constructive comments of three anonymous reviewers.
Author contributions
K.O.Z. Conceptualization, sampled, processed and data curation, Formal analysis, wrote original draft preparation. A.P.R. Conceptualization, methodology, reviewing, and editing. All authors approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.11.007.
Accatino, F., Wiegand, K., Ward, et al., 2016. Trees, grass, and fire in humid savannas-The importance of life history traits and spatial processes. Ecol. Model., 320: 135-144. DOI:10.1016/j.ecolmodel.2015.09.014 |
Anderson, M.J., 2001. Permutation tests for univariate or multivariate analysis of variance and regression. Can. J. Fish. Aquat., 58: 626-639. DOI:10.1139/f01-004 |
Armenteras, D., González, T.M., Ríos, O.V., et al., 2020. Fire in the ecosystems of northern south America: advances in the ecology of tropical fires in Colombia, Ecuador and Peru. Caldasia, 42: 1-16. DOI:10.15446/caldasia.v42n1.77353 |
Azócar, A., Rada, F., García-Nuñez, C., 2017. Aspectos ecofisiológicos para la conservación de ecosistemas tropicales contrastantes. Bot. Sci., 65: 89-94. DOI:10.17129/botsci.1599 |
Bader, M.Y., Van Geloof, I., Rietkerk, M., 2007. High solar radiation hinders tree regeneration above the alpine treeline in northern Ecuador. Plant Ecol., 191: 33-45. DOI:10.1007/s11258-006-9212-6 |
Bilbao, B., Leal, A., Méndez, C., et al., 2009. The role of fire in the vegetation dynamics of upland savannas of the Venezuelan Guayana. In: Cochrane, M.A. (Ed. ), Tropical Fire Ecology, pp. 451-480. https://doi.org/10.1007/978-3-540-77381-8_16.
|
Bond, W.J., Keeley, J.E., 2005. Fire as a global "herbivore": the ecology and evolution of flammable ecosystems. Trends Ecol. Evol., 20: 387-394. DOI:10.1016/j.tree.2005.04.025 |
Bond, W.J., Midgley, J.J., 2001. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol., 16: 45-51. DOI:10.1016/S0169-5347(00)02033-4 |
Borrelli, P., Armenteras, D., Panagos, P., et al., 2015. The implications of fire management in the Andean páaramo: a preliminary assessment using satellite remote sensing. Rem. Sens., 7: 11061-11082. DOI:10.3390/rs70911061 |
CAR, 2016. Modificación al Plan de Manejo Reserva Forestal Protectora de Bosque Oriental de Bogotá. Corporación Autónoma Regional de Cundinamarca. https://www.car.gov.co/vercontenido/173.
|
Cárdenas-Arévalo, G., Vargas-Ríos, O., 2008. Rasgos de historia de vida de especies en una comunidad vegetal alterada en un páramo húmedo (Parque Nacional Natural Chingaza). Caldasia 30, 245-264. https://revistas.unal.edu.co/index.php/cal/article/view/39168.
|
Casanoves, F., Pla, L., Di Rienzo, J.A., 2011. Valoración y análisis de la diversidad funcional y su relación con los servicios ecosistémicos. Informe técnico (CATIE): Número 384. https://repositorio.catie.ac.cr/handle/11554/8190.
|
Castro-Bonilla, M.A., 2015. Estructura y diversidad florística de los matorrales y frailejones del páramo de los valles de Anaime. Bachelor's thesis, Universidad del Tolima. http://repository.ut.edu.co/bitstream/001/2220/1/Trabajo%20de%20Grado.pdf.
|
Cavero, R., Ederra, A., 1999. Evolución de la composición florística post-fuego en un carrascal de Navarra (España). Pirineos, 153-154: 61-100. DOI:10.3989/pirineos |
Clarke, K.R., 1993. Non-parametric multivariate analyses of changes in community structure. Austr. J. Ecol., 18: 117-143. DOI:10.1111/j.1442-9993.1993.tb00438.x |
Clarke, P.J., Lawes, M.J., Midgley, J.J., et al., 2013. Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytol., 197: 19-35. DOI:10.1111/nph.12001 |
Cleef, A.M., 2008. Influencia humana en los páramos. In: Castañeda, J.P. (Ed. ), Panorama y perspectivas sobre la gestión ambiental de los ecosistemas de páramo: memorias. Procuraduría delegada para asuntos ambientales y agrarios,
pp. 26-33. https://hdl.handle.net/11245/1.297709.
|
Cleef, A.M., 1981. The vegetation of the páramos of the Colombian Cordillera Oriental. Inst. Syst. Bot. 481, 1-320. https://repository.naturalis.nl/pub/534752.
|
Cortés-Duque, J., Sarmiento Pinzón, C.E., 2013. Memorias del proceso de definición de criterios para la delimitación de páramos. In: Visión socioecosistémica de los páramos y la alta montaña colombiana. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. http://repository.humboldt.org.co/handle/20.500.11761/31458.
|
Cruz, M., Lasso, E., 2021. Insights into the functional ecology of páramo plants in Colombia. Biotropica, 53: 1415-1431. DOI:10.1111/btp.12992 |
Cuatrecasas, J., 1958. Aspectos de la vegetación natural de Colombia. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat., 10: 221-264. |
Di Pasquale, G., Marziano, M., Impagliazzo, S., et al., 2008. The Holocene treeline in the northern Andes (Ecuador): first evidence from soil charcoal. Palaeogeogr. Palaeoclimatol. Palaeoecol., 259: 17-34. DOI:10.1016/j.palaeo.2006.12.016 |
Díaz, S., Cabido, M., 1997. Plant functional types and ecosystem function in relation to global change. J. Veg. Sci., 8: 463-474. DOI:10.2307/3237198 |
Durbecq, A., Jaunatre, R., Buisson, E., et al., 2020. Identifying reference communities in ecological restoration: the use of environmental conditions driving vegetation composition. Restor. Ecol., 28: 1445-1453. DOI:10.1111/rec.13232 |
Espinoza, I.G., Franco-Gaviria, F., Castañeda, I., et al., 2022. Holocene fires and ecological novelty in the high Colombian Cordillera Oriental. Front. Ecol. Evol., 10: 895152. DOI:10.3389/fevo.2022.895152 |
Falster, D.S., Westoby, M., 2005. Tradeoffs between height growth rate, stem persistence and maximum height among plant species in a post-fire succession. Oikos, 111: 57-66. DOI:10.1111/j.0030-1299.2005.13383.x |
Frantzen, N.M.L.H.F., Bouman, F., 1989. Dispersal and growth form patterns of some zonal páramo vegetation types. Acta Bot. Neerl., 38: 449-465. DOI:10.1111/j.1438-8677.1989.tb01376.x |
Gutiérrez-Salazar, P., Ramsay, P.M., 2020. Physiognomic responses of páramo tussock grass to time since fire in northern Ecuador. Rev. Peru. Biol., 27: 205-214. DOI:10.15381/rpb.v27i2.17876 |
Hofstede, R.G., Rossenaar, A.J., 1995. Biomass of grazed, burned, and undisturbed Páramo Grasslands, Colombia. II. Root mass and aboveground: belowground ratio. Arct. Alp. Res., 27: 13-18. DOI:10.2307/1552063 |
Hofstede, R.G.M., 1995. The effects of grazing and burning on soil and plant nutrient concentrations in Colombian páramo grasslands. Plant Soil, 173: 111-132. DOI:10.1007/BF00155524 |
Horn, S.P., 1989. Post fire vegetation development in the Costa Rican páramos. Madroño, 36: 93-114. http://www.jstor.org/stable/41424741. |
Horn, S.P., 1990. Vegetation recovery after the 1976 páramo tire in Chirripó National. Rev. Biol. Trop., 38: 267-275. http://www.cabdirect.org/abstracts/19920662187.html. |
Horn, S.P., Kappelle, M., 2009. Fire in the páramo ecosystems of central and south America. In: Cochrane, M.A. (Ed. ), Tropical Fire Ecology, pp. 505-539. https://doi.org/10.1007/978-3-540-77381-8.
|
Huang, B., L'Heureux, M., Hu, Z.Z., et al., 2016. Ranking the strongest ENSO events while incorporating SST uncertainty. Geophys. Res. Lett., 43: 9165-9172. DOI:10.1002/2016GL070888 |
IDEAM, 2007. Meteorología Y Estudios Ambientales. In: Estudio de la
caracterización climática de Bogotá y cuenca alta del Río Tunjuelo. Bogotá D.C. Instituto de Hidrología. https://oab.ambientebogota.gov.co/?post_type=dlm_download&p=3678.
|
Kappelle, M., Horn, S.P., 2016. The páramo ecosystem of Costa Rica's Highlands. In: Kappelle, M. (Ed. ), Costa Rican Ecosystems. University of Chicago Press,
pp. 492-524. https://doi.org/10.7208/chicago/9780226121642.003.0015.
|
Kauffman, J.B., 1991. Survival by sprouting following fire in tropical forests of the eastern Amazon. Biotropica, 23: 219. DOI:10.2307/2388198 |
Keating, P.L., 2007. Fire ecology and conservation in the high tropical Andes: observations from northern Ecuador. J. Lat. Am. Geogr., 6: 43-62. DOI:10.1353/lag.2007.0003 |
Keeley, J.E., Fotheringham, C.J., 2009. Role of fire in regeneration from seed. In:
Fenner, M. (Ed. ), Seeds: the Ecology of Regeneration in Plant Communities. Cabi
Publishing, Wallingford UK, pp. 311-330. https://doi.org/10.1079/9780851994321.0311.
|
Keeley, J.E., Pausas, J.G., Rundel, P.W., et al., 2011. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci., 16: 406-411. DOI:10.1016/j.tplants.2011.04.002 |
Knox, K.J.E., Clarke, P.J., 2006. Fire season and intensity affect shrub recruitment in temperate sclerophyllous woodlands. Oecologia, 149: 730-739. DOI:10.1007/s00442-006-0480-6 |
Kraft, N.J.B., Ackerly, D.D., 2014. Assembly of plant communities. J. Ecol. Environ., 8: 67-88. DOI:10.1007/978-1-4614-7501-9_1 |
Laegaard, S., 1992. Influence of fire in the grass paramo vegetation of Ecuador. In:
Balslev, H., L Luteyn, J. (Eds. ), Páramo: an Andean Ecosystem under Human
Influence. Academic Press, London, UK, pp. 151-170.
|
Laliberte, E., Legendre, P., 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology, 91: 299-305. DOI:10.1890/08-2244.1 |
Lamont, B.B., He, T., Yan, Z., 2019. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev., 94: 903-928. DOI:10.1111/brv.12483 |
Lawes, M.J., Clarke, P.J., 2011. Ecology of plant resprouting: populations to community responses in fire-prone ecosystems. Plant Ecol., 212: 1937-1943. DOI:10.1007/s11258-011-9994-z |
Lawes, M.J., Keith, D.A., Bradstock, R.A., 2016. Advances in understanding the influence of fire on the ecology and evolution of plants: a tribute to Peter. J. Clarke. Plant Ecol., 217: 597-605. DOI:10.1007/s11258-016-0625-6 |
Li, K.T., 2012. Physiology and classification of fruits. In: Sinha, N.K., Sidhu, J., Barta, J., et al. (Eds. ), Handbook of Fruits and Fruit Processing. John Wiley & Sons,
pp. 1-12. https://doi.org/10.1002/9781118352533.ch1.
|
Lippok, D., Beck, S.G., Renison, D., et al., 2013. Forest recovery of areas deforested by fire increases with elevation in the tropical Andes. For. Ecol. Manage., 295: 69-76. DOI:10.1016/j.foreco.2013.01.011 |
Londoño, C., Cleef, A., Madriñán, S., 2014. Angiosperm flora and biogeography of the páramo region of Colombia, Northern Andes. Flora, 209: 81-87. DOI:10.1016/j.flora.2013.11.006 |
Mason, N.W.H., MacGillivray, K., Steel, J.B., et al., 2003. An index of functional diversity. J. Veg. Sci., 14: 571-578. DOI:10.1111/j.1654-1103.2003.tb02184.x |
McIntyre, S., Lavorel, S., Landsberg, J., Forbes, T.D.A., 1999. Disturbance response in vegetation – towards a global perspective on functional traits. J. Veg. Sci., 10: 621-630. DOI:10.2307/3237077 |
Melcher, I.M., Bouman, F., Cleef, A.M., 2000. Seed dispersal in páramo plants: epizoochorous and hydrochorous taxa. Plant Biol., 2: 40-52. DOI:10.1055/s-2000-9146 |
Myers, R.L., 2006. Living with fire-sustaining ecosystems & livelihoods through integrated fire management. Global Fire Initiative. Nat. Conserv. 28. https://www.conservationgateway.org/Files/Pages/living-fire.aspx.
|
Mouchet, M.A., Villéger, S., Mason, N.W., Mouillot, D., 2010. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol., 24: 867-876. DOI:10.1111/j.1365-2435.2010.01695.x |
Muscarella, R., Uriarte, M., 2016. Do community-weighted mean functional traits reflect optimal strategies?. Proc. R. Soc. B., 283: 20152434. DOI:10.1098/rspb.2015.2434 |
Naccarella, A., Morgan, J.W., Cutler, S.C., et al., 2020. Alpine treeline ecotone stasis in the face of recent climate change and disturbance by fire. PLoS One, 15: e0231339. DOI:10.1371/journal.pone.0231339 |
Noble, I.R., Slatyer, R.O., 1980. The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio, 43: 5-21. DOI:10.1007/BF00121013 |
Oksanen, J., Blanchet, F.G., Friendly, M., et al., 2020. Package "vegan" Title Community Ecology Package Version 2.5-7. R 2.5, 1–286. https://CRAN.R-project.org/package=vegan
|
Oosterhoorn, M., Kappelle, M., 2000. Vegetation structure and composition along an interior-edge-exterior gradient in a Costa Rican montane cloud forest. For. Ecol. Manage., 126: 291-307. DOI:10.1016/S0378-1127(99)00101-2 |
Otterstrom, S.M., Schwartz, M.W., Velázquez-Rocha, I., 2006. Responses to fire in selected tropical dry forest trees. Biotropica, 38: 592-598. DOI:10.1111/j.1744-7429.2006.00188.x |
Pausas, J.G., 2012. Incendios forestales. Una visión desde la ecología. Catarata-CSIC, Madrid.
|
Pausas, J.G., Bradstock, R.A., Keith, D.A., et al., 2004. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology, 85: 1085-1100. DOI:10.1890/02-4094 |
Pausas, J.G., Keeley, J.E., 2014. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol., 204: 55-65. DOI:10.1111/nph.12921 |
Pausas, J.G., Lamont, B.B., Paula, S., et al., 2018. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol., 217: 1435-1448. DOI:10.1111/nph.14982 |
Pausas, J.G., Pratt, R.B., Keeley, J.E., et al., 2016. Towards understanding resprouting at the global scale. New Phytol., 209: 945-954. DOI:10.1111/nph.13644 |
Pausas, J.G., Ribeiro, E., 2017. Fire and plant diversity at the global scale. Global Ecol. Biogeogr., 26: 889-897. DOI:10.1111/geb.12596 |
Peyre, G., Balslev, H., Font, X., 2018. Phytoregionalisation of the andean páramo. PeerJ, 6: e4786. DOI:10.7717/peerj.4786 |
Premauer, J., Vargas-Ríos, O., 2004. Patrones de diversidad en vegetación pastoreada y quemada en un páramo húmedo (Parque Natural Chingaza, Colombia). Ecotropicos, 17: 52-66. |
R Development Core Team, 2022. R: A Language and Environment for Statistical
Computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
|
Rada, F., 2016. Functional diversity in tropical high elevation giant rosettes. In:
Goldstein, G., Santiago, L. (Eds. ), Tropical Tree Physiology. Tree Physiology.
Springer, Cham, pp. 181-202. https://doi.org/10.1007/978-3-319-27422-5_8.
|
Rada, F., Azócar, A., García-Núñez, C., 2019. Plant functional diversity in tropical Andean páramos. Plant Ecol. Divers., 12: 539-553. DOI:10.1080/17550874.2019.1674396 |
Ramírez-Tixe, M.G., 2013. Diversidad florística a diferente altitud en el ecosistema
páramo en siete comunidades de la OSG UNOCANT. Bachelor's thesis, Escuela
Superior Politécnica de Chimborazo. http://dspace.espoch.edu.ec/handle/123456789/2790.
|
Ramsay, P.M., 1999. Landscape mosaics in the High Andes: the role of fire in páramo communities. Nat. Cult. Landsc. Ecol. Exp. 3rd Millenn. Karolinum Press, Prague. https://www.academia.edu/download/49945566/Landscape_mosaics_in_the_High_Andes_the_20161028-8267-oeas39.pdf.
|
Ramsay, P.M., Oxley, E.R.B., 1996. Fire temperatures and postfire plant community dynamics in Ecuadorian grass páramo. Vegetatio, 124: 129-144. DOI:10.1007/bf00045489 |
Rangel, O. 2000. Colombia Diversidad Biótica III, la región de vida paramuna. 2000. 1 ed. Bogotá D.C. p. 902. https://repositorio.unal.edu.co/handle/unal/81936
|
Reginato, M., Vasconcelos, T.N.C., Kriebel, R., et al., 2020. Is dispersal mode a driver of diversification and geographical distribution in the tropical plant family Melastomataceae?. Mol. Phylogenet. Evol., 148: 106815. DOI:10.1016/j.ympev.2020.106815 |
Rodríguez, P., Pinilla, C., 2022. Relación entre la severidad de quemado y la recuperación de la vegetación post- incendio en un bosque altoandino en el cerro Aguanoso, Cerros Orientales de Bogotá. Bachelor's tesis. Universidad Distrital
Francisco José de Caldas.
|
Rodríguez, W., Vargas, O., 2002. Estrategias de regeneración postquema en áreas de vegetación altoandina tipo matorral. Pérez-Arbelaezia 13, 9-32. https://perezarbelaezia.jbb.gov.co/index.php/pa/article/view/87.
|
Santamaría, C., Rodríguez, W., 2018. Bachelor's Tesis. In: Identificación de rasgos funcionales de especies vegetales del bosque altoandino y páramo relacionados con su respuesta regenerativa post-fuego. Universidad Distrital Francisco José
de Caldas. https://repository.udistrital.edu.co/handle/11349/7614?show=full.
|
Schulze, E. -D., Beck, E., Müller-Hohenstein, K., 2005. Plant Ecology. Springer Science & Business Media.
|
Sklenár, P., Ramsay, P.M., 2001. Diversity of zonal páramo plant communities in Ecuador. Divers. Distrib., 7: 113-124. DOI:10.1046/j.1472-4642.2001.00101.x |
Sturm, H., Rangel, O., 1985. Ecología de los páramos andinos: una visión preliminar integrada. Univ. Nac. Colomb. 1, 292. https://repositorio.unal.edu.co/handle/unal/82357.
|
Tamme, R., Götzenberger, L., Zobel, M., et al., 2014. Predicting species' maximum dispersal distances from simple plant traits. Ecology, 95: 505-513. DOI:10.1890/13-1000.1 |
Torres, R.C., Giorgis, M.A., Trillo, C., et al., 2014. Post-fire recovery occurs overwhelmingly by resprouting in the Chaco Serrano forest of central Argentina. Austral Ecol., 39: 346-354. DOI:10.1111/aec.12084 |
Tovar, C., Melcher, I., Kusumoto, B., et al., 2020. Plant dispersal strategies of high tropical alpine communities across the Andes. J. Ecol., 108: 1910-1922. DOI:10.1111/1365-2745.13416 |
van der Hammen, T., Cleef, A.M., 1986. Development of the high Andean páramo flora and vegetation. In: Vuilleumier, F., Monasterio, M.M. (Eds. ), High Altitude
Tropical Biogeography, pp. 153e201. New York, USA.
|
van der Hammen, T., Andrade, G., 2003. Estructura Ecológica Principal de Colombia: Primera aproximación. Ministerio de Ambiente, Vivienda y Desarrollo Territorial, Instituto de Hidrología, Meteorología y Estudios Ambientales. Bogotá, Colombia. https://observatorio.epacartagena.gov.co/estructura-ecologicaprincipal-de-colombia-primera-aproximacion/.
|
Vargas-Ríos, O., 1997. Un modelo de sucesión-regeneración de los páramos después de quemas. Caldasia 19, 331-345. https://revistas.unal.edu.co/index.php/cal/article/view/17430.
|
Vargas-Ríos, O., Pérez-Martínez, L.V., 2014. Semillas de plantas de páramo: ecología
y métodos de germinación aplicados a la restauración ecológica. Universidad Nacional de Colombia, pp. 17-63.
|
Weiher, E., Werf, A., Thompson, K., et al., 1999. Challenging Theophrastus: a common core list of plant traits for functional ecology. J. Veg. Sci., 10: 609-620. DOI:10.2307/3237076 |
Whelan, R.J., 1986. Seed dispersal in relation to fire. In: Murray, D.R. (Ed. ), Seed
Dispersal. Academic Press, San Diego, pp. 237-271. https://doi.org/10.1016/b978-0-12-511900-9.50011-5.
|
White, S., 2013. Grass páramo as hunter-gatherer landscape. Holocene, 23: 898-915. DOI:10.1177/0959683612471987 |
Zomer, M.A., Ramsay, P.M., 2020. Post-fire changes in plant growth form composition and diversity in Andean páramo grassland. Appl. Veg. Sci., 24: e12554. DOI:10.1111/avsc.12554 |



