b. Research and Collections Center, Illinois State Museum, 1011 East Ash Street, Springfield, IL 62703, USA;
c. Research Center of Global Change and Complex Ecosystems, Zhejiang Tiantong Forest Ecosystem National Observation and Research Station, School of Ecological and Environmental Sciences, East China Normal University, Shanghai 200241, China
Invasive species cause significant loss of biodiversity and ecosystem function worldwide (Williamson, 1996). It has been argued that native species diversity (hereafter diversity or richness) can either resist or facilitate species invasions (Rejmánek, 1996, 2003; Williamson, 1996; Brown and Peet, 2003; Guo et al., 2019; Chen et al., 2021). Most previous studies showing biotic resistance due to native diversity have been done over small scales and sometimes through experimental manipulation based on exotic abundance data (but see Chen et al., 2010; Beaury et al., 2019; Peng et al., 2019; Tomasetto et al., 2019). However, biotic resistance over large or regional scales have been difficult to prove mostly due to the following three factors: (1) the well-known positive species–area relationships (i.e., the number of both native and exotic species increases with area) and associated confounding factors such as environmental heterogeneity (MacArthur and Wilson, 1967; Burns, 2016), (2) the low likelihood of demonstration through experiments, and (3) the lack of large-scale abundance (density, biomass, cover) data.
The number of exotic species (richness) alone may not be a strong or accurate indicator of habitat invasibility and degree of invasion (DI) because most exotic species are not invasive, that is, they are present but with low abundance and restricted distribution (Williamson, 1996; Williamson and Fitter, 1996). Contrary to the frequently claimed negative native−exotic richness relationship over small spatial scales (Shea and Chesson, 2002), a recent review (Guo, 2015) found that of the 43 cases based on richness data, only 8 (19%) showed negative native−exotic correlations, and the remainder had either no, or multiple or variant forms of correlations (see also Guo, 2022). Studies that found negative relationships between native and exotic species have used abundance rather than richness data for exotic plants (e.g., Byrne et al., 2010).
Efforts to quantify invasion success on large scales have been hampered by inadequate information on relative species abundance. To overcome this issue, ecologists and land managers have attempted identifying alternative indicators such as distribution or range size (e.g., Guo et al., 2006). More recently, researchers have tried to examine whether species distribution (range size) is related to species richness (Guo et al., 2022). Such perceived diversity−range size relationships could offer important clues regarding the causes of large-scale species invasions, especially for predicting invasions. Another reason for using range size data is that distribution is usually positively related to abundance (Brown, 1984), and thus may indeed better represent overall resource use. For conservation purposes, if species in species-richer regions indeed have smaller ranges (thus lower abundance), priority should be given to such diversity hotspots when resources are limited (Eeley and Foley, 1999).
In a recent synthesis, Guo et al. (2022) concluded that negative diversity–range relationships are nearly universal. But what do such relationships imply in regional species invasion and conservation? With rapidly accumulated data on species invasions worldwide, this question could be answered by examining two sets of species related to native species diversity: (1) the native species distribution and (2) invading species distribution. If the negative diversity–range size relationship is universal (i.e., richness does play an important role), the invading species should also have smaller ranges in species-rich regions than in species-poor regions. Thousands of species of plants have been transported, both intentionally and accidentally, between north temperate regions of eastern Asia (EA), North America (NA), and Europe (EU). These regions have similar ranges of ecological conditions (Qian and Ricklefs, 2000; Qian, 2002; Guo et al., 2006; Ricklefs et al., 2008) (see also Supplementary data) and share many genera and species of native plants, reflecting long-standing biogeographic connections. Comparisons in the degree of invasion (DI) or invasibility measured by relative range size of exotic species across continents such as Europe (EU)–eastern Asia (EA)–North America (NA) (Fig. S1) would offer a unique opportunity to test the regional diversity–invasibility relationships (Heberling et al., 2017).
In this study, we investigate the role of regional species diversity in resisting or facilitating biotic invasions through regulating the spread and range size of existing and invading species (Batt et al., 2017). We compare DI using distribution (range size) data from EU, EA and NA. The difference in native plant richness is quite large among the three continental regions (EA > NA > EU; Fig. 1). We test the hypothesis that higher regional diversity will constrain the range size of component species, including exotic species, thus, leading to higher resistance (lower invasibility) due to competition for resources and space (Legault et al., 2020). To do this, we compare the distribution patterns (e.g., range size) of the exotics between native and introduced ranges among the three regions. We then discuss the significance and implications of our findings for biotic invasions and conservation.
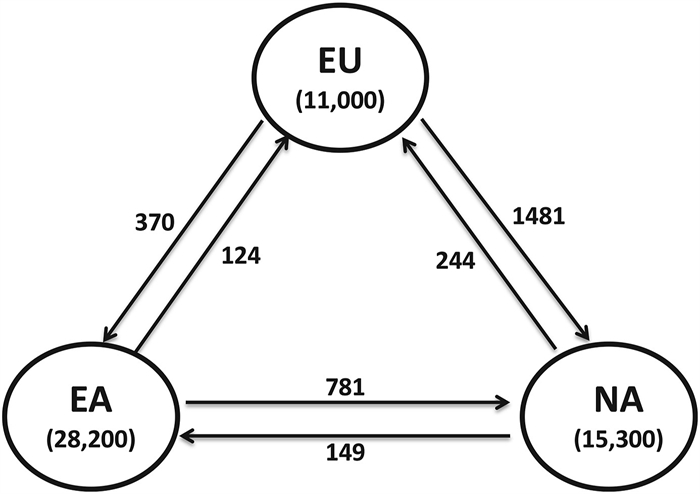
|
| Fig. 1 Native plant species richness and the number of exchanged exotic plants across Europe (EU), Eastern Asia (EA), and North America (NA) used for range size comparisons. |
Our study covered the whole longitudinal breath of the temperate latitudes of the Northern Hemisphere, which was divided into three continental regions (EU, EA, and NA, as shown in Fig. S1). The geographic extent of Asia in our study is the same as that of eastern Asia in Guo et al. (2006). For consistency with Guo et al. (2006), we also called the Asian part of our study area "eastern Asia". Our study included those species of vascular plants that are each exotic in at least one of the three continental regions and native to at least one of the three regions. Thus, species that were exotic to any of the three regions and not native to any of the regions were not included in our study. We only included naturalized exotic species. We made an effort to include all vascular plant species that met the above-described criteria, and avoided bias toward any region in inclusion of species in this study. As a result, we assembled a comprehensive data set with distribution (range size) data for 2872 plant species that are shared by at least two of the three continental regions (EU, EA, and NA; Fig. S1).
Geographic units for measuring species distribution/range size are shown in Fig. S1 and Table S1. Specifically, the geographic units of EA and NA are those used in Guo et al. (2006), and the geographic units of EU are those used in the Flora Europaea (Tutin et al., 1964–1980). Using these geographic units to document species distributions for studies on exotic plants are common (e.g., Guo et al., 2006; Winter et al., 2010). The data of presence or absence and nativity status of each species in each of the geographic units were obtained from multiple sources. Specifically, data for the geographic units in EU were obtained from Tutin et al. (1964–1980); data for the geographic units in NA were obtained from Kartesz (1999); data for the geographic units in eastern Asia were obtained from Wu et al. (1994–2003) for China; Charkevicz (1985–1996) for Russian Far East; Krasnoborov et al. (1988–1997) for Siberia; Lee, 1996, Lee, 1980, Ri and Hoang (1984) for the Korean peninsula; and Grubov (2001) for Mongolia. These data sources have been used in previous inter-continental comparisons of native and exotic plants (e.g., Qian, 2002; Winter et al., 2010). We standardized botanical nomenclature according to The Plant List (http://www.theplantlist.org). Infraspecific taxa were combined with their respective species.
In principle, it would have been desirable to base the analyses on more natural geographic or ecological units, but the data simply are not available. Most of the species included in this study are distributed in more than one ecoregion or climate zone, and do not necessarily occupy the entire area of any one of these. Thus, except for measuring the actual area occupied (range maps are not available for most species in EA), it is not clear that "natural" geographic units would offer any advantage over geographic units in quantifying distribution. Indeed, the borders of geographic units often follow natural geographic boundaries or boundaries between ecoregions. Another approach would have been to use equal-area latitude-longitude grid squares, as is the case in many studies of diversity patterns. However, different ecoregions have different areas within a region and so the distributional area of an introduced species might reflect its typical habitat rather than the extent to which it has occupied potentially suitable habitats. A fortunate feature of geographic units is that they tend to be smaller where the scale of environmental variation is smaller and productivity is greater. Thus, the scale of the geographic units matches the kinds of environments that support the greatest number of introduced species. Furthermore, because the same method of dividing regions into units was used in all three continental regions, there should be no bias with regard to region. Unit area tends to increase toward the north, but this is true of all three regions and most introduced species are concentrated in southern units in all three regions.
The total area was calculated as the sum of all regions on each continent (excluding Greenland, which was not included), that is 22, 042, 751 km2 for EA, 19, 122, 006 km2 for NA, and 9, 900, 000 km2 for EU. Range size measures could only include the part of the range within the area for which diversity was measured (i.e., not the entire range a species actually occupies unless the species only occurs in that particular area). The distribution of each species was calculated as the proportion (ranging from 0 to 1) of total continental area. Detailed information regarding our data compilation is provided in Supplementary data.
The numbers of exotic naturalized plant species in the three regions that were analyzed in this study are given in Fig. 1. Range size (distributional areas) of each species within each region was calculated as the number of geographic units (countries in EU, states or provinces or equivalent geographic units in EA and NA; Table S1) from which they have been reported divided by the total number of geographic units in the region. Thus, the geographic range for each species varies between 0 and 1 within each region (see Supplementary data). Although these values overestimate geographic extent because plant populations need not occupy the entire area of any given political unit, they are not biased with respect to region. We use the proportion of geographic units in a region reported for each species, instead of the area of those units, to avoid biases caused by local occurrence in large units. However, our results were independent of which measure was used.
We compared the averages of proportional area for native and exotic species in each region using t-tests assuming unequal sample sizes and variances. We used analysis of variance (ANOVA) to relate the nine averages for proportional area in Table 1 to source (native) region, target (exotic) region, and native versus exotic status. Multiple regressions of average extent as a function of native vs. exotic, source region, and recipient region were performed by SAS software, GLM procedure (SAS Institute, 2014).
| Native region | |||||
| Exotic region (area, km2) | Native plant richness | Land use | Eastern Asia (EA) | North America (NA) | Europe (EU) |
| Eastern Asia (22.0 × 106) | High (28, 200) | Older | 0.217 ± 0.171 (840) | 0.111 ± 0.131 (149) | 0.147 ± 0.171 (370) |
| North America (19.1 × 106) | Intermediate (15, 300) | Younger | 0.221 ± 0.254 (781) | 0.449 ± 0.273 (350) | 0.251 ± 0.273 (1481) |
| Europe (9.9 × 106) | Low (11, 000) | Older | 0.203 ± 0.210 (124) | 0.197 ± 0.178 (244) | 0.593 ± 0.295 (1524) |
Our intercontinental comparison showed that on average, exotic plants introduced among eastern Asia (EA), North America (NA), and Europe (EU) had proportionally smaller ranges in species-rich regions than in species-poor regions (i.e., EA < NA < EU) (Table 1; see also Fig. 2). Such differences were also evident as revealed by detailed comparisons in average species range sizes between EA and NA; that is, the range sizes of species native to both EA and NA were 0.169 ± 0.150 and 0.521 ± 0.254, t-test, P < 0.0001, respectively; and the range sizes of those exotic to both EA and NA were 0.128 ± 0.145 and 0.320 ± 0.308, t-test, P < 0.0001, respectively (Fig. 3).
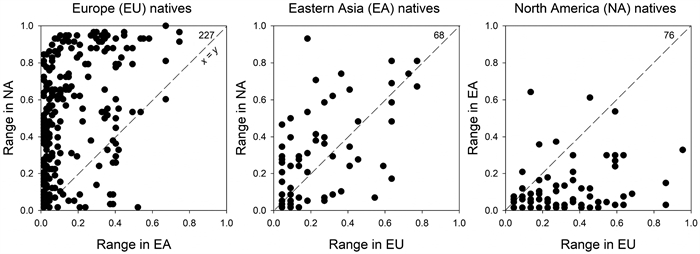
|
| Fig. 2 Comparison of range size of exotic plants introduced among Europe (EU), eastern Asia (EA), and North America (NA) measured as proportional distribution. Note that the number of exotic plants here do not include all exotic plants shared among the three regions. |
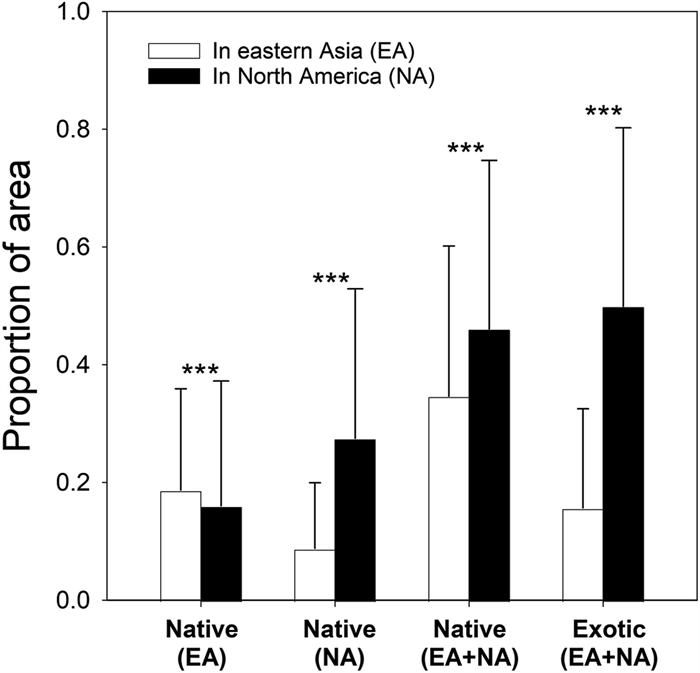
|
| Fig. 3 Comparison of range sizes (proportional distribution) of transpacific exotic plants in eastern Asia (EA) vs. North America (NA) and the plant species either native or exotic to both regions (in all cases, paired t–test, *** indicates P < 0.001). In general, species in all four groups showed broader distribution (ranges) in NA than in EA. |
In general, EU species performed better in NA than in EA (Fig. 2), measured by average species' invaded range sizes. EU natives and NA natives and the shared exotics performed almost equally well in NA and EU, respectively. However, EA natives performed better in EU than the EU natives performed in EA, and the shared exotics performed better in EU than in EA (Table 1). Although the lower diversity in EU (relative to NA) should have allowed exotics from EA to spread more broadly there, the higher latitude and fewer climate zones of EU might limit the distribution of many invasive species, especially those from climate zones that were poorly represented in EU. EU species had larger ranges in NA than in EA. Although EA natives in NA and EU occupied similar ranges, the contrast in diversity between NA and EU also was not nearly as great as it is between EA and the other two regions. Exotics from EU or NA clearly had much smaller ranges in EA than in either NA or EU, respectively (Fig. 2, Table 1).
Analysis of variance (ANOVA) that linked the nine averages for proportional area with native vs. exotic regions and natives vs. exotics (Table 1) explained 93.6% of the variance in average proportion of geographic units occupied, and native vs. exotic (P = 0.016) and target region (P = 0.072) were significant, or marginally significant (P < 0.10), effects. EU did not differ from NA as a target region (P = 0.70). When these regions combined were contrasted with EA and source region was dropped, the model explained 81.9% of the variance (F2, 6 = 13.6, P = 0.006) and both native vs. exotic (P = 0.006) and EA as target region (P = 0.019) showed significant effects: areas occupied by exotics in EA were smaller than those in NA and EU by 0.17 ± 0.05 units of proportional area, and species in their native regions had larger extents than in exotic regions by 0.23 ± 0.05 units.
In detailed comparisons between EA and NA, among our comprehensive inclusion of total 1532 species analyzed, 781 were species introduced from EA to NA, 149 were introduced from NA to EA, 375 were native to both regions, and 227 were exotic to both regions. We observed significant differences in range sizes among the four plant groups and between the species introduced from EA to NA and the ones from NA to EA (Fig. 3, Table 1). Among the four groups of species, only the species native to EA but exotic to NA had greater ranges in EA; the species native to NA but exotic to EA, species native or exotic to both EA and NA all had larger ranges in NA (in all cases, P < 0.0001). However, even among the species introduced from EA to NA, the species with larger ranges (proportion > 0.5) on both continents also had larger ranges in NA while the species with < 0.5 had wider distributions in EA (paired t-tests, P < 0.005). This may indicate that at least some of the EA species with smaller distributions have not had enough time to expand in NA or they naturally lacked the invasiveness. Taking distributional ranges in both EA and NA together, species native to both EA and NA had greater ranges than species native only to EA or NA (Table 1; Fig. 3). In general, the native ranges set a clear control for the native–exotic comparisons.
4. DiscussionMost of the earlier studies showing the role of native species diversity in resisting biotic invasions were conducted at small spatial scales, and studies over large spatial scales are lacking. Indeed, factors that determine successful invasion by exotic plants at the regional scale are still poorly understood and the role of regional diversity in resisting biotic invasions has been difficult to prove. Although native richness is positively related to exotic richness on large spatial scales ("the rich get richer") (Rejmánek, 2003; Stohlgren et al., 2003), the observed negative richness–range size relationships reported here, if proven to be universal or nearly so, would also have strong implications for explaining present, and predicting future, species invasions; that is, even if exotic species can still invade species-rich regions, the chances for their range sizes (thus total population size or overall abundance) to be large are relatively small.
In this study, species (even the same species) usually have smaller distribution ranges in species-rich regions than in species-poor regions (average range size: EA < NA < EU) (Guo et al., 2006), probably indicting some level of biotic resistance. EA plants introduced to NA spread to occupy nearly the same proportion of the region as in their native areas, whereas exotics from NA have restricted distributions in EA. Species introduced to both regions from EU also spread more in NA than in EA, although species native to both regions have similar range sizes. This could be because EA harbors a more diverse flora than does NA, which may to some extent contribute to invasion resistance (Li and Wilcove, 2005). Europe (EU), which has the lowest plant diversity of the three temperate regions, showed the highest degree of invasion (DI; Table 1). Such evidence may serve as an indication of native plant diversity to biotic resistance at the regional scale.
EU has fewer ecological zones, each with relatively larger area, than does either EA or NA, which might at least in part be responsible for larger ranges for species in EU. Nonetheless, EA and NA contain similar diversity of ecological zones, yet the ranges of native species differ (Tables S2–S4). Indeed, our inter-continental comparison shows the product of total species richness and the average proportional area occupied per species varies little among the regions (EA, 5499; NA, 6854; EU, 6582), indicating nearly complete compensation between species richness and geographic range. Such results offer valuable insights that are difficult to achieve regarding the role of regional species diversity in resisting biotic invasions. In addition, NA and EU both have a much larger percentage of areas of temperate climate while EA has more subtropic climate (Tables S2–S4), which could contribute to smaller ranges of NA and EU species in EA but larger ranges of NA species in EU and EU species in NA.
The nearly universal negative richness–range size relationship may shed light on the causes for the role of regional diversity in resisting large-scale species invasions. Also, although the diversity–total population size relationships are generally positive (Storch et al., 2018), the average population sizes of component species may be smaller. The contrasting patterns in regional diversity and proportional range size across the three large regions may be caused either by interspecific competition or by different ecological heterogeneity within regions (Guo et al., 2006).
There is no question that multiple other potential factors, such as geographic barriers, human activity, and patterns of disturbance, among others, might have also contributed to the observed patterns. Unfortunately, we currently do not have adequate data to examine the role of all these factors. This is mainly because, unlike most previous studies that only examine invasibility or DI using exotic (vs. native) richness data, our study uses distribution/range size data. Without knowing the extent to which each exotic species is restricted to human-altered environments or has penetrated natural communities in each region, we cannot judge the roles of other possible factors such as land use and history. For example, most invasive species tolerate disturbance and are associated with human-altered environments in their native regions. Crops and land use in EA differ considerably from EU and NA, which have similar agricultural practice. Thus, disturbed environments in EA might not present suitable conditions for the spread of NA and EU plants, although differences in land use have not hampered the spread of EA plants in EU and NA. New data are needed to examine the possible effects of traits, invasion pathways, climate, land use, and other potential factors on the distribution of each exotic species, so that the role of overall native species diversity in invasion success can become clearer.
In short, regions with higher species diversity may support a large total population size when all coexisting species are combined but generally smaller population size for each component species than species-poorer regions. Therefore, even if certain exotic species may still be able to invade, regions with higher diversity could have higher resistance to species invasions by reducing both the possibility of invasions (invading species need to build their population size to certain level to get established and spread after invasions due to smaller populations sizes limited by available resources).
AcknowledgementsWe thank Barbara Conkling and four anonymous reviewers for constructive comments that greatly improved the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author Contributions
QG initiated the research, HQ provided plant data. HQ and QG analyzed the data, JZ generated the map, and all authors contributed to developing and writing of the manuscript. QG, HQ and JZ participated in revising the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.09.004.
Batt, R.D., Morley, J.W., Selden, R.L., et al., 2017. Gradual changes in range size accompany long-term trends in species richness. Ecol. Lett., 20: 1148-1157. DOI:10.1111/ele.12812 |
Beaury, E.M., Finn, J.T., Corbin, J.D., et al., 2019. Biotic resistance to invasion is ubiquitous across ecosystems of the United States. Ecol. Lett., 23: 476-482. |
Brown, J.H., 1984. On the relationship between abundance and distribution of species. Am. Nat., 124: 255-279. DOI:10.1086/284267 |
Brown, R.L., Peet, R.K., 2003. Diversity and invasibility of southern Appalachian plant communities. Ecology, 84: 32-39. DOI:10.1890/0012-9658(2003)084[0032:DAIOSA]2.0.CO;2 |
Burns, K., 2016. Native–exotic richness relationships: a biogeographic approach using turnover in island plant populations. Ecology, 97: 2932-2938. DOI:10.1002/ecy.1579 |
Byrne, K.M., Lauenroth, W.K., McManus, L., 2010. Impacts of nonnative plant species on production and diversity in the front range of Colorado. Western N. Am. Nat., 70: 288-295. DOI:10.3398/064.070.0302 |
Charkevicz, S., 1985-1996. Plantae Vasculares Orientis Extremi Sovietici, 1-8. Nauka, Leningrad, Russia.
|
Chen, H., Qian, H., Spyreas, G., et al., 2010. Native-exotic species richness relationships across spatial scales and biotic homogenization in wetland plant communities of Illinois, USA. Divers. Distrib., 16: 737-743. DOI:10.1111/j.1472-4642.2010.00679.x |
Chen, S., Chen, Z., Huang, W., et al., 2021. Explaining the geographic pattern of plant invasion in 67 nature reserves in China. Front. Ecol. Evol., 9: 655313. DOI:10.3389/fevo.2021.655313 |
Eeley, H.A., Foley, R.A., 1999. Species richness, species range size and ecological specialisation among African primates: geographical patterns and conservation implications. Biodivers. Conserv., 8: 1033-1056. DOI:10.1023/A:1008831320469 |
Grubov, V.I., 2001. Key to the Vascular Plants of Mongolia. Science Publishers, Enfield, New Hampshire.
|
Guo, Q., 2015. No consistent small-scale native-exotic relationships. Plant Ecol., 216: 1225-1230. DOI:10.1007/s11258-015-0503-7 |
Guo, Q., 2022. Scale dependency in native–exotic richness relationships revisited. Ecol. Evol., 12: e8549. |
Guo, Q., Fei, S., Potter, K.M., et al., 2019. Tree diversity regulates forest pest invasion. Proc. Natl. Acad. Sci. U.S.A., 116: 7382-7386. DOI:10.1073/pnas.1821039116 |
Guo, Q., Qian, H., Zhang, J., 2022. On the relationship between species diversity and range size. J. Biogeogr., 49: 1911-1919. DOI:10.1111/jbi.14477 |
Guo, Q., Qian, H., Ricklefs, R.E., et al., 2006. Distributions of exotic plants in eastern Asia and North America. Ecol. Lett., 9: 827-834. DOI:10.1111/j.1461-0248.2006.00938.x |
Heberling, M.J., Jo, I., Kozhevnikov, A., et al., 2017. Biotic interchange in the Anthropocene: strong asymmetry in East Asian and eastern North American plant invasions. Global Ecol. Biogeogr., 26: 447-458. DOI:10.1111/geb.12551 |
Kartesz, J.T., 1999. A synonymized checklist and atlas with biological attributes for the vascular flora of the United States, Canada, and Greenland. In: Kartesz, T.T., Meacham, C.A. (Eds.), Synthesis of the North American Flora, Version 1.0. North Carolina Botanical Garden, Chapel Hill, North Carolina.
|
Krasnoborov, I., Peschkova, G.A., Malyschev, L., et al., 1988-1997. Flora of Siberiae, 1-14. Nauka, Novosibirsk, Russia.
|
Lee, T. -B., 1980. Illustrated Flora of Korea. Hyangmunsa, Seoul, p. 990.
|
Lee, W. -T., 1996. Lineamenta FLorae Koreae. Academic Publishers, Seoul.
|
Legault, G., Bitters, M.E., Hastings, A., et al., 2020. Interspecific competition slows range expansion and shapes range boundaries. Proc. Natl. Acad. Sci. U.S.A., 117: 26854-26860. DOI:10.1073/pnas.2009701117 |
Li, Y., Wilcove, D.S., 2005. Threats to vertebrate species in China and the United States. Bioscience, 55: 147-153. DOI:10.1641/0006-3568(2005)055[0147:TTVSIC]2.0.CO;2 |
MacArthur, R.H., Wilson, E.O., 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, New Jersey.
|
Peng, S., Kinlock, N.L., Gurevitch, J., et al., 2019. Correlation of native and exotic species richness: a global meta-analysis finds no invasion paradox across scales. Ecology, 100: e02552. |
Qian, H., 2002. A comparison of the taxonomic richness of temperate plants in East Asia and North America. Am. J. Bot., 89: 1818-1825. DOI:10.3732/ajb.89.11.1818 |
Qian, H., Ricklefs, R.E., 2000. Large-scale processes and the Asian bias in species diversity of temperate plants. Nature, 407: 180-182. DOI:10.1038/35025052 |
Rejmánek, M., 1996. Species richness and resistance to invasions. In: Orians, G.H., Dirzo, R., Cushman, J.H. (Eds.), Biodiversity and Ecosystem Processes in Tropical Forests. Springer, Berlin, Germany, pp. 153-172.
|
Rejmánek, M., 2003. The rich get richer - responses. Front. Ecol. Environ., 1: 122-123. |
Ri, J. -D., Hoang, H. -D., 1984. Dictionary of Plant Names. Goahakbaekgoasadzon-Tschulpansa, Pyongyang, North Korea.
|
Ricklefs, R.E., Guo, Q.F., Qian, H., 2008. Growth form and distribution of introduced plants in their native and non-native ranges in Eastern Asia and North America. Divers. Distrib., 14: 381-386. |
SAS Institute, 2014. SAS 9.4 Output Delivery System: User's Guide. SAS institute.
|
Shea, K., Chesson, P., 2002. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol., 17: 170-176. DOI:10.1016/S0169-5347(02)02495-3 |
Stohlgren, T.J., Barnett, D.T., Kartesz, J.T., 2003. The rich get richer: patterns of plant invasions in the United States. Front. Ecol. Environ., 1: 11-14. DOI:10.1890/1540-9295(2003)001[0011:TRGRPO]2.0.CO;2 |
Storch, D., Bohdalková, E., Okie, J., 2018. The more – individuals hypothesis revisited: the role of community abundance in species richness regulation and the productivity – diversity relationship. Ecol. Lett., 21: 920-937. DOI:10.1111/ele.12941 |
Tomasetto, F., Duncan, R.P., Hulme, P.E., 2019. Resolving the invasion paradox: pervasive scale and study dependence in the native – alien species richness relationship. Ecol. Lett., 22: 1038-1046. DOI:10.1111/ele.13261 |
Tutin, T.G., Heywood, V.H., Burges, N.A., et al., 1964-1980. Flora Europaea, 1-5. Cambridge University Press, Cambridge.
|
Williamson, M., 1996. Biological Invasions. New York: Springer.
|
Williamson, M., Fitter, A., 1996. The varying success of invaders. Ecology, 77: 1661-1666. DOI:10.2307/2265769 |
Winter, M., Kühn, I., La Sorte, F.A., et al., 2010. The role of non-native plants and vertebrates in defining patterns of compositional dissimilarity within and across continents. Global Ecol. Biogeogr., 19: 332-342. DOI:10.1111/j.1466-8238.2010.00520.x |
Wu, Z., Raven, P.H., Hong, D., 1994-2003. Flora of China, 1-25. Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, Missouri.
|



