b. CAS Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China;
c. Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Wuhan 430074, China;
d. University of Chinese Academy of Sciences, Beijing 100049, China;
e. Global Change Research Group, Mediterranean Institute for Advanced Studies (IMEDEA; UIB-CSIC), Esporles, Balearic Islands, Spain;
f. The Rocky Mountain Biological Laboratory, Post Office Box 519, Crested Butte, CO 81224, USA;
g. Department of Biology, University of Maryland, College Park, MD 20742, USA
Intraspecific variation, widespread in both plants and animals, is thought to confer resilience to communities in unpredictable ecosystems (Hughes et al., 2008; Jump et al., 2009). Recent research has indicated that the effects of intraspecific variation may be twice as strong as previously thought and that it affects communities and ecosystems more when manipulated by primary producers than when manipulated by consumer species (Raffard et al., 2019). Intraspecific variation should be as important as, or more important than, interspecific diversity, but attracts comparatively less attention (Bangert et al., 2005; Hughes et al., 2008; Johnson et al., 2012). In particular, the effect of individual variation at the intraspecific level on the dynamics of populations, communities and ecosystems still remains a fundamental question in ecology (Sutherland et al., 2012).
Evaluating the effects of intraspecific phenotypic variation relies on identifying and measuring traits that are ecologically important. Floral traits are clear examples of important traits because flowers interact with different floral consumers (i.e., mutualists and antagonists) that influence plant reproduction. Studies of interspecific floral variation have greatly enhanced our understanding of angiosperm evolution (Grant, 1949; Whittall and Hodges, 2007). In addition, floral trait variation across populations has illustrated the evolutionary significance of floral shape in response to biotic and/or abiotic factors (Galen, 1999; Galen and Cuba, 2001). However, the effects of within-population floral trait variation on responses to unpredictable ecological factors need to be further investigated (Delesalle and Mazer, 1995; Mendez-Vigo et al., 2013; Jacquemyn and Brys, 2020). Within-population flower variation may allow mutualists and antagonists to distinguish and select different flowers and thereby reduce the risk that all plants in the population will be attacked by antagonists, and thus, enhance population-level fitness.
In many systems, pollinators (mutualists) and nectar robbers (antagonists) interact simultaneously with flowers in populations. Nectar robbers feed upon nectar not through the floral opening, but by biting or piercing holes in flowers, and thus typically do not transfer pollen (Inouye, 1980; Irwin et al., 2010). Flowering plants with long tubular flowers are most commonly subjected to nectar robbing (Irwin and Maloof, 2002); furthermore, animals that are capable of consuming nectar as legitimate flower visitors may also act as nectar robbers in some systems or contexts (Irwin et al., 2010). Bumble bees are important pollinators and/or nectar robbers for many flowering plants. Several studies have indicated that individual bumble bees display forage consistently either when pollinating or when robbing nectar from flowers (Bronstein et al., 2017; Lichtenberg et al., 2020a, 2020b). However, it still remains unclear why bumble bees exhibit such high tactic constancy. Since flower handling skills are thought to be one of the determinants of bumble bee foraging tactic constancy (Goulson, 1999; Ishii and Kadoya, 2016; Bronstein et al., 2017), floral trait variation may influence their foraging preferences, mediated by their respective handling skills.
In this study, we first tested whether floral trait variation across individuals can be distinguished by pollinators and nectar robbers in Caryopteris divaricata (Sieb. et Zucc.) Maxim. (Verbenaceae), a plant with tube-shaped flowers in which the length of the corolla varies substantially. Individual plants of this herb produce a large number of flowers, and different bumble bee species are both pollinators and nectar robbers in the studied population. We evaluated the influence of nectar robbing on the behavior of legitimate visitors and their effect on plant reproductive success. Our study sheds light on how floral trait variation among individual plants modulates the interactions among plants, pollinators, and nectar robbers.
2. Materials and methods 2.1. Study species and populationCaryopteris divaricata (Verbenaceae), also known as synonym of Tripora divaricata (Maxim.) P.D. Cantino (Lamiaceae) (Cantino et al., 1999), is a perennial, self-compatible herbaceous plant distributed in central and western China, Mongolia, the Korean Peninsula, and Japan (Pei and Chen, 1982). The plant grows 0.7–2.4 m tall and produces numerous stems (2–13) arising from a single root. Each stem supports a verticillaster consisting of a number of cymes (Pei and Chen, 1982). In our study population, individual plants can produce up to 1900 flowers (mean ± SE: 1331.89 ± 66.81, 744–1913, N = 35 individual plants), and flowering lasts for about seven weeks. In a given day, there are 35.13 ± 2.65 blooming flowers within an individual plant (1–145, N = 188 individual plants). Corollas are purple, slightly two-lipped, and have five lobes, among which the lower lobe is the largest and displays dark purple spots. It is an herkogamous flower, as it has four stamens (didynamous) that are exserted far out of the corolla by the filaments, while the style is slightly longer than the filaments, ending with a split stigma (Fig. 1). Each flower produces four ovules. The flowers always open early in the morning and wilt before the evening; thus, flowers are totally different on each observation day and have no chance of receiving visits by nocturnal pollinators. Nectar is hidden in the base of a slender corolla tube with a small diameter at the opening (range: 1.41–2.35 mm, mean ± SE: 1.82 ± 0.03, N = 51 flowers each from different plants). Hand pollinations of flowers from 30 individual plants revealed that outcrossing maximized reproductive output in terms of seed set (seed/ovule: 91.60 ± 1.93%, 119 flowers), whereas self-pollination and autonomous pollination (bagged flowers) yielded fewer seeds (53.06 ± 3.18%, 155 flowers; 18.56 ± 1.86%, 299 flowers, respectively). This suggests that the plant requires an external vector to enhance pollination and subsequent seed set.

|
| Fig. 1 The main flower visitors of Caryopteris divaricata in the studied population and their foraging behavior. (A) Bombus nobilis, primarily robbing nectar from a flower; (B) B. picipes legitimately visiting a flower; (C) the small-sized B. picipes legitimately visiting a flower; and (D) the small-sized B. picipes secondarily robbing nectar from a flower. Scale bar = 10 mm. |
The study was conducted over two summers (2015, 2016) during the blooming period of Caryopteris divaricata in Shaanxi Province, China. After field investigations on population size and the occurrence of nectar robbing, we selected a population near Huangbaiyuan, in Taibai County (33°49′30.1″ N, 107°30′18.9″ E; 1387 m a.s.l.). The population was located in an area of 300–400 m2 along a forest edge and consisted of about 200 individual plants.
2.2. Methods 2.2.1. Field surveys: floral visitors and nectar robbing rateIn 2015, the first robbed flower was found on July 22, about at the peak blooming time of the population (more than 70% of individuals were flowering) that year. We investigated the differences in composition of flower visitors before and after the occurrence of nectar robbing. Observations of flower visitors were made for 15 days (at least 2 h each day), with seven days before and eight days after July 22. We recorded floral visitors during a 15-min observation period of randomly selected individual plants. A total of 215 observation periods were conducted from 7:00 to 16:45 on days with fine weather conditions. For each floral visitor type (i.e., each potential species of legitimate visitor or nectar robber) we recorded visitation frequency for each type of floral visitor, which referred to the number of visits to flowers recorded for the flower visitor on an individual plant within 1 h divided by the total flowers. We also calculated an overall visitation frequency for all visits and legitimate visits, respectively.
After July 22, the nectar robbing rate (i.e., the percentage of flowers with a hole in the corolla tube on an individual plant) ranged from 65% to 100% on any given observation day with more than 60% of the plants in the population having a 100% nectar robing rate. To explore whether differences in nectar robbing among individual plants were maintained across the years, we marked ten individuals with relatively low nectar robbing intensity (hereafter, low robbing intensity plants) and 15 individuals with 100% nectar robbing intensity (hereafter, high robbing intensity plants) for study the following year. We aimed to mark plants with similar height and inflorescence number.
To assess whether, in the absence of nectar robbing, there were differences in legitimate flower visitation rates between plants from these two groups, we compared the total legitimate visitation rate of these groups (5 individuals per group) on two observation days (July 16 and 21) before nectar robbers arrived (July 22 of 2015). Furthermore, flowers that opened during these two days were marked with color string for seed harvesting (more than nine flowers each day for each of the plants).
In 2016, unlike the previous year, flowers were robbed from the beginning of anthesis. We conducted observations on flower visitors following the same protocol as in 2015, but on the 25 plants from the two groups with different nectar robbing rates. A total of 252 observation periods took place on nine sunny days (at least 2 h each day). Additionally, during 12 sunny days, we noted the nectar robbing rate of each day for each plant from the two groups. Bumble bees were the main floral visitors both years. During the field survey, the bumble bees visiting flowers were separately recorded according to differences in body size and/or stripe patterns; we also collected more than 15 individuals of each type for later identification and measurement of body size and proboscis length. Some honeybee individuals (Apis cerana) were recorded as legitimate visitors in both study years.
2.2.2. Floral trait variationCorolla tube length is regarded as an important trait modulating nectar robbing (Lara and Ornelas, 2001; Irwin et al., 2010; Lázaro et al., 2015; Richman et al., 2017; Rojas-Nossa et al., 2021). Nectar traits are also thought to be linked with nectar robbing (Lázaro et al., 2015; Ye et al., 2017). In 2016, we investigated corolla tube length, nectar volume and sugar concentration in the 25 marked individual plants from two groups. On four sunny days, we measured corolla tube length with a caliper to the nearest 0.01 mm, from the floral receptacle to the point of corolla opening. Each observation day, we sampled at least 10 flowers per individual and recorded the nectar robbing rate of the individual. We measured the nectar volume early in the morning, using 2- and 5-μL capillary micropipettes on five randomly selected open flowers from each of the 25 individual plants. Those flowers were enclosed with fine-meshed bags to exclude bee visitation before opening. Additionally, we calculated nectar sugar concentration using a portable hand refractometer (Scale Brix 0–100%) as soon as possible after mingling the samples in an individual plant to get a large enough sample volume. The measurements of nectar volume and sugar concentration were conducted on two sunny days.
2.2.3. Nectar removal by legitimate visitors and nectar robbersTo evaluate the nectar removal efficiency of nectar robbers and legitimate visitors, we compared the nectar standing crop between robbed and legitimately visited flowers. For this, in 2015, we selected two flowers from each individual (N = 30 individuals). One flower was unmanipulated, which allowed it to be naturally robbed (robbed flower), while the other was protected against nectar robbing by the placement of transparent tape around the corolla, which excluded visits by nectar robbers but allowed legitimate visits. We extracted nectar from those flowers within a single day from 7:00 am to 16:00 pm, in intervals of 1 h (a total of 10 times), using 2 μL capillary micropipettes.
2.2.4. Handling time of floral visitorsBumble bees may respond to floral trait variation to optimize foraging behavior for energy return (Goulson, 1999). To detect any difference in handling skills among floral visitors, we investigated flower handling time (seconds from landing to leaving a flower) for the main floral visitors when they were legitimately visiting and/or robbing a flower during four observation days (N > 200 visits of legitimate visitors and robbers) in 2015. Moreover, we recorded the handling time of nectar robbers on eight observation days in 2016 while they visited flowers from groups of high robbing intensity plants (N = 1342) and low robbing intensity plants (N = 169).
2.2.5. Pollen removal and deposition by floral visitorsTo evaluate the pollination efficiency of the main legitimate visitors, we determined pollen deposition and removal per visit. For each type of visitor (different species or individuals of a species with different size), we assessed more than 30 flowers each from different randomly selected plants. To estimate pollen removal and deposition, we enclosed the flowers before opening by means of fine-meshed bags, and when opened, we removed the bags and exposed the flowers to bumble bees on days of fine weather in 2015. After a single visit, the flowers were bagged again, harvested at least 1 h later, and stored in formalin-acetic acid-alcohol (FAA) solution constituted of formalin (37–40%), acetic acid and alcohol (50%) at a ratio of 5:6:89 by volume. In the laboratory, we dissected the stigmas from flowers to count the number of pollen grains on the stigma. To estimate pollen removal, all four anthers of the flowers were also carefully dissected to count the remaining pollen. To count pollen grains, anthers from a flower were pounded and shaken completely to suspend all the pollen grains in 5 mL of water. Pollen production per flower (or remaining in a flower) was then determined by counting pollen grains in ten drops (0.5 mL) of the pollen solution by using a microscope (Nikon E−600); these counts were extrapolated to ascertain the total number of grains in 5 mL. To estimate pollen removal per visit, we first measured the pollen production per flower. Flowers with undehisced anthers from randomly selected individuals were harvested to count the total pollen production per flower (N = 120 flowers each from different individual). Pollen removal per visit was then estimated by subtracting the number of pollen grains remaining after a single legitimate visit to the total pollen production per flower.
2.2.6. Seed productionTo detect the influence of nectar robbing on female reproductive success, we investigated seed production in both study years. In 2015, we compared the number of seeds per fruit for flowers from 13 to 23 individual plants that opened before and after July 22, respectively, when nectar robbing was first recorded. After the occurrence of nectar robbing, we used transparent tape to protect 100 randomly selected flowers (each from a different individual) from robbing, while the robbed flowers in the same individual plants were considered as controls. In 2016, we counted the number of seeds per fruit in each of the 25 individual plants from the two groups; the number of harvested fruits per individual ranged from 120 to 304.
2.3. Statistical analysis 2.3.1. Floral trait variation and nectar robbingWe examined differences in floral traits between plants from the group with high robbing intensity and with low robbing intensity. In addition, we estimated the contribution of robbing intensity, individual differences, and observation days to variance in corolla length, nectar volume, and sugar concentration. For these purposes, we fitted three linear mixed models (LMMs) and then used the custom function 'var_decomp' adapted based on R package partR2 and MuMIn (Bartoń, 2019; Huang et al., 2020; Stoffel et al., 2020). Group and observation day were treated as fixed effects and plant identity as a random factor in these models. Corolla tube length and nectar volume were loge-transformed, whereas we applied a logit transformation to nectar sugar concentration to improve the normality of residuals. To test for the influence of corolla tube length and nectar traits on nectar robbing rate in the plants from the low robbing intensity group (plants from the high robbing intensity group were not analyzed because 100% of these flowers were robbed), we used generalized linear mixed models (GLMMs) with binomial errors and logit link functions. Corolla tube length, nectar volume and sugar concentration were included in the model as fixed effects and plant identity as a random factor. We also assessed the relationship between corolla tube length and nectar traits for all the 25 individuals using two separate LMMs, in which plant identity was a random effect.
2.3.2. Behavior, pollen transfer efficiency and nectar removal for the main floral visitorsWe used separate GLMMs with Gamma errors to detect differences in flower handling time for the main legitimate visitors when visiting robbed and un-robbed flowers. Flower status (robbed vs. un-robbed) was a fixed factor and observation day was a random factor. In addition, to detect differences in flower handling time of nectar robbers when visiting flowers of plants from high robbing intensity and low robbing intensity groups (in case floral traits varied between individuals from these two groups), we used a GLMM assuming Gamma error structure with robbing intensity type as a fixed factor and observation day as a random factor.
To evaluate different dynamics of nectar removal for robbed and legitimately visited flowers, we compared the nectar standing crop during the day by using an LMM with flower status (robbed vs. un-robbed) and extraction hour as fixed factors and plant identity as a random factor. We then compared the nectar standing crop between robbed and legitimately visited flowers for each hour separately. The nectar standing crop was transformed by logarithmic transformations (log(x+1)).
We used a generalized linear regression model (GLM) with Poisson error structure and log link function to evaluate the differences in pollen removal between the main legitimate visitors. Overdispersion was tested using the function 'testDispersion' in R package DHARMa (Hartig, 2021); as the model was overdispersed, a quasi-Poisson distribution was used to improve model fit. For analysis of differences in pollen deposition between the main legitimate visitors, in addition to overdispersion, zero inflation was determined by the function 'testZeroInflation'. Therefore, we used a zero-altered two-part hurdle GLM (Zuur and Ieno, 2016) in R package pscl (Zeileis et al., 2008). The use of the hurdle model was divided into two parts (zero hurdle model and count model). In the first part of our hurdle model, the zero hurdle model was used to test the probability of stigma with pollen deposition by bumble bees. The hurdle was crossed only if the realization was positive, and then the non-zero count data were analyzed by the count model, in which a zero-truncated negative binomial error was applied to deal with overdispersion.
2.3.3. The influence of nectar robbing on the frequency of legitimate visitors and seed productionBecause nectar robbing occurred at different stages of the flowering period in the two study years, we analyzed the data for the two years differently. In 2015, the influence of nectar robbing on legitimate visitation and seed production was investigated on plants flowering before and after July 22, the day that the first flower was recorded to be robbed. In 2016, the influence of nectar robbing on legitimate visitation and seed production was examined in 25 plants from the low robbing intensity and high robbing intensity plants. With these analyses, we aimed to detect whether the visitation frequency of legitimate visitors changed when nectar robbing started (2015 data), and whether it differed between plants subjected to different rates of nectar robbing (2016 data). Further, we evaluated the changes in visitation frequency of each type of legitimate visitor (different species or individuals of a species with different sizes). GLMMs with negative binomial error distribution were used for all analyses on visitation frequencies because overdispersion was detected; as a response variable, we used the number of flower visits observed in a 15-min period, with log (number of observed flowers) as an offset (Benadi and Pauw, 2018); observation day was included as a random factor in 2015, whereas in 2016, observation day and plant identity were both included as random factors.
To assess the effect of nectar robbing on seed production, we used three zero-altered two-part hurdle GLMMs by the function 'glmmTMB' in R package glmmTMB (Brooks et al., 2017) because zero inflation had been verified. In the models, group (before and after occurrence of nectar robbing for 2015 data, low and high robbing intensity groups for 2016 data, and robbed and protected flowers for the treatment experiment, respectively) was treated as a fixed effect and plant identity as a random factor. The hurdle GLMM included a zero-inflation model and a conditional model; the zero-inflation model was used to test the probability of producing fruits without seeds (zero values), whereas the conditional model was for non-zero count data. We fitted all of these hurdle GLMMs by adding zero-inflation and using zero-truncated generalized Poisson distribution for a conditional model, which could handle both over dispersed and under-dispersed data (Consul, 1989; Consul and Famoye, 1992). For seed production between plants from the two groups when there was no influence of nectar robbing (before July 22 in 2015) but later varied in nectar robbing intensity, we tested the difference by using GLMM with Poisson error distribution; group was included as a fixed effect and plant identity as a random factor.
All analyses were conducted in R 3.6.2 (R Core Team, 2019). LMMs (using the function 'lmer'), GLMs ('glm') and GLMMs ('glmer') were performed in R package lme4 (Bates et al., 2015) unless otherwise specified. We used Wald Chi-Square-tests (type Ⅱ) by the function 'Anova' in R package car (Fox and Weisberg, 2019) to determine if overall effects of predictors were significant for GLMs and GLMMs; for LMMs, we used likelihood ratio tests with the function 'lrtest' from the package lmtest (Zeileis and Hothorn, 2002) to compare the full model to a null model that was identical except that it lacked the term for predictors. All averages were reported as means ± standard error.
3. ResultsIn each of the two study years, we recorded four distinct types of bumble bees according to body size and stripes. Two of them had similar stripes but could be clearly distinguished in the field according to a difference in body size (see below). In the laboratory, we identified both as the same species: Bombus picipes. We therefore use the term 'small-sized B. picipes' in this study to refer to the recorded individuals of B. picipes with small body sizes. Among the four types of bumble bees, B. nobilis was the primary nectar robber (Fig. 1A) and never legitimately visited flowers. Individuals of B. picipes (Fig. 1B) with large body sizes were the main legitimate visitors of the study species in both years, while B. trifasciatus was an occasional visitor whose frequency varied across years; these two types of bumble bees neither primarily nor secondarily robbed nectar from the flowers. In addition, small-sized B. picipes were very frequent visitors in both years. They foraged on flowers both as pollinators and robbers, but, when robbing, only played a role as secondary nectar robbers (Fig. 1C and D). Small-sized B. picipes obtained nectar from the flowers via holes previously made by B. nobilis.
Among the main visitors, the robber (Bombus nobilis) had the longest body and proboscis (16.28 ± 0.36 mm, 10.44 ± 0.34 mm, respectively, N = 15). The length of body and proboscis for the main legitimate visitor (B. picipes) was 14.43 ± 0.18 mm, and 10.85 ± 0.45 mm, respectively (N = 13), while small-sized B. picipes was 12.49 ± 0.07 mm for body length and 8.69 ± 0.10 mm for proboscis length (N = 20).
3.1. Flower trait variation and nectar robbingDifferences in intensity of nectar robbing rate (two groups: individuals with high and low robbing rate) accounted for 15.9% variance in corolla tube length, while 0.3% and 5.9% of the variance was explained by the observation day and plant identity, respectively. Most of the variance (77.9%) was not explained by variables involved in this study.
Corolla tubes were significantly longer in individuals from the high robbing intensity group (11.08 ± 0.037 mm) than in those from the low robbing intensity group (10.29 ± 0.044 mm; χ2 = 20.55, df = 1, P < 0.001). In 2016, the nectar robbing rate for all individuals from the high robbing intensity group was 100% on each of the 12 observation days. For any individual from the low robbing intensity group, the robbing rate did not reach 100% on most observation days (45%–100%). For individuals from the low robbing intensity group, the nectar robbing rate for a given observation day was positively related to corolla tube length of the flowers that opened that day (χ2 = 10.87, df = 1, P < 0.001, Fig. 2A).
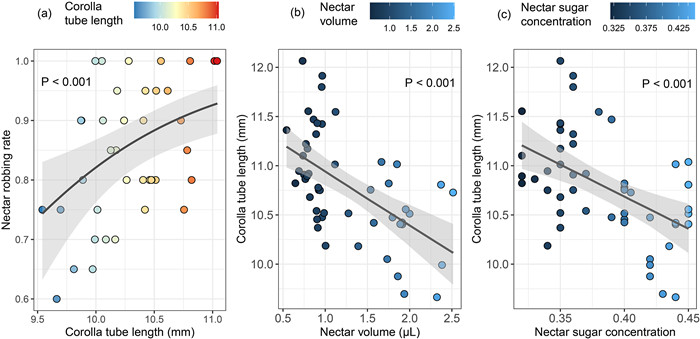
|
| Fig. 2 (A) The relationship between corolla tube length and nectar robbing rate across individuals and observation days in the Caryopteris divaricata population. Each dot represents the average value of corolla tube length of an individual plant on an observation day (x axis) and the nectar robbing rate of the individual plant (the number of flowers with a hole in the corolla tube out of total flowers) on the observation day (y axis), respectively. The relationship between (B) corolla tube length and nectar volume, (C) corolla tube length and sugar concentration across individuals and observation days in the Caryopteris divaricata population. Each dot represents the average value of nectar volume and sugar concentration for an individual plant on an observation day (x axis) and the average value of corolla tube length for the individual plant on the observation day (y axis), respectively. |
Differences in intensity of nectar robbing rate explained 48.2% of the variance in nectar volume, while 0.01% and 1.5% of the variance was explained by the observation day and plant identity, respectively; 50.3% of the variance was not explained by any of the study variables. Differences in intensity of nectar robbing contributed most to the variance (74.0%) in sugar concentration, while 2.5% and 8.2% of the variance was explained by the observation day and plant identity, respectively; about 15.4% of the variance was not explained by any of the study variables. Accordingly, both the volume and sugar concentration of flower nectar was higher in plants with low robbing intensity (volume: 1.88 μL ± 0.074; sugar concentration: 43% ± 0.4%) than in those with high robbing intensity (volume: 0.86 μL ± 0.021; sugar concentration: 35% ± 0.4%; volume, χ2 = 48.65, df = 1, P < 0.001; sugar concentration, χ2 = 37.64, df = 1, P < 0.001).
Corolla tube length was negatively related to both nectar volume (χ2 = 13.53, df = 1, P < 0.001) (Fig. 2B) and sugar concentration (χ2 = 15.15, df = 1, P < 0.001) (Fig. 2C). For individuals in the low robbing intensity group, the nectar robbing rate for a given observation day was not significantly related to either nectar volume (χ2 = 2.44, df = 1, P = 0.119) or sugar concentration (χ2 = 2.44, df = 1, P = 0.118) on that day.
3.2. Behavior, pollen transfer efficiency, and nectar removal for the main floral visitorsThe handling time for the main legitimate visitor, B. picipes, was significantly longer when visiting a robbed flower (1.91 s ± 0.058) than when visiting an un-robbed flower (1.40 s ± 0.030; χ2 = 6.47, df = 1, P = 0.011). For small-sized B. picipes, the handling time when robbing a flower (1.96 s ± 0.035) was significantly shorter than when legitimately visiting a flower (2.43 s ± 0.055; χ2 = 6.37, df = 1, P = 0.012). In addition, the handling time for primary nectar robbers (B. nobilis) was significantly longer in flowers from the low robbing intensity group (2.97 s ± 0.249) than in those from the high robbing intensity group (2.24 s ± 0.027; χ2 = 65.29, df = 1, P < 0.001).
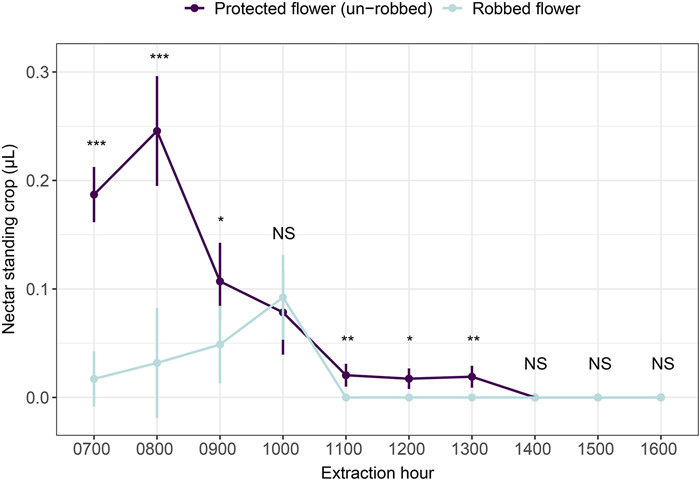
The nectar standing crop was significantly lower in robbed flowers than in those that only received legitimate visits (χ2 = 50.67, df = 1, P < 0.001, Fig. 5). The two main visitors (B. picipes and small-sized B. picipes) did not differ in the extent of pollen removal per visit when legitimately visiting flowers (χ2 = 0.56, df = 1, P = 0.456). The results of the zero-hurdle model indicated that larger B. picipes had a higher probability of depositing pollen grains to a stigma than did small-sized B. picipes (z = -2.44, P = 0.015; Table S1); in contrast, the count model indicated that except for the values of zero, there was no significant difference between these two types of bumble bees (z = -0.89, P = 0.372; Table S1). This indicates that small-sized B. picipes were less likely to touch the stigmas than larger bees but that they deposited similar numbers of pollen grains upon successful contact.

|
| Fig. 3 The overall visitation frequency by flower visitors including and excluding nectar robbers in the two study years in the Caryopteris divaricata population. NS indicates no significant difference; *, P < 0.05; **, P < 0.01; ***, P < 0.001. |
|
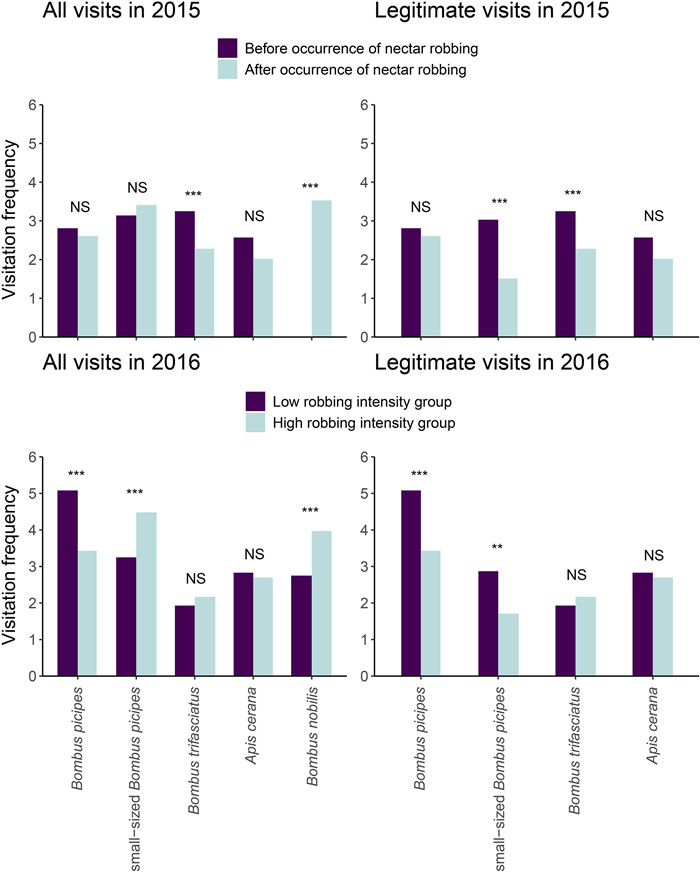
| Fig. 4 Changes in visitation frequency for each flower visitor before and after the occurrence of nectar robbing in 2015, and between the low and high robbing intensity groups in 2016 in the Caryopteris divaricata population. NS indicates no significant difference; *. P < 0.05; **, P < 0.01; and ***, P < 0.001. |
|
| Fig. 5 Comparison of nectar standing crop in robbed Caryopteris divaricata flowers and experimentally protected flowers. *, P < 0.05; **, P < 0.01; ***, P < 0.001; and NS, no significant differences between treatments. |
In 2015, frequency of legitimate visitation was lower in flowers that opened after the occurrence of nectar robbing than in those that opened before (χ2 = 29.52, df = 1, P < 0.001; Fig. 3), although the overall visitation frequency (including nectar robbing) was higher in flowers that opened after the occurrence of nectar robbing than in those that opened before (χ2 = 4.94, df = 1, P = 0.026). As expected, the visitation frequency of most of the visitors changed after the arrival of nectar robbers (Fig. 4). Before the occurrence of nectar robbing, the main floral visitors were small-sized B. picipes, followed by B. trifasciatus and B. picipes (Fig. 4). The main visitors changed to B. nobilis (robber), small-sized B. picipes, and B. picipes when nectar robbing occurred; the dominant legitimate visitors were individuals of B. picipes. Moreover, most of the small-sized B. picipes shifted to secondary nectar robbers when B. nobilis appeared (Fig. 4). Zero-altered two-part hurdle GLMM indicated that the probability of fruits without seeds (zero values) was statistically higher in plants after than before the occurrence of nectar robbers (z = 14.79, P < 0.001; Table S2); and even for those with seeds (non-zero values), seed production per fruit was significantly higher in plants that opened before the occurrence of nectar robbers than in those opened after (z = -6.98, P < 0.001; Table S2; Fig. S1A).
In 2016, the legitimate visitation frequency was significantly higher in individuals from the low robbing intensity group than in those from the high robbing intensity group (χ2 = 150.20, df = 1, P < 0.001), while the overall visitation frequency (including nectar robbing) did not differ significantly between groups (χ2 = 1.35, df = 1, P = 0.245; Fig. 3). For the low robbing intensity group, the dominant visitor was B. picipes, which legitimately visited the flowers, whereas for individuals from the high robbing intensity group, the small-sized B. picipes was the dominant visitor and mainly acted as nectar robber; B. picipes remained as the dominant legitimate visitor (Fig. 4). Plants from the high robbing intensity group had a higher probability of producing fruits without seeds (zero values) than those from the low robbing intensity group (z = 10.48, P < 0.001; Table S2); in contrast, plants from the low robbing intensity group produced more fruits with seeds (non-zero values) than did those with high robbing intensity (z = -6.96, P < 0.001; Table S2; Fig. S1B). When nectar robbing was prevented, the probability of yielding a fruit with seeds (z = 3.15, P = 0.010; Table S2) and the number of seeds per fruit decreased (z = -2.58, P = 0.002; Table S2; Fig. S1C).
In 2015, before nectar robbing began, neither the frequency of legitimate visitors (χ2 = 0.02, df = 1, P = 0.884) nor seed production per fruit (χ2 = 0.006, df = 1, P = 0.940) differed between plants from the two groups.
4. DiscussionOur study indicated that the intensity of nectar robbing varied across individuals within the study population and floral traits such as corolla tube length, nectar volume, and sugar content displayed substantial variation among individuals. Moreover, we found a positive relationship between intensity of nectar robbing and corolla tube length. Nectar robbing significantly influenced legitimate visitation of the plant and decreased plant female reproductive success. However, this negative effect was mitigated in plants with short corolla tubes, because the nectar robbers preferred to forage on long-tubed flowers. Floral trait variation did not influence legitimate visitation frequency or seed production when nectar robbing was absent, suggesting that this variation does not alter plant reproductive capability but does benefit a population by helping plants in it to deal with unpredictable conditions, such as nectar robbing.
Although nectar robbing is ubiquitous, the proportion of robbed flowers can range from 0% to 100% per plant at any given census when measuring population- or community-wide levels of robbing (Irwin and Maloof, 2002). Predicting the occurrence and intensity of nectar robbing remains a challenge (Irwin and Maloof, 2002; Irwin et al., 2010; Rojas-Nossa et al., 2016); it may depend on both changes in composition of the visitor community, and variation in the availability of alternative floral resources (Irwin et al., 2010; Rojas-Nossa et al., 2016; Ye et al., 2018; Kohl and Steffan-Dewenter, 2022). Therefore, it is difficult to study the influence of flower trait variation on interactions among plants, pollinators, and nectar robbers across spatiotemporal scales. However, if the intensity of nectar robbing varies among individuals of a population at a given time, within-population flower trait variation may provide a novel system to study the foraging tactics and floral preferences for both nectar robbers and legitimate visitors.
We found that nectar robbers preferred to forage on flowers with comparatively long corolla tubes. Numerous studies have shown that nectar robbing is frequently found in flowers with long corolla tubes (Lara and Ornelas, 2001; Irwin et al., 2010; Maruyama et al., 2015; Richman et al., 2017; Ye et al., 2017; Rojas-Nossa et al., 2021). Although the link between nectar robbing and long corolla tubes is not well studied, for Bombus nobilis, the primary nectar robber of Caryopteris divaricata, it may be more difficult to handle flowers with short corolla tubes than with long tubes. This was evidenced by the handling time, as the robbers spent more time when foraging on flowers with short corolla tubes. A short corolla tube may result in space limitation for nectar robbers when handling the flower from outside the flower due to its large body size, but may favor the legitimate visitor for its relatively small body size. In this population, the difference in foraging preferences of legitimate visitors and nectar robbers that resulted from across individual variation in corolla tube length, seems to segregate visitors' niches, thus helping the population to maintain reproduction in the presence of nectar robbers.
Lázaro et al. (2015) showed that in Lonicera implexa Aiton (Caprifoliaceae), nectar robbing was related to corolla length, lip size, and nectar volume; in addition, bumble bees may shift to robbing in response to changes of these traits. They found two floral trait optima for plant reproduction: flowers with long corollas and little nectar, and others with short corollas and abundant nectar. Such a floral resource allocation pattern was confirmed in our study. The long-tubed flowers of C. divaricata were preferentially visited by nectar robbers and produced little nectar with low sugar content, while short-tubed flowers invested more resources into nectar and suffered from comparatively less nectar robbing. Nectar volume and sugar concentration are thought to be under complex selection pressure by pollinators and antagonists such as nectar robbers (Chalcoff et al., 2006). Moreover, the foraging preference of bumble bees is influenced not only by energy intake rate but also by the rate of imbibition (Harder, 1986; Nardone et al., 2013) and even by offloading rate (Pattrick et al., 2020), which is highly dependent on nectar sugar concentration. Nardone et al. (2013) reported that, for Bombus impatiens (Cresson), the viscosity significantly affects imbibition rate when nectar sugar concentration is above the threshold of 27%. For flowers with deep corollas, nectar with high viscosity may negatively influence ingestion rate of long-tongued bumble bees (Harder, 1986). In C. divaricata, the combination of long-tubed flowers with diluted nectar and short-tubed flowers with viscous nectar may also help to segregate niches among bumble bee species with different foraging preferences.
In the studied population of Caryopteris divaricata, the visiting frequency by legitimate visitors was much lower in individuals with intense nectar robbing than in those with relatively low intensity of nectar robbing. The flowers of plants with high nectar robbing had lower nectar standing crop, likely because the speed of nectar removal is more rapid for robbers than for legitimate visitors. Moreover, nectar robbing highly changed the composition of legitimate visitors of the plant. Almost all the individuals of the dominant legitimate visitor, small-sized B. picipes, shifted to secondary nectar robbing after the primary robbers worked in the population, even when visiting flowers from the low robbing intensity group. Bumble bees are thought to be easily induced to secondary nectar robbing and maintain tactic constancy as robbers even though the switching costs are low (Barker et al., 2018; Lichtenberg et al., 2020a). Large B. picipes continued to forage as legitimate visitors independent of the intensity of nectar robbing. This finding suggests that switching between foraging strategies might depend on body size for bumble bees (Ye et al., 2017). Moreover, in C. divaricata, large-sized individuals of B. picipes spent less time than small-sized ones when legitimately visiting flowers. The handling time decreased significantly for the small-sized B. picipes when switching to nectar robbing. The switching behavior may be sensitive to the nutritional level of the colony (Lichtenberg et al., 2020a), or result from size-matching to flowers, which could influence the handling skill of the visitor (Ishii and Kadoya, 2016; Bronstein et al., 2017).
For the robbed flowers (always with long corolla tubes), the switching of small-sized B. picipes to nectar robbing may aggravate the negative effect on reproduction by removing nectar without transferring pollen (see also Richman et al., 2017). In contrast, flowers with short corolla tubes may benefit when the small-sized B. picipes switches from legitimate visits to nectar robbing. Although the small-sized B. picipes legitimate visitors remove pollen at a high rate, they are inefficient at depositing pollen, therefore, wasting pollen. This is likely because small-sized B. picipes visitors mismatched with Caryopteris divaricata flower size. In this population of C. divaricata, flower trait variation modulates the complicated impact of nectar robbing on pollination. Although short-tubed flowers benefited as the non-preferable target of nectar robbers when the antagonists are unavoidable, the long-tubed flowers may have potential advantages, especially in the presence of long-tongued pollinators (Lázaro et al., 2015). The long corolla tube may function in filtering pollinators and is adapted to long-tongued pollinators (Armbruster, 2017). Moreover, long corolla tubes (also with a long pistil) may help to enhance male fitness through pollen competition because the long pistil may encourage male competition at the stage of pollen tube growth (Yang and Wang, 2015), promoting outcrossing. In years with low nectar robbing intensity, plants with long-tubed flowers might thus compensate by having higher realized fitness than those with short-tubed flowers. However, further investigation is needed to confirm this contention. Among individuals in the population, the fact that plants with long-tubed flowers produce little nectar whereas those with short-tubed flowers have abundant nectar may infer a trade-off between flower size and nectar production. In fact, because floral trait variation did not change legitimate visitation and seed production when nectar robbers were absent, floral trait variation might not be driven by pollinators.
5. ConclusionOur case study revealed that Caryopteris divaricata is an ideal system to explore how floral trait variation mediates nectar robbing and pollination. Thanks to the variation in floral traits and the associated segregation of floral visitors, many plant individuals can escape robbing and maintain their seed production (and therefore maintain the population) in adverse and unpredictable conditions, such as nectar robbing. Although floral trait variation in this species did not change plant reproductive success in the absence of antagonists, it allows flower visitors to segregate niches, thus helping the population to maintain seed production in the presence of nectar robbers. The variation in floral traits may not only enhance the capability of the plant population to deal with unpredictable nectar robbing, but also helps to maintain the diversity of floral visitors in the community. However, because we could not include all flowers from an individual plant, we were unable to determine the overall fitness of C. divaricata individuals that exhibited floral trait variation. Including more plant species in any given community is necessary to broaden our knowledge of the interplay between flower trait variation, pollinators and nectar robbers.
AcknowledgementsWe thank Ming-Fang Du for assistance in the field, Jia-Xing Huang for identifying the bumble bees, and Michele Dudash for improvement on an earlier manuscript. The research was supported by the National Natural Science Foundation of China (No. 31970253 and 32270243) and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB31010000). AL was also supported by a Ramón y Cajal contract (RYC-2015-19034) from the Spanish Ministry of Science, Innovation and Universities, the Spanish State Research Agency, European Social Funds (ESF invests in your future) and the University of the Balearic Islands, and by the project PRPPID2020-117863RB-I00, financed by the Spanish Ministry of Science and Innovation and the Spanish Research Agency (MCIN/AEI/10.13039/501100011033).
Author contributions
YHG and CFY conceived the project. ST collected data. ST, YDH and CFY designed data analyses. YDH and CFY wrote the first draft and all authors provided input and approved the final manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.11.002.
Armbruster, W.S., 2017. The specialization continuum in pollination systems: diversity of concepts and implications for ecology, evolution and conservation. Funct. Ecol., 31: 88-100. DOI:10.1111/1365-2435.12783 |
Bangert, R.K., Turek, R.J., Martinsen, G.D., et al., 2005. Benefits of conservation of plant genetic diversity to arthropod diversity. Conserv. Biol., 19: 379-390. DOI:10.1111/j.1523-1739.2005.00450.x |
Barker, J.L., Dornhaus, A., Bronstein, J.L., Muth, F., 2018. Learning about larceny: experience can bias bumble bees to rob nectar. Behav. Ecol. Sociobiol., 72: 1-11. DOI:10.1007/s00265-017-2413-2 |
Ramón, K., 2019. MuMIn: Multi-Model Inference. R package version 1.43.15. https://CRAN.R-project.org/package=MuMIn
|
Bates, D., Maechler, M., Bolker, B., et al., 2015. Fitting linear mixed-effects models using lme4. J. Stat. Software, 67: 1-48. DOI:10.1097/COC.0000000000000259 |
Benadi, G., Pauw, A., 2018. Frequency dependence of pollinator visitation rates suggests that pollination niches can allow plant species coexistence. J. Ecol., 106: 1892-1901. DOI:10.1111/1365-2745.13025 |
Bronstein, J.L., Barker, J.L., Lichtenberg, E.M., et al., 2017. The behavioral ecology of nectar robbing: why be tactic constant?. Curr. Opin. Insect. Sci., 21: 14-18. DOI:10.1016/j.cois.2017.05.013 |
Brooks, M.E., Kristensen, K., Van, B.K.J., et al., 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal, 9: 378-400. DOI:10.32614/rj-2017-066 |
Cantino, P.D., Wagstaff, S.J., Olmstead, R.G., 1999. Caryopteris (Lamiaceae) and the conflict between phylogenetic and pragmatic considerations in botanical nomenclature. Syst. Bot., 23: 369-386. |
Chalcoff, V.R., Aizen, M.A., Galetto, L., 2006. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot., 97: 413-421. DOI:10.1093/aob/mcj043 |
Consul, P.C., 1989. Generalized Poisson Distributions: Properties and Applications. Marcel Dekker, Inc, New York, NY.
|
Consul, P.C., Famoye, F., 1992. Generalized Poisson regression model. Commun. Stat. Theor. Methods, 21: 89-109. DOI:10.1080/03610929208830766 |
Delesalle, V.A., Mazer, S.J., 1995. The structure of phenotypic variation in gender and floral traits within and among populations of Spergularia marina (Caryophyllaceae). Am. J. Bot., 82: 798-810. DOI:10.1002/j.1537-2197.1995.tb15692.x |
Fox, J., Weisberg, S., 2019. An {R} Companion to Applied Regression, third ed. Sage, Thousand Oaks CA. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
|
Galen, C., Cuba, J., 2001. Down the tube: pollinators, predators, and the evolution of flower shape in the alpine skypilot, Polemonium viscosum. Evolution, 55: 1963-1971. |
Galen, C., 1999. Why do flowers vary? The functional ecology of variation in flower size and form within natural plant populations. Bioscience, 49: 631-640. DOI:10.2307/1313439 |
Goulson, D., 1999. Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect. Plant Ecol. Evol. Syst., 2: 185-209. DOI:10.1078/1433-8319-00070 |
Grant, V., 1949. Pollination systems as isolating mechanisms in angiosperms. Evolution, 3: 82-97. DOI:10.2307/2405454 |
Harder, L.D., 1986. Effects of nectar concentration and flower depth on flower handling efficiency of bumble bees. Oecologia, 69: 309-315. DOI:10.1007/BF00377639 |
Hartig, F., 2021. DHARMa: residual diagnostics for hierarchical (Multi-Level/mixed) regression models. R package version 0.4.1. https://CRAN.R-project.org/package=DHARMa.
|
Huang, J.G., Ma, Q., Rossi, S., et al., 2020. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. U.S.A., 117: 20645-20652. DOI:10.1073/pnas.2007058117 |
Hughes, A.R., Inouye, B.D., Johnson, M.T., et al., 2008. Ecological consequences of genetic diversity. Ecol. Lett., 11: 609-623. DOI:10.1111/j.1461-0248.2008.01179.x |
Inouye, D.W., 1980. The terminology of floral larceny. Ecology, 61: 1251-1253. DOI:10.2307/1936841 |
Irwin, R.E., Bronstein, J.L., Manson, J.S., et al., 2010. Nectar robbing: ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst., 41: 271-292. DOI:10.1146/annurev.ecolsys.110308.120330 |
Irwin, R.E., Maloof, J.E., 2002. Variation in nectar robbing over time, space, and species. Oecologia, 133: 525-533. DOI:10.1007/s00442-002-1060-z |
Ishii, H.S., Kadoya, E.Z., 2016. Legitimate visitors and nectar robbers on Trifolium pratense showed contrasting flower fidelity versus co-flowering plant species: could motor learning be a major determinant of flower constancy by bumble bees?. Behav. Ecol. Sociobiol., 70: 377-386. DOI:10.1007/s00265-016-2057-7 |
Jacquemyn, H., Brys, R., 2020. Lack of strong selection pressures maintains wide variation in floral traits in a food-deceptive orchid. Ann. Bot., 126: 445-453. DOI:10.1093/aob/mcaa080 |
Johnson, D., Martin, F., Cairney, J.W., et al., 2012. The importance of individuals: intraspecific diversity of mycorrhizal plants and fungi in ecosystems. New Phytol., 194: 614-628. DOI:10.1111/j.1469-8137.2012.04087.x |
Jump, A.S., Marchant, R., Peñuelas, J., 2009. Environmental change and the option value of genetic diversity. Trends Plant Sci., 14: 51-58. DOI:10.1016/j.tplants.2008.10.002 |
Kohl, P.L., Steffan-Dewenter, I., 2022. Nectar robbing rather than pollinator availability constrains reproduction of a bee-flowered plant at high elevations. Ecosphere, 13: e4077. |
Lara, C., Ornelas, J.F., 2001. Preferential nectar robbing of flowers with long corollas: experimental studies of two humming-bird species visiting three plant species. Oecologia, 128: 263-273. DOI:10.1007/s004420100640 |
Lázaro, A., Vignolo, C., Santamaría, L., 2015. Long corollas as nectar barriers in Lonicera implexa: interactions between corolla tube length and nectar volume. Evol. Ecol., 29: 419-435. DOI:10.1007/s10682-014-9736-5 |
Lichtenberg, E.M., Irwin, R.E., Bronstein, J.L., 2020a. Bumble bees are constant to nectar-robbing behaviour despite low switching costs. Anim. Behav., 170: 177-188. DOI:10.1016/j.anbehav.2020.09.008 |
Lichtenberg, E.M., Richman, S.K., Irwin, R.E., et al., 2020b. Competition for nectar resources does not affect bee foraging tactic constancy. Ecol. Entomol., 45: 904-909. DOI:10.1111/een.12866 |
Maruyama, P.K., Vizentin-Bugoni, J., Dalsgaard, B., et al., 2015. Nectar robbery by a hermit hummingbird: association to floral phenotype and its influence on flowers and network structure. Oecologia, 178: 783-793. DOI:10.1007/s00442-015-3275-9 |
Mendez-Vigo, B., Gomaa, N.H., Alonso-Blanco, C., et al., 2013. Among- and within-population variation in flowering time of Iberian Arabidopsis thaliana estimated in field and glasshouse conditions. New Phytol., 197: 1332-1343. DOI:10.1111/nph.12082 |
Nardone, E., Dey, T., Kevan, P.G., 2013. The effect of sugar solution type, sugar concentration and viscosity on the imbibition and energy intake rate of bumblebees. J. Insect Physiol., 59: 919-933. DOI:10.1016/j.jinsphys.2013.06.007 |
Pattrick, J.G., Symington, H.A., Federle, W., et al., 2020. The mechanics of nectar offloading in the bumblebee Bombus terrestris and implications for optimal concentrations during nectar foraging. J. R. Soc. Interface, 17: 20190632. DOI:10.1098/rsif.2019.0632 |
Pei, C., Chen, S.L. (Eds. ), 1982. Verbenaceae. In: Flora Reipublicae Popularis Sinicae, Vol 65. Science Press, Beijing.
|
R Core Team, 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
|
Raffard, A., Santoul, F., Cucherousset, J., et al., 2019. The community and ecosystem consequences of intraspecific diversity: a meta-analysis. Biol. Rev., 94: 648-661. DOI:10.1111/brv.12472 |
Richman, S.K., Irwin, R.E., Nelson, C.J., et al., 2017. Facilitated exploitation of pollination mutualisms: fitness consequences for plants. J. Ecol., 105: 188-196. DOI:10.1111/1365-2745.12657 |
Rojas-Nossa, S.V., Sánchez, J.M., Navarro, L., 2016. Nectar robbing: a common phenomenon mainly determined by accessibility constraints, nectar volume and density of energy rewards. Oikos, 125: 1044-1055. DOI:10.1111/oik.02685 |
Rojas-Nossa, S.V., Sánchez, J.M., Navarro, L., 2021. Nectar robbing and plant reproduction: an interplay of positive and negative effects. Oikos, 130: 601-608. DOI:10.1111/oik.07556 |
Stoffel, M.A., Nakagaw, S., Schielzeth, H., 2020. partR2: partitioning R2 in generalized linear mixed models. bioRxiv. DOI:10.1101/2020.07.26.221168 |
Sutherland, W.J., Freckleton, R.P., Godfray, H.C.J., et al., 2012. Identification of 100 fundamental ecological questions. J. Ecol., 101: 58-67. |
Whittall, J.B., Hodges, S.A., 2007. Pollinator shifts drive increasingly long nectar spursin columbine flowers. Nature, 447: 706-709. DOI:10.1038/nature05857 |
Yang, C.F., Wang, Q.F., 2015. Nectarless flowers with deep corolla tubes in Pedicularis: does long pistil length provide an arena for male competition?. Bot. J. Linn. Soc., 179: 526-532. DOI:10.1111/boj.12331 |
Ye, Z.M., Jin, X.F., Inouye, D.W., et al., 2018. Variation in composition of two bumble bee species across communities affects nectar robbing but maintains pollinator visitation rate to an alpine plant, Salvia przewalskii. Ecol. Entomol., 43: 363-370. DOI:10.1111/een.12509 |
Ye, Z.M., Jin, X.F., Wang, Q.F., et al., 2017. Nectar replenishment maintains the neutraleffects of nectar robbing on female reproductive success of Salvia przewalskii (Lamiaceae), a plant pollinated and robbed by bumble bees. Ann. Bot., 119: 1053-1059. DOI:10.1093/aob/mcw285 |
Zeileis, A., Kleiber, C., Jackman, S., 2008. Regression models for count data in R. J. Stat. Software, 27: 1-25. http://www.jstatsoft.org/v27/i08/. |
Zeileis, A., Hothorn, T., 2002. Diagnostic checking in regression relationships. R. News, 2: 7-10. https://CRAN.R-project.org/doc/Rnews/. |
Zuur, A.F., Ieno, E.N., 2016. Beginner's Guide to Zero-Inflated Models with R. Highland Statistics Ltd., Newburgh.
|



