b. Research Institute of Tropical Forestry, Chinese Academy of Forestry, Guangzhou, 510520, China
Leaf photosynthesis, a fascinating process in transforming atmospheric CO2 into carbohydrate, is fundamental for plant growth and ecosystem functioning (Wright et al., 2004; Terrer et al., 2021). For terrestrial plants, there is a conspicuous tradeoff regarding CO2 assimilation, i.e., the leaf economics spectrum (LES), depicting that leaves with a high photosynthetic rate usually have a short lifespan, and vice versa (Wright et al., 2004; Reich, 2014; Diaz et al., 2016). To maintain photosynthesis, leaves should be supplied with sufficient water. The efficiency of the water supply system in leaves is determined by leaf minor veins which act as 'the super highway' for delivering water to photosynthetic tissues (Feild and Brodribb, 2013). Leaf minor vein traits, such as the minor vein diameter and density (minor vein length per unit leaf area), are closely related to leaf hydraulic conductance (Sack and Frole, 2006; Xiong et al., 2016) and are commonly used as leaf hydraulic traits. Although LES and leaf hydraulics are sparsely reported to be coupled (Blonder et al., 2011), they are increasingly recognized as independent trait dimensions worldwide (see the classical reviewer paper by Sack et al., 2013; Li et al., 2015 and references therein), as such forming a multidimensional trait space in leaves. The multi-dimension relative to the single LES dimension is commonly recognized to confer plants more ecological strategies (i.e., more trait combinations) for adaption to heterogeneous environments.
The decoupling between LES and leaf hydraulics could be related to light and soil water availability which vary independently under natural conditions (Oliveira et al., 2021; Umana et al., 2021) and differently affect the two trait dimensions (Zhang and Cao, 2009; Baraloto et al., 2010; Li et al., 2015). For example, all else being equal, sites with high light intensity usually have high temperature, consequently causing greater water loss through evaporation and low soil moisture (Stuart Chapin Ⅲ et al., 2011). Soil moisture at the high light sites could be high in cases of sufficient water sources such as rivers, groundwater or abundant precipitation. This will cause out-of-sync variations between light and soil water availability and hence multifarious environmental niches. The decoupled light and water availability can affect leaf mesophyll and leaf minor veins separately, thereby driving the formation of decoupled LES and leaf hydraulics (Li et al., 2015).
Leaf size is another important trait dimension reflecting plant adaptation to environmental temperature through an adjustment of the leaf-level energy balance (Sack et al., 2013; Wright et al., 2017; Baird et al., 2021). For example, large leaves have a thick boundary layer that slows sensible heat loss from the leaf surface (Dong et al., 2017). This causes large leaves a great risk of lethal temperature damage in hot and dry environment with limited transpirational cooling. Mounting evidence shows the independence of leaf size from leaf hydraulics represented by minor vein density (Scoffoni et al., 2011; Sack et al., 2012; Sack and Scoffoni, 2013). Such decoupled trait dimensions could also be related to independent variation of light and soil water availability. For example, species with similar leaf vein density under moist conditions could have different light and heat conditions, which allows plants to produce different leaf size to balance leaf-level heat exchange (Sack et al., 2012; Wright et al., 2017).
To better understand how plants adapt to the multifarious environmental niches, it is necessary to concomitantly take into account leaf economics, hydraulics and size dimensions. However, most studies concentrate on only one (e.g., leaf size, Wright et al., 2017; hydraulics, Oliveira et al., 2021) or two of the trait dimensions (e.g., hydraulics and economics, Li et al., 2015; economics and size, Eallonardo et al., 2013; Diaz et al., 2016). Most importantly, our knowledge of the trait relationships comes mainly from interspecific comparisons under unstressed conditions. It remains unclear how the above three trait dimensions vary in a coordinated or decoupled way at the intraspecific level, e.g., comparing a species along a stress gradient.
Mangrove trees usually grow in intertidal zones in the tropics and subtropics suffering from salt stress. One of the conspicuous adverse effects by the salt stress is that it can cause difficulty in water absorption by plant roots, i.e., physiological drought, given the low osmotic potential of the soil solution around the roots (Liang et al., 2021). A salinity gradient is frequently observed in mangrove habitats, with lower salinity in river estuaries and higher salinity along open coasts. Here, we selected a typical mangrove species, Ceriops tagal (Perr.) C.B. Rob., growing at two habitats with similar light conditions but different soil salinities. We hypothesized that leaf economics, hydraulics and size are coupled rather than decoupled along the salinity gradient. This is because salinity-induced physiological drought usually varies coordinately with the soil nutrient content in mangrove habitats. For example, river estuaries generally have lower salinity (lower physiological drought for plants) and higher soil nutrients whereas open coasts have higher salinity (higher physiological drought) and lower soil nutrients (Woodroffe, 1992; Xiong et al., 2018). Under similar sunny and hot conditions, coupled soil water and nutrients could drive leaf economics, hydraulics and size to vary in a coordinated rather than a decoupled manner.
2. Materials and methods 2.1. Study site and sample collectionThe study was conducted in the Dongzhai Harbor National Natural Reserve for the mangrove ecosystem, Hainan Island China (19°51′N-20°01′N, 110°30′E−110°37′E). This location has the richest mangrove species in China (Xiong et al., 2016). For detailed information of the mangrove communities in this area, please can refer to Xiong et al. (2016). The area has a tropical monsoon climate with mean annual precipitation of 1676 mm and mean annual air temperature of 23.5 ℃. Almost 80 percent of the annual precipitation occurs from May to October. Here, we selected a common mangrove species, C. tagal, which naturally grows in mono-specific stands with ages older than 60 years. C. tagal is a typical species in Rhizophoraceae usually growing in coast beach as a shrub or a small tree. C. tagal is well-adapted to the sunny, hot and high salinity coast environment by bearing leaves with thick cuticle, high tannin content and sunken stomata (Li, 2006; Xiong et al., 2018).
In the growing season in May 2015, we collected the roots and leaves of C. tagal at two sites with different soil salinities and nutrient contents. One site was located near the seashore of the outer part of the bay with high salinity and a low soil nutrient content (see Table 2 in Xiong et al., 2016). C. tagal at this site grew as tall as 1–1.5 m. The other site was in the Yanfeng River estuary, located in the inner part of the bay where C. tagal grew as tall as 2–3 m. This site was characterized with lower salinity and a higher soil nutrient content relative to the aforementioned site (Xiong et al., 2016).
At each site, we randomly selected 5 mature C. tagal trees. For each tree, 15–20 intact leaves without herbivory damage were collected; 3–5 leaves were fixed in solution (90 ml 70% ethanol, 5 ml 100% glacial acetic acid and 5 ml 37% methanol) for leaf anatomy, and the remaining leaves were used to determined leaf morphology, nutrients, defensive chemicals and minor vein diameter and density. To test the covariation of the hydraulics between leaves and roots, we also collected the first-order roots along with the leaf samples and immediately placed the roots in the fixation solution for subsequent root anatomy measurements.
2.2. Trait measurementsFor each tree, 3–5 leaves were selected and scanned to calculate individual leaf area using IMAGE J software (NIH Image, Bethesda, MD, USA). Specific leaf area (SLA, leaf area per unit leaf mass) was measured after the leaves were oven-dried (60 ℃, 48 h) and weighed. The leaf nitrogen concentration and isotopic composition (δ13C) were measured by an elemental analyzer interfaced with isotope ratio mass spectrometry (EA1112 coupled with Delta-XP, Thermo Fisher Scientific, Bremen, Germany, Wang et al., 2021). Leaf total phenol, an indicator of leaf defense function, was measured by referring to a classical study (Hättenschwiler and Jørgensen, 2010).
Leaves and the first-order roots were taken out of the fixation solution and processed in a suite of procedures including the following: dehydration, embedded in paraffin, cut into sections (8 μm in thickness), stained and then photographed (Kong et al., 2014). Root and leaf anatomical structures were also measured using IMAGE J software (NIH Image, Bethesda, MD, USA), including the leaf thickness (LT), leaf palisade tissue length and width, leaf spongy tissue thickness. Generally, roots are more or at least equally sensitive to drought as leaves because roots grow in the soil and can sense and respond to soil drought prior to leaves in a suite of root hydraulic traits such as root vessel diameter (RVdia), density (RVden) and the number (n) of the first-order roots. Measurements of the root hydraulics can also be useful for evaluating hydraulic coordination between roots and leaves across the salinity gradient.
To measure the leaf minor vein traits, we first carefully removed the epidermis and the upper layers of the mesophyll to expose the leaf minor veins using a sharp knife. This is because C. tagal leaves are thick and the mesophyll cannot be easily removed using alkali solution. Six leaves per site were then immerged in NaOH solution (5%) for hours, and the residual mesophyll tissues were carefully cleaned by a pin. Finally, leaf veins were dyed red and were photographed using a camera (Eclipse Ni–U; Nikon). Leaf minor vein diameter (μm) and density (mm mm−2) were also calculated for 6 fields of view per leaf using IMAGE J (Li et al., 2015).
2.3. Data analysisWe used the hydraulic weighted conduit diameter (Dh) as the vessel diameter for the first-order roots of C. tagal (Long et al., 2013). Dh was calculated as follows:
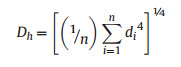
|
where di is the lumen diameter of vessel i and n is the vessel number. Vessel density was expressed as the number of vessels per unit root stele cross-sectional area (Long et al., 2013). Differences in leaf economics, hydraulics and leaf size between the two sites were analyzed using independent t tests in R (v. 3.30, R Core Team, 2016).
3. ResultsCompared with leaves of C. tagal under low soil salinity, leaves under high soil salinity had lower SLA (p = 0.029), LT (p = 0.014) and single leaf area (0.37-fold of the former case, p = < 0.001; Table 1). Both leaf palisade and spongy tissues were thinner under high relative to low soil salinity (p < 0.05). Moreover, leaves at the saltier site had a lower N concentration (p = 0.03), higher δ13C value (p < 0.001) and higher total phenol content (p < 0.001; Table 1).
| Leaf/Root traits | Abbreviation | Unit | High salinity site | Low salinity site | |
| Leaf economics | Specific leaf area | SLA | m−2 g−1 | 48.77±1.00b | 59.64±4.30a |
| Leaf thickness | LT | mm | 1.42±0.04b | 1.74±0.09a | |
| Leaf N content | LN | mg g−1 | 10.10±0.42b | 11.79±1.03a | |
| Leaf carbon isotope composition | δ13C | ‰ | -28.71±0.15a | -30.92±0.10b | |
| Leaf total phenol content | LTP | % | 4.20±0.04a | 4.02±0.04b | |
| Leaf palisade length | LPL | μm | 349.33±27.89b | 495.83±32.84a | |
| Leaf palisade width | LPW | μm | 20.99±0.80b | 25.58±1.07a | |
| Leaf spongy thickness | LST | μm | 792.70±25.60b | 1025.87±44.28a | |
| Leaf hydraulics | Leaf minor vein density | LVden | mm mm−2 | 8.66±0.07a | 4.78±0.16b |
| Leaf minor vein diameter | LVdia | μm | 41.52±1.53b | 52.56±0.61a | |
| Root hydraulics | Root vessel number | RVN | 3.95±0.33a | 4.06±0.49a | |
| Root diameter | RD | μm | 176.09±3.68a | 169.25±6.48a | |
| Root vessel density | RVden | mm mm−2 | 0.011±0.0015a | 0.016±0.0022a | |
| Root vessel diameter | RVdia | μm | 2.45±0.06b | 3.67±0.36a | |
| Leaf size | Single leaf area | LA | m−2 | 8.98±0.22b | 24.24±1.23a |
| Different lowercase letters following a trait of Ceriops tagal indicated a significant difference between the high and the low salinity site at p < 0.05. | |||||
Leaf minor vein density under high salinity was significantly higher (1.81-fold) than that under low salinity (p < 0.001), while the minor vein diameter was significantly lower (0.79-fold) than that under low salinity (p < 0.001). The vessel diameter of the first-order roots in the high salinity soil was lower (0.67-fold) than that in the low salinity soil (p < 0.001). However, there were no differences in the first-order root diameter, root vessel number and density between the two salinities (p > 0.05, Table 1).
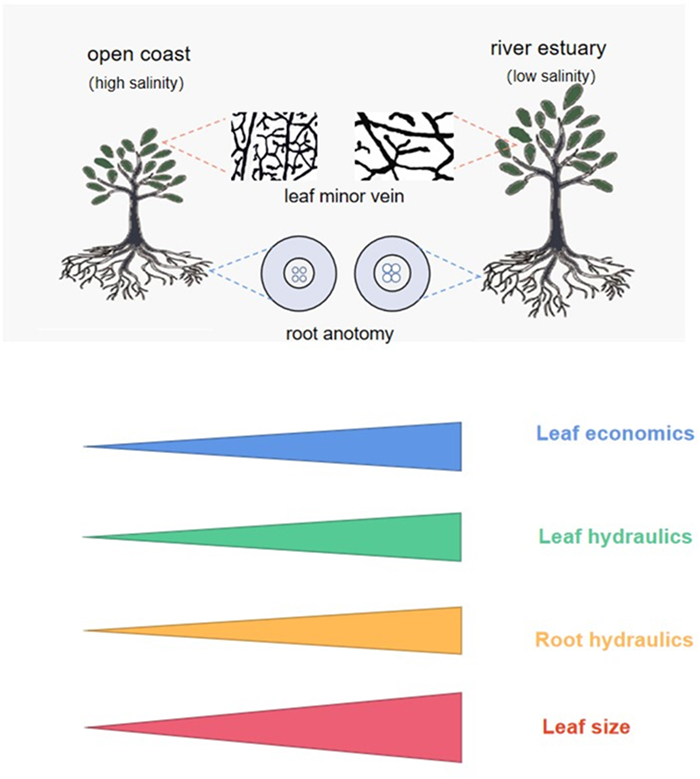
4. DiscussionOur results show coordinated variation among leaf economics, hydraulics, and leaf size along a salinity gradient for a typical mangrove species, Ceriops tagal (Fig. 1). Specifically, compared with leaves under low salinity, leaves under high salinity had lower photosynthetic rate because of lower leaf N content and hence less chloroplast represented in smaller palisade and spongy tissues (Wright et al., 2004; Clarke et al., 2021), higher leaf water use efficiency usually under stressful conditions (indicated by higher leaf δ13C; Li et al., 2015) and more resource allocated to defense function (indicated by higher total phenol content) rather than leaf photosynthesis. In addition, C. tagal leaves under higher salinity also had a lower water transport capacity because a significant reduction of the conduit diameter of the vascular system from roots (i.e., vessels) to leaves (i.e., minor veins; Table 1). Theoretically, water flux in a conduit is proportional to the fourth power of the conduit diameter according to the Hagen–Poiseuille law (Jensen et al., 2016). Finally, leaf size is smaller under high than under low salinity because of the shift of leaf hydraulics associated leaf energy budget (Wright et al., 2017; also see below for details). The coupling of leaf economics, hydraulics and leaf size, as revealed within salt-stressed mangrove plants, is different from the independence of these trait dimensions across plants worldwide (Sack et al., 2012; Li et al., 2015; Diaz et al., 2016; Oliveira et al., 2021). Generally, soil water and nutrient availability are closely coupled in terrestrial ecosystems because moist soils, all else being equal, usually have high plant litter production and fast decomposition rates of litter and soil organic matter (Zhang and Cao, 2009; Wang et al., 2016; Petraglia et al., 2018). Given that soil nutrients, e.g., N, are a critical component of proteins in the CO2 assimilation process (Taiz and Zeiger, 2010), it can be expected that under sufficient light conditions the coordinated variation of soil nutrient and water availability would cause coupled leaf economics and hydraulics. This could underlie the coupling of leaf economics and hydraulics for C. tagal in our study that is featured with coordinated variation of soil water and nutrient availability across the soil salinity under similar sunny and hot condition.
|
| Fig. 1 Conceptional framework for the coordination of leaf hydraulics with leaf economics and leaf size along a salinity gradient for a mangrove species, Ceriops tagal. Leaf and root hydraulics refer to the water transport capacity via minor veins and root vessels and leaf economics refer to the photosynthetic capacity. |
However, when light varies independently from soil water and nutrient availability as is the case in natural conditions, this can cause the light-driven leaf economics to decouple from leaf hydraulics as widely shown in previous studies (Sack et al., 2013; Li et al., 2015). The reason is that nutrient absorption by roots is an energy-demanding process; the energy, in the form of adenosine triphosphate (ATP), is mainly provided by decomposing current photosynthate in root respiration (Swamy, 1998; Lynch et al., 2013; Taiz and Zeiger, 2010). This means that the nutrients absorbed by roots could be closely related to the light-demanding photosynthesis rather than to the availability of nutrients even in fertile and moist soils (Litton et al., 2007; Kong and Fridley, 2019). Therefore, although soil water usually co-varies with nutrient availability, leaf economics can be decoupled with leaf hydraulics because light generally varies independently rather than coordinately with soil water availability in natural conditions.
In addition, the coordinated leaf size and leaf hydraulics could also be due to the change in soil water availability along the salinity gradient. Theoretically, leaf size is dominantly affected by transpirational cooling, with higher such cooling in plants with larger leaves, especially in sunny and hot environments (Dong et al., 2017; Wright et al., 2017). Leaf transpiration is usually affected by soil water conditions (e.g., higher soil moisture may correspond to higher leaf transpiration all else being equal) and air temperature (air temperature is usually heated by, as such, closely related to light; the higher the air temperature, the higher the transpiration when all else being equal; Dong et al., 2017; Wright et al., 2017). The naturally occurring independence of soil water and light, as such, could cause soil water-related leaf hydraulics to decouple from leaf size which is co-driven by air temperature (closely related to light) and soil water (Wright et al., 2017). This could explain the worldwide decoupling of leaf hydraulics from leaf size (Scoffoni et al., 2011; Sack et al., 2012). However, under similar light conditions such as the sunny and hot environments in our study, plants growing under higher salinity with higher physiological drought usually have lower transpiration due to thinner minor veins (Table 1; Baraloto et al., 2010; Li et al., 2015). Less transpirational cooling precludes the formation of large leaves because of the greater risk of lethal temperature damage (Sack et al., 2012; Wright et al., 2017), as such, leading to coupled leaf size and leaf hydraulics.
Here, we suggest a dominant role of salinity-induced physiological drought in driving the coordination of leaf hydraulics with leaf economics and leaf size. Previous studies frequently reported an increase in the leaf minor vein density in response to a drought environment (Baraloto et al., 2010; Mueller et al., 2010; Valverde-Barrantes et al., 2020), which shortens the water transport distance between water supply tissue (i.e., leaf minor veins) and water-demanding mesophyll cells (Brodribb and Feild, 2010; Valverde-Barrantes et al., 2020; Zhou et al., 2021). Assuming leaf minor veins function as a long conduit for water transport, it is usually the diameter of the veins that defines the capacity of water supply to the mesophyll cells. Therefore, the minor vein diameter could be more important than the minor vein density in determining leaf transpiration. The reason is that the minor vein diameter determines the capacity of the water supply to leaves and water loss via transpiration while the minor vein density is only a proxy of how efficiently the water diffuses to the mesophyll and the stomata. More importantly, the reduced capacity of water supply via thinner minor veins under higher salinity is in accordance with the concomitant thinner vessels in roots (Table 1) and fewer absorptive roots (Xiong et al., 2016), with less water transported upward to leaves. This in turn suggests the coordination of hydraulics above- and belowground in response to salinity-induced drought, i.e., ensuring safety at the cost of efficiency in water transport.
In conclusion, our study reveals that leaf economics, hydraulics and leaf size in a tropical mangrove species vary coordinately along a soil salinity gradient. The coordinated trait dimensions could be due to the parallel salinity-induced physiological drought and soil nutrient content between the two mangrove sites with similar sunny and hot conditions. Furthermore, our results suggest that the physiological drought induced concomitant hydraulic changes in leaves and roots, especially the minor vein diameter and root vessel diameter, is important in coupling leaf hydraulics with leaf economics and leaf size. Although variations in leaf trait dimensions were examined across only two salinity gradients, the results here provide an insightful view of our understanding of how plants adapt and respond to changes in the multifarious environmental niches. Future studies could test our conclusion across more environmental gradients as well as in other species.
AcknowledgementsWe thank Mr. Lingqun Kong for their help in collecting plant materials and Chao Guan, Xinyu Lu, Jinqi Tang, Qubing Ran, Song Huang and Haiyan Zhang for their assistance in pre-processing leaves and roots of the mangrove species in this study. We also thanks Dongzhai Harbor mangrove Wetland National Nature Reserve for their support. This study was funded by the National Natural Science Foundation of China (32171746, 31870522 and 31670550), Special Foundation for National Science and Technology Basic Research Program of China (2019FY101300) and the Scientific Research Foundation of Henan Agricultural University (30500854).
Author contributions
D.K. designed this study; J.C. and D.K. wrote the draft of the paper; J.C., Q.Y., X.Y. and W.R. contributed to the data analysis and discussion of the results.
Data availability statement
Data relevant to this study are available on request from the corresponding author, DK.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Baird, A.S., Taylor, S.H., Pasquet-Kok, J., et al., 2021. Developmental and biophysical determinants of grass leaf size worldwide. Nature, 592: 242-247. DOI:10.1038/s41586-021-03370-0 |
Baraloto, C., Timothy Paine, C.E., Poorter, L., et al., 2010. Decoupled leaf and stem economics in rain forest trees. Ecol. Lett., 13: 1338-1347. DOI:10.1111/j.1461-0248.2010.01517.x |
Blonder, B., Violle, C., Bentley, L.P., et al., 2011. Venation networks and the origin of the leaf economics spectrum. Ecol. Lett., 14: 91-100. DOI:10.1111/j.1461-0248.2010.01554.x |
Brodribb, T.J., Feild, T.S., 2010. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol. Lett., 13: 175-183. DOI:10.1111/j.1461-0248.2009.01410.x |
Clarke, V.C., Danila, F.R., von Caemmerer, S., 2021. CO2 diffusion in tobacco: a link between mesophyll conductance and leaf anatomy. Interface Focus, 11: 20200040. DOI:10.1098/rsfs.2020.0040 |
Diaz, S., Kattge, J., Cornelissen, J.H., et al., 2016. The global spectrum of plant form and function. Nature, 529: 167-171. DOI:10.1038/nature16489 |
Dong, N., Prentice, I.C., Harrison, S.P., et al., 2017. Biophysical homoeostasis of leaf temperature: a neglected process for vegetation and land-surface modelling. Global Ecol. Biogeogr., 26: 998-1007. DOI:10.1111/geb.12614 |
Eallonardo, A.S., Leopold, D.J., Fridley, J.D., et al., 2013. Salinity tolerance and the decoupling of resource axis plant traits. J. Veg. Sci., 24: 365-374. DOI:10.1111/j.1654-1103.2012.01470.x |
Feild, T.S., Brodribb, T.J., 2013. Hydraulic tuning of vein cell microstructure in the evolution of angiosperm venation networks. New Phytol., 199: 720-726. DOI:10.1111/nph.12311 |
Hättenschwiler, S., Jørgensen, H.B., 2010. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol., 98: 754-763. DOI:10.1111/j.1365-2745.2010.01671.x |
Jensen, K.H., Berg-Sørensen, K., Bruus, H., et al., 2016. Sap flow and sugar transport in plants. Rev. Mod. Phys., 88. |
Kong, D., Fridley, J.D., 2019. Does plant biomass partitioning reflect energetic investments in carbon and nutrient foraging?. Funct. Ecol., 33: 1627-1637. DOI:10.1111/1365-2435.13392 |
Kong, D., Ma, C., Zhang, Q., et al., 2014. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol., 203: 863-872. DOI:10.1111/nph.12842 |
Li, L., McCormack, M.L., Ma, C., et al., 2015. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett., 18: 899-906. DOI:10.1111/ele.12466 |
Li, Y., 2006. Studies on Leaf Anatomy of Some Mangrove Species. XMU.
|
Liang, X., Liu, S., Wang, T., et al., 2021. Metabolomics-driven gene mining and genetic improvement of tolerance to salt-induced osmotic stress in maize. New Phytol., 230: 2355-2370. DOI:10.1111/nph.17323 |
Litton, C.M., Raich, J.W., Ryan, M.G., 2007. Carbon allocation in forest ecosystems. Global Change Biol., 13: 2089-2109. DOI:10.1111/j.1365-2486.2007.01420.x |
Long, Y., Kong, D., Chen, Z., et al., 2013. Variation of the linkage of root function with root branch order. PLoS One, 8: e57153. DOI:10.1371/journal.pone.0057153 |
Lynch, D.J., Matamala, R., Iversen, C.M., et al., 2013. Stored carbon partly fuels fine-root respiration but is not used for production of new fine roots. New Phytol., 199: 420-430. DOI:10.1111/nph.12290 |
Mueller, K.E., Diefendorf, A.F., Freeman, K.H., et al., 2010. Appraising the roles of nutrient availability, global change, and functional traits during the angiosperm rise to dominance. Ecol. Lett., 13: E1-E6. DOI:10.1111/j.1461-0248.2010.01455.x |
Oliveira, R.S., Eller, C.B., Barros, F.V., et al., 2021. Linking plant hydraulics and the fast-slow continuum to understand resilience to drought in tropical ecosystems. New Phytol., 230: 904-923. DOI:10.1111/nph.17266 |
Petraglia, A., Cacciatori, C., Chelli, S., et al., 2018. Litter decomposition: effects of temperature driven by soil moisture and vegetation type. Plant Soil, 435: 187-200. |
Reich, P.B., 2014. The world-wide 'fast-slow' plant economics spectrum: a traits manifesto. J. Ecol., 102: 275-301. DOI:10.1111/1365-2745.12211 |
Sack, L., Frole, K., 2006. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. J. Ecol., 87: 483-491. DOI:10.1890/05-0710 |
Sack, L., Scoffoni, C., 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol., 198: 983-1000. DOI:10.1111/nph.12253 |
Sack, L., Scoffoni, C., John, G.P., et al., 2013. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot., 64: 4053-4080. DOI:10.1093/jxb/ert316 |
Sack, L., Scoffoni, C., McKown, A.D., et al., 2012. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nat. Commun., 3: 837. DOI:10.1038/ncomms1835 |
Scoffoni, C., Rawls, M., McKown, A., et al., 2011. Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol., 156: 832-843. DOI:10.1104/pp.111.173856 |
Stuart Chapin Ⅲ, F., Matson, P.A., Vitousek, P.M., 2011. Principles of terrestrial Ec cosystem ecology. In: Stuart Chapin Ⅲ, F., Matson, P.A., Vitousek, P.M. (Eds.), Water and Energy Balance, second ed. Springer ScienceþBusiness Media, LLC, USA, pp. 93-122. 233 Spring Street, New York, NY 10013.
|
Swamy, G.S., 1998. How do plants absorb nutrients from the soil?. Resonance, 3: 45-52. |
Taiz, L., Zeiger, E., 2010. In: Taiz, L., Zeiger, E. (Eds.), Plant Physiology, fifth ed. Sinauer Associates, Massachusetts U.S. A, pp. 161-198.
|
Terrer, C., Phillips, R.P., Hungate, B.A., et al., 2021. A trade-off between plant and soil carbon storage under elevated CO2. Nature, 591: 599-603. DOI:10.1038/s41586-021-03306-8 |
Umana, M.N., Swenson, N.G., Marchand, P., et al., 2021. Relating leaf traits to seedling performance in a tropical forest: building a hierarchical functional framework. Ecology, 102: e03385. |
Valverde-Barrantes, O.J., Maherali, H., Baraloto, C., et al., 2020. Independent evolutionary changes in fine-root traits among main clades during the diversification of seed plants. New Phytol., 228: 541-553. DOI:10.1111/nph.16729 |
Wang, D., He, N., Wang, Q., et al., 2016. Effects of temperature and moisture on soil organic matter decomposition along elevation gradients on the Changbai Mountains, Northeast China. Pedosphere, 26: 399-407. DOI:10.1080/10402004.2015.1083064 |
Wang, R., Penuelas, J., Li, T., et al., 2021. Natural abundance of (13) C and (15) N provides evidence for plant-soil carbon and nitrogen dynamics in a N-fertilized meadow. J. Ecol., 102: e03348. |
Woodroffe, C., 1992. Mangrove sediments and geomorphology. In: Robertson, A.I., Alongi, D.M. (Eds.), Tropical Mangrove Ecosystems. American Geophysical Union, pp. 7-41.
|
Wright, I.J., Dong, N., Maire, V., et al., 2017. Global climatic drivers of leaf size. Science, 357: 917-921. DOI:10.1126/science.aal4760 |
Wright, I.J., Reich, P.B., Westoby, M., et al., 2004. The worldwide leaf economics spectrum. Nature, 428: 821-827. DOI:10.1038/nature02403 |
Xiong, Y., Liao, B., Proffitt, E., et al., 2018. Soil carbon storage in mangroves is primarily controlled by soil properties: a study at Dongzhai Bay, China. Sci. Total Environ., 619–620: 1226-1235. |
Xiong, Y., Liu, X., Guan, W., Liao, B., et al., 2016. Fine root functional group based estimates of fine root production and turnover rate in natural mangrove forests. Plant Soil, 413: 83-95. DOI:10.1002/hlca.201500231 |
Zhang, J.-L., Cao, K.-F., 2009. Stem hydraulics mediates leaf water status, carbon gain, nutrient use efficiencies and plant growth rates across dipterocarp species. Funct. Ecol., 23: 658-667. DOI:10.1111/j.1365-2435.2009.01552.x |
Zhou, M., Bai, W., Li, Q., Guo, Y., et al., 2021. Root anatomical traits determined leaf-level physiology and responses to precipitation change of herbaceous species in a temperate steppe. New Phytol., 229: 1481-1491. DOI:10.1111/nph.16797 |



