b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. University of Hong Kong Department of Ecology and Biodiversity, The University of Hong Kong School of Biological Sciences, China;
d. Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Yunnan 666303, China;
e. Minzu University of China, Beijing 100081, China
Myanmar extends nearly 30° in latitude and has a high level of endemic biodiversity. It is estimated that Myanmar has more than 11, 800 gymnosperm and angiosperm species belonging to 273 families and 2371 genera (He et al., 2018), of which about 1071 are endemic. Myanmar is a confluence of four major flora regions, namely India, Malesia (Sundaic), Sino-Himalayan and Indochina (Ministry of National Planning and Economic Development, 2013). The mountains of Myanmar range from north to south, with the highest peak (Mount Hkakaborazi) rising to 5881 m above sea level. Forests cover 43% of the total land area and hold 1.654 million metric tons of carbon (Saatchi et al., 2011; Myint et al., 2021). Climate in Myanmar is variable. The northern and northeastern mountainous regions receive high monsoonal rainfall (Mie Sein et al., 2021), while the lowland areas experience a tropical monsoon climate (Peel et al., 2007). The climate and transitional zones of Myanmar have produced a highly diverse set of plant species. Understanding patterns of plant species diversity is valuable for conservation and biological research in Myanmar.
Large-scale biological inventories and botanical collections of native plants in Myanmar are limited and/or with data unavailable. Data quality and the availability of newly collected data are vital for scientific research and conservation planning. However, available data are plagued by sampling biases and data inaccuracies, ultimately hindering a comprehensive understanding of the patterns of plant diversity in Myanmar. There are also geographical and taxonomic complications persisting in South and Southeast Asia, particularly in Myanmar (Serra-Diaz et al., 2017). For example, some older specimens listed by locality may have persistent name changes, inconsistent spellings, and similar names, making it challenging to identify the origin of these specimens. Numerous species and their characteristics remain undescribed and are not completely understood (Murray et al., 2020). There is an urgent need to assess plant distribution data to enable conservation planning and management of the rich plant diversity in Myanmar.
Existing plant data in Myanmar are unevenly sampled temporally and spatially. Many species were originally collected and described only once by British botanists in the late 19th and early 20th century. Key botanical collections were created by well-known plant collectors, such as Charles Parish, George Forrest, Frank Kingdon-Ward, Frederick Garrett Dickason, John Henry Lace and James Keenan, in the first half of the 20th century (1908–1962). Botanical collections from the 19th to the early 20th centuries were concentrated in Upper Myanmar, Kachin State and Chin State (Tanaka, 2005). Tanaka (2005) has summarized key botanical collections in Myanmar using previously deposited herbarium specimens (Holmgren and Holmgren, 1991). Much work has been done to make biodiversity data freely available, especially during the past decade, and there is a need to compile open data to build a comprehensive and contemporary national database for Myanmar's plants. Here, we collated a dataset of Myanmar's native higher plant species, and analyzed species collection patterns and factors that affect the plant collection density to identify knowledge gaps for better understanding floristic diversity patterns in Myanmar.
2. Materials and methods 2.1. Study areaMyanmar is located between 9°32′N and 28°31′N, and 92°10′E and 101°11′E and is politically divided into seven states, seven regions, one union territory, and 330 townships. Myanmar borders China to the north and northeast, Laos to the east, Thailand to the southeast, and Bangladesh and India to the west (Aye and Clerk, 2007). The country is divided into five topographic regions: the northern mountainous areas (1830 to 5881 m a.s.l.), western mountain ranges (143–3000 m a.s.l), eastern Shan Highlands (71–937 m a.s.l.), Central Myanmar and the Fertile Delta (0–2957 m a.s.l), and the southern lower valley regions of the Irrawaddy and Sittaung rivers (0–1890 m a.s.l) (Beffasti and Galanti, 2011). Myanmar has a tropical monsoon climate with three seasons: the monsoon or rainy season (from May to October), the winter season (from November to February), and the summer or hot season (from March to April) (Aung et al., 2017).
2.2. Herbarium data and online databaseWe first compiled in-country plant data (offline data source) by digitizing specimens and publications (e.g., theses or dissertations, field survey reports and academic papers). We digitized specimens and literature from four herbaria: the Forest Research Institute Herbarium (RAF) in Nay Pyi Taw, the Department of Botany Herbarium at Mandalay University (MAND), the Department of Botany Herbarium (RANG) in Yangon, and the Central Forestry Development Training Center (CFDTC) in Hmawbi. In total, we collected approximately 55, 106 digitized specimens, 448 published papers, 118 theses/dissertations, 44 local research articles, and 21 flora books from the four herbaria. We further included online data sources, including herbaria databases and biodiversity data journals etc. Online data were compiled from 31 international biodiversity websites and two local websites (details Table S1). Our final dataset included a total of 1, 253, 096 occurrence records.
2.3. Standardization of taxonomic namesThe Taxonomic Name Resolution Service includes plant species names from four databases: Tropicos, United States Department of Agriculture, Global Compositae Checklist, and National Center for Biotechnology Information (Dauby et al., 2016). Using Catalogue Of Life 2021 Annual Checklist (https://www.catalogueoflife.org/2021/07/29/release), we quality-checked the accepted and/or unresolved names (Roskov et al., 2013). Species names of angiosperms and gymnosperms were standardized following the Angiosperm Phylogeny Group Ⅳ system and recent advances on phylogenomics of gymnosperms (Yang et al., 2022), respectively. The classification of pteridophytes and bryophytes followed "The Plant List" (Kalwij, 2012; PPG I, 2016).
The names of 22, 151 plant species were matched to taxonomic lists of the Taxonomic Name Resolution Service (Boyle et al., 2013), and though this included synonyms, once removed, this resulted in 15, 061 species and 16, 218 taxa (when subspecies were considered) belonging to 3287 genera and 377 families. We excluded records of exotic species based on the classification in national flora checklist (Yang et al., 2013) and records of native Myanmar species occurring outside natural ranges. After removing 1, 144, 439 invalid records not containing species names or localities, the remaining records were processed for spatial analysis. A resulting dataset of 108, 657 occurrence records for 12, 186 native higher plant species in Myanmar was created.
2.4. Georeferencing verification processesWe first created a spatial database of species occurrence records at the township level. Specimens without clear collection localities that can be used for georeferencing were excluded. All species occurrence records without the township level information, or duplicate records within a township, were removed from the database. We then converted the species occurrence map at township level to a 30-km resolution by assigning the species information to the 30 km × 30 km grid cells within the township it occurred. For records with detailed locality descriptions (e.g., villages and specific sampling sites), we extracted the latitude and longitude of the site from Myanmar's administrative map using a geocoding process in ArcMap (Ormsby et al., 2010). Angiosperms, gymnosperms, pteridophytes, and bryophytes were represented by 102, 632 records for 10, 952 species, 610 records for 70 species, 3404 records for 793 species, and 2011 records for 371 species, respectively.
2.5. Collection density, completeness and explanatory variablesWe calculated collection density (i.e., number of collections per square kilometer) by 30 km × 30 km grid cell and ecoregion (Wang et al., 2020), respectively, using the point density tool in ArcGIS 10.0 (ESRI, 2011). There are 14 ecoregions in Myanmar, including rainforests in the lowlands to alpine meadow along the Eastern Himalayas. We performed a rarefaction analysis for each ecoregion to estimate the total number of species of the ecoregion (Fig. S1) and also calculated sample completeness curves to determine how completely the species had been sampled in each ecoregion using the iNEXT package in R 4.2.1 (Fig. S2) (Chao et al., 2016; R Core Team, 2018).
We used multiple linear regressions to explore collection density against road proximity, population density, altitudinal range, and size of PFEs with data generated at the 30 km resolution in R 4.2.1. PFEs contained Protected Areas Systems (PAs), Protected Public Forests (PPFs), and Reserved Forests (RFs). We obtained the maps of PFEs produced by Ministry of Natural Resources and Environmental Conservation, Myanmar (www.monrec.gov.mm) and digitized these maps. We log-transformed all explanatory variables to improve model fit and ensure a normalized distribution of residuals. As collection density is a zero-inflated response variable, a log10 (n+1) transformation was applied to eliminate extreme discrepancies from the norm (Zuur et al., 2009; Ballesteros-Mejia et al., 2013).
3. Results 3.1. Temporal patterns of collectionInitial records dated back to the year around 1700, but significant levels of collection began after 1900 (Fig. 1). Between 1700 and 1900, 2% of the total specimens were collected, and 98% were collected after 1900. Only 2483 species were discovered between 1700 and 1900; another 9703 species were discovered after 1900. The number of surveys had increased sharply since 2000. The spatial distribution of sampling records varied annually. Gaps in spatial data had narrowed over the last three centuries but considerable spatial gaps still exist (Fig. S3). However, our database is now the largest specimen-based dataset in Myanmar, including 100, 443 specimens of which 55, 106 have been digitized (This dataset is available online now on GBIF : https://www.gbif.org/dataset/d7c3401c-6672-4e4a-ac18-b79e0f51993b and its DOI is: 10.15468/nkwfnd).
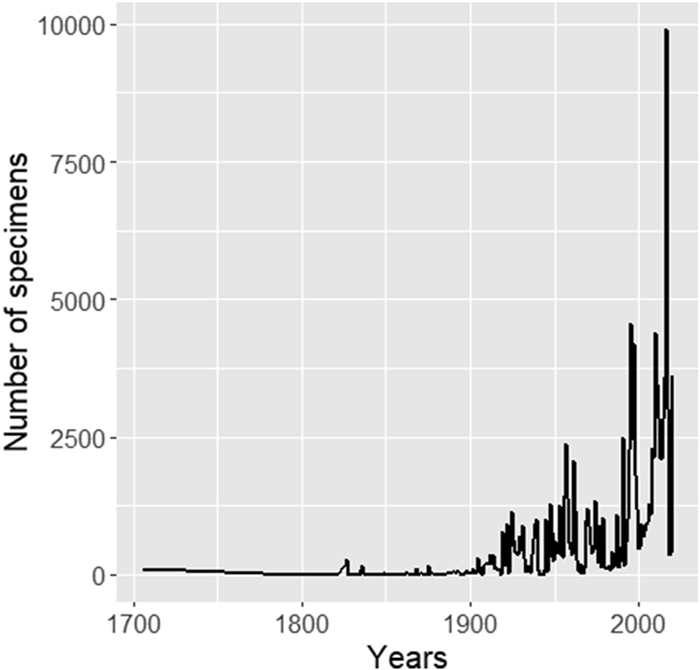
|
| Fig. 1 Number of specimens collected over time in Myanmar between 1700 and 2020. |
The 14 ecoregions differed significantly in the number of records, with the lowest in Nujiang Langcang Gorge alpine conifer and mixed forests near China's border (n = 444), Eastern Himalayan alpine shrub and meadows (n = 836), and Tenasserim-South Thailand semi-evergreen rain forests (n = 1163), and the highest in Irrawaddy dry forests (n = 8369), Northern Indochina subtropical forests (n = 16, 240), and Irrawaddy moist deciduous forests (n = 26, 876); the latter three ecoregions spatially covered more than half of the country. Large number of records (n > 1000) were found in the Mandalay Mountain region, Inn Lay Lake region, Popa Mountain National Park, Nat Ma Taung National Park, Hkakaborazi National Park, and Hlawga National Park.
Collection densities ranged from 0.05 to 0.25 specimens/km2, with approximately 17% of the 30 km × 30 km grid cells having no collection records from 1700 to 2020 (Fig. 2). At the ecoregion level, the collection density was the highest in the Irrawaddy freshwater swamp forests (0.3 specimen/km2), and the lowest in the Kayah-Karen montane rainforests, Tenasserim-South Thailand semi-evergreen rainforests, Mizoram-Manipur-Kachin rainforests, and Nujiang Langcang Gorge alpine and conifer mixed forests (< 0.1 specimen/km2).
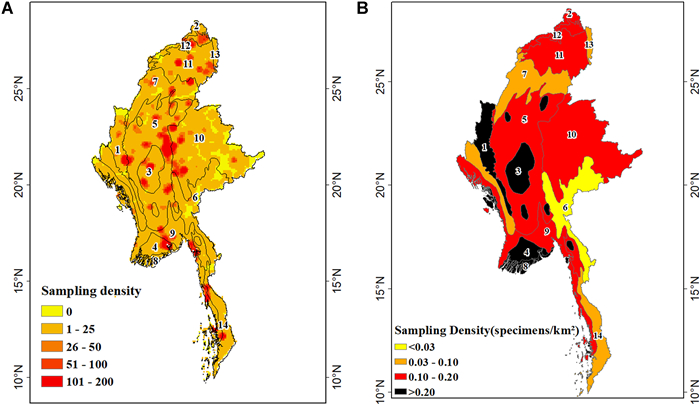
|
| Fig. 2 (A) Density of sampling per cell. (B) Density of sampling per ecoregion. Terrestrial ecoregions classified into (1) Chin Hills-Arakan Yoma Montane Forests(A) Density of sampling per cell. (B) Density of sampling per ecoregion. Terrestrial ecoregions classified into (1) Chin Hills-Arakan Yoma Montane Forests, (2)Eastern Himalayan Alpine Shrub And Meadows, (3) Irrawaddy Dry Forests, (4) Irrawaddy Freshwater Swamp Forests, (5) Irrawaddy Moist Deciduous Forests, (6) Kayah-Karen Montane Rain Forests, (7) Mizoram-Manipur-Kachin Rain Forests, (8) Myanmar Coast Mangroves, (9) Myanmar Coastal Rain Forests, (10) Northern Indochina Subtropical Forests, (11) Northern Triangle Subtropical Forests, (12) Northern Triangle Temperate Forests, (13) Nujiang Langcang Gorge Alpine Conifer And Mixed Forests and (14) Tenasserim-South Thailand Semi-Evergreen Rain Forests. |
In terms of where records are located, and based on both locally available and online data (this is dataset is available online now on GBIF: https://www.gbif.org/dataset/d7c3401c-6672-4e4a-ac18-b79e0f51993b and its DOI is: 10.15468/nkwfnd) Myanmar ranked first in collection efforts (collecting 59% of the total specimens), followed by the United States (11%), China (10%), the United Kingdom (9%), Japan (7%) and South Korea (2%) (Fig. S4). China began collecting specimens in Myanmar in 2010. In recent years, China has been a country where collections had accumulated rapidly. The United Kingdom, United States, and Japan have uploaded digitized specimens from Myanmar on their websites. The specimens collected from Myanmar by China, India, and South Korea remain undigitized. Digitized specimen records across global collections should be linked for the purposes of data sharing and transparency. In Myanmar, foreign collectors are required to deposit duplicate specimens at the Myanmar herbaria. Some specimens were identified to species level, while others were identified to genus and family levels.
3.4. Density of specimen samplingCollection density per ecoregion was uneven. The lowest density recorded was 0.02 specimens/km2 and the highest density was 0.31 specimens/km2. Four ecoregions had sampling densities less than 0.1 specimens/km2. No ecoregion had an average of > 1 specimen/km2. Only two ecoregions had been sampled relatively well: the Irrawaddy Freshwater swamp forests and Myanmar Coast mangroves. Several ecoregions had higher levels of species richness and high heterogeneity but were under-sampled. All ecoregions had low specimen density, whereas only a small fraction of ecoregions had higher density. Furthermore, many ecoregions have high levels of endemism but are undersampled, thus gauging the level of endemism is difficult or impossible. At the township level, 17 counties still had collection gaps, and further work will be needed universally to reconcile this.
3.5. RepresentativenessA total of 17 townships (i.e., Dedaye, Kyaiklat, Dagon Myothit (Seikkan), Hpasawng, Shadaw, Kamma, Munaung, Poke Ba Thi Ri, Myothit, Mongpan, Mongping, Monghsu, Mongyang, Narphan, Kunlong, Konkyan, Kyunhla) still have gaps for specimen collection. The rarefaction curves of all ecoregions were clearly reaching an asymptote (sample completeness > 0.80) except for two ecoregions (i.e., Eastern Himalayan alpine shrub and meadows ecoregion (0.74) and Tenasserim-South Thailand semi-evergreen rainforest ecoregion (0.62)) (Table S2). Collection density percentage of all ecoregions were low, ranging from 1.3% in the Kayah-Karen montane rainforests to 14.7% in the Irrawaddy freshwater swamp forests. Irrawaddy moist deciduous forests covered about 21% of Myanmar's total land area and had a collection density percentage of 8.9% (Fig. 2). This means that whilst the species area curves seemed to reach near-completeness for many ecoregions, these curves in fact represented intensive sampling from a small area that was not representative of the whole ecoregion.
Significant relationships were found between collection density and human population density, road proximity, and elevational range was found (F (4, 635) = 106.8, p < 0.00001), with a R2 of 0.40 (Table S3). We found that collection density was significantly positively correlated (r = 0.58 and 0.42) with population density and road proximity (p < 0.00001) but size of PFEs showed no significant impacts (p > 0.05) (Fig. 3).
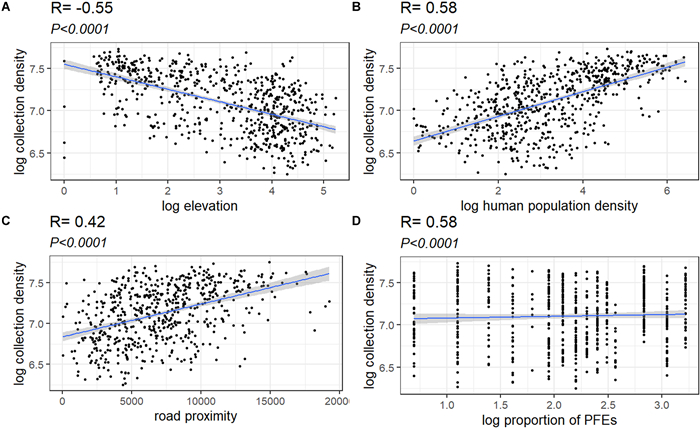
|
| Fig. 3 Single predictor relationships between explanatory variables and collection density in Myanmar (A–D). |
Botanical collection in Myanmar started around 1700 and peaked in the late 2000s, yet the last "complete" inventory of Myanmar's ecoregions is almost 100 years old (Stamp, 1925). Few botanical explorations have recently been conducted in Myanmar leaving current botanical collections insufficient in representing floristic diversity, covering only a tiny proportion of each ecoregion. Our study shows that 17 out of the 330 townships in Myanmar had incomplete specimen collections based on accessible public data sources. The greatest collection gaps were found in townships from states where a variety of ethnic groups and languages are prominent (Fig. S5). This partially is due to political reasons; these townships do not allow for field collections. Different languages also relate to data availability in some regions in other parts of the world (e.g., Africa; Stropp et al., 2016). For gymnosperms, pteridophytes, and bryophytes, the distributional knowledge gained from floristic collection over the past three centuries was still limited (Fig. S6). Hence, gymnosperms and bryophytes in Myanmar are particularly difficult to protect, because research is limited and shows major sampling gaps.
In 2020, 1271 species with 5415 total specimens were published in the Flora of Myanmar Database and aligned with the digitization efforts of the herbaria for the National Museum of Nature and Science (Tokyo), the University of Tokyo, and the RAF. The specimens in the Flora of Myanmar Database were collected between 1999 and 2018. As of 2022, the number of specimens has reached 7227 records. However, more than 20, 000 specimens at MAND remain undigitized. Due to issues associated with data sharing and transparency, determining how many specimens remain undigitized in MAND is difficult and further work is needed to make these specimens information available.
4.2. RepresentativenessAscertaining true species distribution representation is challenging, because higher sampled regions are more likely to document more species as a product of sampling efforts, and no checklists exist to cross-reference completeness. The spatial pattern of species diversity may be influenced by the total number of specimens. The Irrawaddy moist deciduous forests ecoregion was a well-sampled area, possessing 29% of Myanmar's total specimens records. Moderate specimen collection records (between 4% and 5% specimens recorded on average) were found in Mizoram-Manipur-Kachin rainforests and Irrawaddy freshwater swamp forests. Conversely, under-sampling was most prominent in the Nujiang Langcang Gorge alpine conifer and mixed forests (0.4% specimens), Eastern Himalayan alpine shrub and meadows (0.9% specimens), and Tenasserim-South Thailand semi-evergreen rainforests (1.2% specimens) (Fig. S7).
RAF and MAND are located in the Irrawaddy moist deciduous forests ecoregion, the most densely recorded specimen ecoregion in Myanmar as our study show. Due to a lack of research funding and other constraints, majority of students from MAND and RAF concentrate taxonomic research activities in the Irrawaddy moist deciduous forests ecoregion (Chan and Than, 2016), likely due to the accessibility and well-developed transportation infrastructure. RAF is also a forest research institute herbarium under the Forestry Department's umbrella. The Forestry Department generates many international collaborations since the highly diverse forests are well-protected. Collaborators agreed a collection of herbarium samples should be sent to RAF and as a result, RAF's collection is more updated than other herbaria.
Northern Myanmar ecoregions are smaller than the Irrawaddy moist deciduous forests ecoregion, but had a higher number of specimen records, despite being under-sampled. The distribution of Myanmar's specimen record was also uneven in different states and regions. Mandalay Region had the highest number of specimens records in Myanmar (22, 160 specimens), with over half of the territory falling inside the Irrawaddy moist deciduous forest ecoregion (Table S4). The smallest state in Myanmar, Kayah State had the lowest number of specimen records (310 specimens) and is entirely covered by the Kayah-Karen montane rainforest ecoregion. Due to the historic and contemporary political issues of the world's longest-running armed conflict (spanning almost 70 years), no research is allowed in the region (Miliband, 2016). Despite having more plant species than Mandalay Region (4402 species), Kachin State (5417 species) in Northern Myanmar had a lower number of specimen records (Fig. S8). Mandalay region's ease of access may cause the resulting duplicate records and recordings of common species. However, Kachin State is relatively remote and known for its species diversity.
Despite the fact no correlation between PFEs and collection density was found in our study, PFEs still play an important role in plant collecting because nature reserves tend to attract the attention of botanists (Parnell et al., 2003; Reddy and Dávalos, 2003). Sampling efforts were greater at low elevations than at higher elevations likely due to the potential of increased accessibility. Similar trends about the relationship between elevation and sampling effort have been observed in previous studies (Hughes et al., 2021). Our study showed a positive relationship between road proximity and specimen density. Human population and the density of specimens further underscores the need for more comprehensive collection. Based on the species diversity, other explanations for specimen density can be provided. For example, in our analysis, we determined that the western side of Mount Victoria has a higher specimen density than the eastern side. Furthermore, detailed work on Myanmars fauna is needed, but until we fill the spatial gaps, and develop checklists with species occurrences at an administrative level, we cannot conduct higher resolution analyses on species distribution patterns.
4.3. Species latitudinal range sizes and range limitsThe minimum and maximum latitudinal distribution across different species subsets was not homogeneous. Gymnosperms, pteridophytes, and bryophytes share similar minimum and maximum in latitude gradients at 21°N, 26°N and 28°N, respectively. Due to abiotic heterogeneity impacts on species coexistence, different species respond to environments in different ways (Kraft et al., 2015). At latitudes 26°N and 12°N, the minimum and maximum latitudinal zone of angiosperms range patterns were significantly different from the other three groups. The latitudinal gradient of species' range-patterns increased along Myanmar's southern border. Mountain ranges in this ecoregion extend from Himalaya in Nepal to Mt. Hkhakabo Rhazi in Myanmar (5881 m), hosting over 7000 plant species (Yi et al., 2005). Species are more likely to be observed in easily accessible areas, resulting in limited occurrence data for remote areas (Hughes et al., 2021).
Results suggest areas with the highest species richness were predominantly found in temperate rainforest areas with high potential for pteridophyte diversity in Kachin, Chin Hills, and Shan. The minimum and maximum latitude gradient of bryophyte species boundaries were found at 21°N at and 28°N. Bryophyte species richness was especially concentrated in the Kachin montane temperate broadleaf forest, Kachin warm temperate rainforest, Western Shan semi-evergreen forest, and Shan warm temperate rainforest. Past research has well established that species richness increases from higher to lower latitudes (Ekman, 1967). The resulting variation in latitudinal peaks from our dataset may indicate significant ecophysiological and sampling limitations. Angiosperm range boundaries declined significantly above latitude 25°N. Gymnosperm species latitudinal ranges (15°N–19°N) were marginally lower. Pteridophyte range boundaries were minimally found between latitude 10°N and 14°N. Similarly, only a small area of bryophytes latitudinal range existed between 10°N and 14°N and 24°N and 26°N.
Most protected areas have flora and fauna checklists, but the lists are often incomplete and in need of updating. We suggest MAND that should prioritize the species digitization process. MAND houses the largest herbarium in Myanmar and the exact number of specimens is unknown. In addition, university theses and local floral book digitization are also needed to record Myanmar's current floristic status. Taxonomic and geographical gaps in accessible data for species occurrence in Asia should adhere to the Global Biodiversity Information Facility program. Institutions and universities in Myanmar lack funding and equipment necessary for digitization and funding may help increase capacity and coverage. Thirteen projects involving institutions from eleven countries and areas in Asia have recently received funding from the Biodiversity Information Fund for Asia (GBIF: https://www.gbif.org), though this has not yet included projects in Myanmar and more work is needed to make the countries specimens accessible. The grants aim to digitize and publish georeferenced information about specimens from Asian collections. The golden age of exploring biodiversity is beginning at the same time biodiversity is most at risk. Further work is needed to fill knowledge gaps and inform more targeted protection measures (Webb et al., 2010).
5. ConclusionThis study described current knowledge of plant biodiversity across Myanmar and informed conservation policy with important species data. More extensive inventories and surveys for gymnosperms, pteridophytes, and bryophytes are needed, especially in poorly explored ecoregions like Kayah-Karen montane rainforests, and Nujiang Langcang Gorge-alpine conifer and mixed forests. We suggest international research collaboration for the expansion and enhancement of specimen digitization. Many floristic inventories have already been collected from Myanmar by other countries such as China, Germany, Korea, Japan, the United Kingdom, and the United States. Collaboration could facilitate the further collection of specimens and accurate species identification. Such data would enable more representative and comprehensive conservation planning in Myanmar, and a basis for the national inventory essential to National Strategic Biodiversity and Action Plans. In the future, increasing inventories in nature reserves will provide the baseline data needed to map biodiversity. Current biodiversity databases are restrained in biodiversity research utility because they are filled with biases, gaps, and uncertainties. Prioritizing efforts to obtain additional data and improve the quality of data already collected is essential. For the benefit of future investigations on the spatial distribution of species in Myanmar, we strongly recommend the digitization and sharing of specimens from local herbariums, museums, universities, botanical gardens, institutions, and private collectors.
AcknowledgementsThis study was supported the Professional Association of the Alliance of International Science Organizations (grant number ANSO-PA-2020-10), the Strategic Priority Research Program of the Chinese Academy of Sciences, China (grant number XDA19050404). We also want to acknowledge: at Yale University, Elizabeth Tokarz, for practical comments and suggestions on the revision of the manuscript. In Myanmar, Dr. Thaung Naing Oo, Dr. Mu Aung, Mr. U Aung Zaw Moe and Ms. Daw Myint San for their help in specimen digitization and data collection; Dr. Bo Sann for Statistics and R Support.
Author contributions
K.-P.M. framed the study. T.S.A and A.C.H. conducted the analysis. All authors discussed and interpreted the results. A.C.H. and T.S.A. wrote the manuscript with support from all authors.
Declaration of competing interest
No conflict of interest exits with the submission of this manuscript, and it is approved by all authors for publication.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2023.01.008.
Aung, L.L., Zin, E.E., Theingi, P., et al., 2017. Myanmar climate report. Dep. Meteorol. Hydrol. Minist. Transp. Commun. Gov. Myanmar.. |
Aye, W., Clerk, U.D., 2007. Country report of Myanmar. Experts' Meeting on Competence. Republic of the Union of Myanmar, Nay Pyi Taw.
|
Ballesteros-Mejia, L., Kitching, I.J., Jetz, W., et al., 2013. Mapping the biodiversity of tropical insects: species richness and inventory completeness of African sphingid moths. Global Ecol. Biogeogr., 22: 586-595. DOI:10.1111/geb.12039 |
Beffasti, L., Galanti, V., 2011. Myanmar Protected Areas: Context, Current Status, and Challenges. Instituto Oikos Ancora Libri, Milan, pp. 1-86.
|
Boyle, B., Hopkins, N., Lu, Z., et al., 2013. The taxonomic name resolution service: an online tool for automated standardization of plant names. BMC Bioinformatics, 14: 1-15. DOI:10.1090/noti1040 |
Chan, N., Than, Y.M.M., 2016. Review on Research Papers Presented at FRI's Research Congresses. Republic of the Union of Myanmar, Nay Pyi Taw.
|
Dauby, G., Zaiss, R., Blach-Overgaard, A., et al., 2016. RAINBIO: a mega-database of tropical African vascular plants distributions. PhytoKeys, 74: 1-18. DOI:10.3897/phytokeys.74.9723 |
Ekman, S., 1967. Zoogeography of the Sea. Sidgwick & Jackson.
|
ESRI, 2011. ArcGIS Desktop: Release 10. Environ. Syst. Res. Institute, CA.
|
He, Y., Zhuang, H., Wang, Y., 2018. A data set of plant diversity in Myanmar. China Scientific Data, 3. DOI:10.11922/csdata.2017.23.zh |
Holmgren, P.K., Holmgren, N.H., 1991. Index Herbariorum. Taxon: 687-692. DOI:10.1002/j.1996-8175.1991.tb01215.x |
Hughes, A.C., Orr, M.C., Ma, K., et al., 2021. Sampling biases shape our view of the natural world. Ecography, 44: 1259-1269. DOI:10.1111/ecog.05926 |
Kalwij, J.M., 2012. Review of 'The Plant List, a working list of all plant species'. J. Veg. Sci., 23: 998-1002. DOI:10.1111/j.1654-1103.2012.01407.x |
Kraft, N.J.B., Adler, P.B., Godoy, O., et al., 2015. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol., 29: 592-599. DOI:10.1111/1365-2435.12345 |
Mie Sein, Z.M., Ullah, I., Saleem, F., et al., 2021. Interdecadal variability in Myanmar rainfall in the monsoon season (May–October) using eigen methods. Water, 13: 729. DOI:10.3390/w13050729 |
Chao, A., Ma, K.H., Hsieh, T.C., 2016. User's Guide for iNEXT Online: Software for Interpolation and Extrapolation of Species Diversity. Institute of Statistics. http://chao.stat.nthu.edu.tw/wordpress/software_download.
|
Miliband, D., 2016. How to Bring Peace to the World's Longest Civil War Time, United Kingdom, London.
|
Ministry of National Planning and Economic Development, 2013. Millennium Development Goals Report. Republic of the Union of Myanmar. Nay Pyi Taw.
|
Murray, N.J., Keith, D.A., Tizard, R., et al., 2020. Threatened Ecosystems of Myanmar. An IUCN Red List of Ecosystems Assessment, Version 1.0.
|
Myint, Y.Y., Sasaki, N., Datta, A., et al., 2021. Management of plantation forests for bioenergy generation, timber production, carbon emission reductions, and removals. Clean. Environ. Syst., 2: 100029. DOI:10.1016/j.cesys.2021.100029 |
Ormsby, T., Napoleon, E., Burke, R., et al., 2010. Getting to Know ArcGIS Desktop. Citeseer.
|
Parnell, J.A.N., Simpson, D.A., Moat, J., et al., 2003. Plant collecting spread and densities: their potential impact on biogeographical studies in Thailand. J. Biogeogr., 30: 193-209. DOI:10.1046/j.1365-2699.2003.00828.x |
Peel, M.C., Finlayson, B.L., McMahon, T.A., 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci., 11: 1633-1644. DOI:10.5194/hess-11-1633-2007 |
PPG I, 2016. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol., 54: 563-603. DOI:10.1111/jse.12229 |
Reddy, S., Dávalos, L.M., 2003. Geographical sampling bias and its implications for conservation priorities in Africa. J. Biogeogr., 30: 1719-1727. DOI:10.1046/j.1365-2699.2003.00946.x |
R Core Team, 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
|
Roskov, Y., Kunze, T., Paglinawan, L., et al., 2013. Species 2000 & ITIS Catalogue of Life, 2013 Annual Checklist.
|
Saatchi, S.S., Harris, N.L., Brown, S., et al., 2011. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. U.S.A., 108: 9899-9904. DOI:10.1073/pnas.1019576108 |
Serra-Diaz, J.M., Enquist, B.J., Maitner, B., et al., 2017. Big data of tree species distributions: how big and how good?. For. Ecosyst., 4: 1-12. DOI:10.1186/s40663-016-0088-1 |
Stamp, L.D., 1925. The Vegetation of Burma: from an Ecological Standpoint. University of Rangoon.
|
Stropp, J., Ladle, R.J., Malhado, A.C., et al., 2016. Mapping ignorance: 300 years of collecting flowering plants in Africa. Global Ecol. Biogeogr., 25: 1085-1096. DOI:10.1111/geb.12468 |
Tanaka, N., 2005. Plant inventory research: contributions to the flora of Myanmar. Acta Phytotax. Geobot., 56: 21-26. |
Wang, S., Zhou, Y., Musili, P.M., et al., 2020. Inventory incompleteness and collecting priority on the plant diversity in tropical East Africa. Biol. Conserv., 241: 108313. DOI:10.1016/j.biocon.2019.108313 |
Webb, C.O., Slik, J.W., Triono, T., 2010. Biodiversity inventory and informatics in Southeast Asia. Biodivers. Conserv., 19: 955-972. DOI:10.1007/s10531-010-9817-x |
Yang, Y., Ferguson, D.K., Liu, B., et al., 2022. Recent advances on phylogenomics of gymnosperms and an updated classification. Plant Divers., 44: 340-350. |
Yang, W., Ma, K., Kreft, H., 2013. Geographical sampling bias in a large distributional database and its effects on species richness–environment models. J. Biogeogr., 40: 1415-1426. DOI:10.1111/jbi.12108 |
Yi, Y.W., Cilia, L., Yin, Y.M., 2005. Characteristic Flora and Fauna of the Kachin State, Northern Myanmar. Myan. Acad. Arts & Sc. Vol. Ⅲ. No. 4(ⅰ).
|
Zuur, A.F., Leno, E.N., Walker, N., et al., 2009. Zero-truncated and zero-inflated models for count data. In: Mixed effects models and extensions in ecology with R. Statistics for Biology and Health. Springer, New York, pp. 261-293. https://doi.org/10.1007/978-0-387-87458-6_11.
|



