b. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, PR China;
c. School of Environment, Earth and Ecosystem Sciences, The Open University, Milton Keynes, MK7 6AA, UK;
d. Centre of Advanced Study, Department of Botany, University of Calcutta, 35, B.C. Road, Kolkata 700019, India
'Siwalik' sediments comprise a thick (about 7000 m) succession of Neogene predominantly freshwater coarsely bedded sandstone, siltstone, clay, and conglomeratic molassic deposits exposed along the length of the Himalayan foothills from the Potwar Plateau of Pakistan in the west to Assam in the east (Parkash et al., 1980; Bora and Shukla, 2005; Chakrabarti, 2016). They were deposited in a variety of fluvial environments, including piedmonts, outwash plains, channels, floodplains, and oxbow lakes, although some record marine influence (Taral et al., 2019; Debnath et al., 2021). They have accumulated close to sea level in a long but narrow foredeep to the south of the rising Himalayas since the middle Miocene time (Bora and Shukla, 2005; Chakrabarti, 2016). The Siwalik succession is generally subdivided into three subgroups, namely the lower, middle, and upper Siwaliks, and their ages are assigned to middle Miocene, early Pliocene–late Pliocene, and late Pliocene–early Pleistocene respectively (Pilgrim, 1910, 1913; Johnson et al., 1985; Ranga Rao et al., 1988; Valdiya, 2002). During the latest phase of the rise of the Himalayas, in Pleistocene to recent times, 'Siwalik' sediments were uplifted, folded, and faulted to form a continuous mountain range of relatively low height ranging from 1000–1200 m a.s.l., 2400 km in length and 20–25 km in width. From west to east along their length the Siwaliks have been divided into seven sectors: Jammu, Himachal, Uttarakhand, Nepal, Darjeeling, Bhutan, and the Southeastern Himalaya (Fig. 1; Karunakaran and Ranga Rao, 1976; Ranga Rao et al., 1979). Compared to their outcrops in the western and central Himalayas, the eastern Himalayan Siwaliks occur as a thinner and discontinuous belt. The Darjeeling, Bhutan, and Southeastern Himalaya Siwalik sectors of the eastern Himalayas are the focus of the present review.

|
| Fig. 1 Maps showing the seven sectors of the Siwalik belt (modified after Karunakaran and Ranga Rao, 1976) showing the locations of the present study areas. |
Although a rich vertebrate fauna has been reported from eastern Himalayan Siwalik sediments (Medlicott, 1865; Pilgrim, 1910, 1913; Singh, 1975, 1983; Acharyya et al., 1987), which has helped in establishing the stratigraphy, classification, and depositional environments of the Siwalik Group of rocks, comparatively little systematic work has been carried out on the plant fossils entombed in these beds. Here, we explore the floral composition within the different units of the Siwalik succession in the eastern Himalayan sectors and trace how the climate and floras have changed through time from the middle Miocene through the Pleistocene to the present using a common proxy framework that allows direct climatic comparison between fossil sites free of artefacts that might otherwise be introduced by using a range of proxies differently calibrated.
The Siwalik floras offer considerable potential for studies of Neogene vegetation and climate change, including monsoon signatures, elevation changes within the Himalayan and Tibetan region, and plant biogeography since the middle Miocene (Prasad, 2008; Khan et al., 2014a, 2019a). The recovered fossil floras from the different eastern Siwalik sectors comprise mainly leaves of woody dicot angiosperms (Mehrotra et al., 1999; Prasad et al., 1999, 2004; Prasad and Tripathi, 2000; Prasad, 2008; Khan et al., 2014a; Srivastava et al., 2018). Here, we review fossil floras recovered from the Darjeeling, Bhutan, and Southeastern Himalaya Siwalik sectors of the eastern Himalayas and reconstruct the climate by applying a new common proxy and calibration to all the currently available eastern Siwalik fossil floras. The use of a common proxy with the same calibration has been lacking thus far as different authors tend to apply different methodologies and the methodologies themselves have evolved over time. This mix of approaches means detection and quantification of subtle changes in climate over time is hard to achieve and ambiguous at best. By using a common analytical framework, we provide insights into the monsoon evolution during Siwalik depositional period in the eastern Himalayas.
In the present assessment, we compile all palaeobotanical plant data from published literature to document plant diversity in the eastern Himalayas throughout the Siwalik succession. We consider only fossil taxa represented by megafossils (leaves, wood, fruits, fruiting calyx, and seeds). Potential megafossils, especially leaves, cannot travel far from their point of origin before fossilization and remain identifiable (Ferguson, 1985; Spicer and Wolfe, 1987; Spicer, 1991) and are destroyed when the sediments hosting them are reworked. Pollen and spores (microfossils), however, can travel long distances up and down the slope prior to final burial and still appear pristine, and can be reworked many times. Moreover, they often cannot be identified to a fine enough taxonomic resolution using only a light microscope (Ferguson et al., 2007). By focusing on megafossils we ensure that we are reconstructing vegetation local to the fossil site, and so potentially are able to detect fine-scale changes in space and time.
We first briefly introduce the present-day floristic composition in the eastern Himalayas, then present known fossil families, genera, and species to depict the diversity of Siwalik plants and ecosystems, and then analyze the inferred floristic characters and changes. We also summarize the Siwalik climate evolution in the eastern Himalayas. The present review aims to facilitate access to the rich Siwalik palaeobotanical record of the eastern Himalayas. We also re-examine the eastern Himalayan Siwalik Miocene-Pleistocene climate and introduce new quantitative proxy palaeo-humidity measurements in order to characterize better the eastern Himalayan environment during a critical time of Himalayan uplift and associated evolution (Ding et al., 2017; Bhatia et al., 2021, 2022).
2. Geological settingPilgrim (1913) divided the Siwalik succession into three units, namely the lower (middle Miocene), middle (late Miocene to early Pliocene), and upper (late Pliocene to early Pleistocene). The Siwalik sediments are characterized by alternating sandstone and mudstone facies, with the finer sediments very often containing abundant biota (Chakrabarti, 2016). In general Siwalik sediments exhibit a coarsening trend over time, but even the youngest units contain fine-grained beds that are fossiliferous. Here, we represent a generalized lithostratigraphy of the Siwalik sediments exposed in the eastern Himalayas (Table 1; Figs. 1 and 2). In the Darjeeling area, this is based on Ganguly and Rao (1970), Acharya (1994), and Taral et al. (2017); in Bhutan, we refer to Coutand et al. (2016), while in the Southeastern Himalaya we rely on Anand-Prakesh and Singh (2000) and Singh (2007). The ages of the sedimentary succession in different Siwalik sectors of the eastern Himalayas have been quantified by means of magnetostratigraphy (Chirouze et al., 2012; Coutand et al., 2016). Magnetostratigraphic correlations indicate that the Bhutan Siwalik Group was deposited during the latest Miocene and the Pleistocene, between ~7 million years ago (Ma) and ~1 Ma, and that the boundary between lower and middle Siwaliks can be dated to ~6 Ma, the middle to upper Siwalik to 3.8 Ma, and the top of the section as ~1 Ma (Coutand et al., 2016). In the Southeastern Himalaya, the Siwalik Group was deposited between 13 and 2.5 Ma, with the transition between the lower and middle Siwaliks dated at about 10.5 Ma and the middle to upper Siwalik transition at 2.6 Ma (Chirouze et al., 2012). The boundary age differences exhibited among the different parts of the eastern Himalayas could be due to temporal and locational changes in the various loci of deposition as well as local erosion events.
| Group | Sub-group | Generalized Siwalik Lithology | Age | Formation | |||||
| Darjeeling | Bhutan | Southeastern Himalaya | Darjeeling | Bhutan | Southeastern Himalaya | ||||
| SIWALIK | Upper Siwalik | Loosely packed, friable very course-grained grey sandstones with high limonitisation in places and intercalated with claystones and shales. Frequent boulder beds with a sandy matrix also occur in this formation. Remains of wood, leaves and fruits have been recorded | Pliocene | Pliocene–Pleistocene | Late Pliocene–early Pleistocene | Murti boulder bed | Formation Ⅲ | Kimin Formation | |
| Middle Siwalik | Generally weakly indurated, medium to coarse-grained sandstones with salt and pepper texture. Calcareous concretions of various shapes and sizes occur in the sandstones, occasionally associated with grey shales with plant fossils | Late Miocene–Pliocene | Late Miocene–Pliocene | Pliocene | Parbu grit Geabdat sandstone | Formation Ⅱ | Subansiri Formation | ||
| Lower Siwalik | Well-indurated medium to fine-grained generally well-sorted sandstones, subordinate micaceous sandstones, bluish nodular silty shale, claystone, and small lenses of coal; plant fossils occur frequently | Middle Miocene | Late Miocene | Late–Middle Miocene | Formation Ⅰ | Dafla Formation | |||
| Gish Clay/Chunabati formation | |||||||||

|
| Fig. 2 Fossiliferous Siwalik exposures of the eastern Himalayas (a: Darjeeling; b: Bhutan; c: Southeastern Himalaya). |
To contextualize megafossil records from the eastern Himalayas, it is useful to consider the rich and diverse modern flora of the region. At elevations similar to those inferred for the source vegetation of the Siwalik fossil assemblages, modern vegetation close to the fossil localities in the eastern Himalayas is characterized as warm humid tropical (Champion and Seth, 1968; Biswas et al., 1976; Grierson and Long, 1983; Kaul and Haridasan, 1987; Hazra et al., 1996; Baishya et al., 2001).
The eastern Himalayas are considered 'crisis ecoregions' and 'biodiversity hotspots' (Brooks et al., 2006). This region is also a meeting ground for the Indo-Malayan, Palearctic, and Sino-Japanese biogeographical realms, and has diverse biota as well as diverse ecological and elevational gradients (CEPF, 2005, 2007). The complex topography and extreme elevational gradients from less than 300 m (tropical lowlands) to more than 8000 m (high mountains) have led to the development of a variety of floristic as well as vegetation patterns. Climate-dependent vegetation is largely determined by decreasing moisture and temperature with increasing elevations (thermal and hygrometric terrestrial lapse rates), which vary through time, affording opportunities for plant migrations, novel juxtapositions, and speciation. The Himalayan range to the north acts as a barrier to the southwest monsoon from the Bay of Bengal, causing the moisture regime to decrease westwards along the Siwaliks, and comparatively more rain is received in the East. The complex mountain topography creates diverse bioclimatic zones, an exceptionally rich biodiversity assemblage, and 'sky' island conditions for many species. Broadly, vegetation in the eastern Himalayas can be categorized into tropical, sub-tropical, warm temperate, cool temperate, sub-alpine, and alpine types in ascending order based on parameters such as physiognomy, floral composition, habitat conditions, and physiography (WWF and ICIMOD 2001).
Takhtajan (1969) regarded the eastern Himalayas as the 'cradle' of flowering plants. The region is also well known for its botanically curious and rare species (e.g., Sapria himalayana Griff., Rafflesiaceae). Nearly 50% of the total flowering plants recorded in India are from the northeastern region. The genus Rhododendron (Ericaceae) is a remarkable taxon of showy plants, with most confined to this region and a substantial number of endemic species (Pradhan and Lachungpa, 1990). The eastern Himalayan region is rich in endemic floras and many species have value as medicinal or edible plants (Sundriyal, 1999).
Several species, namely Shorea robusta C.F. Gaertn., Mesua ferrea L., Syzygium cumini (L.) Skeels, Terminalia paniculata Roth., are dominant in northern tropical wet evergreen forests (150 m altitude); Magnolia L., Terminalia elliptica Willd., and Bombax ceiba L. in northern sub-tropical semi-evergreen forests (150–230 m); Chukrasia tabularis A. Juss., Gmelina arborea Roxb., Rhododendron arboretum Sm., Dalbergia sissoo Roxb. in North India moist deciduous forests (230–300 m); Madhuca longifolia (J. Konig) J.F. Macbr., Gordonia chilaunia, T. elliptica, Gossypium L., Toona ciliata M. Roem. and Cinnamomum glaucescens (Nees) Hand.-Mazz. in Northern sub-tropical broad-leaved wet forests (300–1650 m altitude); Senegalia catechu (L. f.) P.J.H. Hurter & Mabb., Abies densa Griff., Tsuga canadensis Carrière, Acer L. in northern montane wet temperature forests (1650–3000 m); M. longifolia (J. Konig) J.F. Macbr., Schima wallichii (DC.) Korth., Castanopsis indica (Roxb. ex Lindl.) A. DC., T. elliptica, Duabanga grandiflora Walp., Cassia fistula L., Annona muricata L. in east Himalayan moist temperature forests (1500–1800 m) and Ficus religiosa L., Acer, Exbucklandia populnea (R.Br. ex Griff.) R.W.Br., Ceiba pentandra (L.) Gaertn., Prunus undulata Buch. Ham. ex D. Don, Castanopsis (D. Don) Spach, Rhododendron L., Salix L. in sub-alpine forests (3000–3660 m) (Grierson and Long, 1983; Kaul and Haridasan, 1987; Hazra et al., 1996; Baishya et al., 2001).
In and around the fossil localities the principal constituents of tropical moist semi-evergreen to deciduous forests are Pongamia pinnata (L.) Pierre, Bauhinia purpurea L., Albizia sp. L., Dalbergia sisso (Fabaceae); D. grandiflora (D.C.) Walp., Lagerstroemia parviflora Roxb. (Lythraceae); Terminalia catappa L., T. chebula Retz., T. miocarpa F. Muell. (Combretaceae); Litsea sp. Lam., Cinnamomum bejolghota (Buch.-Ham.) Sweet, Actinodaphne angustifolia (Blume) Nees, A. obovata (Nees) Blume, Phoebe goalparensis Hutch. (Lauraceae); Gynocardia odorata R.Br. (Achariaceae); Calophyllum polyanthum Wall. ex Choisy (Calophyllaceae); Bombax malabaricum D.C. (Malvaceae); Macaranga denticulata (Blume) Müll. Arg., Mallotus Lour. (Euphorbiaceae); Knema Lour. (Myristicaceae); Elaeocarpus aristatus Roxb. (Elaeocarpaceae); Shorea robusta (Dipterocarpaceae); Gynocardia arborea Roxb. ex Sm. (Achariaceae); Vitex quinata (Lour.) F.N. Williams (Lamiaceae); Dillenia pentagyna Roxb. (Dilleniaceae); Sterculia villosa Roxb. ex Sm., Grewia eriocarpa Juss., Grewia zizyphifolia Baill. (Malvaceae); Garuga pinnata Roxb. (Burseraceae); Meliosma simplicifolia (Roxb.) Walp. (Sabiaceae); Spondias axillaris Roxb. B.L. Burtt & A.W. Hill (Anacardiaceae).
4. Research history of Palaeobotany in eastern Himalayan SiwalikThe eastern Himalayas hosts many Cenozoic sedimentary basins that have yielded Siwalik deposits that bear micro and mega plant fossils (Chakrobarty et al., 2020). The Lower, Middle, and Upper Siwalik floral archives range from the middle Miocene to the early Pleistocene (Table 1). The first information about the occurrence of plant fossils in the eastern Himalaya Siwaliks dates back to 1969 when Pathak (1969) initially described a few fragmentary angiosperm leaves from the middle Siwalik sediments of the Mahanadi section of Darjeeling. In subsequent years other geologists and paleobotanists also noted the presence of plant remains in the Siwalik sediments of the eastern Himalayas (Antal and Awasthi, 1993; Antal and Prasad, 1995, 1996a, b, c, 1997, 1998). During the last few decades plant fossil localities have increased and valuable contributions have been made to the knowledge of the Siwalik paleobotany of the eastern Himalayas (Singh and Prakash, 1980; Mehrotra et al., 1999; Prasad and Tripathi, 2000; Joshi and Mehrotra, 2003, 2007; Mitra and Banerjee, 2004; Khan et al., 2007, 2008, 2009, 2011, 2014a, b, c, 2015a, b, 2016, 2017a, b, 2018a, b, 2019a, b; Tripathi et al., 2007; Srivastava and Mehrotra, 2009; Khan and Bera, 2010, 2014a, b, 2016, 2017; Prasad et al., 2015; Mehrotra et al., 2018; More et al., 2018; Srivastava et al., 2018). Rich and varied assemblages of plant megafossil, including leaf impressions, compressions, fruits, seeds, and woods, are now known and provide a sufficient basis for tracking regional environmental changes through time and space. Qualitatively, the present-day distribution of the taxonomically nearest living relatives (NLRs) of the Siwalik plant fossils suggests the existence of a tropical evergreen type of forest throughout the eastern Himalaya lowlands during the period of deposition. In recent years plant-based quantitative techniques (CLAMP - Climate Leaf Analysis Multivariate Program and CoA - Coexistence Approach) have been used to derive past climate parameters (temperature, humidity, and precipitation) and chart monsoon evolution (Khan et al., 2014a, 2019b; Srivastava et al., 2021; Bhatia et al., 2022).
5. Palaeofloristic compositionThis review is based on Siwalik (middle Miocene–early Pleistocene) plant megafossil assemblages recovered from the road and river exposures at different locations within the eastern Himalayas. The fossiliferous localities in the Darjeeling Siwalik sector are those of Oodlabari, Sevok road sections, and the Sevok bridge, Washbari, Gish, Lish, Churanthi, and Ramthi river sections; in Bhutan the Lakshmi and Darranga river sections and in the easternmost Siwalik sector, i.e., Southeastern Himalaya localities, the river cutting sections of the East Kameng district, the Bhalukpong, East Pinjoli and South Pinjoli road cutting sections of the West Kameng district, and the Naharlagun-Banderdewa, Nirjuli-Banderdewa, Itanagar-Banderdewa and Chandernagar-Gohpur road sections of the Papumpare district (Fig. 2). Most leaf impressions were preserved in grey shales. Here, we review 219 plant fossil taxa, of which 102 taxa are from Darjeeling foothills, 9 taxa are from Bhutan and 108 fossil taxa are from the Southeastern Himalaya. Most of the megafossil plant specimens were identified to the species level, but for a few specimens, necessary morphological characters were not preserved. Khan et al. (2015a, 2016, 2017a, b) studied cuticular epidermal features of some compressed leaves from the Southeastern sub-Himalaya and those are the most securely identified specimens.
There have been several contributions describing plant macrofossils from the eastern Himalaya Siwaliks, but so far none has synthesized our knowledge of the past flora and environment throughout Siwalik sedimentation. The recovered megaplant remains include compressions and impressions of leaves, fruiting calyxes, fruits, seeds, petrified and carbonized woods (Figs. 3-5). The Siwalik floral assemblage is rich both in fossil quality and quantity and comprises 219 species belonging to 162 genera and 42 families (Tables 2 and S1–S5). Angiosperms are grouped into 159 genera within 39 families, the most abundant being the Fabaceae (represented by seventeen genera and twenty-four species), Dipterocarpaceae (four genera and thirteen species), Annonaceae (eight genera and eleven species), Lauraceae (seven genera and eleven species), Euphorbiaceae (six genera and seven species), Anacardiaceae (six genera and six species), Flacourtiaceae (four genera and five species), Rubiaceae (four genera and five species), Apocynaceae (four genera and four species) and Meliaceae (three genera and four species) (Tables S4 and S5). The eastern Himalayan Siwalik fossil floras consist of a wide variety of mostly woody plants listed in Tables S1–S3. Arborescent taxa dominate the assemblage, grass, and ferns being in a minority. Additionally, some workers reported in-situ occurrences of characteristic epiphyllous fungi on Siwalik leaf megafossils (Table S6). Reliable identification of the fossils is crucial for reliable palaeoclimatic and palaeoecological interpretation, thus, here some fossil specimens of uncertain affinities have been excluded from the listed fossil floras. The fossil specimens are held in the repository of the Palaeobotany–Palynology Section, Department of Botany, University of Calcutta.

|
| Fig. 3 (a) A fossil leaflet of Dysoxylum miocostulatum Khan and Bera (2007) from Lower Siwalik sediments of Southeastern Himalaya (CUH/PPL/P26) (Scale bar = 1 cm); (b, c) Winged seeds of Pinus arunachalensis Khan and Bera (2017) from Lower Siwalik sediments of Southeastern Himalaya (CUH/PPL/P/f/61a, b) (Scale bar = 1 cm); (d) A fossil leaf Quercus cf. lamellosa Khan et al. (2011) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/46) (Scale bar = 1 cm); (e) Dysoxylum raptiensis Khan et al. (2015a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/17) – abaxial cuticle showing stomata and epidermal cells (Scale bar = 10 μm); (f) Fossil leaf of Shorea mioobtusa Khan et al. (2016) from Lower Siwalik sediments of Southeastern Himalaya (CUH/PPL/P 83) (Scale Bar = 1 cm); (g) Dipterocarpus koilabasensis Khan et al. (2015a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/3) - abaxial cuticle, hyphae with opposite and pointed appressoria of epiphyllous fungi Asterina sp. (Scale bar = 10 μm); (h) Calophylloxylon eoinophyllum Khan et al. (2017a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/W1A) - transverse section (T.S.) showing diffuse vessel distribution and solitary arrangement, and parenchyma bands (Scale bar = 10 μm); (i) Fossil fruit wing of Shorea mioassamica Khan and Bera (2010) from Lower Siwalik sediments of Southeastern Himalaya (CUH/PPL/P14) (Scale bar = 1 cm). |

|
| Fig. 4 (a) A fossil leaf of Actinodaphne palaeoangustifolia Khan et al. (2011) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/40) (Scale bar = 1 cm); (b) A fossil leaf of Calophyllum suraikholaensis Khan et al. (2009) from Middle Siwalik sediments of Southeastern Himalaya (CUH/PPL/B1) (Scale bar = 1 cm); (c) Light micrographs of Gmelina siwalika Khan et al. (2018a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/C3/44) - tangential longitudinal sections of the secondary xylem showing 2–3 seriate ray cells (Scale bar = 50 μm); (d) Gynocardia arunachalensis Khan et al. (2014c) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/f/61a) - fossil seed showing thick, leathery seed coat (Scale bar = 1 cm); (e) Fossil leaf of Glochidion siwalikum Khan et al. (2019a) from Middle Siwalik sediments of Southeastern Himalaya (CUH/PPL/B/64A) (Scale bar = 1 cm); (f) Cyathea siwalika Bera et al. (2014) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/TF/1) - Cyatheoid arrangement of vascular bundles within the leaf scar (scale bar = 1 cm); (g) Calophyllum suraikholaensis Khan et al. (2015a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/19) - lower cuticle showing paracytic stomata and epidermal cells (Scale bar = 10 μm). |

|
| Fig. 5 (a) A fossil leaf of Persea preglaucescens Khan and Bera, 2014b, Khan and Bera, 2014a from Middle Siwalik sediments of Southeastern Himalaya (CUH/PPL/B/19) (Scale bar = 1 cm); (b) A well-preserved wing-like persistent calyx lobe of Shorea bhalukpongensis Khan et al. (2016) from Middle Siwalik sediments of Southeastern Himalaya (CUH/PPL/B/f/19) showing characteristic parallel primary veins (green arrows) (Scale bar = 1 cm); (c) Light micrographs of Meliolinites neogenicus Khan et al. (2019c) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/36/AS1) - Hypha of M. neogenicus showing capitate appressoria with head cells and stalk cells (Scale Bar = 20 μm); (d) Dysoxylum raptiensis Khan et al. (2015a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/17) – SEM of the abaxial cuticle, inner surface, paracytic stomata (Scale bar = 10 μm); (e) Fossil leaf of Shorea Khan et al. (2019b) from Middle Siwalik sediments of Bhutan (CUH/PPL/BH/12A) (Scale bar = 1 cm); (f) Fossil fruit of Dalbergia prelatifolia Khan and Bera, 2014b, Khan and Bera, 2014a from Lower Siwalik sediments of Darjeeling foothill (CUH/PPL/SV/f/1) (Scale bar = 1 cm); (g) Fossil fruit of Elaeocarpus prelancaefolius Bera et al. (2004) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/5/F1) (Scale bar = 1 cm); (h) Zig-zag type leaf mining on the fossil leaf of Terminalia panandhroensis (Lakhanpal and Guleria) Khan et al. (2014b) from Middle Siwalik sediments of Southeastern Himalaya (CUH/PPL/B/54) (Scale bar = 1 cm); (i) Scanning electron micrographs of Gmelina siwalika Khan et al. (2018a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/C3/44) - transverse section of the secondary xylem showing vessel with a prominent tylosis (Scale bar = 50 μm); (j) Dysoxylum raptiensis Khan et al. (2015a) from Upper Siwalik sediments of Southeastern Himalaya (CUH/PPL/IB7/17) - adaxial cuticle with characteristic frass-trail (Scale bar = 10 μm). |
The Lower Siwalik assemblage recovered from sediments exposed near Gish, Ramthi, Oodlabari, Sevok of Darjeeling, East and West Kameng of Southeastern Himalaya comprises mainly angiosperm plant remains attributable to 91 species within 65 genera belonging to 32 families (Table S1; Antal and Awasthi, 1993; Antal et al., 1996; Antal and Prasad, 1996a; Joshi and Mehrotra, 2007; Khan et al., 2008; Srivastava and Mehrotra, 2009; Khan and Bera, 2012, Khan and Bera, 2014a; Khan et al., 2015a, Khan et al., 2015b). In this assemblage, 90 leaf fossils, one dicot fossil wood, one Thelypteridaceae fern, and one gymnosperm taxon have been reported. Of these, 22 species are new to the Siwalik palaeoflora and 19 have been identified as new to the Neogene flora of India. For example, Khan and Bera (2017) described Pinus on the basis of seed remains from the Dafla Formation exposed around the West Kameng district in the Southeastern Himalaya. This report provides the first-ever fossil record of Pinus winged seeds from India. It is obvious from the list of fossil taxa (Table S1) from the lower Siwalik eastern Himalaya assemblages that the family Fabaceae, represented by ten genera (Entada Adans., Dalbergia L. f., Derris Lour., Millettia Wight & Arn., Cynometra L., Bauhinia Plum. ex L., Albizia Durazz., Pongamia Adans., Acacia Mill., and Mastertia L.), is the most dominant, followed by Lauraceae comprising four genera, Flacourtiaceae comprising four genera, Dipterocarpaceae comprising two genera, Annonacaae comprising two genera, Euphorbiaceae comprising of two genera and Combretaceae comprising two genera. The dominance of Fabaceae and the presence of Dipterocarpaceae is very significant from both palaeoecological and phytogeographical contexts.
5.2. The Middle Siwalik flora (late Miocene to Pliocene)The Middle Siwalik assemblages recovered from sediments exposed from the fossil localities of Bhutan, Darjeeling, and Southeastern Himalaya represent mainly angiosperm plant remains currently comprising 81 species of 56 genera within 29 families (Table S2). Assignments are based mainly on leaf impressions. In this assemblage, 67 leaf fossils, four dicot fossil wood specimens, and one Thelypteridaceae fern have been described. They show closed affinity with extant thermophilic taxa such as Mitrephora Hook. f. & Thomson, Dipterocarpus C.F. Gaertn., Combretum Loef l., Millettia, Donax Lour. (Clinogyne grandis accepted name Donax canniformis), Shorea, Meiogyne Miq., Fissistigma Griff., Gynocardia R. Br., Vatica L., and Garcinia L. (Antal and Prasad, 1996a; Mehrotra et al., 1999; Prasad and Tripathi 2000; Tripathi et al., 2007; Prasad et al., 2015; Khan et al., 2016; 2017a, Khan et al., 2019a, Khan et al., 2019b, Khan et al., 2019c). Of these, 17 species are new to the Siwalik flora and 15 have been identified as new to the Neogene flora of India.
Dipterocarpaceae, represented by 13 genera, is most dominant in the assemblage, followed by Annonaceae comprising four genera, Lauraceae comprising four genera, Sterculiaceae comprising two genera, and Calophyllaceae comprising two genera.
5.3. The Upper Siwalik flora (late Pliocene to early Pleistocene)The upper part of the Siwalik assemblage recovered from sediments exposed near Papumpare, East and West Kameng of Southeastern Himalaya is mainly represented by dicots comprising 47 species of 31 genera belonging to 21 families (Table S3). Assignments are based on both leaf impressions and compressions. An exception is that part of a compressed tree fern axis with leaf and adventitious root scars in the unusual arrangement has been described from the Plio-Pleistocene sediments of Southeastern Himalaya (Bera et al., 2014). This was the first macroscopic record of a cyatheaceous fern from the Indian Cenozoic. Other specimens show affinity with extant angiosperm taxa such as Dipterocarpus, Calophyllum L., Actinodaphne Nees, Shorea, Mastixia Blume, Gynocardia, Millettia, Knema Lour., Macaranga Thouars, Canarium L., Quercus L., Terminalia L., Croton L., Gmelina L., Kayea Wall., Elaeocarpus L., and Pongamia (Bera et al., 2004; Joshi et al., 2003; Joshi and Mehrotra, 2007; Khan et al., 2011, Khan et al., 2015a, Khan et al., 2015b, 2016, 2017a, b; Khan and Bera, 2014a; Srivastava et al., 2018; Mehrotra et al., 2018). Lauraceae, represented by four genera, is the most dominant in this assemblage, followed by Fabaceae and Calophyllaceae represented by two genera.
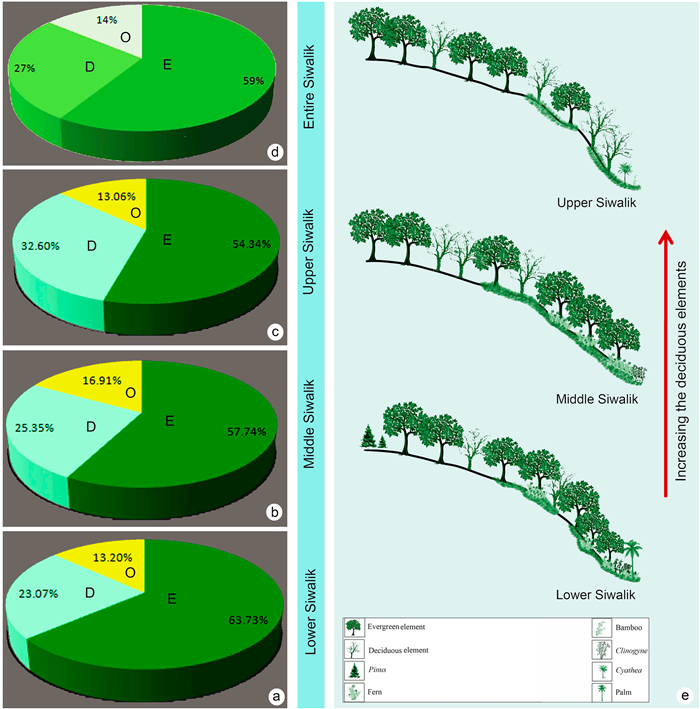
6. Floristic changes throughout the Siwalik successionThe nearest living relatives (NLR) method extrapolates the known climatic requirements of modern taxa back to comparable and related taxa in the past and presupposes that fossil plants and their modern relatives share similar physiological requirements for climate (Mosbrugger and Utescher, 1997). The megafossil assemblages recovered from the Siwalik sediments of the eastern Himalayas have yielded mainly angiosperm taxa (Tables S1–S3) that can be used effectively to interpret palaeoclimate and palaeoflora because the nearest living relatives of these fossil angiosperm taxa are known with high confidence. On the basis of NLRs, the Siwalik floral assemblages of the eastern Himalayas consist of three major forest elements: evergreen (58.60%), deciduous (26.82%), and others (14.39%) (Fig. 6a-d). In the eastern Himalayan Lower Siwalik assemblage 63.73% of the taxa are evergreen, and deciduous elements make up just 23.07% of the taxa (Fig. 6a). In the Middle Siwalik assemblage, 57.74% of the taxa are evergreen and 25.35% of taxa are deciduous (Fig. 6b). In the Upper Siwalik 54.34% and 32.60% are evergreen and deciduous respectively (Fig. 6c). With respect to the present-day distribution pattern of NLR taxa this suggests that wet evergreen forests persisted throughout the period of deposition (Tables S1–3; Fig. 6d). The predominance of evergreen elements in the assemblage along with pteridophytes (ferns) indicates the prevalence of a tropical, warm, humid climate with abundant rainfall in contrast to the relatively dry present-day climate in the area. An increase in deciduous elements is evident towards the close of the Middle Siwalik and the beginning of the Upper Siwalik (Fig. 6e). This change in the vegetation pattern must reflect a climatic change between the lower part (Miocene) and upper part of Siwalik (Plio-Pleistocene) deposition, and seems to indicate an increase in rainfall seasonality. We reconstruct the paleovegetation to better understand the general evolutionary history of floristic patterns in eastern Himalaya during Siwalik sedimentation (Fig. 7).
|
| Fig. 6 (a) Diagrammatic representation of different types of forest elements of the lower Siwalik flora of the eastern Himalayas (E = Evergreen, D = Deciduous, O = Others); (b) Diagrammatic representation of different types of forest elements of middle Siwalik flora of the eastern Himalayas (E = Evergreen, D = Deciduous, O = Others); (c) Diagrammatic representation of different types of forest elements of upper Siwalik flora of the eastern Himalayas (E = Evergreen, D = Deciduous, O = Others); (d) Diagrammatic representation of different types of forest elements of entire Siwalik flora of the eastern Himalayas (E = Evergreen, D = Deciduous, O = Others); (e) Schematic sketch of the floristic pattern changes throughout the Siwalik sediments in the eastern Himalayas. |

|
| Fig. 7 Reconstruction of the palaeovegetation during Siwalik sedimentation of the eastern Himalayas. |
Investigations using different qualitative (NLR, cuticular studies) and quantitative (Co-existence Approach and CLAMP) proxies have revealed the history of past climate, and in particular the evolution of Indian summer monsoon (ISM), during the Siwalik sediments of the eastern Himalayas (Khan and Bera, 2014a, b; Khan et al., 2014a, 2015, 2019a, b; Prasad et al., 2015; Srivastava et al., 2021; Bhatia et al., 2022).
7.1. A qualitative NLR approachThe principal basis of any study of the past is that known as 'uniformitarianism'. This, principle, often summarized as 'the present is the key to the past', implies that the physical and biological processes that operate in today's environment, as well as vegetation, must have functioned in a similar way in the past (Thanukos, 2012). In the case of the NLR approaches used to reconstruct past climates, this extrapolates the known climatic requirements of modern taxa to presumed ancestral taxa in the past. Of the plant fossils recovered from the eastern Himalayas that have nearest living relatives, several still exist in the area today. This suggests some degree of climatic similarity between the past and now and the persistence of a tropical warm and humid climate.
7.2. The coexistent approach (CoA)CoA, developed by Mosbrugger and Utescher (1997), is based on the concept that the climatic requirements of fossil species are similar to those of their NLRs and reconstructs the paleoclimate parameters for a given fossil flora using climatic intervals in which all the NLRs of the fossil flora could coexist coexist (Mosbrugger and Utescher, 1997; Mosbrugger, 1999; Utescher et al., 2014). This is an improvement from many previous NLR analyses that have tended to choose single, or at best, just a few taxa for the analysis. By adopting a whole-population approach, outliers due to misidentification or evolutionary innovation can be isolated and removed from the analysis, thus, improving accuracy and precision. Prasad et al. (2015) first reconstructed the eastern Siwalik palaeoclimate by applying this quantitative method to the middle Siwalik flora of Darjeeling sub-Himalaya. They estimated different climatic variables, such as mean annual temperature (MAT), warmest month mean temperature (WMMT), coldest month mean temperature (CMMT), and mean annual precipitation (MAP) as 22–26.5 ℃, 17.8–20 ℃, 25–30 ℃, and 2650–3200 mm, respectively. However, their methodology differed from that codified by Mosbrugger and Utescher (1997) and Utescher et al. (2014) and did not specify the origins of their plant distribution data. Srivastava et al. (2021) subsequently reconstructed the climate of the Upper Siwalik strata of Southeastern Himalaya quantitatively, based on the more usual form of CoA specified by Mosbrugger and Utescher (1997) and Utescher et al. (2014), and reported that during the late Pliocene–early Pleistocene, the temperature seasonality between warm (27–28.1 ℃) and cold months (22–23.6 ℃) was less pronounced compared with present-day warm (27–27.7 ℃) and cold (14.8–15.4 ℃) month conditions. The reconstructed rainfall data indicated a monsoonal type of climate having a strong wet/dry seasonality during the deposition of the Upper Siwalik sediments. Recently, Bhatia et al. (2022) also applied this methodology to two Siwalik floras recovered from the Lower (middle Miocene) and Middle (late Miocene–Pliocene) Siwalik successions of Darjeeling in the eastern Himalayas. The reconstructed climate data suggested a decrease in both winter temperature and precipitation during the wettest months, and thus an overall drying, from the Lower to Middle part of the Siwalik succession.
7.3. The CLAMP approachThe principal leaf-based palaeoclimate proxy for assessing a range of climate variables is known as CLAMP (Climate Leaf Analysis Multivariate Program; http://clamp.ibcas.ac.cn) (Wolfe, 1993; Kovach and Spicer, 1996; Yang et al., 2011, 2015). CLAMP utilizes the universal relationships that exist between leaf form in woody dicotyledonous plants and an array of climate variables. On a global scale, aggregate leaf form in a stand of vegetation is more strongly determined by climate than by taxonomic composition (Yang et al., 2015). Using a multivariate statistical engine, CLAMP decodes these relationships and, by scoring fossil leaf traits the same way as for living vegetation growing under known climatic regimes, estimates past conditions (http://clamp.ibcas.ac.cn). Five fossil floras (one lower Siwalik mid-Miocene, one middle Siwalik Pliocene, and one upper Siwalik Plio–Pleistocene flora of Southeastern Himalaya; one lower Siwalik mid-Miocene flora of Darjeeling and one latest Miocene-Pliocene middle Siwalik Group of Bhutan sub-Himalaya) ranging in age from the mid-Miocene to the early Pleistocene from the eastern Siwalik near Bhutan, Darjeeling and in Southeastern Himalaya were also subjected to a CLAMP analysis using a calibration data set that includes sites from India, southern China, and Thailand and gridded climate data (Khan et al., 2014a, 2019b) (Tables S7–S11). CLAMP climate retrodictions derived from the PhysgAsia2 calibration for all the fossil sites of the eastern Himalayas are given in Tables 3-5.
| FERNS | Homonoia mioriparia Antal and Prasad (L; D) |
| Thelypterideaceae | Macaranga denticulate Khan et al. (L; SEH) |
| Thelypteridaceophyllum tertiarum (Joshi and Mehrotra) Khan et al. (L; SEH) | M. siwalika Antal and Awasthi (L; D) |
| Mallotus kalimpongensis Antal and Awasthi (L; D) | |
| Cyatheaceae | Fabaceae |
| Cyathea Siwalika Bera et al. (L; SEH) | Acacia miocatechuoides Khan and Bera (F; SEH) |
| GYMNOSPERM | Albizia palaeolebbek Antal and Awasthi (L; D) |
| Pinaceae | Albizinium arunachalensis Mehrotra et al. (W; D) |
| Pinus daflaensis Khan and Bera (L; SEH) | Bauhinia ramthiensis Antal and Awasthi (L; D) |
| ANGIOSPERMS | Bauhinium palaeomalabaricum Antal et al. (L; D) |
| Monocots | B. siwalika Khan et al. (L; SEH) |
| Marantaceae | Callerya precinerea Khan et al. (L; SEH) |
| Clinogyne ovatus Antal and Prasad (L; D) | Cassinium borooahii Mehrotra et al. (L; SEH) |
| C. lishensis Antal and Prasad (L; D) | Cynometra palaeoiripa Prasad et al. (L; D) |
| Poaceae | C. tertiara Antal and Awasthi (L; D) |
| Bambusa sp. Antal and Awasthi (L; D) | Cynometroxylon sp. cf. C. holdenii Mehrotra et al. (W; D) |
| B. siwalika (Joshi and Mehrotra) Khan et al. (L; SEH) | C. holdenii Mehrotra et al. (W; D) |
| Arecaceae | Dalbergia prelatifolia Khan and Bera (F; D) |
| Amesoneuron Joshi and Mehrotra (L; SEH) | D. rimosa Khan et al. (L; SEH) |
| ANGIOSPERMS | Derrisocarpon miocenicum Mitra and Banerjee (F; D) |
| Dicots | Derrisophyllum siwalicum Mitra and Banerjee (L; D) |
| Achariaceae | Entada palaeoscandens Antal and Awasthi (F; D) |
| Gynocardia butwalensis Prasad et al. (L; D) | Milletia extensa Khan et al. (L; SEH) |
| G. arunachalensis Khan et al. (L; SEH) | M. koilabasensis (Prasad and Tripathi) Srivastava and Mehrotra (L; SEH, B) |
| G. mioodorata (Prasad et al.) Khan et al. (L; SEH) | |
| Anacardiaceae | M. miosericea Prasad et al. (L; D) |
| Bouea premacrophylla Antal and Awasthi (L; D) | M. oodlabariensis Antal and Prasad (L; D) |
| Buchanania palaeosessilifolia Prasad et al. (L; D) | M. prakashii Prasad et al. (L; D) |
| Dracontomelum Mangiferum Khan et al. (L; SEH) | M. purniyagiriensis Prasad et al. (L; D) |
| Glutoxylon burmense Mehrotra et al. (W; SEH) | M. sevokensis Prasad et al. (L; D) |
| Mangifera someshwarica Khan et al. (L; SEH) | M. siwalika Khan et al. (L; SEH) |
| Nothopegia eutravancorica Antal and Awasthi (L; D) | Mastertia neoassamica Khan and Bera (F; SEH) |
| Sorindeia subansiriensis Khan et al. (L; SEH) | Pongamia siwalika Antal and Awasthi Khan et al. (L; D, SEH) |
| Annonaceae | P. kathgodamensis Khan et al. (L; SEH) |
| Artabotrys siwalicus Prasad et al. (L; D) | Pahudioxylon bankurensis Mehrotra et al. (W; D) |
| Cerbera miocenica Prasad et al. (L; D) | P. indicum Srivastava et al. (W; SEH) |
| Fissistigma palaeobicolor Joshi and Mehrotra (L; SEH) | Spatholobus siwalicus Prasad et al. (L; D) |
| F. senii Prasad et al. (L; SEH) | Fagaceae |
| Meiogyne sevokensis Prasad et al. (L; D) | Quercus sp. Khan et al. (L; SEH) |
| Mitrephora siwalika (Antal and Awasthi) Prasad and Tripathi (L; D, B) | Quercus lamellosa Khan et al. (L; SEH) |
| Flacourtiaceae | |
| Polyalthia palaeosiamiarum Antal and Prasad (L; D) | Alsodeia palaeoechinocarpa Antal and Prasad (L; D) |
| Polyalthioxylon arunachalensis Srivastava et al. (W; SEH) | A. palaeoracemosa Antal and Prasad (L; D) |
| Pseuduvaria mioreticulata Prasad et al. (L; D) | A. palaeozeylanicum Antal and Awasthi (L; D) |
| Uvaria ghishia Antal and Prasad (L; D) | Casearia pretomentosa Antal and Awasthi (L; D) |
| U. neograndiflora Khan et al. (L; SEH) | Flacourtia tertiara Antal and Prasad (L; D) |
| U. siwalica Prasad et al. (L; SEH) | Hydnocarpus palaeokurzii Antal and Awasthi (L; D) |
| Apocynaceae | H. ghishiensis Prasad et al. (L; D) |
| Alstonia mioscholaris Antal and Awasthi (L; D) | Lamiaceae |
| Chonemorpha miocenica (Prasad et al. Khan et al. (L; D, SEH)) | Premna pliobengalensis Khan et al. (L; SEH) |
| Tabernaemontana precoronaria Srivastava and Mehrotra (L; SEH) | Gmelina siwalika Khan et al. (L; SEH) |
| Lauraceae | |
| Asteraceae | Actinodaphne palaeoangustifolia (Antal and Awasthi) Khan et al. (L; D; SEH) |
| Vernonia palaeoarborea Antal and Awasthi (L; D) | |
| Burseraceae | A. palaeomalabarica Srivastava and Mehrotra (L; SEH) |
| Bursera preserrata Antal and Awasthi (L; D) | A. palaeoobovata Khan et al. (L; SEH) |
| B. serratoides Antal and Awasthi (F; D) | Beilschmiedia plioroxburghiana Khan et al. (L; SEH) |
| Canarium bengalense Khan et al. (L; SEH) | Cinnamomum sp. Antal and Awasthi (L; D) |
| Calophyllaceae | C. palaeobejolghota Khan and Bera (L; D) |
| Calophylloxylon cuddalorense Srivastava et al., (W; SEH) | Litsea preglabrata Srivastava and Mehrotra (L; SEH) |
| C. eoinophyllum Khan et al. (W; SEH) | L. salicifolia Khan et al. (L; SEH) |
| C. suraikholaensis (Antal and Awasthi) Joshi and Mehrotra; Khan et al. (L; D, SEH) | Lindera neobifaria Khan and Bera (L; SEH) |
| L. pulcherrima Khan et al. (L; SEH) | |
| C. siwalikum Khan et al. (L; D) | Persea miogamblei Khan and Bera (L; D) |
| Celastraceae | P. mioparviflora Khan and Bera (L; SEH) |
| Lophopetalumoxylon indicum Srivastava and Mehrotra (L; SEH) | P. neovillosa Khan and Bera (L; D) |
| P. preglaucescens Khan and Bera (L; SEH) | |
| Salacia miocenica Srivastava and Mehrotra (L; SEH) | Lythraceae |
| Clusiaceae | Lagerstroemia sp. Tripathi et al. (L; B) |
| Garcinia eocambogia Prasad et al. (L; D) | L. jamraniensis Khan et al. (L; SEH) |
| Kayeoxylon assamicum Srivastava et al. (W; SEH) | L. deomaliensis Srivastava et al. (L; SEH) |
| Combretaceae | Lagerstroemioxylon deomaliensis Mehrotra et al. (W; SEH) |
| Combretum sahnii (Antal and Awasthi) Khan et al. (L; D, SEH) | Malvaceae |
| C. miocenicum Prasad and Tripathi (L; B) | Bombax palaeomalabaricum Prasad et al. (L; D) |
| C. prechinense Khan et al. (L; SEH) | Grewia ghishia Antal and Awasthi (L; D) |
| Lagerstroemia patelii Antal and Awasthi (L; D) | G. tistaensis Antal and Prasad (L; D) |
| Terminalia miobelerica Antal and Prasad (L; D) | Pterospermum siwalicum Antal and Prasad (L; D) |
| T. palaeocatappa Joshi et al. (L; SEH) | P. palaeoheynianum Antal and Awasthi (L; D) |
| T. palaeochebula Khan et al. (L; SEH) | Sterculia miocolorata Prasad et al. (L; D) |
| Terminalioxylon belericum Mehrotra et al. (W; SEH) | S. siwalica Prasad et al. (L; D) |
| Connaraceae | S. mioparviflora (L; D) |
| Rourea miocaudata Khan and Bera (L; SEH) | Melastomaceae |
| Cornaceae | Memecylon arunachalensis Srivastava and Mehrotra (L; SEH) |
| Mastixia asiatica Khan et al. (F; SEH) | Meliaceae |
| M. siwalika Khan et al. (L; SEH) | Beddomia palaeoindica Antal and Prasad (L; D) |
| Dilleniaceae | Dysoxylum miocostulatum Khan and Bera (L; SEH) |
| Dillenia palaeoindica Antal and Awasthi (L; D) | D. raptiensis Khan et al. (L; SEH) |
| Dipterocarpaceae | Toona siwalika Prasad and Tripathi (L; B) |
| Dipterocarpus siwalicus (Prasad and Tripathi) Antal and Prasad; Joshi and Mehrotra, Khan et al. (L; B, D, SEH) | Moraceae |
| Ficus retusoides Antal and Awasthi (L; D) | |
| D. koilabasensis Khan et al. (L; SEH) | F. oodlabariensis Antal and Awasthi (L; D) |
| Dipterocarpoxylon parabaudii Tripathi et al. (W; B) | F. precunea Prasad et al. (L; D) |
| Hopea kathgodamensis Antal and Prasad (L; D) | Myristicaceae |
| H. siwalika Antal and Awasthi (L; D) | Knema glaucescens Khan et al. (L; SEH) |
| Hopenium kalagarhensis Tripathi et al. (L; B) | Myrtaceae |
| Hopeoxylon speciosum Mehrotra et al. (W; SEH) | Syzygium palaeocuminii Antal and Prasad (L; D) |
| H. eosiamensis Srivastava et al. (W, SEH) | Oleaceae |
| Shorea miocenica Antal and Prasad (L; D, SEH) | Chionanthus siwalicus Prasad et al. (L; D) |
| S. bengalensis Antal and Prasad (L; D) | Rhamnaceae |
| S. bhalukpongensis Khan et al. (FC; SEH) | Rhamnus siwalicus Prasad et al. (L; D) |
| S. chandernagarensis Khan et al. (FC; SEH) | Ventilago tistaensis Antal and Prasad (L; D) |
| S. mioassamica Khan and Bera (FC; SEH) | Ziziphus palaeoapetala Antal and Prasad (L; D) |
| S. mioobtusa Khan and Bera (F; A) | Rubiaceae |
| S. neoassamica Joshi and Mehrotra (L; SEH) | Callicarpa siwalika Antal and Awasthi (L; D) |
| S. nepalensis Khan et al. (L; SEH) | Gardenia precoronaria Prasad et al. (L; D) |
| S. palaeoridleyana Joshi and Mehrotra (L; SEH) | Neolamarckia paleocadamba Khan et al. (L; SEH) |
| S. pinjoliensis Khan and Bera (FC; SEH) | Randia miowallichii (Antal and Awasthi) Srivastava and Mehrotra (L; D, SEH) |
| S. pliotumbuggaia Khan et al. (L; SEH) | |
| S. siwalika (Antal and Awasthi) Khan et al. (L; D, SEH) | R. lishensis Prasad et al. (L; D) |
| Shoreoxylon evidens Mehrotra et al. (W; SEH) | Rutaceae |
| Vatica siwalica Prasad et al. (L; D) | Toddalia miocenica Prasad et al. (L; D) |
| V. prenitida Prasad et al. (L; D) | Sapindaceae |
| Ebenaceae | Euphoria longanoides Antal and Awasthi (L; D) |
| Diospyros palaeoargentea Prasad et al. (L; D) | Cupania oodlabariensis Prasad et al. (L; D) |
| D. koilabasensis Antal and Awasthi (L; D) | Euphorioxylon deccanense Mehrotra et al. (W; SEH) |
| Ebenoxylon miocenicum Antal et al. (W; D) | Filicium koilabasensis Prasad et al. (L; D) |
| E. siwalicus Srivastava et al. (W; SEH) | Paranephelium miocenica Prasad et al. (L; D) |
| Elaeocarpaceae | Sabia eopaniculata Prasad et al. (L; D) |
| Elaeocarpus prelanceaefolius Bera et al. (F; SEH) | Vitaceae |
| Sloanea pliodasycarpa More et al. (L; D) | Vitis siwalicus Prasad et al. (L; D) |
| Euphorbiaceae | Xanthophyllaceae |
| Croton caudatus Khan et al. (L; SEH) | Xanthophyllum mioflavescens Antal and Prasad (L; D) |
| Dicotylophyllum breyniodes Srivastava and Mehrotra (L; SEH) | |
| Abbreviations | |
| Glochidion palaeohirsutum Antal and Prasad (L; D) | L: Leaf; F: Fruit; W: Wood; B: Bhutan; D: Darjeeling; SEH: Southeastern Himalaya |
| G. siwalikum Khan et al. (L. SEH) | |
| G. palaeogamblei Khan et al. (L. SEH) |
| Locality | Siwalik Strata | Age (Formation) | MAT (℃) | WMMT (℃) | CMMT (℃) | MIN_T_W (℃) | MAX_T_C (℃) | THERM. (℃) | GDD_0 | GDD_5 | LGS (months) |
| Darjeeling | Lower Siwalik | Middle Miocene (Gish Clay Formation) | 24.2 (25.37) | 28.2 (28.35) | 18.7 (17.88) | 22.6 | 24.3 | 602 | 107.9 | 107.2 | 12.8 (12.95) |
| Southeastern Himalaya | Upper Siwalik | Late Pliocene to early Pleistocene (Kimin Formation) | 25 (25.38) | 28 (28.05) | 20.3 (20.86) | 22.8 | 25.9 | 646 | 111.1 | 108.9 | 12.7 (12.58) |
| Southeastern Himalaya | Middle Siwalik | Pliocene (Subansiri Formation) | 23.3 (23.67) | 27.3 (28.14) | 18.3 (16.92) | 23.1 | 23.6 | 588 | 102 | 101.5 | 12 (12.1) |
| Southeastern Himalaya | Lower Siwalik | Middle Miocene (Dafla Formation) | 25.2 (25.29) | 28.0 (27.84) | 20.7 (21.29) | 22.9 | 26.4 | 653 | 111.4 | 109.1 | 12.7 (12.48) |
| Bhutan | Middle Siwalik | Late Miocene to Pliocene (Formation Ⅱ) | 24.3 (24.1) | 27.3 (27.8) | 20 (18.9) | 23.1 | 25.4 | 626 | 106.2 | 104.9 | 12.3 (12.1) |
| Standard deviation | ±2.4 | ±2.9 | ±3.6 | ±2.9 | ±3.5 | ±75 | ±11.7 | ±10.4 | ±1.1 | ||
| MAT – mean annual temperature; WMMT – warm month mean temperature; CMMT – cold month mean temperature; MIN_T_W – minimum temperature of the warmest month; MAX_T_C – maximum temperature of the coldest month; THERM. – compensated thermicity index: sum of mean annual temp., min. temp. of coldest month, max. temp. of coldest month, × 10, with compensations for better comparability across the globe; GDD_0 – sum of mean monthly temperature for months with mean temperature greater than 0 ℃ multiplied by number of days; GDD_5 – sum of mean monthly temperature for months with mean temperature greater than 5 ℃ multiplied by number of days and LGS – length of the growing season when mean temperatures are above 10 ℃. | |||||||||||
| Locality | Siwalik Strata | Age (Formation) | GSP (cm) | MMGSP (cm) | 3WET (cm) | 3DRY (cm) | ENTH (kJ/kg) |
| Darjeeling | Lower Siwalik | Middle Miocene (Gish Clay Formation) | 235.3 (242.33) | 21.8 (24.5) | 119.1 (111.73) | 24.9 (28.86) | 353.3 (354.1) |
| Southeastern Himalaya | Upper Siwalik | Late Pliocene to early Pleistocene (Kimin Formation) | 208.7 (189.86) | 18 (15.87) | 107.7 (101.64) | 10.7 (8.97) | 358.3 (356.1) |
| Southeastern Himalaya | Middle Siwalik | Pliocene (Subansiri Formation) | 200.5 (198.12) | 17.5 (17.9) | 102.2 (99.41) | 13.3 (13.78) | 352.8 (351.3) |
| Southeastern Himalaya | Lower Siwalik | Middle Miocene (Dafla Formation) | 198.3 (174.13) | 16.5 (13.97) | 101.5 (96.15) | 9.1 (7.34) | 358.3 (355.8) |
| Bhutan | Middle Siwalik | Late Miocene to Pliocene (Formation Ⅱ) | 189.9 (189.9) | 15.8 (15.4) | 97.4 (95.7) | 10.2 (10.6) | 356.5 (353.3) |
| Standard deviation | ±64.3 | ±6.5 | ±40 | ±9.8 | ±8 | ||
| GSP – precipitation during the growing season; MMGSP – mean monthly precipitation during the growing season; 3WET – precipitation during the three consecutive wettest months; 3DRY – precipitation during the three consecutive driest months; ENTH – annual mean moist enthalpy. | |||||||
| Locality | Siwalik Strata | Age (Formation) | RH. ANNUAL (%) | SH. ANNUAL (g/kg) | VPD.ANN (hPa) | VPD.SUM (hPa) | VPD.WIN (hPa) | VPD.SPR (hPa) | VPD. AUT (hPa) | PET.ANN (mm)/10 | PET.WARM (mm) | PET.COLD (mm) |
| Darjeeling | Lower Siwalik | Middle Miocene (Gish Clay Formation) | 77.2 (80.99) | 14.5 (14.46) | 6.5 | 4.5 | 6.2 | 8.8 | 6 | 137.7 | 127.6 | 85.8 |
| Southeastern Himalaya | Upper Siwalik | Late Pliocene to early Pleistocene (Kimin Formation) | 81.3 (82.37) | 15.5 (14.97) | 7.6 | 4 | 7.1 | 11.5 | 5.4 | 148.6 | 133 | 107.8 |
| Southeastern Himalaya | Middle Siwalik | Pliocene (Subansiri Formation) | 80 (78.84) | 14.5 (14.01) | 6.7 | 4 | 6.5 | 10.2 | 5.1 | 141.3 | 131.2 | 95.3 |
| Southeastern Himalaya | Lower Siwalik | Middle Miocene (Dafla Formation) | 80.4 (81.15) | 15.5 (14.91) | 8.0 | 4.8 | 7.2 | 11.8 | 5.8 | 151.6 | 139.2 | 110.1 |
| Bhutan | Middle Siwalik | Late Miocene to Pliocene (Formation Ⅱ) | 81.7 (80.2) | 15.2 (14.4) | 7 | 3.6 | 6.8 | 10.9 | 4.9 | 147.5 | 134.6 | 106.5 |
| Standard deviation | ±10.2 | ±1.8 | ±2.4 | ±3.5 | ±1.5 | ±4 | ±2 | ±16.2 | ±24.5 | ±13.8 | ||
| RH. ANNUAL – annual mean relative humidity; SH. ANNUAL – annual mean specific humidity; VPD.ANN – annual mean vapour pressure deficit; VPD.SUM – mean VPD for the summer quarter; VPD.WIN – mean VPD for the winter quarter; VPD.SPR – mean VPD for the spring quarter; VPD-AUT – mean VPD for the autumn quarter; PET.ANN – annual mean potential evapotranspiration; PET.WARM – mean potential evapotranspiration for the warmest quarter; PET.COLD – mean potential evapotranspiration for the coldest quarter. | ||||||||||||
The results of these analyses are also consistent with published quantitative climate data (Khan et al., 2014a, 2019b) using CLAMP analysis (Wolfe, 1993; Teodoridis et al., 2011; Yang et al., 2011) on fossil leaf morphotypes (i.e., not assigned taxonomic affiliation) from the eastern Himalayas. Two lower Siwalik mid-Miocene floras of Darjeeling and Southeastern Himalaya yielded almost the same values suggesting mean annual temperatures (MATs) of 25.4 and 25.3 ± 2.8 ℃ (all uncertainties ±2 sigma) with warm month mean temperatures (WMMTs) of 28.4 and 27.8 ± 3.39 ℃ and cold month mean temperatures (CMMTs) of 17.9 and 21.3 ± 4 ℃. Precipitation estimates have high uncertainties but suggest a weak monsoon with growing season precipitations of 181 ± 91 cm for Bhutan, 242 ± 92 cm for Darjeeling, and 174 ± 92 cm for Southeastern Himalaya. Leaves from the middle Siwalik (Pliocene) sediments of Southeastern Himalaya indicate a lowering of the MAT to 23.7 ℃, which appears to be largely a function of cooler winter months (CMMT 16.9 ℃). Southeastern Himalaya's early Pleistocene temperatures and rainfall were similar to those of the mid-Miocene. Khan et al. (2019b) compared palaeoclimate estimates of the latest Miocene–Pliocene Siwalik (ca. 6 to 3.8 Ma) flora of Bhutan with those of Siwalik floras from the Miocene-Pleistocene of Southeastern Himalaya and the Miocene Siwalik flora of Darjeeling. Because all the Siwalik floras of the eastern Himalayas spanning the mid-Miocene to Pleistocene yield almost the same values, they suggested that overall, the eastern Himalayan Siwalik climate appears to have been remarkably uniform over the past 15 million years. The MAT result of the Bhutan Siwalik palaeoflora differs by just 0.6 ℃ from the Southeastern Himalaya, and 1.2 ℃ from the Darjeeling palaeoflora. For all Siwalik fossil assemblages, WMMTs, CMMTs, LGSs (length of the growing season), RH (mean annual relative humidity), and SH (mean annual specific humidity) are similar and consistent (WMMTs around 28 ℃, CMMTs around 18 ℃, LGSs around 12 months, RHs around 80% and SHs around 14 g/kg). Hence, palaeoclimate estimates of the Southeastern Himalaya, Darjeeling, and Bhutan Siwalik flora provide valuable insights into monsoon climatic evolution throughout the eastern Himalayan Siwalik belt during late Cenozoic time and indicate that the Siwalik floras experienced a persistent monsoonal tropical warm humid climate. Changes in the Monsoon index suggest that in both the Bhutan and Southeastern sub-Himalaya, there has been little change in the intensity of the monsoon since mid-Miocene time, while further west in the Darjeeling area precipitation seasonality has increased since the mid-Miocene.
7.3.1. New insights into the thermal and hydrological regime of the eastern Himalayan SiwalikHere, we re-analyze the five well-documented fossil leaf assemblages from across the eastern Himalayas spanning Siwalik time (middle Miocene to early Pleistocene). All have been previously analyzed for the eleven standard CLAMP climate variables (mean annual temperature – MAT; warm month mean temperature – WMMT; cold month mean temperature – CMMT; length of the growing season – LGS; growing season precipitation – GSP; mean monthly growing season precipitation – MMGSP; precipitation during the three consecutive wettest months – 3WET; precipitation during the three consecutive driest months – 3DRY; mean annual relative humidity – RH. ANN; mean annual specific humidity – SH.ANN; and mean annual moist enthalpy – ENTH), calibrated using modern gridded climate data at 10′ spatial resolution (HiResGridMetAsia2) and physiognomic PhysgAsia2 calibration (Table S12). Here, fossil leaf assemblages are subjected to a CLAMP analysis using a new high spatial resolution 30" (~1 km2) WorldClim2 gridded climate data (Fick and Hijmans 2017; http://worldclim.org/version2) (Table S13) with 15 new climate variables (Tables 2-4; Figs. 8 and S1–S4). However, we use the same modern vegetation trait scores as used previously (PhysgAsia2) (Table S12). This calibration data set interpolates average meteorological observations between 1970 and 2000 onto a spatial grid approximating 1 km2. CLAMP climate retrodictions for all the fossil sites of the eastern Himalayas are given in Tables 3-5.

|
| Fig. 8 CLAMP WorldClim2 regression models for (a) mean annual temperature (MAT) and (b) cold month mean temperature (CMMT). The position of the eastern Himalayan fossil flora along the second-order polynomial regression relating the MAT and CMMT vector scores for modern vegetation against the observed MATs and CMMTs for those sites is shown as a red-rimmed circle with a yellow center, with uncertainty bars (1 s.d.) reflecting the scatter of the residuals about the regression line. The vector score represents the relative position of the sites, modern and fossil, along with a vector representing the primary trend of the climate variable in axes 1–4 space. See the CLAMP website (http://clamp.ibcas.ac.cn) for details. |
One advantage of using WorldClim2 for calibration is that numerous environmental variables have been mapped onto the same grid, so for CLAMP, the range of environmental signals decoded from leaf form can be extended. The new temperature-related environmental variables that correlate strongly with leaf form are (1) the compensated thermicity index –THERM (sum of mean annual temperature, minimum temperature of the coldest month, the maximum temperature of the coldest month, × 10, with compensations for better global comparability), (2) growing degree days above 0 ℃ – GDD_0 (sum of mean monthly temperature for months with mean temperature > 0 ℃ multiplied by the number of days this occurs), (3) growing degree days above 5 ℃ – GDD_5 (Sum of mean monthly temperature for months with mean temperature > 5 ℃ multiplied by the number of days this occurs), (4) minimum temperature of the warmest month –MIN_T_W (lowest daily temperature during the warmest month) and (5) maximum temperature of the coldest month – MAX_T_C (warmest daily temperature during the coldest month). The new humidity-related variables are (6) mean annual vapour pressure deficit – VPD.ANN, (7) mean summer vapour pressure deficit – VPD.SUM (average vapour pressure deficit during the three summer months), (8) mean winter vapour pressure deficit – VPD.WIN (average vapour pressure deficit during the three winter months), (9) mean spring vapour pressure deficit – VPD.SPR (average vapour pressure deficit during the three spring months), (10) mean autumn vapour pressure deficit – VPD.AUT (average vapour pressure deficit during the three autumn months), (11) mean annual potential evapotranspiration – PET.ANN (the ability of the atmosphere to remove water through evapotranspiration, given unlimited water supply-- no limits on plant water supply-- averaged over the year), (12) mean monthly potential evapotranspiration during the warmest quarter – PET.WARM and (13) mean monthly potential evapotranspiration during the coldest quarter – PET. COLD.
Tables 3-5 present results obtained for the fossil assemblages using the new WorldClim2 CLAMP calibration, as well as (for comparison) previously obtained results (in parentheses) that used low spatial resolution HiResGridMetAsia2CLAMP calibration. Figs. 8 and S1–S4 illustrate the CLAMP regression models for each of the climate variables to show not only the relative position on the regression of the Siwalik fossil locations but also the scatter of the modern training data and thus the precision of the CLAMP predictions. As used in earlier CLAMP analyses, all regression models are derived from the leaf physiognomy/climate relationships in four-dimensional space (Spicer and Herman, 2010). CLAMP scoresheets for all eastern Himalayan Siwalik fossil assemblages are given in the Tables S7–S11. The new WorldClim2-based climate training set (WorldClim2_3br) and the accompanying modern leaf physiognomic (PhysgAsia2) data files are given in the Tables S12 and S13.
The new calibration and range of climate variables allow us to explore new insights into the hydrological regime. We examine not only precipitation but humidity in terms of specific humidity (SH), relative humidity (RH), vapour pressure deficit (VPD), and potential evapotranspiration (PET). Both VPD and PET are investigated in respect of annual average values and seasonal variations.
Using leaf form (physiognomy) we reconstruct middle Miocene–early Pleistocene thermal and hydrological regimes at five locations in the eastern Himalayas. The new high spatial resolution (~1 km) WorldClim2 calibration yields result similar to previous analyses, but also provides more detailed insights into the hydrological regime through the return of annual and seasonal vapour pressure deficit (VPD), potential evapotranspiration (PET) estimates, as well as new thermal overviews through measures of thermicity and growing degree days. The new results confirm the overall warmth of the region. Palaeo-rainfall estimates have large uncertainties due to moisture not being limiting in the context of the Siwalik assemblages and because fossils are usually preserved in water-lain deposits, suggesting the parent plants were growing in or near year-round wet soils. The new measures of VPD and PET show the persistent high humidity to which the leaves were exposed and adapted, but with notably lower humidity during the summers at all the eastern Himalayan locations.
7.4. Cuticular approachNo proxy is perfect, so a multiproxy approach is always desirable. The examination of fossil leaf cuticles found on compressed leaves can also afford an estimation of past climate. Several cuticular characters indicate a warm, humid tropical climate with non-limiting rainfall, including thin cuticles, undulate to sinuous epidermal lateral walls, non-papillate or smooth leaf external surfaces, few epidermal hairs, unspecialized stomata, and subsidiary cells, all of which are commonly found in the Siwalik assemblages (Khan et al., 2015a). The hypostomatic nature of many Siwalik stomata also reflects heavy precipitation, humidity, and shade. Cuticular micro-morphological features have also helped to confirm the identification of some leaf compressions to the species level, and are clearly indicative of mesophytic ecological conditions that reflect a tropical climate with high precipitation (Khan et al., 2015a, 2016, 2017a, b).
Some workers (Mitra and Banerjee, 2000; Mitra et al., 2002; Das et al., 2007; Mandal et al., 2009, 2011; Vishnu et al., 2017, 2019; Bera et al., 2018, 2019, 2022a, b; Khan et al., 2018b, 2019c) reported in-situ occurrences on leaf megafossils of characteristic epiphyllous fungi such as Meliolinites (fossil Meliolaceae) (comparable to the modern genus Meliola Fr.), Phomites (comparable to the modern genus Phoma Sacc.), Palaeocercospora (comparable to the modern genus Cercospora Fresen. ex Fuckel), Palaeocolletotrichum (comparable to the modern genus Colletotrichum Corda), Palaeoasterina (comparable to the modern genus Asterina Lév.) and Vizellopsidites (comparable to modern genus Vizella Sacc.) on the cuticular surfaces of fossilized leaf cuticle fragments of the different angiosperm taxa recovered from the Siwalik sediments (middle Miocene to early Pleistocene) of Darjeeling, Bhutan, and Southeastern Himalaya (Table S6). They described fossil fungi on the basis of vegetative and reproductive structures. The Siwalik host leaves harboring the fossil fungi so far identified are Shorea, Dipterocarpus (Dipterocarpaceae), Breonia A. Rich. ex D.C. (Rubiaceae), Dysoxylum Blume (Meliaceae), Combretum (Combretaceae), Xylopia L. (Annonaceae), Amherstia Wall. (Fabaceae), Actinodaphne Nees, Lindera Thunb., Persea (Lauraceae), Macaranga Thouars (Euphorbiaceae), Lauraceae, and Poaceae. Based on earlier records, it is also evident that Lauraceae has been a common host for meliolaceous fungi since the early Cenozoic (Khan et al., 2019c). The reported appreciable numbers of foliicolous fungal remains indicate the prevalence of a warm, humid, climate favored by the high rate of precipitation in the eastern Himalayas during the Plio-Pleistocene (Mitra and Banerjee, 2000; Mitra et al., 2002; Das et al., 2007; Mandal et al., 2009, Mandal et al., 2011, Vishnu et al., 2017, Bera et al., 2018, Vishnu et al., 2019, 2019, 2022a, b; Khan et al., 2018b, 2019c). These climatic data are also consistent with published climatic data obtained from the study of the macroscopic plant remains using qualitative and quantitative methods. Thus, for the eastern Himalayan Siwalik, all approaches (CLAMP, NLR, CoA, and cuticle) give broadly similar palaeoclimate outcomes. The in-situ evidence of epiphyllous fungal remains on host leaf cuticles also indicate the possible existence of a host-ectoparasite relationship in the ancient warm and humid tropical evergreen forest of this area during Siwalik sedimentation (Vishnu et al., 2017, 2019; Bera et al., 2018, 2019, 2022a, b; Khan et al., 2018b, 2019c).
8. Comparisons 8.1. Comparisons with other Siwalik florasTo reveal the degree of resemblance to other Siwalik floras (western and central), we make the following comparisons.
8.1.1. Western Siwalik floraThis includes the floras of Jammu and Kashmir, Uttarakhand, and Himachal Pradesh and comprises a large number of fossil woods and leaves (Sahni, 1964a, b, Lakhanpal, 1965, 1967; Varma, 1968; Lakhanpal and Awasthi, 1992; Prasad, 1994a, 2006; Prasad et al., 1997; Shashi et al., 2006, 2008; Srivastava et al., 2015). The NLRs of common fossil taxa are Millettia, Ziziphus Mill., Pongamia, Dalbergia, Diospyros, Fissistigma, Bambusa Schreb., Dipterocarpus, Lagerstroemia, Marantochloa Brongn. ex Gris, Calophyllum, Shorea, Gynocardia, Grewia, Cynometra, Persea, Sterculia, Mallotus, Terminalia, and Hopea Roxb. This indicates that these taxa were widely distributed in both eastern and western Siwalik strata and flourished under a generally equitable climate, at least in terms of temperature.
8.1.2. Central (Nepal) Siwalik floraPlant megafossils (mainly fossil leaves) are known from various localities in Nepal such as Koilabas, Arung Khola, Surai Khola, Tinau Khola, Babai and Surkhet Valleys, Mahendra Highway, Arjun Khola, and Sindhuli (Prakash and Prasad, 1984; Prasad, 1990, 1994b; Prasad et al., 1997, 1999; Tripathi et al., 2002; Dwivedi et al., 2006; Prasad and Dwivedi, 2007). Comparison of the eastern Himalaya fossil floras with those of the central Siwalik fossil flora assemblages shows that most NLRs of the fossil genera Mesua L., Mangifera L., Bouea Meisn., Garcinia, Albizia, Cassia L., Millettia, Ziziphus, Pongamia, Dalbergia, Diospyros, Fissistigma, Bambusa, Dipterocarpus, Lagerstroemia, Marantochloa, Calophyllum, Shorea, Gynocardia, Grewia, Cynometra, Persea, Sterculia, Melilotus, Cinnamomum Schaeff., Mitrephora Hook. f. & Thomson, Hopea, Polyalthia Blume, Uvaria L., Sabia Colebr., Miliusa Lesch. ex A. D.C., Swintonia Griff., Euphorbia L., Entada, Combretum, Dillenia, Randia L., and Flacourtia Comm. ex L'Hér. are common to both regions.
8.2. Comparisons among Siwalik CLAMP data 8.2.1. MethodologyThe CLAMP methodology, its limitations, and its evolution are detailed in Spicer et al. (2021), but in summary, CLAMP reconstructs past climate based on an array of macroscopic leaf traits preserved in leaf megafossils. Organic remains are not required and CLAMP can be applied to mere leaf impressions provided that the leaves retain sufficient trait data across a minimum of 20 taxa or morphotypes in any one fossil assemblage. Also, leaf identification is not required, only an ability to distinguish one taxon (morphotype) from another. Morphotype partitioning is based not only on the CLAMP traits (31 trait states encompassing leaf lobing, margin features size, apex, base forms, and overall shape, but also venation and other taxonomically useful features.
To calibrate CLAMP, a database of trait spectra from modern vegetation stands growing under a wide range of known climate conditions provides a multidimensional framework for identifying correlations between leaf trait combinations and individual climate variables such as temperature and moisture metrics. This physiognomic data set is accompanied by a suite of climate data derived in most cases from gridded observations. There are several such modern gridded data sets available at different spatial resolutions (e.g., New et al., 1999, 2002; Harris et al., 2014; Fick and Hijmans, 2017) and each are slightly different, largely as a function of interpolation artifacts and different observation periods, and so return slightly different retrodictions of past climate. This is shown in Tables 3 and 4 where WorldClim2 calibration results are compared with those based on New et al. (2002), shown in parentheses. These climate calibration anomalies apply to any climate proxy, so it is important when comparing proxy-reconstructed past climates that uncertainties introduced by using different types of climate calibration data are fully appreciated. Here we use a common proxy CLAMP calibration combining the PhysgAsia2 leaf trait data set (Spicer et al., 2021) with the WorldClim2 climate data gridded at ~1 km resolution (Fick and Hijmans, 2017). The trait/climate relationships are decoded using the multivariate statistical engine known as Canonical Correspondence Analysis (ter Braak, 1986; http://clamp.ibcas.ac.cn).
Recently, fossil leaf assemblages from the lower (middle Miocene) and middle (late Miocene-Pliocene) Siwalik sediments exposed in Nepal were subjected to a CLAMP analysis by Bhatia et al. (2021) using the new high spatial resolution (~1 km2) WorldClim2 gridded climate data and PhysgAsia2 calibration. Their analysis indicates a mean annual temperature (MAT) of 22.2 ± 2.3 ℃ and 24.7 ± 2.3 ℃ for the Lower Siwalik and Middle Siwalik assemblages, respectively. Cold month mean temperatures (CMMTs) were 14.7 and 19 ± 3.5 ℃ and warm month mean temperatures (WMMTs) were 28.3 and 28.5 ± 3 ℃ for the Lower and Middle Siwalik assemblages, respectively, showing warming of the cold months being mostly responsible for a slight increase in the MAT over that time interval. Here, we compare palaeoclimate estimates of the middle Miocene to Plio-Pleistocene Siwalik flora from the eastern Himalayas with those of previously investigated Siwalik middle Miocene-Pliocene floras of Nepal.
Tables 3-5 summarize the CLAMP results for the eastern Himalaya Siwaliks. Table 3 focuses on temperature-related metrics while Table 4 provides an overview of rainfall-related metrics and Table 5 presents humidity-related metrics. All middle Miocene (Lower Siwalik of Southeastern Himalaya and Darjeeling) thermal metrics are identical within 1 sigma uncertainty, showing frost-free year-round growth in a climate where the CMMT is > 18 ℃ and thus 'tropical'. The CMMT of the Lower Siwalik of Nepal is somewhat lower at 14 ℃ and just on the margins of the 1 sigma uncertainty difference from the eastern Himalaya value reported here. This marginal difference is also evident in the cooler (21 ℃ for Nepal versus 24.3 and 26.4 ℃ for Darjeeling and Southeastern Himalaya, respectively) maximum temperature of the coldest month metric, but again it is debatable whether these differences can be regarded as real. For the Middle Siwalik assemblages (late Miocene to Pliocene of Southeastern Himalaya and Bhutan) a similar pattern is evident with all thermal metrics showing no discernible differences between assemblages (Table 5). These values are also more or less identical to those from the Middle Siwalik of Nepal reported by Bhatia et al. (2021). There is only one Upper Siwalik (late Pliocene to Pleistocene) assemblage reported here from the eastern Himalayas and that is from the Southeastern Himalaya section. This also shows thermal metrics apparently unchanged from those of earlier times.
Regarding rainfall-related metrics (Table 4), the Lower Siwalik assemblages of Darjeeling and Southeastern Himalaya are identical within uncertainty except for precipitation during the three consecutive driest months, which indicates Darjeeling was markedly wetter than Southeastern Himalaya. This difference is independent of which meteorological data set is used for calibration. The Middle Siwalik sites similarly are indistinguishable from one another, this time across all moisture metrics. The Darjeeling Lower Siwalik assemblage appears to be notably wetter than the other sites, particularly in respect of precipitation during the three consecutive driest months, but apart from that, all assemblages appear very similar in terms of their reconstructed climate metrics.
It is often assumed that rainfall is an important environmental constraint on plant growth, but this need not be the case. What is critical is soil moisture combined with transpirational stresses imposed by atmospheric humidity and wind strength. In situations where leaf fossils are preserved (i.e., near water bodies), soil moisture reflects the proximity to that water body, not necessarily local rainfall, and because of this, the soil moisture in these situations is rarely limiting. It follows that a more useful measure of atmospheric conditions can be found in the way that leaf traits code for humidity metrics, especially vapour pressure deficit (VPD) and potential evapotranspiration (PET) (Spicer et al., 2019, 2021). VPD is a measure of the ease by which a plant can lose moisture to the atmosphere, with low VPDs found when the air is near saturation and there is strong resistance to transpiration, whereas at high VPDs there is no atmospheric constraint on transpirational water loss from the plant. Unlike relative humidity (RH), VPD has a nearly straight-line relationship to the rate of evapotranspiration, and plant distribution (Huffaker, 1942) and leaf physiognomy (Spicer et al., 2019) are more reflective of VPD than RH. PET is similar but is a measure of the ability of the atmosphere to remove water through evapotranspirational processes provided the water supply to the roots is not limiting. Unlike with VPD, atmospheric dynamics (convection and wind) play a role in determining PET.
Table 5 suggests very similar values of humidity metrics among the different assemblages through time. That is to say, there are no discernible step changes in climate features from the mid-Miocene to the Pleistocene. However, there are some important seasonal differences in VPD and PET metrics consistent across all assemblages, with summer being consistently more humid than spring, as with the modern SAM, but Darjeeling shows less seasonal variation in VPD than at Bhutan or Southeastern Himalaya. This reflects the relatively wet dry season in Darjeeling as indicated by rainfall metrics (Table 4). Similarly, the cold month (winter) PET value is more than 1 standard deviation lower than the other cold month PET values, suggesting a markedly wetter dry season.
Because all the Siwalik floras of the eastern Himalayas and Central Himalayas yield almost the same values, we suggest that overall, the eastern and central Himalayan Siwalik climate appears to have remained remarkably uniform from the mid-Miocene to Pleistocene. However, while the modern temperature regime for the eastern Himalayan Siwaliks exhibits cooler winters than evident from the fossil data, the overall temperature regime is similar over time. The most marked differences are in the precipitation regime, with the modern being wetter with a greater seasonality in rainfall (wetter wet seasons and drier dry seasons) (Table S14). However, because uncertainties are also quite large for these metrics, the differences between the past and present may not be genuinely significant. It is unlikely that this consistency over time is representative of the whole SAM region, but it does have important implications for ecosystem evolution in the eastern Himalayas at low elevations, as discussed below.
9. Plant-arthropod associations from Siwalik forestsFossil leaf impressions and compressions from the Siwalik sedimentary strata of the eastern Himalayas provide evidence of a variety of plant–insect interactions that have operated throughout the evolution of monsoon-influenced forests since middle Miocene times (Khan et al., 2014b, 2015b). Five functional feeding groups (FFGs) were identified in this study, namely leaf mining, hole feeding, skeletonizing, galling, and margin feeding. Furthermore, these morphotraces are similar to those found in leaves of extant plant species such as Millettia, Canarium L., Glochidion J.R. Forst. & G. Forst., Callicarpa L., Chonemorpha G. Don, Actinodaphne, Persea, Woodfordia Salisb., Shorea, Artocarpus J.R. Forst. & G. Forst., Albizia, Lagerstroemia and others, suggesting similar interactions have existed in the eastern Himalaya region for at least 15 million years. On the basis of comparison with extant taxa, possible leaf feeders could have belonged to the insect orders Orthoptera, Coleoptera, Lepidoptera, and Diptera, and those plant-arthropod relationships were established by the mid-Miocene and continue to the present, shaping both the present-day flora and fauna. Khan et al. (2014b, 2015b) also compared insect herbivory evident in the Darjeeling Lower Siwalik flora to that of the similarly aged Southeastern Lower Siwalik flora, as well as two younger floras from that area and noted a similar range of FFGs and damage types among all four fossil floras of the eastern Himalayas. They concluded that compared to biotic factors climate had little influence on determining the evolution of plant–insect interactions in the eastern Himalayan region.
10. Phytogeographic patternsThe Siwalik plant fossils date from the late Neogene time (middle Miocene to early Pleistocene), and close relatives of the fossil forms still exist in the tropical forests of India and Southeast Asia today. This allows direct tracking of changes in phytogeographic distributions of these taxa over time. The present-day distributions of NLRs of 219 fossil taxa recovered from the Siwalik sediments (Mio-Pleistocene) of the eastern Himalayas indicate that today they grow in a variety of locations all over India and other adjoining countries (Fig. 9). In India, they are distributed in the northeastern (14.75%) and southern regions (4.35%). In this Siwalik assemblage, the NLRs of 49.04% taxa (Shorea roxburghii G. Don, Uvaria hirsuta A. St. Hil., Dipterocarpus tuberculatus Roxb., D. turbinatus Roxb., Ventilago calyculata Tul., Syzygium cuminii (Gamble) Tenjarla & Kashyapa, Homonoia riparia Lour., Xanthophyllum flavescens Roxb., Mitrephora maingayi Hook. f. & Thomson, Hopea wightiana Roxb., Bauhinia accrescens Killip & J.F. Macbr., Randia wallichii Hook. f., R. densiflora Lou., Fissistigma bicolor Merr., B. malabaricum D.C., Sterculia parviflora Roxb., Buchanania sessilifolia Blume, Cynometra iripa Kostel., C. polyanthum Wall. ex Choisy, Glochidion zeylanicum (Gaertn.) A. Juss., P. pinnata (L.) Merr. occur both in India and the Malaya Peninsula and 22.7% taxa (Pterospermum yunnanense H.H. Hsue, Parashorea buchananii (C.E.C. Fisch.) Symington, Flacourtia inermis Wall., Fordia albiflora (Prain) Dasuki & Schot, Paranephelium macrophyllum King, Diospyros argentea Griff.) are found to generally grow in Malaya. This clearly indicates a free exchange of floral elements during the late Neogene across Southeastern Asia (Fig. 10).

|
| Fig. 9 (a) Diagrammatic representation of Siwalik flora of the eastern Himalayas represented in the present-day flora of different geographical regions (NE = Northeast India; SI = South India; IM = India and Malaya Peninsula; M = Malaya; C = Cosmopolitan; O = Others); (b) Diagrammatic representation of the Siwalik flora of the eastern Himalayas in three different categories (EL = Extant local taxa; ELE = Extant but locally extinct taxa; ERE = Extant but regionally extinct taxa). |

|
| Fig. 10 Map showing hypothetical migratory routes of Siwalik and Southeast Asian tropical elements. |
The late Neogene floral assemblages covering the eastern Himalayas and Southeast Asian countries (Sumatra, Borneo, Indonesia, Vietnam, Malaysia, and Java) not only reflect great diversity with respect to the angiosperm families and variety of taxa but also uniformity and similarity in floristic composition. Thus, the late Neogene may be regarded as the time of maximum proliferation and diversification of tropical vegetation, particularly in the evergreen forests in Southeast Asia (Bande and Prakash, 1986; Awasthi and Mehrotra, 1990, 1997; Guleria, 1992). This floral spread was not unidirectional but suggests cross-migration between the regions as some Southeast Asian taxa must have migrated from the Indian landmass due to the widespread distribution of similar edaphic and climatic factors. Evidence of many southeast Asian elements recovered from the Siwalik exposures of the eastern Himalayas suggest migration into the Indian subcontinent and intermixing and merging with the existing eastern Himalayan flora before expanding further westwards along the entire Himalayan lowlands. Hence, the late Neogene period may be considered an important time in the evolution and speciation of flowering plants in India and Southeast Asia through the introduction and proliferation of new floral elements and the opportunity for subsequent interbreeding.
11. Disappearance of some plant taxaGradual changes in climate and monsoon amplification across Asia during the Neogene (Su et al., 2013; Tang et al., 2013, 2015) have been attributed to a general global cooling (Zachos et al., 2001, 2008), closure of the Tethys, loss of littoral environments along the Himalayan front (Lakhanpal, 1970) and rapid uplift of the Himalayas (Chatterjee and Scotese, 1999; Boos and Kuang, 2010, Molnar et al., 2010 Ding et al., 2017; Farnsworth et al., 2019). These factors are all likely to have played an important role in altering and diversifying floral patterns (Morley, 2000;Huggett, 2004; Mosbrugger et al., 2005). Some plant taxa were able to adapt to the changed circumstances or already had wide environmental tolerances. These have continued to flourish to the present-day in the areas surrounding the fossil localities, whereas others that could not adapt to the new environment either suffered local extinction or shifted to suitable areas with more favourable eco-climatic conditions.
Based on the present-day distribution of NLRs of the eastern Himalayan Siwalik plant fossil species, we categorize the Siwalik species into three main groups: extant local species (65%), extant but locally extinct species (12%), and extant but regionally extinct species (23%) (Fig. 9b). Extant local species have their NLRs (for example, Guatteria australis A. St. Hil., D. tuberculatus Roxb., D. turbinatus C.F. Gaertn., S. cuminii (Gamble) Tenjarla & Kashyapa, Tarennoidea wallichii (Hook. f.) Tirveng. & Sastre, F. bicolor Merr., B. ceiba L., S. parviflora Roxb., C. iripa Kostel., C. polyanthum Wall. ex Choisy, G. zeylanicum (Gaertn.) A. Juss., P. pinnata (L.) Merr.) growing today in or near the fossil localities. Extant but locally extinct species (for example, Mastixia arborea (Wight) C.B. Clarke, Shorea tumbuggaia Roxb., Persea parviflora (Meisn.) Harid. & R.R. Rao, Callerya cinerea (Benth.) Schot, Phyllanthus daltonii Müll. Arg., Calophyllum inophyllum L., Lindera bifaria Hosseus, Premna bengalensis C.B. Clarke etc.) grow in other parts of India, but do not occur in the present-day near the fossil localities. However, extant but regionally extinct NLRs (Millettia extensa (Benth.) Benth. ex Baker, Dysoxylum costulatum Blume, Rourea caudata Planch., Sloanea dasycarpa Hemsl., Shorea leprosula Miq., S. obtusa Wall. ex Blume etc.) have disappeared from India and now grow in other parts of the world. Given the apparent constancy of the climate in this region, we presume that significant changes in biotic interactions might be a reason for the disappearance of taxa from the present-day vegetation, including human activity. However, these taxa are presently growing in other regions of India, including the northeast region and south India, due to the availability of suitable conditions. If climate changes are involved, then they are subtler than can be detected by the CLAMP proxy given the existing statistical uncertainties.
The upper Paleocene record of Calophyllum in India (Bhattacharyya, 1967; Ambwani, 1992) suggests India is the centre of origin of this genus. Subsequently, this genus gradually became a ubiquitous component of the Neogene Siwalik forests of India and moved into other adjoining Southeast Asian regions, Polynesia, and the east coast of Africa as evidenced by later records outside India (Khan et al., 2017a). Khan et al. (2017a) suggested that distinct, but modest, elevation changes in northeastern India, possibly related to Himalayan orogeny since the Miocene, might have caused the disappearance of Calophyllum inophyllum from the entire eastern Himalayas.
Based on fossil records Khan et al. (2016) suggested that Shorea was a common forest element in the Neogene (Miocene time) Siwalik forests of the eastern Himalayas. They also reviewed the historical phytogeography and highlighted the phytogeographic implications of this genus.
Several other low-elevation eastern Himalaya taxa have undergone range shifts since the mid-Miocene. According to Khan and Bera (2007), Dysoxylum costulatum probably migrated to the Malaya region after the Miocene. Khan and Bera (2017) suggested that Pinus was an important component of tropical-subtropical evergreen forest in the West Kameng district of Southeastern Himalaya during the Miocene but this conifer taxon subsequently declined from the local vegetation. At present, Rourea caudata (Connaraceae) does not grow in India and is confined to the tropical evergreen forest of southeast Asian regions (China and Myanmar) where conditions are more suitable. Khan and Bera (2016) suggested that this species of Connaraceae probably migrated to these Southeast Asian regions after lower Siwalik sedimentation (middle-upper Miocene).
Khan et al. (2017b) reported the occurrence of the extant species Mastixia arborea (family Cornaceae) from the middle Miocene to early Pleistocene Siwalik sediments of Southeastern Himalaya. This report provided the first-ever fossil record of Mastixioids from India, as well as Asia. At present, M. arborea does not grow in the eastern Himalayas but is endemic to the tropical evergreen forests of the Western Ghats. They suggested that its extinction from the entire eastern Himalaya and probable movement to the Western Ghats is likely due to climate change (Siwalik forests experienced a weaker monsoon, i.e., less rainfall seasonality than now) in the area, related to the Himalayan Orogeny during Miocene-Pleistocene times. They also suggested that its disappearance from present-day vegetation proximal to the fossil locality may be related to the gradual intensification of rainfall seasonality since the late Miocene. Such intensification is not evident in the CLAMP data. Similarly, More et al. (2018) reported Sloanea dasycarpa of Elaeocarpaceae from the Geabdat Sandstone Formation, Pliocene of Darjeeling foothills. They suggested that after the Pliocene the species might have migrated from the Darjeeling Himalayan region to adjoining southeast Asia (China, Myanmar, and Vietnam), the area of the present-day distribution of modern Sloanea, due to possible increasing aridity and rainfall seasonality, but again this is not evident in the data presented here.
12. ConclusionThis is the first thorough review of the eastern Himalayan Siwalik flora. Based on our overview of published palaeobotanical data from the Siwalik sediments, the following key points emerge:
(1) The eastern Himalaya has a rich Siwalik plant fossil record spanning the mid-Miocene to the Pleistocene, which, over the past two decades, has been investigated extensively with an increasing number of fossil taxa being reported. These fossil taxa are important for answering outstanding questions on plant diversity and floral evolution in this region.
(2) Plant species from the Cenozoic of the eastern Himalayas are diverse. To date, approximately 219 fossil species belonging to 162 genera of 42 families have been documented. They cover ferns, gymnosperm, and angiosperms, of which angiosperms are by far the most diverse, including 216 fossil species grouped into 159 genera of 39 families. In the fossil angiosperms, Fabaceae, Dipterocarpaceae, Lauraceae, Annonaceae, Euphorbiaceae, Calophyllaceae, Anacardiaceae, Apocynaceae, Rubiaceae, and Lythraceae are among the most diverse families; and Shorea, Dipterocarpus, Calophyllum, Millettia, Glochidion, Actinodaphne, Combretum, Bauhinia, Lagerstroemia, Uvaria, Rinorea Aubl., Sterculia, Ficus L., Terminalia, and Persea are among the most species-rich genera.
(3) Most of the families and genera represented in the fossil record are still part of the modern natural vegetation in the eastern Himalayas, but some taxa have disappeared. Thus far, fourteen taxa are known to have become extinct in the eastern Himalayas, namely Mastixia arborea, Shorea tumbuggaia, S. leprosula, S. obtusa, P. parviflora, C. cinerea, P. daltonii, C. inophyllum, L. bifaria, P. bengalensis, M. extensa, D. costulatum, R. caudata, and S. dasycarpa. In addition, phytogeographic exchanges of Siwalik elements of the eastern Himalayas with southeast Asia also occur.
(4) A gradual change in floral composition through the Siwalik succession is apparent. Floras changed significantly from the middle Miocene to the early Pleistocene through to today, but invoking climate change as the explanation is problematic as no distinctive (statistically significant) shift in climate metrics can be detected in foliar adaptations. If the climate contributed to these changes, it was through very subtle changes that affected overall taxon fitness, most likely intensification of rainfall seasonality.
(5) Here, we introduce new quantitative proxy palaeo-humidity measurements and explore new insights into the hydrological regime. Eastern Himalayan Siwalik forests experienced a monsoonal tropical warm humid climate. Tropical Siwalik forests of the eastern Himalaya prior to the Quaternary have a weaker monsoon (less rainfall seasonality) than now.
AcknowledgmentsMK and SM gratefully acknowledge the Department of Botany, Sidho-Kanho-Birsha University for providing infrastructural facilities to accomplish this work. SB acknowledges the Centre of Advanced Study (Phase-Ⅶ), the Department of Botany, the University of Calcutta for providing necessary facilities. We also thank Prof. Paul Valdes, Bristol University, U.K., for providing the modern WorldClim2 climate data for the fossil localities. RAS and TEVS were supported by NERC/NSFC BETR Project NE/P013805/1.
Declaration of competing interest
The authors declare that they have no competitive interest.
Author contributions
Data collection and analysis were performed by MAK, SM, RAS, TEVS, AA, TH and SB. The first draft of the manuscript was written by MAK, SM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.12.003.
Acharya, S.K., 1994. The Cenozoic foreland basin and tectonics of the eastern Sub-Himalaya: problem and prospects. Himal. Geol., 15: 3-21. |
Acharyya, S.K., Bhatt, D.K., Sen, M.K., 1987. Earliest Miocene planktonic foraminifera from Kalijhora area, tista river section, Darjeeling sub-Himalaya. Ind. Miner., 41: 31-37. |
Ambwani, K., 1992. Leaf impressions belonging to the Tertiary age of northeast India. Phytomorphology, 41: 139-146. |
Anand-Prakash, Singh, T., 2000. Nature, composition, rank (maturation) and depositional environment of Siwalik coals from Arunachal Himalaya. Mycol. Prog., 21: 17-29. |
Antal, J.S., Awasthi, N., 1993. Fossil flora from the Himalayan foot-hills of Darjeeling district, West Bengal and its palaeoecological and phytogeographical significance. Palaeobotanist, 42: 14-60. DOI:10.54991/jop.1993.1129 |
Antal, J.S., Prasad, M., 1995. Fossil leaf of Clinogyne Salisb. From the siwalik sediments of Darjeeling district, West Bengal. Geophytology, 24: 2412-2443. |
Antal, J.S., Prasad, M., 1996a. Some more leaf-impressions from the Himalayan foothills of Darjeeling district, West Bengal, India. Palaeobotanist, 43: 1-9. |
Antal, J.S., Prasad, M., 1996b. Dipterocarpaceous fossil leaves from Grish River section in Himalayan foot hills near Oodlabari, Darjeeling district, West Bengal. Palaeobotanist, 43: 73-77. |
Antal, J.S., Prasad, M., 1996c. Leaf-impressions of Polyalthia Bl. in the siwalik sediments of Darjeeling district, West Bengal. Geophytology, 26: 125-127. |
Antal, J.S., Prasad, M., 1997. Angiospermous fossil leaves from the Siwalik sediments (Middle-Miocene) of Darjeeling district, West Bengal. Palaeobotanist, 46: 95-104. DOI:10.54991/jop.1997.1353 |
Antal, J.S., Prasad, M., 1998. Morphotaxonomic study of some more fossil leaves from the lower Siwalik sediments of West Bengal, India. Palaeobotanist, 47: 86-98. |
Antal, J.S., Prasad, M., Khare, E.G., 1996. Fossil woods from the Siwalik sediments of Darjeeling District, West Bengal, India. Palaeobotanist, 43: 98-105. |
Awasthi, N., Mehrotra, R.C., 1990. Some fossil woods from Tipam sandstone of Assam and Nagaland. Palaeobotanist, 38. |
Awasthi, N., Mehrotra, R.C., 1997. Some fossil dicotyledonous woods from the Neogene of Arunachal Pradesh, India. Palaeontograph. Abteilung B, 245: 109-121. |
Baishya, A.K., Haque, S., Bora, P.J., et al., 2001. Flora of Arunachal Pradesh-an overview. Arunachal Floral News, 19: 1-24. |
Bande, M., Prakash, U., 1986. The Tertiary flora of Southeast Asia with remarks on its palaeoenvironment and phytogeography of the Indo-Malayan region. Rev. Paleobot. Palynol., 49: 203-233. DOI:10.1016/0034-6667(86)90028-X |
Bera, S., De, A., De, B., 2004. First record of Elaecarpus Linn. fruits from the upper siwalik sediments (Kimin formation) of Arunachal Pradesh, India. J. Geol. Soc. India, 64: 350-352. |
Bera, S., Gupta, S., Khan, M.A., et al., 2014. First megafossil evidence of Cyatheaceous tree fern from the Indian Cenozoic. J. Earth Syst. Sci., 123: 1433-1438. DOI:10.1007/s12040-014-0460-x |
Bera, M., Khan, M.A., Bera, S., 2018. Two new species of Phomites fritel from the phyllosphere of siwalik. J. Mycopathol. Res., 56: 11-14. DOI:10.17951/j.2017.30.4.11 |
Bera, M., Khan, M.A., Bera, S., 2019. A new foliicolous melioloid fungus from the Pliocene of eastern Himalaya. Mycol. Prog., 18: 921-931. DOI:10.1007/s11557-019-01502-5 |
Bera, M., Khan, M.A., Acharya, K., et al., 2022a. In situ occurrence of Phomites fritel in the phyllosphere of ancient siwalik forests of eastern Himalaya during the miopleistocene. In: Rai, M., et al. (Eds.), Phoma: Diversity, Taxonomy, Bioactivities, and Nanotechnology. https://doi.org/10.1007/978-3-030-81218-8_18.
|
Bera, M., Khan, M.A., Hazra, T., et al., 2022b. A novel fossil-species of Meliolinites Selkirk (fossil Meliolaceae) and its life cycle stages associated with an angiosperm fossil leaf from the Siwalik (Mio-Pliocene) of Bhutan sub-Himalaya. Fungal Biol., 126: 576-586. DOI:10.1016/j.funbio.2022.07.003 |
Bhatia, H., Srivastava, G., Spicer, R.A., et al., 2021. Leaf physiognomy records the Miocene intensification of the south Asia monsoon. Global Planet. Change, 196: 103365. DOI:10.1016/j.gloplacha.2020.103365 |
Bhatia, H., Srivastava, G., Adhikari, P., et al., 2022. Asian monsoon and vegetation shift: evidence from the Siwalik succession of India. Geol. Mag., 159: 1397-1414. DOI:10.1017/s0016756822000243 |
Bhattacharyya, B., 1967. Tertiary plant fossils from cherrapunji and Laitryngew in Khasi and Jainta hills, Assam. Q. J. Geol. Min. Metall. Soc. India, 39: 131-134. |
Biswas, S.K., Ahuja, A.D., Saproo, M.K., et al., 1976. Geology of himalayan foot-hills, Bhutan. In: Cyclostyled Paper Presented at the Himalayan Geology Seminar, New Delhi.
|
Boos, W.R., Kuang, Z., 2010. Dominant control of the South Asian monsoon by orographic insulation versus plateau heating. Nature, 463: 218-222. DOI:10.1038/nature08707 |
Bora, D.S., Shukla, U.K., 2005. Petrofacies implication for the lower Siwalik foreland basin evolution, Kumaun Himalaya, India. Spec. Pub. Palaeontol. Soc. India, 2: 163-179. |
Brooks, T.M., Mittermeier, R.A., da Fonseca, G.A.B., et al., 2006. Global biodiversity conservation priorities. Science, 313: 58-61. DOI:10.1126/science.1127609 |
CEPF, 2005. Ecosystem Profile: Indo-Burman Hotspot, Eastern Himalayan Region. WWF, US-Asian Programme /CEPF, Kathmandu.
|
CEPF, 2007. Ecosystem Profile: Indo-Burma Hotspot, Indo-China Region. Critical Ecosystem Partnership Fund, Birdlife International, UK.
|
Chakrabarti, B.K., 2016. Geology of the Himalayan Belt Deformation, Metamorphism, Stratigraphy. Elsevier, pp. 12-46.
|
Chakraborty, T., Taral, S., More, S., et al., 2020. Cenozoic Himalayan foreland basin: an overview and regional perspective of the evolving sedimentary succession. Geodyn. Ind. Plate: 395-437. DOI:10.1007/978-3-030-15989-4_11 |
Champion, H.G., Seth, S.K., 1968. A Revised Survey of the Forest Types in India. Manager of Publication, Delhi.
|
Chatterjee, S., Scotese, C.R., 1999. The breakup of Gondwana and the evolution and biogeography of Indian plate. Proc. Natl. Acad. Sci. India, 65A: 397-425. |
Chirouze, F., Dupont-Nivet, G., Huyghe, P., et al., 2012. Magnetostratigraphy of the Neogene siwalik group in the far eastern Himalaya: Kameng section, Arunachal Pradesh, India. J. Asian Earth Sci., 44: 117-135. DOI:10.1016/j.jseaes.2011.05.016 |
Coutand, I., Barrier, L., Govin, G., et al., 2016. Late Miocene-Pleistocene evolution of India-Eurasia convergence partitioning between the Bhutan Himalaya and the Shillong Plateau: new evidences from foreland basin deposits along the Dungsam Chu section, eastern Bhutan. Tectonics, 35: 2963-2994. DOI:10.1002/2016TC004258 |
Das, P., Khan, M.A., De, B., et al., 2007. Evidence of exoparasitic relationship between Asterina (Asterinaceae) and Chonemorpha (Apocynaceae) from the upper siwalik (Kimin formation) sediments of arunachal sub-Himalaya, India. J. Mycopathol. Res., 45: 225-230. |
Debnath, A., Taral, S., Mullick, S., et al., 2021. The Neogene Siwalik succession of the Arunachal Himalaya: a revised lithostratigraphic classification and its implication for the regional paleogeography. J. Geol. Soc. India, 97: 339-350. DOI:10.1007/s12594-021-1692-4 |
Ding, L., Spicer, R.A., Yang, J., et al., 2017. Quantifying the rise of the Himalaya orogen and implications for the south Asian monsoon. Geology, 45: 215-218. |
Dwivedi, H.D., Prasad, M., Tripathi, P.P., 2006. Angiospermous fossil leaves from the lower Siwalik sediments of Koilabas area, western Nepal and their significance. J. Appl. Biol. Sci., 32: 135-142. |
Farnsworth, A., Lunt, D.J., Robinson, S.A., et al., 2019. Past east Asian monsoon evolution controlled by paleogeography, not CO2. Sci. Adv., 5: eaax1697. DOI:10.1126/sciadv.aax1697 |
Ferguson, D.K., 1985. The origin of leaf-assemblages: new light on an old problem. Rev. Palaeobot. Palynol., 46: 117-188. DOI:10.1016/0034-6667(85)90041-7 |
Ferguson, D.K., Zetter, R., Paudayal, K.N., 2007. The need for the SEM in palaeopalynology. Comptes Rendus Palevol, 6: 423-430. DOI:10.1016/j.crpv.2007.09.018 |
Fick, S.E., Hijmans, R.J., 2017. WorldClim2: new 1-km spatial resolution climate surfaces for global land surfaces. Int. J. Climatol., 37: 4302-4315. DOI:10.1002/joc.5086 |
Ganguly, S., Rao, D.P., 1970. Stratigraphy and structure of the tertiary foothills of eastern Himalaya. Darjeeling district. West bengal Quart. J. Geol. Min. Metal. Soc. India, 42: 185-195. |
Grierson, A.J.C., Long, D.G., 1983. Flora of Bhutan, vol. 1. Royal Botanical Garden, Edinburgh.
|
Guleria, J.S., 1992. Neogene vegetation of peninsular India. Palaeobotanist, 40: 285-311. |
Harris, I., Jones, P.D., Osborn, T.J., et al., 2014. Updated high-resolution grids of monthly climatic observations - the CRU TS3.10 Dataset. Int. J. Climatol., 34: 623-642. DOI:10.1002/joc.3711 |
Hazra, P.K., Verma, D.M., Giri, G.S., 1996. Materials for the Flora of Arunachal Pradesh, vol. 1. Bot. Sur. India, Calcutta.
|
Huffaker, C.B., 1942. Vegetational correlations with vapour pressure deficit and relative humidity. Am. Midl. Nat., 28: 486-500. DOI:10.2307/2420832 |
Huggett, R.J., 2004. Fundamentals of Biogeography. Routledge, New York, USA.
|
Johnson, N.M., Stix, J., Tauxe, L., et al., 1985. Paleomagnetic chronology, fluvial processes, and tectonic implications of the Siwalik deposits near Chinji village, Pakistan. J. Geol., 93: 27-40. DOI:10.1086/628917 |
Joshi, A., Mehrotra, R.C., 2003. A thelypteridaceous fossil fern from the lower Siwalik of the east Kameng district, Arunachal Pradesh, India. J. Geol. Soc. India, 61: 483-486. |
Joshi, A., Mehrotra, R.C., 2007. Mega remains from the Siwalik sediments of west and east Kameng Districts, Arunachal Pradesh. J. Geol. Soc. India, 69: 1256-1266. |
Joshi, A., Tewari, R., Mehrotra, R.C., et al., 2003. Plant remains from the Upper Siwalik sediments of West Kameng District, Arunachal Pradesh. India. J. Geol. Soc. India, 61: 319-324. |
Karunakaran, C., Ranga Rao, A., 1976. Status of exploration for the Hydrocarbons in the Himalayan region-contributions to the stratigraphy and structure. In: International Himalayan Geological Seminar India. Section Ⅲ. O. N. G. C, pp. 1-72.
|
Kaul, R.N., Haridasan, K., 1987. Forest type of Arunachal Pradesh- a preliminary study. J. Econ. Taxon. Bot., 9: 379-389. |
Khan, M.A., Bera, S., 2007. Dysoxylum miocostulatum sp. nov. -a fossil leaflet of Meliaceae from the lower Siwalik sediments of west Kameng district, Arunachal Pradesh, eastern India. Indian J. Geol., 79: 63-68. |
Khan, M.A., Bera, S., 2010. Record of fossil fruit wing of Shorea Roxb. From the Neogene of Arunachal Pradesh. Curr. Sci., 98: 1573-1574. |
Khan, M.A., Bera, S., 2012. Glochidion palaeogamblei sp. nov. e a new fossil leaf of Euphorbiaceae from the Pliocene sediments of Arunachal Pradesh, eastern India and its palaeoclimatic significance. In: Diversity and conservation of plants and traditional knowledge Bishen Singh Mahendra Pal Singh, Dehradun, pp. 149-154.
|
Khan, M.A., Bera, S., 2014a. On some fabaceous fruits from the siwalik sediments (middle Miocene-lower Pleistocene) of eastern Himalaya. J. Geol. Soc. India, 83: 165-174. DOI:10.1007/s12594-014-0028-z |
Khan, M.A., Bera, S., 2014b. New lauraceous species from the Siwalik Forest of Arunachal Pradesh, eastern Himalaya, and their palaeoclimatic and palaeogeographic implications. Turk. J. Bot., 38: 453-464. DOI:10.3906/bot-1308-35 |
Khan, M.A., Bera, S., 2016. Occurrence of Persea Mill. from the siwalik forest of Darjeeling, eastern Himalaya: paleoclimatic and paleogeographic implications. J. Earth Sci., 27: 882-889. |
Khan, M.A., Bera, S., 2017. First discovery of fossil winged seeds of Pinus L. (family Pinaceae) from the Indian Cenozoic and its paleobiogeographic significance. J. Earth Sci., 126: 1-11. |
Khan, M.A., De, B., Bera, S., 2007. A fossil fern-leaflet of family Thelypteridaceae from the Middle Siwalik sediments of West Kameng district, Arunachal Pradesh. J. Bot. Soc. Bengal, 61: 65-69. |
Khan, M.A., De, B., Bera, S., 2008. Fossil leaves resembling modern Terminalia chebula Retzius from the lower Siwalik sediments of Arunachal Pradesh, India. Pleione, 2: 38-41. |
Khan, M.A., De, B., Bera, S., 2009. Leaf-impressions of Calophyllum L. from the middle Siwalik sediments of Arunachal sub-Himalaya, India. Pleione, 3: 101-106. |
Khan, M.A., Ghosh, R., Bera, S., et al., 2011. Floral diversity during Plio-Pleistocene siwalik sedimentation (Kimin formation) in Arunachal Pradesh, India, and its palaeoclimatic significance. Palaeodivers. Palaeoenviron., 91: 237-255. DOI:10.1007/s12549-011-0059-z |
Khan, M.A., Spicer, R.A., Bera, S., et al., 2014a. Miocene to Pleistocene floras and climate of the eastern himalayan Siwaliks, and new palaeoelevation estimates for the namling-oiyug basin, tibet. Global Planet. Change, 113: 1-10. DOI:10.14201/ADCAIJ201438112 |
Khan, M.A., Spicer, R.A., Spicer, T.E.V., et al., 2014b. Fossil evidence of insect folivory in the eastern Himalayan Neogene Siwalik forests. Palaeogeogr. Palaeoclimatol. Palaeoecol., 410: 264-277. DOI:10.1016/j.palaeo.2014.05.043 |
Khan, M.A., Spicer, T.E.V., Spicer, R.A., et al., 2014c. Occurrence of Gynocardia odorata Robert Brown (Achariaceae, formerly Flacourtiaceae) from the Plio-Pleistocene sediments of Arunachal Pradesh, northeast India and its palaeoclimatic and phytogeographic significance. Rev. Palaeobot. Palynol., 211: 1-9. DOI:10.1016/j.revpalbo.2014.10.002 |
Khan, M.A., Bera, S., Ghosh, R., et al., 2015a. Leaf cuticular morphology of some angiosperm taxa from the Siwalik sediments (middle Miocene to lower Pleistocene) of Arunachal Pradesh, eastern Himalaya: systematic and palaeoclimatic implications. Rev. Palaeobot. Palynol., 214: 9-26. DOI:10.1016/j.revpalbo.2014.10.008 |
Khan, M.A., Bera, S., Spicer, R.A., et al., 2015b. Plant-arthropod associations from the Siwalik forests (middle Miocene) of Darjeeling sub-Himalaya, India. Palaeogeogr. Palaeoclimatol. Palaeoecol., 438: 191-202. DOI:10.1016/j.palaeo.2015.07.019 |
Khan, M.A., Spicer, R.A., Spicer, T.E.V., et al., 2016. Occurrence of Shorea Roxburgh ex C.F. Gaertner (Dipterocarpaceae) in the Neogene siwalik forests of eastern Himalaya and its biogeography during the cenozic of Southeast Asia. Rev. Palaeobot. Palynol., 233: 236-254. DOI:10.1016/j.revpalbo.2016.07.011 |
Khan, M.A., Spicer, R.A., Spicer, T.E.V., et al., 2017a. Evidence for diversification of Calophyllum L. (Calophyllaceae) in the Neogene siwalik forests of eastern Himalaya. Plant Syst. Evol., 303: 371-386. DOI:10.1007/s00606-016-1376-5 |
Khan, M.A., Spicer, R.A., Spicer, T.E.V., et al., 2017b. First occurrence of Mastixioid (Cornaceae) fossil in India and its biogeographic implication. Rev. Palaeobot. Palynol., 247: 83-96. DOI:10.1016/j.revpalbo.2017.08.006 |
Khan, M.A., Bera, M., Spicer, R.A., et al., 2018a. Evidence of simultaneous occurrence of tylosis formation and fungal interaction in a late Cenozoic angiosperm from the eastern Himalaya. Rev. Palaeobot. Palynol., 259: 171-184. DOI:10.1016/j.revpalbo.2018.10.004 |
Khan, M.A., Bera, M., Bera, S., 2018b. Vizellopsidites siwalika, a new fossil epiphyllous fungus from the Plio-Pleistocene of Arunachal Pradesh, eastern Himalaya. Nova Hedwigia, 107: 543-555. DOI:10.1007/s41870-018-0149-5 |
Khan, M.A., Bera, M., Spicer, R.A., et al., 2019a. Floral diversity and environment during the middle Siwalik sedimentation (Pliocene) in the Arunachal sub-Himalaya. Paleobiodivers. Paleoenviron., 99: 401-424. DOI:10.1007/s12549-018-0351-2 |
Khan, M.A., Bera, M., Spicer, R.A., et al., 2019b. Palaeoclimatic estimates for a latest Miocene-Pliocene flora from the Siwalik group of Bhutan: evidence for the development of the south Asian monsoon in the eastern Himalaya. Palaeogeogr. Palaeoclimatol. Palaeoecol., 514: 326-335. DOI:10.1016/j.palaeo.2018.10.019 |
Khan, M.A., Bera, M., Bera, S., 2019c. A new meliolaceos foliicolous fungus from the Plio-Pleistocene of Arunachal Pradesh, eastern Himalaya. Rev. Palaeobot. Palynol., 268: 55-64. DOI:10.22161/ijebm.3.3.1 |
Kovach, W.L., Spicer, R.A., 1996. Canonical correspondence analysis of leaf physiognomy: a contribution to the development of a new palaeoclimatological tool. Palaeoclimates, 2: 125-138. |
Lakhanpal, R.N., 1965. Occurrence of Zizyphus in the Siwaliks near Jawalamukhi. Curr. Sci., 34: 666-667. |
Lakhanpal, R.N., 1967. In: Fossil Rhamnaceae from the Lower Siwalik beds near Jawalamukhi, Himachal Pradesh, 3. Publication of Centre of Advance Study in Geology, Panjab University, Chandigarh, pp. 23-26.
|
Lakhanpal, R.N., 1970. Tertiary floras of India and their bearing on the historical geology of the region. Taxon, 19: 675-694. DOI:10.2307/1219280 |
Lakhanpal, R.N., Awasthi, N., 1992. New species of Fissistigma and Terminalia from the siwalik sediments of Balugoloa, Himachal Pradesh. Geophytology, 21: 49-52. |
Mandal, A., Samajpati, N., Bera, S., 2009. In situ occurrence of epiphyllous fungus Phomites Fritel from the lower Siwalik sediments of Darjeeling foothills. J. Bot. Soc. Bengal, 63: 37-40. |
Mandal, A., Samajpati, N., Bera, S., 2011. A new species of Meliolinites (fossil Meliolales) from the Neogene sediments of sub-Himalayan West Bengal, India. Nova Hedwigia, 92: 435-440. DOI:10.1127/0029-5035/2011/0092-0435 |
Medlicott, H.B., 1865. The coal of Assam, results of a brief visit to the coalfields that province in 1865; with geological note on Assam and the hills to the south of it. Mem. Geol. Sur. India, 4: 388-442. |
Mehrotra, R.C., Awasthi, N., Dutta, S.K., 1999. Study of fossil wood from the upper Tertiary sediments (Siwalik) of Arunachal Pradesh, India and its implication in palaeoecological and phytogeographical interpretations. Rev. Palaeobot. Palynol., 107: 223-247. DOI:10.1016/S0034-6667(99)00029-9 |
Mehrotra, R.C., Srivastava, G., Srikarni, C., 2018. Lagerstroemia L. wood from the Kimin Formation (upper Siwalik) of Arunachal Pradesh and its climatic and phytogeographic significance. J. Geol. Soc. India, 91: 695-699. DOI:10.1007/s12594-018-0925-7 |
Mitra, S., Banerjee, M., 2000. On the occurrence of epiphyllous Deuteromycetous fossil fungi Palaeocercospora siwalikensis gen. et. sp. nov. and Palaeocolletotrichum graminioides gen. et. sp. nov. from Neogene sediments of Darjeeling foothills, Eastern Himalaya. J. Mycopathol. Res., 37: 7-11. |
Mitra, S., Banerjee, M., 2004. Fossil fruit Derrisocarpon miocenicum gen. et. sp. nov. and leaflet Derrisophyllum siwalicum gen. et. sp. nov. cf. Derris trifoliata Lour. of Fabaceae from Siwalik sediments of Darjeeling foothills, eastern Himalaya, India with remarks on site of origin and distribution of the genus. Phytomorphology, 54: 253-263. |
Mitra, S., Bera, S., Banerjee, M., 2002. On a new epiphyllous fungus Palaeoasterina siwalika gen. et. sp. nov. from the Siwalik (middle Miocene) sediments of Darjeeling foothills, India with remarks on environment. Phytomorphology, 52: 285-292. |
Molnar, P., Boos, W.R., Battisti, D.S., 2010. Orographic controls on climate and paleoclimate of Asia: thermal and mechanical roles for the Tibetan Plateau. Annu. Rev. Earth Planet Sci., 38: 77-102. DOI:10.1146/annurev-earth-040809-152456 |
More, S., Rit, R., Khan, M.A., et al., 2018. Record of leaf and pollen cf. Sloanea (Elaeocarpaceae) from the middle siwalik of Darjeeling sub-Himalaya, India and its palaeobiogeographic implications. J. Geol. Soc. India, 91: 301-306. DOI:10.1007/s12594-018-0854-5 |
Morley, R.J., 2000. Origin and Evolution of Tropical Rain Forests. Chichester, UK, p. 27.
|
Mosbrugger, V., 1999. The nearest living relative method. In: Jones, T.P., Rowe), N.P. (Eds.), In fossil plants and spores: Modern techniques. Bath: Geol. Soc., London, pp. 261-265.
|
Mosbrugger, V., Utescher, T., 1997. The coexistence approach-a method for quantitative reconstructions of Tertiary terrestrial palaeoclimate data using plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol., 134: 61-86. DOI:10.1016/S0031-0182(96)00154-X |
Mosbrugger, V., Utescher, T., Dilcher, D.L., 2005. Cenozoic continental climatic evolution of Central Europe. Proc. Natl. Acad. Sci. U.S.A., 102: 14964-14969. DOI:10.1073/pnas.0505267102 |
New, M., Hulme, M., Jones, P., 1999. Representing twentieth-century space-time climate Variability. Part Ⅰ: development of a 1961-90 mean monthly terrestrial climatology. J. Clim., 12: 829-856. DOI:10.1175/1520-0442(1999)012<0829:RTCSTC>2.0.CO;2 |
New, M., Lister, D., Hulme, M., et al., 2002. A high-resolution data set of surface climate over global land areas. Clim. Res., 21: 1-15. DOI:10.3354/cr021001 |
Parkash, B., Sharma, R.P., Roy, A.K., 1980. The Siwalik group (Molasse) sediments shed by collision of continental plates. Sediment. Geol., 25: 127-159. DOI:10.1016/0037-0738(80)90058-5 |
Pathak, N.R., 1969. Megafossils from the foothills of Darjeeling district, India. J. Bot. Soc. Bengal, Calcutta, pp. 379-386.
|
Pilgrim, G.E., 1910. Preliminary note on a revised classification of the Tertiary freshwater deposits of India. Record Geol. Surv. India XL (3), 185-205.
|
Pilgrim, G.E., 1913. The correlation of the Siwaliks with mammal horizons of Europe. Rec. Geol. Sur. India, 43: 264-326. |
Pradhan, U.C., Lachungpa, S.T., 1990. Sikkim-himalayan Rhododendron. Primulaceae Books, Kalimpong, Darjeeling, p. 130.
|
Prakash, U., Prasad, M., 1984. Wood of Bauhinia from the lower Siwalik beds of Uttar Pradesh, India. Paleobotanist, 32: 140-145. DOI:10.54991/jop.1984.1372 |
Prasad, M., 1990. Fossil flora from the siwalik sediments of Koilabas, Nepal. Geohydrology, 19: 79-105. |
Prasad, M., 1994a. Siwalik (middle-Miocene) leaf impressions from the foot hills of the Himalaya, India. Tert. Res., 15: 53-90. |
Prasad, M., 1994b. Plant megafossils from the Siwalik sediments of Koilabas, central Himalaya, Nepal and their impact on palaeoenvironment. Palaeobotanist, 42: 126-156. |
Prasad, M., 2006. Plant fossils from Siwalik sediments of Himachal Pradesh and their palaeoclimatic significance. Phytomorphology, 56: 9-22. |
Prasad, M., 2008. Angiospermous fossil leaves from the Siwalik foreland and their paleoclimatic implication. Paleobotanist, 57: 177-215. DOI:10.54991/jop.2008.238 |
Prasad, M., Dwivedi, H.D., 2007. Systematic study of the leaf impressions from the Churia Formation of Koilabas area, Nepal and their significance. Palaeobotanist, 56: 39-54. |
Prasad, M., Tripathi, P.P., 2000. Plant megafossils from the Siwalik sediments of Bhutan and their climatic significance. Biol. Mem., 26: 6-19. |
Prasad, M., Antal, J.S., Tiwari, V.D., 1997. Investigation on plant fossil from Seria Naka in the Himalayan foot hills of Uttar Pradesh, India. Paleobotanist, 46: 13-30. DOI:10.54991/jop.1997.1344 |
Prasad, M., Antal, J.S., Tripathi, P.P., et al., 1999. Further contribution to the Siwalik flora from the Koilabas area, western Nepal. Palaeobotanist, 48: 49-95. DOI:10.54991/jop.1999.1291 |
Prasad, M., Ghosh, R., Tripathi, P.P., 2004. Floristic and climate during the Siwalik (middle Miocene) near Kathgodam in the Himalayan foothills of Uttaranchal, India. J. Palaeontol. Soc. India, 49: 35-93. |
Prasad, M., Kannaujia, A.K., Alok, Singh, S.K., 2015. Plant megaflora from the Siwalik (upper Miocene) of Darjeeling district, West Bengal, India and its palaeoclimatic and phytogeographic significance. Palaeobotanist, 64: 13-94. DOI:10.54991/jop.2015.103 |
Ranga Rao, A., Venkatechala, B.S., Sastri, V.V., 1979. Neogene/quaternary boundary and the Siwalik. In: Sastri, V.V.E.A. (Ed.), Field Conference on Neogene-Quaternary Boundary, India.
|
Ranga Rao, A., Agarwal, R.P., Sharma, U.N., et al., 1988. Magnetic polarity stratigraphy and vertebrate palaeontology of the upper Siwalik subgroup of Jammu Hills, India. J. Geol. Soc. India, 31: 361-385. |
Sahni, B., 1964a. Revision of Indian Fossil Plants-Monocotyledons. Monograph 1. Birbal Sahni Institute of Palaeobotany, Lucknow.
|
Sahni, B., 1964b. Revision of Indian Fossil Plants-part Ⅲ. Monocotyledons. Monograph 1. Birbal Sahni Institute of Palaeobotany, Lucknow.
|
Shashi, Pandey, S.M., Tripathi, P.P., 2006. Fossil leaf impressions from Siwalik sediments of Himalayan foot hills of Uttaranchal, India and their significance. Palaeobotanist, 55: 77-87. DOI:10.54991/jop.2006.97 |
Shashi, Pandey, S.M., Tripathi, P.P., 2008. Siwalik (middle Miocene) leaf impressions from Tanakpur area, Uttaranchal and their bearing on climate. Geophytology, 37: 99-108. |
Singh, G., 1975. On the discovery of first vertebrate fossil from the Upper Tertiary of Subansiri district, Arunachal Pradesh. Ind. Miner., 29: 65-67. DOI:10.1017/S0014479700006244 |
Singh, T., 1983. On the stratigraphic correlation of upper Tertiary of Arunachal Pradesh. Geol. Sur. India Mis. Publ., 43: 82-84. DOI:10.1378/chest.83.1.82 |
Singh, T., 2007. Geology of Itanagar capital complex, Arunachal Himalaya, with special reference to neotectonics. J. Geol. Soc. India, 70: 339-352. |
Singh, T., Prakash, U., 1980. Leaf-impressions from the Siwalik sediments of Arunachal Pradesh. Geohydrology, 10: 104-107. |
Spicer, R.A., 1991. Plant taphonomic processes. In: Allison, P.A., Briggs, D.E.G. (Eds.), Taphonomy: Releasing the Data Locked in the Fossil Record. Plenum Press, New York, pp. 71-113.
|
Spicer, R.A., Herman, A.B., 2010. The Late Cretaceous environment of the Arctic: a quantitative reassessment using plant fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol., 295: 423-442. |
Spicer, R.A., Wolfe, J.A., 1987. Plant taphonomy of late Holocene deposits in trinity (clair engle) lake, northern California. Paleobiology, 13: 227-245. |
Spicer, R.A., Valdes, P.J., Hughes, A.C., et al., 2019. New insights into the thermal regime and hydrodynamics of the early Late Cretaceous Arctic. Geol. Mag., 157: 1729-1749. |
Spicer, R.A., Yang, J., Spicer, T.E.V., et al., 2021. Woody dicot leaf traits as a palaeoclimate proxy: 100 years of development and application. Palaeogeogr. Palaeoclimatol. Palaeoecol., 562: 110138. |
Srivastava, R., Mehrotra, R.C., 2009. Plant fossils from Dafla Formation, West Kameng district, Arunachal Pradesh. Palaeobotanist, 58: 33-49. DOI:10.54991/jop.2009.80 |
Srivastava, G., Gaur, R., Mehrotra, R.C., 2015. Lagerstroemia L. from the middle Miocene Siwalik deposits, northern India: implication for Cenozoic range shifts of the genus and the family Lythraceae. J. Earth Syst. Sci., 124: 227-239. DOI:10.1007/s12040-014-0526-9 |
Srivastava, G., Mehrotra, R.C., Sirkarni, C., 2018. Fossil wood flora from the Siwalik group of Arunachal Pradesh, India and its climatic and phytogeographic significance. J. Earth Syst. Sci., 127: 1-22. |
Srivastava, G., Farnsworth, A., Bhatia, H., et al., 2021. Climate and vegetation change during the upper Siwalik-a study based on the palaeobotanical record of the eastern Himalaya. Paleobiodivers. Paleoenviron., 101: 103-121. DOI:10.1007/s12549-020-00457-w |
Su, T., Liu, Y.S., Jacques, F.M.B., et al., 2013. The intensification of the east Asian winter monsoon contributed to the disappearance of Cedrus (Pinaceae) in southwestern China. Quat. Res., 80: 316-325. DOI:10.1016/j.yqres.2013.07.001 |
Sundriyal, M., 1999. Distribution, Propagation and Nutritive Value of Some wild Edible Plants in the Sikkim Himalaya. High Altitude Plant Physiology Research Centre, HNB Garjhwal University, Srinagar (Garhwal) and GB Pant Institute of Himalayan Environment and Development, Sikkim Unit, Sikkim, India. PhD Thesis.
|
Takhtajan, A., 1969. Flowering Plants: Origin and Dispersal. Oliver & Body, Edinburgh.
|
Tang, Q., Zhang, X., Yang, X., et al., 2013. Cold winter extremes in northern continents linked to Arctic Sea ice loss. Environ. Res. Lett., 8: 014036. DOI:10.1088/1748-9326/8/1/014036 |
Tang, H., Eronen, J.T., Kaakinen, A., et al., 2015. Strong winter monsoon wind causes surface cooling over India and China in the Late Miocene. Clim. Past, 11: 63-93. DOI:10.5194/cpd-11-63-2015 |
Taral, S., Kar, N., Chakrobarty, T., 2017. Wave-generated structures in the Siwalik rocks of Tista valley, eastern Himalaya: implication for regional palaeogeography. Curr. Sci., 113: 887-901. |
Taral, S., Chakraborty, T., Huyghe, P., et al., 2019. Shallow marine to fluviatransition in the Siwalik succession of the Kameng River section, Arunachal Himalaya and its implication for foreland basin evolution. J. Asian Earth Sci., 184: 103980. |
Teodoridis, V., Kovar-Eder, J., Marek, P., et al., 2011. The integrated plant record vegetation analysis: Internet platform and online application. Acta Musei Natl. Pragae, Ser. B - Hist. Nat., 67: 159-164. |
ter Braak, C.J.F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology, 67: 1167-1179. DOI:10.2307/1938672 |
Thanukos, Anna, 2012. "Uniformitarianism: Charles Lyell". University of California Museum of Paleontology. (Accessed 23 July 2012).
|
Tripathi, P.P., Pandey, S.M., Prasad, M., 2002. Angiospermous leaf impressions from Siwalik sediments of Himalayan foot hills near Jarva, U.P. and their bearing on palaeoclimate. Biol. Mem., 28: 79-90. |
Tripathi, P.P., Pandey, P., Mishra, R.K., 2007. Leaf impressions from the Siwalik beds of south-eastern Bhutan and their climatic significance. Plant Archiv., 7: 169-173. |
Utescher, T., Bruch, A.A., Erdei, B., et al., 2014. The Coexistence Approach - theoretical background and practical considerations of using plant fossils for climate quantification. Palaeogeogr. Palaeoclimatol. Palaeoecol., 410: 58-73. |
Valdiya, K.S., 2002. Emergence and evolution of Himalaya: reconstructing history in the light of recent studies. Prog. Phys. Geogr., 26: 360-399. |
Varma, C.P., 1968. On a collection of leaf-impressions from Hardwar, Uttar Pradesh. J. Palaeontol. Soc. India, 5-9: 83-88. |
Vishnu (née Mandal), A., Khan, M.A., Bera, M., et al., 2017. Fossil Asterinaceae in the phyllosphere of the eastern himalayan Neogene siwalik forest and their palaeoecological significance. Bot. J. Linn. Soc., 185: 147-167. DOI:10.1093/botlinnean/box050 |
Vishnu, A., Khan, M.A., Bera, M., et al., 2019. Occurrence of phoma Sacc. In the phyllosphere of Neogene siwalik forest of arunachal sub-Himalaya and its palaeoecological implications. Fungal Biol., 123: 18-28. |
Wolfe, J.A., 1993. A method of obtaining climatic parameters from leaf assemblages. Geol. Soc. Am. Bull., 2040: 1-73. |
WWF ICIMOD, 2001. Ecoregion-based Conservation in the Eastern Himalaya: Identifying Important Areas for Biodiversity Conservation. WWF-Nepal, Kathmandu.
|
Yang, J., Spicer, R.A., Spicer, T.E.V., Li, C.S., 2011. 'CLAMP online': a new web-based palaeoclimate tool and its application to the terrestrial Paleogene and Neogene of North America. Palaeobiol. Palaeoenvir., 91: 163-183. DOI:10.1007/s12549-011-0056-2 |
Yang, J., Spicer, R.A., Spicer, T.E.V., et al., 2015. Leaf form-climate relationships on the global stage: an ensemble of characters. Global Ecol. Biogeogr., 10: 1113-1125. DOI:10.1111/geb.12334 |
Zachos, J.C., Pagani, M., Sloan, L., et al., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686-693. |
Zachos, J.C., Dickens, G.R., Zeebe, R.E., 2008. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature, 451: 279-283. DOI:10.1038/nature06588 |



