b. Academy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
Fabaceae (Leguminosae) is a large and diverse family that has three subfamilies (Caesalpinioideae, Mimosoideae, and Papilionoideae) and envelops about 770 genera and over 19,500 species (Lewis et al., 2005; LPWG, 2013, 2017). Based on recent molecular phylogenetic studies, six subfamilies, namely Caesalpinioideae, Cercidoideae, Detarioideae, Dialioideae, Duparquetioideae and Papilionoideae, have been recognized (Lavin et al., 2005; Bruneau et al., 2008; LPWG, 2017). Fabaceae is cosmopolitan in distribution, representing one of the most spectacular examples of evolutionary diversification in plants. The family is morphologically, physiologically and ecologically exceptionally diverse and is only behind Asteraceae and Orchidaceae. Fabaceae trails Poaceae in agricultural importance, although the spectrum of legume uses is much greater. Legumes are diverse in areas of varied topography with seasonal climates (Axelrod, 1992; LPWG, 2017), extending from lowland rainforests to deciduous and semi-arid forests and savannas (Schrire et al., 2005a, b; Yahara et al., 2013). In India, the family is represented by 179 genera and 1297 species, of which about 23% are strictly confined to India. Moreover, these species are dominantly found in biodiversity hotspots of India such as northeast India, including eastern Himalaya (about 767 species) and the Western Ghats (Sanjappa, 1991, 1995; Chavan et al., 2013).
Legume fossils have been discovered from the Paleocene sediments across the globe (Table 1). The biogeography of legumes is questionable due to ambiguous fossil records (Ma et al., 2017). The Paleocene legume records can provide an opportunity to better understand the ecological niches during its early divergence when the Earth was globally warm and without polar ice caps (Zachos et al., 2001). Attempts have been made to understand the early evolution, diversification and ecology of legumes but due to high species diversity this is still not clear. The fossil records of legumes from the aforesaid period are important in understanding the early ecology of legumes.
| Fossil species | NLR | Organ | Age | Locality | Lat-Long | Reference |
| Legume fruit and leaflets | Fabaceae | Fruit and leaflets | Earliest Paleocene (~65.35 Ma) | Corral Bluffs section, Colorado, USA | 38.86 N, 104.62 W | Lyson et al. (2019) |
| Legume leaflet | Fabaceae | Leaflet | Early Paleocene | Palacio de Los Loros locality, Chubut, Argentina | 45.91 S, 69.29 W | Iglesias et al. (2007) |
|
Paracacioxylon frenguellii
Brea et al. |
Mimosoideae | Wood | Early Paleocene | Palacio de Los Loros locality, Chubut, Argentina | 45.91 S, 69.29 W | Brea et al. (2008) |
| Fruit morphotype 1, 2, 4, 6, 7 | Fabaceae | Fruit, leaflets | Middle to late Paleocene | Cerrejon coalmine, Guajira Peninsula, Colombia | 11.13 N, 72.5 W | Herrera et al. (2019) |
| Leaf morphotype 1, 4, 5, 6 | ||||||
| Fruit morphotype 3, 5, 8 | Fabaceae | Fruit, leaflets | Middle to late Paleocene | Sabana de Bogotá, Colombia | 5.17 N, 74.06 W | Herrera et al. (2019) |
| Leaf morphotype 2, 3 | ||||||
| Leaf and fruit | Fabaceae | 6 Leaflets and 4 fruit types | Middle to late Paleocene | Cerrejon, Colombia | 11.13 N, 72.5 W | Wing et al. (2009) |
| Leguminocarpon gardneri (Chandler) Herendeen and Crane | Caesalpinioideae | Fruit | Late Paleocene | Cold Ash Quarry, England | 51.43 N, 1.28 W | Herendeen and Crane (1992) |
| Paleobowdichia lamarensis Herendeen, D.B.O.S. Cardoso, F. Herrera and Wing | Papilionoideae | Fruit, leaflets | Latest Paleocene-late early Eocene | Northwestern Bighorn Basin, Wyoming, United States | 44.83 N, 109.07 W | Herendeen et al. (2022) |
| Tobya claibornensis (Herendeen et Dilcher) Herendeen, D.B.O.S. Cardoso, F. Herrera and Wing | Papilionoideae | Fruit | Latest Paleocene-late early Eocene | Northwestern Bighorn Basin, Wyoming, United States | 44.83 N, 109.07 W | Herendeen et al. (2022) |
| Legume fruit | Fabaceae | Fruit and leaflet | Late Paleocene-early Eocene | Southeastern Bighorn Basin, Wyoming, United States | 43.96 N, 107.65 W | Wing et al. (2005) |
| Leguminocarpon albizoides Bhattacharyya, L. derrisoides Bhattacharyya, L. desmodioides Bhattacharyya, L. millettioides Bhattacharyya, L. pongamioides Bhattacharyya | Fabaceae | Fruit | Late Paleocene | Garo Hills, Nangwalbibra, India | 25.44 N, 90.71 E | Bhattacharyya (1985) |
| Leguminocarpum meghalayensis Bhatia, Srivastava et Mehrotra | Fabaceae | Fruit | Late Paleocene | Garo Hills, Damalgiri, India | 25.32 N, 90.07 E | Present study |
| Parvileguminophyllum damalgiriensis Bhatia, Srivastava et Mehrotra | Fabaceae | Leaflet | Late Paleocene | Garo Hills, Damalgiri, India | 25.32 N, 90.07 E | Present study |
Here we report a well-preserved pod and leaflet of legume from the late Paleocene sediments of Tura Formation of Meghalaya, India (Fig. 1). During the deposition of the late Paleocene sediments, the fossil locality was in the southern Hemisphere ~7°S paleolatitude (Scotese, 2001) (www.odsn.de) which is in contrast to the modern latitude (25.44°N).
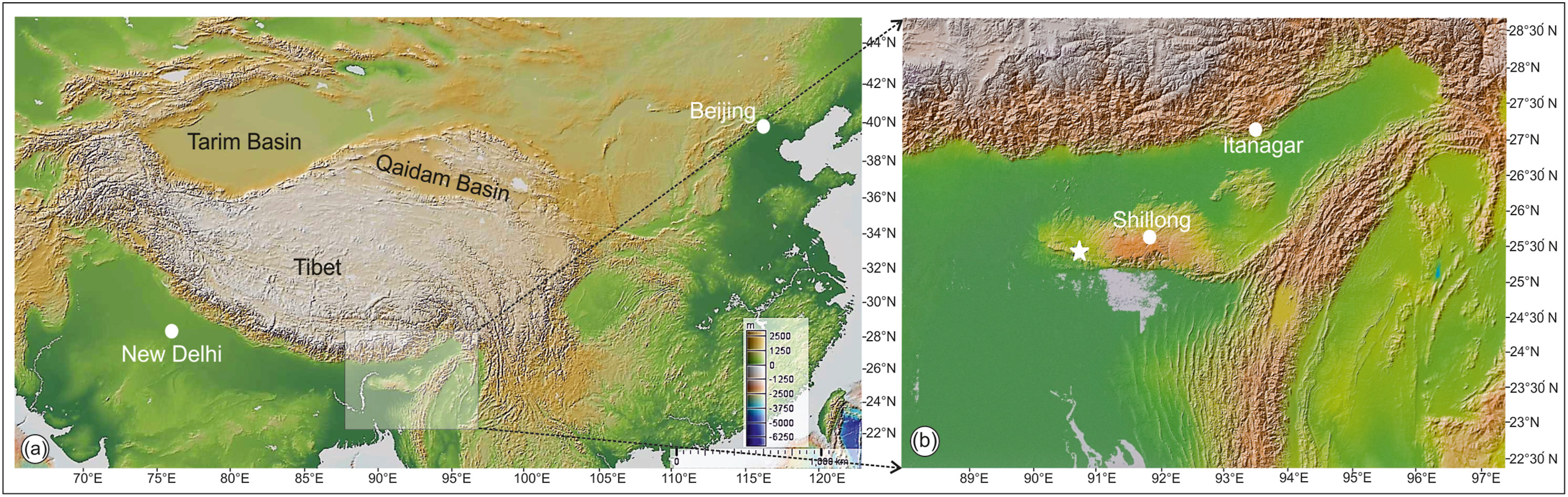
|
| Fig. 1 Physiographic map of south Asia showing the fossil locality. (a) Map of south Asia showing the fossil locality (white asterisk) in shaded area. (b) Enlarged map of shaded area showing the present fossil locality (white asterisk) and nearby cities (white solid circles). |
Fossil fruit and leaflet were collected from the late Paleocene sediments of the Tura Formation, which are well exposed in Damalgiri near Tura, Meghalaya, India (Fig. 1). The exact locality is 16 km southwest of Tura. The fruit and leaflet are preserved as compressions/impressions. The specimens were first cleaned with the help of a fine chisel and hammer and then photographed using a 10-megapixel digital camera (Canon SX110) in low-angled natural light. Details of the fossils were examined under a Leica S8APO stereomicroscope. The terminology used in describing specimens follows Dilcher (1974), Herendeen (1992) and Ellis et al. (2009). To determine the nearest relatives of these fossils, we compared them to specimens at the Central National Herbarium (CNH), Howrah and Forest Research Institute (FRI), Dehradun, India. We also consulted the website of the Royal Botanical Gardens, Kew (apps.kew.org/herbcat/navigator.do; accessed on July 23, September 10, 2020 and August 20, 2021). Systematic relationship of fossils was evaluated through comparison of morphological features such as shape, size, valve striation and venation pattern. The fossils have been deposited in the museum of the Birbal Sahni Institute of Palaeosciences, Lucknow, India.
3. Geological settingsThe Tura Formation is well exposed near Tura, which is situated in the Garo Hills district of Meghalaya, India. The formation is divided into lower, middle, and upper members (Biswas, 1962; Chakarborty and Baksi, 1972; Bakshi, 1974; Raja Rao, 1981). The lower member is composed of massive sandstones with pebbly interbeds, while the middle member consists of argillaceous sandstones and lithomargic clays, along with coal seams. The upper member is arenaceous with few pebbly interbeds (Biswas, 1962; Bakshi, 1974; Raja Rao, 1981). A general stratigraphic succession is provided in Table 2. Plant megafossils are found in the bed of the middle member, which contains greyish-white to buff coloured clay stones (Bhattacharyya, 1985) (Fig. 2). Based on the lithostratigraphy and biostratigraphy, the age of the Tura Formation has been assigned as late Paleocene (Sah and Singh, 1974; Saxena et al., 1996; Ambwani and Kar, 2000; Monga et al., 2014). Saxena et al. (1996) have suggested that the palynofloral assemblage of Tura Formation has taxonomic uniformity with the Cherra and Lakadong Sandstone formations of the Khasi Hills (Dutta and Sah, 1970; Sah and Dutta, 1974; Kar and Kumar, 1986), the Therria Formation of Jaintia Hills, Meghalaya (Tripathi and Singh, 1984), and the Mikir Formation of the North Cachar Hills, Assam (Mehrotra, 1981). Because all the aforesaid formations are considered as Paleocene in age, Saxena et al. (1996) have suggested the same age for the Tura Formation. Ambwani and Kar (2000) have suggested a more specific age, i.e., the late Paleocene, for the Tura Formation based on characteristic pollen taxa previously described by Sah and Singh (1974), including Dandotiaspora telonata, D. pseudoreticulata, D. plicata, Polycolpites speciosus, P. cooksoniae, Lycopodiumsporites paleocenicus, Proxapertites microreticulatus, Matanomadhiasulcites maximus, Retitribrevicolporites matanomadhensis, Tricolpites levis, Neocouperipollis rarispinosus, and N. brevispinosus. The late Paleocene age for the Tura Formation has been subsequently supported by Monga et al. (2014). Characteristic plant megafossils reported from the Tura Formation also support the late Paleocene age (Mehrotra et al., 1998; Mehrotra, 2000; Srivastava et al., 2018).
| Age | Stratigraphic units | Lithology | Thickness |
| Miocene | Angartoli Formation | Fine-grained, non-feldspathic, micaceous sandstone, bluish siltstone and sandy shales | – |
| Boldamgiri Formation | Coarse-grained, gritty, feldspathic, ferruginous sandstone with carbonaceous shale | – | |
| Oligocene | Kherapara Formation | Fine-grained alterations of thinly bedded sandstone and shale, thickly bedded sandstone and carbonaceous shale | – |
| Late Eocene | Rewak Formation | Thinly bedded shale and carbonaceous shale, sandstones with molluscan fauna and coal streaks | 450–500 m |
| Middle Eocene | Siju Formation | Banded alternations of arenaceous, hard foraminiferal limestones with pyrite, carbonaceous shales, Marl with molluscan fauna, massive limestones | 100–160 m |
| Paleocene–Early Eocene | Tura Formation | Medium to coarse-grained and gritty, clayey, dirty white, yellow and reddish, non-feldspathic, current bedded sandstone with intercalations of grey shale, carbonaceous shale, siltstone, lithomargic clay and coal | 189–250 m |
| Precambrian | Basement Complex | Granite and granitic gneisses | – |
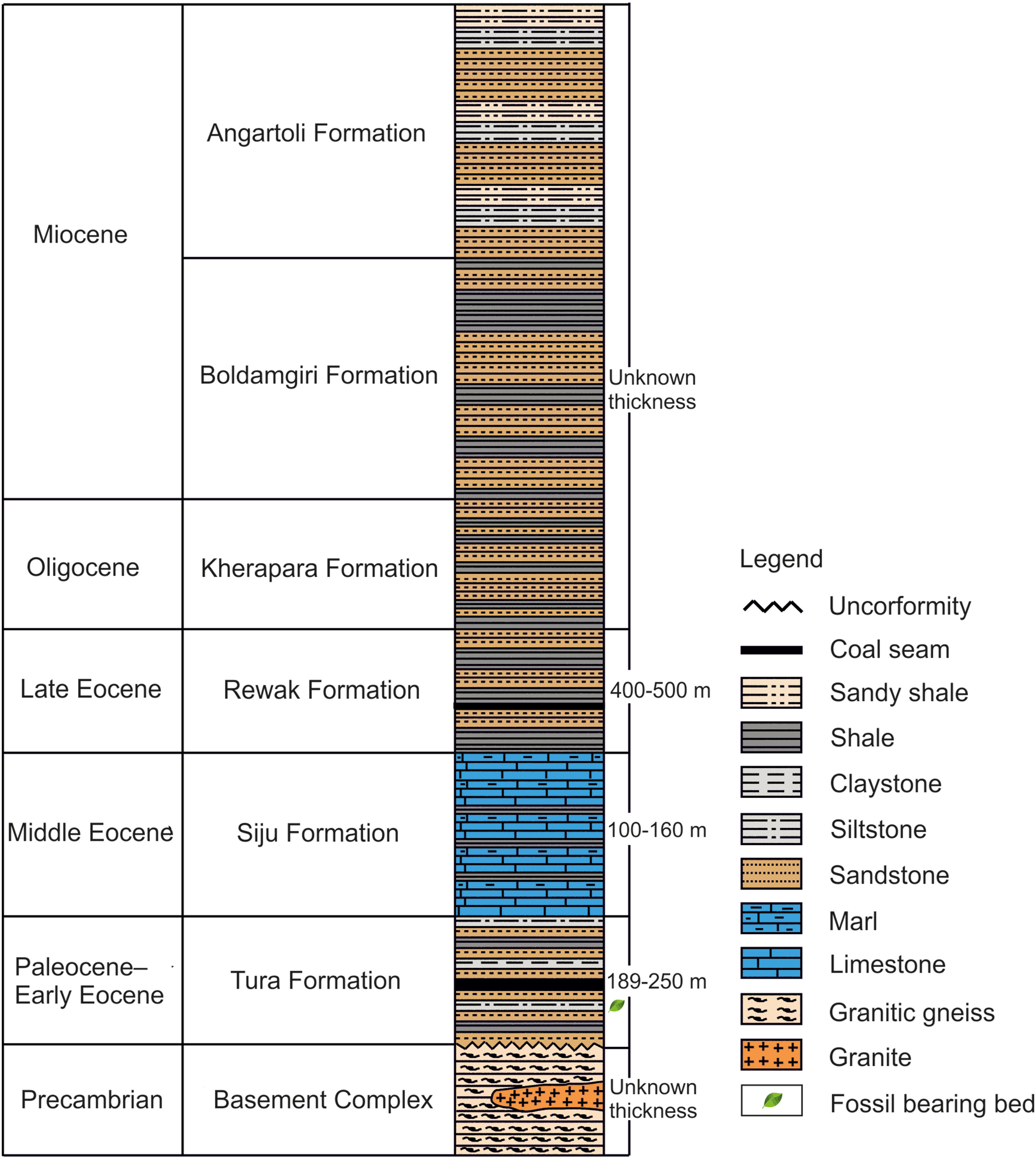
|
| Fig. 2 Generalized stratigraphic succession of the Garo Hills (after Chakraborty and Baksi, 1972). |
Order: Fabales Bromhead.
Family: Fabaceae Lindl.
Genus: Leguminocarpum Dotzler.
Species: Leguminocarpum meghalayensis Bhatia, Srivastava et Mehrotra sp. nov. (Fig. 3).
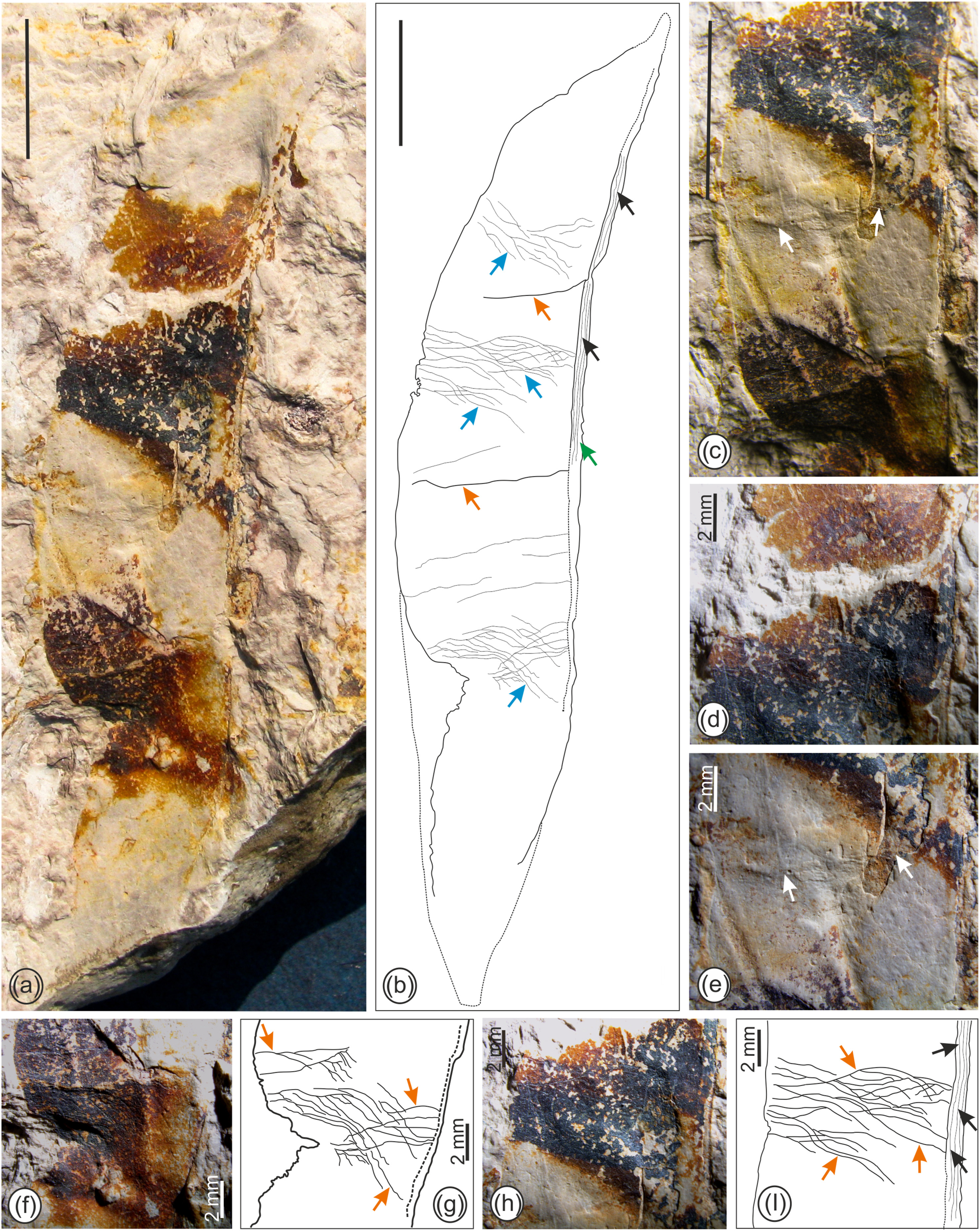
|
| Fig. 3 Leguminocarpum meghalayensis Bhatia, Srivastava et Mehrotra sp. nov. (bar scale = 1 cm, unless specified). (a) Fossil fruit shape, size, valve venation, fracture marks and sutures. (b) Diagram of the fossil fruit showing fascicles of vascular strands (black arrows) preserved on suture with a flange (green arrow), fracture marks (orange arrows) and transverse to slightly oblique striations (sky blue arrows). (c) Magnified middle portion of the fossil showing suture, fracture mark (white arrows) and striations present on the valve. (d) Magnified upper portion of the fossil fruit showing valve venations. (e) Magnified view of the fracture mark (white arrows) nearly perpendicular to the suture present on the fossil. (f) Lower portion of the fossil fruit showing prominent striations present on the valve, closely spaced, transverse to slightly oblique in appearance, coalescing in the center. (g) Diagram of the fossil shown in (f) indicating pattern of striations (orange arrows) preserved on the valve. (h) Magnified middle portion of the fossil fruit showing pattern of striations preserved on the valve arising from the sutures and forming dense reticulate venation in the middle of the valve. (i) Diagram of fossil shown in (h) indicating striations (orange arrows) and vascular fascicles preserved on the suture (black arrows). |
Etymology: The specific epithet “meghalayensis” is named after the fossil locality from where the specimens have been recovered.
Holotype: Specimen no. BSIP 42119.
Repository: Birbal Sahni Institute of Palaeosciences, Lucknow.
Type locality: Damalgiri near Tura, Meghalaya, India.
Horizon: Tura Formation.
Age: Late Paleocene.
Specific diagnosis: Fruit, straight, flattened, broadly linear in shape, wingless; sutures visible, narrow to medium in thickness; valve venation dense, consisting of numerous closely spaced veins, transverse to slightly oblique, anastomosing in the middle, forming a dense reticulum; suture thickened with fascicles of vascular strands and with narrow flange measuring 0.6–1.0 mm in width; fracture marks perpendicular to suture observed.
Description: Fossil well preserved, straight, flattened pod without wings, broadly linear in shape (Fig. 3a and b); the valve texture possibly chartaceous; preserved pod length 6.8 cm and width 1.5 cm; apex and base poorly preserved but seemingly acute and tapered in appearance; margin straight to slightly constricted; seeds or seed chamber not observed; sutures distinct, medium to broad in thickness, with fascicles of vascular strands and thickened into a flange (Fig. 3a–c, h, i) measuring 0.6–1.0 mm wide; difficult to distinguish between placental and non-placental sutures; dense and prominent valve venation present, closely spaced, at some places veins not discernible (Fig. 3b, f–i); transverse to slightly oblique in appearance, veins arising from sutures, dividing several times, at some places bifurcating and generally forming dense reticulate pattern in the middle of the valve; fracture marks present on valves, perpendicular to the suture (Fig. 3b–e).
Affinities: The key characters of the fossil fruit are shape, length, width, apex, base, marginal suture, and striations, which distinctly represent a legume. Morphologically it resembles numerous legume fruits and is difficult to identify to an extant genus. We have compared our fossil fruit with many similar extant and fossil taxa (Appendix A) as shown in Fig. 4. Wang (2012) suggested that fossil fruits earlier assigned to the organ genus Leguminosites Bowebank with uncertain affinities should be assigned to Leguminocarpum Dotzler. Moreover, Leguminocarpon Göppert, Leguminocarpos Göppert, Leguminocarpum Göppert (non Dotzler) and Leguminocarpas Miki used for fossil legume fruits should be abandoned because they are not validly published names (Wang, 2012). Due to insufficient preservation, we follow Wang (2012) and describe the present fossil fruit under the morphogenus Leguminocarpum.

|
| Fig. 4 Comparable extant fruits of Fabaceae (bar scale = 1 cm, unless specified). (A) Acacia xiphocarpa Hochst. ex Benth. (Kew Herbarium sheet no. K000244245); (B) Milletia ovalifolia Kurg. (FRI Herbarium sheet no. 116553); (C) Rhynchosia rojazii Hassl. (Kew Herbarium sheet no. K000836123); (D) Vicia dennesiana H.C. Watson (Kew Herbarium sheet no. K000418313); (E) Milletia auriculata Baker ex Brand. (FRI Herbarium sheet no. 1914/145736); (F) Dioclea virgata (Rich.) Amshoff. (Kew Herbarium sheet no. K000921818); (G) Clitoria aeborea Hoffm. ex Benth. (Kew Herbarium sheet no. K000920635); (H) Millettia auriculata Baker ex Brand. (FRI Herbarium sheet no. 57/104); (I) Erythrina falcata Benth. (Kew Herbarium sheet no. K000930967); J. Acacia alliacea Buch.-Ham. ex Wall. (Kew Herbarium sheet no. K001120305). All the herbarium sheets are © copyright of the Board of Trustees of the Royal Botanic Garden, Kew. |
While comparing the present fossil with previously described fruits, we gave particular attention to key characters such as straight to linear, flattened fruit with a vascularized flange present on one of the sutures and transverse to slightly oblique venation. Herrera et al. (2019) described eight fruit morphotypes from the middle to late Paleocene of Colombia. These fruit morphotypes look similar to the present fruit but differ in shape, valve venation and absence of vascularized flange. Leguminocarpon gardneri (Chandler) Herendeen and Crane from the late Paleocene of England (Herendeen and Crane, 1992) and Paleobowdichia lamarensis (Knowlton) Herendeen, D.B.O.S. Cardoso, F. Herrera and Wing and Tobya claibornensis (Herendeen et Dilcher) Herendeen, D.B.O.S. Cardoso, F. Herrera and Wing from latest Paleocene to late early Eocene of United States (Herendeen et al., 2022) also show some similarity with the present fossil but the presence of wings is the major difference. Bhattacharyya (1985) described a few legume fruits from the Paleocene of Meghalaya, India, such as Leguminocarpon albizoides Bhattacharyya, L. derrisoides Bhattacharyya, L. desmodioides Bhattacharyya, L. millettioides Bhattacharyya, and L. pongamioides Bhattacharyya, which bear some similarity with the present fossil fruit but differ in the absence of vascular fascicles, extended flange, pattern of valve venation, as well as shape and size (Appendix A). Given these circumstances, we propose a new fossil species L. meghalayensis Bhatia, Srivastava et Mehrotra sp. nov.
4.1.2. LeafGenus: Parvileguminophyllum Herendeen and Dilcher.
Type species: Parvileguminophyllum damalgiriensis Bhatia, Srivastava et Mehrotra sp. nov.
Etymology: The specific epithet “damalgiriensis” is named after the fossil locality from where the specimen was unearthed.
Holotype: Specimen no. BSIP 42120.
Repository: Birbal Sahni Institute of Palaeosciences, Lucknow.
Type locality: Damalgiri near Tura, Meghalaya, India.
Horizon: Tura Formation.
Age: Late Paleocene.
Specific diagnosis: Leaflet shape elliptic, seemingly asymmetrical, with obtuse to reflex asymmetric base; primary venation pinnate; major secondaries eucamptodromous, distance between 2° veins irregular; compound agrophic veins present, 1–2 visible; minor 2° veins present; inter-secondary veins course parallel to secondary veins, terminating into percurrent tertiary veins; intercoastal tertiary veins percurrent with dominantly AR–RO angle of origin; epimedial tertiary veins opposite percurrent.
Description: Leaflet seemingly asymmetrical, elliptic to ovate in shape with medial asymmetry, preserved lamina length 8.2 cm, maximum width 4.8 cm (Fig. 5a and b); lamina un-lobed with entire margin; primary vein moderate in thickness, straight to slightly curved; apex missing; base asymmetrical with obtuse to reflex angle and seemingly round to weakly cordate (Fig. 5a and b); primary venation pinnate; compound agrophic veins, 1–2 visible (Figs. 5 and S1); four major secondary veins visible, eucamptodromous with irregular spacing ranging between 1.2 and 2.3 cm, angle of divergence moderate to wide acute (58°–72°) with excurrent attachment to the primary vein (Fig. 5a and b); minor secondary veins present (Figs. 5 and S1) with angle of divergence narrow to moderate acute (42°–63°); inter-secondary veins present, covering <50% length of the subjacent secondaries, proximal course perpendicular to primary vein, distal course of intersecondary veins parallel to secondary veins and terminating into percurrent tertiaries, frequency 1 to >1 (Fig. 5a and b); intercoastal tertiary veins percurrent, straight to slightly wavy, opposite to alternate, dominantly AR–RO angle of origin (Fig. 5a, b, f); epimedial tertiary veins opposite percurrent with proximal course perpendicular to the primary vein and distal course parallel to intercoastal tertiary veins (Fig. 5a, b, e); exterior tertiary veins not visible; areoles poorly preserved.
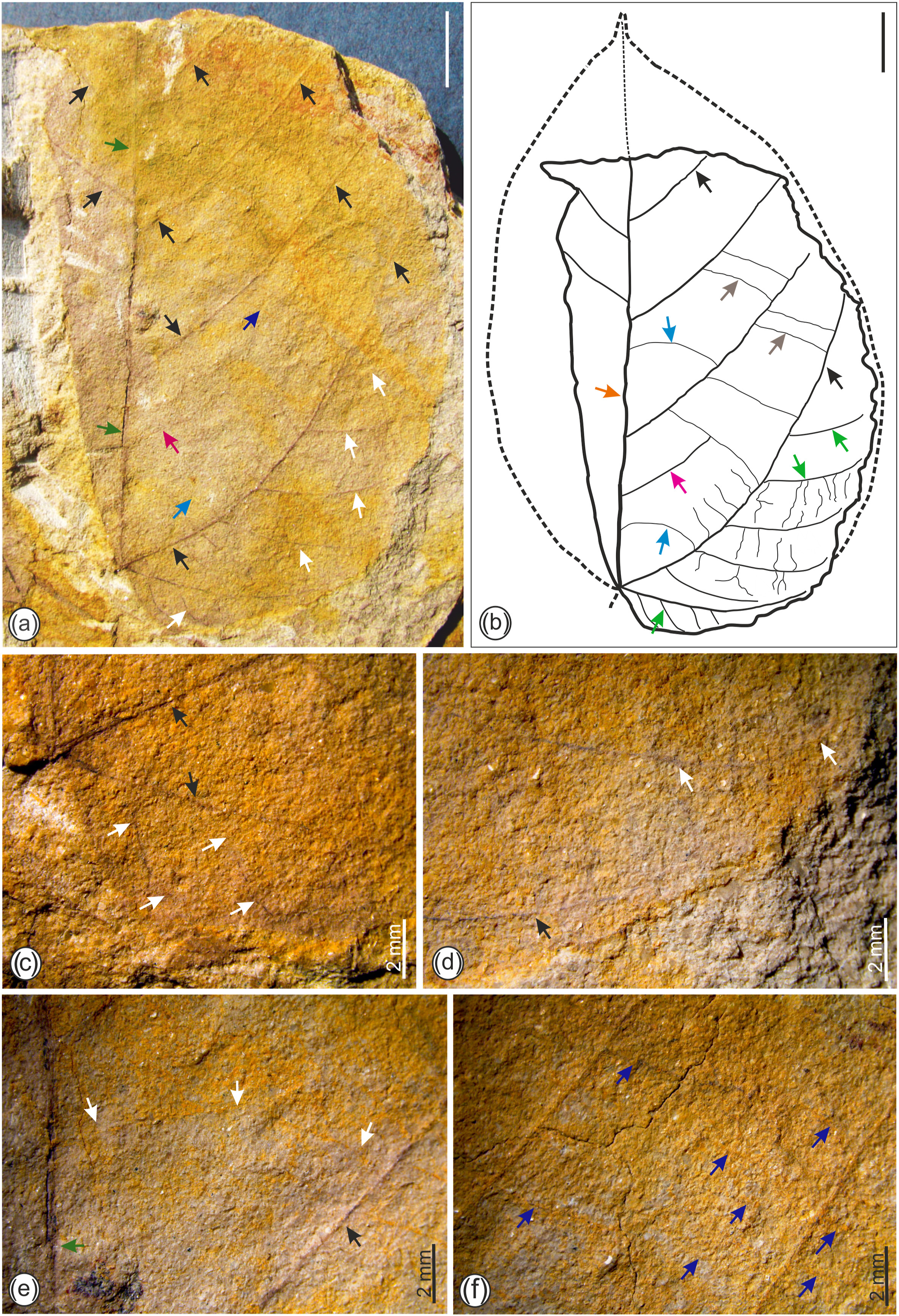
|
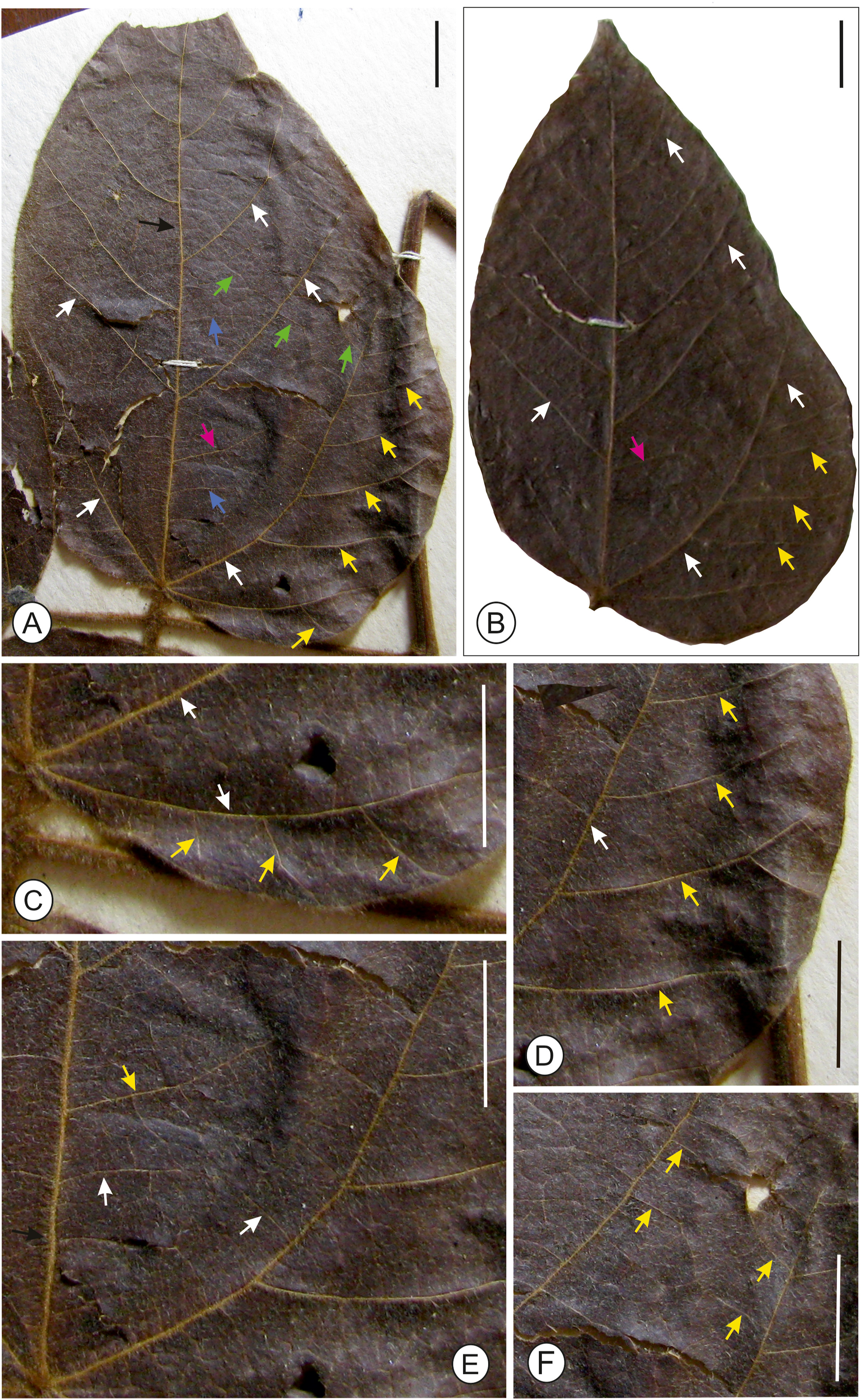
| Fig. 5 Fossil leaf of Parvileguminophyllum damalgiriensis Bhatia, Srivastava et Mehrotra sp. nov. showing shape, size and venation pattern (bar scale = 1 cm, unless specified). (a) Fossil leaf showing pinnate primary vein (dark green arrows), eucamptodromous major secondary veins (black arrows), minor secondary veins (white arrows), intersecondary vein (pink arrow), epimedial tertiary veins (sky blue arrow) and intercoastal percurrent tertiary veins (dark blue arrow); (b) diagram of the fossil leaf showing primary vein (orange arrow), secondary veins (black arrows), minor secondary veins (green arrows), intersecondary vein (pink arrow), epimedial tertiary veins (sky blue arrows) and intercoastal percurrent tertiary veins (dark brown arrows); (c) basal portion of the leaf showing asymmetrical base with compound agrophic veins (black arrows) and minor secondary veins (white arrows); (d) lower part of the fossil leaf showing agrophic vein (black arrow) and minor secondary veins (white arrows); (e) fossil leaf showing course of epimedial tertiary veins (white arrows) and secondary vein (black arrow); (f) part of fossil leaf showing percurrent intercoastal tertiary veins (dark blue arrows). |
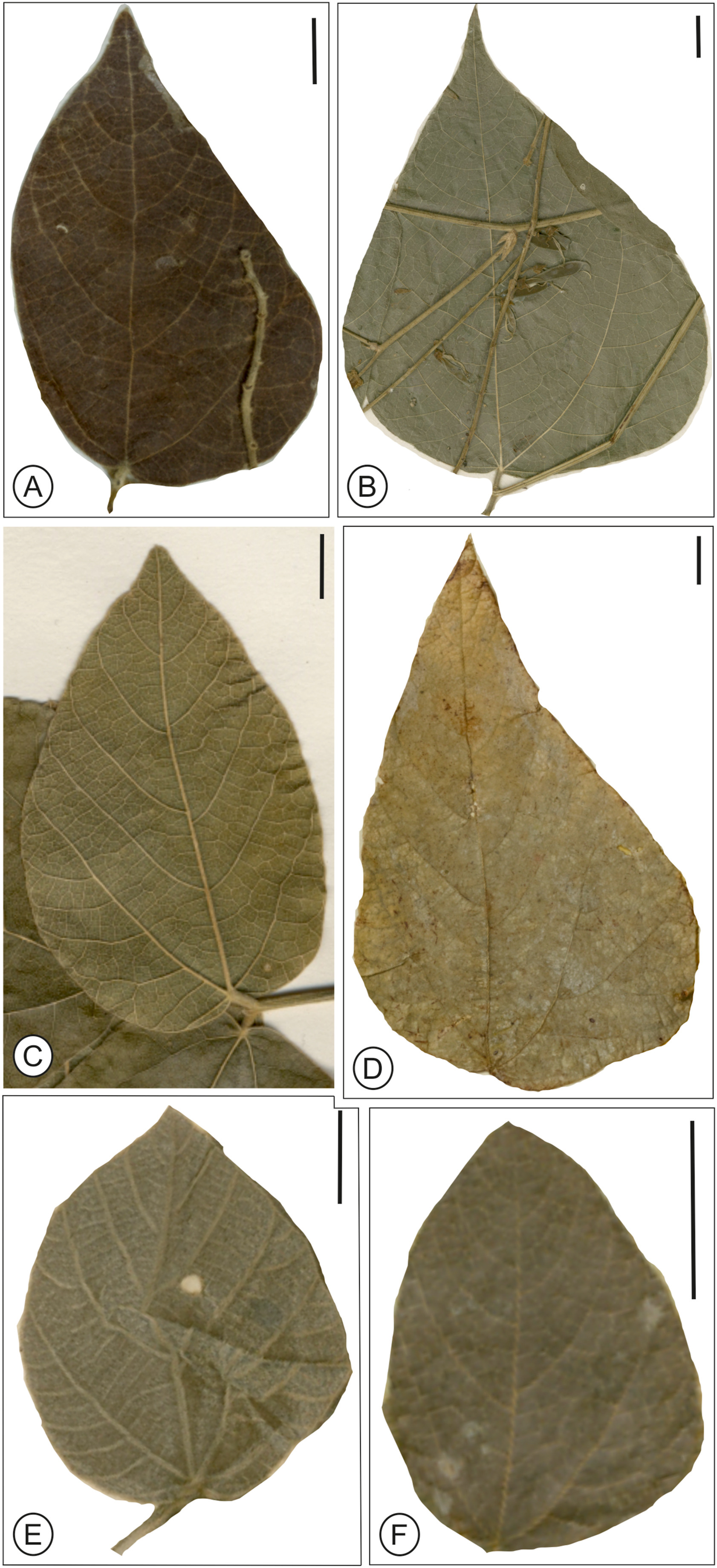
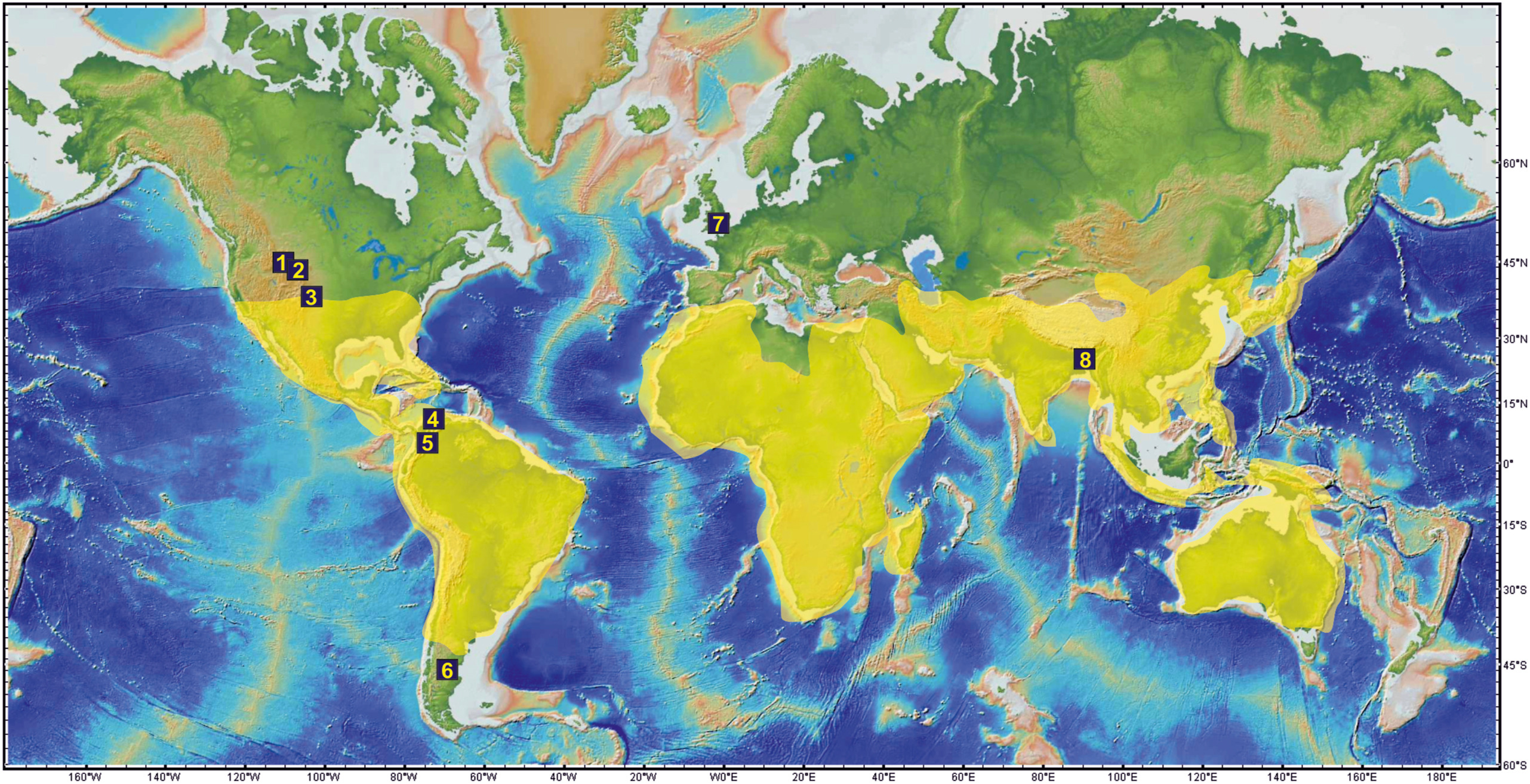
Affinities: The characteristic features of the fossil leaf are elliptic to ovate lamina, compound agrophic vein, minor secondaries, eucamptodromous venation of secondary veins and percurrent tertiaries, which shows its near similarity with the genus Rhynchosia Lour (Fig. 6). of the family Fabaceae. A large number of herbarium specimens were studied and compared with the present fossil to determine its nearest living relatives. The present fossil shows similarity to some of the taxa within the Fabaceae, such as Demodium elegans DC., D. latifolium (Roxb.) DC. Dolichos lablab L., Erythrina humeana Spreng., E. addisoniae Hutch. and Dalziel, Mucuna pruriens DC., Pueraria candollei Benth., P. montana (Lour.) Merr., and some from other families such as Alangium chinense (Lour.) Harms of the Alangiaceae, Byttneria aspera Colebr. ex Roxb., Berrya amonilla Roxb. of the Malvaceae and Macaranga pustulata King ex Hook.f., Mallotus macrostachyus (Miq.) Müll. Arg. of the Euphorbiaceae. However, critical comparison with these extant species and with previously described fossil species [e.g., Pueraria miothunbergiana and P. maxima (Wang et al., 2010), Wisteria taoiana (Wang et al., 2006), Swartzia sp. 1 and Ormosia sp. 1 (Herendeen, 1992), Leaf morphotype 6 (Herrera et al., 2019), and P. lamarensis (Herendeen et al., 2022)] indicates that the present fossil differs in a combination of characters (Appendix A). Moreover, the present fossil leaf most resembles the genus Rhynchosia (Figs. 6, 7 and S1) (Appendix A), which are climbing, prostrate or sometimes erect herbs or small shrubs having a pantropical distribution (c a. 140 spp. in Africa–Madagascar; c a. 55 endemic spp. in tropical and subtropical America and c a. 30–35 spp. in warm temperate to tropical Asia to Australia) (Fig. 8; Lewis et al., 2005). Because the present fossil leaf is not satisfactorily preserved, we describe it under an organ genus Parvileguminophyllum Herendeen and Dilcher.
|
| Fig. 6 Modern leaves of Rhynchosia cyanosperma Benth. ex Baker (FRI herbarium sheet 103357) showing shape, size and venation pattern (bar scale = 1 cm, unless specified). (A) Modern leaf showing pinnate primary vein (black arrow), eucamptodromous major secondary veins (white arrows), minor secondary veins (yellow arrows) similar to fossil leaf, intersecondary vein (pink arrow), epimedial tertiary veins (sky blue arrows) and percurrent intercoastal tertiary veins (green arrows). (B) Extant leaf showing major secondary veins (white arrows), minor secondary veins (yellow arrows) and intersecondary vein (pink arrow). (C) Basal portion of the modern leaf showing compound agrophic veins (white arrows) and minor secondary veins (yellow arrows) similar to the fossil leaf. (D) Magnified portion of the leaf showing agrophic vein (white arrow) and course of minor secondary veins (yellow arrows) similar in appearance to the preserved minor secondary veins in fossil leaf. (E) Magnified lower portion of the leaf showing epimedial tertiary veins (white arrows) and intersecondary vein (yellow arrow) terminating into the intercoastal tertiary veins. (F) Magnified portion of the leaf showing intercoastal tertiary veins (yellow arrows). |
|
| Fig. 7 Modern leaves of Rhynchosia Lour. (bar scale = 1 cm, unless specified). (A) R. candicans Wall. (Kew Herbarium sheet no. K000900366); (B) R. rojasii Hassl. (Kew Herbarium sheet no. K000836123); (C) R. congensis Bak. ssp. Orientalis Verdc. (Kew Herbarium sheet no. K000058903); (D) R. calosperma Warbg. (Kew Herbarium sheet no. K000900413); (E) R. ambacensis (Hiern) K. Schum. (Kew Herbarium sheet no. K000263765); (F) R. clausseni Benth. (Kew Herbarium sheet no. K000836141). All the herbarium sheets are © copyright of the Board of Trustees of the Royal Botanic Garden, Kew. |
|
| Fig. 8 Global map showing the modern distribution of the genus Rhynchosia (shaded area) and Paleocene fossil records of the family Fabaceae. (1) Latest Paleocene-late early Eocene (Herendeen et al., 2022); (2) late Paleocene–early Eocene (Wing et al., 2005); (3) earliest Paleocene (Lyson et al., 2019); (4) middle–late Paleocene (Wing et al., 2009; Herrera et al., 2019); (5) middle–late Paleocene (Herrera et al., 2019); (6) early Paleocene (Iglesias et al., 2007; Brea et al., 2008); (7) late Paleocene (Herendeen and Crane, 1992); (8) late Paleocene (Present study; Bhattacharyya, 1985). |
The origin and evolution of the family Fabaceae, or the legumes, is currently under debate. Phylogenetic studies based on molecular and fossil data have led some researchers to propose that the legumes evolved during the Cretaceous (Wang et al., 2009; Magallón et al., 2015; Koenen et al., 2019). Evidence that supports an early origin of legumes comes in the form of fruits from the late Campanian (about 73.5 Ma) of Mexico (Centeno-Gonzalez et al., 2021), although the affinity of these fossil fruits with legumes has been questioned (Herendeen et al., 2022). In addition, the hypothesized origin of legumes in moist equatorial region of West Gondwanaland during the Late Cretaceous (Raven and Axelrod, 1974; Raven and Polhill, 1981) lacks reliable fossil records (Herrera et al., 2019). Several studies have indicated that the crown node of the family and diversification of multiple major lineages began in the Paleocene (Lavin et al., 2005; Bruneau et al., 2008; Zhao et al., 2021). Abundant fossil records support the early Paleocene origin of legumes (Crepet and Taylor, 1985; Crepet and Herendeen, 1992; Herendeen, 1992; Herendeen et al., 1992; Koenen et al., 2019). Legume fossils have been recorded from the early Paleocene of Colorado, USA in North America and Argentina in South America. Moreover, they have been reported from middle to late Paleocene of Colombia, late Paleocene of England and India and late Paleocene to early Eocene of USA (Table 1). These early Paleogene legume records are important for understanding the family's early divergence and ecology.
Our fossil legumes are consistent with early records of legumes in India, which have previously been discovered in late Paleocene sediments of northeast India (Bhattacharyya, 1985). During the late Paleocene the Indian plate was in the Southern Hemisphere and the fossil locality was near the equator at ~7°S of paleolatitude. This paleolatitude, together with fossil records of legumes from the Paleocene sediments of North America, South America, and Europe suggest that legumes most likely immigrated to India from Africa via the Kohistan-Ladakh Arc during the latest Cretaceous–Paleocene (Chatterjee et al., 2013).
Previous studies have reconstructed climate data from late Paleocene of the Tura Formation (Bhatia et al., 2021). These data suggest that deep time legumes were living in a warm seasonal climate with monsoonal rainfall. In addition, the fossil assemblage of plant taxa at the Tura Formation provides information on the paleoclimate during the late Paleocene. For instance, the presence of Terminalia catappa L. and Nypa fruiticans Wurmb indicate estuarine conditions with large amounts of swampy vegetation (Lakhanpal, 1952; Bhattacharyya, 1983; Mehrotra, 2000). Similarly, the presence of Nelumbo nucifera Gaertn (Bhattacharyya, 1983). and Ipomoea L. (Srivastava et al., 2018) suggest that there were ponds and or other small bodies of water around the fossil locality during the deposition of sediments. Thus, the Tura Formation provides important phytogeographical and climatological information.
6. ConclusionsThe early Paleogene was characterized by a warm global climate which redistributed the habitat of marine and terrestrial biota. Understanding the ecology of biota under these warm climates is important for their future conservation. In this communication, we have reported two new fossils of legumes, namely Leguminocarpum meghalayensis Bhatia, Srivastava et Mehrotra sp. nov. and Parvileguminophyllum damalgiriensis Bhatia, Srivastava et. Mehrotra sp. nov. from the late Paleocene sediments of Tura Formation of Meghalaya, northeast India. The Paleocene records of legumes indicate that they might have immigrated to India from Africa via the Ladakh-Kohistan Arc during the latest Cretaceous–Paleocene. Our fossil discoveries, when taken together with previously reconstructed climate data from the Tura Formation of Meghalaya, indicate that legumes were well adapted to a seasonal climate in the late Paleocene.
AcknowledgementsAuthors are grateful to the Director of the Birbal Sahni Institute of Palaeosciences, Lucknow for providing necessary facilities and encouragement during the research. They are thankful to the Directors of the Forest Research Institute, Dehradun and the Central National Herbarium, Howrah for providing permission to consult their herbarium. They express their gratitude to the Kew Herbarium, London for providing high resolution images of herbarium sheets. The authors are indebted to Profs Tao Su and Xiangchuan Li and one anonymous reviewer for their valuable suggestions in improving the manuscript.
Author contributions
H.B., G.S., and R.C.M. planned and designed the research. H.B., and G.S., participated in the research. H.B., G.S., R.C.M., wrote the manuscript.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.08.001.
Ambwani, K., Kar, R.K., 2000. Occurrence of Anonidium-like pollen in the Tura Formation (Palaeocene) of Meghalaya, India. Palaeobotanist, 49: 219-223. DOI:10.54991/jop.2000.143 |
Axelrod, D.I., 1992. Climatic pulses, a major factor in legume evolution. In: Herendeen, P.S., Dilcher, D.L. (Eds.), Advances in Legume Systematics, Part 4. The Fossil Record. Royal Botanic Gardens, Kew, London, UK, pp. 259-279.
|
Bakshi, S.K., 1974. Significant pollen taxa in the stratigraphical analysis of the Tertiary sediments of Assam. In: Surange, K.R., Lakhanpal, R.N., Bhardwaj, D.C. (Eds.), Aspects and Appraisal of Indian Palaeobotany. Birbal Sahni Institute of Palaeobotany, Lucknow, India, pp. 502-515.
|
Bhatia, H., Khan, M.A., Srivastava, G., et al., 2021. Late Cretaceous–Paleogene Indian monsoon climate vis-à-vis movement of the Indian plate, and the birth of the South Asian Monsoon. Gondwana Res., 93: 89-100. DOI:10.1016/j.gr.2021.01.010 |
Bhattacharyya, B., 1983. FossiI plants from the Tura Formation (Eocene) in the Garo Hills. Meghalaya. Indian J. Earth Sci., 10: 1-10. |
Bhattacharyya, B., 1985. Leguminous fruits from the Eocene of Garo Hills, Meghalaya. Q. J. Geol. Min. Metall. Soc. India, 57: 215-225. |
Biswas, B., 1962. Stratigraphy of the Mahadeo, Langpar, Cherra and Tura formations, Assam, India. Bull. Geol. Min. Metall. Soc. India, 25: 1-48. |
Brea, M., Zamuner, A.B., Matheos, S.D., et al., 2008. Fossil wood of the Mimosoideae from the early Paleocene of Patagonia, Argentina. Alcheringa, 32: 427-441. DOI:10.1080/03115510802417695 |
Bruneau, A., Mercure, M., Lewis, G.P., Herendeen, P.S., 2008. Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany, 86: 697-718. DOI:10.1139/B08-058 |
Centeno-González, N.K., Martínez-Cabrera, H.I., Porras-Múzquiz, H., et al., 2021. Late Campanian fossil of a legume fruit supports Mexico as a center of Fabaceae radiation. Commun. Biol., 4: 41. DOI:10.1038/s42003-020-01533-9 |
Chakraborty, A., Baksi, S.K., 1972. Stratigraphy of the Cretaceous–Tertiary sedimentary sequence, south-west of Shillong plateau. Q. J. Geol. Min. Metall. Soc. India, 44: 109-127. |
Chatterjee, S., Goswami, A., Scotese, C.R., 2013. The longest voyage: tectonic, magmatic, and palaeoclimatic evolution of Indian plate during its northward flight from Gondwana to Asia. Gondwana Res., 23: 238-267. DOI:10.1016/j.gr.2012.07.001 |
Chavan, S., Sardesai, M.M., Pokle, D.S., 2013. Alysicarpus sanjappae (Leguminosae-Papilionoideae): a new species from Western Ghats of India. Kew Bull., 68: 183-186. DOI:10.1007/s12225-012-9425-x |
Crepet, W.L., Herendeen, P.S., 1992. Papilionoid flowers from the early Eocene of southwestern North America. In: Herendeen, P.S., Dilcher, D.L. (Eds.), Advances in Legume Systematics, Part 4. The Fossil Record. Royal Botanic Gardens, Kew, London, UK, pp. 45-55.
|
Crepet, W.L., Taylor, D.W., 1985. The diversification of the Leguminosae: first fossilevidence of the Mimosoideae and Papilionoideae. Science, 228: 1087-1089. DOI:10.1126/science.228.4703.1087 |
Dilcher, D.L., 1974. Approaches to the identification of angiosperm leaf remains. Bot. Rev., 40: 1-157. DOI:10.1007/BF02860067 |
Dutta, S.K., Sah, S.C.D., 1970. Palynostratigraphy of the tertiary sedimentary formations of Assam: 5. Stratigraphy and palynology of south Shillong plateau. Palaeontograph. Abteilung B, 11: 1-72. DOI:10.1177/001946467000700101 |
Ellis, B., Daly, D.C., Hickey, L.J., et al., 2009. Manual of Leaf Architecture. |
Herendeen, P.S., 1992. The fossil history of the Leguminosae from the Eocene of southeastern North America. In: Herendeen, P.S., Dilcher, D.L. (Eds.), Advances in Legume Systematics, Part 4. The Fossil Record. Royal Botanic Gardens, Kew, London, UK, pp. 85-160.
|
Herendeen, P.S., Cardoso, D.B.O.S., Herrera, F., et al., 2022. Fossil papilionoids of the Bowdichia clade (Leguminosae) from the Paleogene of North America. Am. J. Bot., 109: 1-21. DOI:10.1002/ajb2.1813 |
Herendeen, P.S., Crane, P.R., 1992. Early caesalpinioid fruits from the Paleogene of southern England. In: Herendeen, P.S., Dilcher, D.L. (Eds.), Advances in Legume Systematics, Part 4. The Fossil Record. Royal Botanic Gardens, Kew, London, UK, pp. 57-68.
|
Herendeen, P.S., Crepet, W.L., Dilcher, D.L., 1992. The fossil history of the Leguminosae: phylogenetic and biogeographical implications. In: Herendeen, P.S., Dilcher, D.L. (Eds.), Advances in Legume Systematics, Part 4. The Fossil Record. Royal Botanic Gardens, Kew, London, UK, pp. 303-316.
|
Herrera, F., Carvalho, M.R., Wing, S.L., et al., 2019. Middle to late Paleocene Leguminosae fruits and leaves from Colombia. Aust. Syst. Bot., 32: 385-408. DOI:10.1071/sb19001 |
Iglesias, A., Wilf, P., Johnson, K.R., et al., 2007. A Paleocene lowland macroflora from Patagonia reveals significantly greater richness than North American analogs. Geology, 35: 947-950. |
Kar, R.K., Kumar, M., 1986. Palaeocene palynostratigraphy of Meghalaya, India. Pollen Spores, 28: 177-218. |
Koenen, E.J.M., Ojeda, D.I., Steeves, R., et al., 2019. The origin and early evolution of the legumes are a complex paleopolyploid phylogenomic tangle closely associated with the Cretaceous-Paleogene (K-Pg) Boundary. Syst. Biol., 70: 508-526. |
Lakhanpal, R.N., 1952. Nipa sahnii a palm fruit in the Tertiary of Assam. Palaeobotanist I: 289-294. DOI:10.54991/jop.1952.413 |
Lavin, M., Herendeen, P.S., Wojciechowski, M.F., 2005. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Syst. Biol., 54: 575-594. DOI:10.1080/10635150590947131 |
Lewis, G., Schrire, B., Mackinder, B., et al., 2005. Legumes of the World. Royal Botanic Gardens, Kew, Richmond, U.K.
|
LPWG (Legume Phylogeny Working Group), 2013. Legume phylogeny and classification in the 21st century: Progress, prospects and lessons for other species-rich clades. Taxon, 62: 217-248. DOI:10.12705/622.8 |
LPWG (Legume Phylogeny Working Group), 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon, 66: 44-77. DOI:10.12705/661.3 |
Lyson, T.R., Miller, I.M., Bercovici, A.D., et al., 2019. Exceptional continental record of biotic recovery after the Cretaceous–Paleogene mass extinction. Science, 366: 977-983. DOI:10.1126/science.aay2268 |
Ma, F.J., Liu, S., Sun, B.N., et al., 2017. Legume fruits from the Oligocene Ningming Formation of Guangxi, China, and their biogeographical and palaeoclimatic implications. Rev. Palaeobot. Palynol., 244: 192-202. DOI:10.1016/j.revpalbo.2017.05.009 |
Magallón, S., Gómez-Acevedo, S., Sánchez-Reyes, L.L., et al., 2015. A meta calibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol., 207: 437-453. DOI:10.1111/nph.13264 |
Mehrotra, N., 1981. Palynological correlation of Mikir Formation with lower Palaeogene sediments of Shillong plateau. Geophytology, 11: 133-142. |
Mehrotra, R.C., 2000. Study of plant megafossils from the Tura Formation of Nangwalbibra, Garo Hills, Meghalaya, India. Palaeobotanist, 49: 225-237. DOI:10.54991/jop.2000.144 |
Mehrotra, R.C., Dilcher, D.L., Awasthi, N., 1998. A Palaeocene Mangifera – like leaf fossil from India. Phytomorphology, 48: 91-100. |
Monga, P., Srivastava, G., Kumar, M., Mehrotra, R.C., 2014. Further palynological investigation of coaliferous sequences of Tura formation of Nangwalbibra, East Garo Hillls, Meghalaya: Inferences on palaeovegetation and palaeoclimate. Palaeobotanist, 63: 79-85. DOI:10.54991/jop.2014.322 |
Raja Rao, C.S., 1981. Coalfields of India: Coalfields of northeastern India. Bull. Geol. Soc. India Ser A, 45: 1-76. |
Raven, P.H., Polhill, R.M., 1981. Advances in Legume Systematics, Part 1. Royal Botanic Gardens, Kew, London, UK, pp. 27-34.
|
Raven, P.H., Axelrod, D.I., 1974. Angiosperm biogeography and past continental movements. Ann. Mo. Bot. Gard., 61: 539-673. DOI:10.2307/2395021 |
Sah, S.C.D., Dutta, S.K., 1974. Palynostratigraphy of sedimentary formations of Assam: 3. Biostratigraphic zonation of Cherra Formation of south Shillong plateau. Palaeobotanist, 21: 42-47. |
Sah, S.C.D., Singh, R.Y., 1974. Palynological biostratigraphy of the Tura formation in the type area. In: Sah, S.C.D. (Ed.), Symposium on Stratigraphical Palynology, Special Publication 3. Birbal Sahni Institute of Palaeobotany, Lucknow, India, pp. 76-98.
|
Sanjappa, M., 1991. Legumes of India. India: Bishen Singh Mahendra Pal Singh Publication, Dehra Dun.
|
Sanjappa, M., 1995. Leguminosae-papilionoideae: Tribe-Indigofereae. In: Hajra, P.K., Sashtry, A.R.K., Sanjappa, M. (Eds.), Fascicles of Flora of India - 21. Botanical Survey of India, Calcutta, pp. 1-167.
|
Saxena, R.K., Tripathi, S.K.M., Prasad, V., 1996. Palynofloral investigation of the Tura Formation (Palaeocene) in Nongwal Bibra area, East Garo Hills, Meghalaya. Geophytology, 26: 19-31. |
Schrire, B.D., Lavin, M., Lewis, G.P., 2005a. Global distribution patterns of the Leguminosae: insights from recent phylogenies. Biol. Skr., 55: 375-422. |
Schrire, B.D., Lewis, G.P., Lavin, M., 2005b. Biogeography of the Leguminosae. In: Lewis, G., Schrire, B., Mackindera, B., Lock, M. (Eds.), Legumes of the World. Royal Botanic Gardens, Kew, London, UK, pp. 21-54.
|
Scotese, C.R., 2001. Earth System History Geographic Information System, Version 0.2b. PALEOMAP Project, Arlington, TX.
|
Srivastava, G., Mehrotra, R.C., Dilcher, D.L., 2018. Paleocene Ipomoea (Convolvulaceae) from India with implications for an East Gondwana origin of Convolvulaceae. Proc. Natl. Acad. Sci. U.S.A., 115: 6028-6033. DOI:10.1073/pnas.1800626115 |
Tripathi, S.K.M., Singh, H.P., 1984. Palynostratigraphical zonation and correlation of the Jowai-Sonapur road Section (Paleocene-Eocene), Meghalaya, India. In: Tiwari, R.S., Awasthi, N., Srivastava, S.C., Singh, H.P., Sharma, B.B. (Eds.), Proceeding of the Sixth Indian Geophytological Conference. Special Publication of the Palaeobotanical Society, Lucknow, India, pp. 316-328.
|
Wang, Q., 2012. Nomenclatural notes on Leguminosites and several taxonomically relevant names (fossil Leguminosae). Taxon, 61: 871-877. DOI:10.1002/tax.614014 |
Wang, Q., Dilcher, D.L., Zhu, X.Y., et al., 2006. Fruits and leaflets of Wisteria (Leguminosae, Papilionoideae) from the Miocene of Shandong Province, eastern China. Int. J. Plant Sci., 167: 1061-1074. DOI:10.1086/502717 |
Wang, Q., Manchester, S.R., Dilcher, D.L., 2010. Fruits and foliage of Pueraria (Leguminosae, Papilionoideae) from the Neogene of Eurasia and their biogeographic implications. Am. J. Bot., 97: 1982-1998. DOI:10.3732/ajb.1000167 |
Wang, H., Moore, M.J., Soltis, P.S., et al., 2009. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proc. Natl. Acad. Sci. U.S.A., 106: 3853-3858. DOI:10.1073/pnas.0813376106 |
Wing, S.L., Harrington, G.J., Smith, F.A., et al., 2005. Transient floral change and rapid global warming at the Paleocene–Eocene boundary. Science, 310: 993-996. DOI:10.1126/science.1116913 |
Wing, S.L., Herrera, F., Jaramillo, C.A., et al., 2009. Late Paleocene fossils from the Cerrejón Formation, Colombia, are the earliest record of Neotropical rainforest. Proc. Natl. Acad. Sci. U.S.A., 106: 18627-18632. DOI:10.1073/pnas.0905130106 |
Yahara, T., Javadi, F., Onoda, Y., et al., 2013. Global legume diversity assessment: concepts, key indicators, and strategies. Taxon, 62: 249-266. DOI:10.12705/622.12 |
Zachos, J., Pagani, M., Sloan, L., et al., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686-693. DOI:10.1126/science.1059412 |
Zhao, Y., Zhang, R., Jiang, K.-W., et al., 2021. Nuclear phylotranscriptomics and phylogenomics support numerous polyploidization events and hypotheses for the evolution of rhizobial nitrogen-fixing symbiosis in Fabaceae. Mol. Plant, 14: 748-773. DOI:10.1016/j.molp.2021.02.006 |



