b. State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan Agricultural University, Kunming, 650201, China;
c. Key Laboratory of Agro-Biodiversity and Pest Management of Education Ministry of China, Yunnan Agricultural University, Kunming, 650201, China;
d. Southwest Forestry University, Kunming, 650224, China
Monocultures constitute the predominant type of cultivation system currently used in agriculture, as they result in high productivity but decrease biodiversity and increase the crop susceptibility to biotic and abiotic stress (Newton et al., 2008). Currently, the importance of biodiversity in agro-systems has been thoroughly recognized worldwide, which not only ensures global food supplies but also maintains the sustainability of agro-systems (Mundt, 2002; Keesing and Ostfeld, 2015). Many reports have indicated that intercropping by growing two or more crop species within the same field increases biodiversity in farmland, which can promote crop resistance and inhibit pathogens (Gaba et al., 2014; Yang et al., 2014; Zhu and Morel, 2019). Agroforestry ecosystems are widely popular throughout the world, as they reflect a sustainable land use strategy and effective disease management practices (Tscharntke et al., 2011).
Herbaceous plants are important resources for the medicine industry (Zhong et al., 2020). In the past, medicinal herbs were obtained mainly from the wild, but medicinal herbs have now been artificially cultivated on a large scale to meet the increasing demand for health care (Chen and Guo, 2004; Yan, 2012). Monocultures of herb plantations reduce farmland biodiversity and disrupt the balance of ecosystems, which promotes the susceptibility of herbs to insect pests and diseases (Li et al., 2006; Luo et al., 2019, 2020). Furthermore, excessive use of chemical fertilizers and pesticides also leads to issues concerning the quality and safety of medicinal herbs (Maobe et al., 2012). To alleviate these issues associated with herbs monocultures, an innovative planting strategy was created that involved planting shaded medicinal herbs under the forest canopy, which provides a suitable environment with a high level of biodiversity (Donald, 2004; Ivette Perfecto and John Vandermeer, 2008). In recent decades, many medicinal herbaceous species have been successfully planted in forests, including Gastrodia elata, Coptis chinensis, Panax ginseng and Panax notoginseng (Jing et al., 2009; Yang, 2016; Ye et al., 2019). With the high level of biodiversity in forests, the quality of medicinal herbs has been improved, and disease severity has been suppressed (Zhang et al., 2011; Ye et al., 2019; Wu et al., 2020). Many mechanisms, including plant–plant interactions and plant-insect/pathogen interactions, are involved in disease suppression in biologically diverse ecosystems (Poelman et al., 2008; Yang et al., 2014; Ding et al., 2015; Pelissier et al., 2021). Among them, plants utilize allelochemicals, including volatile organic compounds (VOCs), leachates, root exudates and decayed matter, to alter the surrounding environmental conditions, including by inhibiting microbial growth, reducing the fitness of insects or inducing resistance in nearby plants (Albuquerque et al., 2010; Bachheti et al., 2020; Chomel et al., 2020; Ye et al., 2021).
Sanqi (Panax notoginseng (Burk.) F.H. Chen) is a shade-loving medicinal herb that has a long history of use in medicine and important medicinal value, including antioxidative, antiaging, and anticancer effects as well as other health-improving activities (Kim et al., 2015; Mancuso and Santangelo, 2017; Zhang et al., 2018; Zhou et al., 2019). The sustainable development of P. notoginseng plantations is hindered by plant root and leaf diseases associated with infections of various pathogens, including Fusarium oxysporum and Alternaria panax (Yang et al., 2015; Wei et al., 2018; Luo et al., 2019). The necrotrophic pathogen A. panax can cause black spot disease of P. notoginseng, which results in black spots on the leaves and severely reduces yields of traditional monocultures (Yang et al., 2006). Recently, P. notoginseng was shown to be successfully cultivated in pine forests, including those of Pinus yunnanensis, Pinus armandii and Pinus kesiya var. langbianensis (Ye et al., 2019). The allelopathic effects of VOCs and leachates under pine forest are mainly the result of pine needles, and VOCs released by pine needles can alter the proportion of rhizosphere microorganisms (Kato-Noguchi et al., 2011; Ye et al., 2021). The leachates released from or washed off pine needles by rain contain several antimicrobial, defense or growth-regulating compounds (Zeng and Jia, 2009; Verma et al., 2012; Venegas-Molina et al., 2021). Our field observations showed that the leaf disease of P. notoginseng infected with A. panax was relieved in a pine forest, it was speculated to the leachates of pine forest which often adhered to the leaves of P. notoginseng during the rainy period (Fig. 1A); however, the underlying responsible mechanism is still not clear. Based on previous researches (Verma et al., 2012; Ye et al., 2021), we infer that leachates might be act as antifungal substance to directly inhibit the growth of pathogen or inducer to indirectly enhance the plant resistance. Systemic resistance of plants is well studied as systemic acquired resistance (SAR) or induced systemic resistance (ISR) (Vlot et al., 2020). SAR enhances resistance of whole plant to further pathogen attack after infection with pathogens (Conrath, 2006). Alternatively, ISR is triggered by beneficial microbes to protect non-exposed plant parts against future attack by pathogenic microbes or herbivorous insects (Pieterse et al., 2014).

|
| Fig. 1 Effects of Pinus armandii leachates on Panax notoginseng. (A) Leaching model of P. armandii at P. notoginseng. Field observation of pinus leachates (left circular image), field observation of pinus leachates dripping at leaves of P. notoginseng (right circular image). (B) Experimental design of prespraying P. armandii leachates at P. notoginseng with infection of A. panax. (C) Effects of prespraying leachates at concentrations of 0, 1.5, 10 and 50 g/L on the lesion area resulting from infection by A. panax. (D) Effects of leachates on the hyphal growth of A. panax at concentrations of 0, 0.1, 1, 5 and 20 g/L. The data are shown as the means ± SEMs. The different lowercase letters indicate significance differences among treatments according to one-way ANOVA and Duncan's multiple range test (P < 0.05). |
In this study, we used Sanqi in a pine forest as a model to (ⅰ) study the effects of leachates from P. armandii needles on the occurrence of leaf disease caused by A. panax; (ⅱ) identify the constituents of the leachates via GC–MS; and (ⅲ) test the effects of 2, 3-Butanediol from the leachates on the ability to suppress disease and elucidate its underlying mechanism. Based on these analyses, we expect to determine the mechanisms underlying the chemical interactions involved in tree and herbaceous plant disease suppression and then develop potential agents to alleviate disease in agricultural production systems.
2. Materials and methods 2.1. Leachates collection and identificationFresh pine needles of Pinus armandii were collected from pine forests in Shi Ping County (Yunnan Province, China, 23°58′43 ″N, 102°26′46″E, 2010 m above sea level). A total of 600 g of fresh pine needles was soaked in 4 L of sterilized water for 48 h and then filtered with qualitative filter paper (Diameter 18 cm, Jiaojie, Fushun city, China). Afterward, 150 g/L leachates was divided into two parts and stored at 4 ℃ for future component identification and ability to induce disease resistance.
The leachates was extracted and identified via GC–MS according to the methods of previous research (Shettima et al., 2013; Taha and Gadalla, 2017). Briefly, 300 mL of ethyl acetate (Analytical grade, Tianjin Fengchuan Chemical Reagent Co., Ltd., Tianjin, China) was used to extract 100 mL of leachates. Ethyl acetate was subsequently evaporated by R-200 rotary evaporator (BÜCHI, Flawil, Switzerland) at 45 ℃, and the remaining sediment was washed out with 2.5 mL of hexane (EMD Millipore, Billerica, MA, USA). The liquid hexane was then filtered with a Millipore filter (Cartridge filter, Jin Teng, China) for GC–MS analysis (GCMS-QP2010 Ultra, Shimadzu, Kyoto, Japan). The GC–MS system included an SH-Rxi-5Sil mass spectrometer (30 m × 0.25 mm × 0.25 μm, Shimadzu, Kyoto, Japan) and was equipped with a gas chromatography column with a carrier gas helium flow of 3.0 mL/min. The temperature program started from an initial temperature of 40 ℃, followed by an increase of 3 ℃/min to 80 ℃ and then an increase of 5 ℃/min from 80 ℃ to 260 ℃, which was maintained for 30 min. The mass spectra were recorded for the mass range of 35–500 m/z, with a scanning rate of 1 spectrum/0.3 s. Constituents of the leachates were identified via GC–MS software (Shimadzu, Kyoto, Japan) based on the comparison of its mass spectra (MS) with the NIST14 and NIST14s library. 2, 3-Butanediol was selected from the identified compounds for further research based on the high similarity and large relative peak area. Moreover, the synthetic standard sample of 2, 3-Butanediol (98%, Yien Chemical Technology Co., Ltd, Shanghai, China) was analyzed by GC–MS to further verify validity by comparing the MS, retention time (RT) and retention index (RI) in GC–MS solution software.
2.2. Antifungal activity of leachates and 2, 3-ButanediolThe antifungal activity of leachates and 2, 3-Butanediol was measured in accordance with a previous research (De Araujo and Roussos, 2002). Briefly, the antifungal activity of leachates against at A. panax was tested at concentrations of 0, 0.1, 1, 5 and 20 g/L. The antifungal activity of 2, 3-Butanediol against at A. panax was tested at concentrations of 0, 55, 222, 555 and 1110 μM. Collected leachates and synthetic standard 2, 3-Butanediol were added to liquid potato dextrose agar (PDA) media respectively to generate designed concentrations of solutions, which were sterilized then poured into petri dishes. An A. panax mycelial block (6 mm in diameter) was placed in the middle of the petri dishes containing PDA. After four days of incubation at 25 ℃ in darkness, the diameters of the A. panax blocks were measured with a ruler, and the means of the diameters were calculated.
2.3. Induced resistance of the leachates and 2, 3-ButanediolThe seeds of Panax notoginseng were immersed in 1% sodium hypochlorite for 5 min then washed three times with sterile water. Ten seeds were sown in a pot (15.5 cm × 11.5 cm × 13.5 cm) and cultivated for half a year for resistance analysis. All the pots were placed in a greenhouse shaded with a polyethylene net that allowed 10% of full sunlight transmission. After six months, the plants in all the pots were used to assess the induction resistance of the leachates and 2, 3-Butanediol to A. panax. Before treatment with leachates or 2, 3-Butanediol, the pots were wrapped in plastic bags to ensure that the sprayed leachates or 2, 3-Butanediol solution was applied only to the leaves rather than to the soil. Six pots in a plastic storage box (53 cm × 38.5 cm × 32.5 cm; Vengo, Xi'an, China) constituted one experimental unit.
For the leachates-induced resistance assessment (Fig. 1B), leachates at concentrations of 0, 1.5, 10 and 50 g/L were diluted from 150 g/L leachates, which was prepared in a leachates collection section. Afterward, 60 mL of four different concentrations of leachates was sprayed onto the leaves of seedlings each day in a greenhouse (25 ± 2 ℃, 12 h light/12 h darkness). After three days, three out of five leaves of each seedling were inoculated with an A. panax mycelium block (6 mm in diameter). The seedlings were then incubated for five days until black spots appeared, and the lesion area of leaves infected with A. panax was scanned by a scanner (800 dpi resolution; Epson Perfection V850 Pro, Japan). The lesion-affects areas of the leaves were calculated with Adobe Photoshop (Adobe Systems Incorporated, USA).
For induced resistance assessment, synthetic standard 2, 3-Butanediol at concentrations of 0, 11, 55 and 222 μM was sprayed onto leaves, which were then inoculated with or without A. panax. Spraying, inoculation and disease inspection were performed as described in the leachates-induced resistance assessment section. According to the disease inspection results, leaves sprayed with 2, 3-Butanediol at concentrations of 0 and 11 μM with or without A. panax inoculation were collected for RNA-seq.
2.4. RNA-seq of Panax notoginseng leavesRNA isolation, library construction, and sequencing were performed by MAJORBIO Company (Shanghai, China). The library was constructed via an Illumina TruSeq™ RNA Sample Prep Kit (Illumina, Inc., San Diego, CA, USA), in accordance with the following procedure. 1) Total RNA was extracted from the tissue samples, and the concentration and purity of the RNA were detected by a NanoDrop 2000 instrument (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The integrity of RNA was determined via agarose gel electrophoresis, and the RNA integrity number (RIN) was determined by an Agilent 2100 instrument (Agilent, Inc., CA, USA). 2) Magnetic oligo (dT) beads were used to pair A-T bases with poly-A tails, and mRNA was isolated from the total RNA. 3) The mRNA was randomly fragmented with the addition of fragmentation buffer, and a small fragment of approximately 300 bp was isolated by magnetic bead screening. 4) Under the action of reverse transcriptase, random hexamers were added to reverse synthesize first-strand cDNA using mRNA as template, and then two-strand synthesis was carried out to form a stable double-stranded structure. 5) End Repair Mix was added to complete the flat end of the structure of the double-stranded cDNA, and an "A" base was added at the 3′ end to connect the Y-shaped junction. 6) The cDNA was ultimately sequenced on an Illumina NovaSeq 6000 platform (Illumina, Inc., San Diego, CA, USA).
2.5. Sequence data processing, mapping, assembly and annotationThe clean data were obtained as follows: 1) The splice sequences in the reads were removed, and reads without inserted fragments were removed due to the self-ligation of the connectors. 2) Bases with low quality (those with mass value lower than 30) were removed at the end of the sequence (3′ end). If there were still bases with a mass value lower than 10 in the remaining sequence, the whole sequence was removed; otherwise, it was retained. 3) Reads with an N content greater than 10% were removed. Last, 4) sequences shorter than 50 bp after adapter and mass pruning were discarded. The software programs used included SeqPrep (https://github.com/jstjohn/SeqPrep) and Sickle (https://github.com/najoshi/sickle).
The clean reads were then compared to the reference genome (Yang et al., 2021) via HISAT2 (http://ccb.jhu.edu/software/hisat2/index.shtml) to acquire mapped reads (Kim et al., 2015). On the basis of the existing reference genome, the mapped reads were assembled and spliced into transcripts by using StringTie software (http://ccb.jhu.edu/software/stringtie/) (Pertea et al., 2015). Moreover, gene/transcript annotation information was obtained by comparison of the data with the contents of six databases (NR, SwissProt, Pfam, EggNOG, GO and KEGG), the results of which are shown in Table S1. With the use of RSEM software, the read counts of each sample gene/transcript were obtained on the basis of the results of the genome alignment and genome annotation files (Li and Dewey, 2011). The results were then converted to transcripts per million (TPM) to obtain standardized gene/transcript expression levels.
2.6. DEGs, GO, and KEGG enrichment analysesPrincipal component analysis (PCA) was performed using the "princomp" function in R software (Team, 2013). The component loadings were calculated on the basis of correlations between the measured expression values and principal component scores. The top 2 principal components, PC1 and PC2, were visualized using 2-dimensional scatter plots via the ggplot2 package. Differential expression was assessed using the linear model for microarray data (LIMMA) package, which involves a modified t-test that incorporates the Benjamini–Hochberg (BH) multiple hypothesis correction method (Ritchie et al., 2015). To improve the reliability of the identification of differentially expressed genes (DEGs), probe sets with an adjusted P of < 0.05 and a log2(|fold change|) > 2 between two comparison groups were defined as significantly differentially expressed. A Venn diagram of all the DEGs was plotted with the R package "venn" (Dusa, 2019). Functional enrichment analysis was subsequently performed using the ClusterProfile package (Yu et al., 2012), the statistical significance threshold level for all Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses was P < 0.05 (BH corrected for multiple comparisons). Heatmaps of gene expression were generated with the R package "pheatmap" (Raivo, 2019).
2.7. Verification of expression levels via quantitative real-time PCR (qRT-PCR) analysisTwelve DEGs were selected for qRT-PCR verification. The genes and primers used in this study are listed in Table S2. Three technical replicates were performed for each of the three biological replicates. cDNA was synthesized using HiScript Q RT SuperMix for qPCR (Vazyme, Nanjing, China). ChamQ SYBR Color qPCR Master Mix (2X) was used for quantitative PCR (Vazyme, Nanjing, China), and an ABI7500 fluorescence quantification PCR instrument was used (Applied Biosystems, USA). The P. notoginseng 18S gene was used as an internal control (CK). The amplification efficiencies of 12 DEGs were verified, all of which ranged from 90% to 110%. The amplification efficiencies of the target genes and internal reference genes were similar, and the difference between them was less than 5%; thus, it was rational to use the 2−ΔΔCT method to calculate relative expression levels (Livak and Schmittgen, 2001).
2.8. Statistical analysisIBM SPSS Statistics version 25 (SPSS, Inc., Chicago, Illinois, USA) was used for general statistical analyses. One-way analysis of variance (ANOVA) and Duncan's multiple range test (P < 0.05) were used to analyze the mean separations among treatments. Bar charts were drawn with GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA). R software was used for transcriptome analysis.
3. Results 3.1. Pinus armandii leachates reduced Alternaria panax infectionPrespraying leachates from P. armandii at concentrations of 1.5, 10 and 50 g/L significantly reduced the lesion area resulting from infection by A. panax. The lesion size decreased 68.18% at a concentration of 1.5 g/L compared with 0 g/L, but the lesion size was not further decreased when the concentration increased from 1.5 g/L to 50 g/L (Fig. 1C). Further study demonstrated that leachates from P. armandii at concentrations of 0.1–20 g/L did not show antifungal activity against the growth of A. panax (Fig. 1D).
3.2. 2, 3-Butanediol is present in Pinus armandii leachatesThe leachates of P. armandii were collected and analyzed via GC–MS. GC–MS produced 148 peaks that corresponded to the leachates. According to the NIST 14 and NIST 14s library, 14 compounds with similarity greater than 95% and relative peak area greater than 0.1% were identified in the leachates (Table 1). These included mainly alcohols, esters, alkanes and alkenes. The compounds associated with relative peak areas greater than 0.5% included 2, 3-Butanediol, toluene, bis(2-methylpropyl) ester-1, 2-benzenedicarboxylic acid, dibutyl phthalate, acetic acid and (2R, 3R)-Butanediol. Among these compounds, 2, 3-Butanediol was selected for further analysis (Fig. 2A and B), as this compound and its isomer ((2R, 3R)-Butanediol) was associated with a large relative peak area (Total 2.3%) and a high similarity (97%).
| No. | Compounds | Retention time (mins) | Similarities (%) | Relative peak area (%) |
| 1 | 2, 3-Butanediol | 5.185 | 97 | 1.76 |
| 2 | Toluene | 4.747 | 97 | 1.37 |
| 3 | 1, 2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 37.246 | 96 | 1.23 |
| 4 | Dibutyl phthalate | 39.111 | 97 | 0.92 |
| 5 | Acetic acid, butyl ester | 5.874 | 96 | 0.76 |
| 6 | (2R, 3R)-Butanediol | 4.964 | 97 | 0.54 |
| 7 | Dodecane | 20.484 | 96 | 0.33 |
| 8 | Ethylbenzene | 7.281 | 97 | 0.31 |
| 9 | 1-Heptadecene | 28.737 | 95 | 0.24 |
| 10 | Heptacos-1-ene | 37.671 | 96 | 0.23 |
| 11 | Nonadecylcyclohexane | 39.18 | 95 | 0.23 |
| 12 | 3-Hexanol | 5.354 | 97 | 0.21 |
| 13 | 1-Undecanol | 23.332 | 95 | 0.19 |
| 14 | Butyrolactone | 9.091 | 95 | 0.13 |

|
| Fig. 2 Total ion current (TIC) chromatograms of leachates from Pinus armandii and of 2, 3-Butanediol. (A) TIC chromatogram of the sample collected from Shi Ping County (Yunnan Province, China, 23°58′43 ″N, 102°26′46″E, 2010 m above sea level). (B) TIC chromatogram from the synthetic standard sample of 2, 3-Butanediol. RT and RI indicate retention time and retention index, respectively. |
The size of lesions caused by A. panax infection was significantly decreased when the leaves of P. notoginseng were presprayed with 2, 3-Butanediol at concentration of 11, 55 or 222 μM, respectively (Fig. 3A). The lesion size was decreased by 55.53% when the concentration 11 μM compared to 0 μM, but the lesion size did not further decrease when the concentration increased from 11 to 222 μM (Fig. 3B). Further study demonstrated that 2, 3-Butanediol at concentrations of 55–1110 μM did not inhibit hyphal growth of A. panax (Fig. 3C).
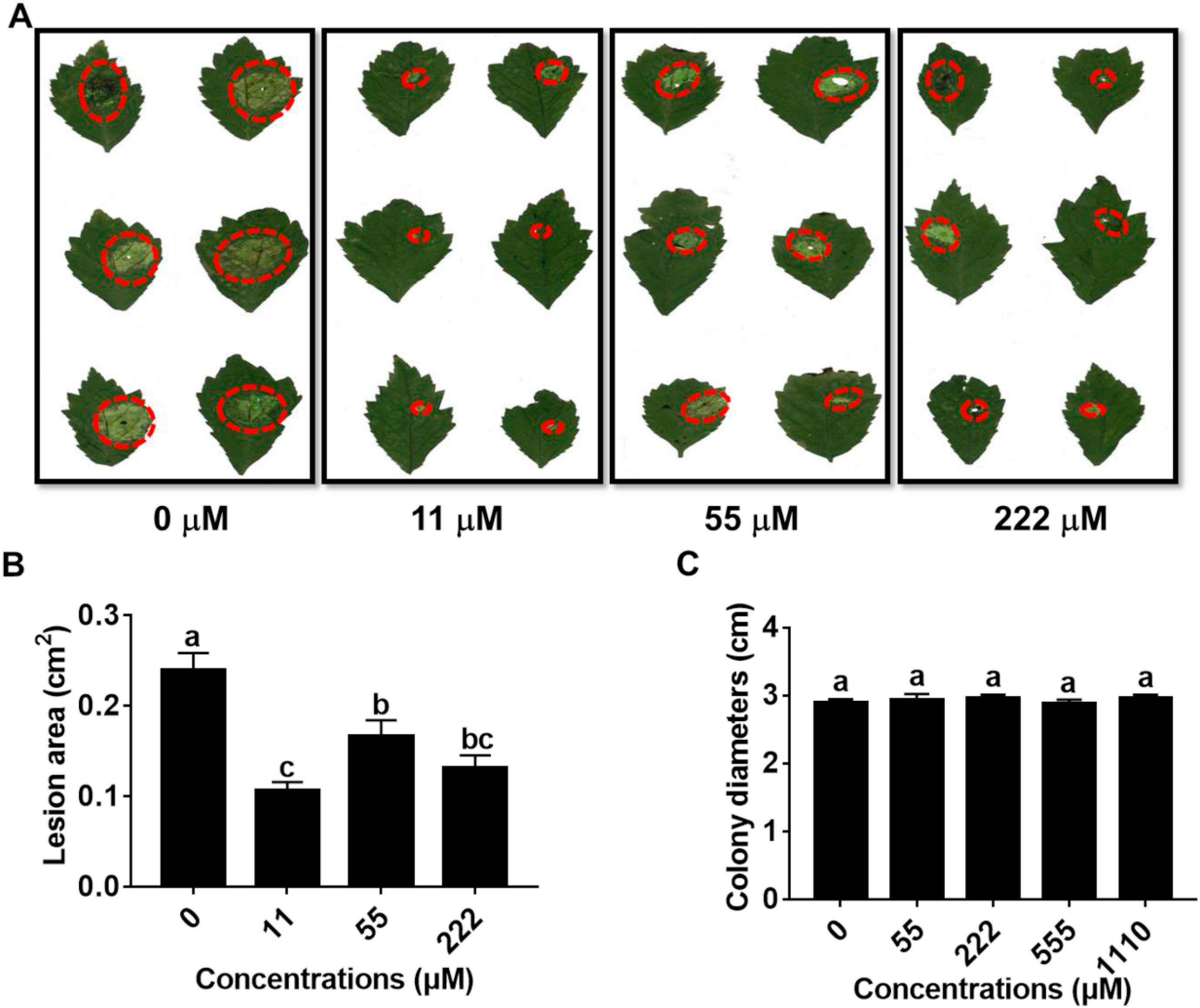
|
| Fig. 3 Effects of 2, 3-Butanediol on Alternaria panax infection and growth. (A) and (B) Effects of prespraying 2, 3-Butanediol at concentration of 0, 11, 55 and 222 μM on the disease lesion area. (C) Effects of 2, 3-Butanediol on the hyphal growth of A. panax at concentration of 0, 55, 222, 555 and 1110 μM. The data are shown as the means ± SEMs. The different lowercase letters indicate significant differences among treatments according to one-way ANOVA and Duncan's multiple range test (P < 0.05). |
Four treatments, including the CK (no foliar prespraying with 2, 3-Butanediol or infection by A. panax), foliar infection with A. panax (i), foliar prespraying with 2, 3-Butanediol (E), and foliar prespraying with 2, 3-Butanediol together with infection by A. panax (Ei), were established for transcriptome analysis. More than 98% clean reads were obtained after filtration of the raw reads which was over 6.26 Gb from each sample and more than 94.82% of the bases had a quality value of 30 (Q30). The transcriptome sequencing data could meet the basic requirements necessary for downstream analysis (Table S3). In total, 54, 672 expressed genes and 89, 363 expressed transcripts were obtained. The resulting high-dimensional dataset was converted into a two-dimensional dataset to analyze the correlations between all the samples via PCA, the results of which showed that all the samples from the three treatments were significantly separate from the CK samples (Fig. 4A). However, the movement direction of the A. panax-infected samples (i) was different from that of the 2, 3-Butanediol-treated samples (E and Ei). Specifically, compared to the CK samples, the samples infected with A. panax (i) moved along with both PC1 and PC2. Compared to the CK and A. panax-infected samples (i), samples treated with 2, 3-Butanediol without A. panax infection (E) moved along with PC1. Compared to the CK and A. panax-infected sample (i), samples treated with 2, 3-Butanediol together with infection with A. panax (Ei) moved along with both PC1 and PC2. In addition, compared to samples subjected to 2, 3-Butanediol treatment (E), samples treated with 2, 3-Butanediol followed by infection with A. panax (Ei) moved largely along PC2, but their (E and Ei) confidence ellipse were largely overlapped. Taken together, these results demonstrated that, compared with CK and infection of A. panax (i), prespraying 2, 3-Butanediol (E and Ei) resulted in different abilities to alter the structure of P. notoginseng at the transcriptome level. Meanwhile, the structure of transcriptome was similar between 2, 3-Butanediol treatments (E and Ei).
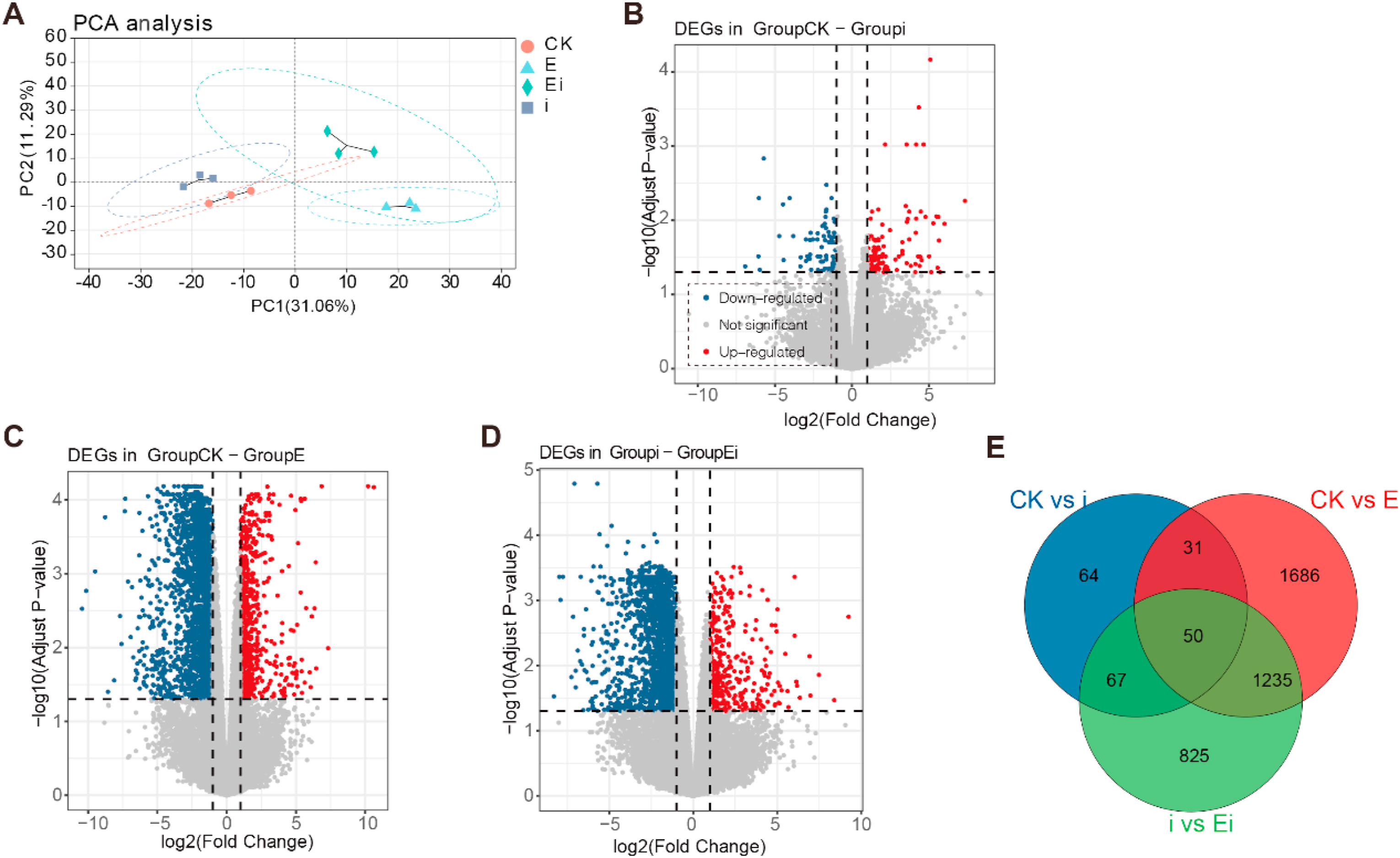
|
| Fig. 4 Overview of the Panax notoginseng transcriptome changes among the four treatments. (A) PCA score plot of four treatments (CK, E, Ei and i). (B) Volcano plot for DEGs between CK and i treatments. (C) Volcano plot for DEGs between CK and E treatments. (D) Volcano plot for DEGs between i and Ei treatments. (E) Venn diagram of DEGs among the CK vs i, CK vs E and i vs Ei comparison groups. CK represents foliar prespraying with water, i represents foliar prespraying with water then infected by Alternaria panax, E represents foliar prespraying with 2, 3-Butanediol, Ei represents foliar prespraying with 2, 3-Butanediol then infected by A. panax. The upregulated and downregulated genes are highlighted with red dots and blue dots, respectively. The X-axis represents the log2-transformed expression changes, and the Y-axis represents the –log10-transformed p-value denoting the significance of differential expression. Probe sets with an adjusted P < 0.05 and a log2(|fold change|) > 2 between two comparison groups were defined as significant differentially expressed. |
Further analysis demonstrated that 80 upregulated DEGs and 132 downregulated DEGs were found in foliar A. panax-infected plants (i) compared with CK plants (Fig. 4B) (Table S4). However, foliar spraying 2, 3-Butanediol with or without A. panax infection (E and Ei) induced more DEGs (Fig. 4C and D). Compared with the CK, prespraying 2, 3-Butanediol (E) onto the leaves of P. notoginseng upregulated 2267 DEGs but downregulated 735 DEGs (Table S4). Prespraying 2, 3-Butanediol followed by infection with A. panax (Ei) resulted in the upregulation of 1811 DEGs but downregulation of 366 DEGs (Table S4). The Venn diagram also demonstrated that foliar prespraying 2, 3-Butanediol together with infection (Ei) or no infection with A. panax (E) could induce more shared and unique genes than could A. panax infection (i) alone (Fig. 4E).
3.5. 2, 3-Butanediol activated the defense response of Panax notoginsengGO enrichment analysis demonstrated that, the DEGs from Alternaria panax infection (i) compared with CK (CK vs i) were enriched primarily in "MF: transcription factor activity, sequence-specific DNA binding", "MF: FAD binding", "MF: transcription regulatory region DNA binding", "BP: gene silencing by RNA", and "MF: sequence-specific DNA binding" (Fig. 5A). However, the DEGs from 2, 3-Butanediol induced (E) compared with the CK (CK vs E) were enriched primarily in "MF: transcription factor activity, sequence-specific DNA binding", "MF: sequence-specific DNA binding", "MF: FAD binding", "MF: heme binding", and "MF: iron ion binding" (Fig. 5C). The DEGs from prior 2, 3-Butanediol induction and infection with A. panax (Ei) compared with A. panax infection (i) (i vs Ei) were enriched primarily in "MF: transcription factor activity, sequence-specific DNA binding", "MF: sequence-specific DNA binding", "MF: protein serine/threonine kinase activity", and "MF: oxidoreductase activity" (Fig. 5E). Overall, A. panax infection and prespraying 2, 3-Butanediol with or without A. panax infection (E and Ei) most significantly regulated "transcription factor activity, sequence-specific DNA binding" in P. notoginseng. Notably, compared with A. panax infection (i) and prespraying with 2, 3-Butanediol only (E), prespraying 2, 3-Butanediol together with A. panax infection (Ei) regulated genes specifically involved in "protein serine/threonine kinase activity" and "oxidoreductase activity".
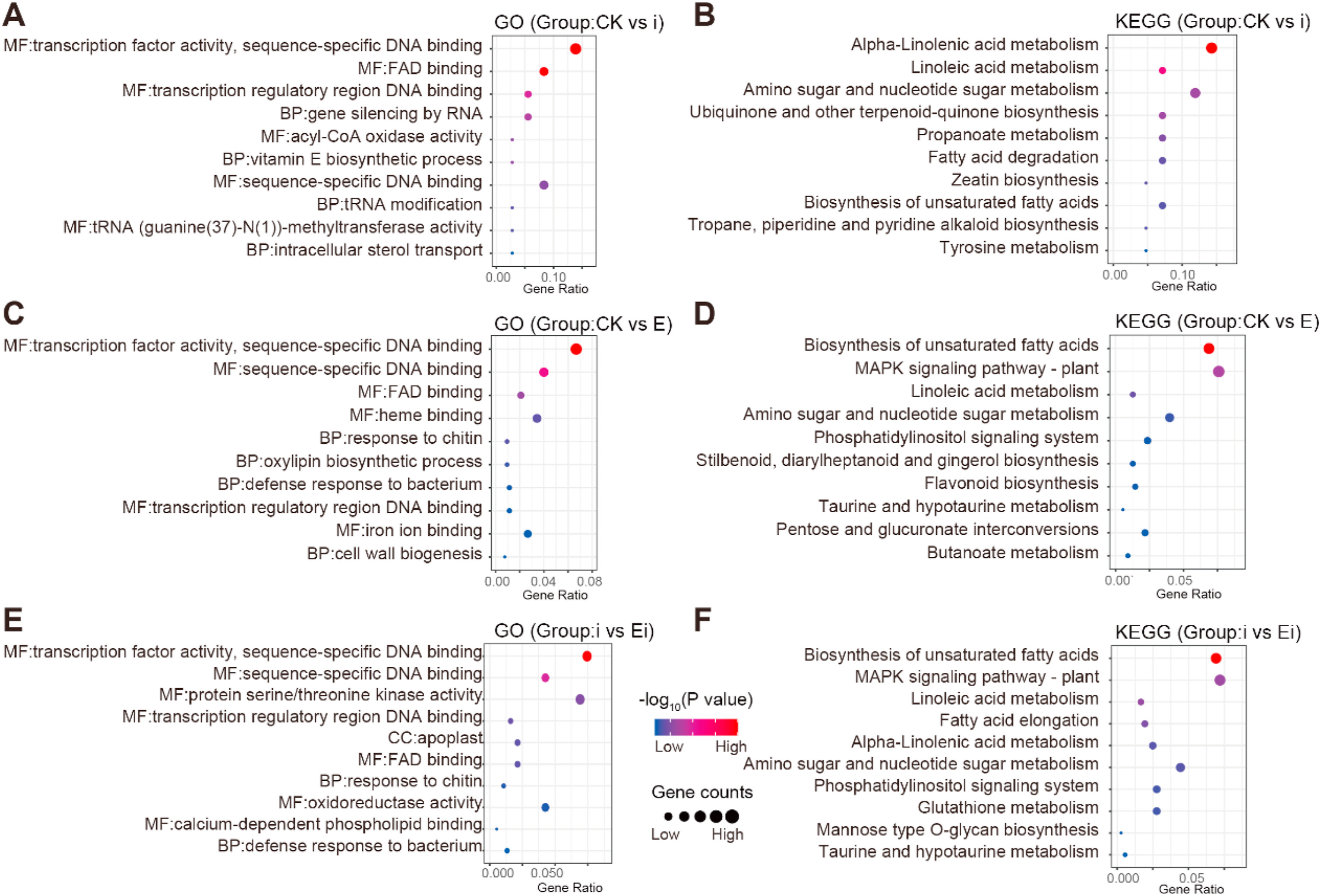
|
| Fig. 5 GO and KEGG enrichment analyses of different comparison groups. (A) GO enrichment and (B) KEGG enrichment between CK and i treatments. (C) GO enrichment and (D) KEGG enrichment between CK and E treatments. (E) GO enrichment and (F) KEGG enrichment between i and Ei treatments. CK represents foliar prespraying with water, i represents foliar prespraying with water then infected by Alternaria panax, E represents foliar prespraying with 2, 3-Butanediol, Ei represents foliar prespraying with 2, 3-Butanediol then infected by A. panax. The dot size corresponds to the gene number, and the dot color corresponds to statistical significance. The statistical significance threshold level for all GO and KEGG enrichment analyses was P < 0.05 (BH corrected for multiple comparisons). |
The treatments with prespraying 2, 3-Butanediol (E and Ei) activated many transcription factors (TFs) from WRKY family and ERF family (Fig. S1). Most of DEGs encoding WRKY family and ERF family were up-regulated in "transcription factor activity, sequence-specific DNA binding" (Table S5). In which, A. panax infection (i) had enriched five TFs, but only NFYA (Pno06G004231) was upregulated. Prespraying 2, 3-Butanediol without A. panax infection (E) had enriched 35 TFs, only six TFs were downregulated. Prespraying 2, 3-Butanediol with A. panax infection (Ei) had enriched 28 TFs, only MYBP (Pno06G007234) was downregulated.
KEGG pathway enrichment analysis demonstrated that, DEGs in the comparison of CK and A. panax infection (CK vs i) were enriched primarily in "alpha-linoleic acid metabolism" and "amino sugar and nucleotide sugar metabolism" (Fig. 5B). DEGs in the comparison of CK and induction with 2, 3-Butanediol (CK vs E) were enriched primarily in the "biosynthesis of unsaturated fatty acids" and "MAPK signaling pathway-plant" (Fig. 5D). DEGs in the comparison of A. panax infection and prior induction of 2, 3-Butanediol and infection with A. panax (i vs Ei) were enriched primarily in "biosynthesis of unsaturated fatty acids" and "MAPK signaling pathway-plant" as well (Fig. 5F). Overall, A. panax infection (i) significantly regulated genes involved in the "alpha-linoleic acid metabolism pathway"; however, 2, 3-Butanediol with or without A. panax infection (E and Ei) significantly regulated genes involved in the "MAPK signaling pathway-plant" (Table S6).
Many DEGs associated with the MAPK signaling pathway in response to 2, 3-Butanediol induction with (Ei) or without (E) A. panax infection were involved in pathogen infection (Fig. 6A and C) and phytohormone transduction (Fig. 6B and D). In pathogen infection, both treatments activated the defense response and camalexin synthesis. The defense response pathway starts from either Rboh D or the FLS2-BAK1 complex and continues with MKK5 and MPK3, subsequently activating the transcription factor WRKY22 to initiate the defense response. At the same time, camalexin synthesis was activated by WRKY33, but upstream MKS1 was activated only in response to 2, 3-Butanediol without A. panax infection (E).
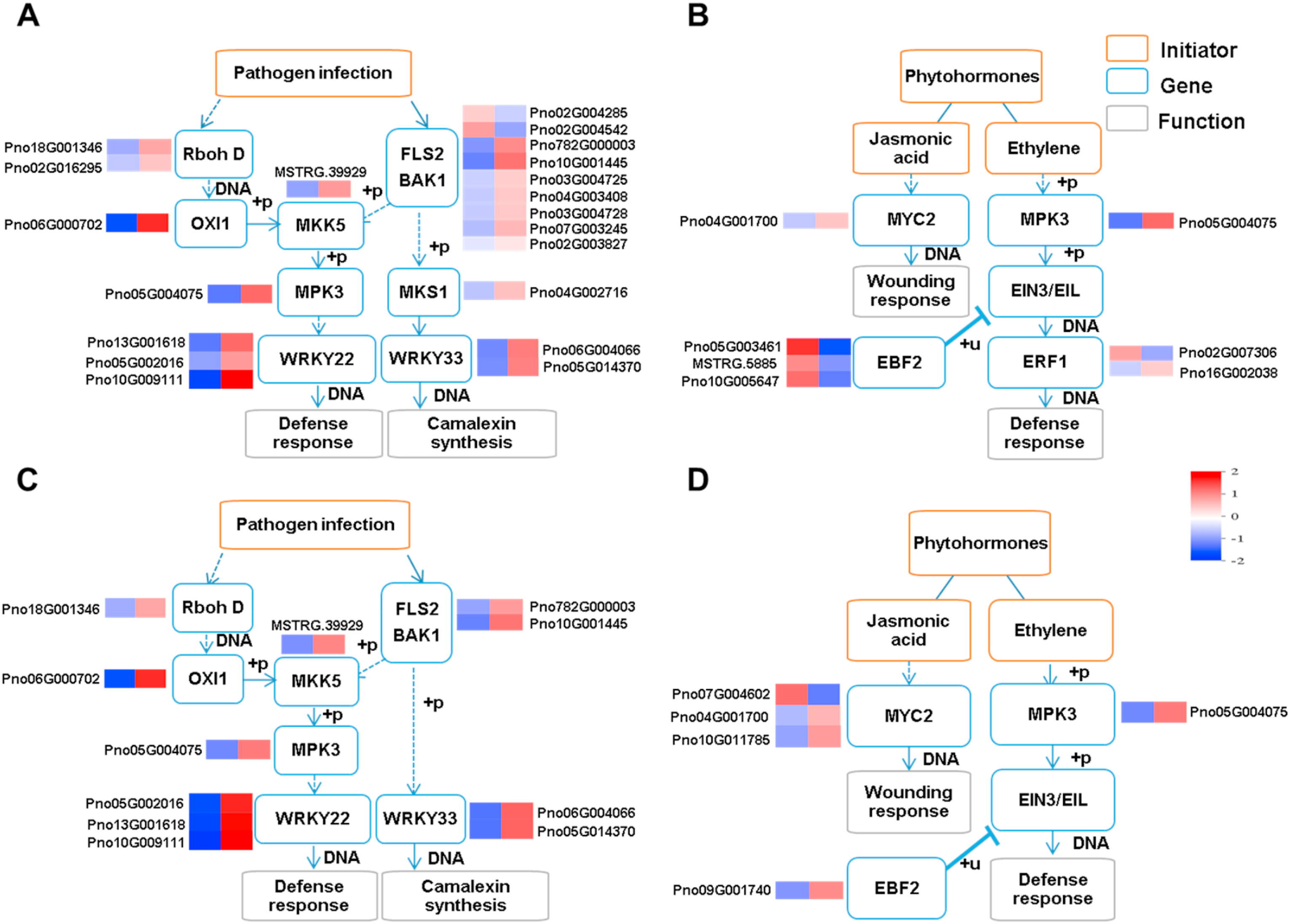
|
| Fig. 6 Pathogen infection and phytohormone cascades of MAPK signaling pathway when Panax notoginseng was treated with 2, 3-Butanediol. (A) DEGs involved in pathogen infection cascades and (B) DEGs involved in phytohormone cascades between E and CK treatments. (C) DEGs involved in pathogen infection cascades and (D) DEGs involved in phytohormone cascades between Ei and i treatments. CK represents foliar prespraying with water, i represents foliar prespraying with water then infected by Alternaria panax, E represents foliar prespraying with 2, 3-Butanediol, Ei represents foliar prespraying with 2, 3-Butanediol then infected by A. panax. Normalized expression levels of DEGs are shown via a heat map. |
In terms of phytohormone transduction, the JA- and ET-mediated pathways were modified by 2, 3-Butanediol with or without A. panax infection (E and Ei). The JA-mediated pathway was activated in response to both treatments, more DEGs encoding MYC2 were upregulated in response to 2, 3-Butanediol followed by A. panax infection (Ei) than 2, 3-Butanediol treatment only (E). In the ET-mediated pathway, both treatments upregulated MPK3 (Pno05G004075). EBF2 related DEGs were downregulated in response to 2, 3-Butanediol without A. panax infection (E) but upregulated in response to 2, 3-Butanediol together with A. panax infection (Ei). As a result, the downstream ERF1 component of the ET pathway was differentially expressed only under 2, 3-Butanediol treatment without A. panax infection (E). Overall, both treatments involving prespraying 2, 3-Butanediol with or without A. panax infection (E and Ei) activated pathogen infection-related defense responses and camalexin synthesis, as well as components of JA-related pathways. However, the ET-related defense response was activated only by 2, 3-Butanediol together without A. panax infection (E).
3.6. Verification of RNA-seq data via qRT-PCRqRT-PCR results demonstrated that, compared with the CK and infection of A. panax (i), prespraying 2, 3-Butanediol (E and Ei) could significantly upregulate the expression of many genes (Fig. 7). The expression patterns of the selected genes were consistent with those of the DEGs identified by RNA-seq analysis, which suggested that the RNA-seq data were reliable and reproducible. Compared to the CK, A. panax (i) infection did not significantly alter the expression of 12 genes. Compared to the prespraying of 2, 3-Butanediol without A. panax infection (E), the prespraying of 2, 3-Butanediol together with A. panax infection (Ei) upregulated gene expression more strongly. Compared to the CK, prespraying of 2, 3-Butanediol without A. panax infection (E) significantly upregulate the expression FLS2 (Pno03G004728), OXI1 (Pno06G000702), WRKY22 (Pno13G001618) (Pno10G009111) and WRKY33 (Pno05G014370). Compared to the A. panax infection (i), prespraying of 2, 3-Butanediol with A. panax infection (Ei) significantly upregulate the expression Rboh D (Pno18G001346), OXI1 (Pno06G000702), MKK5 (MSTRG.39, 929), MPK3 (Pno05G004075), WRKY22 (Pno13G001618) (Pno10G009111), WRKY33 (Pno06G004066) (Pno05G014370) and MYC2 (Pno10G011785).
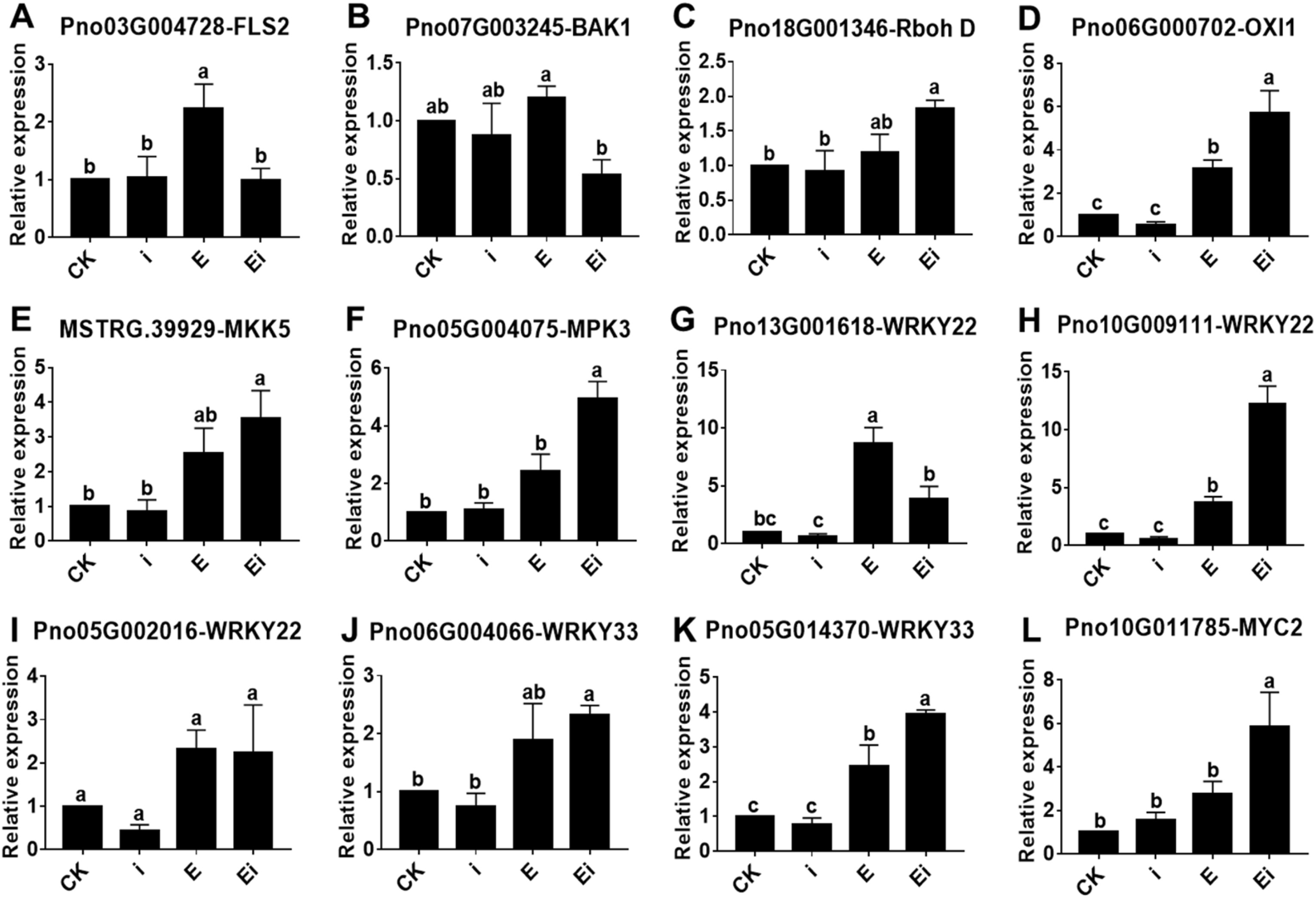
|
| Fig. 7 Relative expression of 12 DEGs (A–L) in Panax notoginseng among four different treatments according to qRT-PCR. CK represents foliar prespraying with water, i represents foliar prespraying with water then infected by A. panax, E represents foliar prespraying with 2, 3-Butanediol, Ei represents foliar prespraying with 2, 3-Butanediol then infected by A. panax. The data are shown as the means ± SEMs. The different lowercase letters indicate significant differences among the treatments according to one-way ANOVA and Duncan's multiple range test (P < 0.05). |
Plant metabolites drive cellular regulatory processes that occur during interactions between plants and a broad range of other organisms (Piasecka et al., 2015). During these processes, numerous functions and multiple biological activities involving substrates, products, cofactors of enzymes, or defense or growth-regulating compounds are carried out (Venegas-Molina et al., 2021). Black spot disease caused by the necrotrophic pathogen A. panax can result in the production of black spots on leaves of P. notoginseng, severely reduce yields (Yang et al., 2006). In this study, we found that prespraying leachates from needles of P. armandii onto leaves could reduce the size of lesions caused by A. panax, at the same time leachates from needles showed no antifungal activity at A. panax (Fig. S2A). The constituents of the leachates from P. armandii were determined via GC–MS, and 2, 3-Butanediol was identified which is an important secondary metabolite that can be derived from the biotransformation of natural resources (Syu, 2001; Zhang et al., 2011). 2, 3-Butanediol was reported to induce systemic resistance by increasing the expression of JA-related genes (Cortes-Barco et al., 2010a, 2010b; Shi et al., 2018). In this study, it was also found that 2, 3-Butanediol was not antagonistic to A. panax (Fig. S2B) but instead could reduce the size of lesions caused by A. panax and significantly induce the resistance of P. notoginseng by activating ISR- and SAR-related genes.
Prespraying 2, 3-Butanediol onto leaves activated the MYC branch of the JA/abscisic acid (ABA) pathway. Transcription factor MYC2 was deemed as the JA signaling 'master switch', which acted additively with its close homologs MYC3 and MYC4 as major regulators of diverse JA responses (Breeze, 2019). MYC2 could be upregulated by ABA and JA transcriptionally, at the same time MYC2 could regulate genes in ABA and JA signaling pathways (Hickman et al., 2017). Our transcriptome results (Fig. 6B and D) showed that the MYC2 was activated in both 2, 3-Butanediol prespraying treatments with or without A. panax infection (E and Ei), which suggested that the defense response dependent on a functional JA pathway was activated. Once 2, 3-Butanediol-treated leaves were infected with A. panax (Ei), more DEGs that encode MYC2 were found, which implied that the MYC2-primed defense response during 2, 3-Butanediol treatment (E) was activated more strongly. This kind of priming could enhance defense responses of plants and make the plants more resistant to upcoming stresses (Mhlongo et al., 2018).
Prespraying of 2, 3-Butanediol onto Panax notoginseng leaves regulated the ERF branches of the JA/ethylene (ET) pathways. MPK3 can activate the biosynthesis of ET through the phosphorylation of ACS6 upon flg22 perception (Liu and Zhang, 2004) or bind to ERF1 to regulate the ET-dependent ISR against necrotrophic fungal pathogens (Meng et al., 2013). Here, MPK3 (Pno05G004075) was significantly upregulated in response to 2, 3-Butanediol with and without A. panax infection (E and Ei). DEGs encoding EBF2 were downregulated in the presence of 2, 3-Butanediol without A. panax infection (E) but upregulated in the presence of 2, 3-Butanediol together with A. panax infection (Ei) (Fig. 6B and D), EBF2 acted as repressor of ERF1 transcription factor (Potuschak et al., 2003). As a result, downstream ERF1 was differentially expressed only under 2, 3-Butanediol treatment (E) and not under 2, 3-Butanediol treatment followed by A. panax infection (Ei) (Fig. 6B and D). Activation of the JA/ET-related defense response relies on the constitutive expression of the ERF1 transcription factor (Solano et al., 1998), suggesting that only the prespraying of 2, 3-Butanediol without A. panax infection (E) could activate the JA/ET pathway.
ERF1 and MYC2 seem to be antagonistic overall, and this reciprocal repression of the ERF branch and MYC branch has been previously reported (Pieterse et al., 2012; Song et al., 2014; Zhang et al., 2014). The results of our transcriptome analysis demonstrated that more DEGs encoding MYC2 were upregulated in the 2, 3-Butanediol treatment together with A. panax infection group (Ei) than in the noninfected treatment group (E), whereas the repressor EBF2 (Pno09G001740) was upregulated to suppress the activation of ERF1 in the 2, 3-Butanediol treatment together with A. panax infection (Ei).
Consistently, the GO enrichment analysis demonstrated that more transcription factors of ERF family were activated after 2, 3-Butanediol treatment without A. panax infection (E) (Fig. S1), members of which were involved into JA/ET-mediated defense response (Lorenzo et al., 2003; Onate-Sanchez et al., 2007). Noticeably, PTI5 transcription factor (Pno03G004610) was upregulated only in the presence of 2, 3-Butanediol without A. panax infection (E) (Table S5), which has a strong priming effect related to the JA/ET pathways (Harel et al., 2014). Therefore, 2, 3-Butanediol induction (E) could activate JA/ET-mediated ISR. However, 2, 3-Butanediol induction followed by A. panax infection (Ei) could activate JA/ABA-mediated ISR. These results were consistent with those of previous findings in which treatment with 2, 3-Butanediol could induce ISR in plants (Cortes-Barco et al., 2010a, 2010b; Shi et al., 2018).
SAR is a form of resistance against a broad spectrum of (hemi-)biotrophic pathogens and can be induced by PTI or ETI (Spoel and Dong, 2012; Vlot et al., 2020). Although 2, 3-Butanediol has rarely been reported to be associated with SAR, our transcriptome analysis demonstrated that prespraying 2, 3-Butanediol could prime plant defenses against future pathogen infection and activates many SAR-related defensive genes via the MAPK signaling pathway. In plants, MAPK cascades are involved in response to various biotic and abiotic stresses, hormones, cell division and developmental processes (Ichimura et al., 2002). After pathogen perception, a H2O2 burst and MAPK pathways are initiated via Rboh D and FLS2-BAK1 complex respectively, and eventually the H2O2 pathway feeds into the MAPK pathway to form a positive feedback loop (Zhang and Klessig, 2001) (Fig. 6A and C).
Under the pathogen- or microbial-associated molecular pattern (P/MAMP) system, flg22 is recognized by a pattern recognition receptor (PRR) FLS2-BAK1 (Chinchilla et al., 2009; Ma et al., 2020), which can subsequently activate downstream MAPK cascades, which include MKK5, MPK3 and WRKY22, and ultimately boost PTI, resulting in enhanced resistance against bacterial and fungal pathogens (Fig. 6A and C) (Asai et al., 2002; Tsuda and Katagiri, 2010). In this study, we found that prespraying 2, 3-Butanediol with or without A. panax infection (E and Ei) could upregulate the expression of genes associated with FLS2-BAK1 complex, MKK5, MPK3 and WRKY22, which implied that prespraying 2, 3-Butanediol could activate PTI.
In the oxidative burst pathway, Rboh D acts as a key reaction oxygen species (ROS) signaling node to promote the SAR-related defense response in plants (Suzuki et al., 2011), activation of Rboh D is required for the production of ROS in the form of H2O2 during both PTI and ETI against avirulent bacteria and virulent necrotrophic fungi (Kadota et al., 2019; Guerra et al., 2020; Wang et al., 2020). In this study, Rboh D was activated in both treatments in which 2, 3-Butanediol was sprayed, with or without A. panax infection (E and Ei) (Fig. 6A and C). As an essential component of signal transduction pathways, OXI1 links oxidative burst signals to various downstream responses (Rentel et al., 2004). One of these responses is activated by the transcription factors MKK5, MPK3, and WRKY22 in the same plant MAP kinase cascade found in the preceding content; in particular, the expression of OXI1 is required for full activation of MPK3 in basal resistance to Peronospora parasitica infection (Rentel et al., 2004). In the present study, OXI1 (Pno06G000702) was upregulated in both treatments in which 2, 3-Butanediol was presprayed, with or without A. panax infection (E and Ei). The upregulation of Rboh D and OXI1 genes demonstrated that prespraying 2, 3-Butanediol with or without A. panax infection (E and Ei) could activate the oxidative burst and downstream activity of the MAPK cascade, which have been shown to be defense responses of PTI (Spoel and Dong, 2012; Yu et al., 2017; Van den Berg et al., 2021). Once pathogens enter the cytoplasm of host cells and activate host resistance protein-dependent responses, a prolonged and robust defense response ETI can be activated (Vlot et al., 2020). ETI activation overlaps with PTI activation in some immune responses and signal transduction-related activities, such as ROS generation and MAPK cascade activation (Thomma et al., 2011; Tsuda et al., 2013; Kadota et al., 2019). Moreover, MPK3 discussed previously was reported to promote ROS accumulation and manipulate hypersensitive response (HR) cell death to increase the robustness of ETI (Su et al., 2018). Thus, ETI might also be activated by prespraying 2, 3-Butanediol.
GO enrichment analysis demonstrated that many transcription factors of WRKY family were activated after 2, 3-Butanediol treatment with or without A. panax infection (E and Ei) (Fig. S1), members of which are involved in defense response mediated by various abiotic and biotic stresses (Guo et al., 2014). Specifically, the oxidative burst-related HSFF and WRKY1 genes were activated in the 2, 3-Butanediol treatments (Table S5). HSFF has been documented to be a key regulator involved in the induction of the defense system, and its transcript levels were shown to significantly increase under several stress conditions or in response to H2O2 (Nishizawa et al., 2006). In this study, HSFF (MSTRG.25, 428) was upregulated in both 2, 3-Butanediol treatments with and without A. panax infection (E and Ei). Overexpression of WRKY1 was associated with a strong oxidative burst and increased resistance in Arabidopsis to avirulent strains of Pseudomonas syringae (Encinas-Villarejo et al., 2009). WRKY1 (Pno01G005034) expression significantly increased only under 2, 3-Butanediol treatment without A. panax infection (E). Taken together, these results demonstrated that 2, 3-Butanediol treatment with or without A. panax infection (E and Ei) could upregulate genes related to the MAPK cascade and ROS accumulation to activate PTI and ETI.
Overall, the prespraying of 2, 3-Butanediol activated both SAR- and ISR-related genes. Although ISR activation in response to 2, 3-bitanediol has been reported in many studies (Cortes-Barco et al., 2010a, 2010b; Shi et al., 2018), this was the first to show that 2, 3-Butanediol induced SAR. Several SAR chemical activators, including 2, 6-dichloroisonicotinic acid and its methyl ester (INA), benzo(1, 2, 3) thiadiazole-7- carbothioic acid S-methyl ester (BTH), 3-acetonyl-3-hydroxyoxindole (AHO) and tiadinil (TDL) have been documented (Colson-Hanks and Deverall, 2000; Conrath, 2006; Maeda and Ishiwari, 2012; Chen et al., 2017). Recently, SAR inducers have been applied to control citrus huanglongbing via foliar sprays (Li et al., 2021).
Camalexin (3-thiazol-2′-yl-indole) is a phytoalexin which played an important role in resisting the invasion of Alternaria brassicicola (Thomma et al., 1999; Ahuja et al., 2012). Our results showed that MKS1 (Pno04G002716) was upregulated only under 2, 3-Butanediol treatment without A. panax infection (E), and WRKY33 (Pno06G004066 and Pno05G014370) was upregulated under 2, 3-Butanediol treatment both with and without A. panax infection (E and Ei) (Fig. 6A and C). MKS1 contributes to MPK4-regulated defense activation by facilitating the binding of the kinase to WRKY33, which is responsible for camalexin synthesis and contributes to plant resistance against necrotrophs, including A. brassicicola and A. brassicae (Glazebrook, 2005; Sellam et al., 2007). In addition, MPK3 regulates the biosynthesis of camalexin by phosphorylating WRKY33 and enhancing its transcriptional activity (Mao et al., 2011). Therefore, 2, 3-Butanediol could activate camalexin biosynthesis to withstand infection of A. panax by upregulating DEGs that encoded MKS1 and WRKY33.
5. ConclusionsThis research demonstrates that proper use of plant diversity in the forests could benefit herb plantations. 2, 3-Butanediol is an important chemical constituent from Pinus armandii leachates and can increase the resistance of Panax notoginseng to Alternaria panax by inducing ISR, SAR and camalexin biosynthesis. In the future, the application of exogenous 2, 3-Butanediol could be a way to reduce the severity of plant diseases.
AcknowledgementsThis work was supported by the National Key Research and Development Program of China (2017YFC1702502), the Major Science and Technology Project in Yunnan Province (202102AE090042; 202102AA310048-2), Science and Technology Project of Kunming (2021JH002), Innovative Research Team of Science and Technology in Yunnan Province (202105AE160016).
Authors contributions
All persons who meet authorship criteria are listed as authors. Y.Y.Z. and S.S.Z. conceived the ideas and directed the project; H.C.H., Y.X.L. and X.Y.M. designed this study; Y.J.Z. performed data analysis and statistics; J.X.Z. provided the samples; S.S.Z., X.H.H. and M.Y. supervised all study; T.Y.L. and C.Y. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Data availability statement
Raw RNA-seq data have been deposited in the Short Read Archive (SRA) at NCBI under accession number PRJNA755455.
Declaration of competing interest
The authors declare no financial and non-financial competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.02.003.
Ahuja, I., Kissen, R., Bones, A.M., 2012. Phytoalexins in defense against pathogens. Trends Plant Sci., 17: 73-90. |
Albuquerque, M.B., Santos, R.C., Lima, L.M., et al., 2010. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev., 31: 379-395. |
Asai, T., Tena, G., Plotnikova, J., et al., 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature, 415: 977-983. |
Bachheti, A., Sharma, A., Bachheti, R.K., et al., 2020. Plant allelochemicals and their various applications. In: Co-Evolution of Secondary Metabolites, Reference Series in Phytochemistry, pp. 1-25.
|
Breeze, E., 2019. Master MYCs: MYC2, the jasmonate signaling 'master switch'. Plant Cell, 31: 9-10. DOI:10.1105/tpc.19.00004 |
Chen, Y., Dong, J., Bennetzen, J.L., et al., 2017. Integrating transcriptome and microRNA analysis identifies genes and microRNAs for AHO-induced systemic acquired resistance in N. tabacum. Sci. Rep., 7: 1-13. |
Chen, S., Guo, B., 2004. Sustainable utilization of Chinese material medicine resources. Moder. Tra. Chinese Medi. Mat. Medi., 6: 1-8. |
Chinchilla, D., Shan, L., He, P., et al., 2009. One for all: the receptor-associated kinase BAK1. Trends Plant Sci., 14: 535-541. |
Chomel, M., Baldy, V., Guittonny, M., et al., 2020. Litter leachates have stronger impact than leaf litter on Folsomia candida fitness. Soil Biol. Biochem., 147: 1-21. |
Colson-Hanks, E.S., Deverall, B.J., 2000. Effect of 2, 6-dichloroisonicotinic acid, its formulation materials and benzothiadiazole on systemic resistance to alternaria leaf spot in cotton. Plant Pathol., 49: 171-178. |
Conrath, U., 2006. Systemic acquired resistance. Plant Signal. Behav., 1: 179-184. DOI:10.4161/psb.1.4.3221 |
Cortes-Barco, A.M., Goodwin, P.H., Hsiang, T., 2010a. Comparison of induced resistance activated by benzothiadiazole, (2R, 3R)-Butanediol and an isoparaffin mixture against anthracnose of Nicotiana benthamiana. Plant Pathol., 59: 643-653. DOI:10.1111/j.1365-3059.2010.02283.x |
Cortes-Barco, A.M., Hsiang, T., Goodwin, P.H., 2010b. Induced systemic resistance against three foliar diseases of Agrostis stolonifera by (2R, 3R)-Butanediol or an isoparaffin mixture. Ann. Appl. Biol., 157: 179-189. DOI:10.1111/j.1744-7348.2010.00417.x |
De Araujo, A.A., Roussos, S., 2002. A technique for mycelial development of ectomycorrhizal fungi on agar media. Appl. Biochem. Biotechnol., 98–100: 311-318. |
Ding, X., Yang, M., Huang, H., et al., 2015. Priming maize resistance by its neighbors: activating 1, 4-benzoxazine-3-ones synthesis and defense gene expression to alleviate leaf disease. Front. Plant Sci., 6: 1-11. |
Donald, P.F., 2004. Biodiversity impacts of some agricultural commodity production systems. Conservat. Biol., 18: 17-38. DOI:10.1111/j.1523-1739.2004.01803.x |
Dusa, A., 2019. Draw Venn Diagrams [R Package Venn, version 1.8.
|
Encinas-Villarejo, S., Maldonado, A.M., Amil-Ruiz, F., et al., 2009. Evidence for a positive regulatory role of strawberry (Fragaria x ananassa) Fa WRKY1 and Arabidopsis at WRKY75 proteins in resistance. J. Exp. Bot., 60: 3043-3065. DOI:10.1093/jxb/erp152 |
Gaba, S., Lescourret, F., Boudsocq, S., et al., 2014. Multiple cropping systems as drivers for providing multiple ecosystem services: from concepts to design. Agron. Sustain. Dev., 35: 607-623. |
Glazebrook, J., 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol., 43: 205-227. DOI:10.1146/annurev.phyto.43.040204.135923 |
Guerra, T., Schilling, S., Hake, K., et al., 2020. Calcium-dependent protein kinase 5 links calcium signaling with N-hydroxy-l-pipecolic acid- and SARD1-dependent immune memory in systemic acquired resistance. New Phytol., 225: 310-325. DOI:10.1111/nph.16147 |
Guo, C., Guo, R., Xu, X., et al., 2014. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J. Exp. Bot., 65: 1513-1528. DOI:10.1093/jxb/eru007 |
Harel, Y.M., Mehari, Z.H., Rav-David, D., et al., 2014. Systemic resistance to gray mold induced in tomato by benzothiadiazole and Trichoderma harzianum T39. Phytopathology, 104: 150-157. |
Hickman, R., Van Verk, M.C., Van Dijken, A.J.H., et al., 2017. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell, 29: 2086-2105. DOI:10.1105/tpc.16.00958 |
Ichimura, K., Shinozaki, K., Tena, G., et al., 2002. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci., 7: 301-308. |
Ivette Perfecto, John Vandermeer, 2008. Biodiversity conservation in tropical agroecosystems: A new conservation paradigm. Ann. N. Y. Acad. Sci., 1134: 173-200. |
Jing, S.Q., Jiang, H.P., Liu, F.Y., et al., 2009. Canparison of seven ginsenoside contents in shengshaishen hongshen and linxiashen. Chinese Arch. Tra. Chinese Medi., 27: 207-209. |
Kadota, Y., Liebrand, T.W.H., Goto, Y., et al., 2019. Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector- and PAMP-triggered immunity in plants. New Phytol., 221: 2160-2175. DOI:10.1111/nph.15523 |
Kato-Noguchi, H., Fushimi, Y., Tanaka, Y., et al., 2011. Allelopathy of red pine: isolation and identification of an allelopathic substance in red pine needles. Plant Growth Regul., 65: 299-304. DOI:10.1007/s10725-011-9601-2 |
Keesing, F., Ostfeld, R., 2015. Is biodiversity good for your health?. Science, 349: 235-236. DOI:10.1126/science.aac7892 |
Kim, D., Langmead, B., Salzberg, S.L., 2015a. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods, 12: 357-360. |
Kim, Y.J., Zhang, D., Yang, D.C., 2015b. Biosynthesis and biotechnological production of ginsenosides. Biotechnol. Adv., 33: 717-735. |
Li, B., Dewey, C., 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12: 323. |
Li, C., He, X., Zhu, S., et al., 2006. Crop diversity for yield increase. PLoS One, 4: 1-6. |
Li, J., Kolbasov, V., Pang, Z., et al., 2021. Evaluation of the control effect of SAR inducers against citrus Huanglongbing applied by foliar spray, soil drench or trunk injection. Phytopathol. Res., 3: 1-15. |
Liu, Y., Zhang, S., 2004. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell, 16: 3386-3399. DOI:10.1105/tpc.104.026609 |
Livak, K., Schmittgen, T., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCt method. Methods, 25: 402-408. |
Lorenzo, O., Piqueras, R., Sanchez-Serrano, J.J., et al., 2003. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell, 15: 165-178. DOI:10.1105/tpc.007468 |
Luo, L., Guo, C., Wang, L., et al., 2019. Negative plant-soil feedback driven by re-assemblage of the rhizosphere microbiome with the growth of Panax notoginseng. Front. Microbiol., 10: 1-13. |
Luo, L.-F., Yang, L., Yan, Z.-X., et al., 2020. Ginsenosides in root exudates of Panax notoginseng drive the change of soil microbiota through carbon source different utilization. Plant Soil, 455: 139-153. DOI:10.1007/s11104-020-04663-5 |
Ma, X., Claus, L.A.N., Leslie, M.E., et al., 2020. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature, 581: 199-203. DOI:10.1038/s41586-020-2210-3 |
Maeda, T., Ishiwari, H., 2012. Tiadinil, a plant activator of systemic acquired resistance, boosts the production of herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi in the tea plant Camellia sinensis. Exp. Appl. Acarol., 58: 247-258. DOI:10.1007/s10493-012-9577-2 |
Mancuso, C., Santangelo, R., 2017. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food Chem. Toxicol., 107: 362-372. |
Mao, G., Meng, X., Liu, Y., et al., 2011. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell, 23: 1639-1653. DOI:10.1105/tpc.111.084996 |
Maobe, M.A.G., Gatebe, E., Gitu, L., et al., 2012. Profile of heavy metals in selected medicinal plants used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. Glob. J. Pharmacol., 6: 245-251. |
Meng, X., Xu, J., He, Y., et al., 2013. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell, 25: 1126-1142. DOI:10.1105/tpc.112.109074 |
Mhlongo, M.I., Piater, L.A., Madala, N.E., et al., 2018. The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci., 9: 1-17. |
Mundt, C., 2002. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol., 40: 381-410. |
Newton, A., Begg, G., Swanston, J., 2008. Deployment of diversity for enhanced crop function. Ann. Appl. Biol., 154: 309-322. |
Nishizawa, A., Yabuta, Y., Yoshida, E., et al., 2006. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J., 48: 535-547. |
Onate-Sanchez, L., Anderson, J.P., Young, J., et al., 2007. AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol, 143: 400-409. DOI:10.1104/pp.106.086637 |
Pelissier, R., Violle, C., Morel, J.B., 2021. Plant immunity: good fences make good neighbors?. Curr. Opin. Plant Biol., 62: 1-10. DOI:10.1002/9781119842644.part1 |
Pertea, M., Pertea, G.M., Antonescu, C.M., et al., 2015. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol., 33: 290-295. DOI:10.1038/nbt.3122 |
Piasecka, A., Jedrzejczak-Rey, N., Bednarek, P., 2015. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol., 206: 948-964. DOI:10.1111/nph.13325 |
Pieterse, C.M., Van der Does, D., Zamioudis, C., et al., 2012. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol., 28: 489-521. DOI:10.1146/annurev-cellbio-092910-154055 |
Pieterse, C.M., Zamioudis, C., Berendsen, R., et al., 2014. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol., 52: 347-375. DOI:10.1146/annurev-phyto-082712-102340 |
Poelman, E., van Loon, J., Dicke, M., 2008. Consequences of plant defense for biodiversity at higher trophic levels. Trend. Plant Sci., 13: 534-541. |
Potuschak, T., Lechner, E., Parmentiert, Y., et al., 2003. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell, 115: 679-689. |
Raivo, Kolde, 2019. Pheatmap: Pretty Heatmaps. R Package, Version 1.0.12.
|
Rentel, M.C., Lecourieux, D., Ouaked, F., et al., 2004. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature, 427: 858-861. |
Ritchie, M.E., Phipson, B., Wu, D., et al., 2015. LIMMA powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res., 43: e47. DOI:10.1093/nar/gkv007 |
Sellam, A., Iacomi-Vasilescu, B., Hudhomme, P., et al., 2007. In vitro antifungal activity of brassinin, camalexin and two isothiocyanates against the crucifer pathogens Alternaria brassicicola and Alternaria brassicae. Plant Pathol., 56: 296-301. DOI:10.1111/j.1365-3059.2006.01497.x |
Shettima, A.Y., Karumi, Y., Sodipo, O.A., et al., 2013. Gas chromatography-mass spectrometry (GC-MS) analysis of bioactive components of ethyl acetate root extract of Guiera senegalensis. J. Appl. Pharm. Sci., 3: 146-150. |
Shi, Y., Liu, X., Fang, Y., et al., 2018. 2, 3-Butanediol activated disease-resistance of creeping bentgrass by inducing phytohormone and antioxidant responses. Plant Physiol. Biochem., 129: 244-250. |
Solano, R., Stepanova, A., Chao, Q., et al., 1998. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev., 12: 3703-3714. DOI:10.1101/gad.12.23.3703 |
Song, S., Huang, H., Gao, H., et al., 2014. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell, 26: 1-17. |
Spoel, S.H., Dong, X., 2012. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol., 12: 89-100. DOI:10.1038/nri3141 |
Su, J., Yang, L., Zhu, Q., et al., 2018. Active photosynthetic inhibition mediated by MPK3/MPK6 is critical to effector-triggered immunity. PLoS Biology, 16: 1-29. DOI:10.11648/j.sjedu.20180601.11 |
Suzuki, N., Miller, G., Morales, J., et al., 2011. Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol., 14: 691-699. |
Syu, M.J., 2001. Biological production of 2, 3-Butanediol. Appl. Microbiol. Biotechnol., 55: 10-18. |
Taha, S.M., Gadalla, S.A., 2017. Development of an efficient method for multi residue analysis of 160 pesticides in herbal plant by ethyl acetate hexane mixture with direct injection to GC-MS/MS. Talanta, 174: 767-779. |
Team, D., 2013. R: A language and environment for statistical computing team. R Foundation for statistical computing, Vienna, Austria.
|
Thomma, B., Nelissen, I., Eggermont, K., et al., 1999. Deficiency in phytoalexin production causes enhanced susceptibilty of Arabidopsis thaliana to the fungus Alternaria brassicola. Plant J., 19: 163-171. |
Thomma, B.P., Nurnberger, T., Joosten, M.H., 2011. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell, 23: 4-15. DOI:10.1105/tpc.110.082602 |
Tscharntke, T., Clough, Y., Bhagwat, S., et al., 2011. Multifunctional shade-tree management in tropical agroforestry landscapes - a review. J. Appl. Ecol., 48: 619-629. DOI:10.1111/j.1365-2664.2010.01939.x |
Tsuda, K., Katagiri, F., 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol., 13: 459-465. |
Tsuda, K., Mine, A., Bethke, G., et al., 2013. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genetics, 9: 1-14. DOI:10.1007/978-1-62703-107-3_1 |
Van den Berg, N., Swart, V., Backer, R., et al., 2021. Advances in understanding defense mechanisms in Persea americana against Phytophthora cinnamomi. Front. Plant Sci., 12: 1-17. |
Venegas-Molina, J., Molina-Hidalgo, F.J., Clicque, E., et al., 2021. Why and how to dig into plant metabolite-protein interactions. Trends Plant Sci., 26: 472-483. |
Verma, S.S., Yajima, W.R., Rahman, M.H., et al., 2012. A cysteine-rich antimicrobial peptide from Pinus monticola (PmAMP1) confers resistance to multiple fungal pathogens in canola (Brassica napus). Plant Mol. Biol., 79: 61-74. DOI:10.1007/s11103-012-9895-0 |
Vlot, A.C., Sales, J.H., Lenk, M., et al., 2020. Systemic propagation of immunity in plants. New Phytol., 229: 1234-1250. |
Wang, R., He, F., Ning, Y., et al., 2020. Fine-tuning of RBOH-mediated ROS signaling in plant immunity. Trends Plant Sci., 25: 1060-1062. |
Wei, W., Yang, M., Liu, Y., et al., 2018. Fertilizer N application rate impacts plant-soil feedback in a sanqi production system. Sci. Total Environ., 633: 796-807. |
Wu, H., Xia, J., Qin, X., et al., 2020. Underlying mechanism of wild Radix pseudostellariae in tolerance to disease under the natural forest cover. Front. Microbiol., 11: 1-16. |
Yan, Z.Y., 2012. Major tasks and challenges for resources science of Chinese medicinal materials. Pharm. Clin. Chin., 3: 1-6. |
Yang, H., 2016. Main practice and effects of Chinese herbal medicine planting under forest development in Xiji county. Moder. Agricul. Sci. Tech., 18: 79. DOI:10.2527/jas.2015-9560 |
Yang, T., Chen, Y.J., Duan, C.L., et al., The methodology for artificial identification of Panax notoginseng resistance to black spot disease. J. Yunnan Agricul. Univ.. |
Yang, M., Zhang, Y., Qi, L., et al., 2014. Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS One, 9: 1-22. DOI:10.1016/s0924-9338(14)77675-8 |
Yang, M., Zhang, X., Xu, Y., et al., 2015. Autotoxic ginsenosides in the rhizosphere contribute to the replant failure of Panax notoginseng. PLoS One, 10: 1-17. DOI:10.3390/nu8010001 |
Yang, Z., Liu, G., Zhang, G., et al., 2021. The chromosome-scale high quality genome assembly of Panax notoginseng provides insight into dencichine biosynthesis. Plant Biotechnol. J., 19: 869-871. DOI:10.1111/pbi.13558 |
Ye, C., Fang, Y., H, J., Liu, H., et al., 2019. Current status of soil sickness research on Panax notoginseng in Yunnan, China. Allelopath J., 47: 1-14. |
Ye, C., Liu, Y., Zhang, J., et al., 2021. α-Terpineol fumigation alleviates negative plant-soil feedbacks of Panax notoginseng via suppressing Ascomycota and enriching antagonistic bacteria. Phytopathol. Res., 3: 1-17. |
Yu, G., Wang, L.G., Han, Y., et al., 2012. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics, 16: 284-287. DOI:10.1089/omi.2011.0118 |
Yu, X., Feng, B., He, P., et al., 2017. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol., 55: 109-137. DOI:10.1146/annurev-phyto-080516-035649 |
Zeng, W., Jia, L., 2009. Antimicrobial activities of pine needle extracts. Food Sci., 30: 87-90. |
Zhang, S., Klessig, D.F., 2001. MAPK cascades in plant defense signaling. Trends Plant Sci., 6: 520-527. |
Zhang, X., Yang, T., Lin, Q., et al., 2011a. Isolation and identification of an acetoin high production bacterium that can reverse transform 2, 3-Butanediol to acetoin at the decline phase of fermentation. World J. Microbiol. Biotechnol., 27: 2785-2790. DOI:10.1007/s11274-011-0754-y |
Zhang, Y., Sun, H., Song, X., et al., 2011b. Studied on soil microbial community structure about wild Ginseng under forest. Res. Soil Water Conservat., 18: 169-173. |
Zhang, X., Zhu, Z., An, F., et al., 2014. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell, 26: 1105-1117. DOI:10.1105/tpc.113.122002 |
Zhang, S., Chen, C., Lu, W., et al., 2018. Phytochemistry, pharmacology, and clinical use of Panax notoginseng flowers buds. Phytother. Res., 32: 2155-2163. DOI:10.1002/ptr.6167 |
Zhong, L.L., Yang, W., Lam, W.C., et al., 2020. Potential targets for treatment of coronavirus disease 2019 (COVID-19): a review of qing-fei-pai-du-tang and its major herbs. Am. J. Chin. Med., 48: 1051-1071. DOI:10.1142/s0192415x20500512 |
Zhou, P., Xie, W., He, S., et al., 2019. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells, 8: 204. DOI:10.3390/cells8030204 |
Zhu, S., Morel, J.B., 2019. Molecular mechanisms underlying microbial disease control in intercropping. Mol. Plant Microbe Interact., 32: 20-24. |



