b. Institute of Environment and Ecology, School of the Environment and Safety Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang, 212013, Jiangsu Province, China;
c. Key Laboratory for Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming 650201, Yunnan, China;
d. East and Central Asia Regional Office, World Agroforestry (ICRAF), Kunming 650201, Yunnan, China
Plant phenology will affect the species distribution, ecosystem carbon cycling, ecosystem functions and services, seed production, and feedback to the climate system (Fu et al., 2014; Piao et al., 2019). Compared to a large number of specific phenophase related phenological studies, few studies have investigated the impact of one phenology on the subsequent phenological events (Fu et al., 2014). Fu et al. (2014) found legacy effects of leaf flushing both on leaf senescence later in the same year, and on the leaf flushing of the next year for two European temperate species. Such effects have been detected in the boreal and temperate trees by other studies (Heide, 2003; Petri and Leite, 2004). The legacy or carryover effect is defined as the effect of present states of plants/vegetation on the subsequent growth (Lian et al., 2021). This legacy or carryover effect has been explained by the theory of chilling and forcing effect: namely, that winter warming causes insufficient chilling, inducing a later onset of endodormancy phase. The failure to meet the forcing requirements for flushing, causes a later leaf flushing date (Fu et al., 2014). The above studies mainly focused on the temperate and boreal trees, while less attention has been paid to the tropical trees (Fu et al., 2014; Piao et al., 2019). To our limited knowledge, no studies in the legacy effects have been conducted on tropical tree phenology (Xu et al., 2014).
Rubber trees (Hevea brasiliensis (Willd. ex A. Juss.) Muell. Arg.), were originally introduced from the Amazon rainforest to Asia. H. brasiliensis Muell. -Arg is almost the exclusive source of commercial natural rubber (Bowers, 1990; Priyadarshan, 2011). Growing demand for natural rubber drove the expansion of rubber plantations beyond their original habitats in Amazon regions to sub-optimal regions (with a lower temperature, precipitation, and lower humidity (Priyadarshan, 2011; Yu et al., 2014)), especially in Southeast Asia countries, where the area expanded ~2 million ha in last decade and this region now produces more than 90% of global natural rubber (Warren-Thomas et al., 2015, 2018). In these sub-optimal cultivating habitats, like Xishuangbanna, southwestern China, where the rubber trees have distinct deciduous phenology. The rubber trees undergo annual leaf shedding (wintering) during January–February, and leaf flushing (refoliation) during February–March in Xishuangbanna (Priyadarshan, 2011; Zhai et al., 2019). Leaf flushing phenology has a significant role in the level of infection of powdery mildew disease (Oidium heveae) and latex yield in March (Zhai et al., 2021; Zhai et al., unpublished data). Additionally, leaf flushing phenology is delayed with higher temperatures during November–December (Zhai et al., 2021), which are subsequently associated with reduced latex yield in March (Zhai et al. unpublished data). During tDecember-February, rubber trees undergo defoliation, with leaves first changing color and then falling. During the defoliation period, local farmers stop tapping latex from their trees, usually by late November or early December. We therefore hypothesized that the defoliation period would be a critical period for rubber growth, disease defense, and also subsequent latex production in March. The defoliation phenology may have legacy effects on later refoliation, disease defence, and latex yield.
We tested this hypothesis in a state farm where we have long-term comprehensive data for rubber trees, including: leaf flushing phenology, powdery mildew disease, latex yield, and also climate data. The primary objectives of this study were (1) to investigate the relationship of duration of defoliation on the refoliation phenology, powdery mildew disease, and latex yield in March; (2) to investigate the influences of temperature on defoliation; and (3) to identify the critical periods during when temperatures affect defoliation.
2. Materials and methods 2.1. Study areaXishuangbanna, one of the sub-optimal regions for rubber cultivation in Southeast Asia, is the second-largest rubber producing region in China (Yu et al., 2014; Zhu et al., 2006). The rubber plantations in China suffer from multiple stresses, e.g. typhoons, cold, and drought (Ahrends et al., 2015). Xishuangbanna has two seasons: the rainy season (May–October) and the dry season (November–April). The dry season is further divided into the cool-dry season (November–February) and the hot-dry season (March–April) (Cao et al., 2006). During the dry season, rubber trees shed leaves (November–February), then flushed leaves (February–April) (Lin et al., 2018; Zhai et al., 2019). During this leaf flushing/refoliation period, the immature leaves are susceptible to infection by powdery mildew disease (Zhai et al., 2020). Farmers usually resume tapping latex in late March/April and end tapping in late November/early December (Yu et al., 2014). Rubber trees are given an extended rest period from tapping ('non-tapping') from December to middle March. During the non-tapping period, rubber leaves first become brown, then are shed in January. They are initially leafless in February, and then over the course of one to three weeks grow new leaves. By March to April, foliage has been fully restored.
2.2. Data and statistical analysisThis study was based on previously published data regarding rubber leaf flushing phenology (Zhai et al., 2019), powdery mildew disease (Zhai et al., 2020), and rubber latex production (Yu et al., 2014).
Rubber leaf flushing phenology data had been collected from 2003 to 2011, with a recording interval of 3 days from mid-January to mid-April, following the guidelines and standards of the Bureau of China State Farm. Powdery mildew data were also collected from 2003 to 2011, with a recording interval of 3 days, from the end of January to the end of March. More detailed information on phenology monitoring and powdery mildew infection monitoring was inferred from Zhai et al., 2019, Zhai et al., 2020 respectively. The dry rubber yield data was collected daily (kg ha−1) in the entire state farm in 2004–2010, from March/April to November/December. The productivity of rubber trees was weighted before further processing out of the farm. The tapping frequency has been reduced from every two days (2/d) in the early 1980s to every four days (4/d) in the late 1980s. A half and quarter spiral are both used. To improve the latex yield, the 2%–3% stimulant Ethephon (ET) was applied (Yu et al., 2014). The state farm applied the 2-chloroethylphosphonic acid (Ethephon) every 15 days, which is a common practice to enhance latex yield in rubber plantations (Silpi et al., 2006). Ethephon was applied two to three weeks after tapping, which is normally around the middle of April for the first application. Rubber trees normally are tapped between 4:30–7:30 am. The data collection was terminated in 2011, when plantation management regimes changed. State farms implemented land reforms, in which rubber plantations were contracted out for management by individual households.
We used the same climatic data for 2003–2011 as in previously published research (Yu et al., 2014; Zhai et al., 2019). The climate data were downloaded from the National Meteorological Information Center of China (http://data.cma.cn/). Temperature difference (TD) was calculated by daily maximum temperature (Tmax) and daily minimum temperature (Tmin).
We used a linear model to investigate the relationship between the duration of defoliation and the other variables.
In our previous researches, we found that both Tmax and Tmean influenced rubber leaf flushing phenology (Zhai et al., 2021), and that TD influenced the latex yield (Yu et al., 2014; Zhai et al. unpublished data). We therefore investigated the influence of the above three temperature variables on the duration of defoliation. Partial Least Square (PLS) regression was employed to analyze the influence of temperatures on the duration of defoliation. The daily temperatures of Tmax, Tmean, and TD between previous September (Year N-1) to January (Year N) were used as independent variables for the PLS regression, while the duration of defoliation was treated as the dependent variable. Temperature variables with VIP ≥0.8 and standardized coefficient confidence intervals significantly different from zero were considered important contributing factors to the duration of defoliation. From the VIP and standardized model coefficients of the PLS regression models, we identified the critical periods (at least 11 days with VIP values > 0.8), during which temperatures showed significant effects on the duration of defoliation (Luedeling et al., 2012). The PLS analysis was mainly based on the package of 'pls' and 'chillR' in R programming language (Luedeling 2017; R Core Team, 2017). The further relationship between the identified two critical periods of the three climatic variables and the duration of defoliation was investigated by a three-dimensional response map using the Kriging technique with the R package 'field' (Nychka et al., 2017).
3. Results 3.1. Legacy effects of the duration of defoliation on rubber treesWe found legacy effects of the duration of defoliation on both the duration of refoliation, leaf disease, and rubber latex yield in March, although the effects on the rubber latex yield in March is marginal (Fig. 1). This indicated that the legacy effects impact not only the phenology, but also leaf disease and latex productivity of rubber trees. However, the duration of defoliation had no apparent legacy effects on either the total yield or the annual mean yield of rubber trees.
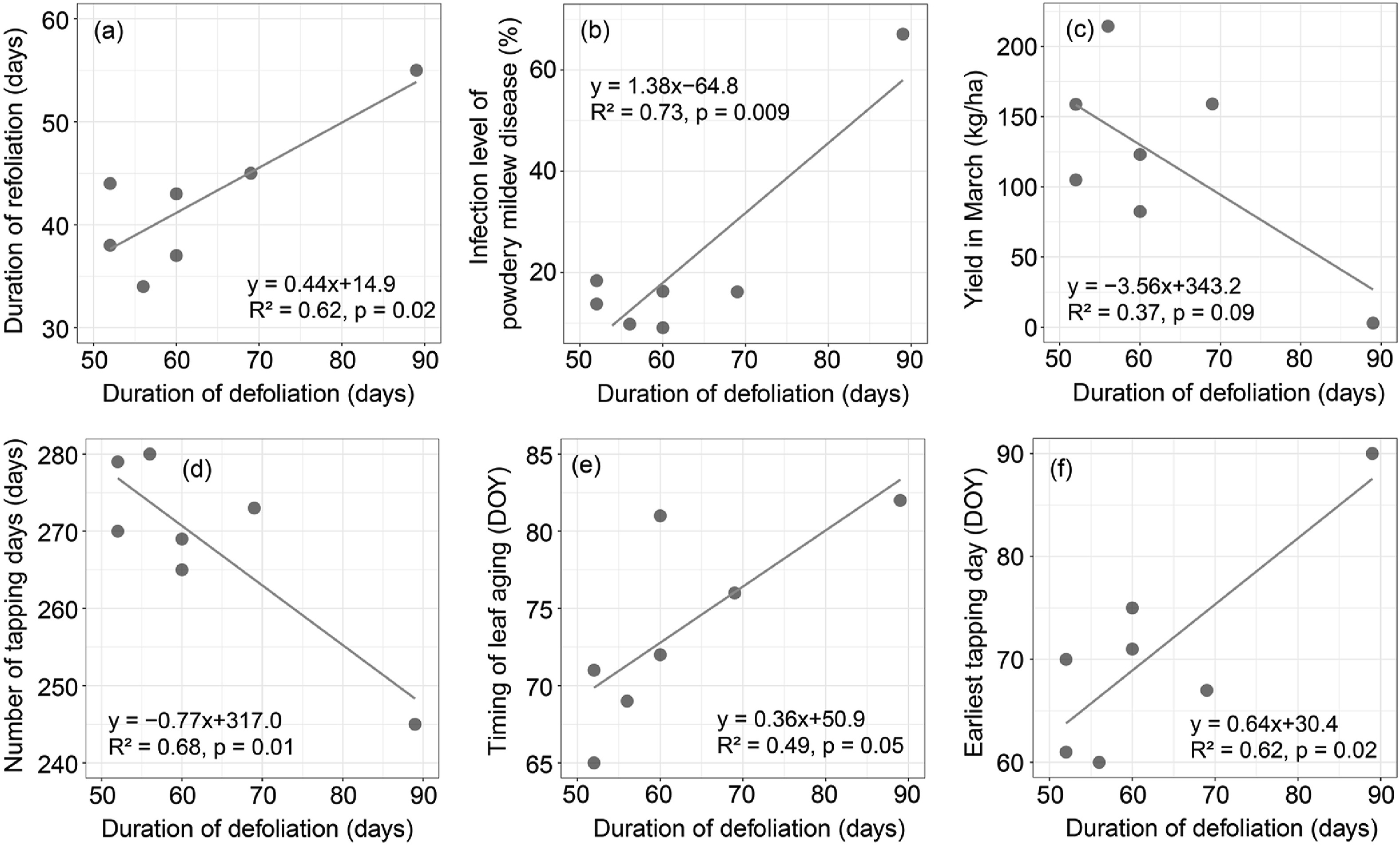
|
| Fig. 1 The relationship between duration of defoliation and the duration of refoliation, powdery mildew disease, latex yield in March, number of tapping days, timing of leaf aging, and earliest tapping day. |
The duration of defoliation also significantly affected the number of tapping days and the timing of earliest tapping (first tapping day) (Fig. 1d and f), both of which significantly affect the rubber latex yield in March (Fig. 2).
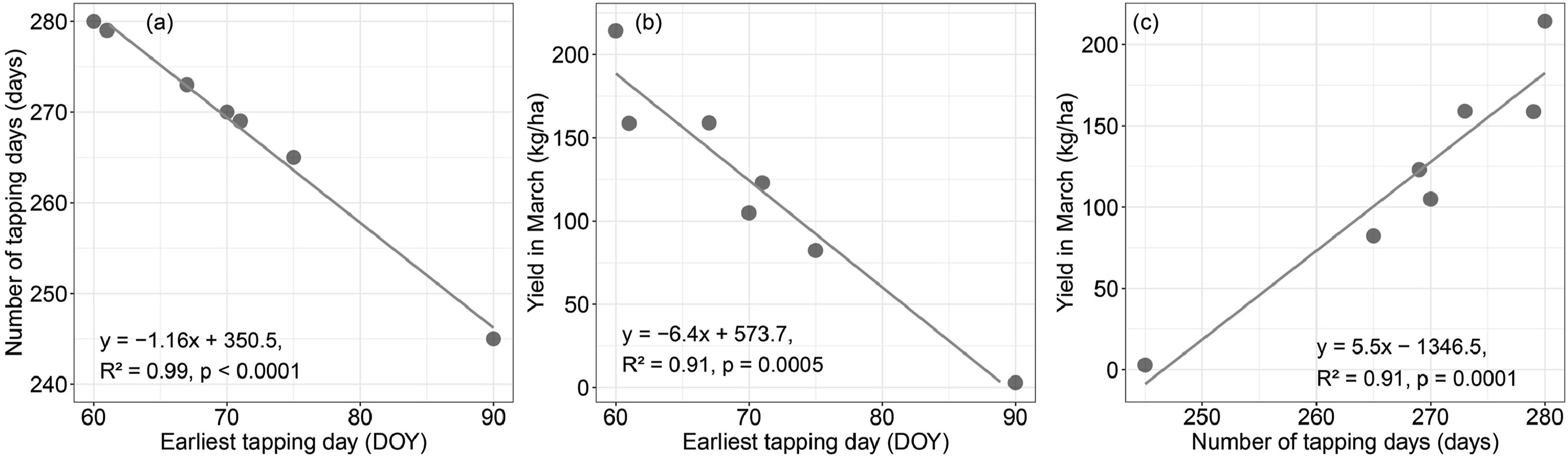
|
| Fig. 2 The relationship between earliest tapping day, number of tapping days, and also yield in March. |
The duration of the defoliation period ranged from 52 days in 2009 and 2010 to 89 days in 2008, with a mean of 62.6 days. The longer the duration of defoliation was related to delayed leaf aging, longer refoliation, a higher infection level of powdery mildew disease, and delayed timing of first tapping day, but fewer total tapping days and a lower latex yield in March (Fig. 1).
An extended period of defoliation delayed the timing of leaf aging, but had no significant effect on the timing of budburst (Fig. 1e). The one day extended duration of defoliation delayed the first tapping day by 0.64 days, which decreased the yield in March by 3.56 kg/ha (Fig. 1f and c). For ten days increase in the defoliation period, the number of tapping days decreased by 7.7 days (Fig. 1d).
3.2. Critical periods of temperature on the duration of defoliationWe found that during October–November which is before the cessation of latex tapping (early December), temperature variables had a strong negative influence on the duration of defoliation, which indicated that a higher Tmax during this period was correlated to a shorter duration of defoliation (Fig. 3). After December, temperature variables had a strong positive influence on the duration of refoliation, which indicated that a higher Tmax during this period was correlated to a longer duration of defoliation (Fig. 3).

|
| Fig. 3 Results of Partial Least Square (PLS) regression correlating the duration of defoliation during 2004–2010 to daily mean temperature (left panel, a/b/c), maximum temperature (middle panel, d/e/f), and the temperature difference (right panel, g/h/i). In the upper panel (a/d/g), blue bars mean that VIP is above 0.8, the threshold for variable importance. In the middle panel (b/e/h), red bars correspond to important and negative model coefficients and VIP values are greater than 0.8, while green bars indicate important positive relationships between the duration of defoliation and climatic variables, and the gray bars indicate no statistical significance. The black line in the bottom panel stands (c/f/i) for the mean temperature of each variable, while the gray, green, and red areas represent the standard deviation of the daily mean temperature of each variable for each day of the year (DOY). |
The pattern of negative and positive associations of temperature with rubber tree phenology was clear and consistent for Tmean, Tmax, and TD, with VIP value > 0.8. We therefore identified two critical periods for Tmean: 21st September to 7th November and 13th December to 3rd January, two for Tmax: 3rd October to 11th November and 10th December to 22nd January, and also for TD: 3rd October to 17th November and 8th December to 21st January (Fig. 3). Further analysis showed that both these two critical periods were important for the duration of defoliation (Fig. 4).
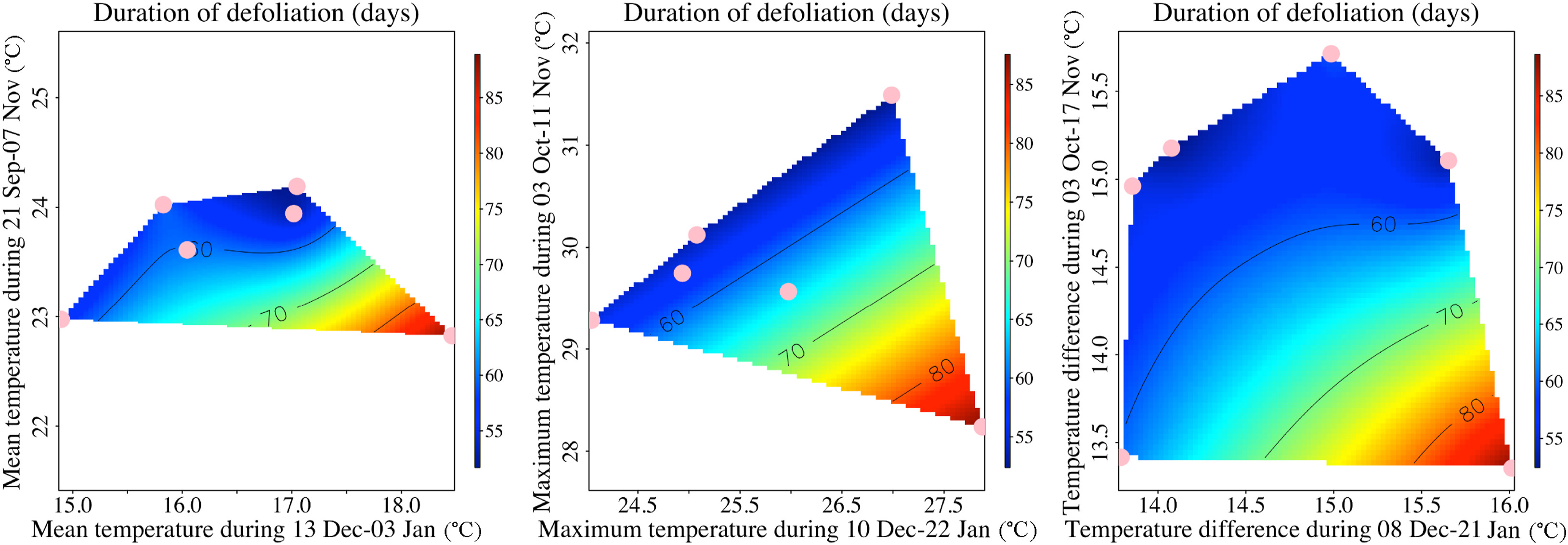
|
| Fig. 4 Response of duration of defoliation to the PLS identified two critical periods. Variation in color reflects variation in duration of defoliation, pink dots show the duration of defoliation observed between 2004 and 2010. |
We found that Tmean, Tmax, and TD, all had negative effects on the latex yield from October to the middle of November (before the cessation of latex tapping), and the positive effects on the latex yield from December to January (after the cessation of latex tapping) (Fig. 3).
4. Discussion 4.1. The legacy effects of defoliation phenology on phenology, powdery mildew disease, and latex productivity of the following seasonThe legacy effects of defoliation phenology might relate to the carbohydrate reserves. During the defoliation period, there is only weak photosynthesis and respiration, but the trees incur a carbon cost due to growth maintenance. Thus, rubber trees must rely on a sufficient pool of reserved carbohydrates to support the self-maintenance and latex regeneration during the defoliation phenology. Silpi et al. (2007) found that the net deposition of the total nonstructural carbohydrates occurred mainly between the vegetation season and the defoliation in January. In Xishuangbanna, rubber trees adjust to cold conditions by shedding leaves and ceasing growth (Lin et al., 2018). With a longer duration of defoliation, rubber trees would consume more carbohydrates thus diminishing the carbohydrate pool available in spring to support resumed and latex production (Chantuma et al., 2009; Silpi et al., 2007). This is a likely mechanism for our observed pattern of longer defoliation periods being associated with longer refoliation periods, later first tapping day, reduced number of latex production days, and lower yield in March. These legacy effects of rubber defoliation have important implications for understanding and modeling leaf phenology, its impact on regional carbon stock, and also potential application in the early-warning of powdery mildew disease. Such impacts should also be included in global land surface models, which to date have not adequately taken into account potential feedback loops due to temperature impacts on leaf phenology within tropical regions (Chen et al., 2020; Wu et al., 2016).
For rubber trees, warming during winter delays the leaf flushing phenology in spring, whereas, cooling advances the leaf flushing dates (Zhai et al., 2021). This response pattern is consistent with that of temperate trees, for which winter cooling is critical for the stimulation of plant endodormancy (Fu et al., 2015; Piao et al., 2019). Later accumulated chilling is required to break endodormancy and enter the second dormant stage or ecodormancy (Fu et al., 2014). However, there is no direct experimental evidence for any dormancy of rubber trees native to the tropical zones, where most of the trees show exchanging leaf phenology (Chen et al., 2020). The deciduous behavior of rubber trees in Xishuangbanna could be considered as a response to less favorable, cooler environmental conditions. Jewaria et al. (2021) reviewed recent research on plant phenology, especially from the subtropics, and found no evidence for dormancy in the tropical trees. Defoliation phenology of rubber plantations has important implications for the rubber industry, distribution of rubber plantations, and the ecosystem carbon cycle (Piao et al., 2019).
4.2. The importance of defoliation periodsThe duration of defoliation significantly affected the duration of refoliation, which indicated that prolonged duration of defoliation would extend the duration of refoliaton. Longer refoliation periods have previously been found to increase the rate of infection by powdery mildew disease (Zhai et al., 2021), which is confirmed by the present study. Our findings of the legacy effects of winter defoliation in the tropical region on next years' leaf flushing and the duration of leaf flushing are of potential importance for the rubber industry and rubber-related leaf disease management. It also indicated that the phenology of plants may have a profound effect on subsequent phenology, disease defense, and other biophysiological factors. Jin et al., 2020 found the lagged effects of spring greening on summer carbon and water cycling in Amazon tropical forests. Further studies of defoliation phenology's impact on tree carbon and water balance should improve the parameterization of land surface models. To knowledge, ours is the first study to show an interconnection between rubber winter phenology to rubber refoliation phenology, rubber powdery mildew, and the latex yield of the first month.
For rubber trees, the phenology of rubber trees might affect photosynthesis and then latex production. During the defoliation period, rubber trees decreased photosynthesis with the leaves shedding, and then increased the photosynthesis with the leaves flushing during the refoliation period (Lin et al., 2018; Wang et al., 2014). The secondary leaf falling during/after the leaf flushing caused by powdery mildew disease decreased the photosynthesis and delayed the date for rubber tapping (Liyanage et al., 2015), and we found during our field survey due to the secondary leaf falling in the year 2021 that there is no latex yield till the recovery of the canopy in June. Therefore, it is critical to further study the influence of defoliation on plants' productivity and disease defense.
The critical period which we identified for December–January is consistent with the cold period found by Lin et al. (2018), which showed a critical one-week cold period during December–January, prior to leaf falling. Our data showed that lower temperatures during December–January shortened the duration of defoliation. Lin et al. (2018) classed their defoliation period as encompassing the leaf senescence, leaf falling, and the leafless period. We covered the same period, with the addition of predefoliation. Although we give no direct evidence for dormancy in rubber trees, low temperatures during December–January were critical to leaf flushing phenology, powdery mildew disease, and the rubber latex yield (Fig. 2; Zhai et al., 2020, 2021). If winter warming trends continue (Zhai et al., 2020), the duration of the defoliation period may lengthen causing delayed refoliation, an increase in the level of powdery mildew disease, and lower latex yields (Fig. 1). Zomer et al. (2014) predicted that expansion of warming in 2050 will mean that greater land areas will have a favorable climate for rubber development. However, the projection of mean warming is insufficient to predict the suitability and distribution of rubber plantations, which will have contrasting responses to changes in winter and spring temperatures (Zhai et al., 2020, 2021).
5. ConclusionThis study has made a major contribution to our understanding of the leaf falling phenology of rubber trees. We found that defoliation phenology plays a critical role in determining both rubber phenology in spring and latex yield, with an additional legacy effect of defoliation on the following year's refoliation, infection by powdery mildew disease, and latex yield. In the current study, we found that autumn (Sep–Nov) and winter (Dec–Jan) temperature had a contrast effect on the duration of defoliation, which significantly affected refoliation, powdery mildew disease, and latex yield. Further research is required regarding the mechanisms by which defoliation phenology impacts rubber tree growth and latex productivity.
AcknowledgmentsThis work was financially supported by the Key Research Program of Frontier Sciences, the Chinese Academy of Sciences (No. QYZDY-SSW-SMC014), and the National Natural Science Foundation of China (No. 32171576). The authors would like to thank the data collectors of Jinghong farm. The authors appreciate the language editing and polishing by Dr. Fiona Worthy, and thanks to her for the comments.
Author contributions
DLZ conducted the analysis and wrote the manuscript. JCX and DLZ collected the data. All authors reviewed and approved the final submission.
Declaration of competing interest
None.
Ahrends, A., Hollingsworth, P.M., Ziegler, A.D., et al., 2015. Current trends of rubber plantation expansion may threaten biodiversity and livelihoods. Global Environ. Change, 34: 48-58. |
Bowers, J.E., 1990. Natural Rubber-Producing Plants for the United States. National Agricultural Library, Beltsville, Maryland pp. VIII + 43 pp.
|
Cao, M., Zou, X.M., Warren, M., et al., 2006. Tropical forests of Xishuangbanna, China. Biotropica, 38: 306-309. DOI:10.1111/j.1744-7429.2006.00146.x |
Chantuma, P., Lacointe, A., Kasemsap, P., et al., 2009. Carbohydrate storage in wood and bark of rubber trees submitted to different level of C demand induced by latex tapping. Tree Physiol., 29: 1021-1031. DOI:10.1093/treephys/tpp043 |
Fu, Y.S.H., Campioli, M., Vitasse, Y., et al., 2014. Variation in leaf flushing date influences autumnal senescence and next year's flushing date in two temperate tree species. Proc. Natl. Acad. Sci. U.S.A., 111: 7355-7360. DOI:10.1073/pnas.1321727111 |
Fu, Y.H., Zhao, H., Piao, S., et al., 2015. Declining global warming effects on the phenology of spring leaf unfolding. Nature, 526: 104-107. DOI:10.1038/nature15402 |
Chen, X., Maignan, F., Viovy, N., et al., 2020. Novel representation of leaf phenology improves simulation of Amazonian evergreen forest photosynthesis in a land surface model. J. Adv. Model. Earth Syst., 12(1): e2018MS001565. |
Heide, O.M., 2003. High autumn temperature delays spring bud burst in boreal trees, counterbalancing the effect of climatic warming. Tree Physiol., 23: 931-936. DOI:10.1093/treephys/23.13.931 |
Jewaria, P., Hänninen, H., Li, X., et al., 2021. A hundred years after: Endodormancy and the chilling requirement in subtropical trees. New Phytol., 231: 565-570. DOI:10.1111/nph.17382 |
Jin, J., Guo, F., Sippel, S., et al., 2020. Concurrent and lagged effects of spring greening on seasonal carbon gain and water loss across the Northern Hemisphere. Int. J. Biometeorol., 64: 1343-1354. DOI:10.1007/s00484-020-01913-0 |
Lian, X., Piao, S., Chen, A., et al., 2021. Seasonal biological carryover dominates northern vegetation growth. Nat. Commun., 12: 983. DOI:10.1038/s41467-021-21223-2 |
Lin, Y., Zhang, Y., Zhao, W., et al., 2018. Pattern and driving factor of intense defoliation of rubber plantations in SW China. Ecol. Indicat., 94: 104-116. |
Luedeling, E., 2017. chillR: Statistical Methods for Phenology Analysis in Temperate Fruit Trees. R Package Version 0.72.5. http://cran.r-project.org/package=chillR. (Accessed 22 October 2020).
|
Luedeling, E., Kunz, A., Blanke, M.M., 2012. Identification of chilling and heat requirements of cherry trees—a statistical approach. Int. J. Biometeorol., 57: 679-689. |
Nychka, D., Furrer, R., Paige, J., Sain, S., 2017. Fields: Tools for Spatial Data, R Package Version 9.0. http://cran.r-project.org/package=fields.
|
Petri, J.L., Leite, G.B., 2004. Consequences of insufficient winter chilling on apple tree bud-break. In: Jindal, K.K., Sharma, R.C., Rehalia, A.S. (Eds. ), Proceedings of the VIIth International Symposium on Temperate Zone Fruits in the Tropics and Subtropics. Acta Horticulturae, pp. 53-60.
|
Piao, S., Liu, Q., Chen, A., et al., 2019. Plant phenology and global climate change: current progresses and challenges. Global Change Biol., 25: 1922-1940. DOI:10.1111/gcb.14619 |
Priyadarshan, P., 2011. Biology of Hevea Rubber. CABI International, Wallingford.
|
R Core Team, 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org.
|
Silpi, U., Lacointe, A., Kasempsap, P., et al., 2007. Carbohydrate reserves as a competing sink: evidence from tapping rubber trees. Tree Physiol., 27: 881-889. DOI:10.1093/treephys/27.6.881 |
Silpi, U., Thaler, P., Kasemsap, P., et al., 2006. Effect of tapping activity on the dynamics of radial growth of Hevea brasiliensis trees. Tree Physiol., 26: 1579-1587. DOI:10.1093/treephys/26.12.1579 |
Wang, L.-F., Wang, M., Zhang, Y., 2014. Effects of powdery mildew infection on chloroplast and mitochondrial functions in rubber tree. Trop. Plant Pathol., 39: 242-250. DOI:10.1590/S1982-56762014000300008 |
Warren-Thomas, E., Dolman, P.M., Edwards, D.P., 2015. Increasing demand for natural rubber necessitates a robust sustainability initiative to mitigate impacts on tropical biodiversity. Conserv. Lett., 8: 230-241. DOI:10.1111/conl.12170 |
Warren-Thomas, E.M., Edwards, D.P., Bebber, D.P., et al., 2018. Protecting tropical forests from the rapid expansion of rubber using carbon payments. Nat. Commun., 9: 911. |
Wu, J., Albert, L.P., Lopes, A.P., et al., 2016. Leaf development and demography explain photosynthetic seasonality in Amazon evergreen forests. Science, 351: 972-976. DOI:10.1126/science.aad5068 |
Xu, G., Luo, S., Guo, Q., et al., 2014. Responses of leaf unfolding and flowering to climate change in 12 tropical evergreen broadleaf tree species in Jianfengling, Hainan Island. Chinese J. Plant Ecol., 38: 585-598. |
Yu, H., Hammond, J., Ling, S., et al., 2014. Greater diurnal temperature difference, an overlooked but important climatic driver of rubber yield. Ind. Crop. Prod., 62: 14-21. |
Zhai, D.-L., Thaler, P., Luo, Y., et al., 2021. The powdery mildew disease of rubber (Oidium heveae) is jointly controlled by the winter temperature and host phenology. Int. J. Biometeorol., 65: 1707-1718. DOI:10.1007/s00484-021-02125-w |
Zhai, D.-L., Wang, J., Thaler, P., et al., 2020. Contrasted effects of temperature during defoliation vs. refoliation periods on the infection of rubber powdery mildew (Oidium heveae) in Xishuangbanna, China. Int. J. Biometeorol., 64: 1835-1845. DOI:10.1007/s00484-020-01969-y |
Zhai, D.-L., Yu, H., Chen, S.-C., et al., 2019. Responses of rubber leaf phenology to climatic variations in Southwest China. Int. J. Biometeorol., 63: 607-616. DOI:10.1007/s00484-017-1448-4 |
Zhu, H., Cao, M., Hu, H.B., 2006. Geological history, flora, and vegetation of Xishuangbanna, southern Yunnan, China. Biotropica, 38: 310-317. DOI:10.1111/j.1744-7429.2006.00147.x |
Zomer, R.J., Trabucco, A., Wang, M., et al., 2014. Environmental stratification to model climate change impacts on biodiversity and rubber production in Xishuangbanna, Yunnan, China. Biol. Conserv., 170: 264-273. |



