b. College of Life and Environmental Sciences, Wenzhou University, Wenzhou, 325035, China;
c. Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, Jiangsu, China;
d. Shanghai Science and Technology Museum, Shanghai, 200127, China;
e. Nanyue Hengshan National Nature Reserve Administration, Hengyang 421900, Hunan, China;
f. Zhejiang Tianmushan National Nature Reserve Management Bureau, Hangzhou, 311311, China;
g. Key Laboratory of Plant Stress Biology, School of Life Sciences, Henan University, Kaifeng 475001, Henan, China;
h. Department of Botany, University of Wisconsin, Madison, WI, 53706, USA
Amana Honda, commonly known as 'East Asian tulips', belongs to the family Liliaceae and is characterized by 2–3 (–4) opposite or verticillate bracts at the upper part of flowering stem and a distinct beak of the fruit (Honda, 1935; Tan et al., 2005). It comprises seven currently recognized species of geophytic, perennial, understory herbs (Ohwi and Kitagawa, 1992; Chen and Mordak, 2000; Shen, 2001; Tan et al., 2007; Han et al., 2014; Wang et al., 2019). All the species are spring ephemerals that occur in temperate deciduous or subtropical evergreen broadleaved/mixed forests of East Asia (Central and Eastern China, Japan and the Korean Peninsula; Fig. 1). Its distribution and diversification centers are in Zhejiang and Anhui provinces of China (Tan et al., 2005). Within the genus, Amana edulis (Miq.) Honda is the most common and widespread species, whereas the other six species are narrow endemics. Although those narrow endemic species are broadly sympatric with A. edulis, five of them rarely co-occur with the widespread A. edulis in intermixed populations due to the different elevations of their natural habitats (Li et al., 2017; Wang et al., 2019). However, A. edulis and A. baohuaensis B.X. Han, Long Wang & G.Y. Lu generally accompany each other within the distribution range of A. baohuaensis.
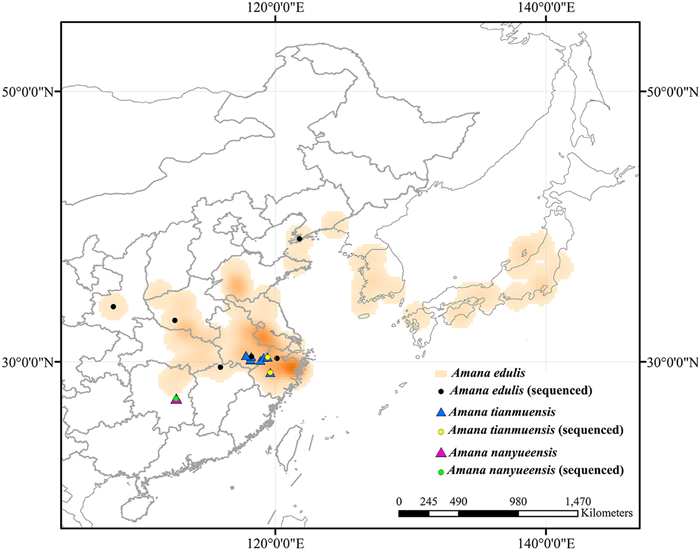
|
| Fig. 1 Distribution of Amana species. Orange represents the distribution area of Amana edulis. The blue and pink triangles represent the known populations of A. tianmuensis and A. nanyueensis, respectively. The black, yellow and green dots represent the individuals of A. edulis, A. tianmuensis and A. nanyueensis sequenced in the present study. |
In 2015 one of the co-authors (Pan Li) noticed some online images (http://ppbc.iplant.cn/tu/1930685) of interesting Amana plants from Hengshan, Hunan Province, China. The plants resemble A. edulis in having two opposite bracts, but differ in their oblanceolate leaves with grayish white midvein and purple anthers. In subsequent herbarium work at NYA (acronyms according to Thiers et al., 2016), a few specimens collected from Hengshan, Hunan were found, but all were misidentified as A. edulis. Since 2017, we have visited Hengshan, Hunan Province several times to observe the plants in the field. Meanwhile, in an excursion to Tianmushan in Zhejiang Province in 2015, an unknown species was also found. This new species resembles Amana erythronioides (Baker) D.Y. Tan & D.Y. Hong, but differs in having a bulb tunic that is yellowish-brown, thinly papery, glabrous inside, and sometimes sparely villous. During fieldwork from 2015 to 2021, more populations were discovered in Tianmushan and the surrounding areas.
Morphological observations, cytological analyses, pollen electron microscope observations, ecological niche modelling and phylogenomic studies all confirmed that both are distinct species. Thus, they are described and illustrated below as new species.
2. Materials and methods 2.1. Sampling and morphological observationsFrom 2015 to 2021, several field excursions and investigations were made to Hengshan in Hunan Province, Jiuhuashan and Huangshan in Anhui Province, Qingliangfeng, Tianmushan and Jinhuashan in Zhejiang Province of China to observe these two suspected new species. At the same time, a large number of phenotypic traits of A. edulis, A. erythronioides, Amana nanyueensis P. Li & L.X. Liu and Amana tianmuensis P. Li & M.Z. Wang were measured, including morphology of bulbs, leaves, bracts, flowers and fruits. The specimens kept in the main Chinese herbaria (CDBI, CSH, HHBG, HIB, HNR, HZU, IBK, IBSC, IFP, JJF, KUN, LGB, NAS, NYA, PE, QFNU, WUK, acronyms according to Thiers et al., 2016) were examined. Based on our field observations, recent collections, and these historical specimens, we documented the morphology of two new species and made comparisons with A. edulis and A. erythronioides.
2.2. Principal component analysisPrincipal component analysis (PCA) was conducted to analyze morphological characters of these four species with Origin 2021 (OriginLab Corporation, Northampton, MA, USA). A total of six traits were used, including the inner side of bulb tunic (glabrous: 0; sparsely villous: 1; densely villous-woolly: 2), lower leaf length/width, upper leaf length/width, bract number (two: 0, three: 1), outer tepal length/width, color of anther (yellow: 0; purple: 1). We measured and analyzed the morphology of 153 individuals, including 69 individuals of A. edulis (from 26 populations), 28 of A. erythronioides (from six populations), 28 of A. nanyueensis (from three populations), and 27 of A. tianmuensis (from nine populations) (Table S1).
2.3. Cytological analysesChromosome numbers were counted in actively growing young dropper (see Fig. 5E1) tips collected in the field. The tips were pretreated with 0.1% colchicine for 4.5 h and fixed in Carnoy's solution (1 glacial acetic acid: 3 absolute alcohol, v/v) for 12–24 h. They were then transferred to absolute ethanol and stored in the refrigerator at −20 ℃. The fixed roots were hydrolyzed in 1 mol/L HCl for 10–15 min, and then washed with distilled water, dyed with Carbol Fuchsin and squashed for observation. Photographs were taken under a SOPTOP DMCX40 microscope (SOPTOP, Ningbo, China).
Karyotype formulae were based on measurements of mitotic metaphase chromosomes taken from photographs. The terminology used for the karyotype position of chromosome composition followed Levan et al. (1964). The degree of karyotype asymmetry was calculated following Arano (1963) "As.K%". The equation: index of Karyotypic Asymmetry (As. K%) = the total of the longest chromosome length/the total of the all chromosome length × 100. Stebbin's asymmetry category was described by karyotypic symmetry division according to Stebbins (1971) and marked as KA. To evaluate karyotype asymmetry, we calculated two parameters suggested by Paszko (2006), Peruzzi et al. (2009), Peruzzi and Eroğlu (2013) and Peruzzi and Altınordu (2014): MCA (Mean Centromeric Asymmetry) and CVCL (Coefficient of Variation of Chromosome Length). Karyotype asymmetry was compared to the CVCI (Coefficient of Variation of Chromosome Index) and karyotype total haploid length (THL) was calculated. To account for these parameters, we used KaryoType software (Altınordu et al., 2016), a cytogenetic tool which can measure karyotype asymmetry indices automatically and efficiently.
2.4. Pollen morphology observationFresh flowers of A. nanyueensis (two populations) and A. tianmuensis (three populations) were collected in the field and then dried in silica gel (Table S2). Pollen grains were then transferred to a Scanning Electron Microscopy (SEM) stub with carbon tape under stereomicroscope, and sputter coated with platinum for 3 min, then finally examined under a FE-SEM TESCAN MAIA3. To assess pollen variation in size and exine sculpture, two specimens from each population were examined. At least 50 pollen grains were measured for pollen polar length (P) and equatorial length (E) with freeware Image J (https://imagej.nih.gov/ij/). To quantify density of lumina per 100 square microns, lumina were counted. The terminology follows Punt et al. (2007) and Halbritter et al. (2018).
2.5. Present distribution modellingEcological niche modelling (ENM) in MaxEnt (v.3.4.4; Phillips et al., 2017) was used to predict the potential distribution of A. edulis, A. nanyueensis and A. tianmuensis under present conditions (1970–2000). We recorded 189 location points for A. edulis, nine for A. nanyueensis and 42 for A. tianmuensis from the records investigated before and from the Global Biodiversity Information Facility (GBIF) Occurrence Download (https://doi.org/10.15468/dl.5kfqbp). A. edulis had a wide distribution and more GPS records, so it was easier to do correlative analysis of climatic factors and filtering. However, it was almost impossible to do so for A. nanyueensis and A. tianmuensis. To obtain more accurate results, no factors were filtered in A. nanyueensis and A. tianmuensis. The species' current climatic niche was modeled at 30 s resolution using 11 (bio 2–3, 6, 8, 10, 13, 15–17, 19, Elev; A. edulis), 20 (bio 1–19, Elev; A. nanyueensis) and 20 (bio1–19, Elev; A. tianmuensis) data layers (Table S2) from WorldClim2 (http://www.wordl clim.org; Fick and Hijmans, 2017). The restricted data set of least-correlated variables of A. edulis was chosen (Pearson's r < |0.8|) to avoid potential overparameterization (Peterson and Nakazawa, 2008). By adopting default settings with 20 replicates, we used 75% of the records to train the model and 25% to test the model. Model accuracy was assessed by evaluating the area under the curve (AUC) of the receiver-operating characteristic plot, where scores higher than 0.7 indicate good performance of the model (Swets, 1988).
2.6. DNA extraction and sequencingTwenty-six plastome sequences were used in the present study (Table 1), including all recognized species of Amana (six accessions of A. edulis, two accessions of A. nanyueensis, three accessions of A. tianmuensis, and two accessions for each of the other species), with three species of Liliaceae (Erythronium sibiricum (Fisch. & C.A. Mey.) Krylov, Tulipa altaica Pall. ex Spreng. and Lloydia tibetica Baker ex Oliv.) selected as outgroups. Voucher specimens were deposited at the Herbarium of Zhejiang University (HZU).
| Accession name | Accession number | Location |
| Amana erythronioides_LP150068-4 | KY401421 | Simingshan, Yuyao City, Zhejiang Province, China |
| Amana erythronioides_LJK26-1 | *MZ561646 | Simingshan, Yuyao City, Zhejiang Province, China |
| Amana kuocangshanica_PNLI20141039-1 | KY401426 | Kuocangshan, Linhai City, Zhejiang Province, China |
| Amana kuocangshanica_LJK22-1 | *MZ561645 | Kuocangshan, Linhai City, Zhejiang Province, China |
| Amana latifolia_LP161225-2 | KY401424 | Koishikawa Botanical Garden, Tokyo, Japan |
| Amana latifolia_LJK70-1 | *MZ561650 | Koshikawa Botanical Garden, Tokyo, Japan |
| Amana baohuaensis_ LP150012 | *MW929176 | Maoshan, Jurong City, Jiangsu Province, China |
| Amana baohuaensis_LJK31-1 | *MZ561647 | Maoshan, Jurong City, Jiangsu Province, China |
| Amana wanzhensis_LP150072-11 | KY401422 | Xianxia Town, Ningguo City, Anhui Province, China |
| Amana wanzhensis_LJK44-1 | *MZ561648 | Xianxia Town, Ningguo City, Anhui Province, China |
| Amana anhuiensis_CMQ2015075-7 | KY401423 | Tianzhushan, Qianshan County, Anhui Province, China |
| Amana anhuiensis_LJK62-1 | *MZ561649 | Tianzhushan, Qianshan County, Anhui Province, China |
| Amana nanyueensis_LP173011-2 | *MW876380 | Hengshan, Hengyang City, Hunan Province, China |
| Amana nanyueensis_LP196219-1 | *MW845753 | Hengshan, Hengyang City, Hunan Province, China |
| Amana tianmuensis_LP161203-2 | *MW876379 | Jinhuashan, Jinhua City, Zhejiang Province, China |
| Amana tianmuensis_LJK42-1 | *MW876378 | Tianmushan, Lin'an City, Zhejiang Province, China |
| Amana tianmuensis_CMQ16198-1 | *MW876377 | Tianmushan, Lin'an City, Zhejiang Province, China |
| Amana edulis_CMQ16213 | *OL351567 | Xilin Temple, Jiujiang City, Jiangxi Province, China |
| Amana edulis_LP173055 | *OL351569 | Dushan, Nanyang City, Henan Province, China |
| Amana edulis_LP173029 | *OL351568 | Cheyu Valley, Zhouzhi County, Shaanxi Province, China |
| Amana edulis_LP161115-1 | AB024388 | Hangzhou Botanical Garden, Hangzhou City, Zhejiang Province, China |
| Amana edulis_LP161235-1 | *MW938051 | Daheishan, Dalian City, Liaoning Province, China |
| Amana edulis_LJK54-1 | *MW929177 | Yuxiang Village, Huangshan City, Anhui Province, China |
| Erythronium sibiricum | NC_035681 | Xinjiang Province, China |
| Tulipa altaica | NC_044780 | Tacheng, Urumqi, Xinjiang Province, China |
| Lloydia tibetica | MK673748 | Taibaishan, Mei County, Shaanxi Province, China |
DNA was extracted from silica gel dried leaves with a modified CTAB reagent (Plant DNAzol, Shanghai, China), according to the manufacturer's protocol. High quality DNA was sheared to yield fragments with lengths less than or equal to 800 bp, and fragment quality was checked using an Agilent Bioanalyzer 2100 (Agilent Technologies). The 500-bp short-insert length paired-end library was prepared and sequenced on an Illumina HiSeq X10 to obtain reads with lengths of 150 bp at Beijing Genomics Institute (BGI, Wuhan, China). The raw reads were first screened for Phred scores < 30 to remove low quality sequences. All the remaining reads were assembled into complete plastome sequences and ribosomal genes in GetOrganelle (Jin et al., 2020). The parameters set in GetOrganelle were as follows: '-R 30 -k 21, 45, 65, 85, 105 –F embplant_pt–mememory-save'. The newly generated sequences of plastomes were all deposited in GenBank (Table 1).
2.7. Phylogenetic analysesThe 76 common plastome CDS (ptCDS) sequences were extracted and concatenated, obtained in Geneious v.4.8.5 (Kearse et al., 2012). Sequence alignments and manual adjustments were performed in MAFFT v.7 (Katoh and Standley, 2013). Phylogenetic analyses were performed with both maximum likelihood (ML) and Bayesian inference (BI) methods based on the ptCDS data set. Gene partition analyses were performed in IQ-TREE 2 (Minh et al., 2020) with Modelfinder (Kalyaanamoorthy et al., 2017) and 17 kinds of partition schemes were found in total. ML analyses were conducted in RAxML-HPC BlackBox v.8.2.12 (Stamatakis, 2014) with 1000 bootstrap replicates; BI analyses were conducted in MrBayes on XSEDE v.3.2.7 (Ronquist and Huelsenbeck, 2003), both on the CIPRES Science Gateway website (Miller et al., 2011). The Markov chain Monte Carlo (MCMC) algorithm was run with two independent chains and default priors for 10, 000, 000 generations, with trees sampled every 1000 generations.
3. Results 3.1. Morphological comparisonsMorphological comparisons of A. edulis (Fig. 2), A. erythronioides (Fig. 3), A. nanyueensis (Figs. 4 and 5) and A. tianmuensis (Figs. 6 and 7) are presented in Table 2. The two new species are different from A. edulis by having oblanceolate leaves (vs. linear) and fewer flowers in a cyme (1–2 vs. 1–5). A. nanyueensis is distinct from A. edulis by having shorter lower leaves (9.8–18.6 cm vs. 13.8–34 cm), leaves with a smaller length/width (LL/LW) ratio (8 < X < 14 vs. 17 < X < 38), a grayish-white midvein (vs. green), and purple anthers (vs. yellow). A. tianmuensis differs from A. edulis by having a bulb tunic that is glabrous inside, sometimes sparsely villous (vs. densely villous-woolly), shorter lower leaves (11.2–22.8 cm vs. 13.8–34 cm), a smaller leaf length/width (LL/LW) ratio (5 < X < 14.5 vs. 17 < X < 38), and three bracts that are verticillate and linear (vs. two, opposite, narrowly lanceolate). A. nanyueensis and A. tianmuensis are similar to A. erythronioides in having oblanceolate leaves. However, A. nanyueensis is different in having bracts two, opposite (vs. three, verticillate) and purple anthers (vs. yellow). A. tianmuensis differs from A. erythronioides in having a yellowish-brown bulb tunic that is thinly papery, glabrous inside, and sometimes sparsely villous (vs. brown, papery, densely villous-woolly), green leaves that are sometimes grayish white in the middle (vs. dark-green or brownish-green, sometimes middle white).

|
| Fig. 2 Amana edulis: (A) Habitat, (B) Individual with a single flower, (C) Individual with two flowers, (D) Whole plant, (E) The front view of the flower, (F) The side view of the flower, (G) Anatomy of flower, (H) Bulb, (I) Leaves, (J) Fruit. |
|
| Fig. 3 Amana erythronioides: (A) Habitat, (B) Population with flowers, (C) Individual with a single flower, (D) Whole plant, (E) The front view of the flower, (F) The side view of the flower, (G) Anatomy of flower, (H) Bulb, (I) Leaves, (J) Fruit (immature). |

|
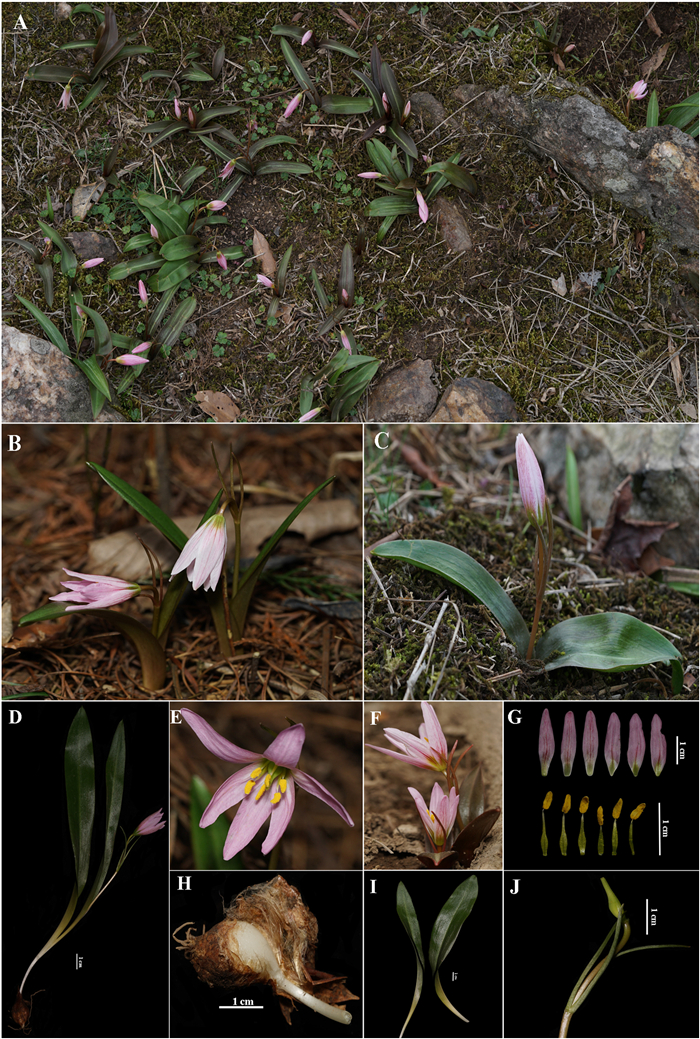
| Fig. 4 Amana nanyueensis: (A) Habitat, (B) Individual with a single flower, (C) Individual with two flowers, (D) Whole plant, (E) The front view of the flower, (F) The side view of the flower, (G) Anatomy of flower, (H) Bulb, (I) Leave, (J) Fruit. |
|
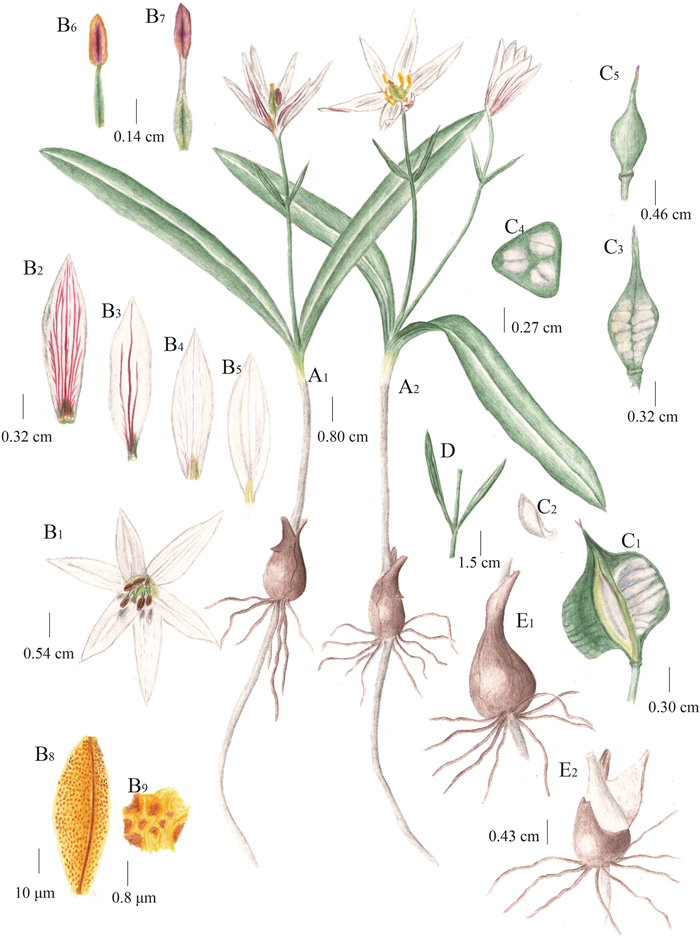
| Fig. 5 Illustration of Amana nanyueensis P. Li & Luxian Liu. (A1-A2) Flowering plants, (B1) Flower, (B2) Abaxial surface of outer tepal, (B3) Abaxial surface of inner tepal, (B4) Adaxial surface of outer tepal, (B5) Adaxial surface of inner tepal, (B6) Outer stamen, (B7) Inner stamen, (B8) Pollen, (B9) Meshed reticulum, (C1) Mature capsule, (C2) Seed, (C3) Longitudinal section of immature capsule, (C4) Transverse section of immature capsule, (C5) Immature capsule, (D) Bracts, (E1) Bulb with a young dropper and several roots, (E2) Tunic. Drawn by Xin-Jie Jin. |

|
| Fig. 6 Amana tianmuensis: (A) Habitat, (B) Individual with a single flower, (C) Individual with two flowers, (D) Whole plant, (E) The front view of the flower, (F) The side view of the flower, (G) Anatomy of flower, (H) Bulb, (I) Leaves, (J) Fruit. |
|
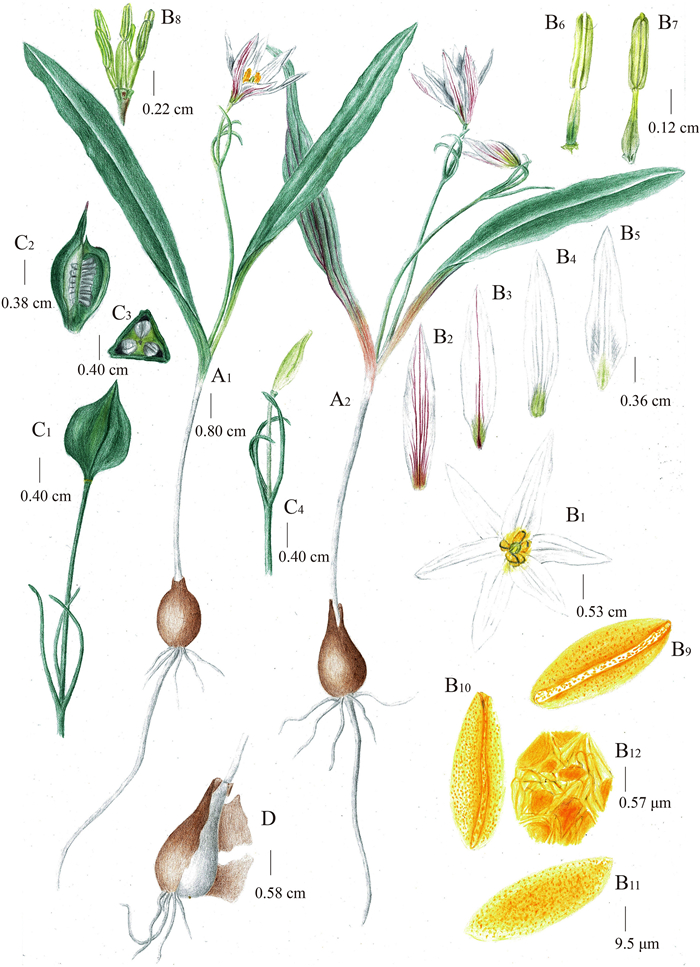
| Fig. 7 Illustration of Amana tianmuensis P. Li & M.Z. Wang. (A1-A2) Flowering plants, (B1) Flower, (B2) Abaxial surface of outer tepal, (B3) Abaxial surface of inner tepal, (B4) Adaxial surface of outer tepal, (B5) Adaxial surface of inner tepal, (B6) Outer stamen, (B7) Inner stamen, (B9–B11) Pollen, (B12) Meshed reticulum, (C1) Mature capsule, (C2) Longitudinal section of immature capsule, (C3) Transverse section of immature capsule, (C4) Immature capsule and bracts, (D) Bulb with several roots and tunic. Drawn by Xin-Jie Jin. |
| Species Characters | Amana edulis | Amana erythronioides | Amana nanyueensis | Amana tianmuensis | |
| Bulb | Diameter | 1.5–4.0 cm | 0.8–1.85 cm | 1.0–1.45 cm | 0.7–1.8 cm |
| Tunic | brown, papery | brown, papery | brown, papery | yellowish-brown, thinly papery | |
| Inside | densely villous-woolly | densely villous-woolly | densely villous-woolly | glabrous, sometimes sparely villous | |
| Leaves | Color | green | green, sometimes midvein white | green, midvein grayish white | green, sometimes midvein grayish white |
| Shape | linear | oblong | oblanceolate | oblanceolate | |
| Lower leaf length (LL) | 13.8–34.0 (23.6) cm | 7.6–25.0 (14.9) cm | 9.8–18.6 (14.2) cm | 11.2–22.8 (16.5) cm | |
| Lower leaf width (LW) | 0.5–1.2 (0.9) cm | 1.1–3.0 (1.8) cm | 0.6–1.9 (1.3) cm | 0.9–2.5 (1.7) cm | |
| Lower LL/LW | 17 < X < 38 | 4 < X < 11 | 8 < X < 14 | 5 < X < 14.5 | |
| Upper leaf length (LL) | 11.4–36.8 (23.1) cm | 7.3–25.6 (14.0) cm | 10.5–18.5 (14.4) cm | 9.5–23.4 (16.1) cm | |
| Upper leaf width (LW) | 0.4–1.0 (0.7) cm | 0.5–2.7 (1.2) cm | 0.5–1.3 (1.0) cm | 0.6–1.9 (1.2) cm | |
| Upper LL/LW | 16.5 < X < 50 | 6 < X < 15 | 8.5 < X < 22.5 | 6.5 < X < 21 | |
| Bracts | 2, opposite, narrowly lanceolate | 3, verticillate, narrowly lanceolate | 2, opposite, narrowly lanceolate | 3, verticillate, linear | |
| Pedicel | 1.0–5.0 cm | 1.1–5.0 cm | 1.5–6.0 cm | 1.1–5.6 cm | |
| Flowers | 1–5 | solitary | mostly solitary, sometimes two | mostly solitary, sometimes two | |
| Anthers | yellow | yellow | purple | yellow | |
| Style | 0.4–0.6 cm long | 0.4–0.7 cm long | 0.3–0.5 cm long | 0.5–0.6 cm long | |
| Length of fruit beak | 0.5–1.2 cm long | 0.5–1.0 cm long | 0.5–1.0 cm long | 0.45–1.1 cm long | |
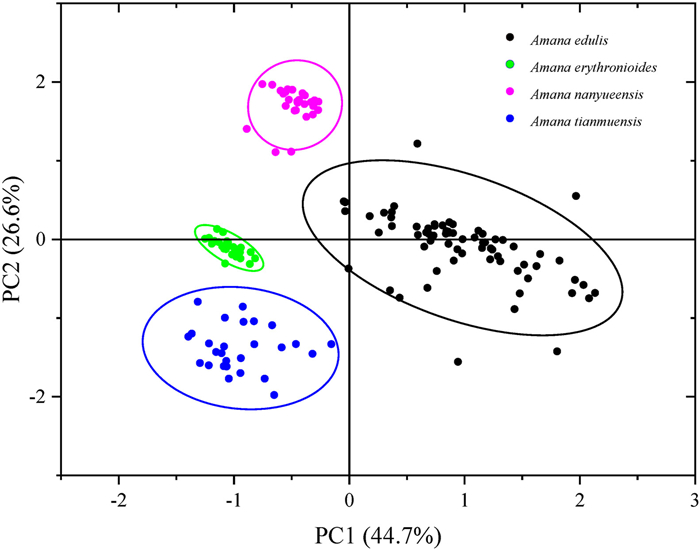
According to the PCA, the first four principal components accounted for 95.8% of the total variance. The PC1 accounted for 44.7%, PC2 for 26.6%, PC3 for 14.0% and PC4 for 10.0% of total variability. Leaf traits (0.58, 0.57) and bracts (−0.43) mostly contributed to PC1, whereas bulb (0.48), bracts (−0.47) and anthers (0.64) contributed to PC2. A. edulis, A. erythronioides, A. nanyueensis and A. tianmuensis were separated from each other based on the scatter plot (Fig. 8).
|
| Fig. 8 Principal components analysis plot of Amana edulis, Amana erythronioides, Amana nanyueensis and Amana tianmuensis. The ellipse represents the 95% confidence interval. |
Two individuals of two populations per species were observed.
The metaphase chromosomes of A. nanyueensis are 2n = 2x = 24 (Table 3, Fig. S1A). The ratio of the longest to the shortest chromosome is 2.00, and the total haploid length of the chromosome set (THL) is 141.42. Coefficient of Variation of Centromeric Index (CVCI) = 16.73. Coefficient of Variation of Chromosome Length (CVCL) = 16.50. Mean Centromeric Asymmetry (MCA) = 63.02. Index of Karyotypic Asymmetry (As.K%) = 81.65. Stebbin's asymmetry category (KA): 4A.
| Parameters | Species | |||||
| Amana nanyueensis | Amana tianmuensis | |||||
| WMZ1464 | WMZ1465-1 | WMZ1465-2 | WMZ1504-1 | WMZ1504-2 | ||
| Ratio LC/SC | 2.00 | 2.75 | 2.16 | 2.34 | 1.74 | |
| As. K% | 81.65 | 74.37 | 72.85 | 72.81 | 73.99 | |
| MCA | 63.02 | 47.86 | 45.01 | 45.57 | 46.89 | |
| CVCL | 16.50 | 23.54 | 14.40 | 18.11 | 10.59 | |
| CVCI | 16.73 | 12.62 | 15.50 | 14.64 | 10.53 | |
| THL | 141.42 | 124.43 | 116.91 | 121.68 | 100.90 | |
| 2n/x/ploidy level | 24/12/2x | 24/12/2x | 24/12/2x | 24/12/2x | 24/12/2x | |
| Karyotype formula | 2sm + 22nd | 16sm + 8th | 2m + 14sm + 8th | 16sm + 8th | 16sm + 8th | |
| KA Type | 4A | 3B | 3B | 3A | 3A | |
The metaphase chromosomes of A. tianmuensis are 2n = 2x = 24 (Table 3, Fig. S1B). The ratio of the longest to the shortest chromosome is 1.74–2.75. The total haploid length of the chromosome set (THL) is 100.90–124.43. Coefficient of Variation of Centromeric Index (CVCI) = 10.53–15.50. Coefficient of Variation of Chromosome Length (CVCL) = 10.59–23.54. Mean Centromeric Asymmetry (MCA) = 45.01–47.86. Index of Karyotypic Asymmetry (As.K%) = 72.81–74.37. Stebbin's asymmetry category (KA): 3A/B.
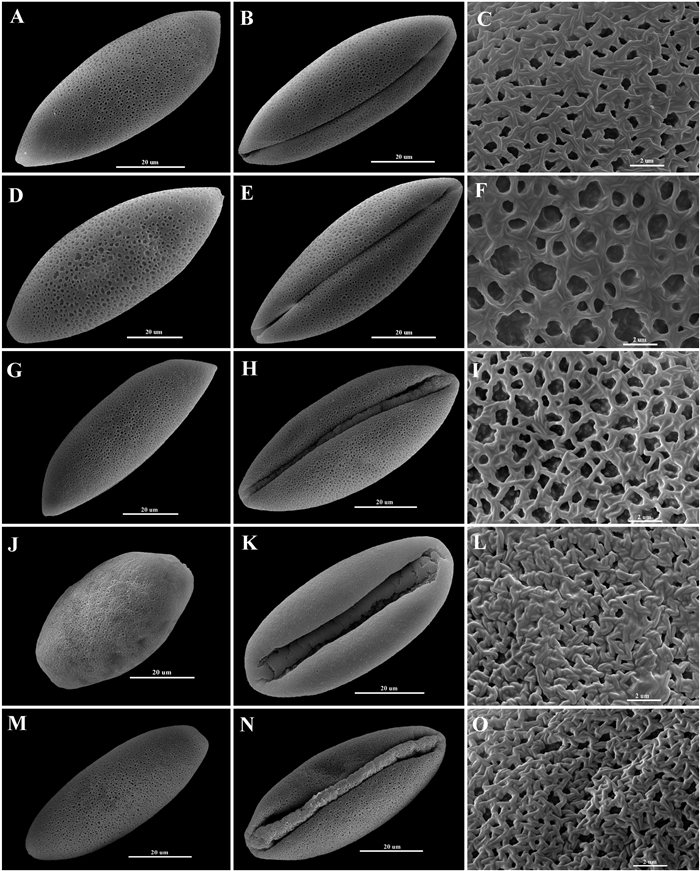
3.4. Pollen morphologyPollen morphology is shown in Fig. 9, with equatorial views of distal and proximal face and sculpture pattern.
|
| Fig. 9 Pollen morphology (Amana nanyueensis: WMZ1463: A-C, WMZ1464: D-F; Amana tianmuensis: WMZ1473: G-I, WMZ1504: J-L, WMZ1506: M-O). Equatorial views of distal (left row) and proximal (mid row) surface; reticulate sculpture patterns (right row: C and F), reticulate-heterobrochate sculpture patterns (right row: I and O) and rugulate with perforate sculpture pattern (right row: L). |
The pollen grains of A. nanyueensis are 1-colpate (sulculate), 76.5 (56.8–82.7) × 27.2 (23.6–30.46) μm, P/E = 2.8, sculpture reticulated, with irregular striates intertwined on muri, forming dense lumina of various sizes, lumina irregularly shaped, with the density of lumina varying between 46.09–81.54/100 μm2. Both pollen sizes and sculptures showed variations, particularly in one specimen (WMZ1464), with some pollen grains abnormally developed in size and ornamentation; in contrast, another specimen (WMZ1463) showed more conservative pollen size and exine sculpture than those of WMZ1464, and only a small range of variation in pollen size.
The pollen grains of A. tianmuensis are 1-colpate (sulculate), with large variations: WMZ1473 70.3 (41.9–82.8) × 28.9 (22.7–29.5) μm, mean ratio of P/E 2.4; WMZ1506 57.6 (25.9–74.9) × 24.5 (9.5–28.8) μm, mean ratio of P/E 2.3; WMZ1504 55.4 (36.8–75.6) × 30.7 (24.5–33.6) μm, mean ratio of P/E 1.8, reticulate-heterobrochate sculpture pattern in the populations WMZ1473 and WMZ1506, rugulate with a perforate sculpture pattern in the population WMZ1504, with irregular striates intertwined on muri forming dense lumina in various sizes, lumina irregularly shaped, with the density of lumina varying between 69.5–99.9/100 μm2 from the population WMZ1473 (terminology not available for another two populations due to the width of muri larger than lumina). Both pollen size and sculptures showed variation, particularly in one specimen (WMZ1504), with a mean P/E ratio of 1.8, much less than those of two other populations; a small number of abnormally small pollen grains were found in the population of WMZ1504.
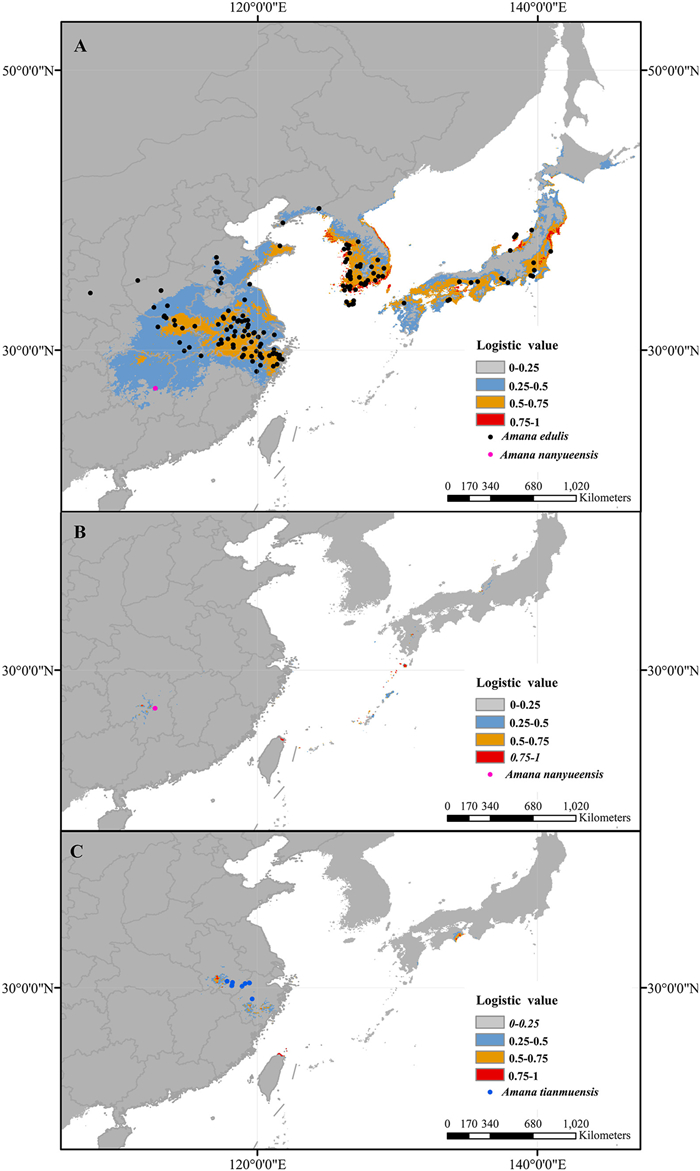
3.5. Present ecological nichesThe high AUC value indicated good predictive model performance of A. edulis (0.975), A. nanyueensis (1.0) and A. tianmuensis (0.999). The niches of A. edulis, A. nanyueensis and A. tianmuensis were differentiated to some extent. The predicted current distribution of A. edulis is generally similar to its actual distribution in Central and East China, the Korean Peninsula and Japan (Fig. 10A). It has a wide range of distribution at low elevations. The predicted current distribution of A. nanyueensis is extremely narrow and mainly concentrated near Hengshan in Hunan Province, where we actually found this new species (Fig. 10B). The predicted current distribution of A. tianmuensis is concentrated in Anhui and Zhejiang provinces, that is, its actual distribution (Fig. 10C). But some predicted distribution areas of A. nanyueensis and A. tianmuensis need to be verified, such as northern Taiwan (China) and central Okinawa and Kyushu (Japan) for A. nanyueensis, and southern Jiangsu (China), eastern Shikoku and eastern Hokkaido (Japan) for A. tianmuensis.
|
| Fig. 10 Potential distributions as probability of occurrence for Amana edulis (A), Amana nanyueensis (B) and Amana tianmuensis (C) in East Asia at the present (1970–2000). |
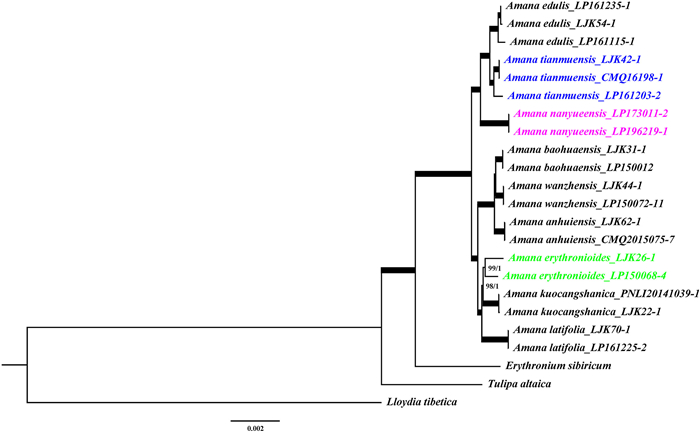
The characteristics of the plastomes used in this study are summarized in Table 4. The total alignment of the ptCDS data set was 73, 021 bp in length and included 3085 variable sites, of which 910 were informative. The ptCDS phylogeny (Fig. 11) showed that three accessions of A. tianmuensis and two accessions of A. nanyueensis form robust monophyletic clades, respectively (Bayesian inference posterior probability, BIPP = 1.0, maximum likelihood bootstrap, MLBS = 100%), and they were sister to A. edulis successively (BIPP = 1.0, MLBS = 100%). This relationship is congruent with our ongoing phylotranscriptomic results (unpublished data).
| Accession name | LSC | SSC | IR | |||
| Length (bp) | GC Content (%) | Length (bp) | GC Content (%) | Length (bp) | GC Content (%) | |
| Amana erythronioides_LP150068-4 | 81, 916 | 34.6 | 17, 075 | 30.1 | 26, 249 | 42.1 |
| Amana erythronioides_LJK26-1 | 81, 916 | 34.6 | 17, 075 | 30.1 | 26, 249 | 42.1 |
| Amana kuocangshanica_PNLI20141039-1 | 81, 872 | 34.6 | 17, 097 | 30.1 | 26, 292 | 42.0 |
| Amana kuocangshanica_LJK22-1 | 81, 880 | 34.6 | 17, 096 | 30.1 | 26, 271 | 42.0 |
| Amana latifolia_LP161225-2 | 81, 241 | 34.7 | 17, 101 | 30.0 | 26, 230 | 42.0 |
| Amana latifolia_LJK70-1 | 81, 241 | 34.7 | 17, 101 | 30.0 | 26, 230 | 42.0 |
| Amana baohuaensis_LP150012 | 81, 553 | 34.6 | 17, 025 | 30.1 | 26, 242 | 42.0 |
| Amana baohuaensis_LJK31-1 | 81, 633 | 34.6 | 17, 025 | 30.1 | 26, 277 | 42.0 |
| Amana wanzhensis_LP150072-11 | 81, 569 | 34.7 | 17, 123 | 30.1 | 26, 246 | 42.0 |
| Amana wanzhensis_LJK44-1 | 81, 569 | 34.7 | 17, 123 | 30.1 | 26, 246 | 42.0 |
| Amana anhuiensis_CMQ2015075-7 | 81, 797 | 34.6 | 17, 126 | 30.1 | 26, 235 | 42.1 |
| Amana anhuiensis_LJK62-1 | 81, 797 | 34.6 | 17, 126 | 30.1 | 26, 235 | 42.1 |
| Amana nanyueensis_LP173011-2 | 81, 782 | 34.6 | 17, 190 | 30.1 | 26, 131 | 42.1 |
| Amana nanyueensis_LP196219-1 | 81, 782 | 34.6 | 17, 190 | 30.1 | 26, 131 | 42.1 |
| Amana tianmuensis_LJK42-1 | 82, 032 | 34.6 | 16, 943 | 30.2 | 26, 257 | 42.0 |
| Amana tianmuensis_CMQ16198-1 | 81, 949 | 34.6 | 16, 944 | 30.2 | 26, 227 | 42.0 |
| Amana tianmuensis_LP161203-2 | 81, 893 | 34.6 | 16, 936 | 30.2 | 26, 189 | 42.0 |
| Amana edulis_CMQ16213 | 81, 860 | 34.6 | 17, 025 | 30.2 | 26, 287 | 42.0 |
| Amana edulis_LP173055 | 81, 862 | 34.6 | 17, 025 | 30.2 | 26, 272 | 42.0 |
| Amana edulis_LP173029 | 81, 828 | 34.6 | 17, 026 | 30.2 | 26, 302 | 42.0 |
| Amana edulis_LP161115-1 | 81, 903 | 34.6 | 17, 020 | 30.2 | 26, 237 | 42.0 |
| Amana edulis_LP161235-1 | 81, 849 | 34.6 | 17, 030 | 30.2 | 26, 238 | 42.0 |
| Amana edulis_LJK54-1 | 81, 815 | 34.6 | 17, 028 | 30.2 | 26, 238 | 42.0 |
| Erythronium sibiricum | 82, 138 | 34.5 | 17, 366 | 30.0 | 25, 765 | 42.3 |
| Tulipa altaica | 78, 433 | 35.1 | 16, 628 | 30.4 | 25, 913 | 42.3 |
| Lloydia tibetica | 82, 045 | 34.7 | 17, 477 | 30.4 | 25, 586 | 42.5 |
|
| Fig. 11 Phylogenetic tree of all Amana species based on CDS from chloroplast sequence. The branches representing 'BIPP = 1.0, MLBS = 100%' are indicated by bold lines. |
Our previous study (Li et al., 2017) included one individual from each of all six recognized Amana species at that time, and showed a sister relationship between the widespread species A. edulis and five other narrow endemics ((Amana wanzhensis, Amana anhuiensis), ((A. erythronioides, Amana kuocangshanica), Amana latifolia)). With a more comprehensive sampling of all nine species (2–6 for each species), including the recently published A. baohuaensis (Wang et al., 2019) and two new species described in the present work, we reconstructed the phylogeny of Amana (Fig. 11). The genus Amana can be divided into three clades: Clade I ((A. edulis, A. tianmuensis), A. nanyueensis), Clade II ((A. baohuaensis, A. wanzhensis), A. anhuiensis) and Clade III ((A. erythronioides, A. kuocangshanica), A. latifolia)). Clade I is sister to Clade II + III. This topology (Fig. 11) is congruent with previous results (Li et al., 2017), and is important for understanding the evolution of Amana.
4.2. Species delimitationA. tianmuensis and A. nanyueensis are closely related to the widespread species A. edulis (Fig. 11) but they dwell in a much higher elevations (A. tianmuensis: 570–1620 m, A. nanyueensis: 950–1110 m vs. A. edulis: 0–400 m). One might think that they could be recently evolved ecotypes of A. edulis and do not merit species recognition. However, this is obviously not the case because both A. tianmuensis and A. nanyueensis form a distinct lineage that is comparable with (or even having a longer evolutionary history than) other accepted species (Fig. 11). They can be easily separated from A. edulis morphologically. They both differ from A. edulis by having oblanceolate leaves and a smaller LL/LW ratio (A. tianmuensis: 5 < X < 14.5, A. nanyueensis: 8 < X < 14 vs. A. edulis: linear, 17 < X < 38). Furthermore, A. tianmuensis has three bracts, and A. nanyueensis has purple anthers, which are both different from A. edulis. The PCA results clearly separated populations of A. tianmuensis, A. nanyueensis and A. edulis into distinct clusters (Fig. 8). Moreover, the pollen from three different A. tianmuensis populations all have a sulcus membrane that creates the illusion of double grooves, which is until now unique in the genus (Tan, 2005). Cytological analyses showed that A. tianmuensis and A. nanyueensis are both diploid (2n = 2x = 24), different from A. edulis, which is either diploid (northern populations, such as Liaoning, N Henan, N Anhui and N Jiangsu; Deng et al., 2016) or tetraploid (southern populations, 2n = 4x = 48, such as S Anhui and Zhejiang; Deng et al., 2016; Zhu et al., 2002). We also conducted a cytogeographic study on the genus Amana, in which we counted the chromosome numbers for 50 populations of A. edulis and found that those A. edulis populations distributed close to A. tianmuensis and A. nanyueensis (i.e., southern populations) are mostly tetraploid (unpublished data) and flowering later. Using an integrative taxonomic approach, we have shown that A. tianmuensis and A. nanyueensis are both distinct new species supported by three different species concepts (phylogenetic species concept, morphological species concept, biological species concept).
4.3. Parallel evolution in AmanaMorphological characteristics such as leaf shape (linear/oblanceolate/oblong), color of midvein (green/white), number of bracts (two/three), bract development (normal, length > 1 cm/degraded, 1–5 mm long), color of anthers (yellow/purple), indumentum on bulb tunics (densely villous-woolly/sparely villous/glabrous) are diagnostic among Amana species. However, it is difficult to identify morphological synapomorphies for either of the three clades (Clades I, II, III) in Amana (Fig. 11). Many morphological characteristics show a mosaic distribution pattern in the genus. For example, A. edulis (Clade I) and A. baohuaensis (Clade II) both have linear leaves; A. baohuaensis (Clade II) and A. latifolia (Clade III) have a broad white band along the midvein; A. tianmuensis (Clade I) and Clade II + III species have three bracts; A. edulis & A. tianmuensis (Clade I), A. wanzhensis (Clade II) and A. erythronioides (Clade III) have yellow anthers. This mosaic pattern means that most morphological characteristics show a complex evolutionary history and have changed multiple times during the evolution of Amana. Parallel evolution, which describes the evolution of similar phenotypes or genotypes in independent populations/species as a result of natural selection and adaption (Harvey and Pagel 1991; Bolnick et al., 2018), is likely to be the cause of this mosaic phenomenon. Parallel evolution has been reported in diverse groups of plant and animal, such as Rheum alexandrae (Liu et al., 2012), Senecio (James et al., 2021), Convolvulaceae (Eserman et al., 2018), marine and freshwater fishes (Friedman et al., 2021), as well as bats and toothed whales (Shen et al., 2012). To better understand the underlying mechanisms of this evolutionary pattern within the genus Amana, further genomic data and more analyses are needed.
5. Taxonomical treatment 5.1. Amana nanyueensis P. Li & L.X. Liu, sp. nov. (南岳老鸦瓣, Figs. 4 and 5).Diagnosis. The new species resembles A. edulis in possessing densely villous-woolly bulb tunics and two opposite bracts, but differs by having oblanceolate leaves (vs. linear), a lower ratio of leaf length (LL)/leaf width (LW), 8 < X < 14 (vs. 17 < X < 38), a grayish-white midvein (vs. green), and purple anthers (vs. yellow).
Type. CHINA. Hunan Province: Hengyang City, Nanyue District, Hengshan, Cangjingdian, in evergreen-deciduous broadleaved mixed forest, N27°16′36.09″, E112°40′28.65″, 1065 m, 1 March 2021, Pan Li et al. WMZ1463 (holotype HZU; isotype CSH, KUN, NAS, PE).
Description. Perennial herb. Bulb ovoid, 1–1.45 cm in diameter, tunic brown, papery, densely villous-woolly inside. Stem 2–15 cm tall, glabrous, simple. Leaves usually 2, opposite, green, midvein grayish white, oblanceolate, glabrous; the lower leaf 9.8–18.6 × 0.6–1.9 cm, the upper 10.5–18.5 × 0.5–1.3 cm. Scape 5.5–11 cm tall, glabrous. Bracts usually 2, opposite, very rarely 3 and whorled, narrowly lanceolate, green, 1.6–4.2 × 0.2–0.8 cm; pedicels 1.5–6 cm. Flowers solitary, sometimes two, funnel-shaped; tepals 6, white, with a green or yellowish-green blotch at the very base inside and purple-red streaks on the back; outer tepals lanceolate, acute, 1.8–2.7 × 0.3–0.7 cm, inner tepals narrow elliptic, bluntly acute, 1.6–2.6 × 0.3–0.7 cm. Stamens 6, two-wheeled, the inner three slightly longer than the outer; filaments yellowish-green, 4–9 mm long, proximally dilated, gradually attenuate towards apex, glabrous; anthers purple, 3–6 mm long. Oval ovaries, yellowish-green, constricted below the style, 0.3–0.5 cm long, styles 0.3–0.5 cm long. Capsule subglobose, triquetrous, 0.9–1.5 cm in diameter, apex long beaked, 0.5–1 cm long.
Phenology. Flowering from March to April, fruiting from April to May.
Etymology. The specific epithet is named after its type locality, Hengshan, Hunan Province, China. Being one of five famous mountains in China, which are called "Wuyue", Hengshan is also called "Nanyue", meaning the South Mountain.
Distribution and habitat. So far, A. nanyueensis is only known from several peaks of Hengshan in Hunan Province, China. It mostly grows in moist deciduous broadleaf forests on the mountain slope at elevations of 900–1200 m.
Conservation status. A. nanyueensis exists at three sites in Hengshan, occupying an area less than 12 km2, but the population is not declining and there are no current threats. Thus, we suspect that A. nanyueensis could be categorized as Near Threatened (NT) according to IUCN criteria (IUCN Standards and Petitions Committee, 2022).
Additional specimens examined (paratypes). CHINA. Hunan Province: Hengyang City, Nanyue District, Hengshan, Nantianmen, 950 m, 6 March 2013, Fl., Yonghong Xie 201305 (NYA); ibidem, 1060 m, 20 March 2015, Fl., Jianglin Xia 2015010 (NYA); Hengyang City, Nanyue District, Hengshan, Cangjingdian, 1055 m, 16 March 2017, Fl., Luxian Liu LP173011 (HZU); ibidem, 27 March 2019, Fl., Pan Li LP196219 (HZU); ibidem, 21 April 2019, Fr., Zhangshichang Zhu RZ201902 (HZU); Hengyang City, Nanyue District, Hengshan, Zushidian, 1105 m, 1 March 2021, Fl., Pan Li et al. WMZ1464 (HZU); ibidem, 13 April 2021, Fr., Meizhen Wang & Minghong Li WMZ1586 (HZU).
5.2. Amana tianmuensis P. Li & M.Z. Wang, sp. nov. (天目老鸦瓣, Figs. 6 and 7).Diagnosis. The new species resembles A. erythronioides in possessing three verticillate bracts and yellow anthers, but differs in having leaves that are oblanceolate (vs. oblong), and a bulb tunic that is yellowish-brown, thinly papery, glabrous inside, sometimes sparely villous (vs. brown, papery, densely villous-woolly).
Type. CHINA. Zhejiang Province: Hangzhou City, Lin'an District, Tianmushan, Longfengjian to Xianrending, in evergreen-deciduous broadleaved mixed forest, N30°20′58.94″, E119°25′34.38″, 1433 m, 21 March 2021, Meizhen Wang & Shenglu Zhang WMZ1504 (holotype HZU; isotype CSH, KUN, PE).
Description. Perennial herb. Bulb ovoid, 0.7–1.8 cm in diameter, tunic yellowish-brown, thinly papery, glabrous inside, sometimes sparsely villous. Stem usually simple, 3.3–13.2 cm, slender, glabrous. Leaves usually 2, glaucous, oblanceolate, glabrous; the lower leaf 11.2–22.8 × 0.9–2.5 cm, the upper 9.5–23.4 × 0.6–1.9 cm. Scape 6–12.8 cm tall, glabrous. Bracts usually 3, verticillate, linear, green, 1.5–3.5 × 0.1–0.3 cm; pedicels 1.1–5.6 cm. Flowers solitary, sometimes two, funnel-shaped; tepals 6, white, with a green or yellowish-green blotch at the very base inside, streaked with purple-red on the back, outer tepals lanceolate, acute, 1.8–3.4 × 0.4–0.6 cm, inner tepals lanceolate, acute, 1.8–3.2 × 0.4–0.7 cm. Stamens 6, two-wheeled, the inner three slightly longer than the outer; filaments yellowish-green, 3–10 mm long, proximally dilated, gradually attenuate towards apex, glabrous; anthers 6, yellow, 2–10 mm long. Oval ovaries, yellowish-green, 0.3–1 cm long, styles 0.3–0.7 cm long. Capsule subglobose, triquetrous, 0.6–1.2 cm in diameter, apex long beaked, 0.5–1.2 cm long.
Phenology. Flowering from March to April, fruiting April to May.
Etymology. A. tianmuensis was first discovered in Tianmushan, Zhejiang Province, China, from which the epithet tianmuensis is derived.
Distribution and habitat. So far, A. tianmuensis is known from Jiuhuashan, Huangshan in Anhui Province, and Qingliangfeng, Tianmushan, Jinhuashan in Zhejiang Province, China. It grows in evergreen-deciduous broadleaved mixed forests or moist deciduous broadleaf forests on the mountain slope, at elevations of 500–1700 m.
Conservation status. A. tianmuensis is known from several mountains in Anhui and Zhejiang provinces, with thousands of individuals at each site and grows well with relatively little human disturbance. Thus, we think that A. tianmuensis could be categorized as Least Concern (LC) according to IUCN criteria (IUCN Standards and Petitions Committee, 2022).
Additional specimens examined (paratypes). CHINA. Anhui Province: Chizhou City, Qingyang County, Jiuhuashan, Daosengdong to Tiantai, 1170 m, 4 April 1910, Fl., anonymous collector 3328 (PE00035344); ibidem, 7 April 2018, Fr., Pan Li et al. LP185061 (HZU); ibidem, 5 March 2021, Fl., Pan Li et al. WMZ1473 (HZU); Huangshan City, Huangshan District, Qiaoshancun, Yuxiang, 692 m, 19 March 2017, Fl., Zhechen Qi LP173012 (HZU); ibidem, 23 March 2019, Fl., Pan Li & Junke Li LJK51 (HZU); ibidem, 4 March 2021, Fl., Pan Li et al. WMZ1470 (HZU); Huangshan City, Huangshan District, Huangshan, Yuanlinchang, 620 m, 23 March 2019, Fl., Pan Li & Junke Li LJK49 (HZU); ibidem, 4 March 2021, Fl., Pan Li et al. WMZ1472 (HZU); Huangshan City, Huangshan District, Huangshan, Xiandufeng, 1620 m, 22 March 2021, Fl., Meizhen Wang & Shenglu Zhang WMZ1506 (HZU); ibidem, 28 April 2021, Fr., Hesheng Yao WMZ1626 (HZU); Zhejiang Province: Hangzhou City, Lin'an District, Tianmushan, Longfengjian to Xianrending, 1173 m, 27 April 2015, Fr., Yonghua Zhang LP150063 (HZU); ibidem, 21 April 2021, Fr., Meizhen Wang & Kaijie Gu WMZ1442 (HZU); ibidem, 6 May 2021, Fr., Pan Li et al. WMZ1627 (HZU); Hangzhou City, Lin'an District, Tianmushan, Laodian, 1104 m, 16 March 2016, Fl., Minqi Cai CMQ16198 (HZU); ibidem, 22 March 2019, Fl., Pan Li & Junke Li LJK42 (HZU); ibidem, 27 March 2020, Fl., Pan Li LP207918 (HZU); ibidem, 3 March 2021, Fl., Pan Li et al. WMZ1465 (HZU); Hangzhou City, Lin'an District, Qingliangfeng, Qianqingtang, 1120 m, 22 March 2019, Fl., Pan Li & Junke Li LJK45 (HZU); Hangzhou City, Lin'an District, Qingliangfeng, Qingliangfeng Botanical Garden, 872 m, 10 March 2021, Fl., Pan Li et al. WMZ1502 (HZU); Jinhua City, Wucheng District, Jinhuashan, Double-Dragon Scenic Area, 577 m, 2 April 2016, Fr., Pan Li LP161203 (HZU); ibidem, 26 February 2021, Fl., Pan Li et al. WMZ1452 (HZU).
Key to the species of Amana.
1. Bracts usually two, opposite.
2
1. Bracts usually three, verticillate, or not verticillate (in this case degraded).
3
2. Lower leaves linear; flowers 1–5; anthers yellow at maturity (before releasing pollens).
A. edulis
2. Lower leaves oblanceolate; flower mostly solitary, sometimes two; anthers purple at maturity (before releasing pollens).
A. nanyueensis
3. Bracts usually not verticillate, degraded, 1–5 mm long.
A. wanzhensis
3. Bracts verticillate, not degraded, longer than 1.5 cm.
4
4. Lower leaves linear, or linear to oblanceolate in the same population, always with a broad white band along the midvein above.
5
4. Lower leaves oblong or oblanceolate, sometimes with white veins or midvein, but not a broad white band.
6
5. Leaves green, linear, 0.4–1 cm wide; purple-red on the back of outer tepals.
Amana baohuaensis
5. Leaves green or brownish-green to purple, linear to oblanceolate, 1–2 cm wide; purple-red streaks on the back of outer tepals.
A. latifolia
6. Anthers purple at maturity (before releasing pollens).
7
6. Anthers yellow at maturity (before releasing pollens).
8
7. Leaves green, with white veins; bulb tunic sparsely villous inside.
A. anhuiensis
7. Leaves green, dark-green or purplish-green, without white veins; bulb tunic glabrous inside.
A. kuocangshanica
8. Leaves green, oblanceolate; bulb tunic glabrous or sparely villous inside.
Amana tianmuensis
8. Leaves green, dark-green or brownish-green, oblong; bulb tunic densely villous-woolly inside.
A. erythronioides
We sincerely thank Mr. Hong-Wei Zhang (Zhejiang Qingliangfeng National Natural Reserve Bureau) and Yong-Fu Xu (Central South University of Forestry and Technology) for providing location information of the new species, He-Sheng Yao and Jun Wu (Huangshan Scenic Area Management Committee) for collection, Xin-Jie Jin (Wenzhou University) for colored pencil illustration, and Jun-Jie Wu (Zhejiang University) for helping with ENM analysis. The research is supported by the National Natural Science Foundation of China (Grant No. 31970225), the Zhejiang Provincial Natural Science Foundation (Grant No. LY19C030007), and the NSFC-NSF Dimensions of Biodiversity Program (Grant No. 31461123001).
Author contributions
MZW, XKF, JW, SLZ, MQC, MHL, ZSCZ, MSZ, YHZ, LXL and PL conducted the sampling. MZW did the morphological and ecological niche modelling analyses. XKF conducted the phylogenetic analyses. JW performed the karyotypic study. LMM examined the pollen morphology. SLZ took the photograph. MZW, XKF, JW, LMM and PL wrote the manuscript. SLZ, MQC, MHL, ZSCZ, MSZ, YHZ, LXL and KC revised the manuscript. All authors approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.03.001.
Altınordu, F., Peruzzi, L., Yu, Y., et al., 2016. A tool for the analysis of chromosomes: KaryoType. Taxon, 65: 586-592. DOI:10.12705/653.9 |
Arano, H., 1963. Cytological studies in subfamily Carduoideae (Compositae) of Japan IX. Bot. Mag., 76: 32-39. DOI:10.15281/jplantres1887.76.32 |
Bolnick, D.I., Barrett, R.D.H., Oke, K.B., et al., 2018. (Non)Parallel evolution. Annu. Rev. Ecol. Evol. Syst., 49: 303-330. DOI:10.1146/annurev-ecolsys-110617-062240 |
Chen, X.Q., Mordak, H.V., 2000. Tulipa linnaeus. In: Wu, Z.Y., Raven, P.H. (Eds. ), Flora of China, vol. 24. Science Press, Beijing, pp. 123-126. St. Louis: Missouri Botanical Garden Press.
|
Deng, A.H., Li, K., Cheng, Y., et al., 2016. Karyotype analysis of different populations of Tulipa edulis. J. Chin. Med., 39: 493-498. |
Eserman, L.A., Jarret, R.L., Leebens-Mack, J.H., 2018. Parallel evolution of storage roots in morning glories (Convolvulaceae). BMC Plant Biol., 18: 95. DOI:10.1186/s12870-018-1307-4 |
Fick, S.E., Hijmans, R.J., 2017. WorldClim 2: new 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol., 37: 4302-4315. DOI:10.1002/joc.5086 |
Friedman, S.T., Collyer, M.L., Price, S.A., et al., 2021. Divergent processes drive parallel evolution in marine and freshwater fishes. Syst. Biol. syab080. DOI:10.1093/sysbio/syab080 |
Halbritter, H., Ulrich, S., Grímsson, F., et al., 2018. Illustrated Pollen Terminology, second ed. Springer Nature, p. 487.
|
Han, B.X., Z, K., H, L.Q., 2014. Amana wanzhensis (Liliaceae), a new species from Anhui, China. Phytotaxa, 177: 118-124. DOI:10.11646/phytotaxa.177.2.3 |
Harvey, P.H., Pagel, M.D.
, 1991. The Comparative Method in Evolutionary Biology. New York: Oxford University Press.
|
Honda, M., 1935. Amana a new genus of Liliaceae. Bull. Chem. Soc. Jpn., 6: 19-21. |
IUCN Standards, Petitions Committee, 2022. Guidelines for Using the IUCN Red List Categories and Criteria. Version 15. Prepared by the Standards and Petitions Committee. Downloadable from. https://www.iucnredlist.org/documents/RedListGuidelines.pdf.
|
James, M.E., Wilkinson, M.J., Bernal, D.M., et al., 2021. Phenotypic and genotypic parallel evolution in parapatric ecotypes of Senecio. Evolution, 75: 3115-3131. DOI:10.1111/evo.14387 |
Jin, J.J., Yu, W.B., Yang, J.B., et al., 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol., 21: 241. DOI:10.1186/s13059-020-02154-5 |
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., et al., 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods, 14: 587-589. DOI:10.1038/nmeth.4285 |
Katoh, K., Standley, D.M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol., 30: 772-780. DOI:10.1093/molbev/mst010 |
Kearse, M., Moir, R., Wilson, A., et al., 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. DOI:10.1093/bioinformatics/bts199 |
Levan, A., Fredga, K., Sandberg, A.A., 1964. Nomenclature for centromeric position on chromosomes. Hereditas, 52: 201-220. |
Li, P., Lu, R.S., Xu, W.Q., et al., 2017. Comparative genomics and phylogenomics of East Asian tulips (Amana, Liliaceae). Front. Plant Sci., 8: 451. |
Liu, B.B., Opgenoorth, L., Miehe, G., et al., 2012. Molecular bases for parallel evolution of translucent bracts in an alpine "glasshouse" plant Rheum alexandrae (Polygonaceae). J. Syst. Evol., 51: 134-141. DOI:10.1111/j.1442-2042.2011.02906.x |
Miller, M.A., Pfeiffer, W., Schwartz, T., 2011. The CIPRES science gateway: a community resource for phylogenetic analyses. In: Proceedings of the 2011 TeraGrid Conference on Extreme Digital Discovery-TG '11. ACM Press, Salt Lake City, Utah p. 1.
|
Minh, B.Q., Schmidt, H.A., Chernomor, O., et al., 2020. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol., 37: 1530-1534. DOI:10.1093/molbev/msaa015 |
Ohwi, J., Kitagawa, M., 1992. New Flora of Japan. Shibundo Co., Ltd. Publishers, Tokyo, p. 1716.
|
Paszko, B., 2006. A critical review and a new proposal of karyotype asymmetry indices. Plant Syst. Evol., 258: 39-48. DOI:10.1007/s00606-005-0389-2 |
Peruzzi, L., Altınordu, F., 2014. A proposal for a multivariate quantitative approach to infer karyological relationships among taxa. Comp. Cytogenet., 8: 337. DOI:10.3897/CompCytogen.v8i4.8564 |
Peruzzi, L., Eroğlu, H.E., 2013. Karyotype asymmetry: again, how to measure and what to measure?. Comp. Cytogenet., 7: 1-9. DOI:10.3897/compcytogen.v7i1.4431 |
Peruzzi, L., Leitch, I.J., Caparelli, K.F., 2009. Chromosome diversity and evolution in Liliaceae. Ann. Bot., 103: 459-475. DOI:10.1093/aob/mcn230 |
Peterson, A.T., Nakazawa, Y., 2008. Environmental data sets matter in ecological niche modelling: an example with Solenopsis invicta and Solenopsis richteri. Global Ecol. Biogeogr., 17: 135-144. |
Phillips, S.J., Anderson, R.P., Dudík, M., et al., 2017. Opening the black box: an open-source release of Maxent. Ecography, 40: 887-893. DOI:10.1111/ecog.03049 |
Punt, W., Hoen, P.P., Blackmore, S., et al., 2007. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol., 143: 1-81. DOI:10.1016/j.revpalbo.2006.06.008 |
Ronquist, F., Huelsenbeck, J.P., 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572-1574. DOI:10.1093/bioinformatics/btg180 |
Shen, X.S., 2001. A new species of Tulipa (Liliaceae) from China. Syst. Bot., 23: 39-40. |
Shen, Y.Y., Liang, L., Li, G.S., Murphy, R.W., Zhang, Y.P., 2012. Parallel evolution of auditory genes for echolocation in bats and toothed whales. PLoS Genet., 8: e1002788. DOI:10.1371/journal.pgen.1002788 |
Stamatakis, A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Stebbins, G.L., 1971. Chromosomal Evolution in Higher Plants. Addison-Wesley, London, pp. 87-93.
|
Swets, J.A., 1988. Measuring the accuracy of diagnostic systems. Science, 240: 1285-1293. DOI:10.1126/science.3287615 |
Tan, D.Y., 2005. The Systematics of Tulipa L. (s. l. ) from China. PhD Thesis. Institute of Botany, Chinese Academy of Sciences, pp. 55-67.
|
Tan, D.Y., Zhang, Z., Li, X.R., et al., 2005. Restoration of the genus Amana Honda (Liliaceae) based on a cladistic analysis of morphological characters. Acta Phytotaxon. Sin., 43: 262-270. |
Tan, D.Y., Li, X.R., Hong, D.Y., 2007. Amana kuocangshanica (Liliaceae), a new species from south-east China. Bot. J. Linn. Soc., 154: 435-442. DOI:10.1111/j.1095-8339.2007.00660.x |
Thiers, B.M., Tulig, M.C., Watson, K.A., 2016. Digitization of the New York botanical garden herbarium. Brittonia, 68: 324-333. DOI:10.1007/s12228-016-9423-7 |
Wang, L., Xing, Q., Lu, G.Y., et al., 2019. Amana baohuaensis (Liliaceae), a new species from East China. Phytotaxa, 427: 43-50. DOI:10.11646/phytotaxa.427.1.5 |
Zhu, X.N., Jin, X.F., Gao, Z., 2002. Karyotypic analysis on three species of Liliaceae. J. Zhejiang Forest. Sci. Technol., 22: 22-25. |



