b. State Key Laboratory of Systematic and Evolutionary Botany, Chinese Academy of Sciences, Beijing, 100093, China
The evolutionary dynamics that underlie the intercontinental distribution of biotas have long attracted the attention of biogeographers (Raven and Axelrod, 1974; Wolfe and Toshimasa, 1980; Manchester and Tiffney, 2001; Wen and Ickert-Bond, 2009; Wu et al., 2011; Richardson et al., 2012; Jiang et al., 2019). Biotic interchange among continents has been proposed as a factor that formed these distributions (Vermeij, 1991; Jiang et al., 2019). For example, biotic interchange events through the Bering land bridge during the late Eocene and middle Miocene contributed to the disjunct distribution of floras between Eurasia and North America (Tiffney, 1985; Xiang et al., 2000; Donoghue and Smith, 2004; Manchester et al., 2009; Jiang et al., 2019). Additionally, the formation of the Isthmus of Panama (late Eocene–Pliocene) allowed biotic interchange between the North and South America biota (Jaramillo, 2018; Carrillo et al., 2020). Tropical Asia is characterized by an abundance of seed plants, and contains multiple biodiversity hotspots (such as: Indo–Burma, South–Central China, Sundaland, Wallacea, and Philippines). However, in neighboring Australasia, plant richness is relatively lower and there are few biodiversity hotspots (Mittermeier et al., 2005). Despite this disparity in biodiversity hotspots, there is a close relationship between the floras of tropical Asia and Australasia.
The geological history of the insular regions in tropical Asia and Australasia is notably complex due to the deformation of the plates and volcanic activities (Fig. 1; de Boer and Duffels, 1996; Metcalfe, 1998; Ali and Heaney, 2021). Gondwanaland began to separate in the early Jurassic (180 million years ago, Ma) and then divided into four continental plates during the early Cretaceous (130 Ma) (Lomolino et al., 2006). Sundaland (including the islands of Sumatra, Java, and Borneo among the other surrounding islands and the mainland cape on the Asian mainland) was assembled from Gondwana fragments by the early Mesozoic (Hall, 2013). During the Eocene, rifting of the SE Sundaland margin probably separated West Sulawesi from Borneo. Further, Oceania began to move north in the early Eocene (ca. 45 Ma) and collided with Sundaland in the late Oligocene (ca. 23–25 Ma) (Hall, 2011). In the early Miocene, Australia began to collide with southeastern Asia, and subsequently formed a region that is currently known as Wallacea. Since the middle Miocene, volcanic activity and subduction-related deformation may have contributed to the elevation of Sumatra and extension in Sulawesi (Hall, 1998, 2011, 2013). Almost simultaneously, the westernmost Australian promontory collided with Sulawesi, resulting in land emergence in central and eastern parts of Sulawesi (de Bruyn et al., 2014). A series of complex geological events such as collisions and volcanic activities resulted in islands extension and fragmentation, gradually forming island chains (Hall, 1998, 2011, 2013).
|
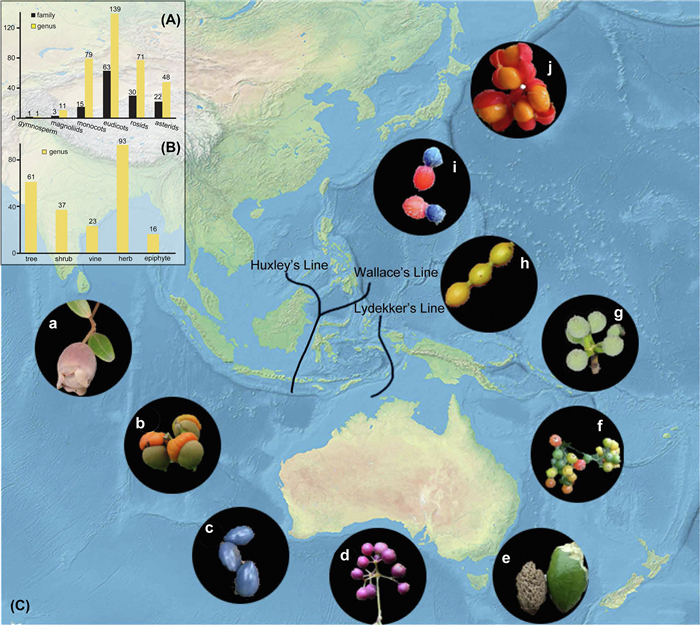
| Fig. 1 The disjunct distributions between tropical Asia and Australasia and topography of the region. (A) Numbers of 81 families and 225 genera plant lineages in APG IV major clades; (B) Numbers of these groups in different habits; (C) Complex topography of tropical Asia and Australasia. Inset images show representatives of the 10 seed plants in this study (a, Rhodomyrtus; b, Semecarpus; c, Cerbera; d, Tetrastigma; e, Elaeocarpus; f, Glycosmis; g, Pseuduvaria; h, Desmos; i, Dacrycarpus; j, Harpullia). Map also indicating landmasses, straits, faunal lines and zoogeographical realms. |
Paleobiogeography has demonstrated that the distributions of organisms in tropical Asia and Australasia are closely related to plate tectonics and climate change (Wallace, 1857; Hooker, 1859; Lieberman, 2005). Wallace's line and Lydekker's line were originally proposed as faunistic boundaries (e.g. mammals, birds) between Asia and Australasia, and were generally uncrossable (Stelbrink et al., 2012). However, both these lines do not seem to apply to plants (Richardson et al., 2012). Palynological studies inferred that the Australasian rainforest flora migrated from southeastern Asia in the late Miocene (Truswell et al., 1987). Fossil observations in southern China also provided reliable evidence for the long-distance spread of Dacrycarpus from Australia to Asia during the Miocene (Wu et al., 2019). In addition, the dated phylogenies of Loranthaceae and Citrus (Rutaceae) suggest that plants were dispersed between Asia and Australia during the Miocene and later through a series of island chains or by birds (Schwartz et al., 2016; Liu et al., 2018). Generally, the paleontological and historical biogeography of special plant groups suggests that dispersal events played significant roles in flora exchanges between tropical Asia and Australasia since the Miocene. An estimated 81 families and 225 genera of seed plants, which cover the main clades of angiosperm and different habits, exhibit a typical distribution between tropical Asia and Australasia (Wu et al., 2011) (Fig. 1a and b). Nevertheless, the evolutionary dynamics of this disjunction and the main factors that contribute to it are unclear.
The present study used 29 lineages of seed plants, comprising trees, shrubs, epiphytes and herbs to clarify the floristic interchange between tropical Asia and Australasia. For each group, phylogenetic tree, molecular dating and ancestral range reconstruction were integrated and the timing and mode of dispersal were inferred. Finally, the 'maximal number of potential dispersal events' (MDE) per million years was calculated to determine the magnitude and temporal pattern of dispersal between tropical Asia and Australasia.
2. Materials and methods 2.1. Taxon selection criterionWe selected 29 seed plant lineages to analyze taxon distribution between tropical Asia and Australasia. Plant lineages represented gymnosperm (Dacrycarpus), magnoliids (Cinnamomum, Desmos, Neolitsea, and Pseuduvaria), monocots (Caryota, Cymbidium, Dendrobium, and Livistona), basal eudicots (Helicia), cores eudicots (Tetrastigma), asterids (Breynia, Cerbera, Cyrtandra, and Mazus) and rosids (Acronychia, Ailanthus, Buchanania, Cudrania, Dysoxylum, Elaeocarpus, Glycine, Glycosmis, Harpullia, Melastoma, Rhodomyrtus, Semecarpus, Toona, and Trichosanthes). The plant lineages we selected had to meet two criteria: (i) sufficient molecular data available to infer a phylogeny; (ii) fossil data suitable for molecular clock calibration or secondary calibration points inferred from fossil dated phylogenies. Thus, detailed information for each group is presented in Table S1.
2.2. Divergent time estimationFor each group, we first downloaded sequence data from GenBank and aligned sequences using ClustalW as implemented in BioEdit v.7.2.5 (Hall, 1999). Divergent time estimations were conducted under an uncorrelated relaxed molecular clock model implemented in BEAST v.1.8.4 (Drummond et al., 2012). The log files were visualized and checked in Tracer v.1.5 (Rambaut and Drummond, 2007) to ensure that the effective sample sizes (ESS) of most parameters were greater than 200. The maximum clade credibility tree was computed using TreeAnnotator v.1.8.4 (Drummond et al., 2012). The details of each calibration scheme are presented in Supplementary data.
2.3. Ancestral area estimationThe distribution range of each species was collected from Plants of the World Online (https://powo.science.kew.org/), Tropicos (https://www.tropicos.org/home) and Global Biodiversity Information Facility (GBIF, https://gbif.org/). Only the natural distribution area was retained before the ancestral area reconstruction analysis.
Based on the distribution of ingroups and outgroups, six biogeographic regions were delimited: Asia (including tropical Asia), Australasia, Africa, North America, South America, Europe (Fig. S1). All ancestral area reconstructions were inferred using the statistical dispersal extinction cladogenesis (S-DEC) model implemented in RASP v.4.0 (Yu et al., 2015). S-DEC exports the estimated likelihood of all possible biogeographic scenarios and summarizes biogeographic processes (including dispersal, vicariance and extinction) at given nodes (Yu et al., 2015). The maximum clade credibility tree obtained from BEAST was chosen as the input tree. A total of 1, 000 random trees after burn-in obtained from BEAST were used to estimate the probabilities of the ancestral range at each node. The maximum number of areas in each node was unconstrained.
2.4. Biogeographical meta-analysesCredibility intervals of divergence times when dispersal events occurred were determined using ancestral range analysis (Table S2). MDE was calculated using the method described by Klaus et al. (2016). Based on the credibility intervals (95% highest posterior densities, HPD) of the interested nodes, the MDE was calculated by summing up potential dispersal events across all data sets with one million years (Myr) slices. According to the principles of biogeographical meta-analyses, dispersal events occurred likely under the same abiotic conditions with overlapping of the corresponding divergent time intervals. All dispersal events from Asia to Australasia and vice versa were treated as independent. Additionally, a sliding window analysis was used with a time frame of 5 Myr to smooth the data to avoid over-weight changes in MDE. The R package "ecp" (James and Matteson, 2014) was used to visually examine the change points in the MDE under a divisive hierarchical estimation algorithm (Székely and Rizzo, 2005). The 'e.divisive' function was run with the following parameters: maximum number of random permutations = 500; significance level = 0.05; k = NULL; minimum number of observations between change points = 5 Ma; moment index used to determine the distance between and within segments = 1.0.
2.5. Ancestral state reconstructionsThe dispersal types were divided into the following two categories: (1) biotic dispersal corresponding to fleshy fruits (druplets enveloped by a fleshy calyx, fleshy berry-like drupe, drupe with fleshy hypocarp, berry, seed with fleshy aril, berry-like capsules and monocarps with fleshy aril); and (2) abiotic dispersal corresponding to non-fleshy fruits (capsules with small seeds, capsules with winged seeds, samara, follicles with winged seeds, and pods). The habitats were also divided into two categories: forests and non-forests. The fruit types and habitat of each taxon were mainly collected from the recorded in Global Biodiversity Information Facility (GBIF, https://gbif.org/), iPlant (http://iplant.cn/), and relevant references (see Table S1). Both the evolutionary histories of dispersal types and habitats were reconstructed under the likelihood method implemented in the software Mesquite v.3.01 (Maddison and Maddison, 2014). For the lineages with polymorphic states (Glycine and Mazus), we adopted the likelihood method in BayesTraits v.2.0 (Pagel et al., 2004) to reconstruct their ancestral states.
3. ResultsTime-calibrated phylogenies and biogeographic reconstructions of 29 groups of seed plants indicated that a total of 98 migrations have occurred between tropical Asia and Australasia since the middle Eocene (Figs. S1–5 and S7–28), of which 30 migration events occurred recently (Figs. S1, S2, S5, S9–12, S15–18, S20, S22, S24, S25 and S28). Additionally, 37 vicariance events occurred immediately or lagging after migrations (Figs. S3, S6, S7, S9–11, S14, S15, S17, S19–24 and S26–29).
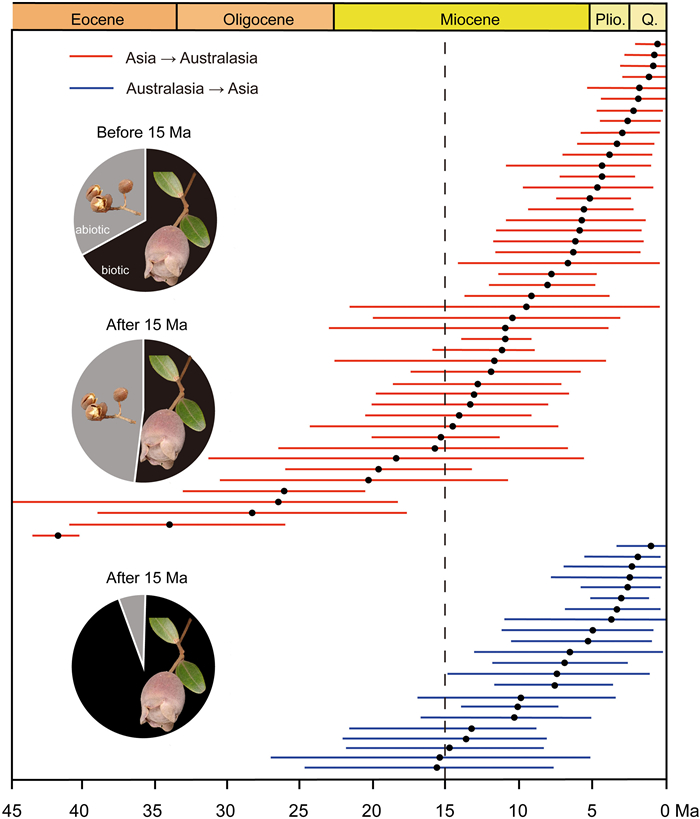
Aside from recent migration events, 68 migrations have occurred between tropical Asia and Australasia (Table S1; Figs. S2–4, S7–11, S13–17 and S19–28), with most early events occurring during the middle Eocene (41.94 Ma, 95% HPD: 40.7–43.46) and the latest occurring in the Pleistocene (0.61 Ma, 95% HPD: 0–1.94). Interestingly, only 12 migrations occurred before the middle Miocene (15 Ma); the remaining 56 events occurred after that time (Fig. 2). Of these ancient migrations, 46 have occurred from tropical Asia to Australasia since the Eocene, mostly during the middle Miocene to Pleistocene (15.38–0.61 Ma), although a few migrations could be dated to the middle Eocene (41.94 Ma) (Fig. 2). The remaining 22 migration events occurred from the Australasia to tropical Asia (Fig. 2), and were concentrated during the middle Miocene to Pleistocene (15.51–1.31 Ma).
|
| Fig. 2 Time of migration events in tropical Asia and Australasia. Red lines: from Asia to Australasia; blue lines: from Australasia to Asia. The two pie charts at the top show the ratio of abiotic and biotic dispersal of plant groups that migrated from Asia to Australasia before and after 15 Ma, respectively. The pie chart at the bottom shows the ratio for abiotic and biotic dispersal of plant groups in the opposite direction after 15 Ma. |
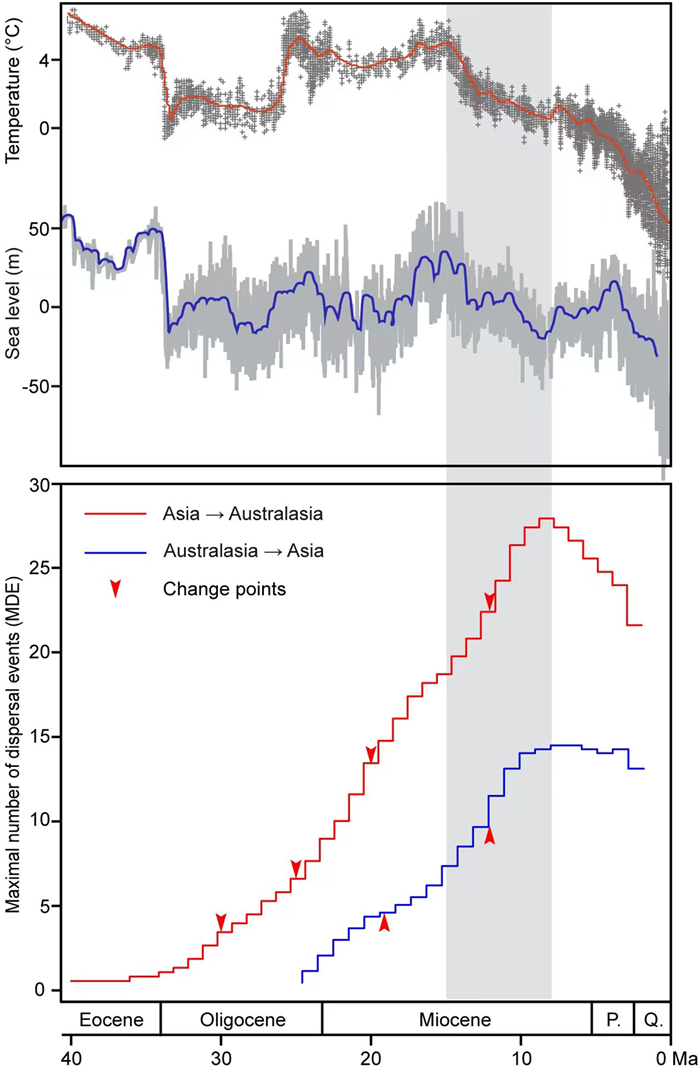
Overall, MDE from tropical Asia to Australasia has increased in four stages (30 Ma, 25 Ma, 20 Ma, and 12 Ma, respectively), reaching its extreme value during the late Miocene (7 Ma), and subsequently slowed down (Fig. 3). MDE from Australasia to the tropical Asia has changed twice (19 Ma and 12 Ma) and reached its extreme value during the late Miocene (5 Ma), after which it began to decline slowly (Fig. 3).
|
| Fig. 3 Development of the MDE for biotic interchange between tropical Asia and Australasia. Arrowheads indicate estimated change points. Estimates of climatic oscillations through time are based on Zachos et al. (2001) and estimates of sea level through time are based on Miller et al. (2020). P., Pliocene; Q., Quaternary. |
The ancestral reconstructions of the dispersal types indicate that the ancestors of 86% of the groups were taxa with biotic dispersal, whereas those of the remaining groups were taxa with abiotic dispersal. Among the immigration events from tropical Asia to Australasia, taxa with biotic dispersal accounted for 67% and 51% before and after the middle Miocene, whereas the taxa with abiotic dispersal increased from 33% to 49% around the middle Miocene (Fig. 2). Of the migrations from Australasia to tropical Asia, our statistics show that the groups with biotic dispersal are dominant (> 95%) in migration events since Miocene (Fig. 2). Overall, up to 72% of migration events occurred in taxa with biotic dispersal. Further, our habitat reconstructions showed that 27 of 29 groups were dominant in forests and there were no habitat shifts during their evolutionary history (Figs. S1–5, S7–19 and S21–29). Only Mazus and Glycine existed both in forest and non-forest habitat, but the habitat shifts did not correspond to the migration events (Figs. S6 and S20).
4. Discussion 4.1. The asymmetrical floristic interchange between tropical Asia and AustralasiaOur results indicate that previous floristic migrations between tropical Asia and Australasia were unequal. Excluding the terminal migrations, a total of 68 migration events occurred from the middle Eocene to the late Pleistocene, and approximately 84% events occurred after the middle Miocene (Fig. 2). Of these migration events, approximately 68% of migrations occurred from tropical Asia to Australasia since the Eocene, whereas 32% of migrations occurred from the Australasia to tropical Asia since the Miocene (Fig. 2). MDE analysis also showed a predominance of southward dispersal (Fig. 3). Crayn et al. (2015) indicated that migrations of angiosperm clades from Sunda to Sahul predominated over the reverse direction 2.4 times. Our results are consistent with those reported in previous studies on plant groups that migrated from Asia to Australasia (e.g., Grudinski et al., 2014; Crayn et al., 2015; Peng et al., 2021).
The pattern of biotic interchange is generally highly unilateral, with movement in one direction exceeding that in the other direction (Vermeij, 1991). This is likely because of high extinction events in one direction. For example, high extinction rates of mammals in South America drove the asymmetry of the Great American Biotic Interchange (Carrillo et al., 2020). However, neither Asia nor Australasia has had a mass extinction event since the Miocene. Ecosystem stability and habitat preferences of taxa can also promote dispersal asymmetry, i.e., organisms tend to migrate from biotas with a larger species pool and more saturated ecological systems to biotas with fewer species and young ecological systems (Vermeij, 1991; Yap et al., 2018, 2020). The warm and humid climates dominating much of tropical Asia during the early Miocene facilitated the establishment of moist forest vegetation, and led to its abundant seed plants (Zachos et al., 2001; Mittermeier et al., 2005; Richardson et al., 2012). However, the plant richness in Australasia is much lower than that in tropical Asia (Mittermeier et al., 2005). The rainforest in the Australian continent contracted in response to significant climate shifts caused by the opening of the Southern Ocean after the Eocene (Byrne et al., 2011; Crisp and Cook, 2013; Thornhill et al., 2016); also, the decline in area and extent of rainforest continued due to the Australian continent aridity during the Quaternary (Lomolino et al., 2006). Following the expansion of forests in tropical Asia and the dramatic climatic and geographic changes in Australia during the Neogene, the Australian regions may have provided more opportunities for aliens to establish (Byrne et al., 2011; Crisp and Cook, 2013; Yap et al., 2020). Most of our sampled lineages (27/29) are components of tropical forests, and there is no evidence of habitat shift during their evolutionary histories. Studies on Tetrastigma have found the establishment of suitable habitats facilitated the southward expansion of species, and their restricted distribution in wet forests of Australia likely reflects the low adaptability of Tetrastigma to new environmental conditions outside their ancestral niche (Yap et al., 2018; Peng et al., 2021). In addition, the limited northward dispersal of Tetrastigma might be attributed to competition pressure in the already established northern flora (Peng et al., 2021). The rates of migrations slowed down since the latest Pliocene were possibly due to contraction of rainforests (Sniderman et al., 2007; Sniderman and Jordan, 2011) (Fig. 3). We hypothesize that environmental conditions might have affected the rates and direction of floristic interchanges between tropical Asia and Australasia.
4.2. The biotic and abiotic drivers of the floristic interchangeOur results show that nearly 72% of migration events occurred in groups with biotic dispersal. Among the migration events from tropical Asia to Australasia, groups with biotic dispersal mainly contributed before and after middle Miocene (67% and 51%, respectively). Of the migrations from Australasia to tropical Asia, our findings show that taxa with biotic dispersal have been dominant (> 95%) since the Miocene (Fig. 2). Crayn et al. (2015) indicated that localized animal dispersal has contributed to floristic exchange between Sahul and Sunda since 12 Ma. Additionally, many previous studies have shown that groups with fleshy fruits are more likely to be eaten by birds or small mammals and can be dispersed through long distance (e.g., Phoon, 2015; Liu et al., 2018). For instance, Zona and Henderson found that birds, bats, reptiles, insects and fish feed on palm fruits and scatter the seeds. Similarly, studies have shown that Elaeocarpus dispersal from New Guinea to Malesia was driven by frugivorous birds during the Miocene (19.77–12.28 Ma) (Phoon, 2015). Interestingly, the timeframes of mass migrations (< 15 Ma) are slightly lagging behind the Miocene collision of the Sahul and Sunda plates (ca. 23–25 Ma) (Hall, 1998, 2011; Lomolino et al., 2006).
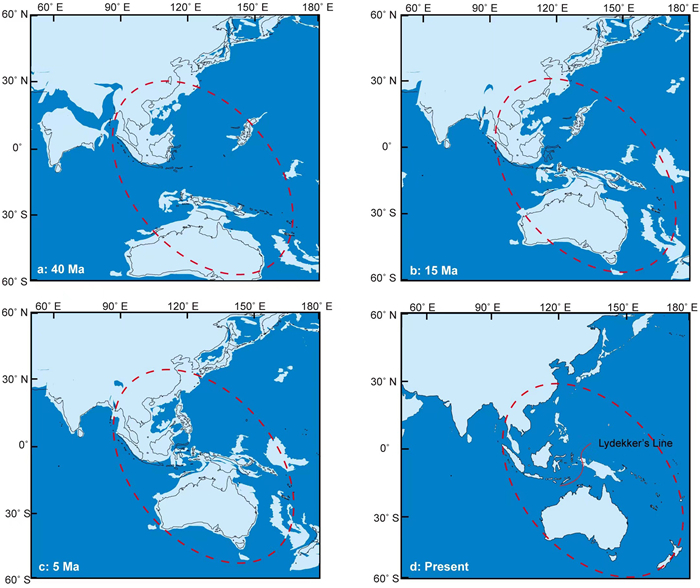
Palaeogeographical data revealed that the Australian continent rafted northward after separating from Antarctica during the Eocene (ca. 38 Ma) and collided with the Southeast Asian plate (ca. 20–25 Ma; Hall, 2002, 2012). This tectonic collision event resulted in the change of the SE Asia plate boundaries and caused fragments of the Australian micro-continent to reach Sulawesi (Hall, 1998) (Fig. 4). The formation of stepping stones since the middle Miocene has provided possible routes for seed plant migration between tropical Asia and Australasia (Lomolino et al., 2006). The spread of several Vitaceae plants such as Tetrastigma and Cayratia through dispersal by birds was one result of the stepping stones between tropical Asia and Australasia (Parmar et al., 2021; Peng et al., 2021). Furthermore, the sea level has declined and fluctuated more frequently (Fig. 2; Miller et al., 2020), which benefits plant dispersal in this region. During the late Miocene–Pleistocene, periodical sea-level fluctuations may have further enhanced land connections across the Sunda region and promoted more common dispersals by birds or small mammals (Gorog et al., 2004; Lohman et al., 2011; de Bruyn et al., 2014). The MDE value, which was used to represent the dynamic process of migration, indicated that migration increased significantly in both directions after 15 Ma. Thus, our results support the idea that formation of island chains in the late Miocene–Pleistocene and sea level fluctuations strengthened the floristic interchange between tropical Asia and Australasia, particularly for the animal-mediated dispersal groups. More significantly, these events coincided with the forest expansion in tropical Asia and are characterized by a major period of biotic exchange (Sniderman and Jordan, 2011; Crayn et al., 2015).
|
| Fig. 4 Palaeogeographical maps of the Asia–Australasia from 40 Ma to present. The dark blue represents the ocean, the black line represents the land area now. The light blue represents the land area of different reconstruction periods (a: 40 Ma; b: 15 Ma; c: 5 Ma; d: Present). Data of paleomaps are available at ODSN Plate Tectonic Reconstruction Service (https://www.odsn.de/). |
Biotic exchange of floras was crucial for tropical Asia and Australasia throughout the late Cenozoic, with the peak rates of dispersal occurring after the middle Miocene (ca. 15 Ma). Migrations between tropical Asia and Australasia were asymmetrical, and influx from Australasia into tropical Asia was lower than in the opposite direction throughout the same periods. This pattern can be attributed to a strong association between dispersal events and environmental changes. The formation of island chains after the Australian–Sundaland collision and climate changes have been beneficial for seed plants migrations since the middle Miocene. Furthermore, biotic dispersal and stable habitat may be crucial for floristic interchange among tropical Asia and Australasia. However, this speculation remains to be further tested by studying more plant and animal lineages, through integration of palynology, dating phylogenies and paleo-environments.
AcknowledgementsThis research was supported by the National Natural Science Foundation of China (31670212, 32060056, and 31300181), and the National Natural Science Foundation of China–Yunnan Joint Fund Project (U1802242), and Guangxi Key Laboratory Construction Project (19–185–7).
Author contributions
X.G.X., J.R., and Y.Z. conceived the idea; L.G.Z., X.G.X. and Y.J.L. collected the data; L.G.Z. and X.Q.L. analyzed the data; L.G.Z. and X.G.X. wrote the manuscript; and X.G.X., W.T.J., J.R., and Y.Z. revised the draft.
Declaration of competing interest
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.05.006.
Ali, J.R., Heaney, L.R., 2021. Wallace's line, Wallacea, and associated divides and areas: history of a tortuous tangle of ideas and labels. Biol. Rev. Camb. Phil. Soc., 96: 922-942. DOI:10.1111/brv.12683 |
Byrne, M., Steane, D.A., Joseph, L., et al., 2011. Decline of a biome: evolution, contraction, fragmentation, extinction and invasion of the Australian mesic zone biota. J. Biogeogr., 38: 1635-1656. DOI:10.1111/j.1365-2699.2011.02535.x |
Carrillo, J.D., Faurby, S., Silvestro, D., et al., 2020. Disproportionate extinction of South American mammals drove the asymmetry of the Great American biotic interchange. Proc. Natl. Acad. Sci. U.S.A., 117: 26281-26287. DOI:10.1073/pnas.2009397117 |
Crayn, D.M., Costion, C., Harrington, M.G., 2015. The Sahul–Sunda floristic exchange: dated molecular phylogenies document Cenozoic intercontinental dispersal dynamics. J. Biogeogr., 42: 11-24. DOI:10.1111/jbi.12405 |
Crisp, M.D., Cook, L.G., 2013. How was the Australian flora assembled over the last 65 million years? A molecular phylogenetic perspective. Annu. Rev. Ecol. Evol. Syst., 44: 303-324. DOI:10.1146/annurev-ecolsys-110512-135910 |
de Boer, A.J., Duffels, J.P., 1996. Historical biogeography of the cicadas of Wallacea, new Guinea and the west pacific: a geotectonic explanation. Palaeogeogr. Palaeoclimat. Palaeoecol., 124: 153-177. DOI:10.1016/0031-0182(96)00007-7 |
de Bruyn, M.D., Stelbrink, B., Morley, R.J., et al., 2014. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Syst. Biol., 63: 879-901. DOI:10.1093/sysbio/syu047 |
Donoghue, M.J., Smith, S.A., 2004. Patterns in the assembly of temperate forests around the Northern Hemisphere. Philos. Trans. R. Soc. Lond. B Biol. Sci., 359: 1633-1644. DOI:10.1098/rstb.2004.1538 |
Drummond, A.J., Suchard, M.A., Xie, D., et al., 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol., 29: 1969-1973. DOI:10.1093/molbev/mss075 |
Gorog, A.J., Sinaga, M.H., Engstrom, M.D., 2004. Vicariance or dispersal? Historical biogeography of three Sunda shelf murine rodents (Maxomys surifer, Leopoldamys sabanus and Maxomys whiteheadi). Biol. J. Linn. Soc., 81: 91-109. DOI:10.1111/j.1095-8312.2004.00281.x |
Grudinski, M., Wanntorp, L., Pannell, C.M., et al., 2014. West to east dispersal in a widespread animal-dispersed woody angiosperm genus (Aglaia, Meliaceae) across the Indo-Australian Archipelago. J. Biogeogr., 41: 1149-1159. DOI:10.1111/jbi.12280 |
Hall, R., 1998. The plate tectonics of Cenozoic SE Asia and the distribution of land. In: Hall, R., Holloway, J.D. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys Publishers, Leiden, The Netherlands, pp. 99-131.
|
Hall, T.A., 1999. BioEdit: a user–friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser., 41: 95-98. |
Hall, R., 2002. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computerbased reconstructions, model and animations. J. Asian Earth Sci., 20: 353-434. DOI:10.1016/S1367-9120(01)00069-4 |
Hall, R., 2011. Australia–SE Asia collision: plate tectonics and crustal flow. Geol. Soc. Spec. Publ., 355: 75-109. DOI:10.1144/SP355.5 |
Hall, R., 2012. Sundaland and Wallacea: geology, plate tectonics and palaeogeography. In: Gower, D.J., Johnson, K.G., Richardson, B.R., et al. (Eds.), Biotic evolution and environmental change in Southeast Asia. The Systematics Association, Cambridge University Press, pp. 32-78.
|
Hall, R., 2013. The palaeogeography of Sundaland and Wallacea since the late jurassic. J. Limnol., 72: 1-17. |
Hooker, J.D.S., 1859. On the Flora of Australia, its Origin, Affinities and Distribution; Being an Introductory Essay to the Flora of Tasmania. Lovell Reeve, London.
|
James, N.A., Matteson, D.S., 2014. ecp: an R package for nonparametric multiple change point analysis of multivariate data. J. Stat. Software, 62: 1-25. |
Jaramillo, C., 2018. Evolution of the Isthmus of Panama: biological, palaeoceanographic and palaeoclimatological implications. In: Hoorn, C.P.A., Antonelli, A. (Eds.), Mountains, Climate and Biodiversity. John Wiley and Sons, New Jersey, pp. 323-338.
|
Jiang, D.C., Sebastian, K., Zhang, Y.P., et al., 2019. Asymmetric biotic interchange across the Bering land bridge between Eurasia and North America. Natl. Sci. Rev., 6: 739-745. DOI:10.1093/nsr/nwz035 |
Klaus, S., Morley, R.J., Plath, M., et al., 2016. Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat. Commun., 7: 12132. DOI:10.1038/ncomms12132 |
Lieberman, B.S., 2005. Geobiology and paleobiogeography: tracking the coevolution of the Earth and its biota. Palaeogeogr. Palaeoclimat. Palaeoecol., 219: 23-33. DOI:10.1016/j.palaeo.2004.10.012 |
Liu, B., Le, C.T., Barrett, R.L., et al., 2018. Historical biogeography of Loranthaceae (Santalales): diversification agrees with emergence of tropical forests and radiation of songbirds. Mol. Phylogenet. Evol., 124: 199-212. DOI:10.1016/j.ympev.2018.03.010 |
Lohman, D.J., de Bruyn, M., Page, T., et al., 2011. Biogeography of the Indo–Australian archipelago. Annu. Rev. Ecol. Evol. Syst., 42: 205-226. DOI:10.1146/annurev-ecolsys-102710-145001 |
Lomolino, M.V., Riddle, B.R., Brown, J.H., 2006. Biogeography (3th edition). Sinauer Associates, Inc. Publishers, Sunderland (Massachusetts).
|
Maddison, W.P., Maddison, D.R., 2014. Mesquite: a modular system for evolutionary analysis. Version 3.01. [online]. Available at. http://mesquiteproject.org.
|
Manchester, S.R., Tiffney, B.H., 2001. Integration of paleobotanical and neobotanical data in the assessment of phytogeographic history of Holarctic angiosperm clades. Int. J. Plant Sci., 162: S19-S27. DOI:10.1086/323657 |
Manchester, S.R., Chen, Z.D., Lu, A.M., et al., 2009. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the Northern Hemisphere. J. Syst. Evol., 47: 1-42. DOI:10.1111/j.1759-6831.2009.00001.x |
Metcalfe, I., 1998. Palaeozoic and Mesozoic geological evolution of the SE Asian region: multidisciplinary constraints and implications for biogeography. In: Hall, R., Holloway, J.D. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys Publishers, Leiden, pp. 25-41.
|
Miller, K.G., Browning, J.V., Schmelz, W.J., et al., 2020. Cenozoic sea-level and cryospheric evolution from deep-sea geochemical and continental margin records. Sci. Adv., 6: eaaz1346. DOI:10.1126/sciadv.aaz1346 |
Mittermeier, R.A., Fonseca, P., Gil, R., et al., 2005. Hotspots Revisited: Earth's Biologically Richest and Most Endangered Terrestrial Ecoregions. Cermex, Mexico City.
|
Pagel, M., Meade, A., Barker, D., 2004. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol., 53: 673-684. DOI:10.1080/10635150490522232 |
Parmar, G., Dang, V.C., Rabarijaona, R.N., et al., 2021. Phylogeny, character evolution and taxonomic revision of Causonis, a segregate genus from Cayratia (Vitaceae). Taxon, 70: 1188-1218. DOI:10.1002/tax.12562 |
Peng, D.X., Dang, V.C., Habib, S., et al., 2021. Historical biogeography of Tetrastigma (Vitaceae): insights into floristic exchange patterns between Asia and Australia. Cladistics, 37: 803-815. DOI:10.1111/cla.12462 |
Phoon, S.N., 2015. Systematics and Biogeography of Elaeocarpus (Elaeocarpaceae). Doctoral Dissertation. James Cook University.
|
Rambaut, A., Drummond, A.J., 2007. Tracer v1.5 [online]. Available online at: http://beast.bio.ed.ac.uk/Tracer. (Accessed 30 November 2009). accessed.
|
Raven, P.H., Axelrod, D.I., 1974. Angiosperm biogeography and past continental movements. Ann. Mo. Bot. Gard., 61: 539-673. DOI:10.2307/2395021 |
Richardson, J.E., Costion, C.M., Muellner, A.N., 2012. The Malesian floristic interchange: plant migration patterns across Wallace's Line. In: Gower, D., John, K., Richardson, J., Rosen, B., Rüber, L., Williams, S. (Eds.), Biotic Evolution and Environmental Change in Southeast Asia. Cambridge University Press, Cambridge, pp. 138-163.
|
Schwartz, T., Nylinder, S., Ramadugu, C., et al., 2016. The origin of oranges: a multi-locus phylogeny of Rutaceae subfamily Aurantioideae. Syst. Bot., 40: 1053-1062. DOI:10.1600/036364415X690067 |
Sniderman, J.M.K., Jordan, G.J., 2011. Extent and timing of floristic exchange between Australian and Asian rain forests. J. Biogeogr., 38: 1445-1455. DOI:10.1111/j.1365-2699.2011.02519.x |
Sniderman, J.M.K., Pillans, B., O'Sullivan, P.B., et al., 2007. Climate and vegetation in southeastern Australia respond to Southern Hemisphere insolation forcing in the late Pliocene–early Pleistocene. Geology, 35: 41-44. |
Stelbrink, B., Albrecht, C., Hall, R., et al., 2012. The biogeography of Sulawesi revisited: is there evidence for a vicariant origin of taxa on Wallace's "anomalous island"?. Evolution, 66: 2252-2271. DOI:10.1111/j.1558-5646.2012.01588.x |
Székely, G.J., Rizzo, M.L., 2005. Hierarchical clustering via joint between–within distances: extending Ward's minimum variance method. J. Classif., 22: 151-183. DOI:10.1007/s00357-005-0012-9 |
Thornhill, A.H., Mishler, B.D., Knerr, N.J., et al., 2016. Continental-scale spatial phylogenetics of Australian angiosperms provides insights into ecology, evolution and conservation. J. Biogeogr., 43: 2085-2098. DOI:10.1111/jbi.12797 |
Tiffney, B.H., 1985. Perspectives on the origin of the floristic similarity between Eastern Asia and eastern North America. J. Arnold Arbor., 66: 73-94. DOI:10.5962/bhl.part.13179 |
Truswell, E., Kershaw, A., Sluiter, I., 1987. The Australian-south-east Asian connection: evidence from the palaeobotanical record, Biogeographical evolution of the Malay Archipelago. In: Whitmore, T.C. (Ed.), Biogeographical Evolution of the Malay Archipelago. Oxford University Press, New York, pp. 32-49.
|
Vermeij, G.J., 1991. When biotas meet: understanding biotic interchange. Science, 253: 1099-1104. DOI:10.1126/science.253.5024.1099 |
Wallace, A.R., 1857. On the natural history of the Aru Islands. Ann. Mag. Nat. Hist., 20: 473-485. DOI:10.1080/00222935708680675 |
Wen, J., Ickert-Bond, S.M., 2009. Evolution of the madrean–tethyan disjunctions and the North and South American amphitropical disjunctions in plants. J. Syst. Evol., 47: 331-348. DOI:10.1111/j.1759-6831.2009.00054.x |
Wolfe, J.A., Toshimasa, T., 1980. The Miocene Seldovia Point Flora from the Kenai Group. United States Government Printing Office, Washington, Alaska.
|
Wu, Z.Y., Sun, H., Zhou, Z.H., et al.
, 2011. Floristics of Seed Plants from China. Beijing: Science Press.
|
Wu, X.K., Liu, X.Y., Kodrul, T., et al., 2019. Dacrycarpus pattern shedding new light on the early floristic exchange between Asia and Australia. Natl. Sci. Rev., 6: 1086-1090. DOI:10.1093/nsr/nwz060 |
Xiang, Q.Y., Soltis, D.E., Soltis, P.S., et al., 2000. Timing the eastern Asian–eastern North American floristic disjunction: molecular clock corroborates paleontological estimates. Mol. Phylogenet. Evol., 15: 462-472. DOI:10.1006/mpev.2000.0766 |
Yap, J.-Y.S., Rossetto, M., Costion, C., et al., 2018. Filters of floristic exchange: how traits and climate shape the rain forest invasion of Sahul from Sunda. J. Biogeogr., 45: 838-847. DOI:10.1111/jbi.13143 |
Yap, J.-Y.S., van der Merwe, M., Ford, A.J., et al., 2020. Biotic exchange leaves detectable genomic patterns in the Australian rain forest flora. Biotropica, 52: 627-635. DOI:10.1111/btp.12776 |
Yu, Y., Harris, A.J., Blair, C., et al., 2015. RASP (reconstruct ancestral state in phylogenies): a tool for historical biogeography. Mol. Phylogenet. Evol., 87: 46-49. DOI:10.1016/j.ympev.2015.03.008 |
Zachos, J., Pagani, M., Sloan, L., et al., 2001. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science, 292: 686-693. DOI:10.1126/science.1059412 |



