b. CIFOR-ICRAF China Program, World Agroforestry (ICRAF), Kunming 650201, Yunnan, China;
c. Honghe Center for Mountain Futures (CMF), Kunming Institute of Botany, Chinese Academy of Sciences, Honghe County 654400, Yunnan, China;
d. Faculty of Agroforestry, Lumbini Buddhist University, Lumbini, Nepal
For plant populations to persist, seedlings must be recruited to the next generation. This requires seeds to germinate, survive and grow. The age of a plant at the onset of adverse events is a critical determinant of its survival (Grime et al., 1997; Moles and Westoby, 2004a). The most vulnerable stage during the life history of a plant is the seedling stage (Giménez-Benavides et al., 2008; Orians et al., 2011). During this stage, two of the most conspicuous factors that lead to mortality are grazing and drought (Moles and Westoby, 2004b). The youngest seedlings are usually the most sensitive to both grazing and drought (Warner and Cushman, 2002; Moles and Westoby, 2004b; Elger et al., 2009), lack available energy reserves for recovery (Warner and Cushman, 2002) and are most likely to be completely consumed by herbivores (Hanley and Fegan, 2007; Hanley et al., 2013).
Plant strategies to survive grazing can be divided into defence, escape and tolerance. Defences can present in chemical or structural forms, such as spines (Hanley and Fegan, 2007). Escape includes physiological traits, such as prostrate growth forms and external traits like growth within refuges (e.g., between rocks or beneath spiny bushes and poisonous plants) (Milchunas and Noy-Meir, 2002; Cheng et al., 2014). Grazing tolerance can be mediated by shifts in plant physiology and biomass allocation (Long, 2003; Sun et al., 2014). These include reduced allocation of resources to shoots (Ferraro and Oesterheld, 2002) coupled with increased allocation to roots (Orians et al., 2011; Sun et al., 2014; Cranston et al., 2016) as well as reallocation of resources from roots to shoots to facilitate compensatory shoot growth (Barton, 2008). Grazing-intolerant species suffer reduced shoot and root biomass (Koerner and Collins, 2014; Zhu et al., 2015), potentially resulting in increased plant mortality. The frequency of grazing events can be as influential as grazing intensity (Deleglise et al., 2015; Davis et al., 2014). The impacts can differ not only between plant functional groups, but also vary due to factors such as elevation and soil moisture conditions (Davis et al., 2014).
Plant strategies to survive drought comprise either escape, avoidance or tolerance (Salehi-Lisar and Bakhshayeshan-Agdam, 2016). Plants can escape drought by germinating only when conditions are favourable (Li et al., 2013) and following an annual or ephemeral life history strategy (Qian et al., 2007). Drought avoidance relies on preserving high tissue water potential via reduced stomatal water loss, deep root systems, hairy leaves, succulence or small size (Salehi-Lisar and Bakhshayeshan-Agdam, 2016). Drought tolerance can utilize the above physiological features, which are characterized by plasticity in drought response. At the onset of drought, plant responses include stomatal closure, changed metabolism and reduced shoot growth as well as increased investment in root growth (Orians et al., 2011; Comas et al., 2013; Salehi-Lisar and Bakhshayeshan-Agdam, 2016). Severe drought can trigger above-ground senescence that may be survivable by species with high meristematic tissue tolerance to drought (Deléglise et al., 2015).
Complex interactions can exist between the stressors of grazing and drought (Zavaleta et al., 2003; Shibel and Heard, 2016). In general, grazing is expected to be more damaging in drought periods (Yu et al., 2004; Deléglise et al., 2015). The impacts of grazing and drought on plant survival, recruitment and productivity are especially relevant for plant populations on grazed land in arid regions.
Our study is based on plants from the arid region of the Pamir Mountains of northwestern China. The remoteness of the Pamirs has hindered much research regarding the flora of this region. For plant species from the Pamirs, their ability to escape, avoid or tolerate grazing and drought will be increasingly critical to their future survival. Excessive livestock grazing is currently degrading many Pamiri pastures (Joshi et al., 2013), and droughts are projected to increase in frequency and intensify (Su et al., 2018). Rising temperatures will reduce water availability due to changing precipitation patterns and glacial retreat (Kassam, 2009; Zhang et al., 2016). In the western Pamirs, herders are already beginning to migrate earlier to summer pastures (Joshi et al., 2013) and using progressively higher pastures (Kassam, 2009). Similar changes could be expected in the timing and extent of grazing in the eastern Pamirs.
We examined the perennial forb Saussurea glacialis Herder. (Asteraceae) and the annual forb Plantago lessingii Fisch. & C.A. Mey. (Plantaginaceae). The former grows at the snowline and the latter several hundred meters below it. We selected these species based on their medical, economic and agricultural importance to local Pamiri people. Plantago species are nutritious plants that provide fodder for livestock (Rahim and Maselli, 2012). Saussurea species are used in local medicinal treatments and can also be sold. Their population is vulnerable due to potential overharvesting, high habitat specificity, small populations and limited ranges (Shurupova and Zverev, 2017). For both S. glacialis and P. lessingii, understanding the stress responses of their seedlings to simulated grazing and drought can inform conservation efforts.
The overall goal of this study was to establish how grazing and drought could impact seedling survivability and productivity for two forb species of Pamiri mountain plants. We tested the following three hypotheses regarding the impact of two potential stressors, simulated grazing and drought:
1. Younger seedlings were expected to have higher mortality rates after grazing or drought events.
2. Seedlings that survive grazing or drought were expected to experience either a reduction in biomass or reallocation of biomass between roots and shoots.
3. Interactions between repeated grazing or drought events were expected to have an additive effect on reducing seedling survival and growth.
We produced a mathematical model that integrates the results of our study to determine the relative impact of grazing and drought, the importance of seedling age at the onset of these events and implications for the sustainability of Saussurea glacialis and Plantago lessingii populations under potential future soil moisture conditions and grazing regimes. Seed production, viability and germination rates provided starting parameters for this model.
2. Materials and methods 2.1. Study sitesWe selected study sites on three ridges in the Pamir mountains within the Taxkorgan Nature Reserve, Taxkorgan County, Xinjiang Province, China. The ridges include one to the west and two to the east of the major pass through the Pamirs (Appendix A, Fig. A1). At each site we selected two elevations, both within high summer pastures subject to seasonal grazing by the livestock of transhumant pastoralists. We surveyed these sites to provide the background for our study and to help us establish realistic conditions for our greenhouse experiment. The 1st and 3rd transects were within 1 km of the nearest pastoralist family and all vegetation was heavily grazed with extensive bare patches of soil. The top of the 2nd transect was over 5 km from the nearest pastoralist family and had not been recently grazed.
We analysed past meteorological data to detect variation in regional water availability and predict at what point in the growing season our species may potentially face water deficit conditions. This analysis revealed clear water deficit conditions in the region prior to the growing season of our focal plants (Appendix B, Figs. B1 & B2). Our microclimate station data from 2018 confirmed this trend.
2.2. Plant surveyWe conducted a plant survey in July 2017 in order to select appropriate species for our greenhouse experiment. Local people contributed traditional ecological knowledge regarding plant use. First, we selected Saussurea glacialis, a medicinal plant locally used as a painkiller. Its range was just below the snowline, at elevations of 4530–4580 m. On the 1st and 3rd transect, S. glacialis was present on dry ground at the top of a ridge, whereas on the 2nd transect, it was at the foot of a glacier. Second, we selected Plantago lessingii, which was found at elevations of 4150–4260 m, on dry ground at the top (2nd transect) or side (1st and 3rd transect) of a ridge. Elsewhere, Plantago species provide nutritious fodder for livestock (Rahim and Maselli, 2012). Both species grow in patches under 100 m wide, among sparse, grazed vegetation on rocky ground. We observed no grazing damage on S. glacialis, possibly indicating that it is not a preferred forage plant for livestock.
2.3. Microclimate stations and soil samplesWe used six HOBO microclimate stations to record microclimatic factors alongside patches of S. glacialis and P. lessingii at each study site. We placed an additional two HOBO microclimate stations at 3585 m and 3107 m elevation, to compare microclimatic factors down an elevation gradient (Appendix A). Each of these stations had loggers (HOBO U30 NRC), sensors for soil water content (S-SMC-M005) and soil temperature (S-TMB-M002), air temperature (S-THB-M002) and rainfall (S-RGD-M002). In September 2017, we built an additional four stations on the other two transects, which had loggers (HOBO U30) with sensors for soil water content (S-SMC-M005) and soil temperature (S-TMB-M002). All sensors for soil temperature and soil moisture content were placed at depths of 5 cm, 10 cm, 20 cm and 30 cm. At each location, soil samples were taken to calibrate the HOBO soil moisture sensors and determine the soil conditions in which our study plants had been found (Appendix C, Table C1, Fig. C1).
2.4. Seed collection, storage and X-ray screening of seed viabilityIn September 2017, we collected seeds from three sites per species. We removed all seeds from ten Saussurea glacialis and Plantago lessingii individuals per site, leading to a total sample of 30 plants per species. Due to small population size, we avoided taking an unnecessarily large sample. All seeds were air-dried and transported back to the Kunming Institute of Botany's Germplasm Bank of Wild Species. All seeds were stored overwinter at 15 ℃ in dry conditions, reducing the relative humidity of the seeds to 15%. In February 2018, all seeds were screened, using X-ray facilities to determine the seed viability rate. These X-ray images showed whether seeds were filled by an embryo (solid white image) or empty (grey or translucent image). Filled seeds were deemed to be potentially viable, whereas empty seeds were non-viable. Low seed viability limited the seeds available for our experiment to 6 seeds per pot for S. glacialis and 12 seeds per pot for P. lessingii.
2.5. Greenhouse experimentOn the 13th March 2018, seeds were sown under greenhouse conditions at the Kunming Institute of Botany, Yunnan, China. This initial time of first sowing is hereafter referred to as Tsow 1. Seeds sown 21 days later are hereafter referred to as Tsow 2. Viable seeds were planted in transparent Perspex cuboid pots (10 cm × 20 cm × 15 cm depth) that enabled ongoing observation of soil moisture and root development. The potting medium was a 1:1 mix of fine peat and coir fibre. Soil parameters, including soil nutrients, are shown in Appendix C, Table C2. We sparsely hand-watered on alternating days. The sequence of treatments and harvests is shown in Fig. 1. No water was given during simulated drought treatments (D). The simulated grazing treatment (G) involved mechanical defoliation. Plants were either cut with scissors to a height of 2 cm (level with the top of the pot) or left uncut. During recovery periods, plants were watered with 50 ml daily and not defoliated. Greenhouse temperatures and relative humidity are shown in Appendix C, Fig. C2.
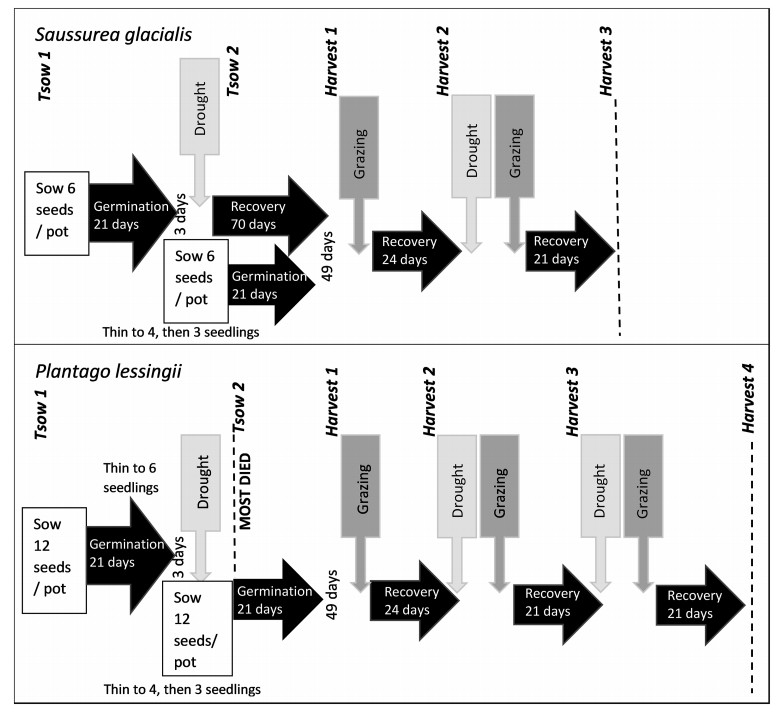
|
| Fig. 1 Timeline of study for Saussurea glacialis (top) and Plantago lessingii (bottom). At the first time point Tsow 1 seeds were sown. A second set of seeds were sown at time point Tsow 2. A subset of plants was harvested and measured at four time points within the study period: Harvest 1, 2, 3 and 4. 'Drought' represents no water addition, 'Grazing' represents seedlings trimmed to 2 cm high. During 'recovery' or control periods, 50 ml water was supplied per day. The first drought period was terminated after 5 days. Thereafter, during 'Drought Grazing' periods, a sequential set of treatments were applied. For S. glacialis either: control, 8 days drought, 16 days drought, or 8 days drought followed by grazing. For P. lessingii either: control, 8 days drought, 16 days drought, grazing only, 16 days drought followed by grazing or 8 days drought followed by two grazing events. |
By the fifth day of the first drought treatment, a high proportion of Plantago lessingii seedlings had died, forcing us to terminate the drought treatment and exclude these P. lessingii pots from the experiment. Consequently, there were two age categories for Saussurea glacialis (40 pots for Tsow 1– and 38 pots for Tsow 2) but only one age category for P. lessingii (80 pots for Tsow 2). A low proportion of S. glacialis seedlings survived the simulated grazing treatment, which limited the number of possible future treatments. All pots with surviving plants were reassigned to four blocks. They were subjected to a factorial combination of sequential treatments: control '_' (no grazing, 50 ml water per day), drought 'D' (no water) and grazing 'G' (seedlings cut to 2 cm high) treatments, with each drought treatment lasting 8 days 'D' or 16 days 'DD'. Treatment sequences are referred to by the order of events, e.g., drought followed by grazing then control is written as 'DG_'.
The treatments for Saussurea glacialis were __, D__, DD_ and DG_. The treatments for Plantago lessingii were __, D__, DD_, D_D, G__, DG_, D_G, DDG and DGG. After a 21-day recovery period, all of the S. glacialis and half of the P. lessingii pots were harvested (Harvest 3). The remaining P. lessingii pots were subjected to a second set of the same drought and grazing treatments. After a 21-day recovery period, all remaining pots were harvested (Harvest 4).
At each harvest, each plant was removed from the pot and all loose soil shaken off. Remaining soil was rinsed off and plants were dried at 60 ℃ for 24 h. Shoot and root length were both recorded separately for each plant, but we have not included shoot length in the analysis, as shoots were directly cut in the defoliation treatment. Because the mass of individual plants was low, we weighed plants from the same pot together.
2.6. Data analysisThe difference in shoot and root mass between harvests was used to calculate the Relative Growth Rate, following Hunt (1978).
Where W1 = weight at time 1; W2 = weight at time 2; time = number of days between harvest events:
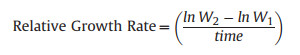
|
(1) |
The absolute shoot and root mass per pot was used to calculate the Shoot Mass Fraction, following Hunt (1978):
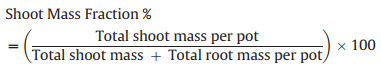
|
(2) |
Analyses were conducted separately for each species. Mean values are quoted in the text as mean ± SD (standard deviation). Survival was calculated as the proportion of plants surviving out of number of plants per pot. Plant survival data were analysed using Generalized Linear Models (GLMs) with binomial errors. The number of leaves per plant was a count variable that was analysed using GLMs with poisson errors. Length of root, shoot mass, root mass and Shoot Mass Fraction were continuous variables. When necessary, log transformations were applied to obtain normal error distributions, allowing analysis using ANOVA. The small data sets for Relative Growth Rate required analysis with the non-parametric Wilcoxon Rank Sum Test.
In all GLM and ANOVA analyses, a model simplification approach was taken, following Crawley (2005). ANOVA was used to test for a significant increase in deviance with each reduction in the model. Terms were only retained if their removal caused a significant reduction in the explanatory power of the model. If no other model was significantly better than the null model in accounting for the variance in the data, we treat this as exerting no significant effect on any model terms, and values are not quoted in the results section. All analyses were conducted in R v.3.4.1 (R Core Team, 2017).
2.7. Mathematical model of age-dependent seedling mortalityWe modelled the results of our experiments in order to determine the relative importance of simulated grazing and drought on seedling mortality. The impact of each mortality factor is proportional to the number of plants to which it is applied. Thus, an earlier high death rate, when more plants are still alive, numerically reduces a population more than the same rate applied later to remaining survivors of a cohort. The classic way to approach the relative importance of multiple mortality factors applied to a population over time is through key-factor analysis (Varley and Gradwell, 1960). However, in this case, we wished to contrast the importance of two mortality factors at multiple time points across two different plant species. Therefore, we mathematically modelled plant survival for a cohort of plants, beginning at their germination and terminating at the end of the first growing season, using the seeds of a single parent plant. All parameters were estimated based on those measured in field, lab and greenhouse studies. The equations were solved numerically to estimate the proportional contribution of both early and late grazing and drought on the number of plants surviving from this cohort at the end of the first growing season.
For the purposes of the model, the time period ran for the length of our experiment, namely from March T1 to end September T7 – although the growing season in the field may be shorter. The ability of a plant population to persist was determined by its replacement rate. On average, each individual must have at least one surviving offspring over its lifetime for the population to persist, so we considered whether the number of descendants per parent plant exceeds one. The number of viable seeds per plant was fixed as the initial cohort size and treated as a constant, calculated from a likely upper limit of cohort size. We made the assumption that the actual number of seeds produced per plant will not exceed the maximum number of seeds observed per plant in the field, and that the actual viability rate will never exceed the maximum viability rate determined by X-ray observations. Thus:
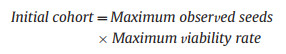
|
(3) |
As seedling emergence in the field can be far below that obtained under laboratory conditions (Giménez-Benavides et al., 2008), this is most likely an overestimation for initial cohort size. In the greenhouse, the first seedlings germinated after one week of sowing, but more continued to germinate over the following three weeks. However, for the purposes of our calculations, we pooled all seeds into two germination categories; early (those sown in March) or late (those sown in April), with their age being the time between sowing and onset of a drought or grazing event. All seeds entered the model at T1, allowing the impact of each mortality event to be calculated at two age intervals. This meant that only one germination rate could be applied; therefore, we selected the higher mean germination rate obtained in April as the more likely representation of the cumulative number of seeds that would eventually germinate as conditions became progressively more favourable.
We included two mortality factors: drought D and grazing G. Each operated at a fixed set of monthly time intervals with values determined by the experimental data. The time intervals were T1 to T7, with harvest at T7. At time Tx, the applicable mortality rates are Dx and Gx
At T1:
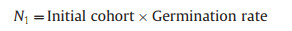
|
(4) |
From T2 onwards:
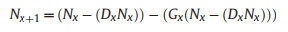
|
(5) |
Using these rates, the model parameters were applied to calculate the values for N at each time interval, and the proportion of total deaths was accounted for by each mortality factor.Where total mortality Nm is the difference between the initial number of seedlings N1 and the final number of surviving seedlings N7:
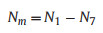
|
(6) |
And proportion of contribution to mortality:
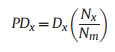
|
(7) |
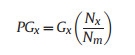
|
(8) |
The proportional contributions to mortality were determined in rank order, with the highest rank being the most important determinant of plant survival.
3. Results 3.1. Water deficit conditionsOur mountain sites receive both precipitation and spring snowmelt, making them generally moister than northwestern China's lower elevation deserts. However, there were water deficit conditions in the summer months. The lower the elevation, the greater and more prolonged were the conditions of water deficit (Appendix B, Fig. B3A). In 2017/2018, soil water content was low at all depths between November and May (Appendix B, Figs. B3 & B4). Thus, there were water deficit conditions in the early growing season. Precipitation levels at lower elevation sites were much lower than at higher elevation sites. The number of days with rainfall increased with elevation (Appendix B, Fig. B3C). The highest soil moisture was in late summer and early autumn (Appendix B, Figs. B3D & B4), likely because the most rainfall is usually received from May until September (Appendix B, Fig. B2). Soil bulk density was greatest at the low elevation site, and soils with higher soil bulk density contained lower soil moisture content (Appendix C, Fig. C1).
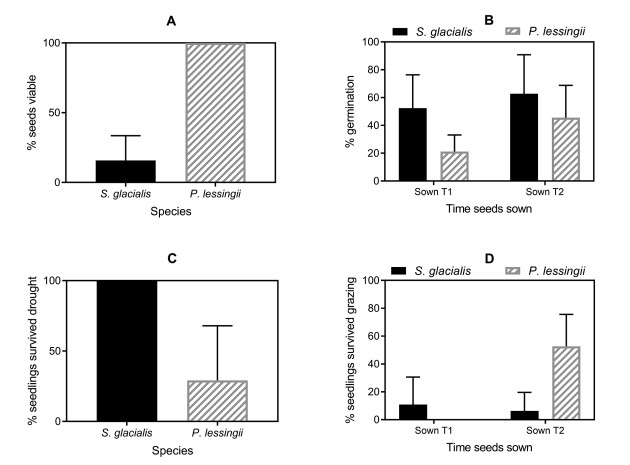
3.2. Estimating the initial cohort of seedlings to enter our modelX-ray images showed that 15.8 ± 17.7% (mean ± SD) of Saussurea glacialis seeds (seeds: N = 1543) were full and potentially viable. Plantago lessingii had a surprisingly high viability rate, with all whole seeds (seeds: N = 1265) being full and potentially viable (Fig. 2A). However, not all full seeds germinated. At Tsow 1, germination rates were 52.3 ± 24.9% (mean ± SD) for S. glacialis (pots: N = 57, seeds: N = 342) and 21.2 ± 11.9% (mean ± SD) for P. lessingii (pots: N = 57, seeds: N = 684). Germination rates were higher at Tsow 2, when temperatures were warmer and humidity was lower, with germination rates of 62.9 ± 28.4% (mean ± SD) for S. glacialis (pots: N = 38, seeds: N = 228) and 45.6 ± 23.2% (mean ± SD) for P. lessingii (pots: N = 80, seeds: N = 960) (Fig. 2b). We used the above figures to fix the starting parameters for our model.
|
| Fig. 2 For Saussurea glacialis (solid black bars) and Plantago lessingii (striped grey bars): A) % seeds viable, B) % of viable seeds that germinated, C) % seedlings that survived the first simulated drought condition, D) % seedlings that survived the first simulated grazing event. |
Overall, the youngest seedlings had the highest mortality rates. However, age-dependent mortality differed between the two species. When the 1st simulated drought treatment was imposed, all Saussurea glacialis seedlings survived, but there was high mortality among P. lessingii seedlings, with only 29.2 ± 38.8% surviving (Fig. 2C). After the 1st simulated grazing event, very few S. glacialis seedlings from Tsow 1 survived (10.9 ± 19.7% survived (mean ± SD)), significantly fewer than in the control condition. Survival rates appeared lower for the younger seedlings from Tsow 2 (6.25 ± 13.4% survived (mean ± SD)), but this effect was not statistically significant (Treatment: z = 3.42, p < 0.001; Tsow: z = -0.928, p = 0.353). P. lessingii seedlings from Tsow 2 were best at surviving simulated grazing, with 57.1 ± 20.4% (mean ± SD) surviving (Fig. 2D), but this was still significantly less than in the not-grazed control (Treatment: z = -391, p < 0.0001). Survival rates for the remaining seedlings were significantly higher than this, after subsequent simulated grazing and drought treatments. No further differences between the treatment conditions made any significant difference to seedling survival. Mean values for all mortality rates are given in Table 1.
| Mortality factor | Saussurea glacialis | Plantago lessingii | Mortality factor | Saussurea glacialis | Plantago lessingii | N | Saussurea glacialis | Plantago lessingii |
| D1 | 0 | 0.708 | G1 | N1 | 164 | 156 | ||
| D2 | G2 | 0.891 | N2 | 164 | 45.6 | |||
| D3 | G3 | 0.938 | 0.429 | N3 | 17.9 | 45.6 | ||
| D4 | 0.833 | G4 | 0.667 | N4 | 1.11 | 26.0 | ||
| D5 | 0.667 | 0.111 | G5 | 0.867 | 0 | N5 | 0.0616 | 26.0 |
| D6 | 0.167 | G6 | 0.083 | N6 | 0.00273 | 23.1 | ||
| N7 | 0.00273 | 17.7 |
For S. glacialis, the maximum number of seeds observed per plant in the field was 438, and the maximum viability rate was 0.593, so the initial cohort size was fixed at 260. The germination rate was fixed at 0.629, giving an initial number of plants N1 of 164. For P. lessingii, the maximum number of seeds observed per plant in the field was 343, and the maximum viability rate was 1, so the initial cohort size was fixed at 343. The germination rate was fixed at 0.456, giving an initial number of plants N1 of 156.
The two mortality factors, drought D and grazing G, were estimated separately for each time interval. These are displayed in Table 1. The highest levels of D were D4 for Saussurea glacialis and D1 for Plantago lessingii. The highest mortality rate due to any factor was G3 for S. glacialis. However, the greatest total reduction in N in a single time interval was from N2 to N3 for S. glacialis and from N1 to N2 for P. lessingii.
When the proportion of total mortality due to each mortality factor was calculated (Table 2), it was apparent that for P. lessingii, the drought mortality factor D1 operating on N1 was indeed the most important cause of mortality. However, the model revealed that for S. glacialis, the most important cause of mortality was not in fact G3. Due to the reduction in N over T, the grazing mortality factor G2 acting on the earlier cohort of N2, accounted for both the highest proportion of mortality overall and for S. glacialis alone. Thus, early grazing was the most important mortality factor for S. glacialis, whereas early drought caused the highest proportion of mortality for P. lessingii.
| Mortality factor | Saussurea glacialis | Mortality factor | Plantago lessingii |
| G2 | 0.891 | D1 | 0.798 |
| G3 | 0.102 | G3 | 0.141 |
| D4 | 0.00562 | D6 | 0.0279 |
| G4 | 0.00451 | D5 | 0.0209 |
| G5 | 0.000326 | G6 | 0.0139 |
| D5 | 0.000251 |
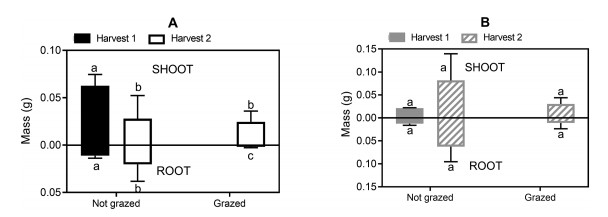
For S. glacialis, between the 1st and 2nd harvest (Fig. 3A), the control plants (not grazed) developed significantly lower shoot mass but greater root mass. Simulated grazing had no additional effect on log shoot mass (Harvest: F1 = 10.8, p < 0.01), but caused shorter log root length (Harvest: F1 = 25.5, p < 0.001; Treatment: F1 = 6.19, p < 0.05) and lighter log root mass (Harvest: F1 = 7.75, p < 0.05; Treatment: F1 = 30.7, p < 0.001). There were no significant changes in number of leaves per plant or Relative Growth Rate between any conditions. By the 2nd harvest, Shoot Mass Fraction was significantly lower, but with higher values for grazed plants than for the control (Harvest: F1 = 27.2, p < 0.01; Tsow: F1 = 62.2, p < 0.001; Treatment: F1 = 415, p < 0.001). By the 3rd harvest (Fig. 4A), there were no further significant effects of any of the treatment conditions on these larger, better-established plants.
|
| Fig. 3 The impact of the 1st simulated grazing event on dry mass of A) Saussurea glacialis and B) Plantago lessingii with harvest 1 being prior to and harvest 2 after simulated grazing. Values for shoots are displayed ascending from the intercept of the y axis. Values for roots are displayed descending from the intercept of the y axis. Different letters denote values that are significantly different. |
|
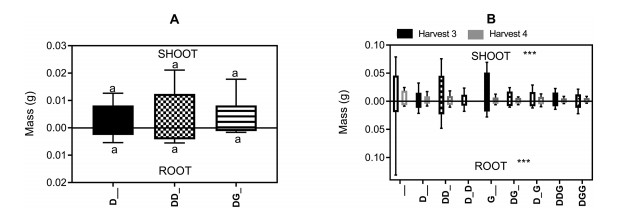
| Fig. 4 The impact of the factorial combination of simulated drought (D) and simulated grazing (G) and no drought or grazing (_) events on the dry mass of A) Saussurea glacialis and B) Plantago lessingii. Harvest 3 (black bars) was after the first set of events and harvest 4 (grey bars) after the second set of events. The order of 'D', 'G' and '_' represents the sequence in which the treatments were applied. Values for shoots are displayed ascending from the intercept of the y axis. Values for roots are displayed descending from the intercept of the y axis. Different letters denote values that are significantly different. |
In contrast, for Plantago lessingii, there was no significant difference in the parameters measured in the control plants (not grazed) between the 1st and 2nd harvest (Fig. 3B), with the exception of an increase in the number of leaves (Harvest: z = 2.74, p < 0.01). However, grazing significantly affected the seedlings, resulting in reduced log root length (Harvest: F1 = 89, p < 0.001; Treatment: F1 = 5.08, p < 0.05). Root mass and shoot mass appeared to also decline, but this difference was not statistically significant.
The 3rd and 4th harvests for Plantago lessingii each came after simulated drought and grazing treatments that had been followed by a recovery period. The block, harvest date and treatments affected multiple plant parameters (Fig. 4B). Both shoot and root mass declined between the 3rd and 4th harvest. They were greatest at the 3rd harvest for the control plants, those under severe drought and those that had been subjected to grazing but not drought (log shoot mass; Block: F3 = 5.26, p < 0.05; Block/Harvest: F4 = 30.6, p < 0.001; Block/Harvest/Treatment: F5 = 6.91, p < 0.01; log root mass: Harvest: F1 = 28.3, p < 0.001; Treatment: F8 = 2.22, p < 0.05). There was a marginally significant effect of harvest date and treatment on root length, with the longest roots after the first harvest with severe drought (Harvest: F1 = 3.17, p = 0.08; Treatment: F8 = 1.85, p < 0.07). The number of leaves per plant did not change significantly between harvests. The fewest leaves were on plants given the drought treatment only (D__: z = -2.26, p < 0.05).
3.3.4. No additive effects on survival and growthFor survival data we found no support for our third hypothesis. The age-dependent mortality effect described above was so strong that no significant additional additive effects were found. Neither were there any significant additive effects on biomass allocation. However, the low number of surviving S. glacialis seedlings reduced the strength of the analysis. For P. lessingii, for the seedlings in every treatment condition, both shoot and root mass declined between the 3rd and 4th harvests (see analysis above). However, biomass reduction was no more severe for plants that had been subjected to multiple drought or grazing events than for those that had only been subjected to a single drought or grazing event (Fig. 4B).
4. Discussion 4.1. Younger seedlings have higher mortality ratesAge-dependent impacts of simulated grazing and drought differed between the two species. Five days without water addition caused no mortality to Saussurea glacialis but resulted in the death of over 70% of Plantago lessingii seedlings. In contrast, over half of young P. lessingii seedlings were able to survive severe simulated grazing, whereas for S. glacialis, this resulted in the death of the majority of seedlings.
4.2. Mathematical model of age-dependent seedling mortalityOur model of seedling mortality and survival allowed us to address our 1st hypothesis in more detail. The highest rate of mortality was due to simulated grazing for Saussurea glacialis. But mortality rates due to simulated drought were high for both species. After all mortality factors had been applied, the surviving number of S. glacialis seedlings per cohort was below the replacement rate – potentially causing extinction. Fortunately, S. glacialis is a perennial species capable of reproducing over many years. Thus, over the course of its lifetime, one plant may be able to replace itself in the population. It is common for entire cohorts of seedlings to die due to grazing for several consecutive years, yet the overall plant population continues to persist (Crawley 1997). As an annual plant, P. lessingii has only one chance to exceed the replacement rate. Although our model showed an average 17.7 surviving offspring per parent P. lessingii plant, it should be remembered that under field conditions, plants would be exposed to additional stressors.
For both species, survival may be higher than we have calculated, simply because not all seedlings will be equally exposed to grazing. Specific microhabitats (e.g., emerging from a rocky outcrop (Milchunas and Noy-Meir, 2002) or beside a poisonous plant (Cheng et al., 2014)) may allow escape from grazing at the critical early stages of seedling development. Our field observations indicate that Saussurea glacialis may not be a favoured forage plant for livestock, and accordingly, its seedlings might experience fewer grazing events.
Surprisingly, P. lessingii, which has been recorded in arid desert steppes (Pei et al., 2008), showed high mortality following early water deficit. However, some Xinjiang desert plants that are highly drought-resistant as mature plants are unable to tolerate extended droughts as young seedlings. They must germinate and establish during a short period of snow melt and precipitation that provides favourable soil moisture conditions in early spring (Li et al., 2013). Seasonal microclimate patterns could help P. lessingii survive at our study sites. Although in winter and early spring, general soil moisture deficit is present, in P. lessingii's mid-elevation sites, soil is moister at 5 cm than at greater depths (Appendix B).
In contrast, S. glacialis seedlings are expected to experience the driest conditions when seedlings are youngest. Thus, its seedlings need to be water stress-tolerant from their first emergence onwards. While not succulents, they do have swollen leaves that would allow some water storage – a classic feature of adaptation to an arid environment (Grime, 1977). This water-stress tolerance makes future persistence of S. glacialis more likely. Despite its position on the snowline, it may be less affected than expected by the reductions in soil moisture content that are likely to result from retreating snowlines.
Likewise, it is a positive outcome that P. lessingii seedlings can survive early simulated grazing. Due to their lower position on the mountains, they are likely to be grazed earlier than S. glacialis. If herders noted a decline in S. glacialis populations, one adaptation may be to move their livestock only up to mid-level pastures in the early spring, delaying their migration to higher pastures. This could potentially increase the survival of S. glacialis seedlings.
4.3. Reduction or reallocation of biomass between roots and shoots 4.3.1. Response to grazingInitially, Plantago lessingii invested more in roots than did Saussurea glacialis. However, its roots were shorter after initial simulated grazing. Some resources may have been shifted away from roots to allow shoot recovery. By the third and fourth harvests, these better-established seedlings had shorter shoots after recovery from simulated grazing but showed no response in roots. This is intriguing, as we cut leaves at a fixed height, so as shoots grew longer, larger seedlings actually lost a progressively greater proportion of tissue.
For Saussurea glacialis, Shoot Mass Fraction values showed that the youngest seedlings reallocated biomass to shoots after simulated grazing. However, for larger, better-established plants, there was no significant treatment effect. It may be presumed that S. glacialis seedlings have low tolerance to grazing and that their resources were already too over-depleted by defoliation to have sufficient remaining resources to invest more biomass into roots (Bloom et al., 1985).
The small biomass of our final harvested specimens precluded testing whether plants might have invested more in chemical defence in response to simulated grazing to deter further herbivory (Herms and Mattson, 1992). It would be worthwhile to repeat our study with quadruple the sample size, so that even after plants suffered from high mortality rates, sufficient seedlings would survive to measure both growth and chemical parameters (e.g., silicon, cyanogenic and phenolic content (Richards and Fletcher, 2002; Huitu et al., 2014)).
4.3.2. Response to droughtFor Saussurea glacialis, simulated drought had no significant effect on any measured growth parameter. The swollen leaves of our seedlings probably retained higher moisture content than did their roots. Thus, maintaining shoot growth may be effective in retaining moisture. For established, mature S. glacialis, investment in broad tap roots may be more important. Functional plant attributes can differ between regenerative and established phases (Grime et al., 1997). To our knowledge, ours is the first study to examine the effects of water deficit on biomass allocation in Saussurea. Repeating this study with other species of Saussurea could determine if this genus uses water storage in leaves to achieve drought-tolerance.
For P. lessingii in general, simulated drought treatments (with no defoliation) produced plants with the fewest leaves, and the most severe drought condition led to shorter shoots. This appeared to be a mostly negative effect on growth (Casper, 1996), rather than reallocation of resources. By the end of the experiment, shoot and root mass had declined for all plants, possibly as reduction in daylight hours had begun to trigger senescence (Lang et al., 2019). The exception to this trend was that after the third harvest of P. lessingii under the most severe drought condition, P. lessingii seedlings had the longest roots of any plants in the study. Possibly only these largest plants had stored sufficient resources to permit downward growth to reach water in response to water deficit. Size-dependent drought tolerance could be of increasing importance for survival, as warming climate conditions can lead to disproportionally greater soil moisture at lower depths, giving an additional advantage to longer roots (Xu et al., 2015).
4.4. No additive effects on survival and growthWe found no evidence of additive negative effects of grazing and drought on survival or growth for either species. Plants were consistently more likely to survive simulated grazing or drought when they were older, despite having survived previous drought or grazing events. This makes persistence of our two focal species more likely. If reduced soil moisture conditions do not lead to progressively worse grazing impacts, there will be no need for radical changes in pasture use by pastoralists to avoid the use of pastures at times when soil moisture is lowest. Grazing should continue to be spread across all current pastures (Joshi et al., 2013; Ning et al., 2013) to avoid increasing grazing intensity in any given area.
However, all such applications should be treated with caution, as the study was conducted under greenhouse conditions outside the study region. Further in situ studies of these species are needed in order to determine the extent to which our experimental results can be applied to field conditions.
5. ConclusionsThe contrasting resilience to simulated grazing and drought of our study species has important implications for their survival. Even the youngest Saussurea glacialis seedlings are comparatively robust under dry conditions. If livestock do not select S. glacialis as a preferred forage plant, it may experience low rates of herbivory. However, the low seed viability and limited range of S. glacialis means that any other threat could easily wipe out local populations. In contrast, Plantago lessingii seedlings should survive the early grazing to which they are exposed in spring pastures. However, if spring soil moisture declines, there could be a high seedling death rate, threatening its persistence in its current range.
Author contributionsFW and SG designed the study. JX selected study sites in Taxkorgan and obtained access to the region. FW conducted the fieldwork and experiments. FW and SR analysed the data. FW wrote the manuscript with SG, SR and JX. All authors approved the final article.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgementsWe thank the Taxkorgan Nature Reserve for permission to work in the region and for field assistance from their staff. From the Kunming Institute of Botany's Centre for Mountain Ecosystem Studies; Wang Zheng Hong and Mu Zhi Lin assisted in seed collection and installing microclimate stations. Yang Tian Suo gave greenhouse assistance. Hu Xiao Jian assisted with seed storage and X-ray screening at the Kunming Institute of Botany's Germplasm Bank of Wild Species. Austin G. Smith commented on the final version of the manuscript. This work was supported by a Yunnan Provincial Human Resources and Social Security Bureau PostDoctoral Grant, Chinese Academy of Sciences President's International Fellowship Initiative grant [grant number 2020FYC0003], the National Sciences Foundation China [grant number 41661144001] and the Key Research Program of Frontier Sciences [grant number QYZDY-SSW-SMC014].
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.07.003.
Barton, K.E., 2008. Phenotypic plasticity in seedling defense strategies: compensatory growth and chemical induction. Oikos, 117: 917-925. DOI:10.1111/j.0030-1299.2008.16324.x |
Bloom, A.J., Chapin, I.F.S., Mooney, H.A., 1985. Resource limitation in plants - an economic analogy. Annu. Rev. Ecol. Systemat., 16: 363-392. DOI:10.1146/annurev.es.16.110185.002051 |
Casper, B.B., 1996. Demographic consequences of drought in the herbaceous perennial Cryptantha flava: effects of density, associations with shrubs, and plant size. Oecologia, 106: 144-152. DOI:10.1007/bf00328593 |
Cheng, W., Sun, G., Du, L. -F., et al., 2014. Unpalatable weed Stellera chamaejasme L. provides biotic refuge for neighboring species and conserves plant diversity in overgrazing alpine meadows on the Tibetan Plateau in China. J. Mt. Sci., 11: 746-754. DOI:10.1007/s11629–013–2729–y |
Comas, L., Becker, S., Cruz, V.M., et al., 2013. Root traits contributing to plant productivity under drought. Front. Plant Sci., 4: 442. DOI:10.3389/fpls.2013.00442 |
Cranston, L.M., Kenyon, P.R., Morris, et al., 2016. Morphological and physiological responses of plantain (Plantago lanceolata) and chicory (Cichorium intybus) to water stress and defoliation frequency. J. Agron. Crop Sci., 202: 13-24. DOI:10.1111/jac.12129 |
Crawley, M.J., 1997. Plant-herbivore dynamics. In: M.J. (Ed.), Plant Ecology, Crawley, second ed. Wiley-Blackwell.
|
Crawley, M.J., 2005. Statistics. An Introduction Using R. Wiley, Imperial College London, U.K.
|
Davis, S.C., Burkle, L.A., Cross, W.F., et al., 2014. The effects of timing of grazing on plant and arthropod communities in high-elevation grasslands. PloS One, 9: e110460. DOI:10.1371/journal.pone.0110460 |
Deleglise, C., Meisser, M., Mosimann, E., et al., 2015. Drought-induced shifts in plants traits, yields and nutritive value under realistic grazing and mowing managements in a mountain grassland. Agric. Ecosyst. Environ., 213: 94-104. DOI:10.1016/j.agee.2015.07.020 |
Elger, A., Lemoine, D.G., Fenner, M., et al., 2009. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos, 118: 767-773. DOI:10.1111/j.1600–0706.2009.17206.x |
Ferraro, D.O., Oesterheld, M., 2002. Effect of defoliation on grass growth. Oikos, 98: 125-133. DOI:10.1034/j.1600-0706.2002.980113.x |
Giménez-Benavides, L., Escudero, A., Iriondo, J.M., 2008. What shapes the altitudinal range of a high mountain Mediterranean plant? Recruitment probabilities from ovule to seedling stage. Ecography, 31: 731-740. DOI:10.1111/j.0906-7590.2008.05509.x |
Grime, J.P., 1977. Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat., 111: 1169-1194. DOI:10.1086/283244 |
Grime, J.P., Thompson, K., Hunt, R., et al., 1997. Integrated screening validates primary axes of specialisation in plants. Oikos, 79: 259-281. DOI:10.2307/3546011 |
Hanley, M.E., Fegan, E.L., 2007. Timing of cotyledon damage affects growth and flowering in mature plants. Plant Cell Environ., 30: 812-819. DOI:10.1111/j.1365–3040.2007.01671.x |
Hanley, M.E., Girling, R.D., Felix, A.E., et al., 2013. Olfactory selection of Plantago lanceolata by snails declines with seedling age. Ann. Bot., 112: 671-676. DOI:10.1093/aob/mct003 |
Herms, D.A., Mattson, W.J., 1992. The dilemma of plants - to grow or defend. Q. Rev. Biol., 67: 283-335. DOI:10.1086/417659 |
Huitu, O., Forbes, K.M., Helander, M., et al., 2014. Silicon, endophytes and secondary metabolites as grass defenses against mammalian herbivores. Front. Plant Sci., 5: 10.3389/fpls. 2014.00478. DOI:10.3389/fpls.2014.00478 |
Hunt, R., 1978. Plant Growth Analysis: Studies in Biology No. 96. London, UK.
|
Joshi, S., Shrestha, R.M., Ismail, M., et al., 2013. High-altitude Rangelands and Land Use Practices in the Karakoram-Pamir Landscape. International Centre for Integrated Mountain Development, Kathmandu, Nepal.
|
Kassam, K. -A., 2009. Viewing change through the prism of indigenous human ecology: findings from the Afghan and Tajik Pamirs. Hum. Ecol., 37: 677. DOI:10.1007/s10745–009–9284–8 |
Koerner, S.E., Collins, S.L., 2014. Interactive effects of grazing, drought, and fire on grassland plant communities in North America and South Africa. Ecology, 95: 98-109. DOI:10.1890/13–0526.1 |
Lang, W.G., Chen, X.Q., Qian, S.W., et al., 2019. A new process-based model for predicting autumn phenology: how is leaf senescence controlled by photoperiod and temperature coupling?. Agric. For. Meteorol., 268: 124-135. DOI:10.1016/j.agrformet.2019.01.006 |
Li, H., Li, X., Zhang, D., et al., 2013. Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (Fabaceae). EXCLI J., 12: 89-101. |
Long, R.J., 2003. Alpine rangeland ecosystems and their management in the Qinghai-Tibetan Plateau. In: Cai, L., Wiener, G., Han, J.L., et al. (Eds.), The Yak, 2nd Edition FAO Regional Office for Asia and the Pacific, Bangkok, Thailand.
|
Milchunas, D.G., Noy-Meir, I., 2002. Grazing refuges, external avoidance of herbivory and plant diversity. Oikos, 99: 113-130. DOI:10.1034/j.1600–0706.2002.990112.x |
Moles, A.T., Westoby, M., 2004. Seedling survival and seed size: a synthesis of the literature. J. Ecol., 93: 372-383. |
Moles, A.T., Westoby, M., 2004. What do seedlings die from and what are the implications for evolution of seed size?. Oikos, 106: 193-199. DOI:10.1111/j.0030-1299.2004.13101.x |
Ning, W., Rawat, G., Joshi, S., et al., 2013. High-Altitude Rangelands and Their Interfaces in the Hindu Kush Himalayas. ICIMOD, Kathmandu.
|
Orians, C.M., Thorn, A., Gómez, S., 2011. Herbivore-induced resource sequestration in plants: why bother?. Oecologia, 167: 1-9. DOI:10.1007/s00442–011–1968–2 |
Pei, S., Fu, H., Wan, C., 2008. Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agric. Ecosyst. Environ., 124: 33-39. DOI:10.1016/j.agee.2007.08.008 |
Qian, Y., Wu, Z., Zhang, L., et al., 2007. Spatial patterns of ephemeral plants in Gurbantünggüt Desert. Chin. Sci. Bull., 52: 3118-3127. DOI:10.1007/s11434–007–0465–9 |
R Core Team, 2017. R: A Language and Environment for Statistical Computing [Online]. Vienna, Austria: R foundation for Statistical Computing. Available: https://www.R-project.org/[Accessed 2017].
|
Rahim, I., Maselli, D., 2012. Herders' Manual for Western Pamir. University of Central Asia, Bishkek, Kyrgyz Republic.
|
Richards, A.J., Fletcher, A., 2002. The effects of altitude, aspect, grazing and time on the proportion of cyanogenics in neighbouring populations of Trifolium repens L. (white clover). Heredity, 88: 432-436. DOI:10.1038/sj.hdy.6800075 |
Salehi-Lisar, S.Y., Bakhshayeshan-Agdam, H., 2016. Drought stress in plants: causes, consequences, and tolerance. In: Hossain, M.A., Wani, S.H., Bhattacharjee, S., et al. (Eds.), Drought Stress Tolerance in Plants, Physiology and Biochemistry, vol. 1. Springer International Publishing, Cham, pp. 1-16.
|
Shibel, Z., Heard, S.B., 2016. Synergistic and additive effects of drought stress and simulated herbivory on two goldenrods, Solidago altissima and S. gigantea. Botany, 94: 635-642. DOI:10.1139/cjb–2016–0060 |
Shurupova, M.N., Zverev, A.A., 2017. Conservation categories and rarity types of Siberian Saussurea species. Int. J. Environ. Stud., 74: 724-731. DOI:10.1080/00207233.2017.1283937 |
Su, B., Huang, J., Fischer, T., et al., 2018. Drought losses in China might double between the 1.5 ℃ and 2.0 ℃ warming. Proc. Natl. Acad. Sci. U. S. A, 115: 10600-10605. DOI:10.1073/pnas.1802129115 |
Sun, J., Wang, X., Cheng, G., et al., 2014. Effects of grazing regimes on plant traits and soil nutrients in an alpine steppe, Northern Tibetan Plateau. PloS One, 9: e108821. DOI:10.1371/journal.pone.0108821 |
Varley, G.C., Gradwell, G.R., 1960. Key factors in population studies. J. Anim. Ecol., 29: 399-401. DOI:10.2307/2213 |
Warner, P.J., Cushman, H.J., 2002. Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia, 132: 77-85. DOI:10.1007/s00442–002–0955–z |
Xu, M., Peng, F., You, Q., et al., 2015. Year-round warming and autumnal clipping lead to downward transport of root biomass, carbon and total nitrogen in soil of an alpine meadow. Environ. Exp. Bot., 109: 54-62. DOI:10.1016/j.envexpbot.2014.07.012 |
Yu, F., Price, K., Ellis, J., et al., 2004. Interannual variations of the grassland boundaries bordering the eastern edges of the Gobi Desert in Central Asia. Int. J. Rem. Sens., 25: 327-346. DOI:10.1080/0143116031000084297 |
Zavaleta, E.S., Shaw, M.R., Chiariello, N.R., et al., 2003. Additive effects of simulated climate changes, elevated CO2, and nitrogen deposition on grassland diversity. Proc. Natl. Acad. Sci. U.S.A., 100: 7650-7654. DOI:10.1073/pnas.0932734100 |
Zhang, Z., Xu, J. -L., Liu, S. -Y., et al., 2016. Glacier changes since the early 1960s, eastern Pamir, China. J. Mt. Sci., 13: 276-291. DOI:10.1007/s11629-014-3172-4 |
Zhu, L., Johnson, D.A., Wang, W., et al., 2015. Grazing effects on carbon fluxes in a Northern China grassland. J. Arid Environ., 114: 41-48. DOI:10.1016/j.jaridenv.2014.11.004 |



