b. Geological Institute of Russian Academy of Sciences, Moscow, 119017, Russia;
c. Contract Affiliation: Kazan Federal University, Republic of Tatarstan, Russia;
d. School of Ecology, Sun Yat-sen University, Guangzhou 510275, China;
e. School of Geography, South China Normal University, Guangzhou 510631, China
The potential of using ferns as palaeoenvironmental proxies has been repeatedly discussed by various authors in broad palaeobotanical contexts (Meyen, 1987; Collinson, 2002; Deng, 2002; DiMichele and Phillips, 2002), and specifically applied to Paleozoic (Pant and Khare, 1974; Lele et al., 1981; Wagner and Lemos de Sousa, 1985; Cúneo, 1988; Barthel and Rössler, 1995; Yang et al., 1997; Césari et al., 1998; Cúneo et al., 2000; Naugolnykh, 2013, 2016), Mesozoic (Litwin, 1985; Serbet and Rothwell, 1999; Naugolnykh and Pronin, 2015), and Cenozoic (Budantsev, 1983; Collinson, 2001, 2002; Naugolnykh et al., 2016; Naugolnykh, 2017; Spicer et al., 2020) fossil materials. Ferns, as a plant group with a long and complicated evolutionary history, can be used as an important tool for reconstructing, in a general sense, environmental constraints from the middle Paleozoic up to the present time. Moreover, many fern families and genera have strict ecological and climatological preferences (Collinson, 2002; Kluge and Kessler, 2006), and so can be used as effective palaeoenvironmental proxies for tracing climatic changes.
Woodwardia Sm., established by Smith in 1793, is one of the most ancient examples of the "chain-ferns" within the family Blechnaceae. Woodwardioid ferns were previously treated as a single genus, Woodwardia, or divided into as many as four genera: Anchistea C. Presl, Lorinseria C. Presl, Woodwardia and Chieniopteris Ching (Cranfill and Kato, 2003). In the Pteridophyte Phylogeny Group I (PPG I) classification, the woodwardioid ferns are separated into three genera based on morphological, cytological and molecular phylogenetic studies: Anchistea, Lorinseria and Woodwardia (Gasper et al., 2016; 2017; PPG I, 2016). These three genera comprise the Subfamily Woodwardioideae of the family Blechnaceae (PPG I, 2016). Additionally, the genus Chieniopteris is not supported for a segregated genus because of a lack of molecular data and is included in the genus Woodwardia (Gasper et al., 2016; 2017; PPG I, 2016). The genera Anchistea and Lorinseria both contain only one species endemic to North America, while Woodwardia contains thirteen species with disjunct distributions across Central and North America, Europe and temperate to tropical areas in Asia (Cranfill and Kato, 2003; Gasper et al., 2016; 2017; PPG I, 2016).
Fossil records of the genus Woodwardia occur in the Paleogene and the Neogene of the Northern Hemisphere. Reliable fossil records of Woodwardia have been reported from as early as the Paleocene (Knowlton, 1919; Hickey, 1977; Collinson, 2001; Wang et al., 2006; Manchester, 2014), while Eocene records are known from North America and Asia (Knowlton, 1899, 1919; Endo, 1962; Collinson, 2001). By contrast Oligocene Woodwardia records are rare and have only been reported from Bohemia and Japan (Tanai, 1970; Kvaček et al., 2018), but Miocene and Pliocene finds come from North America, the intersection of eastern Europe and western Asia, and Asia (Knowlton, 1910, 1919; Smith, 1938; Li and Yang, 1984; Shatilova et al., 2011). The Woodwardia fossil records of the Northern Hemisphere show that the basis for this distribution was attained during the early Paleogene, and extended to high latitudes in the Paleocene to Eocene (Collinson, 2001).
The material newly collected and described here from the middle Eocene deposits of Hainan Island includes the fertile parts of a well-preserved blechnacean fern. It is relatively rare for a fossil fern to be described on the basis of an almost completely preserved fertile pinna, together with both the macromorphological and micromorphological structure of the sori, sporangia and spores preserved in situ. Such well-preserved morphological features allow us to clarify its taxonomical position, and attribute it to a new species within the genus Woodwardia. The present paper adds new data to the origin and evolutionary development of the ferns and discusses the climate of Hainan Island in the middle Eocene.
2. Material and methodsThe fossil pinna of Woodwardia described here was collected from the Changchang Formation of the Changchang Basin, which is located near Jiazi Town, within the administrative region of the city of Qiongshan, in northeastern Hainan Island, China (19°38′03″N and 110°27′04″E, Fig. 1). This locality is well known as a source of numerous well-preserved plant mega- and microfossils (Jin, 2009; Yao et al., 2009; He et al., 2010; Wang et al., 2014). The Changchang Formation is stratigraphically attributed to the middle Eocene (Lutetian–Bartonian, ~48–~38 Ma) by Spicer et al. (2014) based on previously published palaeobotanical (megafossils) and palynological data.
|
| Fig. 1 Geographical and stratigraphical position of the studied locality (after Yao et al., 2009 and Spicer et al., 2014). |
The material in hand includes a single, but well-preserved, representative fragment of a last order pinna. The material studied is preserved as a subcrustation (Krassilov and Makulbekov, 1996). This type of preservation is untypical for Eocene floras of Hainan and this is the first recorded occurrence of such preservation from this location. Subcrustation is a mode of preservation resulting from the precipitation secondary minerals (calcite, siderite, quartz and others) on cell walls normally inside the empty space of inner cavities of the epidermal cells.
A small fragment of the basal part of the pinna containing a well-preserved sorus was detached mechanically by means of a small knife and then macerated for a week in concentric nitric acid. The obtained macerated plant remains were washed, and after that the products of oxidation were removed using a strong solution of ammonia. The obtained sporangia, spores and isolated indusial tissues, and their fragments, were again washed with distilled water and then mounted on a mini-stub. Photomicrographs were taken using a digital camera (Photometries Cool Snap) and the scanning electron microscope (JSM-633) at the State Key Laboratory of Biocontrol and Guangdong Provincial Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou, China, and using the Vega Tescan MV 2300 scanning electron microscope at the Geological Institute of the Russian Academy of Sciences, Moscow. The two parts of the specimen are deposited at the Museum of Biology, Sun Yat-sen University under numbers CCJ 171A and CCJ 171B.
3. ResultsClass: Lycopodiopsida Bartling, 1830
Order: Polypodiales Link, 1833
Family: Blechnaceae Newman, 1844
Subfamily: Woodwardioideae Gasper, V.A.O. Dittrich et Salino, 2016.
Genus: Woodwardia Sm., 1793
Species: Woodwardia changchangensis Naugolnykh et Song, sp. nov. (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7)

|
| Fig. 2 Woodwardia changchangensis Naugolnykh et Song, sp. nov. A, general morphology of the holotype (CCJ 171A, part); B, interpretative line-tracing of the holotype; C, general morphology of the holotype (CCJ 171B, counterpart). Scales are 1 cm (A–C). |
|
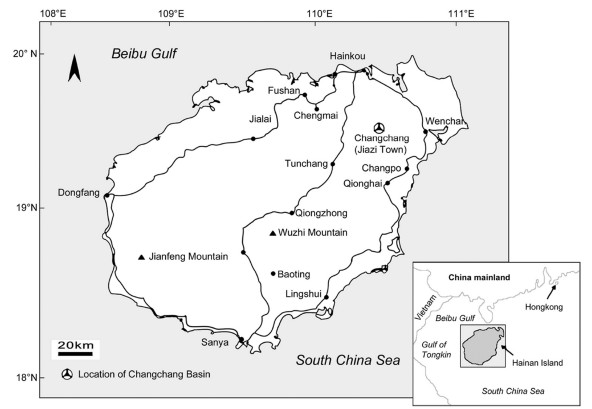
| Fig. 3 Woodwardia changchangensis Naugolnykh et Song, sp. nov. A, epidermal cells of the costal field of the pinnule; B, two neighbouring fertile pinnules showing the sori and venation patterns; C, epidermal cells and a stoma; D, a sorus covered by a relatively thick indusium. Scales are: 10 μm (A); 1 mm (B, D); 100 μm (C). |
|
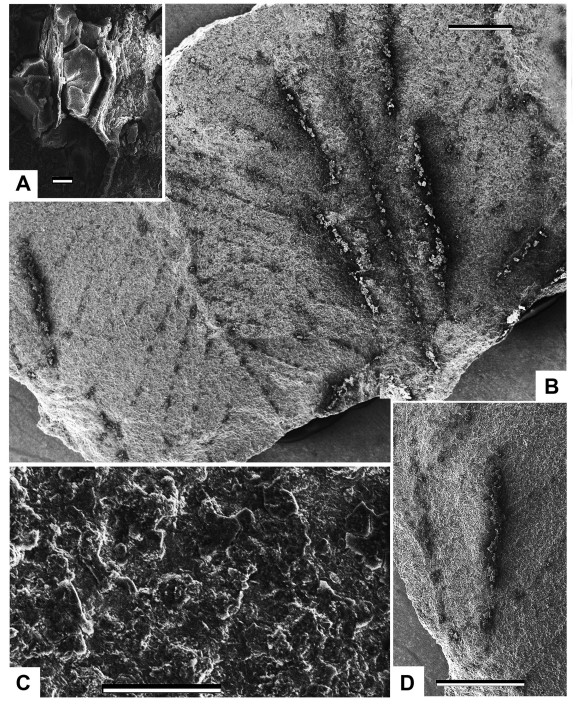
| Fig. 4 Line drawing reconstruction of Fig. 3. A, epidermal cells of the costal field of the pinnule; B, two neighbouring fertile pinnules showing the sori and venation patterns; C, epidermal cells and a stoma; D, a sorus covered by a relatively thick indusium. Scales are: 10 μm (A); 1 mm (B, D); 100 μm (C). |

|
| Fig. 5 Woodwardia changchangensis Naugolnykh et Song, sp. nov. A, an individual sporangium with the clearly visible annulus with stair-like thick-walled cells (left side of the sporangium); B, C, spores with wing-like folds on the surface; D, stalked opened sporangium (Sp – a spore; St – a stalk); E, sporangial stalk (=sporangiophore); F, group of three fragmented stalked sporangia. RSt – reconstructed stalk of the sporangium. Scales are: 50 μm (A, D, F); 10 μm (B); 20 μm (C, E). |
|
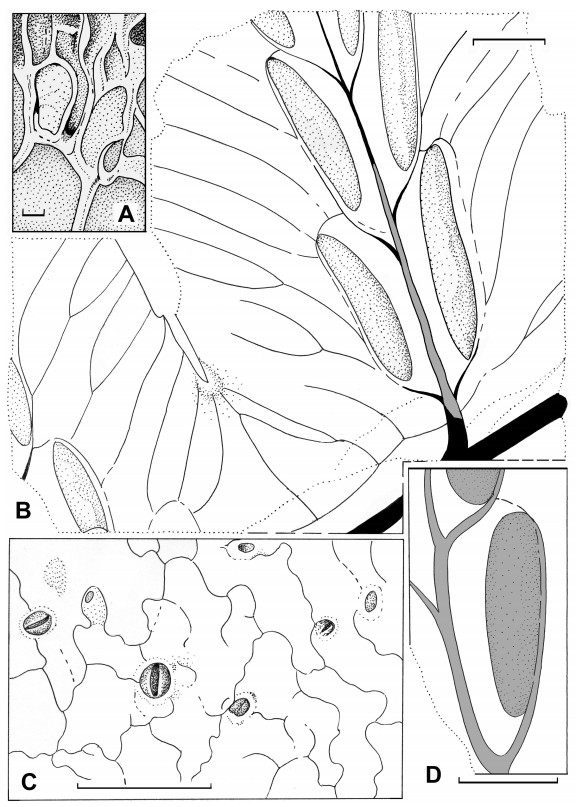
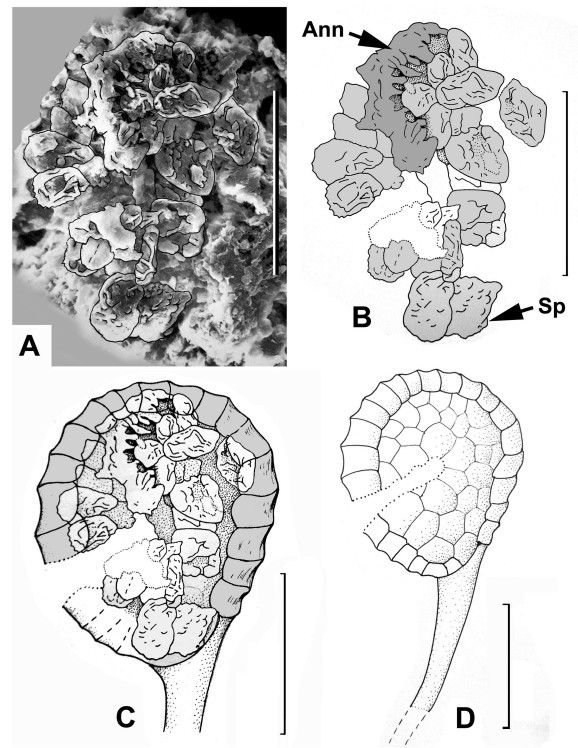
| Fig. 6 Woodwardia changchangensis Naugolnykh et Song, sp. nov. A, an aggregation of spores (spore mass) corresponding to the content of one individual sporangium with an interpretative line-tracing; B, same as A, with the fragmented preserved annulus (Ann) and spores (Sp); C, suggested position of the spore content inside a sporangium; D, general morphology of the sporangium based on Fig. 5A and D; 6A–C; 7C. Scales are: 50 μm (A–D). |

|
| Fig. 7 Reconstruction of the fertile pinnules of Woodwardia changchangensis Naugolnykh et Song, sp. nov. A, a portion of the fertile pinna; B, a sorus covered by the indusium; C, a spore; D, a stalked sporangium. Scales are: 1 cm (A); 500 μm (B); 10 μm (C); 50 μm (D). |
Etymology: The specific epithet "changchangensis" is named after Changchang Basin where the specimens were collected.
Holotype: Figured here (Fig. 2A and C); No. CCJ 171A (part) and CCJ 171B (counterpart).
Type stratum: Changchang Formation, middle Eocene.
Locality: Changchang Basin, Hainan Island, China.
Diagnosis: Pinna rachis straight. Pinnules alternate, subtriangular in shape. Pinnule apex acute, not round. Pinnules fused on the basal parts. Pinnule midvein thin, slightly undulating. Venation basically pinnate, but sometimes with elements of a net-pattern (anastomosing). Pinnule margins almost entire or slightly undulate. Sori long-ovoid in shape, orientated in two rows along midvein. Indusium present. Sporangia with annulus, stalked, round to ovoid in shape. Spores ellipsoidal with low wing-like folds on the surface.
Description: The length of the pinna is more than 70 mm and the width of the pinna in its basal part is about 30 mm (Fig. 2A–C). The pinna rachis is straight and thick (Fig. 2A–C). The width of the pinna rachis in its widest part in the base of the pinna is approximately 0.9 mm (Fig. 2A–C). The pinnules are attached to the pinna rachis in alternate order (Fig. 2A–C). The angle of the pinnule attachment is about 65° (Fig. 2B). The pinnules are of subtriangular shape, slightly curved in apical areas towards the pinna apex (Fig. 2B). The length of the most well-developed pinnule disposed in the basal part of the pinna is 15 mm. The width of the largest pinnule base is 5.5 mm (Fig. 2B). The size of the pinnules slowly decreases towards the pinna top (Fig. 2C). The pinnules are fused by their basal parts forming a wide wing along the pinna rachis (Fig. 2B). The pinnule margin is smooth in the proximal parts of the pinnules, but slightly undulate in their distal parts (Fig. 2B). The pinnule apex is obtuse to slightly acute (Fig. 2A and B). The venation pattern of the pinnules is basically pinnate, but with the net-like elements in the basal areas of the pinnules (Fig. 2B). The midvein is thin, but somewhat thicker than the lateral (secondary) veins (Fig. 2B). The midvein is slightly undulated (Fig. 2B). The lateral veins form the wide chain-like pattern (areoles) along the midvein and are forked, extending to the margin (Fig. 2B). Each fertile pinnule includes two rows of elongated sori disposed along the midvein (Fig. 2A–C). The sori are of long-ovoid shape and are orientated parallel, or slightly oblique, to the pinnule midvein (Fig. 2A and B). The distance between the distal part of the obliquely disposed sorus and the midvein is more than the distance of the proximal part of the same sorus and the midvein (Fig. 2B). As a rule, but not always, the larger sori are disposed in the proximal part of the fertile pinnule, and the smaller sori are disposed in the distal part of the pinnule (Fig. 2B). The length of the largest sorus is 2 mm, while its width is about 0.7 mm. The number of sori on one fertile pinnule varies from ten to fifteen (Fig. 2A and B). Some of the long sori are disposed along the pinna rachis in the basal part of the pinna (Fig. 2A and B). Each sorus is covered by a relatively thick indusium (Fig. 3, Fig. 4D). The indusium is thick and seems to have a concave shape with a shallow prolonged depression in its medial area (Fig. 3, Fig. 4D).
SEM imagery revealed epidermal-cuticular features of the studied pinnules. The cell wall pattern is preserved due to the subcrustation mode of preservation (see above). Some epidermal cells of the costal field of the pinnule are mineral filled (Fig. 3, Fig. 4A). The epidermal cells have undulating or waved centriclinal (= radial) walls (Fig. 3, Fig. 4C). The average size of the common epidermal cells is about 25 × 40 to 40 × 60 μm (Fig. 3, Fig. 4C). The periclinal cell walls are smooth (Fig. 3, Fig. 4C). The stomata are round, with a slit-like guard cell aperture (Fig. 3, Fig. 4C). The diameter of the stoma is about 20 μm including the guard cells (Fig. 3, Fig. 4C).
Several stalked sporangia and spores were obtained by maceration (Fig. 5A–F). A group of three stalked and opened sporangia were also found (Fig. 5F). Numerous spores extracted from the sporangia are visible located near the sporangia (Fig. 5B and C).
The sporangium is ovoid, with a clearly visible stair-like structure (on the left in Fig. 5A) formed by the thick-walled cells that form the annulus that opens the sporangium when it is mature and ready to release the spores. An already opened sporangium with almost all spores released is shown on Fig. 5D. One spore is still preserved nearby the distal end of the stalk of the sporangium (Fig. 5D). The stalk of the sporangium (= sporangiophore) has an irregular fine sculpture on the surface (Fig. 5E).
The spores are ellipsoidal bearing characteristic wing-like folds on the surface (Fig. 5B and C). The spores measure about 11 × 18 μm on average. The same structural peculiarities are characteristic of extant Woodwardia spores (Tryon and Lugardon, 1991).
A compact aggregation of the spores preserved together was also found by maceration (Fig. 6A and B). It is obvious that this spore aggregation is the content of one individual sporangium. Furthermore, just above the spore mass there is one fragment of the series of thick-walled cells that form the annulus (Fig. 6A and B). Based on the size and shape of the spore mass/spore aggregation, which matches the size and shape of the extracted sporangia described above, we offer a reconstruction of the stalked sporangium (Fig. 6C and D). Reconstructions of all the organs illustrated above are shown in Fig. 7.
4. Discussion 4.1. Comparison with extant and fossil speciesThe four genera of the Blechnaceae, Anchistea, Lorinseria, Woodwardia, belonging to the subfamily Woodwardioideae and Doodia R. Br. of the subfamily Blechnoideae (Gasper et al., 2016), as in the present fossil, have so-called chain-like sori. This means the sori follow the areolar veins, interrupted, forming a continuous row (Gasper et al., 2016). The present fossil can be distinguished from Doodia in several ways. For example, species of Doodia have deeply pinnatifid to pinnate blades, serrate-dentate pinnae margins, and veins with one or three series of areoles (Gasper et al., 2016). Lorinseria includes only one species, L. areolate (L.) C. Presl, and one of its characteristics is its deeply pinnatifid sterile blades, which are markedly different from the present fossil (Gasper et al., 2016). Anchistea also contains only one species, Anchistea virginica (L.) C. Presl, and has pinnae generally similar to those of the current fossil, but there are key differences in detail. The veins of A. virginica are anastomosing, forming single rows of areoles along costae and costules (Gasper et al., 2016), whereas the veins of the present fossil form a series of areoles along the costae and possess net-type elements in the basal areas of the pinnules (Fig. 2B). In addition, A. virginica has pinnules with clearly obtuse apices (Fig. 8A), a feature obviously different from the present fossil.

|
| Fig. 8 Morphological diversity of modern fertile pinnae found in the genera Woodwardia Sm. and Anchistea C. Presl. A, Anchistea virginica (L.) C. Presl (=Woodwardia virginica (L.) J. E. Smith), fertile pinnule with sori, the Herbarium at Kunming Institute of Botany (KUN), No. 1221957; B, W. harlandii Hook., the Herbarium of South China Botanical Garden (IBSC), No. 0680309; C, W. kempii Copel. (=W. hetroplanta B.S. Wang), the Herbarium of Sun Yat-sen University, No. 172799; D, Isotype of W. fimbriata Sm., Natural History Museum (BM), Barcode: BM000769837, downloaded and modified from Global Plants Database; E, Isotype of W. martinezii Maxon ex Weath, University of Wisconsin Herbarium (WIS), Barcode: WISv0253435WIS, downloaded and modified from Global Plants Database; F, W. unigemmata (Makino) Nakai, TAIF herbarium of Taiwan Forestry Research Institute No.467444, downloaded and modified from: https://taifdb.tfri.gov.tw/search/specimen.php?SpcmID=356491&DupID=1&SeriID=1&DetID=490637&l=Cht; G, Unspecified W. prolifera Hook. et Arn., Royal Botanic Garden Edinburgh (E), Barcode: E00280161, downloaded and modified from Global Plants Database; H, Holotype of W. auriculata Bl., Naturalis Biodiversity Centre, formerly Leiden University (L), Barcode: L0052402, downloaded and modified from Global Plants Database; I, Holotype of W. magnifica Ching et P. S. Chiu, PE herbarium, Catalogue number: 00044780, downloaded and modified from: http://petype.myspecies.info/taxonomy/term/873; J, W. orientalis Sw., the Herbarium of Sun Yat-sen University, No. SYS 00154035; K, Lectotype of W. radicans (L.) Sm., Linnean Society of London Herbarium (LINN), Barcode: LINN-HL1247-4, downloaded and modified from Global Plants Database; L, Holotype of W. semicordata Mickel et Beitel, The William and Lynda Steere Herbarium of the New York Botanical Garden (NYBG), Barcode: NY00179355, downloaded and modified from Global Plants Database; M, Lectotype of W. spinulosa M. Martens et Galeotti, the Herbarium of the Jardim Botânico do Rio de Janeiro (RB), Barcode: RB00603390, modified from Palacios-Rios and Arana (2018); N, Isotype of W. japonica (L.f.) Sm., Barcode: K001092752, downloaded and modified from: http://specimens.kew.org/herbarium/K001092752. Scales are: 1 cm. |
The pinnae of extant Woodwardia are characterised by veins that anastomose to form a series of areoles along costae and costules, ultimate veins that are free, and sori that are long-linear, sunken, usually confined to costular areoles, and arranged on each side of the costae and costules (Gasper et al., 2016). The general pinna shape of W. changchangensis is obviously distinct from Woodwardia harlandii Hook. and Woodwardia kempii Copel. W. harlandii is characterised by an elongated pinna almost without basal lobes (Fig. 8B) and W. kempii is characterised by an exceptionally well-developed limb (a near-rachis wing) connecting neighboring widely-spaced pinnules (Fig. 8C). The present fossil is different from Woodwardia fimbriata Sm. in that the pinnule shape of W. fimbriata is narrowly deltate to lanceolate, and pinnatifid (Fig. 8D). Woodwardia martinezii Maxon ex Weath. has pinnules that are triangular in shape and bear long linear sori that occur alongside costae and costules (Fig. 8E), which is obviously different from the present fossil. Woodwardia unigemmata (Makino) Nakai, Woodwardia prolifera Hook. et Arn. have oblique, oblong-lanceolate pinnules with acuminate apices and crescent-shaped or elliptical sori (Fig. 8F and G) and Woodwardia auriculata Bl. has oblique lanceolate pinnules and elliptical sori (Fig. 8H). The pinnules of the present fossil are subtriangular with long-ovoid sori, and clearly distinct from the above three extant species. The present W. changchangensis also differs from Woodwardia magnifica Ching et P. S. Chiu, which has lanceolate or narrowly triangular-lanceolate pinnules and linear sori (Fig. 8I). Compared with the present fossil, Woodwardia orientalis Sw., Woodwardia radicans (L.) Sm., Woodwardia semicordata Mickel et Beitel and Woodwardia spinulosa M. Martens et Galeotti have more acute apices and finely-toothed margins (Fig. 8J–M). Moreover, the venation of these four species is more complicated than in our fossil, forming two or three rows of areoles along costules (Fig. 8J–M).
Among the thirteen extant species in Woodwardia, the present fossil pinna is similar to that of Woodwardia japonica (L.f.) Sm. in having subtriangular pinnules with long-ovoid sori and anastomosing veins that are distally free, simple or forked (Fig. 8N). However, the newly established W. changchangensis sp. nov. is different from W. japonica because W. japonica normally possesses well-pronounced terminal teeth on each pinnule (Fig. 8N).
We also compared our material with fossil species of Woodwardia. Woodwardia kamtschatica Budantsev is an early Cenozoic species from the Paleogene deposits of Kamchatka, northern-eastern Russia (Budantsev, 1983, 2006). The new species described here is different from W. kamtschatica as W. kamtschatica has well-formed reticulate (anastomosing) venation (Budantsev, 2006, Plate 70, Fig. 3), which is observed for W. changchangensis only occasionally, and a large distance between well-developed neighboring pinnules (Budantsev, 2006, Plate 70, Fig. 7), which is also characteristic of the recent species W. kempii (=Woodwardia hetroplanta B.S. Wang; see here Fig. 8C).
Four Eocene species of Woodwardia are also compared. Woodwardia areolate Ôishi et Huzioka from the Eocene of the Ishikari coal basin in Hokkaido, Japan, is characterised by a deltoid to oblong basal lobe on each basal pinnule and linear to oblong sori slightly oblique to the midvein (Ôishi and Huzioka, 1941). These characters are absent in the present fossil. In addition, the sori of W. areolate are smaller and are fewer in number on each pinnule compared with the present fossil. Woodwardia decurrens Ôishi et Huzioka, from the same site as W. areolate, is characterised by an acuminate, slightly falcate, apex and basal pinnules that are strongly decurrent below (Ôishi and Huzioka, 1941). These characters are not evident in the present fossil. Subsequently, these two species were assigned to W. japonica Sw. var. eocenica, because of their resemblance to the extant W. japonica (Endo, 1962). Except for the difference in the shape of the pinnules mentioned above, the macroscopic morphology of the present fossil is very similar to them. Another two species are from North America—Woodwardia preareolata Knowlton from the Eocene of Yellowstone National Park, Wyoming (Knowlton, 1899) and Woodwardia maxoni Knowlton from the Eocene of Rock Springs, Wyoming (Knowlton, 1910). The former is characterised by having a broad wing on the rachis (Knowlton, 1899) and the latter is characterised by having the pinnule apices finely serrate (Knowlton, 1910). The present fossil is distinguished from these two American species by possessing more closely arranged pinnules that do not form such a broad wing, and its pinnule margins are almost entire or slightly undulate.
Some other fossil species of Woodwardia, such as Woodwardia arctica (Heer) R.W. Brown, Woodwardia crenata Knowlton and Woodwardia gravida Hickey are difficult to compare with our newly collected material because they lack some essential features for comparison, such as venation and soral details and sporangial anatomy.
4.2. Palaeogeography and palaeoclimate implicationsAmong the Cenozoic ferns, Woodwardia is one of the special genera that shows similar distributions to those of their living relatives (Collinson, 2001). The recent representatives of Woodwardia have a disjunct distribution across Central and North America, Europe and the temperate to tropical areas of Asia, and their fossil records also appear in these areas (Fig. 9). Most Paleocene and Eocene Woodwardia fossils have been recorded in North America (W. arctica (Heer) R.W. Brown from the Paleocene of Wyoming and North Dakota, USA (Manchester, 2014); W. gravida Hickey from the Paleocene of western North Dakota, USA (Hickey, 1977); Woodwardia latiloba Lesquereux from the Paleocene of Colorado, USA (Knowlton, 1919); W. crenata Knowlton from the Eocene of Wyoming and Colorado, USA (Knowlton, 1919); W. preareolataKnowlton and W. maxoniKnowlton from the Eocene of Wyoming, USA (Knowlton, 1899, 1910). In addition, W. areolate L. from the late Paleocene of western North Dakota, USA (Pigg et al., 2006) should be assigned to Lorinseria in the PPG I classification. A small number of Woodwardia fossils are recorded in eastern Asia (Woodwardia bureiensis Fedotov from the Paleocene of Heilongjiang Province, China (Wang et al., 2006); W. kamtschatica Budantsev from the Paleocene of Kamchatka, Russia (Budantsev, 2006); W. arctica (Heer) R.W. Brown from the Paleocene/Eocene of Zeya-Bureya depression of Russia and the Eocene of Japan (Collinson, 2001); W. japonica Sw. var. eocenica from the Eocene of Hokkaidao, Japan (Endo, 1962) and W. changchangensis Naugolnykh et Song from the Eocene of Hainan Island, South China (this study)).

|
| Fig. 9 Distribution of the extant species of the genus Woodwardia based on data from Global Biodiversity Information Facility (GBIF.org (9 August 2021) GBIF Occurrence Download https://doi.org/10.15468/dl.hk6phj) and fossil records of the genus Woodwardia. |
Only two reliable Oligocene Woodwardia records have been reported—Woodwardia sasae Oishi et Huzioka from the Harutori flora in Hokkaidao, Japan (Ôishi and Huzioka, 1942; Tanai, 1970) and Woodwardia muensteriana (C. Presl in Sternberg) Kräusel from the Oligocene flora of North Bohemia (Kvaček et al., 2018).
Miocene and Pliocene Woodwardia fossil records are distributed throughout North America (Woodwardia deflexipinna H.V. Smith from the Miocene of southeastern Oregon, USA (Smith, 1938); Woodwardia florissantia Cockerell from the Miocene of Colorado, USA (Knowlton, 1919) and Woodwardia columbiana Knowlton from the Pliocene of Oregon, USA (Knowlton, 1910)). Additionally, in the PPG I classification, W. areolate L. from the Miocene of western Washington, USA (Pigg et al., 2006) should be assigned to Lorinseria and Woodwardia virginica (L.) J.E. Smith from the Miocene of central Washington, USA (Pigg and Rothwell, 2001) should be assigned to Anchistea. Miocene and Pliocene Woodwardia fossils have also been found in the intersection of eastern Europe and western Asia (Woodwardia roessneriana (Ung.) Heer from the Miocene of Georgia (Shatilova et al., 2011); W. radicans (L.) Smith. and W. roessneriana (Ung.) Heer from the Pliocene of Georgia (Shatilova et al., 2011)), and Asia (W. sp. from the Miocene of Jilin Province, China (Li and Yang, 1984)). The full fossil records are listed in Table 1.
| Species | Locality | Age | Reference |
| Woodwardia arctica (Heer) R.W. Brown | Wyoming, USA | Paleocene | Manchester (2014) |
| North Dakota, USA | Paleocene | Manchester (2014) | |
| Zeya-Bureya depression, Russia | Paleocene/Eocene | Collinson (2001) | |
| W. bureiensis Fedotov | Heilongjiang Province, China | Paleocene | Wang et al. (2006) |
| W. gravida Hickey | Western North Dakota, USA | Paleocene | Hickey (1977) |
| W. kamtschatica Budantsev | Kamchatka, Russia | Paleocene | Budantsev (2006) |
| W. latiloba Lesquereux | Colorado, USA | Paleocene | Knowlton (1919) |
| W. arctica (Heer) R.W. Brown | Japan | Eocene | Collinson (2001) |
| W. crenata Knowlton | Wyoming, USA | Eocene | Knowlton (1919) |
| Colorado, USA | Eocene | Knowlton (1919) | |
| W. japonica Sw. var. eocenica | Hokkaidao, Japan | Eocene | Endo (1962) |
| W. changchangensis Naugolnykh et Song | Hainan Island, China | Eocene | This study |
| W. maxoni Knowlton | Wyoming, USA | Eocene | Knowlton (1910) |
| W. preareolata Knowlton | Wyoming, USA | Eocene | Knowlton (1899) |
| W. muensteriana (C. Presl in Sternberg) Kräusel | České středohoří Mts., North Bohemia | Oligocene | Kvaček et al. (2018) |
| W. sasae Ôishi et Huzioka | Southeastern Hokkaido, Japan | Oligocene | Tanai (1970) |
| W. deflexipinna H.V. Smith | Southeastern Oregon, USA | Miocene | Smith (1938) |
| W. florissantia Cockerell | Colorado, USA | Miocene | Knowlton (1919) |
| W. roessneriana (Ung.) Heer | Georgia | Miocene | Shatilova et al. (2011) |
| W. sp. | Jilin Province, China | Miocene | Li and Yang (1984) |
| W. columbiana Knowlton | Cascades of Columbia, Oregon, USA | Pliocene | Knowlton (1910) |
| W. radicans (L.) Smith | Georgia | Pliocene | Shatilova et al. (2011) |
| W. roessneriana (Ung.) Heer | Georgia | Pliocene | Shatilova et al. (2011) |
Based on fossil evidence, Kvaček (1994) hypothesized that the ancestors of Woodwardia first arose in North America and migrated to eastern Asia via Beringia during the Eocene, finally arriving in Europe at the beginning of the early Miocene warming. According to the phylogeny of Woodwardia estimated using rbcL and rps4 sequences and divergence time estimations based on fossil records, Li et al. (2016) proposed that Woodwardia species diverged initially in the Paleogene of North America, then underwent a greater diversification and expansion in Eurasia during the Eocene before finally arriving in Europe directly from Asia during the Miocene. However, the discovery of W. muensteriana from the Oligocene in North Bohemia (Kvaček et al., 2018) indicates that Woodwardia species arrived in Europe before the Miocene. That means the previous hypotheses need to be further tested and more fossil records are needed to determine the migration and evolution of Woodwardia species. In addition, the discovery of the present fossil indicates that Woodwardia species were present in low-latitude tropical areas of South China as early as the middle Eocene, which has never before been reported.
Many authors have also noted that some Cenozoic ferns are very similar to the present-day representatives of this group, and could be assigned directly to the same genera, or even species (Pigg and Rothwell, 2001; Conran et al., 2010; Homes et al., 2015). There are also a few Cenozoic ferns that cannot be assigned to extant genera, for example, Makotopteris princetonensis Stockey, H. Nishida et Rothwell from the middle Eocene of Canada (Stockey et al., 1999). In the case of W. changchangensis we compared our specimen not only to other fossil forms, but to some of the living species that share some important features with W. changchangensis. As a result, we found that the present fossil most closely resembles W. japonica, as mentioned above. Because of the morphological similarities, it is reasonable to believe that the growth environment of W. changchangensis was similar to that of extant W. japonica, which is found on ridges, exposed slopes, and shaded forests, and is largely restricted to warm or moist locations (Wang et al., 2013; Xu and Deng, 2017). Together with the new observations on our fossil fern, and the previous study of the thelypteridacean fern Cyclosorus scutum Naugolnykh, Wang, Han et Jin recovered from the same locality (Naugolnykh et al., 2016), the climate of the Changchang Basin during the middle Eocene is interpreted to have been warm and humid, more or less similar to the climatic conditions of this region today.
Author contributionsJHJ, XYL and SVN designed the research. HZS, JHJ and XKW collected fossil and modern specimens. SVN and HZS prepared and studied the sporangia and spores with SEM. HZS, SVN and JHJ analyzed and interpreted the results. All authors contributed feedback on drafts, revised and approved the manuscript.
Declaration of competing interestThere is no conflict of interest.
AcknowledgementsThis study was supported by the National Natural Science Foundation of China (Grant Nos. 41872015, 42111530024, 41820104002, 41661134049 to JHJ and XYL), a grant from the UK Natural Environment Research Council (Grant No. NE/P013805/1 to XYL), the Fundamental Research Funds for the Central Universities (Grant No. 2021qntd18 to XKW), the Scientific Research Fund, Hongda Zhang, Sun Yat-sen University, and a subsidiary of the Russian Government that supports a Program of Competitive Growth of Kazan Federal University among World's Leading Academic Centers and the State Program of Geological Institute of RAS (Grant No. 0135-2019-0044 to SVN). We would like to express our sincere gratitude to Prof. Robert A. Spicer (The Open University, UK) for linguistic improvement of the manuscript.
Barthel, M., Rössler, R., 1995. Rotliegend Farne in weisen Vulkan-Aschen "Tonsteine" der Dohlen formation als palaeobotanische Fundschichten. Veroff. Mus. Naturk. Chemnitz, 18: 5-24. |
Bartling, F.G., 1830. Ordines Naturales Plantarum Eorumque Characteres Et Affinitates Adjecta generum enumeratione. Sumptibus Dieterichianis, Gottingae.
|
Budantsev, L.Y., 1983. History of Arctic Flora of the Early Kainophyte Epoch.
Komarov Botanical Institute of Russian Academy of Sciences, Leningrad.
|
Budantsev, L.Y., 2006. Early Paleogene Flora of Western Kamchatka. Nauka Publishing Office, Sant-Petersburg.
|
Césari, S.N., Cúneo, N.R., Archangelsky, S., 1998. Una nueva probable gleicheniácea del Pérmico inferior de Patagonia, Argentina. Rev. Esp. Paléontol., 13: 81-92. |
Collinson, M.E., 2001. Cainozoic ferns and their distribution. Brittonia, 53: 173-235. DOI:10.1007/BF02812700 |
Collinson, M.E., 2002. The ecology of Cainozoic ferns. Rev. Palaeobot. Palynol., 119: 51-68. DOI:10.1016/S0034-6667(01)00129-4 |
Conran, J.G., Kaulfuss, U., Bannister, J.M., et al., 2010. Davallia (Polypodiales: Davalliaceae) macrofossils from early Miocene Otago (New Zealand) with in situ spores. Rev. Palaeobot. Palynol., 162: 84-94. DOI:10.1016/j.revpalbo.2010.06.001 |
Cranfill, R., Kato, M., 2003. Phylogenetics, biogeography, and classification of the
woodwardioid ferns (Blechnaceae). In: Chandra, S., Srivastava, M. (Eds. ),
Pteridology in the New Millennium. Springer Netherlands, Dordrecht,
pp. 25-48.
|
Cúneo, N.R., 1988. Hallazgo de una filice leptosporangida en el Lubeckiano de Chubut, Argentina. Bol. Asoc. Latinoam. Paleobot. Palinol., 11: 12-19. |
Cúneo, N.R., Archangelsky, S., Césari, S.N., 2000. Asterotheca frenguellií (Archangelsky y de la Sota) nov. comb., helecho pérmico de Patagonia, Argentina. Ameghiniana, 37: 363-367. |
Deng, S., 2002. Ecology of the early cretaceous ferns of Northeast China. Rev. Palaeobot. Palynol., 119: 93-112. DOI:10.1016/S0034-6667(01)00131-2 |
DiMichele, W.A., Phillips, T.L., 2002. The ecology of Paleozoic ferns. Rev. Palaeobot. Palynol., 119: 143-159. DOI:10.1016/S0034-6667(01)00134-8 |
Endo, S., 1962. On the Eocene plants from the Woodwardia formation of the Ishikari group. Trans. Proc. Paleontol. Soc. Japan. New Ser., 45: 206-208. |
Gasper, A.L., Almeida, T.E., Dittrich, V.A.O., et al., 2017. Molecular phylogeny of the fern family Blechnaceae (Polypodiales) with a revised genus-level treatment. Cladistics, 33: 429-446. DOI:10.1111/cla.12173 |
Gasper, A.L., Dittrich, V.A.O., Smith, A.R., et al., 2016. A classification for Blechnaceae (Polypodiales: polypodiopsida): new genera, resurrected names, and combinations. Phytotaxa, 275: 191-227. DOI:10.11646/phytotaxa.275.3.1 |
He, X.Y., Shen, R.J., Jin, J.H., 2010. A new species of Nelumbo from South China and its palaeoecological implications. Rev. Palaeobot. Palynol., 162: 159-167. DOI:10.1016/j.revpalbo.2010.05.006 |
Hickey, L.J., 1977. Stratigraphy and Paleobotany of the Golden Valley Formation
(Early Tertiary) of Western North Dakota. Geological Society of America,
Colorado.
|
Homes, A.M., Cieraad, E., Lee, D.E., et al., 2015. A diverse fern flora including macrofossils with in situ spores from the late Eocene of southern New Zealand. Rev. Palaeobot. Palynol., 220: 16-28. DOI:10.1016/j.revpalbo.2015.04.007 |
Jin, J.H., 2009. Two Eocene fossil fruits from the Changchang Basin of Hainan Island, China. Rev. Palaeobot. Palynol., 153: 150-152. DOI:10.1016/j.revpalbo.2008.07.010 |
Kluge, J., Kessler, M., 2006. Fern endemism and its correlates: contribution from an elevational transect in Costa Rica. Divers. Distrib., 12: 535-545. DOI:10.1111/j.1366-9516.2006.00231.x |
Knowlton, F.H., 1899. Fossil Flora of the Yellowstone National Park. Government
Printing Office, Washington.
|
Knowlton, F.H., 1910. Descriptions of fossil plants from the mesozoic and cenozoic of North America. Smithsonian Misc. Collect., 52: 489-496. |
Knowlton, F.H., 1919. A Catalogue of the Mesozoic and Cenozoic Plants of North
America. Government Printing Office, Washington.
|
Krassilov, V.A., Makulbekov, N.M., 1996. Plant subcrustations. Paleontol. J., 2: 125-128. |
Kvaček, Z., 1994. Connecting links between the arctic palaeogene and European
tertiary floras. In: Boulter, M.C., Fisher, H.C. (Eds. ), Cenozoic Plants and Climates
of the Arctic. Springer-Verlag Berlin Heidelberg, New York, pp. 251-266.
|
Kvaček, Z., Teodoridis, V., Radoň, M., 2018. Review of the late Oligocene flora of Matrý near Sebuzín (ceské středohoří mts., the Czech republic). Foss. Impr., 74: 292-316. DOI:10.2478/if-2018-0018 |
Lele, K.M., Maithy, P.K., Mandal, J., 1981. In situ spores from Lower Gondwana ferns-their morphology and variation. Palaeobotanist, 28–29: 128-154. DOI:10.54991/jop.1981.1408 |
Li, C.X., Lu, S.G., Ma, J.Y., et al., 2016. Phylogeographic history of the woodwardioid ferns, including species from the Himalayas. Palaeoworld, 25: 318-324. DOI:10.1016/j.palwor.2014.10.004 |
Li, H.M., Yang, G.Y., 1984. Miocene qiuligou flora in Dunhua county, Jilin Province. Acta Palaeontol. Sin., 23: 204-213. |
Link, H.F., 1833. Hortus Regius Botanicus Berolinensis descriptus. Apud G. Reimer,
Berolini.
|
Litwin, R.J., 1985. Fertile organs and in situ spores of ferns from the late Triassic Chinle Formation of Arizona and New Mexico, with discussion of the associated dispersed spores. Rev. Palaeobot. Palynol., 44: 101-146. DOI:10.1016/0034-6667(85)90030-2 |
Manchester, S.R., 2014. Revisions to roland Brown's North American Paleocene flora. Acta Mus. Natl. Pragae Ser. B Hist. Nat., 70: 153-210. |
Meyen, S.V., 1987. Fundamentals of Palaeobotany. Chapman & Hall, London.
|
Naugolnykh, S.V., 2013. Permian ferns of western Angaraland. Paleontol. J., 47: 1379-1462. DOI:10.1134/S0031030113120010 |
Naugolnykh, S.V., 2016. Palaeobotrychium gen. nov., the first discovery of an ophioglossalean fern in the Carboniferous deposits of Russia. Wulfenia, 23: 147-161. |
Naugolnykh, S.V., 2017. Spores of Pteridium aquilinum (L.) Kuhn and methodical aspects of comparative analysis of recent and fossil plants. Environ. Hum.: Ecol. Stud., 2: 9-26. |
Naugolnykh, S.V., Wang, L., Han, M., et al., 2016. A new find of the fossil Cyclosorus from the Eocene of South China and its paleoclimatic implication. J. Plant Res., 129: 3-12. DOI:10.1007/s10265-015-0765-0 |
Naugolnykh, S.V., Pronin, A.P., 2015. A new matoniaceous fern from the Upper Triassic of the Caspian Depression in the context of florogenetic processes of transition from the Paleozoic to Mesozoic. Paleontol. J., 49: 326-336. DOI:10.1134/S0031030115030090 |
Newman, E., 1844. A history of British ferns, ed. 2. J. Van Voorst, London.
|
Ôishi, S., Huzioka, K., 1941. Studies on the cenozoic plants of hokkaidô and karahuto: I. Ferns from the Woodwardia sandstone of hokkaidô. J. Fac. Sci. Hokkaido Univ. - Ser. 4 Geol. Mineral., 6: 177-192. |
Ôishi, S., Huzioka, K., 1942. New species of Woodwardia and Metasequoia from the Harutori Bed, kusiro coal-field, Hokkaido. J. Geol. Soc. Jpn., 49: 319-324. DOI:10.5575/geosoc.49.319 |
Palacios-Rios, M., Arana, M.D., 2018. A synopsis of Woodwardia (Blechnaceae) in veracruz state, Mexico, and typification of W. spinulosa. Willdenowia, 48: 23-28. DOI:10.3372/wi.48.48102 |
Pant, D.D., Khare, P.K., 1974. Damudopteris gen. nov. – a new genus of ferns from the Lower Gondwanas of the Raniganj Coalfield, India. Proc. R. Soc. Lond. Ser. B Biol. Sci., 186: 121-135. |
Pigg, K.B., Rothwell, G.W., 2001. Anatomically preserved Woodwardia virginica (Blechnaceae) and a new filicalean fern from the Middle Miocene Yakima Canyon flora of central Washington, USA. Am. J. Bot., 88: 777-787. DOI:10.2307/2657030 |
Pigg, K.B., Devore, M.L., Wehr, W.C., 2006. Filicalean ferns from the tertiary of western North America: osmunda L. (Osmundaceae: Pteridophyta), Woodwardia sm. (Blechnaceae: Pteridophyta) and onocleoid forms (Filicales: Pteridophyta). Fern Gaz., 17: 279-286. |
Ppg, I., 2016. A community-derived classification for extant lycophytes and ferns. J. Systemat. Evol., 54: 563-603. DOI:10.1111/jse.12229 |
Serbet, R., Rothwell, G.W., 1999. Osmunda cinnamomea (Osmundaceae) in the Upper Cretaceous of Western North America: additional evidence for exceptional species longevity among filicalean ferns. Int. J. Plant Sci., 160: 425-433. DOI:10.1086/314134 |
Shatilova, I., Mchedlishvili, N., Rukhadze, L., et al., 2011. The History of the Flora and
Vegetation of Georgia (South Caucasus). Georgian National Museum, Tbilisi.
|
Smith, H.V., 1938. Some new and interesting late tertiary plants from sucker creek, Idaho- Oregon boundary. Bull. Torrey Bot. Club, 65: 557-564. DOI:10.2307/2480794 |
Spicer, R.A., Farnsworth, A., Su, T., 2020. Cenozoic topography, monsoons and biodiversity conservation within the Tibetan region: an evolving story. Plant Divers., 42: 229-254. DOI:10.1016/j.pld.2020.06.011 |
Spicer, R.A., Herman, A.B., Liao, W.B., et al., 2014. Cool tropics in the middle Eocene: evidence from the Changchang flora, Hainan Island, China. Palaeogeogr. Palaeoclimatol. Palaeoecol., 412: 1-16. DOI:10.1016/j.palaeo.2014.07.011 |
Stockey, R.A., Nishida, H., Rothwell, G.W., 1999. Permineralized ferns from the Middle Eocene Princeton Chert. I. Makotopteris princetonensis gen. et sp. nov. (Athyriaceae). Int. J. Plant Sci., 160: 1047-1055. DOI:10.1086/314191 |
Tanai, T., 1970. The Oligocene floras from the Kushiro coal field, Hokkaido, Japan. J. Fac. Sci. Hokkaido Univ. - Ser. 4 Geol. Mineral., 14: 383-514. |
Tryon, A.F., Lugardon, B., 1991. Spores of the Pteridophyta: Surface, Wall Structure,
and Diversity Based on Electron Microscope Studies. Springer-Verlag, New York.
|
Wagner, R.H., Lemos de Sousa, M.J., 1985. Oligocarpia leptophylla (Bunbury), nomenclatorial history and description of the lectotype. Contrib. Carbon. Geol. Palaeontol. Iber. Penin. An. Cienc. Porto. Suppl., 64: 481-490. |
Wang, F.G., Xing, F.W., Dong, S.Y., et al., 2013. Blechnaceae. In: Wu, Z.Y., Raven, P.H.,
Hong, D.Y. (Eds. ), (Pteridophytes), Flora of China, vols. 2-3. Science Press, Beijing, pp. 411-417. Missouri Botanical Garden Press, St. Louis.
|
Wang, L., Xu, Q.Q., Jin, J.H., 2014. A reconstruction of the Fossil Salvinia from the Eocene of Hainan Island, South China. Rev. Palaeobot. Palynol., 203: 12-21. DOI:10.1080/07060661.2013.868370 |
Wang, Q., Ablaev, A.G., Wang, Y.F., et al., 2006. Paleocene Wuyun flora in Northeast China: Woodwardia bureiensis, Dryopteris sp. and Osmunda sachalinensis. Acta Phytotaxon. Sin., 44: 712. DOI:10.1360/aps040129 |
Xu, Z.H., Deng, M.H., 2017. Blechnaceae. In: Xu, Z., Deng, M. (Eds. ), Identification
and Control of Common Weeds, Volume 2. Springer Netherlands, Dordrecht,
pp. 71-73.
|
Yang, G.X., Sheng, A., Wang, H.S., 1997. A new species: Szea (Cladophlebis) henanense sp. nov. in Henan Province and its evolutional significance. Chin. Sci. Bull., 42: 1023-1028. DOI:10.1007/BF02882625 |
Yao, Y.F., Bera, S., Ferguson, D.K., et al., 2009. Reconstruction of paleovegetation and paleoclimate in the early and middle Eocene, Hainan Island, China. Climatic Change, 92: 169-189. DOI:10.1007/s10584-008-9457-2 |



