b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Research Associate, Missouri Botanic Garden, Mytholmroyd, West Yorkshire, UK;
d. Center of Plant Ecology, Core Botanical Gardens, Chinese Academy of Sciences, Mengla, Yunnan, 666303, China
Berberis L. (Berberidaceae), as circumscribed by Yu and Chung, 2014, Yu and Chung, 2017, has two main centers of distribution; the Eurasian landmass, with a particular concentration in eastern and southeastern Asia, and Latin America, particularly along the Andean spine. The total number of species is about 500–600 (Ahrendt, 1961; Landrum, 1999; Harber, 2020). It is likely that more than half of these are in the Himalaya-Hengduan Mountains (HHM). These two global biodiversity hotspots harbor a remarkable diversity of endemic species (Wu, 1988; Xing and Ree, 2017; Ding et al., 2020) and new species of other genera from these and adjacent areas are being reported at a rapid pace (Sun et al., 2017; Du et al., 2020; Chang et al., 2021; Li et al., 2021; Olonova et al., 2021; Ya et al., 2021; Ye et al., 2021; Zheng et al., 2021). It has been noted, however, that more than half of the species diversity is unevenly concentrated in particular taxa, Berberis being one of them (others include Aconitum L., Gentiana L., Pedicularis L., Primula L., Rhododendron L. and Saxifraga L.; Ding et al., 2020). This distinct species diversity pattern within the HHM region has been shown to be explained by accelerated diversification rates of the montane clades and is closely related to the uplift history of the Hengduan Mountains and the evolution of regional monsoon climate (Xing and Ree, 2017; Ding et al., 2020). Harber (2020) reported about 200 species in the Chinese part of the HHM, yet the diversification history of Berberis spp. in this region is still poorly understood.
Many species of Berberis are valued in rural areas in western China for their medicinal uses (see e.g. comments gathered from local informants recorded on specimens of Berberis at MO collected in 2003 by D.M. Anderson et al. in Dêqên Xian in northwest Yunnan).
Until Ahrendt's (1961) study, all species of Berberis reported for China were based on collections made between the late 18th century and 1947 by European or American plant and/or seed collectors (e.g. Potanin, Delavay, Wilson, Forrest and Rock) or by Chinese collectors they employed or funded. Although those specimens were collected over a wide area of China, there were significant gaps in that few or no collections were made in northwestern Sichuan north or west of Kangding or in much of northern Sichuan, and none at all were collected in what is now Yushu and Golog prefectures, Qinghai. After 1947, collections of Berberis were made in both of the above areas of Sichuan and sparse collections were made in Yushu and Golog. Before Harber's (2020) study only three new species, B. luhuoensis Ying, B. sichuanica Ying and B. batangensis Ying, were published from those areas, all from Sichuan and all by Ying (2011) — the last named being treated by Harber as a synonym of B. sichuanica. Harber (2020) published 13 new species from those areas — nine from Sichuan, three from Yushu and one from Golog. All 13 novelties published by Harber are deciduous. It is clear to us that a significant number of species from those parts of China remain undescribed.
As a contribution to describing and mapping Berberis, seven new species are proposed here. The floral structure and overall morphology of the seven new species was compared with similar taxa from neighboring areas, and a series of morphological differences were noted both qualitatively and quantitatively. Also, considering that the plastid genomic data provided better support for the backbone of the Berberidaceae (Kreuzer et al., 2019; Hsieh et al., 2022), phylogenetic analyses based on ptDNA and nuclear ribosomal DNA (nrDNA) sequences were used to evaluate the genetic divergences of these species.
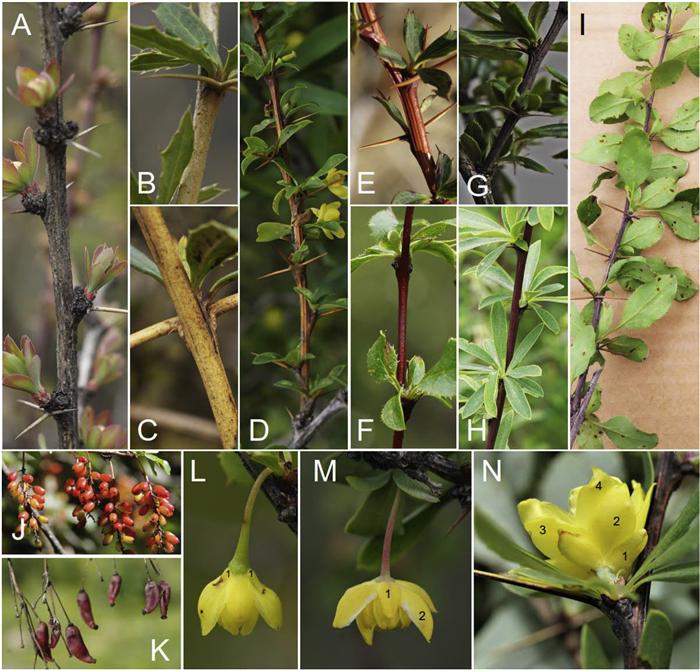
2. Materials and methodsDelimitation of the species of Berberis has always been difficult and often controversial. The first attempt at defining explicit criteria for distinguishing the species of Berberis with universal application was made in a series of articles by Schneider (1916, 1939, 1942). They were all in German and appear to have been overlooked by successive students of Berberis until Harber (2020) drew attention to them and provided a summary in English. Schneider's criteria included the color of mature (second year) stems (Fig. 1A–I), the shape and color of ripe fruit (Fig. 1J and K), the venation and serrations of evergreen species, but also (most explicitly emphasized in his 1942 comments) the importance of flower structure (Fig. 1L–N). (For a useful visual demonstration of the variation in the petals and the shape of the anther shape between various species of Berberis in Nepal see Adhikari et al., 2012). Schneider's criteria, especially those concerning flower structures (with those for leaves extended to deciduous species), largely determined Harber's recognition of species, synonyms and reversals of previous synonyms and to differentiate between nine deciduous species from northwestern Yunnan and southwestern and western Sichuan, all with similar leaves, similar pedicels and fruit bearing two ovules. This study is also based on Schneider's criteria. The type specimens of the proposed new species will be deposited in HITBC, KUN and A.
|
| Fig. 1 (A–I) Color variations of mature stems of Berberis. (A) gray stems more than two years old in most species; (B) mature stems yellowish gray (B. levis); (C) mature stems pale yellowish brown (B. deinacantha); (D) mature stems brown (B. longquensis); (E) mature stems reddish brown (B. gaoshanensis); (F) mature stems reddish purple (B. dictyoneura); (G) mature stems blackish purple (B. sp.); (H) mature stems dark purplish (B. purpureocaulis); (I) mature stems purple (B. jinshajiangensis); (J–K) Variation in inflorescences and fruits. (J) inflorescences racemose, fruit ellipsoid (B. dasystachya); (K) inflorescences sub-fascicled or sub-umbels, fruit oblong with bent apex (B. jiajinshanensis); (L–N) Bracteoles and variation of sepals. (L) bracteoles indicated by number 1 (B. tianbaoshanensis); (M) flower with two whorls of sepals (without bracteoles; B. beimanica); (N) flower with four whorls of sepals (without bracteoles; B. baiyuensis). |
In addition to the species newly described below, 21 other species of Berberis were sampled for the phylogenetic reconstruction (Table S1). Two species of Mahonia were also included as the outgroup taxa.
Total genomic DNA was extracted from leaves dried with silica gel, using a modified CTAB procedure. About 4 Gb of genomic data were generated by an Illumina NovaSeq 6000 platform with a reading length of 150bp. The clean reads were de novo assembled for the whole plastome and nrDNA sequences (i.e., partial ETS, and complete ITS, 28S, 5.8S, 18S sequences) by the GetOrganelle toolkit v.1.7.5 (Jin et al., 2020) and CPGAVAS2 (Shi et al., 2019). Subsequent annotations for all DNA scaffolds/complete contigs were made via GeSeq online portal (Tillich et al., 2017), and then manually edited by Geneious (Kearse et al., 2012). The ptDNA and nrDNA datasets were aligned by MAFFT (Katoh and Standley, 2013). The resulting genomic matrix was subsequently used to reconstruct the maximum likelihood tree through RAxML under a GTRGAMMA + I model with 1000 bootstraps (Stamatakis, 2014) on the CIPRES online portal (https://www.phylo.org/). Final topologies were visualized with FigTree v.1.4.4 (Rambaut, 2010), and edited and polished by iTOL's online portal (Letunic and Bork, 2021). All genomic DNA alignments and original DNA contigs/scaffolds used in this study were deposited and accessible in Dryads via https://doi.org/10.5061/dryad.nzs7h44sg.
3. Results and discussionWith slight variation of the GC content (38.0–38.2%), all newly generated plastomes of Berberis were assembled into circular molecules with sizes ranging from 153, 547 to 168, 208 bp, with a typical quadripartite structure consisting of a large single copy (LSC) ranging from 72, 349 to 73, 669 bp, a small single copy (SSC) ranging from 16, 194 to 18, 932 bp, and two IRs ranging from 36, 445 to 39, 343 bp. We identified 111 unique genes, 80 CDS (coding sequences), 34 transfer RNAs (tRNAs), and four ribosomal RNAs (rRNAs), which are all consistent with previously published Berberis plastomes. As for the nrITS sequences, the lengths of two ITS regions for the sampled Berberis ranged from 230 to 245 bp for the ITS-1 region, and from 228 to 239 bp for the ITS-2 region, respectively. The average GC contents were 45.8% and 55.1% for the ITS-1 and ITS-2 regions, respectively.
As rooted by two Mahonia species, the clades resulting from phylogenetic analyses based on both the plastome (ptDNA) and the nrDNA sequences are depicted in Fig. 2. Given that the low support values of the major clades in the nrDNA phylogram, the topological conflict between the two datasets is most likely due to the influence of concerted evolution of the nrITS sequence (Nieto Feliner and Rossello, 2007), as shown in previous phylogenetic studies of Berberis (Kim et al., 2004b; Yu and Chung, 2017).

|
| Fig. 2 Best-scoring maximum likelihood (ML) phylograms of ptDNA (whole plastome sequences; left) and nrDNA (right) datasets. Numbers next to nodes are bootstrap support values from ML analysis with RAxML. The large-leaved taxa are highlighted in dark grey. |
The plastid phylogeny revealed two main clades – a clade of northern temperate taxa (NT clade) and a Hengduan Mountains clade (HM clade). The species in the former were all large-leaved (> 2.5 cm × 1.5 cm) species from Sichuan with only Berberis elliptifolia being recorded from Qinghai. Those in the Hengduan Mountains clade were mostly, but not exclusively, small-leaved species (< 2.5 cm × 1.5 cm) because Berberis jiajinshanensis, Berberis tachiensis and Berberis tischleri are all large-leaved. One of the new species, Berberis riparia, was in the NT clade; the other six were all in the HM clade and were genetically distinct from both published species and from each other. Interestingly, though, we used nearly all whole plastome sequences to maximize the phylogenetic resolution, one of our new species Berberis chinduensis, which showed relatively slight genetic divergence from Berberis yushuensis. Given the clear morphological differences between them, further studies using more variable markers (e.g., single or low copy nuclear genes) are needed to test species delimitation. Two other northern large-leaved temperate species, Berberis amurensis and Berberis koreana, were outside the NT clade. For the HM clade, it is noteworthy that the small-leaved and single-flowered species did not form a monophyletic group (see e.g. the differing positions of the Qinghai species Berberis qinghaiensis and Berberis bouffordii both being in different positions from the Sichuan species Berberis ambrozyana and Berberis epedicellata). It is also interesting that although B. tachiensis was shown to be close to Berberis longipedicellata it was further from B. tischleri with which it has been frequently confused (Harber, 2020).
In the nrDNA phylogeny despite the overall poor phylogenetic resolution, two major clades (Clades 1 and 2 in Fig. 2), which were comparable to the NT and HM clades of the ptDNA phylogeny, albeit weak support, were recognizable. However, the outgroup species, Berberis amurensis and B. koreana, were positioned in Clade 1 and Clade 2, respectively, in contrast to their position in topology of ptDNA tree as shown in this and previous phylogenetic studies (Kim et al., 2004).
It is important to note that the molecular phylogeny reported here was used only to investigate sequence differences between the new species and closely related species. For that purpose, we did not include species not apparently closely related (e.g., those having paniculate inflorescences) from Sichuan, Qinghai and Yunnan as members of the ingroup, nor distantly related taxa as outgroups (e.g., evergreen species of Berberis or Mahonia).
4. Taxonomic treatments and key to the new species 4.1. The keysThe keys presented here include all of our new proposed species and morphologically similar species from the same areas of Sichuan and Qinghai. It should be stressed that these keys are not definitive for identification. Without doubt, a significant number of unrecognized species with similar characteristics remains, but floral material is needed before they can be published. This applies to species with all of the various types of inflorescences in the keys below. In particular it should be noted that are numerous one-fruited species in the Chinese Virtual Herbarium (CVH) identified as Berberis dictyophylla var. epruinosa (or more rarely simply as B. dictyophylla) appear to be neither of the six already published species listed below nor of the four new one-flowered species proposed here.
4.1.1. Keys to deciduous species of Berberis from• Aba Prefecture, Sichuan (excluding Jiuzhaigou, Mao and Songpan Xian)
• Ganzi Prefecture, Sichuan (excluding Daocheng, Derong, Jiulong and Xiangcheng Xian)
• Yushu Xian and Jiuzhi Xian, Qinghai
4.1.2. List of keysFlowers solitary, sometimes paired or 1–4 fascicled ………....Key 1.
Flowers with a combination of fascicles, sub-fascicles, umbels, sub-umbels or sub-racemes … … … … … … … … … … … ….Key 2.
Flowers in racemes … … … … … … … … … … … … … ….Key 3.
Key 1. Flowers solitary or sometimes paired or 1–4 fascicled.
1a Flowers solitary … … … … … … … … … … … … … … … …2.
1b Flowers paired or 1–4 fascicled … … … … … … … … … ….10.
2a Shrubs to 30 cm tall … … … … ……..Berberis kangdingensis.
2b Shrubs > 30 cm tall … ….… … … … … … … … … … … …. 3.
3a Flowers sessile or pedicel to 3 mm long …..… … … … … ….4.
3b Flower pedicel more than 3 mm long.… … … … … … … …7.
4a Mature stems pale or dark purple, mostly pruinose; leaf blade abaxially mostly pruinose … … ……………..Berberis epedicillata.
4b Mature stems reddish brown or reddish purple, epruinose; leaf blade abaxially epruinose … … … … … … … … … … … … ….5.
5a Leaf blade obovate or narrowly elliptic, 2–2.5 × 1–2 cm … … … …………………………………………….….Berberis barkamensis.
5b Leaf blade obovate, narrowly obovate or oblong-obovate (0.7–) 0.8–1.2 × 0.15–0.7 cm … ……………………………… …....6.
6a Leaf blade narrowly obovate or oblong-obovate, 0.8–1.2 × 0.15–0.4 cm; pedicel 2–3 mm long; ovules/seeds 5 … … … … … … … … … … … … … … … ….Berberis ambrozyana.
6b Leaf blade obovate (0.7–)1–1.7 × 0.3–0.7 cm; pedicel absent or to 2 mm long; ovules/seeds 3 or 4 …… … Berberis baiyuensis.
7a Mature stems pale yellowish brown … … … … … … ……8.
7b Mature stems brown or reddish brown … … … … … … …9.
8a Leaf blade narrowly obovate or very narrowly elliptic, 1.3–1.7 × 0.4–0.6 cm; sepals in two whorls; outer sepals obovate to broadly obovate; inner sepals, elliptic … …. Berberis qinghaiensis.
8b Leaf blade oblanceolate or very narrowly elliptic (0.7–)1.4–1.5 × 0.2–0.3 cm; sepals in three whorls; outer sepals oblong to oblong ovate; median sepals elliptic; inner sepals broadly obovate … … … … … … … … … … … … …..Berberis bouffordii.
9a Leaf blade narrowly obovate or obovate-lanceolate, 0.9–1.3 (–1.6) × 0.3–0.5 cm; pedicel 4–7 mm long; sepals in two whorls … … …..… … … ………………………. Berberis longquensis
9b Leaf blade obovate, narrowly obovate or elliptic obovate, 1.1–2.4 × 0.4–0.8 cm;
pedicel 9–11 mm long; sepals in four whorls … … … … … … … … ………………………………………………. Berberis jinwu
10a Flower solitary or sometimes in pairs … …..… … … … …11.
10b Flowers 1–4 fascicled … … … … … … … … … … … …..13.
11a Shrub to 60 cm tall; margin of leaf blade prominently spinose with 4–14 teeth on each side or sometimes entire; pedicel 7–10 mm long …. … … … … ………………....Berberis yalongensis.
11b Shrub to 2 m tall; margin of leaf blade minutely spinulose with 12–20 teeth on each side; pedicel 15–30 (–35) mm long …...… … … … … … … … … … … … … … … … ……………………12.
12a Mature stems pale yellowish gray; leaf blade obovate, 2.5–3.5 (–4) × 1.2–1.6 cm, margin minutely spinose with 12–20 teeth on each side or entire.……………..Berberis longipedicellata.
12b Mature stems reddish brown: leaf blade narrowly elliptic to narrowly obovate, 1.7–2.3 × 0.3–0.6 cm, margin entire … … … … … … … … … …..………………………..Berberis litangensis.
13a Mature stems partially pruinose; leaf blade often abaxially pruinose; flowers 1–3; pedicel 5–7 mm long … … … … … … … … … … … … … … … … … … …. …………….Berberis pruinosifolia.
13b Mature stems epruinose; leaf blade abaxially epruinose; pedicel 7–16 mm long … … … … … … … … … … … … … … …14.
14a Mature stems pale yellowish gray; flowers 1–3; sepals in 3 whorls; ovules 5 or 6 … ………………Berberis bawangshanensis.
14b Mature stems dark reddish-purple or dark purple; flowers 2–4; sepals in 2 whorls; ovules 3....Berberis degexianensis sp. nov.
Key 2. Flowers with a combination of fascicles, sub-fascicles, umbels, sub-umbels or sub-racemes.
1a Inflorescences to 5.5 cm long … … ….. ….… … … … … …. 2.
1b Inflorescences 3 cm long or less … ….… … … … … … … ….5.
2a Mature stems dark purple, reddish purple, reddish brown or pale reddish brown …..… … … … … … … … … … … … … … …. 3.
2b Mature stem pale yellowish brown … … … … … … … … 4.
3a Mature stems dark purple, reddish purple or reddish brown; leaf margin entire, very rarely spinulose with 1–8 inconspicuous teeth on each side; inflorescences sub-racemes, sometimes fascicled at base or sub-fascicles toward apex of stems … … … … … … … … … … … … … … … … … … … … … …...Berberis mouillacana.
3b Mature stems pale reddish brown; leaf margin spinose with 3–13 conspicuous teeth on each side or entire on young twigs; inflorescences fascicles, sub-fascicles, umbels or sub-umbels; pedicel 5–15 mm long; petals broadly elliptic, apex emarginate … … … … … … … … … … … … …. Berberis jiajinshanensis sp. nov.
4a Leaf blade obovate or obovate-elliptic, 1.5–2.4 (–4.7) × 1–2.3 cm; outer sepals broadly elliptic or elliptic-ovate; inner sepals broadly elliptic or elliptic-ovate; anther connective extended, apiculate; ovules 3 or 4 … ………… Berberis tachiensis.
4b Leaf blade obovate, elliptic-obovate, or oblong-obovate, 2.2–3.6 × 1.1–1.7 cm; outer sepals narrowly obovate; inner sepals broadly obovate or elliptic-obovate; anther connective not extended, truncate; ovules 3–5 … … … … … … Berberis tischleri.
5a Mature stems reddish brown, dark reddish purple of dark reddish brown … … …...……………………………………………….6.
5b Mature stems pale yellowish or grayish brown … … … ….9.
6a Inflorescences sub-fascicled racemes; leaf blade elliptic or elliptic-obovate, margin spinose with 10–20 very conspicuous teeth on each side … … … … ………………. Berberis dictyoneura.
6b Inflorescences fascicles, sub-fascicles, sub-umbels, sub-racemes or racemes fascicled at base; leaf blade obovate or elliptic-obovate, margin entire or spinulose with 1–8 (–10) inconspicuous teeth on either side … … … … … … … … … … ….7.
7a Leaf blade obovate, often narrowly so, 1.5–2.6 × 0.4–0.7 cm; inflorescences to 1.7 cm long, often dense fascicles sub-fascicles or sub-racemes … … ……………………….Berberis yarigongensis.
7b Leaf blade obovate or obovate elliptic 1–3 × 0.6–1.3 cm; inflorescences to 2.2 cm long, fascicles, sub-fascicles, sub-umbels or sub-racemes sometimes fascicled at base … … … … … … … ….8.
8a Inflorescences sub-fascicles, sub-racemes, or racemes, sometimes fascicled at base; flowers 2–5 (–7); pedicel 8–16 mm … … … … … … … … … … … … … ….………………Berberis yaanica.
8b Inflorescences fascicles, sub-fascicles, sub-umbels or sub-racemes; flowers 3–12; pedicel 4–8 mm long … … … … … … … … … … … … … … … … … … … … …. ………Berberis ngawaica.
9a Inflorescences fascicles, or sub-fascicle with single or paired flowers frequently toward end of branches; ovules 4–6 … … … … … … … … …..… … … … … … …………………Berberis diaphana.
9b Inflorescences a fascicles, sub-fascicles, umbels, sub-umbels, racemes or sub-racemes; ovules 2 … … … … … … … … …. … …..10.
10a Sepals in 3 whorls … … … … … … … … … … … … … …11.
10b Sepals in 2 whorls … … … … … … … … … … … … … …12.
11a Leaf blade obovate, often narrowly so; inflorescences a fascicles or sub-fascicles; flowers 2–5 (–9); outer sepals oblong-lanceolate; median sepals oblong-ovate; inner sepals oblong-ovate or obovate-elliptic … … … … … … …. Berberis abbreviata.
11b Leaf blade oblanceolate or obovate-elliptic; inflorescences fascicles, rarely a sub-fascicles, sub-umbels, or sub-racemes, outer sepals lanceolate; median sepals elliptic; inner sepals elliptic … … … … … … … … … … … … … … … … …...… … … …Berberis yui.
12a Leaf blade narrowly obovate to narrowly oblong-obovate or obovate-spatulate, margin spinose with 2–10 teeth on each side … … … … … … … … … … …. ……………………Berberis dawoensis.
12b Leaf blade obovate, broadly obovate, broadly obovate-elliptic, obovate-orbicular or oblanceolate, margin entire or spinulose with 1–6 teeth on each side … … … … ….…………… 13.
13a Inflorescences fascicles, or sub-umbels sometimes fascicled at base; flowers 1–4 (–6); pedicel 5–8 mm long … …..… … … … … … … … … … … … … … … … … … … …....Berberis yushuensis.
13b Inflorescences fascicles, sub-fascicles, umbels, sub-umbels or rarely sub-racemes; flowers 3–7 (–9); pedicel 6–15 mm long … … … … … … … … … … … … …...………………………………14.
14a Leaf blade obovate or obovate-elliptic, 1.5–2.8 (–3.5) × (0.4–)0.8–1.1 (–1.6) cm; pedicel 8–15 mm long; outer sepals narrowly elliptic; petals obovate-elliptic … … … …… … … … … … … … … … … … … … … … … … … Berberis lixianensis.
14b Leaf blade broadly obovate, broadly obovate-elliptic or obovate-orbicular, (0.5–)0.8–1.4 (–2) × (0.4–)0.6–1.2 (–1.8) cm; outer sepals ovate; petals narrowly obovate … … … … … … …..… … … … … … … … … … … … … … … … …..Berberis heishuiensis.
Key 3. Flowers in racemes.
1a Leaf blade abaxially villous, adaxially rugose; inflorescences spike-like … … …...… … … … … … … … … … … … … … … … … … … … … … … … … … … ……………………Berberis brachypoda.
1b. Leaf blade abaxially glabrous, adaxially flat; inflorescence more or less open racemes or sometimes partially paniculate, sometimes partially fascicled at base … … … … … … … … … …2.
2a Inflorescence to 20 cm long; flowers 15–50 … … … … ….3.
2b Inflorescences 0.9–5.5 cm long; flowers 3–15 … … … …..4.
3a Leaf blade obovate, suborbicular, oblong-elliptic, or broadly elliptic, 3–6 (–8) × 2.5–4 cm; inflorescences solely racemose; pedicel erect … … … … … … …………………Berberis dasystachya.
3b Leaf blade ovate to elliptic or sometimes oblong-lanceolate, 2–7 × 1–3 cm; inflorescences often partially paniculate; pedicel reflexed …. …………………………...Berberis francisci-ferdinandi.
4a Leaf blade to 5.5 cm long, to 2.5 cm wide.… … … … … ….5.
4b Leaf blade to 3.5 cm long, to 1.8 cm wide … … … … … … ….7.
5a Leaf blade elliptic or sometimes elliptic-lanceolate, 2–5.5 × 1–2.2 cm; inflorescences sometimes fascicled at base; pedicel 5–10 mm long; sepals in 3 whorls; immature berry translucent..… … … … … … … … … … … … … … Berberis elliptifolia.
5b Leaf blade elliptic, obovate or obovate-elliptic 1.5–5.5 × (0.6–)1.5–2 (–2.6) cm; inflorescences not fascicled at base; pedicel 4–15 mm long; sepals in 2 whorls; immature berry opaque … … … … … … … … … … … … … … … … … … … …. 6.
6a Leaf blade elliptic or obovate-elliptic, 1.5–4 (–5.4) × (0.6–)1.5–2 (–2.6) cm; margin entire, rarely spinose with 1–7 teeth on each side; flowers 4–12; pedicel 8–15 mm long; inner sepals elliptic orbicular … … … … … …..… ……..Berberis emeishanensis.
6b Leaf blade obovate or obovate-elliptic 3.5–5.5 × 2–2.5 cm; margin spinose with 8–26 teeth on each side, flowers 12–15; pedicel 4–5 mm long; inner sepals obovate … …… … … … … … … … … … … … … … … … … … …..… ….. Berberis riparia sp. nov.
7a Shrubs to 3 m tall; axillary spines absent or 0.2–0.3 cm long; petiole to10 mm or almost absent; leaf blade obovate or sometimes obovate-elliptic 1.3–3.5 × 0.6–1.8; margin entire, rarely spinulose with 4–16 teeth on each side; pedicel (5–)12–22 mm long; ovules 2 … … … … … … … … … … … ……….Berberis wenchuanensis.
7b Shrub to 2 m tall; axillary spines 1–3–(–or 5)-fid, 0.5–1.8 (−2.5) cm long; petiole nearly absent to −3 mm long; leaf blade entire or spinose with 1–25 teeth on each side; pedicel 3–12 mm long; ovules 2–4 …...… … … … … … … … … … … … … … … … 8.
8a Mature stems brownish; leaf blade obovate (mostly very narrowly so) or oblanceolate, 1.5–1.8 × 0.4–0.7 cm; anther connective distinctly extended … … …...Berberis chinduensis sp. nov.
8b Mature stems reddish brown or dark purplish red; leaf blade obovate or obovate elliptic, 0.5–2 (–3.5) × 0.5–1.2 cm; anther connective not extended, rounded … … …. ………………………..9.
9a Mature stems reddish brown; leaf margin entire or spinose with 1–5 teeth on each side; inflorescences sometimes partially paniculate; flowers 6–15; pedicel 3–6 mm long; apex of petal entire … … …..… … … … … … ……………... Berberis sichuanica.
9b Mature stems dark purplish red; leaf margin entire or spinose with 10–24 teeth on each side; inflorescences racemes, or flowers sometimes 2- or –3-fascicled at base; flowers 3–10; pedicel 5–12 mm long, apex of petal incised …. …....…………………………………………..Berberis tenuipedicellata.
4.2. Taxonomic treatments 4.2.1. Berberis chinduensis Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 4). 称多小檗

|
| Fig. 3 Distribution of seven new proposed species of Berberis. Circular images show characteristics of each species and indicate type locality. |

|
| Fig. 4 Berberis chinduensis (A–D) and Berberis degexianensis (E–G). (A) Racemose inflorescences and immature fruit; (B) flowers and inflorescence; (C) branches and leaves; (D) mature fruit; (E) leaf; (F) flowers; (G) fruiting branches. |
Type. CHINA. Qinghai, Yushu Prefecture, Chengduo (Chindu) Xian, nr. E-Er Xiang, 32.986222°N, 97.345361°E, 3800 m, 2 July 2020, C.C. Yu & Z.Q. Du yxing 4288 (holotype HITBC 0057625, isotypes A, KUN, PE).
Diagnosis. Berberis chinduensis is similar to B. yushuensis, but is racemose versus the more diverse inflorescences of B. yushuensis and has a different flower structure including a distinctly extended anther connective and 2–4 ovules per ovary; B. yushuensis has a truncate anther connective and 2 ovules.
Shrub, deciduous, to 1.5 m tall; mature stems brownish, sparsely verruculose, sulcate; spines 3-fid, concolorous, 0.5–1 cm long, terete, sometimes abaxially slightly sulcate. Petiole almost absent; leaf blade, abaxially pale green or sometimes grayish-white or glaucous, adaxially dull green, mostly very narrowly obovate, or oblanceolate 1.5–1.8 × 0.4–0.7 cm, thinly leathery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially, conspicuous adaxially, base attenuate, margin entire or spinose with 1–7 teeth on each side, apex acute, mucronate. Inflorescences racemes, 7- to 13-flowered, 0.9–3.5 cm long; pedicels 6–7 mm long, slender, yellow or pinkish yellow; bracts lanceolate 1.5 mm long. Sepals in 2 whorls; outer sepals elliptic, 2–3 × 1.5–2 mm, apex acute; inner sepals obovate, 3–5 × 3–3.5 mm; petals obovate, 2 × 1 mm, apex rounded, incised. Stamens 2 mm long; anther connective distinctly extended. Pistil 3 mm long; ovules 2–4 per ovary. Immature berry turning red, ellipsoid, 8 × 3.5 mm; style deciduous or persistent.
Phenology. Berberis chinduensis has been collected in flower in July and with immature fruit in August.
Distribution and habitat. Berberis chinduensis is known only from the type and one other collection in the same location in Chindu Xian in southern Qinghai; the type at 3800 m, Ho et al. 1340 at 3700 m. Both collections were growing among Ribes and Salix, on the lower grassy slopes of a river valley.
Proposed IUCN conservation status. Berberis chinduensis is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Note. Ho et al. 1834 (details below) was mistakenly identified as Berberis yushuensis by Harber (2020).
Additional specimens examined. Qinghai. Chindu Xian: Xiewu Cun, Shang Saiba, E of Chumda, 32.983333°N, 97.35°E, 3700 m, 15 Aug. 1996, T. N. Ho et al. 1834 (BM001010938, CAS 940714, E E00059996, GH 00280127, HNWP 00001390, MO 5190726, PE 01840140).
4.2.2. Berberis degexianensis Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 4). 德格县小檗Type. CHINA. Sichuan, Garzê Prefecture, Dege Xian, near G317 highway, 31.954583°N, 98.857417°E, 4028 m, 3 July 2020, C.C. Yu & Z.Q. Du yxing 4291 (holotype HITBC 0057623, isotypes, A, KUN, PE).
Diagnosis. The shape of the leaves and leaf margins of Berberis degexianensis are somewhat similar to Berberis dahaiensis which also has purple mature stems. It differs from B. dahaiensis by having longer pedicels and 3 ovules per ovary (the flowers of B. dahaiensis are unknown but it has 2 seeds per berry).
Shrub, deciduous, to 1.5 m tall; mature stems dark reddish-purple or dark purple, sparsely verruculose, terete to slightly sulcate; spines 3-fid, pale yellow, 0.5–1 cm long. Petiole almost absent; leaf blade, abaxially pale green or sometimes grayish-white, adaxially dull green, narrowly obovate or narrowly elliptic, 1.3–2.3 × 0.5–1.0 cm, papery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially and adaxially, base attenuate, margin spinose with 1–12 teeth on each side or entire, apex acute. Inflorescences a solitary flower or 2- to 4-flowered fascicles; pedicels 7–12 mm long, slender. Sepals in 2 whorls; outer sepals lanceolate or narrowly ovate, 3–4 × 2 mm, apex acute; inner sepals oblong-elliptic, 4 × 2 mm; petals obovate, 2.5–3 × 1 mm, apex acute, deeply incised. Stamens 2.5–3 mm long; anther connective apiculate. Pistil 3–4 mm long; ovules 3 per ovary. Berry scarlet, ellipsoid, 6 × 3.5 mm; style persistent ca. 1 mm long.
Phenology. Berberis degexianensis has been collected in flower in late June and July and in fruit in August.
Distribution and habitat. Berberis degexianensis is known from Dege Xian (Sichuan, China) along national highway G317 and provincial highway 217 at 3800–4028 m. It has been collected from roadside thickets at 3800–4028 m.
Proposed IUCN conservation status. Berberis degexianensis is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Sichuan. Dege Xian: Dongfong power station, 3800 m, 1 July 1973, Sichuan Vegetation Team 4230 (CDBI 0027986-87); same details but Sichuan Vegetation Team 4232 (CDBI 0028006, SM 704800542). Provincial highway S217 at Murico lake between Manigange and Gengda, 31.999444°N, 99.066111°E, 4050 m. 18 Aug. 2006, D.E. Boufford et al. 36, 839 (A, CAS, E E00947153, KUN, MO, P, TI). Manigange Cun, 31.912278°N, 99.159556°E, 3969 m, 25 Sept. 2011, Southwest China Natural Resource Survey ZhangDC-07ZX-2298 (KUN 1021537). Near G317 highway, 31.954583°N, 98.857417°E, 4028 m, 10 Sep. 2021, Z.Q. Du yxing 4738 (HITBC).
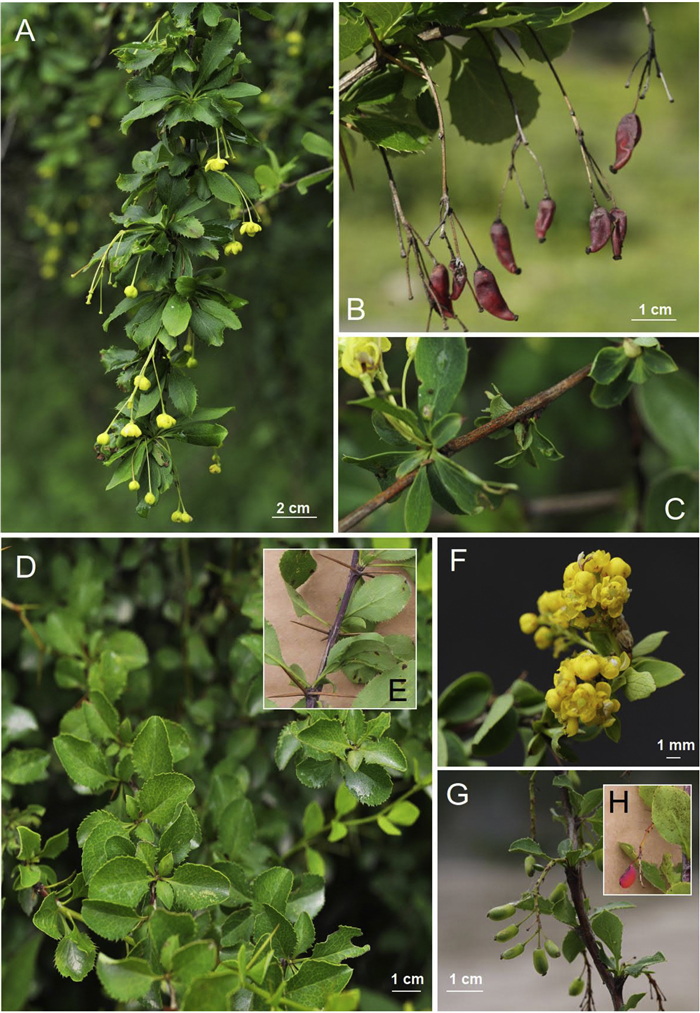
4.2.3. Berberis jiajinshanensis Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 5). 夹金山小檗
|
| Fig. 5 Berberis jiajinshanensis (A–C) and Berberis riparia (D–H). (A) Flowering branches; (B) fruit and inflorescence; (C) color of mature stem; (D) leaf; (E) color of the mature stem; (F) flowers; (G) racemose inflorescences and immature fruit; (H) scarlet mature fruit. |
Type. CHINA. Sichuan, Ngawa Prefecture, Xiaojin Xian, by provincial highway S217, 30.885417°N, 102.650972°E, 3570 m, 26 June 2020, C.C. Yu & Z.Q. Du yxing 4245 (holotype HITBC 0057620, isotypes A, KUN, PE).
Diagnosis. Berberis jiajinshanensis is similarities to B. tachiensis and B. tischleri but differs from both inter alia by its umbellate or sub-umbellate inflorescences and never fascicled from base as in unlike B. tachiensis and B. tischleri. The flower structure also differs from both of those species.
Shrub, deciduous, to 2 m tall; mature stems pale reddish brown, sparsely verruculose, slightly sulcate or terete; spines 3-fid, pale brown, 1–1.5 cm, slender. Petiole almost absent or to 12 mm long; leaf blade, abaxially pale green, adaxially dull darkish green, obovate (sometimes widely so), obovate-elliptic or obovate-lanceolate, 1.6–3.8 (–4.4) × 0.7–1.6 (–2.5) cm, papery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially and adaxially, base attenuate, margin spinose with 3–13 conspicuous teeth on each side or entire on young twigs, apex acuminate or obtuse, sometimes minutely mucronate. Inflorescences fascicles, sub-fascicles, umbels or sub-umbels, 1 to7-flowered, to 5.5 cm long; pedicels 5–15 mm long, slender; bracteoles (when present) lanceolate-triangular. Sepals in 2 whorls; outer sepals elliptic, 2 × 1.5 mm, apex acute; inner sepals obovate, 2.5–3 × 1.5–2 mm; petals broadly elliptic, 2.5–3 × 1.5–2 mm, apex rounded, emarginate. Stamens ca. 1.5 mm long; anther connective apiculate. Pistil ca. 1.5 mm long; ovules 4 or 5 per ovary. Berry red, oblong, apex somewhat bent, 7 × 4 mm; style deciduous.
Phenology. Berberis jiajinshanensis has been collected in flower in June and July and with immature fruit at the end of July.
Distribution and habitat. Berberis jiajinshanensis is known from the N side of Jiajin Shan. It has been found in grassy areas, mixed forests, river and roadsides at 3300–3700 m.
Proposed IUCN conservation status. Berberis jiajinshanensis is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Sichuan. Xiaojin Xian: Jiajin Shan, 3700 m, 9 July 1958, X.S. Zhang & Y.X. Ren 5803 (PE 01030901, CDBI 0028143-44); Jiajin Shan ca. 16 km from "Dengsheng", 30.875°N, 102.631194°E, 3351 m, 7 July 2004, C.S. Chang et al. SI0055 (PE 01839957, SNUA); Jiajin Shan, Jiajindang along provincial highway S210, 30.872778°N, 102.651389°E, 3510–3585 m, 31 July 2018, D.E. Boufford et al. 44301 (A, CAS, E, K, KUN, MO, TI); Jiajin Shan, south of Dawei Cun near Xiamu Cheng along provincial highway S210, 30.881389°N, 102.641667°E, 3300 m, 31 July 2018, D.E. Boufford et al. 44306 (A, CAS, E, K, KUN, MO, TI).
4.2.4. Berberis riparia Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 5). 河岸小檗Type. CHINA. Sichuan, Ganzi Prefecture, Baiyu Xian, by Jinsha Jiang, 31.268583°N, 98.77975°E, 2958 m, 4 July 2020, C.C. Yu & Z.Q. Du yxing 4293 (holotype HITBC 0057618, isotypes A, KUN, PE).
Diagnosis. Berberis riparia has leaves that are somewhat similar in shape to those of B. heteropoda but the latter has an inflorescences that is more varied and has flowers with 5 or 6 ovules per ovary and black berries.
Shrub deciduous, to 3.5 m tall; young shoots reddish brown, mature stems dark purplish or dark purple becoming paler purple with age, slightly sulcate or terete; spines 3- to5-fid, or spine solitary toward the apex of stems, brown or orangish-brown, 1–1.5 cm long. Petiole almost absent to 10 mm long; leaf blade abaxially pale green, sometimes grayish-white, adaxially green or bluish green, obovate or obovate-elliptic 3.5–5.5 × 2–2.5 cm, thinly leathery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous adaxially and abaxially, base attenuate or truncate, margin spinulose with 8–26 teeth on each side or entire on young twigs, apex obtuse, sometimes minutely mucronate. Inflorescences racemes, 12–15-flowered, 2.5–4 cm long; pedicel 4–5 mm long; bracts triangular 0.5 mm long. Sepals in 2 whorls; outer sepals ovate, 1.5 × 1 mm, apex acuminate; inner sepals obovate, 2 × 1.5 mm; petals broadly elliptic, 2 × 1.5 mm, apex rounded, emarginate or incised. Stamens ca. 1.5 mm long; anther connective truncate or slightly extended. Pistil 2 mm long; ovules 2 per ovary. Berry red, oblong, 5–6 × 3–3.5 mm; style deciduous.
Phenology. Berberis riparia has been collected with the remnants of flowers and immature fruit in June and in fruit in August; its flowering and fruiting periods are otherwise not completely known.
Distribution and habitat. Berberis riparia is known from Baiyu and Dege Xian in W Sichuan. It has been found on the margins of Pinus forests and fields and on open slopes by riverbanks.
Etymology. From Latin riparius-frequenting banks of streams or rivers.
Proposed IUCN conservation status. Berberis riparia is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Sichuan. Baiyu Xian: Baiyu, near far bank of Jinshajiang, 3040 m, 11 Mar. 1986, College of Chengdu Chinese Herbal Medicine 0001 (CDCM0000849); NW of the town of Baiyu along the Jinshajiang, downstream from Baji Cun; ca. 3.1 km (by road) downstream from confluence with the Ou Qu, 31.263333°N, 98.754444°E, 2960–2975 m, 24 Aug. 2018, D.E. Boufford et al. 44900 (A, CAS, E, KUN, PE). Dege Xian: Without location 3800 m, 27 June 1973, Sichuan Vegetation Survey Team 4225 (CDBI 0027691-96); Gongya Qu, 3200 m, 20 June 1974, Sichuan Vegetation Survey Team 7038 (CDBI 0027452-54, PE 01037329).
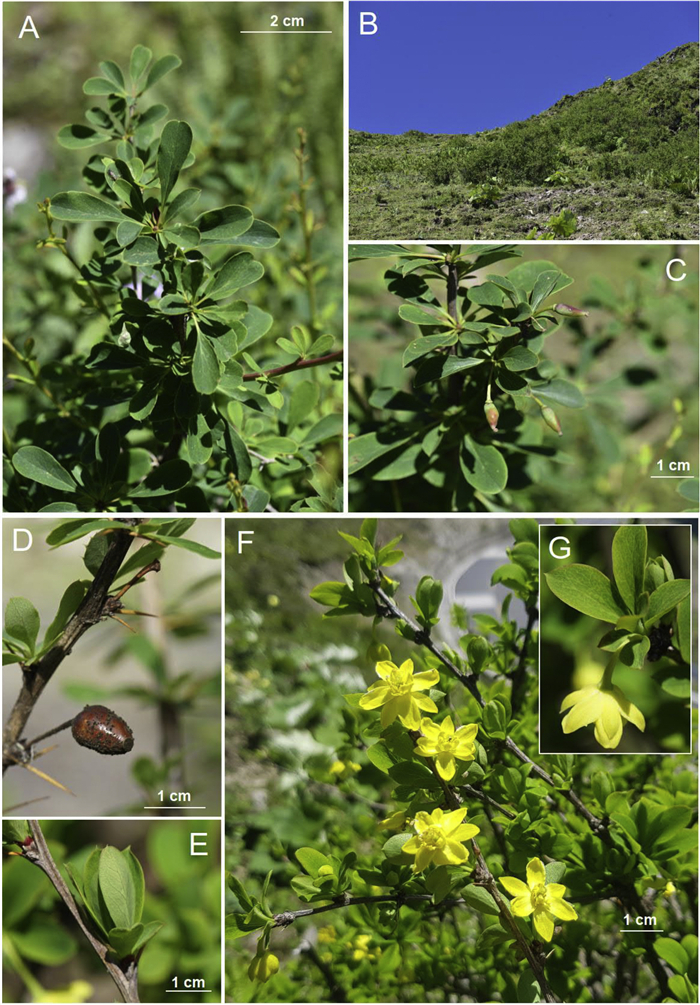
4.2.5. Berberis jinwu Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 6). 金乌小檗
|
| Fig. 6 Berberis jinwu. (A) Mature leaves; (B) habitat; (C) immature fruit; (D) reddish brown fruit from the previous year; (E) young leaves; (F) flowering branches; (G) flowers, exterior view, showing sepals. |
Type. CHINA. Sichuan, Ya'an Shi, Baoxing Xian, S side of Jiajin Shan pass, beside provincial road S210, 30.852944°N, 102.693861°E, 3872 m, 25 June 2020, C.C. Yu & Z.Q. Du yxing 4241 (holotype HITBC 0057619, isotypes A, KUN, PE).
Diagnosis. The leaves of Berberis jinwu are somewhat similar to those of B. cornuta, but the flowers are bright yellow versus orangish yellow in the latter. The flower structure also differs, having four rows of sepals – the only deciduous species in China with this characteristic.
Shrubs, deciduous, to 1 m tall; mature stems brown or reddish-brown, sparsely verruculose, sulcate; spines 3-fid, brown, 0.6–1.6 cm long, slender. Petiole almost absent to 4 mm long; leaf blade abaxially pale green, adaxially dull green, obovate, narrowly obovate or elliptic obovate, 1.1–4.3 × 0.4–1.6 cm, papery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially and adaxially, base attenuate, margin entire or spinose with 1–13 teeth on each side, apex obtuse, sometimes minutely mucronate. Inflorescences a solitary flower; pedicels 9–23 mm long, slender, bracteoles when present, triangular. Flowers bright yellow. Sepals in 4 whorls; outmost sepals lanceolate 8 × 3 mm; outer sepals elliptic, 8.5 × 3.5 mm; inner sepals elliptic, 8–9 × 5 mm; innermost sepals elliptic, 8–9 × 5 mm; petals narrowly elliptic, 5 × 2.5 mm, apex rounded, incised; stamens 4 mm long; anther connective apiculate. Pistil 4 mm long, ovules 3–5 per ovary. Berry color unknown (reddish brown in previous year's fruit), ovoid, 7 × 5.5 mm; style deciduous.
Phenology: Berberis jinwu has been collected with flowers in June. Its fruiting period is unknown.
Distribution and habitat. Berberis jinwu is known from the S side of Jiajin Shan close to the border between Baoxing and Xiaojin Xian. It is mostly in alpine thickets, but sometimes in mountain valleys.
Etymology. Berberis jinwu is named after a Chinese mythological bird— Jinwu, also known as the three-legged crow, which in ancient Chinese culture was believed to inhabit the sun. The flowers of Berberis jinwu have four whorls of eye-catching sepals, that look like a nine-rayed sun.
Proposed IUCN conservation status. Berberis jinwu is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Sichuan. Ngawa Prefecture: beside provincial road S303, 30.951578°N, 102.882472°E, 3946 m, 2 Aug. 2021, C.C. Yu & C. Peng yxing 4687 (HITBC); Ya'an Shi, Baoxing Xian, S side of Jiajin Shan pass, beside provincial road S210, 30.852944°N, 102.693861°E, 3872 m, 1 Aug. 2021, C.C. Yu & C. P. yxing 4678 (HITBC); S side of Jiajin Shan pass, beside provincial road S210, 30.845056°N, 102.707986°E, 3746 m, 1 Aug. 2021, C.C. Yu & C. P. yxing 4675 (HITBC).
4.2.6. Berberis litangensis Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 7). 理塘小檗

|
| Fig. 7 Berberis litangensis (A–D) and Berberis longquensis (E–G). (A) Color of mature stem; (B) scarlet fruit; (C) leaves; (D) front view of flower; (E) flowering branches; (F) front view of view; (G) color of mature stems; (H) immature fruit. |
Type. CHINA. Sichuan, Litang Xian by national highway G318, 30.148194°N, 100.040083°E, 4089 m, 5 July 2020, C.C. Yu & Z.Q. Du yxing 4303 (holotype HITBC 0057624, isotypes A, KUN, PE).
Diagnosis. Berberis litangensis has mature stems reddish brown, the same color as those of B. ambrozyana. The leaves are also similar in shape, but larger. It also differs from B. ambrozyana in having much longer pedicels and flowers sometimes in pairs and a different flower structure, including obovate inner sepals, a truncate anther connective and 2 or 3 ovules per ovary versus the lanceolate inner sepals, apiculate anther connective and 5 ovules per ovary of B. ambrozyana.
Shrubs, deciduous, to 1 m tall; mature stems reddish-brown, sulcate; spines 3-fid, pale brown, 1–1.5 cm long, slender. Petiole almost absent; leaf blade, abaxially pale green, adaxially dull green, narrowly elliptic to narrowly obovate, 1.7–2.3 × 0.3–0.6 cm, thinly leathery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially and adaxially, base cuneate, margin entire, apex acuminate. Inflorescences solitary flowers or sometimes a pair of flowers; pedicels 15–34 mm long, slender. Sepals in 2 whorls; outer sepals ovate, 5–6 × 3–4 mm, apex acute; inner sepals obovate, 6–7 × 3.5–4 mm; petals broadly elliptic, 4.5 × 3 mm, apex acuminate, incised. Stamens ca. 5 mm; anther connective truncate. Pistil ca. 5 mm long; ovules 2 or 3 per ovary. Berry red, or scarlet, oblong, 10 × 5 mm; style persistent, ca. 1 mm long.
Phenology. Berberis litangensis has been collected in flower in July and in fruit in September.
Distribution and habitat. Berberis litangensis is known only from the type and one other collection from the same area in Litang Xian in W Sichuan. It was found in roadside thickets at 4089 m.
Proposed IUCN conservation status. Berberis litangensis is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Sichuan. Litang Xian by national highway G318, 30.148194°N, 100.040083°E, 4089 m, 9 Sep. 2021, Z.Q. Du yxing 4737 (HITBC).
4.2.7. Berberis longquensis Y.K. Li, Harber, Y.W. Xing & C.C. Yu, sp. nov. (Figs. 3 and 7). 隆曲小檗Type. CHINA. Qinghai, Yushu Xian, by national highway G214, 32.708417°N, 96.616306°E, 4002 m, 1 July 2020, C. C. Yu & Z. Q. Du yxing 4284 (holotype HITBC 0057621, isotypes A, KUN, PE).
Diagnosis. Berberis longquensis has leaves that are somewhat similar to those of B. qinghaiensis, but somewhat smaller. It has reddish brown mature stems as against the pale yellowish ones of B. qinghaiensis. The flower structure is also different.
Shrubs, deciduous to 1.6 m tall; mature stems brown or reddish brown, sparsely verruculose, sulcate; spines 3-fid, concolorous, 1–2 cm long, slender. Petiole almost absent; leaf blade abaxially pale green or sometimes grayish white, adaxially dull green, narrowly obovate or obovate-lanceolate, 0.9–1.3 (–1.6) × 0.3–0.5 cm, papery, midvein slightly raised abaxially, slightly impressed adaxially, venation and reticulation inconspicuous abaxially, conspicuous adaxially, base attenuate, margin entire or spinose with 1–4 teeth on each side, apex acute or obtuse. Inflorescences solitary flowers; 4–7 mm long, slender. Sepals in 2 whorls; outer sepals narrowly obovate, 3 × 1–2 mm, apex acuminate or round; inner sepals oblong-elliptic, 5 × 3 mm; petals broadly elliptic, 3 × 2 mm, apex acuminate, incised. Stamens ca. 4 mm long; anther connective slightly extended. Pistil ca. 4 mm long. Ovules 3–5 per ovary. Immature berry oblong or ovoid, color unknown, 8 × 4 mm; style persistent.
Phenology. Berberis longquensis has been collected in flower in late June and with immature fruit in August.
Distribution and habitat. Berberis longquensis is currently known only from Yushu Xian, Qinghai. The type specimens was collected on a roadside at the base of a rocky slope at 4002 m and by Boufford et al. 44585 on a scrubby hillside at 4080 m. Xizang Herbal Medicine Survey Team 1257 was collected on a slope at 3840 m.
Etymology. Berberis longquensis is named after the river "Longqu" which passes through the type locality.
Proposed IUCN conservation status. Berberis longquensis is assessed as DD or Data Deficient, according to IUCN (2012) criteria.
Specimens examined. Qinghai. Yushu Xian: Ang Qian Lin Chang, 3840 m, 1 Aug. 1972, Xizang Herbal Medicine Survey Team 1257 (HNWP 28546, PE 01037822); on road to monastery near Gaimugan, ca. 4.5 km from national highway G214, 32.691944°N, 96.647778°E, 4080 m, 13 Aug. 2018, D.E. Boufford et al. 44585 (A, CAS, E, KUN, PE).
Author contributionsY.-W. X. managed the project; C.C. Y., J. H. and Y.-W. X. conceived this research; C.C. Y. and Z.-Q. D. collected the plant material, C.C. Y. and Y.-K. L. sequenced the raw data; C.C. Y. and C. P. processed the raw data and analyzed the data; C.C. Y. and Y.-K. L. wrote the manuscript; J. H. revised this paper. All authors reviewed and approved the final submission.
Declaration of competing interestThe authors declare no conflict of interests.
AcknowledgementsOur grateful thanks to Mrs. Hong Xi Zhang and Hai Lei Zheng for their assistance in the field during 2019–2021. This study was supported by the National Natural Science Foundation of China (U1802242), and the Chinese Academy of Sciences Taiwan Young Talent Programme (2018TW2SB0002).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2022.03.002.
Adhikari, B., Pendry, C.A., Pennington, R.T., et al.
, 2012. A revision of Berberis s.s. (Berberidaceae) in Nepal. Edinb. J. Bot., 69: 447-522. DOI:10.1017/S0960428612000261 |
Ahrendt, L.W.A.
, 1961. Berberis and Mahonia. A taxonomic revision. Bot. J. Linn. Soc., 57: 1-410. DOI:10.1111/j.1095-8339.1961.tb00889.x |
Chang, Y.H., Yao, G., Neilsen, J., et al.
, 2021. Rhododendron kuomeianum (Ericaceae), a new species from northeastern Yunnan (China), based on morphological and genomic data. Plant Divers., 43: 292-298. DOI:10.1016/j.pld.2021.04.003 |
Du, C., Liao, S., Boufford, D.E., et al.
, 2020. Twenty years of Chinese vascular plant novelties, 2000 through 2019. Plant Divers., 42: 393-398. DOI:10.1016/j.pld.2020.08.004 |
Ding, W.N., Ree, R.H., Spicer, R.A., et al.
, 2020. Ancient orogenic and monsoon-driven assembly of the world's richest temperate alpine flora. Science, 369: 578-581. DOI:10.1126/science.abb4484 |
Harber, J., 2020. The Berberis of China and Vietnam: A Revision, Monogr. Syst. Bot. Missouri Bot. Gard. pp. 359
|
Hsieh, C.L., Yu, C.C., Huang, Y.L., et al.
, 2022. Mahonia vs. Berberis unloaded: generic delimitation and infrafamilial classification of Berberidaceae based on plastid phylogenomics. Front. Plant Sci., 12: 720171. DOI:10.3389/fpls.2021.720171 |
IUCN, 2012. IUCN Red List Category and Criteria. Version 3.1, second ed., Gland and Cambridge, pp. 32
|
Jin, J.J., Yu, W.B., Yang, J.B., et al.
, 2020. Get Organelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol., 21: 241. DOI:10.1186/s13059-020-02154-5 |
Katoh, K., Standley, D.M.
, 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol., 30: 772-780. DOI:10.1093/molbev/mst010 |
Kearse, M., Moir, R., Wilson, A., et al.
, 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. DOI:10.1093/bioinformatics/bts199 |
Kim, Y.D., Kim, S.H., Landrum, L.R.
, 2004. Taxonomic and phytogeographic implications from ITS phylogeny in Berberis (Berberidaceae). J. Plant Protect. Res., 117: 175-182. DOI:10.4258/jksmi.2004.10.2.175 |
Kreuzer, M., Howard, C., Adhikari, B., et al.
, 2019. Phylogenomic approaches to DNA barcoding of herbal medicines: developing clade specific diagnostic characters for Berberis. Front. Plant Sci., 10: 586. DOI:10.3389/fpls.2019.00586 |
Li, L., Zhang, J., Lu, Z.Q., et al.
, 2021. Genomic data reveal two distinct species from the widespread alpine ginger Roscoea tibetica Batalin (Zingiberaceae). J. Syst. Evol., 59: 1232-1243. DOI:10.1111/jse.12596 |
Landrum, L.R.
, 1999. Revision of Berberis (Berberidaceae) in Chile and adjacent southern Argentina. Ann. Mo. Bot. Gard., 86: 793-834. DOI:10.2307/2666170 |
Letunic, I., Bork, P.
, 2021. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res, 49: W293-W296. DOI:10.1093/nar/gkab301 |
Nieto Feliner, G., Rossello, J.A.
, 2007. Better the devil you know? Guidelines for insightful utilization of nrDNA ITS in species-level evolutionary studies in plants. Mol. Phylogenet. Evol., 44: 911-938. DOI:10.1016/j.ympev.2007.01.013 |
Olonova, M.V., Sylvester, S.P.
, 2021. Poa liuliangii and P. kengii (Poaceae), two new species from the Hengduan Mountains of southwest China. Taiwania, 66: 251-257. |
Rambaut, A., 2010. FigTree v1.3.1. Edinburgh, Scotland, Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree.
|
Schneider, C.K.
, 1916. Weitere Beiträge zur Kenntnis der chinesischen Arten der Gattung Berberis (Euberberis). Oesterr. Bot. Z., 66: 313-326. DOI:10.1007/BF01634312 |
Schneider, C.
, 1939. Neue Berberis der sect. Wallichianae. Repert. Spec. Nov. Regni Veg., 46: 245-267. |
Schneider, C.
, 1942. Die Berberis der section Wallichianae. Mitt. Deutsch. Dendrol. Ges., 55: 1-60. |
Shi, L., Chen, H., Jiang, M., et al.
, 2019. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res., 47: W65-W73. DOI:10.1093/nar/gkz345 |
Stamatakis, A.
, 2014. RAxML version 8: a tool for phylogenetic analysis and post- analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Sun, H., Zhang, J.W., Deng, T., et al.
, 2017. Origins and evolution of plant diversity in the Hengduan Mountains, China. Plant Divers., 39: 161-166. DOI:10.1016/j.pld.2017.09.004 |
Tillich, M., Lehwark, P., Pellizzer, T., et al.
, 2017. GeSeq – versatile and accurate annotation of organelle genomes. Nucleic Acids Res, 45: W6-W11. DOI:10.1093/nar/gkx391 |
Wu, Z.Y.
, 1988. Hengduan mountain flora and their significance. J. Jpn. Bot., 63: 297-311. |
Xing, Y.W., Ree, R.H.
, 2017. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A., 114: E3444-E3451. |
Ya, J.D., Lin, D.L., Han, Z.D., et al.
, 2021. Three new species of Liparis s.l. (Orchidaceae: Malaxideae) from Southwest China based on morphological characters and phylogenetic evidence. Plant Divers., 43: 401-408. |
Ye, X.Y., Zhang, Y.X., Li, D.Z.
, 2021. Two new species of Yushania (Poaceae: Bambusoideae) from South China, with a taxonomic revision of related species. Plant Divers., 43: 492-501. |
Ying, J., 2011. Berberis L. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds. ), Flora of China, vol. vol. 19. Science Press & Missouri Botanical Garden, Beijing & St. Louis, pp. 715-771
|
Yu, C.C., Chung, K.F.
, 2014. Systematics of Berberis sect. Wallichianae (Berberidaceae) of Taiwan and Luzon with description of three new species, B. schaaliae, B. ravenii, and B. pengii. Phytotaxa, 184: 61-99. DOI:10.11646/phytotaxa.184.2.1 |
Yu, C.C., Chung, K.F.
, 2017. Why Mahonia? Molecular recircumscription of Berberis s.l., with the description of two new genera. Alloberberis, 66: 1371-1392. DOI:10.12705/666.6 |
Zheng, Q.J., Yu, C.C., Xing, Y.W., et al.
, 2021. A new Rorippa species (Brassicaceae), R. hengduanshanensis, from the hengduan mountains in China. Phytotaxa, 480: 210-222. DOI:10.11646/phytotaxa.480.3.1 |



