b. College of Ecology and Resources Engineering, Wuyi University, Wuyishan, Fujian, 354300, China
Forest gaps provide suitable environmental conditions for plant regeneration (Yamamoto, 2000) and community succession (Feldmann et al., 2018). The gap partitioning hypothesis (GPH) indicated that gap creates the large resource gradients from gap centers to the understory (Lu et al., 2018a). Forest gaps improve light intensity, air temperature, and relative humidity through the action of underground soil (Sevillano et al., 2016; Kern et al., 2013), and reduce the seedling mortality caused by pathogens (Lemoine et al., 2017; Bayandala et al., 2016), thus influencing plant regeneration, species composition and community structure (Velázquez and Wiegand, 2020; Guo et al., 2019). Seedling grows faster in forest gaps than understory make them important for seedling regeneration (Lu et al., 2018a). Moreover, seedlings are difficult to survive in understory with high canopy closure and low light intensity (Chmura et al., 2017).
With the increasing of gap size, the environmental heterogeneity expresses an increasing trend, thus affecting the plant regeneration (Zhang et al., 2018; Vahabinia et al., 2019). The effect of forest gaps showed positive effects on seedlings establishment of shade-intolerant species (Lhotka, 2013). However, several results showed that gap size increases with higher light intensities increase the probability of seedlings being burned, and decrease soil moisture by enhancing soil evaporation rate, thereby it is not conducive to promoting shade-tolerant plant species regeneration (Čater et al., 2014). Thus, it is very important to understand the function of gap size, which could be used as a practical measurement for plant regeneration.
Beside the gap size, within-gap position influences tree regeneration and species diversity (Zhang et al., 2018). The redistribution of environmental resources at different within-gap position affect species composition and community structure (Diaci et al., 2020; Pełechaty et al., 2015). Seedling emergence could be significantly related to within-gap positions in larger gaps rather than smaller gaps due to different degrees of environmental heterogeneity. Lu et al. (2018b) observed seedling survival in the gap center, which was significantly higher than that of gap edges and non-gap. Moreover, the interaction between gap size and within-gap position were found to have a significant effect on seedling growth (Lu et al., 2018a). Therefore, investigating the combined effect of gap size and within-gap position on seed germination and seedlings growth could improve our understanding of the role of forest gaps for forest regeneration.
Castanopsis kawakamii, a tertiary endangered relict plant species of Fagaceae, occurs in a comparatively narrow region and is found in the subtropical forest of China and Viet Nam (Liu et al., 2011, Temperate Broadleaved Tree Specialist Group, 1998). The C. kawakamii Nature Reserve, located in the Fujian province of China, with 700 hm2 of natural forest dominated by the C. kawakamii population (He et al., 2015). This population is a rare natural community with the largest area and highest purity of C. kawakamii in the world, and its population age is above 100 years. However, this natural population is difficult to regeneration as human disturbance, seed provenance, thus their age structure has an "inverted pyramid" shape (He et al., 2019). Recently, due to the comprehensive effect of biological characteristics, environment and human activities, forest canopy fragmented seriously and the number of gaps increased constantly. This affects microenvironment heterogeneity, including climate and soil properties (He et al., 2015), influences the seedling growth (He et al., 2018) and species distribution patterns (He et al., 2019). However, the effect of gap size and within-gap position on seed germination and radicle growth of C. kawakamii is still unclear. Thus, we hypothesized that seed germination and radicle growth would be affected by different sizes of forest gaps and different within-gap positions. We aimed to answer three questions: (1) Which forest gap sizes were suitable for seed germination and radicle growth of C. kawakamii? (2) Which positions in forest gaps and non-gap were favorable for seed germination and radicle growth of C. kawakamii? (3) Which environmental factors were the main factor influencing seed germination and radicle growth of C. kawakamii. We expected that our results would help in projects aimed to promote seed germination of this endangered plant species as well as provide a theoretical basis for natural regeneration of C. kawakamii forests.
2. Materials and methods 2.1. Study siteThis study was conducted in the Castanopsis kawakamii Nature Reserve, located in the southwest of Sanming City, Fujian Province, China (26°07′–26°12′ N, 117°24′–117°29′ E). The area is an extension of Mount Wuyi at altitude of 180–604 m. The study site has a subtropical monsoon climate, with the average annual temperature of 19.5 ℃ and the average annual precipitation of 1500 mm. The soil type is typical zonal soil which mainly consists of ferric acrisols with abundant humus (Wang et al., 2021b). The forest type is dominated by C. kawakamii population, and besides this species, the main species of this forest include Litsea subcoriacea and Schima superba in the tree layer, followed by Diplospora dubia and Clausena dentata in the shrub layer, and Woodwardia japonica and Alpinia japonica in the herb layer (Buajan et al., 2018).
2.2. Sample plot selection and seed collection 2.2.1. Sample plot settingOn a cloudy and windless day in March 2018, we used an unmanned aerial vehicle (UAV DJI Phantom 4 Pro, SZ DJI Technology Co., Ltd., Shenzhen, China), and combined with field investigations to obtain high-resolution remote sensing images of forest gaps in the investigated area. Using the Nikon camera D7200 with a fisheye lens (NIKON DX AF FISHEYE NIKKOR 10.5 mm 1:2.8G ED, NIKON, Tokyo, Japan), we photographed forest gaps in their centers at 1 m above the ground, and calculated the gap size by the hemispherical image method (Hu et al., 2009). The photographs were analyzed using the Gap Light Analyzer software (GLA) to assess canopy openness (Xu et al., 2016). According to gap characteristics and size (Lu et al., 2020), we selected three large gaps (LG, > 200 m2), three medium gaps (MG, 50–100 m2), three small gaps (SG, 30–50 m2), and non-gap (NG, 400 m2) for comparison (Fig. 1; Table S1), and recorded the causes of gap formation (tree fall, natural death, branch broken and trunk broken), slope and altitude.

|
| Fig. 1 Distribution of large gaps, medium gaps, small gaps and non-gap in Castanopsis kawakamii natural forest. |
The seeds of Castanopsis kawakamii were collected from the field in October 2018. All collected seeds were placed in two well-ventilated storage tanks on 10 cm thick layer of fine sand and sprayed with 1.25 g. L−1 trichlorfon to sand storage at room temperature. Before the experiment, the seeds were removed from storage tanks and washed with distilled water. Then, they were soaked in 0.5% KMnO4, disinfected for 30 min, and the intact and pest free seeds (> 2 g per grain) were selected for the germination experiment.
2.3. Experimental designIn January 2019, we set the seed germination plots (2 m × 2 m × 5 cm) in the east, west, south, north, and center positions in the forest gaps and non-gap. A seed germination frame (1.5 m × 1.5 m) was placed in each germination plot (Fig. 2A). In addition, each germination frame was divided into three small rectangles (0.5 m × 1.5 m), which were counted as three replicates (Fig. 2B). We sowed 50 intact Castanopsis kawakamii seeds in each replicate, with a total of 150 seeds in each germination plot. In order to eliminate the effect of shrub, herb, and litter layers on the seed germination and radicle growth of C. kawakamii, we removed them from the germination plots before the experiment.

|
| Fig. 2 (A) Diagram of plot distribution in large gaps, medium gaps, small gaps, non-gap and within-gap positions; (B) The illustration of planting in gap sizes and within-gap positions. |
The seed germination frame (Fig. 2B) was divided into two layers (upper frame and lower frame), and the details of them as follows: we cleared the litter on the soil surface, and placed the germination frame (lower frame) with nylon mesh (aperture 2 mm), and put the seed to be determined and soil (about 2 cm) on it. Then we placed the upper germination frame, then covered the soil and litter, and ensured the combination of the upper and lower germination frame. The function of the lower nylon mesh was to reduce the probability of the radicle penetrating into the soil, and make it grow in the germination frame. The aim was to facilitate measurement and reduce the damage caused by measurement. The function of the upper nylon mesh frame was to facilitate the observation of seed germination and record data, and reduce the probability of animals feeding on seeds.
2.4. Environment factors monitoringAn iButton data logger (Maxim Intergrated, iButton, San Jose, USA) was put in the center of each forest gaps and non-gap. The air temperature and relative humidity recorder (iButton DS1923-F5) was placed 1.5 m above the soil surface, and the soil temperature recorder (iButton DS1922L-F50) was placed at the depth of 5 cm below the soil surface. These recorders automatically recorded and stored air temperature, relative humidity, and soil temperature data every 4 h at the same time of day in the period from January to April 2019.
2.5. Determination of the seed germination indexWe observed the germination of seeds about 10 days after sowing. The standard for the determination of seed germination is that the radicle length has to break 2 mm through the seed coat (Guo et al., 2018). The germinated seeds were marked with a tag, which was more convenient for counting the number of germinating seeds and measuring seed radicle length every week. At the peak of seed germination, we selected the radicle with the same length (5 grains in each replicate) and measured the radicle length of gaps and within-gap position with electronic vernier caliper (accuracy 0.01 mm). The seed germination experiment was ended when the total number of germinated seeds did not increase for two continuous weeks. To reduce adverse effects on late seedling emergence during measurement, we measured the radicle length five times. The germination experiment lasted for 9 weeks and the whole experiment lasted for 70 days. At the end of the experiment, the number of seed germination and radicle length were calculated. The germination rate was calculated as follows:
| Germination rate (%) = the number of germinated seeds / total number of tested seeds × 100% | (Germination rate % = the number of germinated seeds / total number of tested seeds × 100%) |
SPSS 21.0 was used for data analysis in our study. Significant main effects and interactions were investigated using Ducan' test to make comparisons among means, so as to examine the effects of gap size and within-gap position on seed germination and radicle growth of Castanopsis kawakamii. Based on one-way ANOVA, Duncan's test was used to explore the effects of gap size and within-gap position on air temperature, air relative humidity and soil temperature, seed germination and radicle growth of C. kawakamii (P < 0.05).
In order to investigate the main factor affecting seed germination and radicle growth of Castanopsis kawakamii, we carried out a comprehensive analysis of the relationship among the environmental factors in the gaps, seed germination, and radicle growth. Among the environmental variables, forest gaps and non-gap were non-numeric variables which should be evaluated and quantified before the correlation analysis. The non-gap was not completely closed, which made the non-gap area unable to be assigned a value. Here, the canopy openness was affected by gap size, and it gradually increased with the increasing of gap size (R2 = 0.899). Thus, we used canopy openness to replace gap size. In case of significant correlation among gap sizes, environmental factors, seed germination and radicle length, generalized linear models (GLM) based on normal distribution were used to explore the key factors affecting seed germination and radicle growth of the investigated species. Therefore, we used canopy openness, position, air temperature, relative air humidity, soil temperature, slope and altitude as independent variables. According to the AIC criterion for optimizing the model, we used variance expansion factor VIF (Variance Inflation Factor) to diagnose the collinearity of the regression model (Luo et al., 2019). When VIF was above 10, we eliminated the variable with the highest VIF value and then stimulated the model again until all the VIF values were below 10 for the variables.
Generalized linear models combined with R version 3.6.1 (R Core Team, 2018) were used to analyze the relationship between seed germination, radicle growth, and environmental factors. The 'broom' package in R was used to analyze the relationship among seed germination rate, radicle length, and environmental factors (Luo et al., 2019). Data with residuals that did not conform to assumptions of normality and/or homogeneity of variances were transformed using Box–Cox transformations (Sevillano et al., 2016). The 'car' and 'MASS' packages in R were used for normality and homoscedasticity tests (Fox and Weisberg, 2011). The 'ggplot2' package in R was used to declare the input data frame for a graphic and to specify the set of plot aesthetics intended to be common throughout all subsequent layers (Nicholas, 2015).
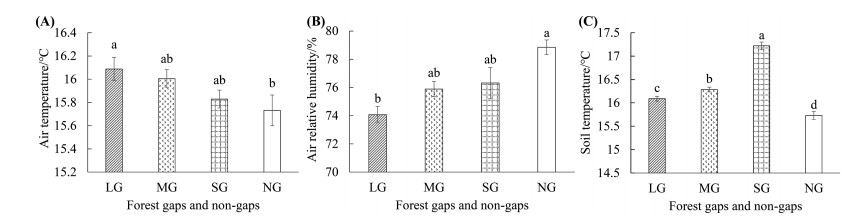
3. Results 3.1. Environmental factors in forest gaps and non-gapThe mean air temperature in the forest gaps was significantly higher than that in non-gap (Fig. 3A). With the increasing of gap size, mean air temperature gradually increased (LG > MG > SG > NG). The large gaps had the highest mean air temperature (16.09 ℃), whereas the non-gap had the lowest temperature (15.73 ℃).
|
| Fig. 3 The environmental factors of C. kawakamii in the forest gaps and non-gap. LG, MG, SG and NG represent large gaps, medium gaps, small gaps and non-gap. (A) The mean air temperature in forest gaps and non-gap; (B) The mean air relative humidity in forest gaps and non-gap; (C) The mean soil temperature in forest gaps and non-gap. Data (mean ± SE) marked with different letters (a, b) in plots were significantly different under P < 0.05 in the same figure. |
The mean relative air humidity in the forest gaps was lower than that in non-gap (Fig. 3B). From the non-gap to large gaps, relative air humidity demonstrated a decreasing trend (NG > SG > MG > LG), and the mean relative air humidity in non-gap was significantly higher than large gaps.
There was a significant difference in mean soil temperature between the forest gaps and non-gap (Fig. 3C) (SG > MG > LG > NG). Small gaps had the highest mean soil temperature (17.22 ℃), whereas non-gap was the lowest (15.73 ℃). This indicated that the presence of forest gaps could improve mean soil temperature conditions in this forest.
3.2. Effect of gap size and position on seed germination of Castanopsis kawakamiiThe results of the two-way ANOVA for gap size, within-gap position, and their interaction on the seed germination rate of Castanopsis kawakamii was shown in Table 1. Forest gap sizes had a significant effect on seed germination rate of C. kawakamii (P < 0.001), whereas no significant effect of gap position and the interaction between gap size and gap position on these factors were observed (P > 0.05).
| Model | df | F | P | |
| Seed germination rate/% | Position | 4 | 0.257 | 0.904 |
| Gap size | 3 | 19.729 | < 0.001*** | |
| Position × Gap size | 12 | 0.824 | 0.625 | |
| Notes: df: degree of freedom; F: the F value; P: the P value. *** represent significant correlation at the 0.001 level. | ||||
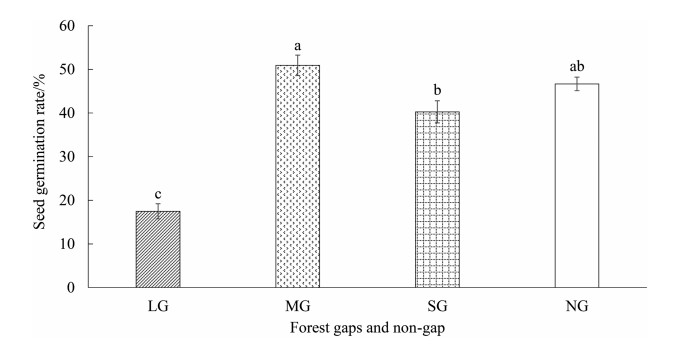
From highest to lowest, the seed germination rate of Castanopsis kawakamii was medium gaps, non-gap, small gaps and large gaps (Fig. 4). C. kawakamii had the highest seed germination rate (51%) and the highest number of viable seeds in medium gaps. The germination rate was 47% in non-gap, 40% in small gaps, and 17% in large gaps. The seed germination rate in medium gaps was significantly higher than that in small gaps and large gaps, whereas in large gaps, it was significantly lower than in medium gaps, small gaps, and non-gap. Medium gaps were the probably most suitable for seed germination, whereas large gaps had an inhibitory effect on the seed germination of C. kawakamii.
|
| Fig. 4 The seed germination rate of C. kawakamii in the large gaps, medium gaps, small gaps and non-gap. Data (mean ± SE) marked with different letters (a, b) in plots were significantly different under p < 0.05 in the same figure. |
Among the gaps and non-gap within the same position (Fig. 5), there was a significant difference in the effect of gap size on the seed germination rate of Castanopsis kawakamii (P < 0.05). The seed germination rate of C. kawakamii was the highest in small gaps (54.22%), and that of large gaps was significantly lower than that of the other gaps in the east position. With the exception of the east position, the seed germination rate of Castanopsis kawakamii in the other positions in medium gaps was higher than that in the other positions in other gaps. We concluded that the seed germination rate of large gaps was lower than that of other gaps in all gap positions.

|
| Fig. 5 The seed germination rate of Castanopsis kawakamii in the within-gap positions. E, S, W, N and C in the figure represent east, south, west, north, center position. Different letters in the figure represent the significant differences of seed germination rate among gap sizes and non-gap in the same position (P < 0.05). |
Gap size, time, and their interaction significantly influenced radicle growth (Table 2). There were significant differences between the influences of forest gaps and non-gap on radicle growth of C. kawakamii (Fig. 6). In the early stage of radicle growth (< 15 d), radicle length was highest in large gaps, and it was lower in non-gap than that in other gaps. In the later stage of radicle growth (> 15 d), radicle length was significantly higher in medium gaps than those in non-gap. The difference in radicle length between large gaps, small gaps, and non-gap was not significant. In addition, it can be seen that radicle length of C. kawakamii was higher in forest gaps than that in non-gap, and medium gaps had the most significant effect on radicle growth of C. kawakamii.
| Between groups | df | F | P | Within groups(time) | df | F | P |
| Position | 4 | 0.374 | 0.826 | Time | 4 | 960.677 | < 0.001*** |
| Gap size | 3 | 3.811 | < 0.017* | Time × position | 16 | 1.312 | 0.195 |
| Position × gap size | 12 | 0.847 | 0.604 | Time × gap size | 12 | 3.109 | < 0.001*** |
| Time × position × gap size | 48 | 1.11 | 0.311 |

|
| Fig. 6 The radicle length of Castanopsis kawakamii in the large gaps, medium gaps, small gaps and non-gap. Different letters in the figure represent the differences of radicle length among gap sizes and non-gap in the same day (P < 0.05). |
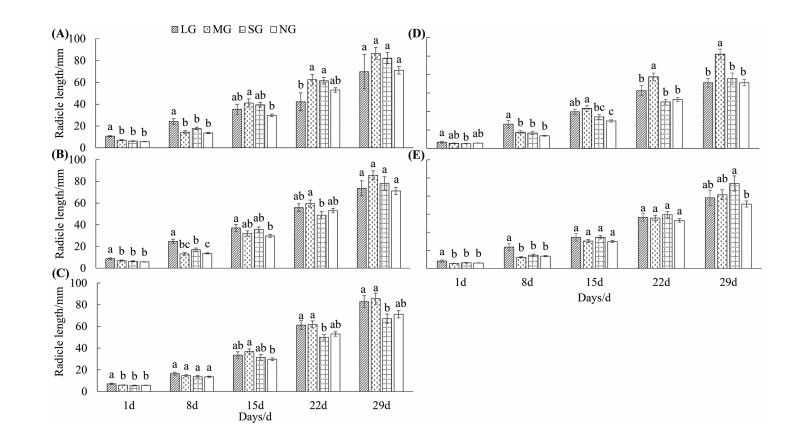
In the same positions, different gap sizes had significantly different effects on radicle growth (Fig. 7A–E). In the early stage of radicle growth (< 15 d), radicle length of Castanopsis kawakamii in large gaps in the east and south positions was significantly higher than that of the same positions in other gaps. In the later stage of radicle growth (29 d), there was no difference in radicle length among large gaps, medium gaps, small gaps, and non-gap (Fig. 7A and B). In the early stage of radicle growth (< 8 d), the radicle length of large gaps was the highest in the west position (Fig. 7C). Radicle length of C. kawakamii in small gaps was significantly lower than that of medium gaps (> 15 d), and no significant difference in radicle length of C. kawakamii was observed among large gaps and non-gap, the trend of radicle length, from highest to lowest, was medium gaps, large gaps, non-gap and small gaps. In the north position (Fig. 7D), plants grown in large gaps with the highest radicle length, and the difference in radicle length between C. kawakamii in medium gaps, small gaps, and non-gap was not significant (< 8 d). On day 15 of radicle growth, C. kawakamii in medium gaps had the longest radicle length, and it was significantly different from that of plants in non-gap. In the later stage of radicle growth (> 15 d), radicle length of C. kawakamii in medium gaps was significantly higher than those of C. kawakamii in large gaps, small gaps, and non-gap. In the center position (Fig. 7E), the radicle length of C. kawakamii in large gaps was significantly higher than that of C. kawakamii in other gaps (< 8 d). On the day 15 and 22, gaps sizes were found to have no significant influence on radicle length. In the later stage of radicle growth (29 d), the radicle length of small gaps was significantly higher than that in other gaps.
|
| Fig. 7 The radicle length of Castanopsis kawakamii in the within-gap positions. (A) East position; (B) South position; (C) West position; (D) North position; (E) Center position. Different letters in the figure represent the differences of radicle length among gap size and non-gap in the same day. |
Canopy openness, air temperature, soil temperature and slope had significant influences on the seed germination rate of Castanopsis kawakamii (Fig. 8). Seed germination rate was positively correlated with air temperature and slope, whereas it was negatively correlated with soil temperature and canopy openness (gap size). Radicle length of C. kawakamii was positively related with air temperature. However, canopy openness had significantly negative correlation with radicle growth. The slope was only found to has a influence on the seed germination rate in the forest gaps and non-gap. Therefore, these results indicated that air temperature and canopy openness were the main factors affecting the seed germination and radicle growth of C. kawakamii.

|
| Fig. 8 GLM analysis of the relationship among the seed germination rate and radicle length and environmental factors. (A) The relationship between seed germination rate and environmental factors; (B) The relationship between radicle length and environmental factors. ST: Mean soil temperature; Posi: Position; CO: Canopy openness; AT: Mean air temperature; Alti: Altitude. The relative influence of environmental factors on the seed germination rate and radicle length in gaps of different sizes. Effect sizes are standardized coefficients from linear mixed models estimated separately for each predictor variable. The lines indicate 95% confidence interval, solid red circles indicate significant positive effects, solid green circles indicate significant negative effects, and solid blue circles indicate non-significant effect. |
The gap sizes play a crucial role in the seed germination by changing the environmental factors. Our filed survey indicated that gap size (canopy openness) is the main factor affecting seed germination, the seed germination rate of Castanopsis kawakamii in forest gaps was, from highest to lowest, medium gaps, non-gap, small gaps and large gaps, which are not in accordance with the findings of previous study in which seedling growth and survival were enhanced in large gaps (Clarke, 2004). The reason may be that the differences of species response to light. In the early growth stage, C. kawakamii are the shade-tolerant tree species, the moderate shady environment is beneficial to promote seed germination and growth. Forest gap receives more light with increasing of gap size, which leads to plant transpiration and soil water evaporation to be enhanced (Wu et al., 2012). This result may reduce seeds vigor, and thus inhibiting the seed germination to some extent (Bakhshandeh and Gholamhossieni, 2019). Our results demonstrated that air temperature was the main factor affecting seed germination rate of C. kawakamii among gap sizes. The reason is probably that forest canopy received fewer light conditions when canopy coverage increased, thus affects the heating rate in understory. Whereas, the heating rate in understory increased when the canopy coverage decreased, which affected the imbalance between seed germination and climate change (Zellweger et al., 2020). Hence, the suitable microclimate conditions may be causing the seed germination rate of C. kawakamii in medium gaps were higher than other gaps. Soil moisture may also be an important factor affecting seed germination. Previous studies have shown that soil moisture in small gaps were higher than large gaps, medium gaps, and non-gap (He et al., 2015). Higher soil moisture in small gaps could hinder the gas exchange between seed and its external environment (Obroucheva et al., 2017), whereas lower soil moisture cannot meet the seed needs for water to sprout (Dong et al., 2011). Therefore, these conditions in large gaps and small gaps could unfavorable for seed germination. Medium gaps provide suitable temperature conditions, and ensure sufficient water to promote seed germination, thereby causing the seed germination rate of C. kawakamii was significantly higher in medium gaps than other gaps.
4.2. Effects of gap positions on seed germination of Castanopsis kawakamiiThe gap partitioning hypothesis claimed that tree species would regenerate along gap environmental gradients due to the gap formation (Lu et al., 2018a). Light availability varies asymmetrically in gaps, thus leading to the difference of resource allocation (Willis et al., 2015). In each forest gap, different positions had significantly different influences on the seed germination of C. kawakamii. The seed germination rates in the east of small gaps and in the south, west, north, and center of medium gaps were higher than those in other gaps, and they were the lowest in all positions in large gaps, which may be caused by the high amounts of light intensities in all positions in the large gaps. Lu et al. (2018b) demonstrated that the light-demanding species Juglans mandshurica survived better in large gaps and medium gaps than in small gaps, and it also survived better in the gap center, north edge, and east edge than in other within-gap positions, which is contrary to our findings. This may be related to the living habits of the species. Light density is often at its maximum near the gap center (Lu et al., 2018a), which influences the distribution of light and heat resources in forest gaps. Excessive or low light may not be conducive to seed germination. In large gaps, the gap center had relatively higher light intensity and air temperature than those in other positions in large gaps. This may cause arid environmental conditions, which are unfavorable for seed germination of C. kawakamii. In certain positions in forest gaps, higher light availability can promote seed germination (Čater et al., 2014). Moreover, the effects of within-gap position on seed germination will vary with gap size as the variations of light intensity (Zhang and Yi, 2021). The higher light intensities in large gaps decrease soil moisture by enhancing evaporation rate (He et al., 2019), which may further reduce the probability of seed germination. Moreover, the temperature difference between day and night, especially the decrease of nighttime temperature, delayed seed germination under lack of canopy protection (Denslow, 1980). Similarly, the results of seed germination rate in large gaps were lower than other gaps in same position, which verified the suitable temperature could be promote seed germination of C. kawakamii.
4.3. Effects of gaps size on radicle growth of Castanopsis kawakamiiDifferent gap sizes had significantly different influences on radicle growth of C. kawakamii. The average radicle length of C. kawakamii was, from highest to lowest, medium gaps, small gaps, large gaps and non-gap. The reason may be that forest gaps increase the soil temperature and promote seed enzyme activity, thus facilitating the seeds germination (Rao et al., 2019). Our results indicated that soil temperature in large gaps and non-gap was significantly lower than that in other gaps. Low soil temperature decreased the water absorption capacity and enzyme activity in the seeds (Shahverdi et al., 2019). The results demonstrated that the soil temperature in small gaps was relatively higher than that of other gaps, which could lead to enzyme degeneration in the seeds, thereby affecting radicle growth of C. kawakamii. In addition, light intensity in forest gaps was positively correlated with gap size, and its increase directly enhances the duration and intensity of light reaching the forest floor, thus changing the light environment within gap (Lu et al., 2021). In the early stage of radicle growth, suitable amounts of light promoted the radicle growth of C. kawakamii in large gaps, and radicle length was significantly higher in large gaps than that in other gaps. On the contrary, in later stages of radicle growth in large gaps, higher light intensity improves direct irradiation, and increases the probability of radicle injuries, thus leading the radicle length was lower in plants in large gaps than that in plants in medium gaps and small gaps. Thus, large gaps are not suitable for the recruitment of C. kawakamii seedlings. Plants in non-gap did not receive enough light for radicle growth, which is why the growth trend of radicle length was slower than that in other gaps. The suitable soil temperature and light intensity in medium gaps could enhance respiration in enzymatic processes, which may increase the solubility of the nutrients stored in the seeds, thereby increasing radicle growth rate (Gu et al., 2019). Radicle growth of C. kawakamii may be affected by soil moisture. An appropriate water stress level can promote radicle growth. The radicle needs absorb appropriate water to dissolve some storage materials become available nutrients, and to meet the needs of radicle growth to a certain extent (Chamorro and Moreno, 2019). The effects of forest gaps on soil aeration may be an important factor affecting seed germination. He et al. (2015) found that forest gaps could significantly improve soil aeration by accelerating gas exchange and improving soil oxygen content during seed germination, thus promoting radicle growth. Li et al. (2019) confirmed this point that radicle length was significantly higher in forest gaps than that in non-gaps. Consequently, we concluded that medium gaps were the most suitable gap size for improving radicle growth of C. kawakamii.
4.4. Effects of gap position on radicle growth of Castanopsis kawakamiiDifferent within-gap positions receive different amounts of solar radiation, which leads to the differences in temperature, water conditions, and vegetation coverage in the forest gaps (Lu et al., 2018b), and causes significant differences in the soil physical, chemical, and biological processes between different within-gap positions (Wang et al., 2021a). Thus, different positions led to the variation in soil nutrient resources, and indirectly influenced the radicle growth of C. kawakamii in the forest gaps. Our results showed that in the same within-gap position, the radicle length of plants in medium gaps were significantly higher than that of other gaps, with the exception of the center position in small gaps. The difference may be caused by the variations of soil moisture and light conditions among different forest gaps. Gap size directly influence the spatial and temporal distribution of soil moisture (maximum and minimum), and the areas of maximum soil moisture (Gálhidy et al., 2006). The previous study indicated that the amounts of water needs to be appropriate for dissolving enough storage materials to promote radicle growth (Shahverdi et al., 2019). With the increasing of gap size, the ratio of crow inclination could influence the light resources redistribution in forest gaps (Orman et al., 2021). Small gaps may be too narrow to produce resource gradients, canopy closure and lower light level will further inhibit the radicle growth (Zhang and Yi, 2021), which resulting in the radicle length was lower than that of other gaps in the same position. Higher canopy openness in large gaps meet the plant needs for amounts of light in all positions, thus leading to the radicle length in large gaps was higher than that in other gaps in early growth stage. However, the radicles were permanently exposed to sunlight, and the excessive light intensity burns radicle to death, which causing the radicle length in large gaps were lower than that of medium gaps of C. kawakamii.
4.5. Implications for the regeneration of Castanopsis kawakamii populationsBy systematically evaluating along gap resource gradients, our study showed that gap size affected the seed germination and radicle growth of C. kawakamii, but does not support the gap partitioning hypothesis for the growth of C. kawakamii. As C. kawakamii is a shade-tolerant species, it needs a relatively shady environment for its growth. Large gaps not suitable for seed germination and radicle growth of this species due to relatively higher light intensities, higher air temperature, and lower soil moisture. On the other hand, higher canopy closure and lower air temperature in small gaps and non-gap, which also inhibited the seed germination and radicle growth of C. kawakamii. Among the investigated gap sizes, we found that medium gaps had the suitable light intensity (canopy openness) and air temperature conditions (Fig. 3), which are conducive to the early growth of C. kawakamii. Results of the GLM analysis demonstrated that the interaction of air temperature and gap size (i.e., canopy openness) significantly influence the seed germination and radicle growth of C. kawakamii. In future forest management, our results could be used for sowing C. kawakamii seeds in forest gaps of suitable sizes and in appropriate within-gap positions by artificial transplantation or cultivation. In summary, along with providing a theoretical basis for forest regeneration, this study will be beneficial for establishing a seedling bank and promoting the sustainable development of C. kawakamii populations.
5. ConclusionsOur results showed that gap sizes affect the environmental conditions, and which have significant effects on the seed germination and radicle growth of C. kawakamii. The seed germination rate of C. kawakamii varied among different gap sizes, and it was higher in medium gaps than in the other gaps. Regarding the within-gap position, medium gaps might be more beneficial for seed germination when plants are grown in the south, west, north, and center position, whereas small gaps may be more beneficial when plants are grown in the east position. Regarding radicle length, our results indicated that forest gaps could significantly promote radicle growth (medium gaps > small gaps > large gaps > non-gap). The radicle length was higher in medium gaps in the east, south, west and north positions than that in large gaps, small gaps and non-gap. In summary, medium gaps (50–100 m2) were found to be the suitable for the seed germination and radicle growth of C. kawakamii. In the future, we can manipulate medium gaps and expand the areas of small gaps to promote the regeneration of C. kawakamii in the natural forest.
Author contributionsJing Zhu conceived and designed the research; Jing Zhu, Cong Xing and Meng-Ran Jin installed the experiment and collected the data; Lan Jiang and De-Huang Zhu performed the analysis; Jing Zhu wrote the manuscript; Jin-Fu Liu and Zhong-Sheng He discussed the results and commented on the manuscript. All authors approved the final version of the manuscript.
Declaration of competing interestThere are no conflicts of interest to declare.
AcknowledgementsWe wish to express our thanks for the support received from the Castanopsis kawakamii Nature Reserve in Sanming City, Fujian province to allow us to collect samples. The authors wish to thank Xin-Guang Gu, Meng-Jia Li, Yao-Shun Lu and Yuan-Zhi Wu for the field and experimental work. We also thank Yu-Xin Chen from Xiamen University for helpful discussions and valuable suggestions during the revision of the manuscript. The authors also sincerely appreciate the helpful and constructive comments provided by the reviewers of the draft manuscript. This research was funded by the National Natural Science Foundation of China, grant numbers 31700550 and 31770678; the Nature Science Fund of the Fujian Province Science and Technology of China, grant number 2019J01367; the Science and Technology Promotion of Project Forestry Bureau of the Fujian Province, grant number 2018TG14-2; and the Innovation and Technology Fund of Fujian Agriculture and Forestry University, grant number CXZX2018125.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.10.003.
Bakhshandeh, E., Gholamhossieni, M., 2019. Modelling the effects of water stress and temperature on seed germination of radish and cantaloupe. J. Plant Growth Regul., 38: 1402-1411. DOI:10.1007/s00344-019-09942-9 |
Bayandala, Fukasawa, Y., Seiwa, K., 2016. Roles of pathogens on replacement of tree seedlings in heterogeneous light environments in a temperate forest: a reciprocal seed sowing experiment. J. Ecol., 104: 765-772. DOI:10.1111/1365-2745.12552 |
Buajan, S., Liu, J.F., He, Z.S., et al., 2018. Effects of gap size and locations on the regeneration of Castanopsis kawakamii in a subtropical natural forest, China. J. Trop. For. Sci., 30: 39-48. |
Čater, M., Diaci, J., Roženbergar, D., 2014. Gap size and position influence variable response of Fagus sylvatica L. and Abies alba Mill. For. Ecol. Manag., 325: 128-135. DOI:10.1016/j.foreco.2014.04.001 |
Chamorro, D., Moreno, J.M., 2019. Effects of water stress and smoke on germination of Mediterranean shrubs with hard or soft coat seeds. Plant Ecol., 220: 511-521. DOI:10.1007/s11258-019-00931-2 |
Chmura, D.J., Modrzyński, J., Chmielarz, P., et al., 2017. Plasticity in seedling morphology, biomass allocation and physiology among ten temperate tree species in response to shade is related to shade tolerance and not leaf habit. Plant Biol., 19: 172-182. DOI:10.1111/plb.12531 |
Clarke, P.J., 2004. Effects of experimental canopy gaps on mangrove recruitment: lack of habitat partitioning may explain stand dominance. J. Ecol., 92: 203-213. DOI:10.1111/j.0022-0477.2004.00861.x |
Denslow, J.S., 1980. Gap partitioning among tropical rainforest trees. Biotropica, 12: 47-55. DOI:10.2307/2388156 |
Diaci, J., Rozman, J., Rozman, A., 2020. Regeneration gap and microsite niche partitioning in a high alpine forest: are Norway spruce seedlings more drought-tolerant than beech seedlings?. For. Ecol. Manag., 455: 117688. DOI:10.1016/j.foreco.2019.117688 |
Dong, S.L., Zhang, Y., Chen, N.L., et al., 2011. Study on characteristics of seeds dormancy and germination of parasitic plant Cynomorium songaricum Rupr. Med. Plant, 2: 2, 1-4+12. |
Feldmann, E., Drößler, L., Hauck, M., et al., 2018. Canopy gap dynamics and tree understory release in a virgin beech forest, Slovakian Carpathians. For. Ecol. Manag., 415–416: 38-46. |
Fox, J., Weisberg, S., 2011. An R Companion to Applied Regression, second ed. Sage, Thousand Oaks, CA. URL: http://socserv.socsci.mcmasterca/jfox/Books/Companion.
|
Gálhidy, L., Mihók, B., Hagyó, A., et al., 2006. Effects of gap size and associated changes in light and soil moisture on the understory vegetation of a Hungarian beech forest. Plant Ecol., 183: 133-145. DOI:10.1007/s11258-005-9012-4 |
Gu, X.B., Cai, H.J., Du, Y.D., 2019. Effects of film mulching and nitrogen fertilization on rhizosphere soil environment, root growth and nutrient uptake of winter oilseed rape in northwest China. Soil Till. Res., 187: 194-203. DOI:10.1016/j.still.2018.12.009 |
Guo, G.H., Liu, X.Y., Sun, F.L., et al., 2018. Wheat miR9678 affects seed germination by generating phased siRNAs and modulating abscisic acid/gibberellin signaling. Plant Cell, 30: 796-814. DOI:10.1105/tpc.17.00842 |
Guo, Y.X., Zhao, P., Yue, M., 2019. Canopy disturbance and gap partitioning promote the persistence of a pioneer tree population in a near-climax temperate forest of the Qinling Mountains, China. Ecol. Evol., 9: 7676-7687. DOI:10.1002/ece3.5319 |
He, Z.S., Liu, J.F., Su, S.J., et al., 2015. Effects of forest gaps on soil properties in Castanopsis kawakamii nature forest. PLoS One, 10: e0141203. DOI:10.1371/journal.pone.0141203 |
He, Z.S., Liu, J.F., Zheng, S.Q., et al., 2018. Diurnal variation of photosynthetic rates of Castanopsis kawakamii seedlings and their relationships with meteorological factors in forest gaps and non-gaps. Pak. J. Bot., 50: 1361-1368. |
He, Z.S., Wang, L.J., Jiang, L., et al., 2019. Effect of Microenvironment on species distribution patterns in the regeneration layer of forest gaps and non-gaps in a subtropical natural forest, China. Forests, 10: 90. DOI:10.3390/f10020090 |
Hu, L.L., Gong, Z.W., Li, J.S., et al., 2009. Estimation of canopy gap size and gap shape using a hemispherical photograph. Trees, 23: 1101-1108. DOI:10.1007/s00468-009-0353-9 |
Kern, C.C., Montgomery, R.A., Reich, P.B., et al., 2013. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol., 6: 101-112. DOI:10.1093/jpe/rts016 |
Lemoine, N.P., Burkepile, D.E., Parker, J.D., 2017. Insect herbivores increase mortality and reduce tree seedling growth of some species in temperate forest canopy gaps. PeerJ, 5: e3102. DOI:10.7717/peerj.3102 |
Lhotka, J.M., 2013. Effect of gap size on mid-rotation stand structure and species composition in a naturally regenerated mixed broadleaf forest. New Forest., 44: 311-325. DOI:10.1007/s11056-012-9319-7 |
Li, Y., Niu, W.Q., Cao, X.S., et al., 2019. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biol., 19: 331. DOI:10.1186/s12870-019-1927-3 |
Liu, J.F., He, Z.S., Hong, W., et al., 2011. Conservation ecology of endangered plant Castanopsis kawakamii. J. Beijing Forestry Univ., 33: 136-143. |
Lu, D.L., Wang, G.G., Yan, Q.L., et al., 2018. Effects of gap size and within-gap position on seedling growth and biomass allocation: is the gap partitioning hypothesis applicable to the temperate secondary forest ecosystems in Northeast China?. For. Ecol. Manag., 429: 351-362. DOI:10.1016/j.foreco.2018.07.031 |
Lu, D.L., Wang, G.G., Yu, L.Z., et al., 2018. Seedling survival within forest gaps: the effects of gap size, within-gap position and forest type on species of contrasting shade-tolerance in Northeast China. Forestry, 91: 470-479. DOI:10.1093/forestry/cpy007 |
Lu, D.L., Zhu, J.J., Wang, X.Y., et al., 2021. A systematic evaluation of gap size and within-gap position effects on seedling regeneration in a temperate secondary forest, Northeast China. For. Ecol. Manag., 490: 119140. DOI:10.1016/j.foreco.2021.119140 |
Lu, Y.S., He, Z.S., Luo, L.J., et al., 2020. Using UAV images to explore quantitative characteristics and influencing factors of forest gaps in Castanopsis kawakamii natural forest. Guihaia, 40: 1869-1876. |
Luo, Y.H., Cadotte, M.W., Burgess, K.S., et al., 2019. Greater than the sum of the parts: how the species composition in different forest strata influence ecosystem function. Ecol. Lett., 22: 1449-1461. DOI:10.1111/ele.13330 |
Nicholas, H., 2015. An extension to 'ggplot2', for the creation of ternary diagrams [R package ggtern version 2.2.1]. Anesthesiology, 64: 72-86. |
Obroucheva, N.V., Sinkevich, I.A., Lityagina, S.V., et al., 2017. Water relations in germinating seeds. Russ. J. Plant Physiol., 64: 625-633. DOI:10.1134/S102144371703013X |
Orman, O., Wrzesiński, P., Dobrowolska, D., et al., 2021. Regeneration growth and crown architecture of European beech and silver fir depend on gap characteristics and light gradient in the mixed montane old-growth stands. For. Ecol. Manag., 482: 118866. DOI:10.1016/j.foreco.2020.118866 |
Pełechaty, M., Ossowska, J., Pukacz, A., et al., 2015. Site-dependent species composition, structure and environmental conditions of Chara tomentosa L. meadows, western Poland. Aquat. Bot., 120: 92-100. DOI:10.1016/j.aquabot.2014.06.015 |
R Core Team, 2018. R: a language and environment for statistical computing. R
Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
|
Rao, G.S., Ashraf, U., Kong, L.L., et al., 2019. Low soil temperature and drought stress conditions at flowering stage affect physiology and pollen traits of rice. J. Integr. Agr., 18: 1859-1870. DOI:10.1016/S2095-3119(18)62067-2 |
Sevillano, I., Short, I., Grant, J., 2016. Effects of light availability on morphology, growth and biomass allocation of Fagus sylvatica and Quercus robour seedlings. For. Ecol. Manag., 374: 11-19. DOI:10.1016/j.foreco.2016.04.048 |
Shahverdi, M.A., Omidi, H., Mosanaiey, H., et al., 2019. Effects of light and temperature treatments on germination and physiological traits of stevia seedling (Stevia rebuadiana Bertoni). J. Plant Nutr., 42: 1125-1132. DOI:10.1080/01904167.2019.1567781 |
Temperate Broadleaved Tree Specialist Group, 1998. Castanopsis kawakamii. The IUCN Red List of Threatened Species: e.T32387A9695771. |
Vahabinia, F., Pirdashti, H., Bakhshandeh, E., 2019. Environmental factors' effect on seed germination and seedling growth of chicory (Cichorium intybus L.) as an important medicinal plant. Acta Physiol. Plant., 41: 27. DOI:10.1007/s11738-019-2820-2 |
Velázquez, E., Wiegand, T., 2020. Competition for light and persistence of rare light-demanding species within tree-fall gaps in a moist tropical forest. Ecology, 101: e03034. |
Wang, Q., Yang, W.Q., Li, H., et al., 2021. Changes in carbon, nitrogen and phosphorus stoichiometry in decaying logs with gap positions in a subalpine forest. J. Plant Ecol., 14: 692-701. DOI:10.1093/jpe/rtab023 |
Wang, X.L., Liu, J.F., He, Z.S., et al., 2021. Forest gaps mediate the structure and function of the soil microbial community in a Castanopsis kawakamii forest. Ecol. Indic., 122: 107288. DOI:10.1016/j.ecolind.2020.107288 |
Willis, J.L., Walters, M.B., Gottschalk, K.W., 2015. Scarification and gap size have interacting effects on northern temperate seedling establishment. For. Ecol. Manag., 347: 237-247. DOI:10.1016/j.foreco.2015.02.026 |
Wu, S.H., Jansson, P.E., Kolari, P., 2012. The role of air and soil temperature in the seasonality of photosynthesis and transpiration in a boreal Scots pine ecosystem. Agr. For. Meteorol., 156: 85-103. DOI:10.1016/j.agrformet.2012.01.006 |
Xu, J.X., Xue, L., Su, Z.Y., 2016. Impacts of forest gaps on soil properties after a severe ice storm in a cunninghamia lanceolata stand. Pedosphere, 26: 408-416. DOI:10.1111/ijd.12839 |
Yamamoto, S.I., 2000. Forest gap dynamics and tree regeneration. J. Forestry Res., 5: 223-229. DOI:10.1007/BF02767114 |
Zellweger, F., De Frenne, P., Lenoir, J., et al., 2020. Forest microclimate dynamics drive plant responses to warming. Science, 368: 772-775. DOI:10.1126/science.aba6880 |
Zhang, T., Yan, Q.L., Wang, J., et al., 2018. Restoring temperate secondary forests by promoting sprout regeneration: effects of gap size and within-gap position on the photosynthesis and growth of stump sprouts with contrasting shade tolerance. For. Ecol. Manag., 429: 267-277. DOI:10.1016/j.foreco.2018.07.025 |
Zhang, M.M., Yi, X.F., 2021. Seedling recruitment in response to artificial gaps: predicting the ecological consequence of forest disturbance. Plant Ecol., 222: 81-92. DOI:10.1007/s11258-020-01089-y |



