b. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, 666303, China;
c. Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Viet Nam;
d. Graduate Academy of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi, Viet Nam;
e. CAS Key Laboratory of Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650204, China;
f. Birbal Sahni Institute of Palaeosciences, Lucknow, 226 007, India
The Arecaceae is a large and diverse pantropical monocotyledonous family, comprising 181 genera and approximately 2600 species (Dransfield et al., 2008; Baker and Dransfield, 2016; Faurby et al., 2016). Species in this family are naturally distributed throughout tropical and subtropical regions worldwide and are considered an important constituent of tropical rainforests (Dransfield et al., 2008). This family is sensitive to temperature with limited frost tolerance, largely owing to the inability of their cells to enter physiological dormancy, a typical solitary apical meristem, and abundant parenchyma cells (Larcher and Winter, 1981; Greenwood and Wing, 1995; Reichgelt et al., 2018).
Palms have a rich fossil record that can be dated back to the early Late Cretaceous (Harley, 2006; Dransfield et al., 2008). The stems of Palmoxylon andevagense Crié, P. guillieri Crié and P. ligerinum Crié, recovered from the Turonian of France (Crié, 1892) are considered to be the oldest record until now (Kvaček and Herman, 2004). Subsequently, different fossil palm organs have been reported, such as leaves, fruits, stems, etc (Harley, 2006; Dransfield et al., 2008; Hartwich et al., 2010; Allen, 2015; Greenwood and West, 2017). Abundant palm fossil records from the Cenozoic are found in the Americas, Europe, Asia and Africa from the Cenozoic (Dransfield et al., 2008).
Southeast Asia represents one of the global biodiversity hotspots, particularly with regard to plants (Böhme et al., 2011). Plant fossils reveal the evolution of biodiversity and paleoenvironmental background in the geological past (Taylor et al., 2008). Nevertheless, studies of the diversity and the evolutionary history of plants in Southeast Asia are scarce compared with many other parts of the Northern Hemisphere (Xing et al., 2016; Kooyman et al., 2019). Even though Cenozoic sedimentary basins are widely distributed in Southeast Asia (Friederich et al., 2016), only a few paleofloras have been investigated in this region since the 19th century (Zeiller, 1903; Colani, 1920; Bande and Prakash, 1986). As far as palms are concerned, Nypa fruits are known from the Eocene of Borneo (Krausel, 1923), Trinh, (1986) identified four taxa of the Arecaceae, including Livistona sp., Flabellaria sp., Sabalites sp., and Palmacites sp., from the Miocene of northern Vietnam and in northeastern Thailand, petrified palm tree stipes and woods of cf. Corypha have been found from the upper Miocene and lower Pleistocene, respectively (Pramook, 2003; Chaimanee et al., 2004). Aside from these macrofossil records, palynological evidence for fossil palms has been extensively recognized in Southeast Asia, such as pollen grains reported from Java, Borneo, Brunei, Southern Sulawesi, the Khorat Plateau and central Myanmar from the Paleocene to Pleistocene (Muller, 1968, 1979; Harley and Morley, 1995; Morley, 1998, 2000; Huang et al., 2020). However, these fossil records are still far from adequate compared with the current high diversity of palms, which number approximately 1600 species in Southeast Asia (Dransfield et al., 2008).
Well-preserved fossil palm leaves with cuticles were recovered from the Oligocene of Hoanh Bo Basin, northern Vietnam, belonging to a costapalmate palm (Sabalites G. Saporta emend Read et Hickey, subfamily Coryphoideae). These fossil palm leaves provide detailed morphological information for their systematic identification. In this study, we describe the morphology of these fossils and assign them to a new species, namely Sabalites colaniae A. Song, T. Su, T.V. Do et Z.K. Zhou sp. nov., and discuss the paleoclimate background in northern Vietnam and its adjacent areas during the Oligocene.
2. Materials and methods 2.1. Geological settingThe palm fossils described in this study were collected from Yen My Village, Le Loi Commune, Hoanh Bo Basin, Quang Ninh Province, northern Vietnam (21°2'42.73″ N, 107°1'56.23″ E) (Fig. 1). Hoanh Bo Basin is a small strike-slip fault basin along the Red River Fault System (Tha et al., 2017). The fossil site is located in the northern part of the basin (Fig. 1). The Cenozoic sediments in the basin consist of the Dong Ho Formation, Tieu Giao Formation, and Quaternary deposits from the bottom to upper parts (Petersen et al., 2005; Tha et al., 2017). The outcrop of the Dong Ho Formation is approximately 80 m thick (Fig. 2) and dominated by gray to black weakly or non-laminated to structureless organic-rich mudstones interbedded with thin layers of muddy sandstones, representing a low energy, long-lasting lacustrine environment (Petersen et al., 2005; Tha et al., 2017). This formation yields numerous plant megafossils, spores and pollen (Nhan and Danh, 1975; Trung et al., 1999a, b, 2000). The Dong Ho Formation is assigned to an Oligocene age according to detailed lithostratigraphic correlation (Traynor and Sladen, 1997; Clift and Sun, 2006) and confirmed by biostratigraphy (Trung et al., 1999a, b). This age assignment is supported by palynological studies. The floristic assemblage is dominated by Fagaceae and gymnosperms (Trung et al., 1999a, b), similar to other Oligocene palynofloras from north Vietnam (Wysocka et al., 2018) and South China (Herman et al., 2017; Wei et al., 2020). Palm fossils were collected from the upper part of the Dong Ho Formation (Fig. 2).
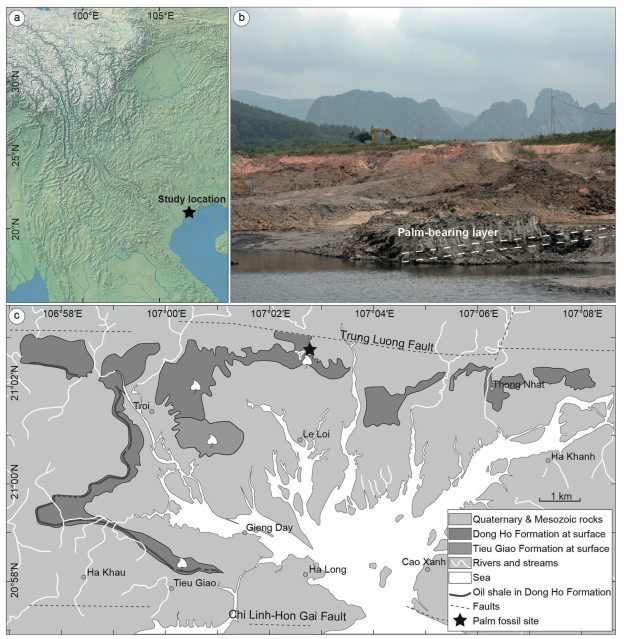
|
| Fig. 1 Geological map with the location of the fossil site (modified from Petersen et al., 2005; Tha et al., 2017). a, Location of the study area. b, Picture in the field showing the palm-bearing layer; c, Location of the fossil site and geological map of the Hoanh Bo Basin showing the distribution of Dong Ho Formation and Tieu Giao Formation. |
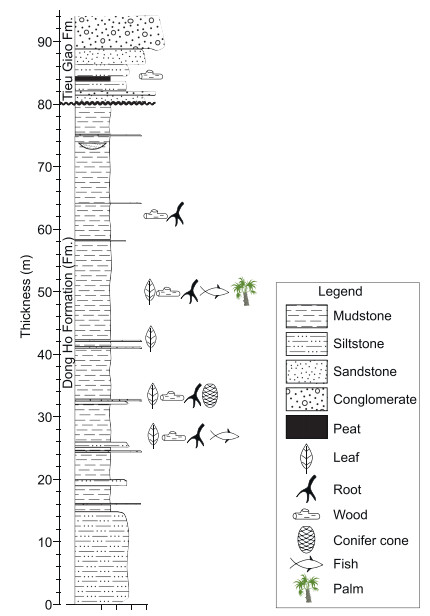
|
| Fig. 2 Sedimentological profile and fossil assemblage of the Dong Ho Formation and Tieu Giao Formation in the Hoanh Bo Basin. |
The fossil palm leaves were photographed using a Nikon D850 DSLR camera with an aperture of F20 (Nikon Corporation, Tokyo, Japan). A stereo-microscope (Leica S8APO, Wetzlar, Germany) was used to examine the morphology in detail. The morphometric data were obtained using ImageJ software v.1.53 (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). The specimens are described using morphological terminology for palm fossils (Read and Hickey, 1972; Zhou et al., 2013; Wang et al., 2015; Greenwood and West, 2017; Su et al., 2019) and modern palm leaves (Palmweb, http://www.palmweb.org/node/2).
Fossil cuticles were prepared using the methods outlined by Wang et al. (2019). Small parts were collected from leaf fossils (specimen No. XTBG-VNMN-1-10395a) and placed in 10% hydrochloric acid (HCl) for 8 h and 40% hydrofluoric acid (HF) for 12 h, respectively, to remove the carbonates and silicates. Afterwards, the fossil cuticles were rinsed at least three times in distilled water and macerated in 3.5% sodium hypochlorite (NaClO) for 0.5–2 h, followed by treatment with 5% ammonium hydroxide (NH3·H2O) for 5 min. In addition, we observed a large number of modern palm leaves from the herbaria of the Xishuangbanna Tropical Botanical Garden (XTBG), the Kunming Institute of Botany (KIB), and the databases of Royal Botanic Garden, Kew, UK (https://www.kew.org) and Palmweb (http://www.palmweb.org/node/2). We also collected leaves from extant palm species of Sabal Adans. and Trachycarpus fortunei (Hook.) H. Wendl. with unarmed petioles and costapalmate blades from the Xishuangbanna Tropical Botanical Garden (XTBG) and Guangxi Botanical Garden. Cleaned leaf fragments from palm leaves of extant species were immersed for approximately 6 h in a mixed solution of acetic acid (CH3COOH) and hydrogen peroxide (H2O2) (1:1) that was heated in a 70 ℃ water bath. The samples were washed thoroughly at least three times in distilled water until white or translucent cuticles appeared. These cuticles were then dyed with an aqueous solution of safranin and mounted on glass slides, and photographed using a Zeiss Axioscope A1 microscope (Oberkochen, Germany). Both unstained fossil cuticles and cuticles of extant T. fortunei leaves were mounted on scanning electron microscope (SEM) stubs, with the inside of the cuticles upwards. Then specimens were sent to the Public Technology Service Center of XTBG to examine cuticle morphology by SEM (ZEISS EVO LS10, Germany).
3. SystematicsFamily: Arecaceae Bercht. & J. Presl.
Subfamily: Coryphoideae Burnett.
Genus: Sabalites G. Saporta emend Read et Hickey.
Species: Sabalites colaniae A. Song, T. Su, T.V. Do et Z.K. Zhou sp. nov.
Holotype: XTBG-VNMN-1-10395.
Paratype: XTBG-VNMN-1-10396.
Repository: Vietnam National Museum of Nature (VNMN), Vietnam Academy of Science and Technology (VAST), Hanoi, Vietnam.
Locality: Yen My Village, Le Loi Commune, Hoanh Bo Basin, Quang Ninh Province, Vietnam (21°2'42.73″ N, 107°1'56.23″ E).
Age and stratigraphy: Oligocene, Dong Ho Formation.
Etymology: The specific epithet 'colaniae' was to commemorate the pioneering work of the French archeologist Madeleine Colani in Vietnam in the 1920s.
Diagnosis: Leaves costapalmate, large fan-shaped, induplicate and slightly asymmetrical at the base with a recognizable stout costa. Costa slightly enlarged at the base and tapering distally. Blade asymmetrically attached to the stout costa. Petiole is 0.38 m long and unarmed. Leaf segments are plicate and connate, emerging at an acute angle from the costa and fusing at an emerging point. The ordinary epidermal cells elongated to polygonal, arranged in rows. Stomata surrounded by two polar subsidiary cells and 2–4 lateral subsidiary cells. The guard cells, consisting of a pair of counter cells, sunken below the surface of the epidermis.
Description: The lamina of the fossil palm has a maximum preserved width of 0.55 m and a maximum preserved length of 0.31 m (Fig. 3a and b). The costapalmate leaf blade is induplicate and slightly asymmetrical at the base with a recognizable stout costa, approximately 56 mm long and 31 mm wide, which gradually tapers distally (Fig. 3d). There are approximately 54–56 pairs of leaf segments, which emerge asymmetrically from the petiole apex and along both sides of the costa (Fig. 3a–d). The leaf segments are narrow and plicate, no more than 14 mm wide and up to 0.42 m long (Fig. 3a). Each leaf segment has a strong, uniform 1° vein (mid-vein) and two nearly parallel marginal veins (Fig. 3e). Lamina pieces show several plicate and connate leaf segments aligned parallel to each other, with a strong 1° vein and 2° veins parallel to the 1° vein (Fig. 3a, b, e). The unarmed petiole is 0.38 m long and 40 mm wide (Fig. 3c).
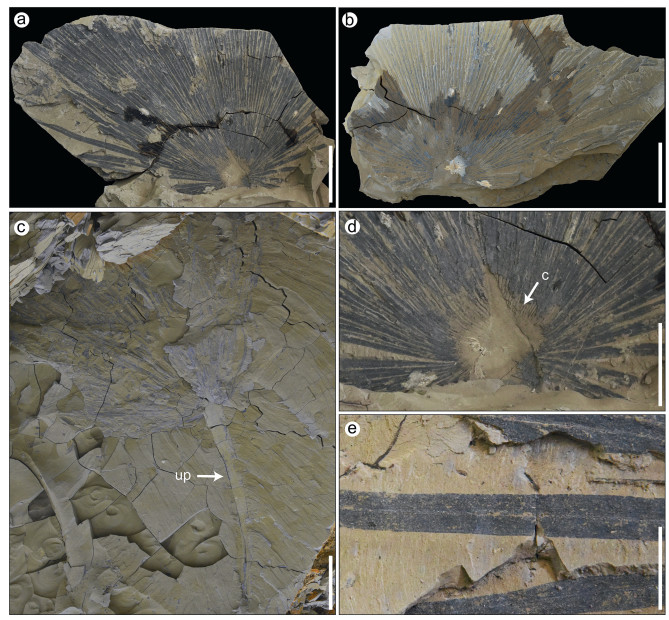
|
| Fig. 3 Leaf blade of Sabalites colaniae. a, b, Gross morphology of the cospalmate blade (XTBG-VNMN-1-10395a/b); c, Leaf blade with a long, unarmed petiole (XTBG-VNMN-1-10396); d, Enlargement of the basal part of leaf blade: costa; e, Segments of the palm fossil. c, coast; up, unarmed petiole. Scale bar = 100 mm (a, b, c), 50 mm (d), 20 mm (e). |
Leaves are amphistomatic (Fig. 4b). Small and dense stomata are recognizable on both adaxial and abaxial cuticles and distributed in bands with some stomata clustered together (Fig. 4b and c). The ordinary epidermal cells and stomatal bands are intermittently distributed regularly (Fig. 4c). The ordinary epidermal cells are elongated to polygonal arranged in rows (Fig. 4c), 3.2–8.4 μm wide and 20.6–44.3 μm long with straight anticlinal walls. Most stomata are elliptic and composed of 4–6 subsidiary cells, two polar cells, and 2 to 4 lateral cells (Fig. 4c and d). Polar subsidiary cells are wide and occasionally short, ca. 10.3 (7.5–11.8) μm wide and ca. 7.5 (6.2–9.1) μm long in average. Guard cells are elongated, 5.2–6.8 μm wide and 16.9–22.8 μm long, and slightly sunken below the surface of the subsidiary cells.
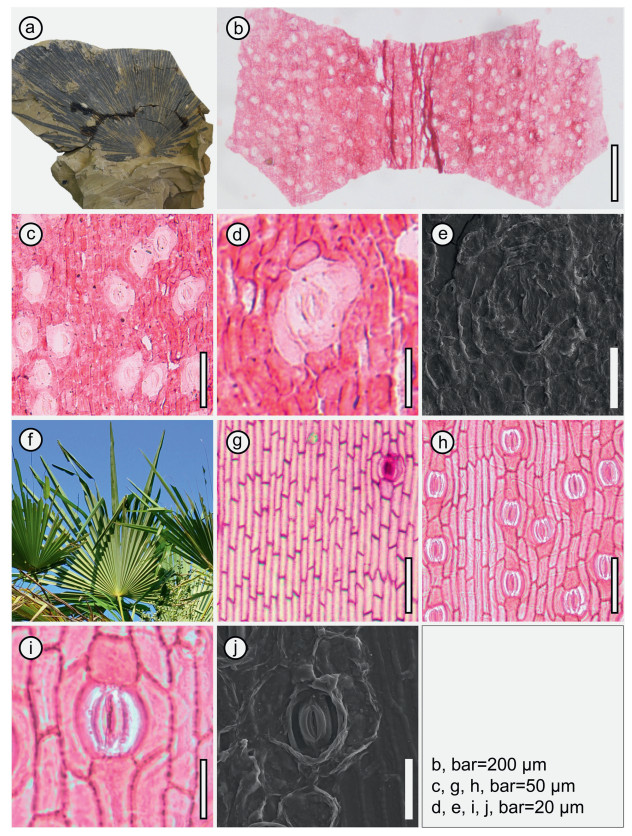
|
| Fig. 4 Comparison of leaf morphology and cuticular micromorphology of Sabalites colaniae (a–e) and living species Trachycarpus fortunei (f–j). a, f, leaf morphology showing the cospalmate blade, single-fold segments; b, c, g, h, amphistomatic cuticles showing the ordinary epidermal cells and stomatal bands; d, e, i, j, enlargement of the stomatal complex by light microscope and SEM. |
There are four types of leaf blades in palms: palmate, pinnate, bipinnate, and entire leaves (Tomlinson et al., 2011). Palmate leaves contain the truly palmate type that lack a costa and the costapalmate type with an extension of the petiole into the blade, forming the costa (Dransfield et al., 2008; Zhou et al., 2013). Species with palmate leaves are predominantly found within the Coryphoideae, including forty-four genera from six different tribes. Some others are found in Calamoideae such as Lepidocaryum Mart., Mauritia L. f., Mauritiella Burret with reduplicate leaves (Table 1), which differ considerably from the fossils described here. Leaves of most palmate palms in Coryphoideae are fan-shaped/orbicular and induplicate, with normal/unrecognizable costa, or a strong costa which is long and tapering, reaching nearly to the end of the blade (Table 1). The petiole is armed or unarmed, robust or slender (Table 1). Morphological features of the segments are different in number, length and shape (Table 1).
| Subfamily | Tribe | Genus | Species | Blade | Segments | Petiole | Modern distribution | |||||
| Shape | Costa | Segments divided | Numbers | Shape | ||||||||
| Calamoideae | Lepidocaryeae | Lepidocaryum Mart. | 1 | fan-shaped or orbicular | reduplicate | palmate | South America | |||||
| Mauritia L.f. | 2 | orbicular | reduplicate | costapalmate | unarmed | South America | ||||||
| Mauritiella Burret | 4 | orbicular | reduplicate | costapalmate | deeply divided, more than 2/3 | unarmed | South America | |||||
| Coryphoideae | Sabaleae | Sabal Adans. | 15 | fan-shaped | induplicate | shortly to prominently costapalmate | unarmed | Central and North America | ||||
| Corypheae | Corypha L. | 5 | fan-shaped | induplicate | costapalmate | ca. 1/2 | armed | South and Southeast Asia, Malesia to Australia | ||||
| Chuniophoeniceae | Chuniophoenix Burret | 3 | induplicate | palmate | unarmed | East and Southeast Asia | ||||||
| Chuniophoenix hainanensis Burret | fan-shaped | no costa | deeply divided | 36-45 segments | linear, 50 × 1.8–2.5 cm | unarmed | ||||||
| Chuniophoenix humilis C.Z.Tang & T.L.Wu | fan-shaped | almost completely divided | 4-7 segments | broad and hooded, 25–35 × 3–7 cm | ||||||||
| Chuniophoenix nana Burret | almost completely divided | |||||||||||
| Kerriodoxa J.Dransf. | 1 | fan-shaped | induplicate | palmate | ca. 1/4 to 1/3 | unarmed | Southeast Asia | |||||
| Kerriodoxa elegans J.Dransf. | unrecognizable costa | unarmed and slender | ||||||||||
| Nannorrhops H.Wendl. | 1 | fan-shaped | induplicate | briefly costapalmate | unarmed | West Asia | ||||||
| Tahina J.Dransf. & Rakotoarin. | 1 | fan-shaped | induplicate | costapalmate | ca.1/2 | unarmed | Madagascar, Africa | |||||
| Tahina spectabilis J.Dransf. & Rakotoarin. | ca.1/2 | 110-122 segments | ||||||||||
| Trachycarpeae | Johannesteijsmannia H.E.Moore | 4 | diamond-shaped | – | strongly costapalmate | armed | Southeast Asia | |||||
| Lanonia A.J. Hend. & C.D. Bacon | 8 | – | palmate | almost completely divided | East and Southeast Asia | |||||||
| Licuala Wurmb | 148 | fan-shaped | – | palmate | almost completely divided | armed or unarmed | Southeast Asia to the western Pacific and Australia | |||||
| Livistona R.Br. | 28 | fan-shaped | induplicate | palmate or costapalmate | armed | Africa and Asia | ||||||
| Pholidocarpus Blume | 6 | induplicate | costapalmate | ca. 2/3 or 1/2 | armed | Southeast Asia | ||||||
| Saribus Blume | 9 | fan-shaped/orbicular | – | – | armed | Southeast Asia and Oceania | ||||||
| Chamaerops L. | 1 | fan-shaped | induplicate | palmate | ca. 1/2 to 2/3 | armed | Mediterranean | |||||
| Guihaia J.Dransf., S.K.Lee & F.N.Wei | 2 | orbicular or cuneate | induplicate | palmate | ca. 3/4 to 4/5 | unarmed | East and Southeast Asia | |||||
| Maxburretia Furtado | 3 | fan-shaped | induplicate | palmate | ca. 2/3 | unarmed | Southeast Asia | |||||
| Rhapidophyllum H.Wendl. & Drude | 1 | fan-shaped | induplicate | very shortly costapalmate | deeply divided, more than 2/3 | North America | ||||||
| Rhapis L.f. ex Aiton | 11 | fan-shaped | induplicate | palmate | ca. 3/4 | unarmed | East and Southeast Asia | |||||
| Trachycarpus H.Wendl. | 9 | fan-shaped to almost circular | induplicate | palmate | ca.1/2 | armed or unarmed | South, East and Southeast Asia | |||||
| Acoelorrhaphe H.Wendl. | 1 | blade nearly orbicular | induplicate | very briefly costapalmate | armed | Central America | ||||||
| Brahea Mart. | 11 | blade nearly orbicular | induplicate | shortly costapalmate | armed or unarmed | Central America | ||||||
| Colpothrinax Schaedtler | 3 | fan-shaped or orbicular | induplicate | shortly costapalmate | unarmed | Central America | ||||||
| Copernicia Mart. ex Endl. | 11 | wedge-shaped or orbicular | induplicate | palmate to shortly costapalmate | ca. 1/4 to 1/3 | armed | Central America | |||||
| Pritchardia Seem. & H.Wendl. | 28 | fan-shaped | induplicate | costapalmate | ca. 1/3 to 1/2 | unarmed | western Pacific islands | |||||
| Serenoa Hook.f. | 1 | nearly orbicular | induplicate | palmate | armed | North America | ||||||
| Washingtonia H.Wendl. | 2 | fan-shaped | induplicate | costapalmate | ca. 1/3 | armed | North America | |||||
| Cryosophileae | Chelyocarpus Dammer | 4 | fan-shaped | induplicate | palmate, or shortly costapalmate | unarmed | South America | |||||
| Chelyocarpus chuco (Mart.) H.E.Moore | crown | ca. 1/4 to 3/4 | 15-24 segments | lanceolate | ||||||||
| Chelyocarpus dianeurus (Burret) H.E.Moore | almost completely divided | |||||||||||
| Chelyocarpus repens F.Kahn & K.Mejia | almost completely divided | |||||||||||
| Chelyocarpus ulei Dammer | blade divided almost to the base into two large halves | |||||||||||
| Coccothrinax Sarg. | 53 | fan-shaped | induplicate | palmate | some species almost completely divided | unarmed or partly armed | Americas in low latitudes | |||||
| Cryosophila Blume | 10 | fan-shaped | induplicate | palmate | more than 2/3 | unarmed | Central America and northern South America | |||||
| Hemithrinax Hook.f. | 3 | fan-shaped | induplicate | palmate | ca. 1/2 to 2/3 | Central America | ||||||
| Itaya H.E.Moore | 1 | fan-shaped or orbicular | induplicate | palmate | ca. 3/4 | unarmed | South America | |||||
| Leucothrinax C.Lewis & Zona | 1 | fan-shaped | induplicate | palmate | ca. 1/2 | Central America | ||||||
| Leucothrinax morrisii (H.Wendl.) C.Lewis & Zona | lanceolate | |||||||||||
| Sabinaria R. Bernal & Galeano | 1 | fan-shaped | induplicate | palmate | South America | |||||||
| Sabinaria magnifica Galeano & R. Bernal | divided almost to the base into two large halves | ca. 1/10 | 36-42 single-fold segments | |||||||||
| Schippia Burret | 1 | fan-shaped | induplicate | palmate to very shortly costapalmate | more than 2/3 | unarmed | Central America | |||||
| Thrinax L.f. ex Sw. | 3 | fan-shaped | induplicate | palmate, unrecognizable costa | lanceolate | unarmed | Central America | |||||
| Trithrinax Mart. | 4 | fan-shaped to nearly circular | induplicate | not or only slightly costapalmate | ca. 1/2 | South America | ||||||
| Zombia L.H.Bailey | 1 | induplicate | palmate | ca. 1/2 to 2/3 | unarmed | Central America | ||||||
| Borasseae | Bismarckia Hildebr. & H.Wendl. | 1 | fan-shaped | induplicate | costapalmate | ca. 1/4 to 1/3 | unarmed | Madagascar, Africa | ||||
| Bismarckia nobilis Hildebr. & H.Wendl. | strong costapalmate, costa 43 cm | 50-77 segments | ||||||||||
| Hyphaene Gaertn. | 8 | fan-shaped | induplicate | costapalmate | ca. 1/3 | armed | Africa, South and West Asia | |||||
| Medemia Wurttenb. ex H.Wendl. | 1 | fan-shaped | induplicate | costapalmate | ca. 2/3 | armed | North Africa | |||||
| Satranala J.Dransf. & Beentje | 1 | fan-shaped | induplicate | costapalmate | ca. 1/4 to 1/3 | Madagascar, Africa | ||||||
| Satranala decussilvae Beentje & J.Dransf. | strong costapalmate, costa to 33 cm | 54-57 segments | ||||||||||
| Borassodendron Becc. | 2 | fan-shaped | induplicate | costapalmate | ca. 1/4 to 2/3 | unarmed | Southeast Asia | |||||
| Borassus L. | 5 | fan-shaped | induplicate | strongly costapalmate | ca. 1/2 | armed | Asia and Africa | |||||
| Latania A.J. Hend. & C.D. Bacon | 3 | fan-shaped | induplicate | costapalmate | ca. 1/3 to 1/2 | lanceolate | margin smooth or with a few shallow teeth | Africa islands in the Indian Ocean east of Madagascar | ||||
| Lodoicea Comm. ex DC. | 1 | induplicate | strong costapalmate | ca. 1/4 to 1/3 | Africa islands in the Indian Ocean east of Madagascar | |||||||
| Morphological comparison between fossils and extant palmate palms are at genus or species level depending on their similarity of leaves. Numbers represent the total amount of species used for comparison. | ||||||||||||
The fossil palm leaves from Vietnam are induplicate, costapalmate and have large leaf blades and an unarmed petiole (Fig. 3). There are approximately 54–56 pairs of segments, and each of them is linear with a width of no more than 14 mm, as well as opposite margins that are nearly parallel except the apex and base (Fig. 3a). We compared the leaf morphological characteristics of extant palmate palms especially those with unarmed petioles and costapalmate blades to our fossil palm from Vietnam (Table 1). Some species, including Bismarckia nobilis Hildebr. & H. Wendl., Johannesteijsmannia H.E. Moore, Satranala decussilvae Beentje & J. Dransf., have costapalmate leaves with a long costa tapering and reaching nearly to the end of the blade. The costa of Lodoicea maldivica (J.F. Gmel.) Pers. ex H. Wendl. extends almost to the leaf apex. Hence, palms in Coryphoideae with too many/few or too long/short segments, and segments deeply divided more than half or with lanceolate/broad segments are obviously different from our fossils, such as Tahina spectabilis J. Dransf. & Rakotoarin, Chuniophoenix humilis C.Z. Tang & T.L. Wu, Chelyocarpus dianeurus (Burret) H.E. Moore and so on. As a result, Sabal Adans. from America and Trachycarpus H. Wendl. from Asia are most morphologically similar to the fossils described herein.
Sabal and Trachycarpus have moderately similar blades, a recognizable stout costa and an unarmed petiole compared with our fossils. However, the segments of the present fossils are narrower than those in living species of Sabal and Trachycarpus (Fig. 4). Leaf cuticular characteristics provide additional information for the systematic identification of fossil palm leaves (Van Der Burgh, 1984; Hartwich et al., 2010; Zhou et al., 2013; Wang et al., 2015, 2017). We compared the fossil palm leaves from Vietnam described in this study to extant Sabal and Trachycarpus. The fossil leaves from Vietnam are amphistomatic (Fig. 4b), ordinary epidermal cells are elongated to polygonal, and arranged in rows (Fig. 4b and c). Elliptical stomata on both the adaxial and abaxial cuticles are abundant and arranged in bands, which are distributed intermittently with the ordinary epidermal cells (Fig. 4b and c). The leaves of Sabal bermudana L.H. Bailey, S. palmetto (Walter) Lodd. ex Schult. & Schult.f., and T. fortunei are also typically amphistomatic (Fig. 4g and h, and Figs. S1), and the stomatal bands, as well as several non-stomatal zones, are visible on both the abaxial and adaxial leaf epidermis (Figs. 4 and S1). However, the ordinary epidermal cells of S. bermudana and S. palmetto are distinctively polygonal and the stomatal complexes are plum-shaped, which differ considerably from our fossils (Fig. S1). The ordinary epidermal cells and stomata of T. fortunei show some similar morphological features to the present fossils such as the distribution of elongated to polygonal ordinary epidermal cells and elliptic stomata, while the biggest difference is that the stomata of T. fortunei are distributed sporadically on the adaxial cuticle rather than in bands (Fig. 4g). In view of the morphological differences mentioned above, the present fossils are hard to assign to Coryphoideae or Calamoideae, and are best classified as Sabalites based on their costapalmate fossil leaves and unarmed petiole (Read and Hickey, 1972).
Many palm fossil records have previously been reported worldwide (Harley, 2006; Dransfield et al., 2008; Srivastava et al., 2014; Allen, 2015; Wang et al., 2015; Greenwood and West, 2017). In this study, we selected fossils with similar costapalmate blades for a detailed morphological comparison from the adjacent regions, including these from Changchang, Hainan (Guo, 1965; Zhou et al., 2013), Maoming, Guangdong (Guo, 1965), and Ningming and Shangsi of Guangxi, China (Guo, 1965; Wang et al., 2015, 2017) (Table S1). Some fossil species have strong costapalmate blades with a costa that is more than 75 mm in length (Table S1), such as Sabalites szei Guo, S. asymmetricus Jin et Zhou, S. tenuifolius Jin et Zhou, S. cf. asymmetricus Jin et Zhou, and Sabalites sp. (Guo, 1965; Zhou et al., 2013; Wang et al., 2017); however, the costa of the fossil palm from northern Vietnam is approximately 56 mm long. Furthermore, S. changchangensis Guo from Hainan and Sabalites sp. from Guangdong differ from our fossils by the smaller size of their costa (Table S1). Sabalites guangxiensis Q.J. Wang et B.N. Sun and Sabalites sp. 2 (Table S1) from the Ningming Formation of Guangxi differ from our palm fossils owing to both their smaller costa and more slender petioles, as well as the longer ordinary epidermal cells (Wang et al., 2017). Since the leaf blades of S. robustus Jin et Zhou from the Changchang Formation of Hainan Province are incomplete, we could not obtain more morphological information (Zhou et al., 2013). The ordinary epidermal cell sizes of the palm species from Vietnam are larger than those of S. robustus, particularly the guard cells, which are almost twice as large as those from S. robustus (Zhou et al., 2013). Sabalites tibetensis T. Su et Z.K. Zhou was recently reported from the Lunpola Basin in central Tibetan Plateau (Su et al., 2019). The leaf of S. tibetensis has a relatively similar shape to the fossil leaves described in this study, but S. tibetensis has a spine-like structure on the base of the leaf blade, which is absent in our fossils. Moreover, no cuticle is preserved on the lamina of S. tibetensis, which restricts further morphological comparisons. Given the dissimilarities with extant palmate palms and fossil Sabalites from adjacent localities, we propose a new species Sabalites colaniae A. Song, T. Su, T.V. Do et Z.K. Zhou sp. nov.
4.2. Paleoclimatic implications in northern Vietnam during the OligoceneThe Arecaceae is a typical pantropical family (Dransfield et al., 2008). Owing to the abundant solar radiation and water resources, most palm species are found today in tropical rainforests around the world (Svenning et al., 2008). This is particularly true for regions between 5°N and 5°S latitudes that contain up to 90% of the living palm species (Couvreur et al., 2011; Srivastava et al., 2014). Previous studies have shown that climate, particularly temperature, is the main factor restricting palm distribution (Larcher and Winter, 1981; Greenwood and Wing, 1995; Bjorholm et al., 2006; Eiserhardt et al., 2011; Greenwood and West, 2017; Reichgelt et al., 2018). Greenwood and Wing (1995) proposed a coldest month mean temperature of 5 ℃ as the limit for palms. Based on a comprehensive analysis of the climatic conditions of the natural distribution of modern-day palms, Reichgelt et al. (2018) derived the survival threshold of the Arecaceae as being a coldest month mean temperature (CMMT) of no less than 5.2 ℃.
Palms are thus good indicators of warm climates in the geological past (Greenwood and Wing, 1995; Greenwood and West, 2017; Su et al., 2019). For example, the pollen grains of palms and Bombacoideae demonstrated the existence of highly diverse, near-tropical forests in Antarctica during the early Eocene (Pross et al., 2012). Our palm fossils were collected from the Oligocene in the Hoanh Bo Basin of northern Vietnam, as well as some other fossil leaves, such as the Dipterocarpaceae Blume, Cinnamomum Schaeff., and Cyclobalanopsis Oerst. (personal observation, unpublished data), which are primarily distributed together and are diverse in tropical and warm subtropical zones in Asia. The occurrence of these fossils indicates that the climate of northern Vietnam was warm and humid during the Oligocene. In addition, paleontological evidence from the Oligocene Na Duong coal mine (21°42.2 N, 106°58.6 E) in Lang Son Province (approximately 70 km northeast of the Hoanh Bo Basin) suggests a warm and humid climate during the Oligocene (Böhme et al., 2011). The occurrence of a large-sized arborescent Osmundaceae stem, vertebrate and invertebrate faunal elements, such as mollusks (representatives of genus Tarebia and Brotia), aquatic turtles, and the skull of a large-sized longirostrine crocodile from the Na Duong Formation also indicate that the climate was warm and humid during the Oligocene (Böhme et al., 2011). In addition, the paleoclimate reconstruction of the Oligocene flora from the Ningming Basin (22°08′20.6″N, 107°01′47.3″E), Guangxi Province in South China suggests that the mean annual temperature and precipitation during the growing season (months with mean temperature greater than 10 ℃) are 19.8 ℃ and 1657 mm, respectively (Shi et al., 2012), and the planosol paleosoils and the absence of pedogenic carbonate concretions or evaporates in the sedimentary basins of northern Vietnam indicate a paratropical and humid climate (Böhme et al., 2011). Therefore, a warm climate prevailed in northern Vietnam and adjacent areas during the Oligocene.
Author contributionsA.S., J.L., and T.S. planned and designed the research. A.S., J.L., S-Q L., T-V D., H–B N., W–Y-D D., participated in the research. T.S. and J.H. conducted the research. A.S. wrote the manuscript. T.S., J.H., L.J., T-V D., L-B J., C.D.R., G.S. Z.F., and Z-K Z. commented and refined the paper.
Declaration of competing interestWe have no competing interest.
AcknowledgementsWe thank members of Paleoecology Research Group (PRG) in Xishuangbanna Tropical Botanical Garden (XTBG), Chinese Academy of Sciences for field trips in northern Vietnam; People's Committee of Hoanh Bo District, Quang Ninh Province, Vietnam and Vietnam National Museum of Nature (VNMN), Vietnam Academy of Science and Technology for field permission and assistance; the Public Technology Service Center in XTBG for imaging assistance. We thank Mrs. Teresa Spicer for polishing English which makes our manuscript a great improve. GS thanks to Dr. Vandana Prasad (Director), Birbal Sahni Institute of Palaeosciences, Lucknow for providing necessary facilities during the research work. This work is supported by National Natural Science Foundation of China (NSFC) (31800183, 41922010, 42002020, 41661134049), Yunnan Basic Research Projects (202001AU070137, 2019FB026), Chinese Academy of Sciences "Light of West China" Program (2020000023), the CAS 135 program (2017XTBG-T03), and Project "Study, collection of fossil woods in Vietnam for exhibition in System of Vietnam National Museum of Nature" (CT0000.01/19-21).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.08.003.
Allen, S.E., 2015. Fossil palm flowers from the Eocene of the rocky mountain region with affinities to Phoenix L. (Arecaceae: Coryphoideae). Int. J. Plant Sci., 176: 586-596. DOI:10.1086/681605 |
Baker, W.J., Dransfield, J., 2016. Beyond Genera Palmarum: progress and prospects in palm systematics. Bot. J. Linn. Soc., 182: 207-233. DOI:10.1111/boj.12401 |
Bande, M.B., Prakash, U., 1986. The Tertiary flora of Southeast-Asia with remarks on its paleoenvironment and phytogeography of the Indo-Malayan region. Rev. Palaeobot. Palynol., 49: 203-233. DOI:10.1016/0034-6667(86)90028-X |
Bjorholm, S., Svenning, J.C., Baker, W.J., et al., 2006. Historical legacies in the geographical diversity patterns of New World palm (Arecaceae) subfamilies. Bot. J. Linn. Soc., 151: 113-125. DOI:10.1111/j.1095-8339.2006.00527.x |
Böhme, M., Prieto, J., Schneider, S., et al., 2011. The Cenozoic on-shore basins of Northern Vietnam: biostratigraphy, vertebrate and invertebrate faunas. J. Asian Earth Sci., 40: 672-687. DOI:10.1016/j.jseaes.2010.11.002 |
Chaimanee, Y., Suteethorn, V., Jintasakul, P., et al., 2004. A new orang-utan relative from the Late Miocene of Thailand. Nature, 427: 439-441. DOI:10.1038/nature02245 |
Clift, P.D., Sun, Z., 2006. The sedimentary and tectonic evolution of the Yinggehai-Song Hong basin and the southern Hainan margin, South China Sea: implications for Tibetan uplift and monsoon intensification. J. Geophys. Res., 111: B06405. |
Colani, M., 1920. Etude sur les flores tertiaires de quelques gisements de lignite de l'Indochine et du Yunnan. Imprimerie d'Extrême-Orient, Hanoi-Haiphong, p. 517.
|
Couvreur, T.L., Forest, F., Baker, W.J., 2011. Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biol., 9: 44. DOI:10.1186/1741-7007-9-44 |
Crié, L., 1892. Recherches sur les palmiers silicifiée des terrains crétacés de l'Anjou. Bulletin de la Socíeté d'Études Scientifiques d'Angers, 21: 97-103. |
Dransfield, J., Uhl, N.W., Lange, C.B.A., et al., 2008. Genera Palmarum: the Evolution and Classification of Palms. Kew Publishing.
|
Eiserhardt, W.L., Svenning, J.C., Kissling, W.D., et al., 2011. Geographical ecology of the palms (Arecaceae): determinants of diversity and distributions across spatial scales. Ann. Bot., 108: 1391-1416. DOI:10.1093/aob/mcr146 |
Faurby, S., Eiserhardt, W.L., Baker, W.J., et al., 2016. An all-evidence species-level supertree for the palms (Arecaceae). Mol. Phylogenet. Evol., 100: 57-69. DOI:10.1016/j.ympev.2016.03.002 |
Friederich, M.C., Moore, T.A., Flores, R.M., 2016. A regional review and new insights into SE Asian Cenozoic coal-bearing sediments: why does Indonesia have such extensive coal deposits?. Int. J. Coal Geol., 166: 2-35. DOI:10.1016/j.coal.2016.06.013 |
Greenwood, D.R., West, C.K., 2017. A fossil coryphoid palm from the Paleocene of western Canada. Rev. Palaeobot. Palynol., 239: 55-65. DOI:10.1016/j.revpalbo.2016.12.002 |
Greenwood, D.R., Wing, S.L., 1995. Eocene continental climates and latitudinal temperature gradients. Geology, 23: 1044-1048. DOI:10.1130/0091-7613(1995)023<1044:ECCALT>2.3.CO;2 |
Guo, S., 1965. On the discovery of fossil palms from the Tertiary formation of Kwangtung and Kwangsi. Acta Palaeontol. Sin., 13: 598-601. |
Harley, M.M., 2006. A summary of fossil records for Arecaceae. Bot. J. Linn. Soc., 151: 39-67. DOI:10.1111/j.1095-8339.2006.00522.x |
Harley, M.M., Morley, R.J., 1995. Ultrastructural studies of some fossil and extant palm pollen, with notes on the geological history of subtribes Iguanurinae and Calaminae. Rev. Palaeobot. Palynol., 85: 153-182. DOI:10.1016/0034-6667(94)00133-5 |
Hartwich, S.J., Conran, J.G., Bannister, J.M., et al., 2010. Calamoid fossil palm leaves and fruits (Arecaceae: Calamoideae) from late Eocene Southland, New Zealand. Aust. Syst. Bot., 23: 131-140. DOI:10.1071/sb09027 |
Herman, A.B., Spicer, R.A., Aleksandrova, G.N., et al., 2017. Eocene–early Oligocene climate and vegetation change in southern China: evidence from the Maoming Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol., 479: 126-137. DOI:10.1016/j.palaeo.2017.04.023 |
Huang, H., Morley, R., Licht, A., et al., 2020. Eocene palms from central Myanmar in a South-East Asian and global perspective: evidence from the palynological record. Bot. J. Linn. Soc., 194: 177-206. DOI:10.1093/botlinnean/boaa038 |
Kooyman, R.M., Morley, R.J., Crayn, D.M., et al., 2019. Origins and assembly of Malesian rainforests. Annu. Rev. Ecol. Evol. Syst., 50: 119-143. DOI:10.1146/annurev-ecolsys-110218-024737 |
Krausel, R., 1923. Nipadites borneensis n. sp. eine fossile palmenfrucht aus Borneo. Senckenbergiana, 5: 3-4. |
Kvaček, J., Herman, A.B., 2004. Monocotyledons from the early Campanian (Cretaceous) of Grünbach, lower Austria. Rev. Palaeobot. Palynol., 128: 323-353. DOI:10.1016/S0034-6667(03)00154-4 |
Larcher, W., Winter, A., 1981. Frost susceptibility of palms: experimental data and their interpretation. Principes, 25: 143-152. |
Morley, R.J., 1998. Palynological evidence for Tertiary plant dispersals in the SE Asian region in relation to plate tectonics and dispersal. In: Hall, R., Holloway, J.D. (Eds.), Biogeography and Geological Evolution of SE Asia. Backhuys, Leiden, pp. 211-234.
|
Morley, R.J., 2000. Origin and Evolution of Tropical Rain Forests. Wiley, Chichester.
|
Muller, J., 1968. Palynology of the pedawan and plateau sandstone formations (Cretaceous-Eocene) in Sarawak, Malaysia. Micropaleontol., 14: 1-37. DOI:10.2307/1484763 |
Muller, J., 1979. Reflections on fossil palm pollen. In: Proceedings of 4th International Palynological Conference, vol. 1. Birbal Sahni Institute of Palaeobotany Lucknow, pp. 568-579.
|
Nhan, T.D., Danh, T., 1975. The new discovers about biostratigraphy of the Neogene sediments in the east of Bac Bo. In: Nhan, T.D., Danh, T. (Eds.), Stratigraphic Works. Science and Technology Publishing House, Hanoi, pp. 244-283.
|
Petersen, H., Tru, V., Nielsen, L., et al., 2005. Source rock properties of lacustrine mudstones and coals (Oligocene Dong Ho formation), onshore Song Hong basin, northern Vietnam. J. Petrol. Geol., 28: 19-38. DOI:10.1111/j.1747-5457.2005.tb00068.x |
Pramook, B., 2003. Petrified Wood of Northeastern Thailand and its Implication on Biodiversity and the Ecosystem during the Cenozoic Era (Ph.D. Thesis). Nakhon Ratchasima, Thailand (In Thai).
|
Pross, J., Contreras, L., Bijl, P.K., et al., 2012. Persistent near-tropical warmth on the Antarctic continent during the early Eocene epoch. Nature, 488: 73-77. DOI:10.1038/nature11300 |
Read, R.W., Hickey, L.J., 1972. A revised classification of fossil palm and palm-like leaves. Taxon, 21: 129-137. DOI:10.2307/1219237 |
Reichgelt, T., West, C.K., Greenwood, D.R., 2018. The relation between global palm distribution and climate. Sci. Rep., 8: 4721. DOI:10.1038/s41598-018-23147-2 |
Shi, G.L., Zhou, Z., Xie, Z., 2012. A new Oligocene Calocedrus from South China and its implications for transpacific floristic exchanges. Am. J. Bot., 99: 108-120. DOI:10.3732/ajb.1100331 |
Srivastava, R., Srivastava, G., Dilcher, D.L., 2014. Coryphoid palm leaf fossils from the Maastrichtian–Danian of Central India with remarks on phytogeography of the Coryphoideae (Arecaceae). PLoS One, 9: e111738. DOI:10.1371/journal.pone.0111738 |
Su, T., Farnsworth, A., Spicer, R.A., et al., 2019. No high Tibetan plateau until the Neogene. Sci. Adv., 5: eaav2189. DOI:10.1126/sciadv.aav2189 |
Svenning, J.C., Borchsenius, F., Bjorholm, S., et al., 2008. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr., 35: 394-406. DOI:10.1111/j.1365-2699.2007.01841.x |
Taylor, T.N., Taylor, E.L., Krings, M., 2008. Paleobotany: the Biology and Evolution of Fossil Plants. second ed. New York: Academic Press.
|
Tha, H.V., Wysocka, A., Cuong, N.Q., et al., 2017. Sedimentary petrology characteristics and their implications for provenance of Hoanh Bo Basin Neogene system in Quang Ninh province, north-eastern Vietnam. Geol. Geophys. Environ., 43: 69-87. DOI:10.7494/geol.2017.43.1.69 |
Tomlinson, P.B., Horn, J.W., Fisher, J.B., 2011. The Anatomy of Palms. Oxford University Press.
|
Traynor, J.J., Sladen, C., 1997. Seepage in Vietnam-onshore and offshore examples. Mar. Petrol. Geol., 14: 345-362. DOI:10.1016/S0264-8172(96)00040-2 |
Trinh, D., 1986. Neogene flora of North Vietnam and its stratigraphic significance (Ph.D. Thesis). In: Associate Doctoral Thesis in Geography-Geology. Vietnam Institute of Geosciences and Mineral Resources (In Vietnamese).
|
Trung, P., Bat, D., An, N., et al., 1999a. The new documentation of spore and pollen fossil in the Dong Ho formation. J. Petrol. Geol., 3: 2-8. |
Trung, P., Quynh, P., Bat, D., et al., 2000. New palynological investigation in the Na Duong mine. Oil Gas J., 7: 18-27. |
Trung, P.Q., Bat, D., An, N.Q., et al., 1999. New palynological find in the Dong Ho formation. Petrovietnam Rev, 3: 14-20. |
Van Der Burgh, J., 1984. Some palms in the Miocene of the lower rhenish plain. Rev. Palaeobot. Palynol., 40: 359-374. DOI:10.1016/0034-6667(84)90016-2 |
Wang, L., Kunzmann, L., Su, T., et al., 2019. The disappearance of Metasequoia (Cupressaceae) after the middle Miocene in Yunnan, Southwest China: evidences for evolutionary stasis and intensification of the Asian monsoon. Rev. Palaeobot. Palynol., 264: 64-74. |
Wang, Q.J., Ma, F.J., Dong, J.L., et al., 2015. Coryphoid palms from the Oligocene of China and their biogeographical implications. C. R. Palevol., 14: 263-279. |
Wang, Q.J., Ma, F.J., Dong, J.L., et al., 2017. New costapalmate palm leaves from the Oligocene Ningming Formation of Guangxi, China, and their biogeographic and palaeoclimatic implications. Hist. Biol., 29: 594-606. DOI:10.1080/08912963.2016.1218487 |
Wei, X., Yan, D., Luo, P., et al., 2020. Astronomically forced climate cooling across the eocene–Oligocene transition in the pearl river Mouth basin, northern South China Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol., 558: 109945. |
Wysocka, A., Pha, P.D., Durska, E., et al., 2018. New data on the continental deposits from the cao bang basin (cao bang-tien yen fault zone, NE Vietnam)–biostratigraphy, provenance and facies pattern. Acta Geol. Pol., 68: 689-709. |
Xing, Y., Gandolfo, M.A., Onstein, R.E., et al., 2016. Testing the biases in the rich Cenozoic angiosperm macrofossil record. Int. J. Plant Sci., 177: 371-388. DOI:10.1086/685388 |
Zeiller, R., 1903. Flore fossile des gites de charbon du Tonkin: Texte. Imprimerie Nationale, Paris.
|
Zhou, W., Liu, X., Xu, Q., et al., 2013. New coryphoid fossil palm leaves (Arecaceae: Coryphoideae) from the Eocene Changchang Basin of Hainan Island, South China. Sci. China Earth Sci., 56: 1493-1501. DOI:10.1007/s11430-013-4681-7 |



