b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Xinping Branch, Yuxi Tobacco Company, Xinping, 653400, China;
d. Guangnan Forestry and Grassland Bureau, Guangnan, 663300, China;
e. Traditional Chinese Medicine College, Yunnan University of Chinese Medicine, Kunming, 650500, China;
f. Center of Economic Botany, Core Botanical Gardens, Chinese Academy of Sciences, Menglun, Mengla, Yunnan, 666303, China
Nervonic acid (NA; C24:1△15, cis-tetracos-15-enoic acid) is a very long-chain monounsaturated fatty acid (Sargent et al., 1994) important to cell motility, growth, senescence, differentiation, and cell fate in the white matter of the brain and myelinated nerve fibers (Merrill et al., 1997; Liu et al., 2021). NA deficiency is closely associated with the risk of developing neurological disorders and neurodegenerative diseases (Amminger et al., 2012; Vozella et al., 2017). One effective treatment for symptoms of neurological diseases, such as Alzheimer's disease, is the use of NA-containing oils (Lewkowicz et al., 2019). However, NA is largely harvested from sharks (Liu et al., 2021), which is destructive and unsustainable. Recently, NA-containing plant seed oils have been identified in approximately 200 plant species belonging to 136 genera and 54 families. In most species, NA content is less than 2% of the total seed fatty acids. In fact, only five species have been reported with NA levels greater than 20% of the total seed fatty acids (He et al., 2021; https://plantfadb.org/). Two woody species, Malania oleifera Chun & S.K. Lee and Macaranga adenantha Gagnep, have NA levels over 50% of seed total fatty acids (Ma et al., 2004; Jia and Zhou, 1987; Tang et al., 2013).
Malania oleifera belongs to the monotypic genus Malania of the Olacaceae family and is endemic to restricted areas in western Guangxi Province and southeastern Yunnan Province, China (Wu et al., 2003), ranging between 23°23′N and 24°28′N and 105°30′E and 107°30′E (Xu et al., 2019). However, it grows naturally at elevations from 300 to 1640 m under various environmental and soil conditions, and genetic differentiation has been found among populations in different habitats (Lai, 2006). A number of studies have reported that M. oleifera kernel oil content varies from 58.0% to 63.0% and that NA levels vary from 55.7% to 67.0% of total kernel fatty acids (Jia and Zhou, 1987; Tang et al., 2013). However, a comprehensive and systematic evaluation of M. oleifera germplasm is still lacking.
Macaranga adenantha (Euphorbiaceae) is endemic to central and western Guangdong Province, Guangxi Province, southwestern Guizhou Province and southeastern Yunnan Province, China, and northern Vietnam (Qiu, 1996). It is a fast-growing tropical pioneer tree species that is usually found in disturbed fields, especially forest gaps, stream banks, and forest margins in primary forests. NA content in M. adenantha is approximately 56% of the total seed fatty acids and kernel oil content is approximately 60% (Ma et al., 2004; Jia and Zhou, 1987). However, germplasm diversity remains unclear. The genus Macaranga includes approximately 280 dioecious species that are distributed from Africa through Southeast Asia to some remote Pacific islands (Whitmore, 2008). Whether other Macaranga species are similarly enriched with NA should be investigated. Macaranga indica Wight is a closely related species of M. adenantha, which is also a fast-growing tropical pioneer tree species widely distributed in southeastern Yunnan Province, China, India, Sri Lanka, Malaysia, and Thailand. However, research on M. indica is limited, and its seed oil content and fatty acid profiles have not been recorded.
In the present study, the seed fatty acid composition of Malania oleifera and five Macaranga species (M. adenantha, M. indica, M. denticulata, M. henryi, and M. kurzii) were analyzed. Seeds were collected from six populations of M. oleifera, four populations from M. adenantha, and four populations from M. indica. The fatty acid composition, seed oil content, and seed weight were analyzed to evaluate germplasm diversity. The results will contribute to germplasm resource nursery building and selection and breeding program construction to improve the development of NA-enriched plants.
2. Materials and methods 2.1. Plant materialMature seeds were collected from wild populations in China. Details on plant materials are given in Table 1. To ensure that seeds were mature, we collected black seeds of Macaranga species from dehisced capsules and fruits of Malania oleifera with softening mesocarp. Seeds were dried in a ventilated oven to a constant weight at 40 ℃.
| Species | Serial number | Acquisition time | Location | Co-ordinates | Elevation (m) |
| Macaranga adenantha | MaP1 | 16-Nov-20 | Yanjia Town, Ceheng, Guizhou | 105°24′E 24°59′N | 850 |
| MaP2 | 17-Nov-20 | Luomu Town, Luodian, Guizhou | 106°31′E 25°11′N | 660 | |
| MaP3 | 18-Nov-20 | Liupai Town, Tian'e, Guangxi | 107°12′E 25°01′N | 500 | |
| MaP4 | 20-Nov-20 | Lizhou Town, Tianlin, Guangxi | 106°24′E 24°22′N | 700 | |
| M. indica | MiP1 | 24-Dec-20 | Menglun Town, Mengla, Yunnan | 101°07′E 21°31′N | 1300 |
| MiP2 | 24-Dec-20 | Yiwu Town, Mengla, Yunnan | 101°27′E 21°59′N | 800 | |
| MiP3 | 24-Dec-20 | Xishuangbanna Tropical Botanical Garden, Mengla, Yunnan | 101°15′E 21°55′N | 570 | |
| MiP4 | 28-Dec-20 | Yuping, Pingbian, Yunnan | 103°25′E 23°01′N | 1400 | |
| M. henryi | Mh | 27-Sep-18 | Damenglong, Jinghong, Yunnan | 100°33′E 21°30′N | 1200 |
| M. kurzii | Mk | ||||
| M. denticulata | Md | 20-Jun-18 | Xishuangbanna Tropical Botanical Garden, Mengla, Yunnan | 101°15′E 21°55′N | 570 |
| Malania oleifera | MoP1 | 26-Sep-21 | Shuguang Town, Guangnan, Yunnan | 105°10′E 23°43′N | 1360 |
| MoP2 | 25-Sep-21 | Jiumo Town, Guangnan, Yunnan | 104°54′E 23°55′N | 1270 | |
| MoP3 | 26-Sep-21 | Babao Town, Guangnan, Yunnan | 105°26′E 23°52′N | 1050 | |
| MoP4 | 27-Sep-21 | Banlun Town, Funing, Yunnan | 105°43′E 23°28′N | 850 | |
| MoP5 | 27-Sep-21 | Zhesang Town, Funing, Yunnan | 106°05′E 23°45′N | 440 | |
| MoP6 | 20-Sep-20 | Nalao Town, Xilin, Guangxi | 105°31′E 24°10′N | 1060 |
Malania oleifera fruits possess thin exocarps, thick mesocarps, and hard endocarps (Tang et al., 2013). The seeds were obtained after cracking open the hard endocarp. As M. oleifera seeds are large, ten seeds were randomly selected to determine the average single seed weight, and then the thousand-seed weight (TSW) was determined. For Macaranga species, 100 seeds were randomly selected to determine seed weight. Subsequently, the TSW was calculated based on the average 100-seed weight.
2.3. Oil contentThe oil content of the seeds was measured using time-domain nuclear magnetic resonance (TD-NMR). TD-NMR determination was carried out using a Minispec mq-one Seed Olive Analyzer (Bruker Optik GmbH, Germany). The calibration curve was obtained from the seed oil of Malania oleifera and Macaranga indica, respectively. Seed oil extraction was performed as previously described (Tian et al., 2020). Approximately 10 g of seeds were heated in isopropanol with 0.01% butylated hydroxytoluene at 85 ℃ for 10 min to inactivate lipases and then homogenized with a Superfine Homogenizer (FLUKO, Germany). Chloroform: methanol (2:1, v/v) was added for extraction. After re-extracting twice, the combined chloroform phase (lower phase) was washed with 0.9% (w/v) NaCl to remove proteins and carbohydrates. The chloroform phase was dried in a vacuum-drying oven.
2.4. Fatty acid compositionSeed samples (~15 mg) were homogenized with a Superfine Homogenizer (FLUKO, Germany) and methylated with 2 mL of 2% sulfuric acid methanolic solution. The mixture was then placed in an 85 ℃ water bath for 2 h. After cooling, 2 mL of aqueous 0.9% NaCl was added, 4 mL of hexane was used for two consecutive extractions, and the supernatant was used to recover fatty acid methyl esters. Analysis of fatty acid methyl esters by gas chromatography (PerkinElmer Clarus 680, Singapore) with flame ionization detection by a 30 m × 0.25 μm × 0.32 mm (inner diameter) Elite-225 column (PerkinElmer, Singapore). The temperature program was 150 ℃ for 3 min, 10 ℃/min to 180 ℃ for 9 min, and 5 ℃/min to 210 ℃ for 8 min. The carrier gas was nitrogen at a flow rate of 1.5 mL/min. The injector temperature was set to 250 ℃, the injection volume was 1 μL, and a split injection mode with a split ratio of 30:1 was used. The retention time of the fatty acid peaks depended on the fatty acid methyl ester standards (Sigma–Aldrich, USA).The relative percentages of fatty acids were calculated from the peak areas. All samples were analyzed in triplicate.
2.5. Data analysisData were subjected to one-way ANOVA, Duncan's multiple range test and the least significant difference (LSD) with the level of significance set at 0.05 (p < 0.05). All values are expressed as the mean (±standard error) of three biological replicates. Statistical analysis was conducted using IBM SPSS statistics 20 (IBM Corp, Armonk, NY, USA). Origin Pro 8.6 (Origin Lab Corporation, Northampton, MA, USA) was used for generating graphics.
3. Results and discussion 3.1. Fatty acids composition of six plant speciesThe seed fatty acid (FA) composition was analyzed in six plant species, including M. oleifera and five Macaranga species (M. indica, M. adenantha, M. denticulata, M. kurzii, and M. henryi). Ten FAs were detected in the M. oleifera seeds (Table 2). Total monounsaturated FA (MUFA) content was 91.64%, of which 35.01% was oleic acid (OA, C18:1), 12.93% erucic acid (EA, C22:1), and 42.74% nervonic acid (NA, C24:1). Low levels of saturated and polyunsaturated fatty acids were detected. The FA species detected were similar to those in previous reports, but the contents of OA and NA were significantly different (Jia and Zhou, 1987; Tang et al., 2013). This may be because the materials were collected from different populations, which suggests high diversity in wild populations of Malania oleifera.
| Species | Fatty acids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C20:1 | C22:0 | C22:1 | C22:2 | C24:0 | C24:1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Malania oleifera | 0.96 | 0.31 | 35.01 | 1.66 | 0.72 | 0.96 | 1.10 | 12.93 | – | 1.97 | 42.74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macaranga adenantha | 10.10 | 0.09 | 25.18 | 11.54 | 1.12 | 0.75 | 0.93 | 11.74 | 1.12 | 0.89 | 36.77 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. indica | 7.45 | 0.50 | 10.51 | 15.00 | 2.83 | 1.51 | 2.61 | 21.50 | 3.41 | 2.12 | 32.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. denticulata | 17.94 | 3.20 | 40.09 | 7.15 | 1.12 | 2.06 | 2.94 | 16.26 | 1.47 | 1.26 | 6.50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. kurzii | 4.97 | 1.28 | 10.93 | 37.33 | 0.89 | 15.09 | 2.78 | 22.59 | 1.54 | 0.54 | 2.06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| M. henryi | 3.99 | 1.27 | 1.39 | 5.60 | 1.19 | 8.67 | 4.26 | 57.51 | 7.61 | 0.90 | 7.35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| –: Undetected or the content is less than 0.01%. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Previous studies have suggested that the production of unusual fatty acids is of taxonomic significance at the family and genus levels (Jia and Zhou, 1987; Ghada et al., 2018). Macaranga adenantha has been reported to have high levels of NA in seed oil (Jia and Zhou, 1987). To determine if NA is enriched in the seeds of other Macaranga species, seeds were collected from five Macaranga species in China, including M. adenantha (Table 1), and GC/MS was used to identify FAs. In all species, eleven FAs were detected. Eleven FAs were detected in all species (Table 2). The contents of different FAs varied among species. In seeds of M. adenantha, MUFA content (74.44%) were significantly lower than that in the seeds of M. oleifera. NA was the most abundant FA (36.77% of total), followed by OA (25.18%) and EA (11.74%). In contrast to Manalia oleifera, Macaranga adenantha seeds contained higher levels of linoleic acid (LA, C18:2) and palmitic acid (PA, C16:0) with 11.54% and 10.10%, respectively. It is worth noting that the seeds of M. indica also contained high levels of NA (32.17% of total FAs), similar to M. adenantha, and the total MUFA was 65.69%. The three other Macaranga species contained low levels of NA (6.50% in M. denticulata, 2.06% in M. kurzii, and 7.35% in M. henryi) (Table 2). These findings indicate that M. indica is a potential novel NA resource plant.
All five species of Macaranga were found to contain high levels of EA, especially M. henryi (57.51%). Additionally, the seeds of M. kurzii contained high levels of LA (37.33%) and eicosenoic acid (EIA, 15.09%), and M. denticulata contained high levels of OA (40.09%) and PA (17.94%) (Table 2). The results indicate that all Macaranga species may contain NA, but the content varies greatly, and different species in the same genus may have evolved different enzymes for FA synthesis, elongation and modification, which likely contributes to differences in FA accumulation. Although NA was abundant in the three species, the FA profiles also varied. Compared with Malania oleifera, Macaranga species contained more PA and LA, and less OA and NA, indicating that the mechanism of NA accumulation differs between plant genera.
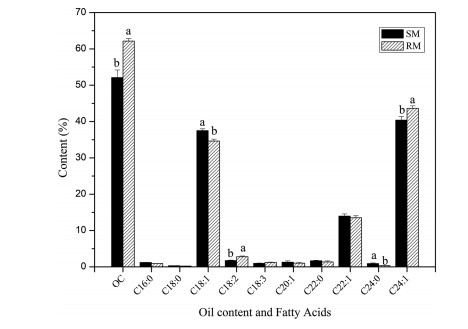
3.2. Seed weight and oil content diversityWe examined seed weight and oil content diversity in the three NA-enriched plant species (Malania oleifera, Macaranga indica and M. adenantha). M. oleifera produces large fruit (approximately 40 g per fresh fruit) with a thick mesocarp and hard endocarp (Guo et al., 2018; Lv et al., 2016). Most wild individuals have low fruit yield, and the seeds cannot germinate after drying; therefore, the average thousand-seed weight (TSW) and oil content of individuals were determined based on the average of ten randomly selected seeds per individual. Seeds were collected from six M. oleifera populations (MoP1–MoP6) that covered most of their natural distribution. The average TSW and oil content of each population was calculated based on the average of every individual. The number of collected fruit-yielding individuals was different in the wild (Table 3). Because only one yielding plant was found, all the values of MoP6 are the mean of five single seeds from the same tree. The average TSW per population ranged from 5749.25 g (MoP3) to 8516.00 g (MoP4), and the seed weight varied greatly among single seeds (ranging from 4.81 g to 10.59 g), but the average TSW varied slightly among individuals and among populations (Table 3). The average oil content in M. oleifera seeds ranged from 46.88% (MoP6) to 63.32% (MoP1) per population, with an average of 58.71% (Table 3). Substantial variation was also detected among single seeds as well among individuals and among populations. We identified three populations [MoP1 (63.32%), MoP2 (61.70%), and MoP3 (60.86%)] with high average oil seed content (62.16%) (Table 3; Fig. 1). This finding is consistent with previous reports (Jia and Zhou, 1987; Ma et al., 2004). The oil content of M. oleifera seeds is likely associated with environmental factors, particularly soil characteristics. M. oleifera is distributed in the karst areas; however, the bareness of rock percentage varies among populations. Plants in the MoP1, MoP2, and MoP3 populations grow in areas with a rock bareness between 70 and 85%, which we refer to as rock mountain; in contrast, the MoP4, MoP5, and MoP6 populations grow in areas with less than 5% rock bareness, referred to here as soil mountain (Fig. 2). Our results suggest that a high bareness of rock percentage may reflect high levels of light, which is beneficial for M. oleifera seed oil accumulation.
| Species | Population | TSW | OC | No. of Individual (n) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Manalia oleifera | MoP1 | 7026.63 ± 515.31ab | 63.32 ± 1.49a | 8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoP2 | 8272.44 ± 226.88a | 61.70 ± 0.76a | 9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoP3 | 5749.25 ± 301.13b | 60.86 ± 0.93a | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoP4 | 8516.00 ± 1082.98a | 54.70 ± 3.64b | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoP5 | 8438.33 ± 876.93a | 58.29 ± 1.90ab | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoP6 | 6429.20 ± 694.20b | 46.88 ± 2.24c | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macaranga adenantha | MaP1 | 20.10 ± 3.07b | 6.26 ± 0.63b | 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MaP2 | 22.71 ± 1.59ab | 18.97 ± 1.78a | 11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MaP3 | 29.43 ± 1.47a | 22.63 ± 1.06a | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MaP4 | 23.12 ± 3.57ab | 20.78 ± 3.84a | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Macaranga indica | MiP1 | 24.35 ± 0.90b | 26.10 ± 0.98c | 11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MiP2 | 27.61 ± 0.79b | 29.46 ± 0.52bc | 8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MiP3 | 31.73 ± 2.01a | 31.09 ± 3.47ab | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MiP4 | 26.12 ± 1.07b | 34.19 ± 1.34a | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Values with the same letter superscripts in the same column within species are not significantly different based on one-way ANOVA and Duncan's multiple range test at p < 0.05. TSW, thousand-seed weight; OC, oil content. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| Fig. 1 Comparison of the average seedoil content and fatty acid content of Malania oleifera populations growing on rock mountains (RM) and soilmountains (SM). Bars with different letters represent values that are significantly different from each other based on one-way ANOVA and Duncan's multiple range test at p < 0.05. |

|
| Fig. 2 Habitat images of Malania oleifera. A. Rock Mountain habitat; B. SoilMountain habitat. |
Malania indica and M. adenantha have small seeds; thus, the TSW was calculated based on the average of 100 randomly selected seeds per individual, which was further used to calculate the average of the population. Seeds were collected from four populations of M. indica (MiP1- MiP4) and four populations of M. adenantha (MaP1- MaP4), representing the major natural distributions of these species.
The average TSW of Macaranga adenantha and M. indica populations ranged from 20.10 g (MaP1) to 29.43 g (MaP3) and from 24.35 g (MiP1) to 31.73 g (MiP3), respectively. M. indica showed a slightly larger TSW (26.65 g) than M. adenantha (24.17 g). The average oil content for M. adenantha and M. indica populations ranged from 6.26% (MaP1) to 22.63% (MaP3) and from 26.10% (MiP1) to 34.19% (MiP4), respectively (Table 3). Oil content was significantly higher in M. indica than in M. adenantha (29.22% vs. 18.50%; Fig. 3). The TSW of M. adenantha and M. indica and the oil content of M. indica are here for the first time. The oil content of M. adenantha in the present study was significantly lower than that in previous reports. This may be because the previous study examined the kernel, but not the seed (Jia and Zhou, 1987).

|
| Fig. 3 Comparison of average seedoil content and fatty acid content among three species. Mo: Malania oleifera, Ma: Macaranga adenantha, Mi: Macaranga indica. Bars with different letters represent values that are significantly different from each other based on one-way ANOVA and Duncan's multiple range test at p < 0.05. |
In all seed samples of Malania oleifera, the major fatty acids were OA, EA, and NA. These three FAs composed over 90% of total FAs in all populations of this species, ranging from 90.68% (MoP3) to 93.11% (MoP5). NA was the most abundant fatty acid, ranging from 37.85% (MoP6) to 44.53% (MoP5), followed by OA, which ranged from 33.73% (MoP1) to 37.92% (MoP4) and from 11.80% (MoP3) to 14.59% (MoP1) (Table 4). Significant differences in FA levels were detected among the populations. It is worth noting that the rock mountain populations showed higher amounts of NA and LA but lower amounts of OA (Fig. 1). These results suggest that a high percentage of rock bareness is probably beneficial to M. oleifera NA accumulation.
| Fatty Acid | Population | |||||
| MoP1 | MoP2 | MoP3 | MoP4 | MoP5 | MoP6 | |
| C16:0 | 0.94 ± 0.08b | 0.92 ± 0.06b | 0.95 ± 0.09b | 1.08 ± 0.06b | 1.05 ± 0.04b | 1.35 ± 0.07a |
| C18:0 | 0.23 ± 0.08 | 0.18 ± 0.06 | 0.29 ± 0.03 | 0.30 ± 0.01 | 0.31 ± 0.03 | 0.31 ± 0.03 |
| C18:1 | 33.73 ± 0.62b | 34.59 ± 1.02b | 36.22 ± 0.29ab | 37.92 ± 0.60a | 36.62 ± 0.75ab | 37.78 ± 0.93a |
| C18:2 | 2.39 ± 0.44ab | 3.01 ± 0.25a | 3.01 ± 0.15a | 2.13 ± 0.29ab | 1.88 ± 0.25b | 1.39 ± 0.14b |
| C18:3 | 1.07 ± 0.15bc | 1.09 ± 0.07bc | 1.73 ± 0.17a | 1.25 ± 0.21b | 0.76 ± 0.07c | 0.90 ± 0.15bc |
| C20:1 | 0.92 ± 0.43b | 1.24 ± 0.33b | 0.77 ± 0.08b | 0.32 ± 0.08b | 0.33 ± 0.03b | 2.42 ± 0.23a |
| C22:0 | 1.50 ± 0.57 | 1.10 ± 0.44 | 1.67 ± 0.10 | 2.07 ± 0.08 | 1.99 ± 0.05 | 1.26 ± 0.05 |
| C22:1 | 14.59 ± 1.01ab | 13.48 ± 0.77abc | 11.80 ± 0.29c | 13.45 ± 1.01abc | 11.96 ± 0.84bc | 15.52 ± 0.41a |
| C24:0 | 0.27 ± 0.09b | 0.33 ± 0.09b | 0.47 ± 0.12b | 0.61 ± 0.19b | 0.24 ± 0.06b | 1.48 ± 0.09a |
| C24:1 | 44.12 ± 1.49ab | 43.75 ± 1.00ab | 42.66 ± 0.79ab | 40.48 ± 0.69bc | 44.53 ± 0.48a | 37.85 ± 0.94c |
| ΣMUFA | 92.44 | 91.82 | 90.68 | 91.85 | 93.11 | 91.15 |
| ΣMUFA: Total of three major monounsaturated fatty acids = C18:1 + C22:1 + C24:1. Values with the same letter superscripts in the same column are not significantly different based on one-way ANOVA and Duncan's multiple range test at p < 0.05. |
||||||
In all seed samples of Macaranga adenantha, five major FAs were detected, including PA, OA, LA, EA, and NA. NA and OA were the most abundant FAs, with NA ranging from 23.89% (MaP4) to 37.78% (MaP3) and OA from 23.44% (MaP3) to 35.87% (MaP2). The PA levels ranged from 9.23% (MaP3) to 16.05% (MaP1), LA from 10.34% (MaP2) to 13.61% (MaP4), and EA from 9.79% (MaP4) to 13.20% (MaP3) (Table 5). The total MUFAs ranged from 66.73% (MaP1) to 74.42% (MaP3), which was significantly lower than that of M. oleifera. The FA levels varied significantly among populations, suggesting that M. adenantha displays great germplasm diversity. It is worth noting that population MaP3 showed a higher NA level and a larger TSW than other populations. The MaP3 population may be the best M. adenantha germplasm resource. Similar to M. adenantha, M. indica seeds contained five major FAs, including PA, OA, LA, EA, and NA. NA and OA were the most abundant FAs, with NA ranging from 20.59% (MiP1) to 24.46% (MiP4) and OA from 23.45% (MiP2) to 32.06% (MiP1). The PA levels ranged from 10.94% (MiP4) to 13.98% (MiP2), LA from 13.08% (MiP4) to 17.41% (MiP2), and EA from 16.04% (MiP1) to 19.14% (MiP3). The total MUFAs ranged from 61.73% (MiP2) to 69.48% (MiP4) (Table 6), which was similar to that of M. adenantha (Table 5). The natural distribution of M. indica ranged from an elevation of 560 m–1468 m (Table 1). No significant differences were detected in major FA levels among populations, which is indicative of slight germplasm variation among populations; thus, the elevation of populations was not associated with the diversity of germplasm. The two Macaranga species have similar FA profiles, but compared with M. adenantha, M. indica had lower NA levels and higher LA and EA levels (Fig. 3).
| Fatty Acid | Population | ||||
| MaP1 | MaP2 | MaP3 | MaP4 | ||
| C16:0 | 16.05 ± 1.27a | 11.77 ± 0.56bc | 9.23 ± 0.65c | 13.42 ± 1.39ab | |
| C18:0 | 1.09 ± 0.47a | 0.64 ± 0.12ab | 0.17 ± 0.06b | 1.10 ± 0.18a | |
| C18:1 | 30.43 ± 1.51a | 35.87 ± 1.16a | 23.44 ± 1.78b | 34.42 ± 3.02a | |
| C18:2 | 11.68 ± 2.16ab | 10.34 ± 0.46b | 11.88 ± 0.28bc | 13.61 ± 0.61a | |
| C18:3 | 0.83 ± 0.24b | 0.74 ± 0.05b | 1.18 ± 0.08a | 0.84 ± 0.05b | |
| C20:1 | 0.70 ± 0.12 | 1.06 ± 0.15 | 0.87 ± 0.04 | 0.87 ± 0.10 | |
| C22:0 | 2.00 ± 0.30a | 0.46 ± 0.08b | 0.68 ± 0.18b | 0.35 ± 0.11b | |
| C22:1 | 12.12 ± 0.77ab | 11.47 ± 0.72ab | 13.2 ± 0.76a | 9.79 ± 0.47b | |
| C22:2 | 1.32 ± 0.49 | 1.07 ± 0.06 | 1.31 ± 0.14 | 1.07 ± 0.06 | |
| C24:0 | 1.41 ± 0.10a | 0.93 ± 0.12b | 0.59 ± 0.09b | 0.64 ± 0.10b | |
| C24:1 | 24.18 ± 2.72b | 25.69 ± 1.09b | 37.78 ± 2.13a | 23.89 ± 3.32b | |
| ΣMUFA | 66.73 | 73.03 | 74.42 | 68.10 | |
| ΣMUFA: Total of three major monounsaturated fatty acids = C18:1 + C22:1 + C24:1. Values with the same letter superscripts in the same column are not significantly different based on one-way ANOVA and Duncan's multiple range test at p < 0.05. |
|||||
| Fatty Acid | Population | |||
| MiP1 | MiP2 | MiP3 | MiP4 | |
| C16:0 | 12.07 ± 0.89 | 13.98 ± 1.18 | 11.45 ± 2.45 | 10.94 ± 1.07 |
| C18:0 | 0.57 ± 0.03b | 0.93 ± 0.15a | 0.39 ± 0.03b | 0.70 ± 0.18 ab |
| C18:1 | 32.06 ± 1.28 | 23.45 ± 4.46 | 24.26 ± 5.07 | 27.68 ± 1.49 |
| C18:2 | 13.41 ± 0.76 | 17.41 ± 1.50 | 16.08 ± 4.00 | 13.08 ± 0.43 |
| C18:3 | 0.93 ± 0.07 | 0.90 ± 0.08 | 1.19 ± 0.35 | 0.90 ± 0.04 |
| C20:1 | 1.23 ± 0.13b | 1.45 ± 0.13 ab | 1.96 ± 0.46a | 1.48 ± 0.3 ab |
| C22:0 | 1.07 ± 0.07 | 1.20 ± 0.24 | 0.74 ± 0.05 | 0.91 ± 0.21 |
| C22:1 | 16.04 ± 0.60 | 16.81 ± 1.02 | 19.14 ± 4.11 | 17.34 ± 2.36 |
| C22:2 | 1.37 ± 0.07 | 1.58 ± 0.13 | 1.77 ± 0.43 | 1.57 ± 0.23 |
| C24:0 | 0.68 ± 0.09b | 1.27 ± 0.15a | 1.04 ± 0.15 ab | 0.95 ± 0.10 ab |
| C24:1 | 20.59 ± 1.06 | 21.47 ± 1.18 | 22.28 ± 2.60 | 24.46 ± 0.98 |
| ΣMUFA | 68.69 | 61.73 | 65.68 | 69.48 |
| ΣMUFA: Total of three major monounsaturated fatty acids = C18:1 + C22:1 + C24:1. Values with the same letter superscripts in the same column are not significantly different based on one-way ANOVA and Duncan's multiple range test at p < 0.05. |
||||
Malania oleifera presented significantly higher NA levels and significantly lower LA and PA levels than the two Macaranga species (Fig. 3). High NA enrichment in M. oleifera seeds may be related to OA content, as OA can be converted to NA via three consecutive steps of carbon chain elongation (Taylor et al., 2009; Blacklock and Jaworski, 2002; Salas and Ohlrogge, 2002; Huai et al., 2015). OA is a limiting substrate in the production of LA. Thus, it is likely that PA is used to efficiently produce OA in M. oleifera seeds; if so, these seeds would contain high levels of beta-ketoacyl-ACP synthase II (KASⅡ), stearoyl-acyl carrier protein-desaturase (SAD) activation, and low levels of oleate delta-12 desaturase (FAD2) activation. Furthermore, OA is also elongated efficiently to NA by a special β-ketoacyl-CoA synthase (KCS). Further studies are needed at the molecular and biochemical levels to confirm this hypothesis.
3.4. Three species of woody plants identified as new potential NA resourcesPlant species with high levels of NA in their seed oil (e.g., Cardamine graeca L., 54%; Lunaria annua L., 24.2%; and Tropaeolum speciosum Poepp. & Endl., 45.4%) have attracted recent interest (Jart, 1978; Taylor et al., 2009; Mastebroek and Marvin, 2000; Litchfield, 1970). However, C. graeca is a small crucifer that grows on special Mediterranean red soil (terra rosa) and has low oil content (13%) in its mature seeds. L. annua is a poor-yielding biennial containing very high levels of EA (50%), which is a well-known unhealthy FA (Vajreswari et al., 1991; Hui, 1996; Parke and Parke, 1999). In addition, T. speciosum seeds are difficult to obtain and cultivate (Taylor et al., 2009; Li et al., 2019b; Liu et al., 2021). Therefore, these species have not been utilized to develop NA products.
In the present study, the germplasms of three woody plants enriched with NA were investigated. Malania oleifera had the largest seeds, highest oil content, highest level of NA, and lowest EA (Table 2, Table 3; Fig. 3). In the wild, a few mature individuals with full bearing age can yield up to approximately 150 kg per individual. M. oleifera has been of particular interest in the recent production of NA by institutions, companies, and governments in China. Some NA products have been developed using the seed oil of M. oleifera. However, further research is necessary to resolve some of the following problems: (1) M. oleifera is a rare and endangered arbor with a long juvenile phase (estimated 10–15 years) and root hemiparasitic habit (Li et al., 2019a), which hinders successful seedling regeneration and planting; and (2) malanin, a novel plant toxin in seeds of M. oleifera (Yuan et al., 2009), also limits its development and industrialization.
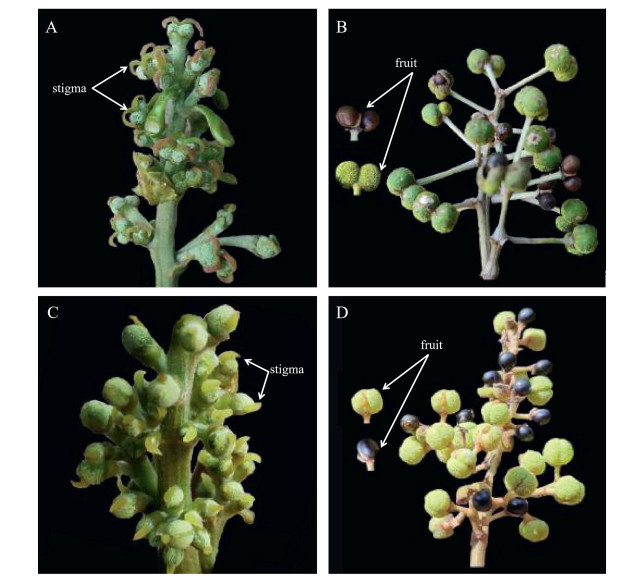
Macaranga adenantha and M. indica, two closely related species, are fast-growing tropical pioneer tree species. As most phenotypes were similar, M. adenantha was lumped into M. indica in Flora of China (Qiu and Michael, 2008). However, our results show that the two species have significant differences in oil content and FA profiles (Table 2, Table 5, Table 6; Fig. 3). M. indica showed higher oil content and higher levels of LA and EA but lower levels of NA. In addition, field investigations found that the two species have distinct natural distributions. In China, the distribution of M. adenantha extends from southeastern Yunnan Province (near 104°42′E) to the center of Guangdong Province (near 112°18′E), whereas M. indica extends from southeastern Yunnan Province (near 103°48′E) to southeastern Tibet Autonomous Region (near 95°12′E). Female flowers of M. adenantha have two stigmas and two ovaries that yield two seeds, but M. indica has one stigma and one ovary yielding one seed (Fig. 4). Meanwhile, M. indica yielded more fruit than M. adenantha (Fig. 5). These phenotypes indicate that M. adenantha is distinct from M. indica. Further studies using additional data, such as DNA sequences, are needed to confirm this conclusion. Compared with M. adenantha, M. indica may be a better NA resource plant candidate owing to its high yield and oil content, albeit with a slightly lower NA level. M. adenantha and M. indica are closely related species with different dominant traits. Both are dioecious, so the germplasm could be improved by interspecific hybridization. Additionally, M. adenantha and M. indica are tropical pioneer tree species that are light-demanding and lack shade tolerance, resulting in their gradual replacement by other tree species after forest establishment. Most individuals were found sporadically in roadside ditches. Furthermore, the sex ratio (female/male plants) of both species was estimated to be approximately 1:1, yet yielding female plants were lacking. The germplasms of M. adenantha and M. indica should therefore be conserved.
|
| Fig. 4 Femaleflowers and fruits of Macaranga adenantha and M. indica. A. Female flowers of M. adenantha have two stigmas, B. Fruits of M. adenantha have two seeds, C. Female flowers of M. indica have one stigma, D. Fruits of M. indica have one seed. |

|
| Fig. 5 Infructescence of Macaranga adenantha (A) and M. indica (B). |
Three woody plants enriched with NA-enriched seed oil were identified, and germplasm diversity was evaluated. Malania oleifera has large seeds enriched with oil and NA. The germplasm variation is probably related to habitat. Populations growing on rock mountains had higher oil content and NA levels. Seeds of Macaranga indica are known to accumulate high levels of oil and NA. Germplasm diversity was detected among populations of Macaranga adenantha, but no significant differences were detected among populations of M. indica. Compared with M. adenantha, although M. indica contained a slightly lower level of NA in seed oil, it had significantly higher oil content and seed yield. M. indica is a tropical pioneer tree species that is easy to cultivate and grows rapidly, thus making it a potential candidate for the development of NA products. The wild germplasm resources of the three species should be methodically utilized and conserved. To develop NA products, further studies should examine nursery construction, discover the mechanisms of NA accumulation in plant seeds, and build a feasible breeding program.
Author contributionsBT conceived research. XH, TL, and JL performed the experiments and wrote the article with contributions from all the authors. LZ, PM, GZ collected the materials. All authors contributed to data analysis and reviewed and approved the final manuscript.
Declaration of competing interestThe authors declare no potential conflicts of interest. All the authors agreed to submit this manuscript.
AcknowledgmentWe thank Dr. Jin Yanqiang and Funing Forestry and Grassland Bureau for their help in seed collection, and thank Dr. Tang Mingyong for his help in measuring seed oil content. This work was supported by the Applied Basic Research Key Project of Yunnan, China (Grant No. 202101AS07001), Reserve Talents for Yunnan Young and Middle-aged Academic and Technical Leaders, China (Grant No. 202105AC160083), National Natural Science Foundation of China (Grant No. 31671732).
Amminger, G.P., Schäfer, M.R., Klier, C.M., et al., 2012. Decreased nervonic acid levels in erythrocyte membranes predict psychosis in help-seeking ultra-high-risk individuals. Mol. Psychiatr., 17: 1150-1152. DOI:10.1038/mp.2011.167 |
Blacklock, B.J., Jaworski, J.G., 2002. Studies into factors contributing to substrate specificity of membrane-bound 3-ketoacyl-CoA synthases. Eur. J. Biochem, 269: 4789-4798. DOI:10.1046/j.1432-1033.2002.03176.x |
Ghada, K., Mohamed, H., Sabrine, S., et al., 2018. A systematic comparison of 25 Tunisian plant species based on oil and phenolic contents, fatty acid composition and antioxidant activity. Ind. Crop. Prod., 123: 768-778. DOI:10.1016/j.indcrop.2018.07.008 |
Guo, F.B., Wang, S.H., Wang, J., et al., 2018. Fruit yield and characters of wild Malania oleifera, a rare plant species in southwest China. Guihaia, 38: 57-64. DOI:10.1117/12.2505217 |
He, X., Li, D.Z., Tian, B., 2021. Diversity in seed oil content and fatty acid composition in Acer species with potential as sources of nervonic acid. Plant Divers., 43: 86-92. DOI:10.1016/j.pld.2020.10.003 |
Huai, D., Zhang, Y., Zhang, C., et al., 2015. Combinatorial effects of fatty acid elongase enzymes on nervonic acid production in Camelina sativa. PLoS One, 10: e0131755. DOI:10.1371/journal.pone.0131755 |
Hui, Y.H., 1996. Bailey's Industrial Oil and Fat Products. vol. 1. Wiley-Interscience, New York, USA
|
Jart, A., 1978. The fatty acid composition of various cruciferous seeds. J. Am. Oil Chem. Soc., 55: 873-875. DOI:10.1007/BF02671410 |
Jia, L., Zhou, J., 1987. The Oil Plants in China. Beijing: Science Press.
|
Lai, J., 2006. Study on Conservation Biology of Rear and Precious Plant Malania oleifera. dissertation, Sichuan University
|
Lewkowicz, N., Pitek, P., Namieciñska, M., et al., 2019. Naturally occurring nervonic acid ester improves myelin synthesis by human oligodendrocytes. Cells, 8: 786. DOI:10.3390/cells8080786 |
Li, A.R., Mao, P., Li, Y.J., 2019. Root hemiparasitism in Malania oleifera (Olacaceae), a neglected aspect in research of the highly valued tree species. Plant Divers., 41: 347-351. DOI:10.1016/j.pld.2019.09.003 |
Li, Q., Chen, J., Yu, X., et al., 2019. A mini review of nervonic acid: source, production, and biological functions. Food Chem., 301: 125286. DOI:10.1016/j.foodchem.2019.125286 |
Litchfield, C., 1970. Tropaeolum speciosum seed fat: a rich source of cis-15-tetracosenoic andcis-17-hexacosenoic acids. Lipids, 5: 144-146. DOI:10.1007/BF02531110 |
Liu, F., Wang, P., Xiong, X., et al., 2021. A review of nervonic acid production in plants: prospects for the genetic engineering of high nervonic acid cultivars plants. Front. Plant Sci., 12: 626625. DOI:10.3389/fpls.2021.626625 |
Lv, S., Wei, C., Huang, F., et al., 2016. Fruit and seed traits and adaptability to rocky desertification mountain of rare tree species Malania oleifera. Chinese. J. Ecol., 35: 57-62. |
Ma, B.L., Liang, S.F., Zhao, D.Y., et al., 2004. Study on plants containing nervonic acid. Acta Bot. Boreali Occident. Sin., 24: 2362-2365. |
Mastebroek, H.D., Marvin, H.J.P., 2000. Breeding prospects of Lunaria annua L. Ind. Crop. Prod., 11: 139-143. DOI:10.1016/S0926-6690(99)00056-4 |
Merrill, A.H., Schmelz, E.M., Wang, E., et al., 1997. Importance of sphingolipids and inhibitors of sphingolipid metabolism as components of animal diets. J. Nutr., 127(5 Suppl. l): 830S-833S. DOI:10.1093/jn/127.5.830s |
Parke, D.V., Parke, A.L., 1999. Rape seed oil - an autoxidative food lipid. J. Clin. Biochem. Nutr., 26: 51-61. DOI:10.3164/jcbn.26.51 |
Qiu H, Michael G.G., 2008. Flora of China. Science Press, Beijing, (and Missouri Botanical Garden Press, St. Louis)
|
Qiu, H., 1996. Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita, Flora Reipublicae Popularis Sinicae 44, Science Press, Beijing
|
Salas, J.J., Ohlrogge, J.B., 2002. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys., 403: 25-34. DOI:10.1016/S0003-9861(02)00017-6 |
Sargent, J.R., Coupland, K., Wilson, R., 1994. Nervonic acid and demyelinating disease. Med. Hypotheses, 42: 237-242. DOI:10.1016/0306-9877(94)90122-8 |
Tang, T.F., Liu, X.M., Ling, M., et al., 2013. Constituents of the essential oil and fatty acid from Malania oleifera. Ind. Crop. Prod., 43: 1-5. DOI:10.1016/s0924-9338(13)75771-7 |
Taylor, D.C., Francis, T., Guo, Y., et al., 2009. Molecular cloning and characterization of a KCS gene from Cardamine graeca and its heterologous expression in Brassica oilseeds to engineer high nervonic acid oils for potential medical and industrial use. Plant Biotechnol. J., 7: 925-938. DOI:10.1111/j.1467-7652.2009.00454.x |
Tian, B., Sun, M., Jayawardana, K., et al., 2020. Characterization of a PLDζ2 homology gene from developing castor bean endosperm. Lipids, 55: 537-548. DOI:10.1002/lipd.12231 |
Vajreswari, A., Rao, P.S., Tulpule, P.G., 1991. Short-term effects of low erucic acid rapeseed oil and high erucic mustard oil on myocardial lipidosis of CFY strain of rats. J. Oil Technol.Assoc. India, 23: 2-5. |
Vozella, V., Basit, A., Misto, A., et al., 2017. Age-dependent changes in nervonic acid-containing sphingolipids in mouse hippocampus. Biochim. Biophys. Acta, 1862: 1502-1511. DOI:10.1016/j.bbalip.2017.08.008 |
Whitmore, T.C., 2008. The Genus Macaranga, a Prodromus. Kew, Royal Botanic Gardens
|
Wu, Z., Raven, P., Hong, D., 2003. Flora of China. Vol. vol. 5 (Ulmaceae through Basellaceae). Science Press, Beijing, and Missouri Botanical Garden Press, St. Louis
|
Xu, C.Q., Liu, H., Zhou, S.S., et al., 2019. Genome sequence of Malania oleifera, a tree with great value for nervonic acid production. GigaScience, 8: 1-14. |
Yuan, Y., Xiao, H., Kang, H., et al., 2009. Fluorescence spectra study of a new toxic protein from Malania oleifera. Spectrosc. Spectr. Anal., 29: 777-780. |



