b. Co-Innovation Center for Sustainable Forestry in Southern China, College of Biology and the Environment, Key Laboratory of State Forestry and Grassland Administration on Subtropical Forest Biodiversity Conservation, Nanjing Forestry University, 219937, Nanjing, China;
c. Department of Biology Education, Chonnam National University, 61186, Gwangju, Republic of Korea;
d. School of Life Sciences, Yunnan Normal University, 650001, Kunming, China;
e. Faculty of Health and Life Sciences, INTI International University, 71800, Nilai, Malaysia
The family Thymelaeaceae comprises 45 genera and about 800 species widely distributed in both temperate and tropical regions (Herber, 2003). Recent taxonomic work on Thymelaeaceae has divided the family into two subfamilies: Octolepidoideae and Thymelaeoideae (Herber, 2003). The latter includes the tribes Aquilarieae, Daphneae, and Synandrodaphneae. The tribe Daphneae, synonym Thymelaeoideae sensu Domke (1934), is further subdivided into four groups: the Daphne, Gnidia, Linostoma, and Phaleria groups. Members of the Daphne group can be easily distinguished by their lack of interxylary phloem; also, no genera except Diarthron Turcz. have unarticulated floral tubes. Fourteen genera have been recorded in the Daphne group: Daphne L., Daphnopsis Mart. & Zucc., Diarthron, Dirca L., Edgeworthia Meisn., Funifera Leandro ex C.A. Mey., Goodallia Benth., Lagetta Juss., Ovidia Meisn., Rhamnoneuron Gilg., Schoenobiblus Mart. & Zucc., Stellera L., Thymelaea Mill., and Wikstroemia Endl. (Herber, 2003).
The genus Daphne is one of the largest genera in the Daphne group, comprising ca. 95 species of well-known ornamental plants distributed in Eurasian and North African (Herber, 2003; Wang et al., 2007a). Thus far, the International Union for Conservation of Nature (IUCN) has only addressed the conservation status of six Daphne species, classifying Daphne rodriguezii Texidor and D. sophia Kolenicz. as "Endangered", D. ludlowii D.G. Long & Rae and D. petraea Leyb. as "Least Concern", and D. altaica Pall. and D. arbuscular Celak. as "Data Deficient" in the IUCN Red List (IUCN, 2020). Because Daphne spp. are commonly cultivated in parks and gardens (Brickell and Mathew, 1998) and are known to have medicinal properties (Sovrlić and Manojlović, 2017), most studies of this genus have focused on either horticultural or medicinal properties. Although genetic studies have been conducted on Daphne jezoensis Maxim., D. laureola L., and D. rodriguezii (Alonso and Herrera, 2011; Castilla et al., 2012; Kameyama and Hirao, 2014; García-Verdugo et al., 2019), species demarcation in Daphne is based solely on floral characteristics (Wang et al., 2007a). However, Daphne species are morphologically similar, suggesting that molecular approaches are required to elucidate their taxonomy.
The circumscription of Daphne, as well as its phylogenetic relationship with allied genera, has long been controversial. For instance, the boundary between Daphne and Wikstroemia remains in dispute (Hamaya, 1963; Wang et al., 2007a), prompting some to propose transferring Wikstroemia into Daphne and further treating it as a subgenus (Halda, 1999, Halda, 2001; Wang et al., 2007a; Zhang et al., 2007). The ambiguity in phylogenetic relationships is largely because the features traditionally used to distinguish species (i.e., the shape of hypogynous disc, the type of fruit, and the leaf arrangement) are exhibited across the genus (Domke, 1934; Hamaya, 1963; Halda, 1999; Wang et al., 2007b), and because of the lack of molecular resources to test phylogenetic inferences. Although molecular studies have been conducted on Thymelaeaceae at the genus-level, these studies have mostly focused on the subfamily Thymelaeoideae, with particular emphasis on a few genera (Gnidia L., Passerina L. mainly from South Africa, Thymelaea from circum-Mediterranean area, and Pimelea Banks ex Sol. from the Asia–Pacific region) (Van der Bank et al., 2002; Galicia-Herbada, 2006; Beaumont et al., 2009; APG IV, 2016; Foster et al., 2018). Such inadequate taxonomic sampling has failed to yield insights into phylogenetic relationships within Daphne or between Daphne and other related genera (Galicia-Herbada, 2006; Wang et al., 2007b; Beaumont et al., 2009; Zhang et al., 2010).
The plastid genome, or plastome, is an ideal tool for molecular taxonomic studies. Plastomes are small, haploid, inherited uniparentally, possess low nucleotide substitution rates, and have highly conserved sequences. Nuclear ribosomal internal transcribed spacer (nrITS) regions, which unlike chloroplast DNA are inherited biparentally, are also known to be useful in assessing genetic variation and reconstructing phylogenetic relationships between closely related species (Neves and Forrest, 2011). For members of Thymelaeaceae, publicly available nrITS sequences far outnumber plastome sequences, likely because nrITS regions are easier to amplify (Alvarez and Wendel, 2003). Used in conjunction, plastid genomes and nrITS sequences promise to advance our understanding of the phylogenetic relationships of Daphne in Thymelaeaceae.
To test the monophyly of Daphne, we first assembled complete plastomes of five Daphne taxa, namely D. championii, D. genkwa Siebold & Zucc., D. kiusiana var. atrocaulis (Rehder) F. Maek., D. odora Thunb., and D. papyracea Wall. ex G. Don. Although these five Daphne species grow in the wild and are domesticated for medicinal or ornamental purposes, their respective genetic identities still remain ambiguous. We analyzed and compared these Daphne plastomes to published plastomes to determine the molecular identities, genetic divergence, and phylogenetic relationships of these species. We also identified highly variable gene regions that may be useful DNA barcodes for Daphne species. The findings of this study will provide future reference for taxonomic studies of Daphne and help elucidate the taxonomy of Thymelaeaceae.
2. Material and methods 2.1. Plant materials and DNA extractionFresh leaves from five Daphne taxa were collected from natural populations and arboreta in China. Samples of D. odora were collected from the Germplasm Resource Nursery of Ornamental Plants of Guangzhou Institute of Forestry and Landscape Architecture in the Guangdong Province, while D. kiusiana var. atrocaulis and D. papyracea were collected from their natural habitat in Mount Luoxiao, Hunan Province and Mount Yunkai, Guangdong Province, respectively. D. championii was collected from Lianzhou, Guangdong Province, and D. genkwa was collected from Mount Tiantai, Hubei Province and Anqing, Anhui Province. Leaves were stored with silica gel in aluminum sealed ziplock bags until DNA extraction. The voucher specimens were deposited at the Herbarium of Sun Yat-sen University (SYS) and Herbarium of Yunnan Normal University (YNUB) (Table 1).
| Species (Sample number) | Collector name and voucher record | Number of individuals examined | Sampling location | GenBank accession numbers | |
| Complete chloroplast genome | nrITS | ||||
| Daphne championii (1–4) | Shiou Yih Lee & Xinjian Zhang, LSY-THY-4001 | 4 | Lianzhou, Guangdong, China | MT648376 | MT623676-MT623679 |
| Daphne genkwa | Yonghong Zhang, SBK12. | 1 | Mount Tiantai, Hubei, China | MN563133 | – |
| Daphne genkwa (1–4) | Shiou Yih Lee, LSY-THY-4013 | 4 | Anqing, Anhui, China | – | MT623680-MT623683 |
| Daphne kiusiana var. kiusiana (1–4) | Jung-Hyun Lee, LJH-GU01 | 4 | Ryuhosan, Kumamoto, Japan | – | MT623684-MT683687 |
| Daphne kiusiana var. kiusiana (5–8) | Jung-Hyun Lee, LJH-SP01 | 4 | Sinpyeong goj-jawal, Jeju-do, Korea | KY991380 | MT623688-MT623691 |
| Daphne kiusiana var. atrocaulis (1–3) | Wenbo Liao, LXP6488 | 3 | Mount Luoxiao, Hunan, China | MT627481 | MT623692-MT623694 |
| Daphne odora (1–4) | Shiou Yih Lee, LSY-THY-4009 | 4 | Guangzhou Institute of Forestry and Landscape Architecture, Guangdong, China | MT627479 | MT623695-MT623698 |
| Daphne papyracea (1–4) | Shiou Yih Lee & Zhihui Chen, LSY-THY-4005 | 4 | Mount Yunkai, Guangdong, China | MT627480 | MT623699-MT623702 |
Total genomic DNA extraction was carried out using the DN15-Plant DNA Mini Kit (Aidlab, China) according to the manufacturer's protocol. The quantity and quality of the DNA extracts for next-generation and Sanger sequencing were determined using Qubit™ 4 Fluorometer (Thermo Fisher Scientific, USA) and Nanodrop™ 2000 spectrophotometer (Thermo Fisher Scientific, USA), respectively.
2.2. Plastid genome sequencing, assembly, and annotationA ~350-bp insert size genomic library was prepared using a TruSeq DNA Sample Prep Kit (Illumina, USA) and sequencing was conducted on an Illumina NovaSeq platform (Illumina, USA) to obtain 6 Gb of 150-bp pair-end reads. Adapter sequences were removed using NGSToolkit (Patel and Jain, 2012), and the raw reads were fed into the NOVOPlasty 2.7.2 pipeline (Dierckxsens et al., 2017) for de novo assembly. Using the rbcL sequences for Daphne papyracea (LC527404) as the seed sequence, a single contig was obtained at the end of the process for each taxon. The assembled genomes were annotated through the online GeSeq annotation tool (Tillich et al., 2017) and manually checked for errors. The GC content was analysed using MEGA 7 (Kumar et al., 2016) and the circular map of each plastome was created in OGDRAW 1.3.1 (Greiner et al., 2019). The plastome sequences were deposited in the NCBI GenBank database under the accession numbers MT648376 (D. championii), MN563133 (D. genkwa), MT627481 (D. kiusiana var. atrocaulis), MT627479 (D. odora), and MT627480 (D. papyracea).
2.3. Sequence repeats, codon usage, and RNA editing site predictionForward, reverse, and palindromic repeat sequences were identified using REPuter (Kurtz et al., 2001), with the Hamming distance set at 3 and the minimum repeat size at 30 bp. The nucleotide sequence of each protein-coding gene in the plastome was extracted for subsequent analyses using FeatureExtract 1.2L Server (Wernersson, 2005). The relative synonymous codon usage (RSCU) for each protein-coding gene was calculated using the Codon Usage Calculator function available in the Sequence Manipulation Suite (Stothard, 2000), and the potential RNA editing sites were predicted using the PREP-Cp function available in the PREP Suite (Mower, 2009) based on default settings.
2.4. Genetic pairwise distance, IR border analysis, and genome comparisonThe plastome sequences of four Daphne species (D. kiusiana var. kiusiana (KY991380), D. giraldii (MN080709), D. laureola (MN201546), and D. tangutica (MK455900)) were downloaded from NCBI GenBank and were included in subsequent analyses. All nine plastomes were aligned using MAFFT (Katoh et al., 2019) and genetic pairwise distance was calculated based on the Kimura 2-parameter DNA substitution model, with 1000 bootstrap replicates, using MEGA 7 (Kumar et al., 2016). Gaps and missing data in the alignment were not included in the analyses (complete deletion). The borders of the four different regions in the plastomes (large single-copy (LSC), small single-copy (SSC), and inverted repeat A and B (IRA and IRB)) of the nine Daphne species were plotted using IRscope to analyse the exact IR border positions and identify adjacent genes (Amiryousefi et al., 2018). The nine plastomes were also aligned and visualised using mVISTA program (Frazer et al., 2004) in Shuffle-LAGAN mode, using D. laureola (MN201546) as the reference genome.
2.5. Polymerase chain reaction (PCR) and Sanger sequencingThree to four biological replicates from each species were selected for Sanger sequencing to obtain their nrITS sequences. PCR amplification of the nrITS region was carried out using primers ITS-p5: 5′-CCT TAT CAY TTA GAG GAA GGA G-3' (Cheng et al., 2016) and ITS-S3R: 5′-GAC CGT TCT CCA GAC TAC AAT-3' (Chiou et al., 2007). PCR was conducted in a final reaction volume of 20 μL, containing 10 μL of 2 × Taq PCR Starmix with loading dye (Genstar Biosolutions, China), 0.4 μM of each primer, and 20 ng of genomic DNA as template. PCR amplification was conducted in a T100™ Thermal Cycler (Bio-Rad, USA), with thermal settings programmed as follows: initial denaturation at 94 ℃ for 5 min; 40 cycles of 94 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 30 s; and a final extension at 72 ℃ for 7 min. PCR products were sent for direct sequencing for both ends on an ABI 3730 DNA Analyzer (Applied Biosystems, USA).
2.6. Phylogenetic inferencePrior to phylogenetic analyses, all nine Daphne plastome sequences and the nrITS sequences of 39 accessions representing 17 Daphne taxa were aligned using MAFFT (Katoh et al., 2019), separately. Based on the findings of Lee et al. (2018), Eucalyptus grandis (Myrtaceae; HM347959 and AF390472) and Gossypium hirsutum (Malvaceae; DQ345959 and KC404827) were included as outgroups. Both alignments were trimmed using trimAl v.1.2 (Capella-Gutiérrez et al., 2009) with the gappyout method to reduce the systematic errors produced due to poor alignment. Phylogenetic analyses of plastome sequences were carried out using maximum likelihood (ML) and Bayesian inference (BI). Maximum likelihood (ML) tree analysis was conducted with RAxML (Stamatakis, 2014), available in the CIPRES Science Gateway (Miller et al., 2010), and using the general time-reversible (GTR) with gamma distribution (+G) (=GTR + G) nucleotide substitution model and 1000 bootstrap replicates for each branch node. Bayesian inference (BI) tree analysis was conducted using MrBayes (Ronquist et al., 2012), available in the CIPRES Science Gateway (Miller et al., 2010), based on default parameters, with minor adjustments: a mixed substitution type (Nst) was selected for the likelihood model and 2, 000, 000 generations were set for the Markov chain Monte Carlo (MCMC), with data sampling collected every 100 generations. The final tree results from both analyses were visualized using FigTree (Rambaut, 2018). Phylogenetic analyses of nrITS sequences was carried out using ML and maximum parsimony (MP) methods that are embedded in MEGA 7 (Kumar et al., 2016). The optimum DNA substitution model for the ML tree was calculated using the "Find Best DNA/Protein Model (ML)" function available in MEGA 7 and ML tree was constructed using the GTR + G with invariant included (+I) (GTR + G + I) nucleotide substitution model and 1000 bootstrap replicates for each branch node. The MP tree was constructed by means of 1000 bootstrap replicates, under the subtree-pruning-regrafting search method. Gaps and missing data were included in the construction of both trees.
2.7. Identification of divergence hotspot and potential DNA barcoding regionsTo detect the nucleotide variability (Pi) in the Daphne plastomes, the plastome sequences were aligned using MAFFT (Katoh et al., 2019). Analyses on singleton variable sites and sliding windows, with 500-bp step size and 1000-bp window length, were performed on DnaSP v.5.1 (Librado and Rozas, 2009).
3. Results 3.1. Plastid genome features of five Daphne speciesThe total plastome sizes for the five Daphne taxa ranged from 132, 869 (D. genkwa) to 174, 773 bp (D. championii) (Fig. 1). All five plastomes exhibit a typical quadripartite structure consisting of a pair of IRs (9372–43, 948 bp) that separate the LSC (84, 681–85, 728 bp) and SSC (1557–28, 397 bp) regions. Total plastome gene number ranged from 106 (D. genkwa) to 141 (D. championii). Plastomes included from 71 (D. genkwa) to 95 (D. championii) protein-coding genes, 31 (D. genkwa) to 38 (D. championii, D. odora, and D. papyracea) tRNA genes, and four (D. genkwa) or eight (D. genkwa, D. kiusiana var. atrocaulis, D. odora, and D. papyracea) rRNA genes. Of these genes, four are duplicated in the plastome of D. genkwa, while 28 are duplicated in the plastomes of D. kiusiana var. atrocaulis, D. odora, and D. papyracea, and 31 are duplicated in the plastome of D. championii, all in the IR regions. The GC content of the plastomes ranged from 36.3% (D. genkwa) to 36.8% (D. papyracea).
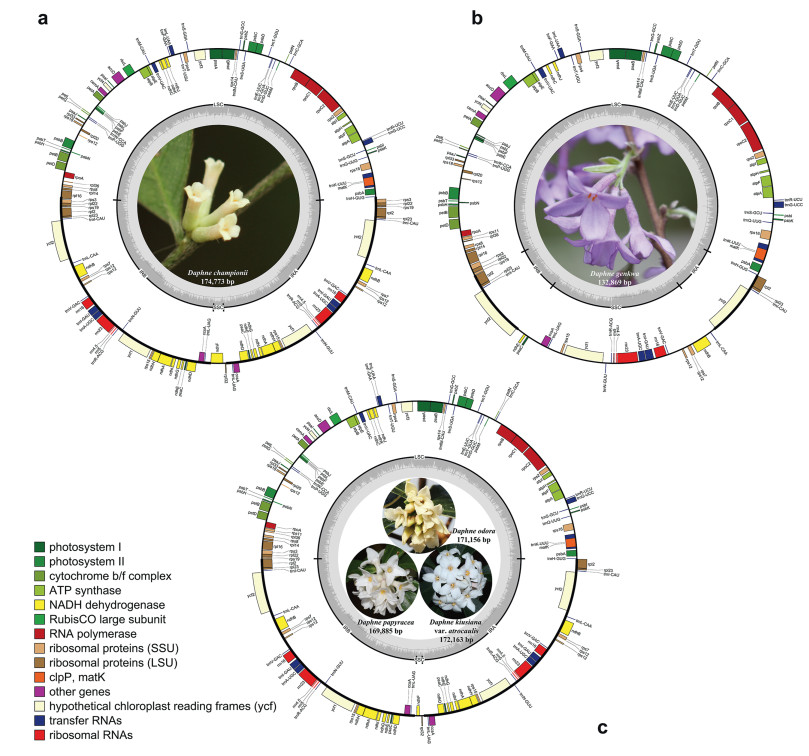
|
| Fig. 1 Plastidgenome maps of four species and one variety in genus Daphne. (a) D. championii, (b) D. genkwa, (c) D. kiusiana var. atrocaulis, D. odora, and D. papyracea. Genes are color-coded according to function. Genes inside the circle are transcribed clockwise, while genes outside the circle are transcribed counterclockwise. |
Large repeats were detected in the plastomes of all five Daphne taxa, including between 24 (D. genkwa) and 26 (D. championii and D. kiusiana) forward repeats and between 16 (D. genkwa) and 25 (D. odora) palindrome repeats (Fig. S1). Only four reverse repeats were recorded, all in the plastome of D. genkwa, and no complementary repeats were detected.
3.3. Relative synonymous codon usage and predicted RNA editing sitesThe protein-coding sequences of Daphne plastomes had between 24, 654 (D. genkwa) and 34, 003 (D. championii) codons (Table S1). Among the encoded amino acids, leucine was most frequent in the plastomes of D. genkwa, D. kiusiana var. atrocaulis, and D. odora, and serine was most frequent in plastomes of D. championii and D. papyracea. While methionine was least frequent in the plastomes of D. championii, D. odora, and D. papyracea, tryptophan was the least frequent in the plastomes of D. genkwa, and D. kiusiana var. atrocaulis. Between 56 (D. genkwa) and 66 (D. kiusiana) potential RNA editing sites were predicted from the protein-coding genes of Daphne plastomes (Fig. 2). The most frequent amino acid conversion in all five Daphne species was serine-to-leucine (S-L). The least frequent amino acid conversions (i.e., occurred only once) differed among Daphne species. For instance, the least frequent amino acid conversion in D. championii was arginine-to-cysteine (R-C); in D. genkwa it was arginine-to-tryptophan (R-W). D. kiusiana var. atrocaulis had three one-time amino acid conversions (alanine-to-valine (A-V), R-C, and R-W), whereas D. odora (R-C and R-W) and D. papyracea (A-V and R-C) each had two.
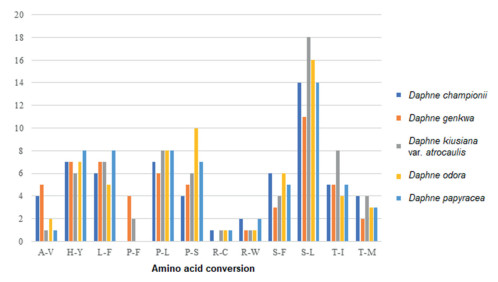
|
| Fig. 2 Amino acid conversions in potential RNAediting sites of the plastidgenomes of five Daphne taxa, including alanine-to-valine (A-V), histidine-to-tyrosine (H-Y), leucine-to-phenylalanine (L-F), proline-to-phenylalanine (P-F), proline-to-leucine (P-L), proline-to-serine (P-S), arginine-to-cysteine (R-C), arginine-to-tryptophan (R-W), serine-to-phenylalanine (S-F), serine-to-leucine (S-L), threonine-to-isoleucine (T-I), and threonine-to-methionine (T-M). |
Genetic pairwise distance based on plastome sequences of nine Daphne taxa was highest between D. championii and D. genkwa (0.0338), whereas the genetic distance was lowest between D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis (0.0003) (Table 2). IR border analysis indicated that in all Daphne species except D. championii, rps19 and rpl2 genes were adjacent to the LSC/IRb junction (JLB); in D. championii rpl16 is located at the JLB (Fig. 3). NdhF is located at the SSC/IRb junction (JSB) in all Daphne species except in three. In D. kiusiana var. atrocaulis and D. papyracea, the ndhF gene is located adjacent to the JSB; in D. genkwa the gene adjacent to the JSB is ycf2, which is located in the IRb region. In the other eight Daphne species the ycf2 gene is adjacent to the SSC/IRa junction (JSA) in the IRa region, whereas the rpl32 gene is adjacent to the JSA within the SSC. In all Daphne taxa, the trnH gene is located in the LSC region adjacent to the LSC/IRa junction (JLA). In D. championii the rpl2 gene located in the IRa region adjacent to the JLA is not present; instead the rps3 gene is located adjacent to the JLA.
| Empty Cell | DC | DGi | DKk | DKa | DP | DO | DT | DL |
| Daphne championii (DC) | – | – | – | – | – | – | – | – |
| Daphne giraldii (DGi) | 0.0295 | – | – | – | – | – | – | – |
| Daphne kiusiana var. kiusiana (DKk) | 0.0288 | 0.0051 | – | – | – | – | – | – |
| Daphne kiusiana var. atrocaulis (DKa) | 0.0289 | 0.0052 | 0.0003 | – | – | – | – | – |
| Daphne papyracea (DP) | 0.0288 | 0.0051 | 0.0017 | 0.0018 | – | – | – | – |
| Daphne odora (DO) | 0.0286 | 0.0049 | 0.0016 | 0.0017 | 0.0016 | – | – | – |
| Daphne tangutica (DT) | 0.0285 | 0.0049 | 0.0015 | 0.0016 | 0.0014 | 0.0012 | – | – |
| Daphne laureola (DL) | 0.0286 | 0.0075 | 0.0069 | 0.0070 | 0.0068 | 0.0067 | 0.0066 | – |
| Daphne genkwa (DGe) | 0.0338 | 0.0229 | 0.0226 | 0.0226 | 0.0226 | 0.0224 | 0.0222 | 0.0220 |
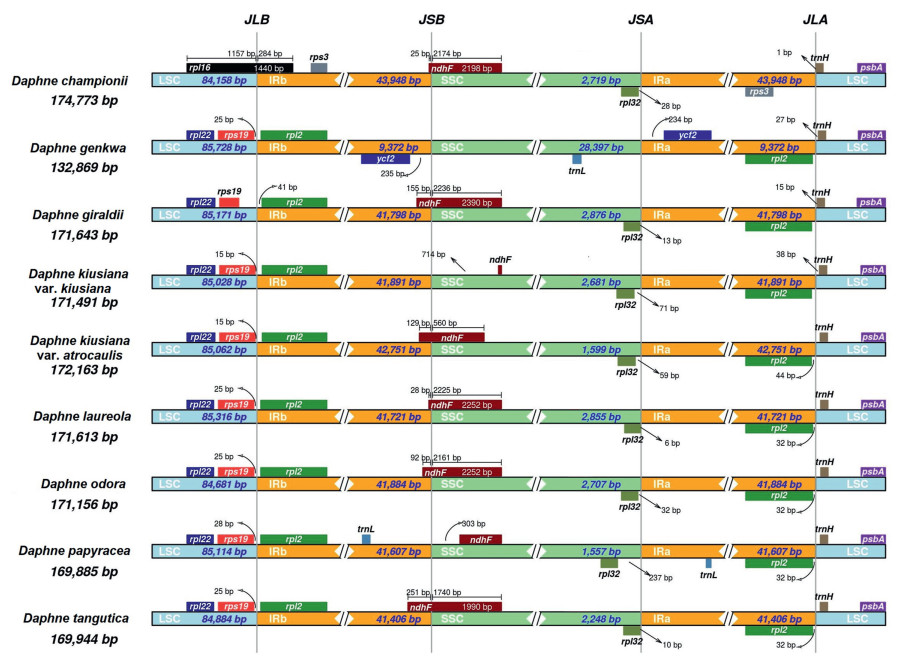
|
| Fig. 3 Inverted repeat (IR) border analysis based on the complete plastidgenomes of nine Daphne taxa, including D. championii, D. genkwa, D. giraldii, D. kiusiana var. kiusiana, D. kiusiana var. atrocaulis, D. laureola, D. odora, D. papyracea, and D. tangutica. |
The plastome alignment of nine Daphne taxa, with the plastome sequence of D. laureola as the reference genome, revealed high sequence conservatism across the plastomes of six Daphne taxa, including D. giraldii, D. kiusiana var. atrocaulis, D. kiusiana var. kiusiana, D. odora, D. papyracea, and D. tangutica (Fig. 4). Hyper-variable regions in the form of continuous distinct small gaps were detected in the LSC region of D. championii and D. genkwa, while three large gaps were detected in the SSC region of D. genkwa, when compared to D. laureola.
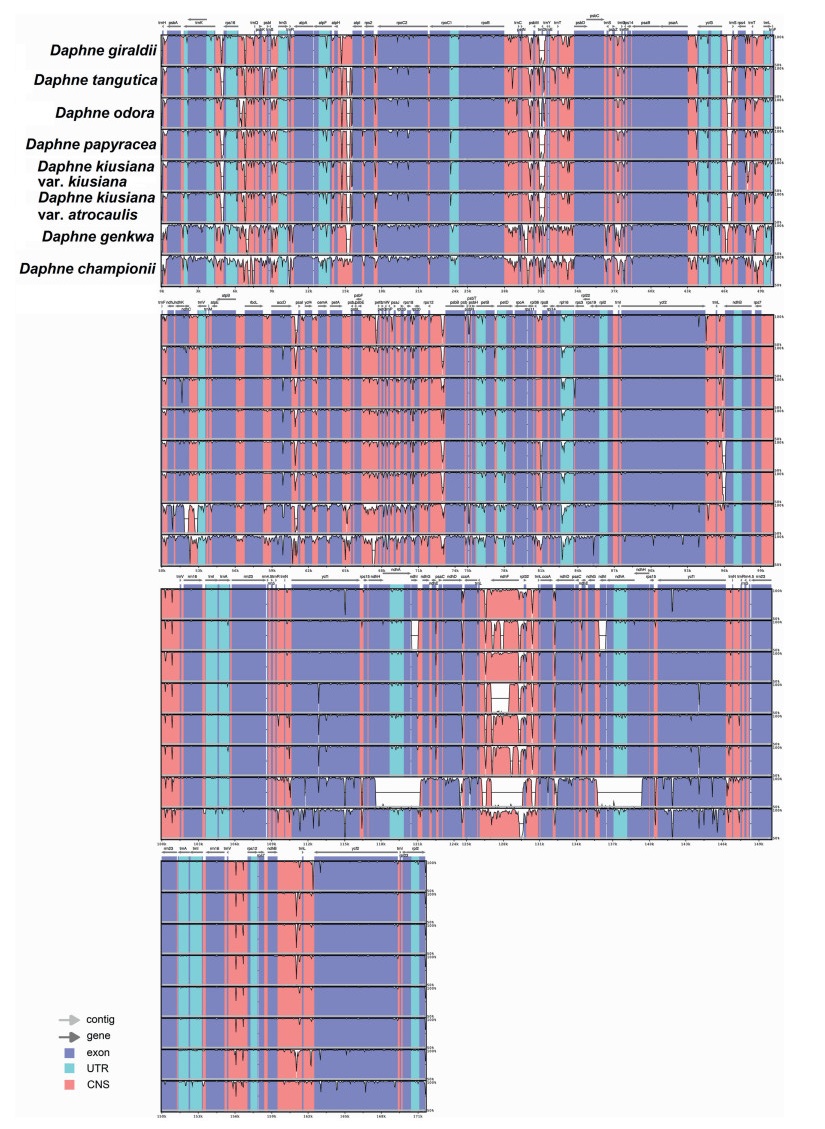
|
| Fig. 4 Comparative plastidgenome analysis of nine Daphne taxa using mVISTA under Shuffle-LAGAN mode. Figure legend describes the direction and types of gene regions using color codes. Probability threshold was set at 50% and the plastid genome of Daphne laureola (MN201546) was selected as the reference genome. |
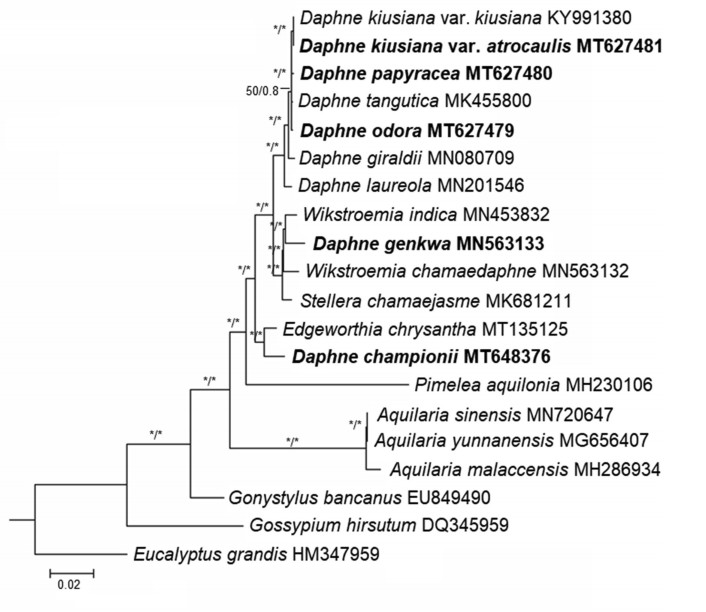
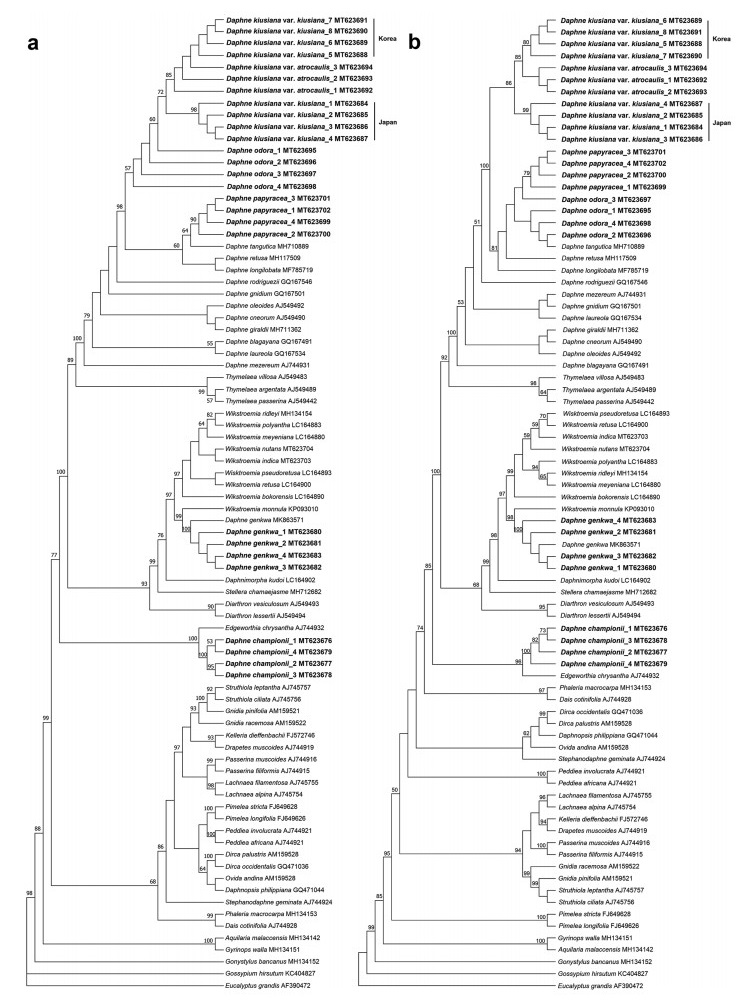
ML and BI analyses based on the complete plastome sequences of nine Daphne taxa strongly suggested a paraphyletic relationship within Daphne, with three well-supported clades (BS ≥ 95%; PP ≥ 0.90) (Fig. 5). Daphne championii formed an independent clade and was sister to a species of another genus, Edgeworthia chrysantha Lindl. D. genkwa clustered with the Wikstroemia clade, sister to Wikstroemia indica (L.) C.A. Mey. D. kiusiana var. atrocaulis, D. odora, and D. papyracea, along with the four other Daphne species, formed a monophyletic group. ML and MP analyses based on nrITS sequences also revealed a paraphyletic relationship in Daphne (Fig. 6). The ML tree had a reliable backbone that was well-supported (BS ≥ 75%) for its major clades (Fig. 6a), but the major clades on the backbone of the MP tree was only partially supported (BS ≥ 75%) (Fig. 6b). Both ML and MP trees revealed similar clustering patterns for the five Daphne species. D. championii was sister to E. chrysantha; D. genkwa was nested within the Wikstroemia clade and was sister to W. monnula Hance; and D. kiusiana var. atrocaulis, D. odora, and D. papyracea formed a monophyletic clade with the other 12 Daphne species.
|
| Fig. 5 Phylogenetic relationships of Daphne and its allied genera in family Thymelaeaceae based on the plastidgenome sequences of 18 taxa representing seven genera of the family Thymelaeaceae. The phylogenetic tree was constructed using both maximum likelihood (ML) and Bayesian inference (BI). All branch nodes were calculated with 1000 bootstrap replicates and reliable bootstrap supports (ML: BS ≥ 95%; BI: PP ≥ 90%) are indicated with an asterisk (∗). Sequences obtained through this study are indicated in bold and two species, Eucalyptusgrandis (HM347959) and Gossypiumhirsutum (DQ345959), were included as outgroups. |
|
| Fig. 6 Phylogenetic analyses of family Thymelaeaceae based on the nuclear ribosomal internal transcribed spacer (nrITS) gene sequences of 58 taxa representing 24 genera from the family Thymelaeaceae. (a) maximum likelihood (ML), and (b) maximum parsimony (MP) tree analyses were conducted in 1000 bootstrap replicates. Trees are presented in consensus mode and sequences obtained through this study are indicated in bold. Only bootstrap support values that exceeded 50% are shown in both trees. Two species, Eucalyptusgrandis (AF390472) and Gossypiumhirsutum (KC404827), were included as outgroups. |
Because our phylogenetic analysis placed Daphne championii and D. genkwa in unexpected positions, we excluded these species from our efforts to identify divergence hotspots. Genome alignment of seven species of Daphne indicated that Pi-values ranged from 0 to 0.02376 and had an average Pi-value of 0.00379. Two gene regions recorded Pi-values greater than our cut-off point (> 0.015; Fig. S2). The two highly divergent regions were located at the psaI gene region (61, 755 to 62, 934 bp) of the LSC and the ndhF-rpl32 gene region (130, 710 to 133, 470 bp) of the SSC.
4. Discussion 4.1. Gene variations due to IR contraction and expansionThis study is the first to describe plastome sequences of Daphne championii, D. genkwa, D. kiusiana var. atrocaulis, D. odora, and D. papyracea. The total number of genes present in the plastomes ranges between 135 and 141 except for in D. genkwa, which has only 106. In addition, D. genkwa has an IR 4.5 times shorter than that of the average IR length in Daphne species. Furthermore, D. genkwa has an SSC region approximately 12 times longer than that of the average SSC of other Daphne taxa. Contraction and expansion at the IR borders are common during evolution and may cause variations in the size of each region or the plastome as a whole (Knox and Palmer, 1999). Analysis of gene order at the IR border allowed us to categorize the plastomes of the nine Daphne taxa examined into three types (Fig. 3) that we have named Type I, II, and III. Type I is the most common type and is found in the plastomes of D. giraldii, D. kiusiana var. kiusiana, D. kiusiana var. atrocaulis, D. laureola, D. odora, D. papyracea, and D. tangutica. Type II is exclusive to D. genkwa, whereas Type III is exclusive to D. championii. Type I and Type III plastomes have only two genes in the SSC region. In contrast, Type II plastomes have 20 genes in the SSC region. The plastomes of other members of Thymelaeaceae (e.g., Aquilaria malaccensis Lam., E. chrysantha, Stellera chamaejasme L., and Wikstroemia chamaedaphne (Bunge) Meisn.) exhibit gene content near the IR boundaries similar to that of Type I (Lee et al., 2018, Lee et al., 2020; Yun et al., 2019; Qian et al., 2020). However, due to the scarceness of Thymelaeaceae plastomes available in GenBank, we cannot conclude that Type I is the dominant gene order pattern at the IR borders.
4.2. Inter- and intraspecific plastome diversity of Daphne kiusiana complexThe taxonomic status of the Daphne kiusiana complex (D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis) has been a long-standing debate. The D. kiusiana complex was initially treated as a variety of D. odora (Keissler, 1898; Rehder, 1916). However, it has since been proposed that D. kiusiana var. atrocaulis is closer to D. papyracea and D. bholua Buch.-Ham. ex D. Don than to D. odora and D. kiusiana var. kiusiana (Mathew, 1989). Taxonomic revisions of the D. kiusiana complex based on extensive morphological analysis have indicated that D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis are distinct taxa (Wang et al., 2007a). We found that the inter- and intraspecific pairwise distance between the plastome sequences of D. odora and D. kiusiana complex were 0.0016 and 0.0017, respectively (Table 2). However, intraspecific pairwise analysis indicated that the genetic distance between D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis was much smaller (0.0003). Although 64 singleton variable sites were detected between D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis plastome sequences, the genetic distance between the two D. kiusiana varieties was much lower than the genetic distance between other species, e.g., Cycas debaoensis Y.C. Zhong & C.J. Chen (Cycadaceae; 0.0056) (Jiang et al., 2016). Moreover, the singletons found across two accessions of the same species were higher than those reported in the plastome sequences of Camellia japonica L. (Theaceae; 25 singletons) and Dysphania pumilio (R.Br.) Mosyakin & Clemants (Amranthaceae; 25 singletons) (Park and Kim, 2019; Park et al., 2019). This low nucleotide variation and small genetic distance are consistent with our observation that D. kiusiana var. atrocaulis is homogeneous with its original.
NrITS sequence analysis established that the genetic distance between Daphne odora and D. kiusiana var. kiusiana from Japan, D. kiusiana var. kiusiana from Korea, and D. kiusiana var. atrocaulis were 0.0040, 0.0013, and 0.0013 respectively. The genetic distance between D. kiusiana var. atrocaulis and each the Japanese D. kiusiana var. kiusiana and the Korean D. kiusiana var. kiusiana was 0.0026 for both accessions; the genetic distance between the Korean D. kiusiana var. kiusiana and D. kiusiana var. atrocaulis was zero. Five singleton variable sites were detected in the plastome alignment of three D. kiusiana accessions. All these sites were detected in sequences of two D. kiusiana accessions and the number of sites detected in the sequences for both the Japanese and Korean D. kiusiana var. kiusiana against D. kiusiana var. atrocaulis were four and one, respectively.
Phylogenetic analysis based on plastome sequences indicated that Daphne kiusiana var. atrocaulis is a well-supported sister to D. kiusiana var. kiusiana (Fig. 5); however, phylogenetic trees based on nrITS sequence data indicated that the intraspecific relationships are not well-defined. Our analysis did not consistently recognize Daphne kiusiana var. kiusiana as a monophyletic group, although samples from Korea and Japan were unequivocally established as two distinct monophyletic clades. Furthermore, MP and ML analyses did not establish D. kiusiana var. atrocaulis as monophyletic; also, the relationship between the D. kiusiana complex and D. odora was not uniform in either tree (Fig. 6). Discrepancies between the nrITS trees are likely because the computational approaches used for each model are influenced by evolutionary factors, including reversals, convergence, and homoplasy (Downie and Katz-Downie, 1996). The taxonomic status of the D. kiusiana complex derived from nuclear sequence data requires more extensive sampling and additional nuclear genes. Our phylogenetic analyses based on plastome sequences support morphological evidence that separates D. kiusiana from D. odora, although this finding is only partly supported by nrITS sequence data.
In our study, complete plastomes showed higher resolution in resolving species relationships than nrITS sequences (Fig. 5, Fig. 6). This implies that super-barcoding (or ultra-barcoding) of Daphne species using complete plastome sequences is reliable and effective (Kane et al., 2012; Li et al., 2015). However, although the cost of next-generation sequencing has become affordable in many countries, performing high-throughput DNA sequencing for diverse genera, such as Daphne, may be cost-prohibitive. Therefore, a cost-effective alternative such as using selected highly polymorphic and relatively shorter plastid barcodes is advisable. Previous studies suggested that identifying barcodes would require aligning eight to ten closely related plastome sequences from different species (Li et al., 2015), although five to seven plastomes have proven sufficient in many cases (Mwanzia et al., 2020; Liu et al., 2021). Accordingly, the Daphne DNA barcodes we identified, which are based on seven plastome sequences, should be reliable. Thus, we propose two potential DNA barcoding regions for Daphne species: the psaI gene and ndhF-rpl32 intergenic spacer region. DNA barcodes and gene markers derived from divergence hotspots in the plastome have been reported to be effective in identifying interrelated species within the plant kingdom (Li et al., 2015). To confirm the efficacy of these potential barcoding regions in delimitation and identification of Daphne species, their discrimination rate should be determined.
4.3. Current circumscription of the genus Daphne is polyphyleticMolecular evidence based on both plastome and nrITS sequences clustered Daphne genkwa within the Wikstroemia clade and removed D. championii from the Daphne clade, placing it into an independent clade that has an affinity with Edgeworthia chrysantha. The phylogenetic positions of D. championii and D. genkwa have been a topic of debate for quite some time (Dute et al., 1996). Despite being under the same genus, the interspecific pairwise genetic distances between D. championii and D. genkwa were greater than those of other Daphne species (Table 2). The two species have long been considered morphologically, closely related and were grouped within the section Genkwa (Bentham and Hooker, 1880), a grouping that is still generally accepted. However, taxonomists have highlighted several morphological features in D. championii and D. genkwa that raised doubts about this treatment. For instance, the presence of long styles and filaments as well as short and upright calyx teeth suggest that D. championii should be grouped in the genus Eriosolena (Domke, 1934). Daphne genkwa exhibit the opposite leaf arrangement, which rarely occurs among Daphne species, and have disks at the base of the floral tube that divide into individual scales or threads, suggesting that the members of this genus should be reassigned to Wikstroemia (Domke, 1934).
The taxonomic delimitation of Daphne and Wikstroemia is challenging, with many Wikstroemia species previously known as members of Daphne and vice versa (Wang and Gilbert, 2007a; Wang et al., 2007a). Although the major morphological features that delimit Daphne from Wikstroemia are the shape of its disk and type of fruit, these features are not consistent across a number of species in either genus (Zhang et al., 2016). Furthermore, anatomical features, such as the presence of tori in the intervascular pit membrane (Dute et al., 1996) and leaf epidermal microfeatures (Zhang et al., 2015), are also unsuitable to delimit the two genera because tori are found in both genera and there is no significant variation in leaf epidermal microfeatures between members of Daphne and Wikstroemia. The Daphne section Eriosolena, which was initially proposed to belong to the genus Daphne (Bentham and Hooker, 1880), is now considered a distinct genus Eriosolena in the family Thymelaeaceae (Wang and Gilber, 2007b). Molecular evidence from our study showed that the strong sister relationship between D. championii and E. chrysantha has raised doubts about whether D. championii should be a member of Eriosolena. Researchers have proposed that Daphne can be delimited from the genera Eriosolena and Edgeworthia by the absence of bicollateral vascular bundles in their midribs (Domke, 1934). The close relationship between Eriosolena and Edgeworthia is supported by the absence of tori in Eriosolena wallichii Meisn., which was once considered a synonym of Eriosolena involucrata (Wall.) Tiegh (synonym Eriosolena composita (L.f.). Merr.) and is reported to have identical wood features as Daphne pendula Sm. (synonym E. composita) (The Plant List, 2010, The Plant List, 2013) and Edgeworthia papyrifera Siebold & Zucc. (synonym E. chrysantha) (Dute et al., 1996). Unfortunately, studies on the presence of tori in D. championii are not available and molecular information of the monotypic genus Eriosolena is scarce. Therefore, work to verify the presence of tori in D. championii and DNA sequencing of E. composita would be useful in providing insights to the taxonomic status of D. championii.
Taxonomic classification is primarily based on morphological characteristics (Greenman, 1940). However, our molecular data for Daphne championii and D. genkwa contradict morphology-based classifications. Specifically, we recommend that D. championii should be removed from Daphne and, after further study, D. genkwa should be reinstated to Wikstroemia.
4.4. Daphne and Wikstroemia are independent generaThe merging of Wikstroemia into Daphne was previously proposed based on traditional distinguishing characteristics (Halda, 2001); however, this treatment has not been universally accepted due to a lack of both morphological and molecular evidence, as well as the vast alteration on taxonomic nomenclature (Wang et al., 2007b; Zhang et al., 2007). Although our sample size was small, our results clearly demonstrate that Daphne and Wikstroemia are each monophyletic. Furthermore, molecular evidence in this study combined with previously identified morphological features (Domke, 1934; Dute et al., 1996) support the separation of D. championii and D. genkwa from Daphne. Taken together, these findings imply that the taxonomic confusion in Daphne, Wikstroemia and allied genera may have been caused by long-term misplacement of certain problematic taxa. If Daphne and Wikstroemia are not sister groups, as shown by both plastome and nrITS trees, the combination of both genera is not suitable and should be excluded. On the contrary, the independent taxonomic status of Daphne and Wikstroemia should be retained. Reasonable transfer of Daphne species to Wikstroemia, and vice versa, based on the morphological characteristics and molecular evidence will be appropriate under current genera delimitation.
Finally, we would like to iterate that evolutionary assessments based on limited sampling sizes, either molecular or morphological, can be elucidated when the context of other biological attributes is duly considered. However, further research that includes more taxa from Thymelaeaceae is essential to validate these hypotheses.
Author contributionsSYL, WL and YZ conceived and designed this study. SYL, CH and JHL conducted the experiments. SYL and KX analyzed the data. SYL wrote the manuscript. KX, JHL, WL, YZ and QF edited the manuscript. All authors read and approved the manuscript.
Declaration of competing interestThe authors declare that they have no conflict of interest.
AcknowledgementsThis work was supported by the Fundamental Research Funds for the Central Universities (33000-31611215), the Guangzhou Science and Technology Program (201903010076) as well as the National Natural Science Foundation of China (31760048).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.11.001.
Alonso, C., Herrera, C.M., 2011. Back-and-forth hermaphroditism: phylogenetic context of reproductive system evolution in subdioecious Daphne laureola. Evolution, 65: 1680-1692. DOI:10.1111/j.1558-5646.2011.01246.x |
Alvarez, I.J.F.W., Wendel, J.F., 2003. Ribosomal ITS sequences and plant phylogenetic inference. Mol. Phylogenet. Evol., 29: 417-434. DOI:10.1016/S1055-7903(03)00208-2 |
Amiryousefi, A., Hyvönen, J., Poczai, P., 2018. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics, 34: 3030-3031. DOI:10.1093/bioinformatics/bty220 |
APG IV, 2016. An update of the Angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc., 181: 1-20. DOI:10.1111/boj.12385 |
Beaumont, A.J., Edwards, T.J., Manning, J., et al., 2009. Gnidia (Thymelaeaceae) is not monophyletic: taxonomic implications for Thymelaeoideae and a partial new generic taxonomy for Gnidia. Bot. J. Linn. Soc., 160: 402-417. DOI:10.1111/j.1095-8339.2009.00988.x |
Bentham, G., Hooker, J.D., 1880. Thymelaeaceae. Gen. Pl., 3: 186-201. |
Brickell, C.D., Mathew, B., 1998. Daphne: the Genus in the Wild and in Cultivation. Alpine Garden Society, Birmingham.
|
Capella-Gutiérrez, S., Silla-Martínez, J.M., Gabaldón, T., 2009. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics, 25: 1972-1973. DOI:10.1093/bioinformatics/btp348 |
Castilla, A.R., Alonso, C., Herrera, C.M., 2012. Genetic structure of the shrub Daphne laureola across the Baetic Ranges, a Mediterranean glacial refugium and biodiversity hotspot. Plant Biol., 14: 515-524. DOI:10.1111/j.1438-8677.2011.00521.x |
Cheng, T., Xu, C., Lei, L., et al., 2016. Barcoding the kingdom Plantae: new PCR primers for ITS regions of plants with improved universality and specificity. Mol. Ecol. Resour., 16: 138-149. DOI:10.1111/1755-0998.12438 |
Chiou, S.J., Yen, J.H., Fang, C.L., et al., 2007. Authentication of medicinal herbs using PCR-amplified ITS2 with specific primers. Planta Med., 73: 1421-1426. DOI:10.1055/s-2007-990227 |
Dierckxsens, N., Mardulyn, P., Smits, G., 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res., 45: e18. DOI:10.1093/nar/gkw955 |
Domke, W., 1934. Untersuchungen über die systematische und geographische-Gliederung der Thymelaeaceen nebst einer Neubeschreibung-ihrer Gattung. Bibl. Bot., 111: 1-151. |
Downie, S.R., Katz-Downie, D.S., 1996. A molecular phylogeny of Apiaceae subfamily Apioideae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. Am. J. Bot., 83: 234-251. DOI:10.1002/j.1537-2197.1996.tb12701.x |
Dute, R.R., Freeman, J.D., Henning, F., et al., 1996. Intervascular pit membrane structure in Daphne and Wikstroemia-Systematic implications. IAWA J., 17: 161-181. DOI:10.1163/22941932-90001446 |
Foster, C.S., Henwood, M.J., Ho, S.Y., 2018. Plastome sequences and exploration of tree-space help to resolve the phylogeny of riceflowers (Thymelaeaceae: Pimelea). Mol. Phylogenet. Evol., 127: 156-167. DOI:10.1016/j.ympev.2018.05.018 |
Frazer, K.A., Pachter, L., Poliakov, A., et al., 2004. VISTA: computational tools for comparative genomics. Nucleic Acids Res., 32: W273-W279. DOI:10.1093/nar/gkh458 |
Galicia-Herbada, D., 2006. Origin and diversification of Thymelaea (Thymelaeaceae): inferences from a phylogenetic study based on ITS (rDNA) sequences. Plant Syst. Evol., 257: 159-187. DOI:10.1007/s00606-005-0371-z |
García-Verdugo, C., Illera, J.C., Traveset, A., 2019. Characterization of microsatellite markers for the endangered Daphne rodriguezii (Thymelaeaceae) and related species. Appl. Plant Sci., 7: e11274. DOI:10.1002/aps3.11274 |
Greenman, J.M., 1940. The concept of the genus: III. Genera from the standpoint of morphology. Bull. Torrey Bot. Club, 67: 371-374. DOI:10.2307/2481070 |
Greiner, S., Lehwark, P., Bock, R., 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res., 47: W59-W64. DOI:10.1093/nar/gkz238 |
Halda, J.J., 1999. Some taxonomic problems in the genus Daphne L. II. Acta Mus. Richnov. Sect. Nat., 6: 195-233. |
Halda, J.J., 2001. The Genus Daphne. (Sen, Dobre).
|
Hamaya, T., 1963. Taxonomical notes on Daphne (Thymelaeaceae). Acta Horti Gotob., 26: 64-99. |
Herber, B.E., 2003. Thymelaeaceae. In: Kubitzki K. (ed) The Families and Genera of Vascular Plants, vol. vol. 5. Springer, Berlin, pp. 373-396.
|
IUCN, 2020. The IUCN Red List of Threatened Species. Version 2020-1. https://www.iucnredlist.org. Accessed 5 June 2020.
|
Jiang, G.F., Hinsinger, D.D., Strijk, J.S., 2016. Comparison of intraspecific, interspecific and intergeneric chloroplast diversity in Cycads. Sci. Rep., 6: 31473. DOI:10.1038/srep31473 |
Kameyama, Y., Hirao, A.S., 2014. Development and evaluation of microsatellite markers for the gynodioecious shrub Daphne jezoensis (Thymelaeaceae). Appl. Plant Sci., 2: 1400001. DOI:10.3732/apps.1400001 |
Kane, N., Sveinsson, S., Dempewolf, H., et al., 2012. Ultra-barcoding in cacao (Theobroma spp.; Malvaceae) using whole chloroplast genomes and nuclear ribosomal DNA. Am. J. Bot., 99: 320-329. DOI:10.3732/ajb.1100570 |
Katoh, K., Rozewicki, J., Yamada, K.D., 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings Bioinf., 20: 1160-1166. DOI:10.1093/bib/bbx108 |
Keissler, K.V., 1898. Die Arten der Gattung Daphne aus der Section Daphnanthes. Bot. Jahrb. Syst., 25: 29-125. |
Knox, E.B., Palmer, J.D., 1999. The chloroplast genome arrangement of Lobelia thuliniana (Lobeliaceae): expansion of the inverted repeat in an ancestor of the Campanulales. Plant Syst. Evol., 214: 49-64. DOI:10.1007/BF00985731 |
Kumar, S., Stecher, G., Tamura, K., 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol., 33: 1870-1874. DOI:10.1093/molbev/msw054 |
Kurtz, S., Choudhuri, J.V., Ohlebusch, E., et al., 2001. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res., 29: 4633-4642. DOI:10.1093/nar/29.22.4633 |
Lee, S.Y., Ng, W.L., Mohamed, R., et al., 2018. The complete chloroplast genome of Aquilaria malaccensis Lam. (Thymelaeaceae), an important and threatened agarwood-producing tree species. Mitochondrial DNA B, 3: 1120-1121. DOI:10.1080/23802359.2018.1519382 |
Lee, S.Y., Xu, K., Liao, W., et al., 2020. The complete chloroplast genome of Edgeworthia chrysantha (Thymelaeaceae). Mitochondrial DNA B, 5: 3657-3658. DOI:10.1080/23802359.2020.1832600 |
Li, X., Yang, Y., Henry, R.J., et al., 2015. Plant DNA barcoding: from gene to genome. Biol. Rev., 90: 157-166. DOI:10.1111/brv.12104 |
Librado, P., Rozas, J., 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25: 1451-1452. DOI:10.1093/bioinformatics/btp187 |
Liu, L., Zhang, Y., Li, P., 2021. Development of genomic resources for the genus Celtis (Cannabaceae) based on genome skimming data. Plant Divers., 43: 43-53. DOI:10.1016/j.pld.2020.09.005 |
Mathew, B., 1989. Daphne kiusiana: Thymelaeaceae. Kew Mag., 6: 112-115. DOI:10.1111/j.1467-8748.1989.tb00331.x |
Miller, M.A., Pfeiffer, W., Schwartz, T., 2010. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. in: Proceedings of the Gateway Computing Environments Workshop (GCE). Institute of Electrical and Electronics Engineers (IEEE), Piscataway, pp. 1-8.
|
Mower, J.P., 2009. The PREP suite: predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res., 37: W253-W259. DOI:10.1093/mar/gkp337 |
Mwanzia, V.M., He, D.X., Gichira, A.W., et al., 2020. The complete plastome sequences of five Aponogeton species (Aponogetonaceae): insights into the structural organization and mutational hotspots. Plant Divers., 42: 334-342. DOI:10.1016/j.pld.2020.02.002 |
Neves, S.S., Forrest, L.L., 2011. Plant DNA sequencing for phylogenetic analyses: from plants to sequences. In: Cagney, G., Emili, A. (eds) Network Biology. Methods in Molecular Biology (Methods and Protocols), vol vol. 781. Humana Press, New Jersey. pp. 183-235.
|
Park, J., Kim, Y., Xi, H., et al., 2019. The complete chloroplast genome of common camellia tree in Jeju island, Korea, Camellia japonica L. (Theaceae): intraspecies variations on common camellia chloroplast genomes. Mitochondrial DNA B, 4: 1292-1293. DOI:10.1080/23802359.2019.1591214 |
Park, J., Kim, Y., 2019. The second complete chloroplast genome of Dysphania pumilio (R. Br. ) Mosyakin & Clemants (Amranthaceae): intraspecies variation of invasive weeds. Mitochondrial DNA B, 4: 1428-1429. DOI:10.1080/23802359.2019.1598807 |
Patel, R.K., Jain, M., 2012. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One, 7: e30619. DOI:10.1371/journal.pone.0030619 |
Qian, S., Zhang, Y., Li, G., 2020. The complete chloroplast genome of a medicinal plant, Wikstroemia chamaedaphne (Thymelaeaceae). Mitochondrial DNA B, 5: 648-649. DOI:10.1080/23802359.2019.1711228 |
Rambaut, A., 2018. FigTree v1.4. http://tree.bio.ed.ac.uk/software/figtree.
|
Rehder, A., 1916. Thymelaeaceae. Pl. Wilson., 2: 530-551. |
Ronquist, F., Teslenko, M., Van der Mark, P., et al., 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol., 61: 539-542. DOI:10.1093/sysbio/sys029 |
Sovrlić, M.M., Manojlović, N.T., 2017. Plants from the genus Daphne: a review of its traditional uses, phytochemistry, biological and pharmacological activity. Serbian J. Exp. Clin. Res., 18: 69-80. DOI:10.1515/sjecr-2016-0024 |
Stamatakis, A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Stothard, P., 2000. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques, 28: 1102-1104. DOI:10.2144/00286ir01 |
The Plant List, 2010. Version 1 (superseded). http://www.the.plantlist.org/1/. Accessed 5 June 2020.
|
The Plant List, 2013. Version 1.1. http://www.theplantlist.org. Accessed 5 June 2020.
|
Tillich, M., Lehwark, P., Pellizzer, T., et al., 2017. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res., 45: W6-W11. DOI:10.1093/nar/gkx391 |
Van der Bank, M., Fay, M.F., Chase, M.W., 2002. Molecular phylogenetics of Thymelaeaceae with particular reference to African and Australian genera. Taxon, 51: 329-339. DOI:10.2307/1554930 |
Wang, Y., Gilbert, M.G., 2007a. Wikstroemia Endlicher. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (eds) Flora of China, vol. vol. 13. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 215-230.
|
Wang, Y., Gilber, M.G., 2007b. Eriosolena Blume. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (eds) Flora of China, vol. vol. 13. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 246.
|
Wang, Y., Gilbert, M.G., Mathew, B., et al., 2007a. Daphne Linnaeus. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (eds) Flora of China, vol. vol. 13. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 230-245.
|
Wang, Y., Gilbert, M.G., Mathew, B., et al., 2007b. Thymelaeaceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (eds) Flora of China, vol. vol. 13. Science Press & Missouri Botanical Garden Press, Beijing & St. Louis, pp. 213-250.
|
Wernersson, R., 2005. FeatureExtract—extraction of sequence annotation made easy. Nucleic Acids Res., 33: W567-W569. DOI:10.1093/nar/gki388 |
Yun, N., Park, J., Oh, S.H., 2019. The complete chloroplast genome of the traditional medicinal plant Stellera chamaejasme L. (Thymelaeaceae). Mitochondrial DNA B, 4: 1796-1797. DOI:10.1080/23802359.2019.1612296 |
Zhang, Y., Sun, H., Boufford, D.E., 2007. Two new species of Wikstroemia (Thymelaeaceae) from Western Sichuan, China. Rhodora, 109: 448-455. DOI:10.3199/0035-4902(2007)109[448:TNSOWT]2.0.CO;2 |
Zhang, Y., Sun, W., Gao, C., et al., 2015. Character of leaf epidermis and their systematic significance of Daphne and Wikstroemia (Thymelaeaceae). Plant Divers. Resour., 37: 493-512. DOI:10.7677/ynzwyj201515019 |
Zhang, Y., Sun, W., Jiang, X., et al., 2016. Numerical taxonomy of the genera Daphne and Wikstroemia. Guihaia, 36: 61-72. DOI:10.11931/guihaia.gxzw20150402 |
Zhang, Y., Volis, S., Sun, H., 2010. Chloroplast phylogeny and phylogeography of Stellera chamaejasme on the Qinghai-Tibet Plateau and in adjacent regions. Mol. Phylogenet. Evol., 57: 1162-1172. DOI:10.1016/j.ympev.2010.08.033 |



