b. University School of Environment Management, Guru Gobind Singh Indraprastha University, Dwarka 16C, New Delhi, 110075, India;
c. Department of Basic Sciences and Social Sciences, North-Eastern Hill University, Shillong, 793022, Meghalaya, India
Population explosion, industrialization, agricultural expansion and deforestation have led to major changes in land use and destruction of natural resources. These have accelerated the rate of habitat loss (Kardol and Wardle, 2010), modified the distribution of plant species (Dawson et al., 2011) and have triggered the sixth major extinction event in the history of life (Vetaas et al., 2012). The important causes of forest destruction are shifting cultivation (Castellanos et al., 2001), logging for timber (Richards, 1996; Mir et al., 2016) and extraction of fuel wood (Upadhaya et al., 2013). According to the World Conservation and Monitoring Centre, more than 8000 tree species are endangered worldwide and conservation biologists estimate that about 25% of the total life forms may become extinct during the next few decades (IUCN, 2003). The fast disappearance of forests has not only endangered many important plant species and their habitats (Defries, 2010; Kushwaha and Nandy, 2012) but also jeopardies the loss of many vital services on which human beings depend (Khandel et al., 2012). Loss of habitat and species has aroused the attention of conservation scientists (Myers et al., 2000). Therefore, a major challenge before conservation biologists is to effectively protect those species that are on the verge of extinction.
A recent approach for conservation has been to maintain the plant species in extensive natural landscapes (Behera and Roy, 2010; Roy et al., 2012). Of particular concern are those places with special biological feature comprising of high diversity and endemism (Margules and Pressey, 2000). In order to retain biodiversity, priority areas needs to be identified, based on the conservation value of sites, assessed in terms of their irreplaceability and vulnerability levels (Bottrill and Joseph, 2008). The identification of conservation zones requires exhaustive knowledge of species diversity and their distribution (Upadhaya et al., 2013). The characteristics used to identify priority areas for conservation include rapid biodiversity assessment (Oliver and Beattie, 1996), identification of hotspots of biodiversity (Dobson et al., 1997), the use of indicator and surrogate species (Curnutt et al., 1994), identification of biologically valuable eco-region (Olson and Dinerstein, 1998), development of rarity and threat levels (Williams et al., 2002), cost-minimizing or land-values analyses (Ando et al., 1998) and remote sensing and GIS (Behera and Roy, 2010). Identification of priority conservation areas depends on a number of factors such as the distribution and vegetation type (Williams et al., 2002), species richness (Scott et al., 1987), endemism level (Kier and Barthlott, 2001), exposure to threats and concentration of red-listed plants (Ahmedullah, 2000) and change in vegetation with environmental variables (Margules et al., 2002).
One of the first approaches towards conservation prioritization was by Myers (1988), and the principles of irreplaceability and vulnerability was used to identified ten priority conservation areas, in order to guide conservation planning on a global scale. This was further elaborated by Brooks et al. (2006) to identified areas for priority conservation at global level and the areas were divided into regions of high-vulnerability and irreplaceability. Using the concept of vulnerability and irreplaceability, a total of 228 key biodiversity sites were identified in Philippines that aimed at conserving about 855 species (Ambal et al., 2012). Based on concentration of rare, endemic and threatened species, 13 habitats, 23 forest communities and 24 alpine communities were identified as priority areas for conservation in the Himalayas (Rana and Samant, 2009). A model based technique using heuristic integer linear programming (HILP) in Guangdong Province, China led to formulation of 19 priority conservation sites (Wang et al., 2009). Therefore, identification and selection of priority areas ensure conservation with minimum species loss and supports most species for the least costs (Wang et al., 2009). This approach ensures that the efforts of conservation evade the adverse human effects, safeguarding aspirations of indigenous communities (Natarajan et al., 2004) and promote conservation goals (Hoekstra et al., 2005).
The state of Meghalaya in northeast India harbours different types of vegetation, viz, tropical evergreen, tropical semi-evergreen, tropical moist and dry deciduous, subtropical broad leaved hill forest, subtropical pine forests, grasslands and temperate forests (Champion and Seth, 1968; Rao and Hajra, 1986). The state has 1, 711, 879 ha of forest cover and accounts to 76.32% of its geographical area. Of these, only 48, 898 ha falls under very dense category, while 926, 729 and 736, 252 ha are characterized as moderately dense- and open-forest (ISFR, 2019). A total of 3331 plant species have been reported from the state, of which 1236 species are endemic (Khan et al., 1997). About 80% of the land belongs to the people and the forest resources are owned by local communities and are being managed by traditional institutions. This traditional forest management is mostly prevalent in Khasi Hills of the state where it is tightly interwoven with religious beliefs, customs and folklore and has sustained the cultures and livelihoods of indigenous communities for centuries (Tiwari et al., 1998). The community forests have been classified into different types depending on their use regime and consists of a) Group of village forest (Law Raid): forests belonging to two or more villages, b) village forest (Law Shnong): forests belonging to a particular village, c) reserve or village restricted forest (Law Adong): forests similar to village forests, but full access to forest resources is restricted and d) sacred forests (Law-Lyngdoh/Law-Kyntang/Law-Niam): forests considered as sacred with a strict prohibition of extraction (Rodgers, 1994; Tiwari et al., 1998; Mir and Upadhaya, 2017). In addition, to the above categories of forests there are also forests which are either private- or clan-owned and only the owners have full access to it.
Due to human activities like urbanization, mining, extraction of -timber, -fuel wood, -non-wood forest products, grazing and shifting cultivation, the forest resources of the area are under severe threat (Upadhaya et al., 2013). Many of the larger forest patches have been fragmented into small patches (Pao and Upadhaya, 2017). Even the sacred forests, which were once considered as pristine, are now affected by human disturbances (Upadhaya et al., 2003; Ormsby and Bhagwat, 2010; Mir and Upadhaya, 2017). Since the community forests are managed differently and are exposed to various levels of extraction and other human disturbances, all of them may not require equal conservation efforts. Hence, attempts should be made to identify and prioritize those community forests, where immediate intervention for biodiversity conservation is required. Most of the plant diversity studies carried out so far are site-specific and there are hardly any studies at the landscape level. Therefore, the present study was carried out to (i) assess the plant diversity of the various community forests at the landscape level in Khasi Hills and (ii) identify and prioritize community forests on the basis of species richness, irreplaceability, vulnerability and risk factors for conservation measures.
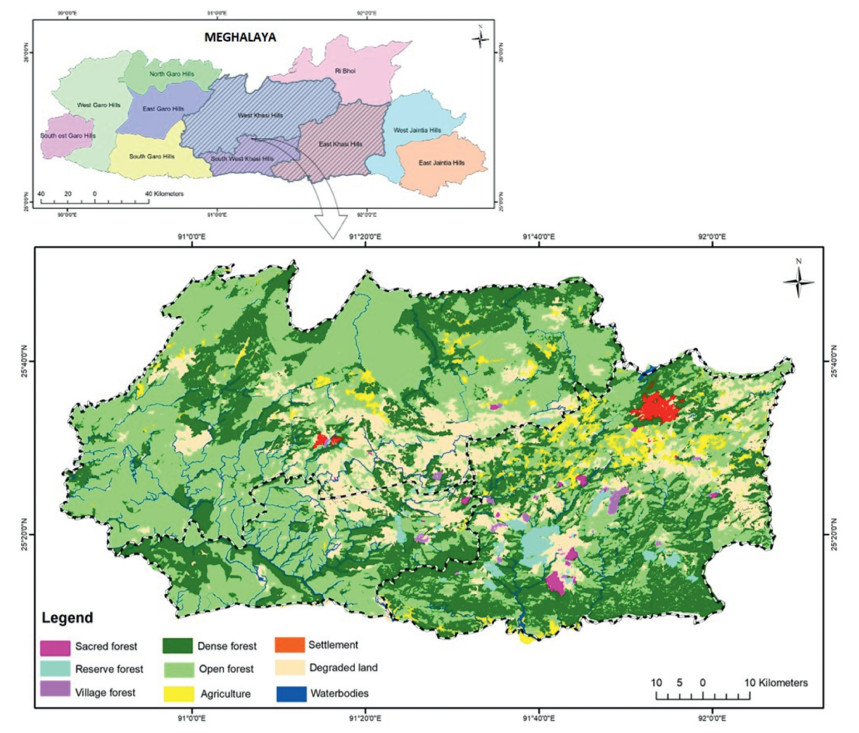
2. Materials and methods 2.1. Study area descriptionThe current study was carried out in community forests of Khasi Hills of Meghalaya, northeast India, and the vegetation is represented by subtropical broad-leaved forest types (Champion and Seth, 1968). These community forests are managed and controlled by the traditional management institutions and the government has no control over it. The area covered by individual forest ranged from 3.7 to 2282.8 ha and were distributed along an altitudinal range of 800–1933 m asl. The Khasi hills in the present study comprised of East, West and South West districts covering an area of 805, 687 ha (Fig. 1). East Khasi Hills has the unique distinction of having the wettest place on earth i.e. Mawsynram with an average annual rainfall of about 12, 270 mm followed by Cherrapunjee (11, 600 mm/year). The average monthly maximum and minimum temperature is 22 ℃ (summer) and 12 ℃ (winter) (en.climate-data.org). The West Khasi Hills district has mildly tropical climate in the southern and northern foothills, while in the central zone, the climate is temperate and places at medium altitude in the northern, western and southern parts experience sub-tropical climate. The average annual rainfall ranges from 1200 to 3000 mm/year. The South West Khasi Hills shares geological and climatic features with both East and West Khasi Hill districts.
|
| Fig. 1 Map showing the distribution of various types of community forests in Khasi Hills of Meghalaya. |
For assessing the land use of the study area (Khasi hills) satellite imagery of Landsat TM and OLI (Landsat 8) data of 2000, 2010 and 2020 were used (https://earthexplorer.usgs.gov/) (Table 1). The relevant topographic maps and imagery were geometrically rectified in 1:50, 000 scale using geographic projection system UTM (Universal Transverse Mercator); spheroid and datum used were WGS 84 with UTM zone 46 N. Based on the reference data acquired in the field, visual image interpretation technique (Garg et al., 1988) was performed to delineate land use and land cover viz., dense forest, open forest, degraded forest/grassland, agriculture and built up area. For spatial distribution of changes, matrix union of different land use land cover (LULC) classes was performed with assigned pixel size of 30 m (Shimrah et al., 2019). The GIS and image processing software used include ArcGIS 10.1 and Erdas Imagine 2014.
| Year | ID | Date of acquisition | Path | Row |
| 2020 (Landsat 8) | LC08_L1TP_137, 042_20, 201, 125_20, 210, 316_02_T1 | 2020/11/25 | 137 | 042 |
| 2010 (Landsat 5) | LT05_L1TP_137, 042_20, 101, 216_20, 200, 823_02_T1 | 2010/12/16 | 137 | 042 |
| 2000 (Landsat 5) | LT05_L1TP_137, 042_20, 001, 102_20, 200, 906_02_T1 | 2000/11/02 | 137 | 042 |
A thorough survey was carried out from 2013 to 2018 in Khasi Hills (East, West and South West Districts) to compile a list of all the community managed forests. All the community forests represented in the form of reserve, village and sacred forests were considered for the study. A total of 87 community forests comprising 42 reserved forests, 20 village forests and 25 sacred forests were surveyed for floristic diversity and community characteristics (Fig. 1; Table S1). For vegetation sampling, in each forest, a belt transect of 20 m wide and 250 m long (0.50 ha) was laid. The transect was further sub-divided into 50 plots of 10 m2 for sampling of trees, 5 × 5 m for shrubs and 1 × 1 m for herbs. The plant species were identified with the help of published literature (Kanjilal et al., 1934–1940; Joseph, 1982; Kataki, 1986; Balakrishnan, 1981, 1983; Haridasan and Rao, 1985, 1987) and consultation of herbaria at Botanical Survey of India (BSI), Eastern Regional Circle, Shillong.
For each site, the disturbance index (DI) was computed following Mir and Upadhaya (2017). The disturbances occurring in each forest were identified using both the quantitative data from the sampling plots and the landscapes surrounding the forests. The disturbances were classified into seven types including extraction-of timber/poles, -fuel wood, -NTFP's (fodder, fruits, medicinal plants, and craft making materials), grazing, encroachment of forest land for agriculture, building roads and fire. For each of these disturbances, a score of 10 was considered as high, 5 as intermediate, 1 as low and 0 as negligible. For a particular forest all the disturbances were summed to obtain a total disturbance score. Collection of NTFPs and fuel wood was calculated based on harvest percentage. Timber extraction was estimated by counting the number of cut stumps in the sampling plots. Percentage density of cut stumps (density of cut individuals divided by total density) of 1–5% was considered as low, 5–10% as intermediate and > 10% as high. Fire (based on signs of fire and the damage caused in each plot) and grazing (based on animal sighting, presence of cow dung and trampling) was assessed in each sampling plot. The presence of signs of fire/grazing in 1–5% plots was considered as low, 5–10% as intermediate and > 10% as high. The effect of agriculture and road construction was assessed at forest patch level. The occurrence of agricultural land away from forest border (≥100 m), 50–100 m and close to the forest edge (< 50 m), were assigned a score of low, intermediate and high, respectively. Similarly, road building impacts were assessed using a measuring tape where the trails (≤1 m width) were considered to have a low impact, footpath (1–3 m width) as intermediate and motorable roads (> 3 m wide) as high impact.
The data on species richness, basal area, density and the number of rare and threatened species were compared between the forest categories using analysis of variance (ANOVA). Assumptions of ANOVA were met through test for normality of variables (Kolmogorov–Smirnov test), and homogeneity of group variances (Levene's test). Since there were an unequal number of forests under different category, therefore a Bonferroni post hoc test was used, as it effectively deals with both equal and unequal sample sizes. Pearson correlations and linear regression analysis was also used to examine the relationships among various community parameters (basal area, density, number of rare and endemic species) and disturbance. All statistical analysis was performed using the software SPSS v.13.
For identification of community forests for priority conservation, the criteria of vulnerability and irreplaceability were applied (Langhammer et al., 2007). The vulnerability was evaluated by the confirmed presence of threatened and rare species. Species that were reported as having low populations in the state of Meghalaya by earlier workers (Haridasan and Rao, 1985, 1987; Nayar and Sastry, 1987, 1988, 1990; Walter and Gillett, 1998; Ved et al., 2005; Upadhaya et al., 2013; Mir et al., 2014) were considered as 'Rare'. Threatened [Critically Endangered (CR), Endangered (E), and Vulnerable (V) and Near threatened (NT)] categories include those plants that are classified as per the IUCN Red List. Owing to the lesser degree of threats, IUCN categories like least concern (LC), data deficient (DD) and not evaluated (NE) were excluded from the study. Irreplaceability was calculated by the confirmed presence of local or exclusively endemic and near or regional endemic species. Species, whose distributions are restricted to Meghalaya, were considered as narrowly endemic. The species restricted to the Northeastern region, Eastern Himalaya and/or Indo-Burma Hotspots were considered as endemic. Based on the levels of disturbance, the community forest under various risk categories was identified.
All the community forests were categorized into low, medium and high priority according to the ranges of species richness, risk levels, endemism (irreplaceability) level and concentration of rare and threatened species (vulnerability level) by assigning appropriate scoring (Margules et al., 2002; Samant et al., 2002; Balaguru et al., 2006; Pant and Samant, 2007). The number of species recorded per unit sampled area (0.5 ha) in each forest represents species richness. According to the ranges of species richness values the forests were regrouped and arbitrarily categorized into low (< 120 species), medium (120–160) and high (> 160) categories. Species that are exclusively restricted to Meghalaya (endemic) were assigned a score of two and Regional/Near-Endemic (restricted to the North-East India or Indo-Burma or Eastern Himalaya) species was assigned a score of one. The number of scores for both the above categories were summed up and each forest was classified into low (< 15), medium (15–30) and high categories (> 30). For vulnerability level, the scoring was given on the basis of the degree of threat status of a species such as: a) Critically Endangered (CR) were assigned a score of six, b) Endangered (EN) = 5, c) Vulnerable (VU) = 4, d) Near Threatened (NT) = 3, e) rare = 7. Finally, all the above scores were summed up for each forest and further classified into low (< 100), medium (100–150) and high (> 150) vulnerability classes. A disturbance score of < 25, 25–50 and > 50 indicates low, medium and high risk level of the studied forests.
GIS softwares (ArcGIS and QGIS) were used to prepare the models for conservation priority zones for each forest category (Fig. 2). For the analysis, google earth imagery was used. The boundary tracing was done using field information by moving along the edges of the forests along with the local Village heads and the polygon was marked using GPS (Global Positioning System). GCP (Ground Control Points) collected with the help of GPS during the field survey were then used to identify the individual community forest on google earth. Polygons for each community forests were digitized in Google Earth software and the whole study area was extracted and transferred to ArcGIS software. All the individual forests were given attributes and weightages for each (species richness, endemic species richness, the concentration of rare and threatened species and disturbance level) parameters as described above. This was followed by the rasterization of each individual vector layers of all the parameters. Except community risk zone layer, all other layers were subjected to weighted overlay analysis using Spatial analyst tool. To run the overlay analysis all the three layers (species richness, vulnerability level and irreplaceability level) were assigned a scale value of 1, 2 and 3 for low, medium and high category, respectively. Later, all the layers were subjected to a weighted overlay analysis, with an overlay scale of 1-3-1, followed by union/integration of all the layers to generate the conservation-priority map.

|
| Fig. 2 Schematic diagram representing the assignment of scores and classification of parameters. |
In the present study, an analysis of LULC showed that the dense and open forests in the area have undergone deforestation and converted to other land uses such as built up area, grassland and agricultural lands. Out of the total geographical area of Khasi Hills (805, 687 ha), an area of 744.88 and 661.39 ha were converted to open forest, grassland, agricultural land and built up area during the period 2000–2010 and 2011–2020, respectively (Table 2).
| Changes in land use land cover | Periods | |
| 2000–2010 | 2011–2020 | |
| Dense forest to built up area | 1.24 | 9.70 |
| Grassland to built up area | 5.47 | 9.70 |
| Grassland to forest | 13.18 | 0 |
| Grassland to agriculture | 17.65 | 2.24 |
| Agriculture to forest | 29.09 | 0 |
| Dense forest to grassland | 137.74 | 31.82 |
| Open forest to grassland | 290.89 | 73.59 |
| Dense forest to forest | 297.60 | 562.89 |
| Dense forest to agriculture | 308.30 | 56.94 |
| Open forest to agriculture | 1111.36 | 159.62 |
| No change | 803, 474.48 | 804, 780.46 |
| Total area (ha) | 805, 687.00 | 805, 687.00 |
A total of 87 community managed forests (42 reserve, 20 village and 25 sacred forests) were recorded in Khasi hills of Meghalaya. Of the total area (805, 687 ha) of Khasi hills, the community managed forests covered 12, 101.7 ha. This accounts for about 1.5% of the total geographical area of Khasi Hills. It represents 0.54% of the total geographic area (2, 242, 900 ha) and 0.77% of the total forest cover (1, 565, 700 ha) of the state. Of all the community forests, the reserve forests covered 7960.7 ha (65.7%), followed by 2131.3 ha (17.6%) of village and 2009.8 ha (16.6%) of sacred forests, respectively (Fig. 1).
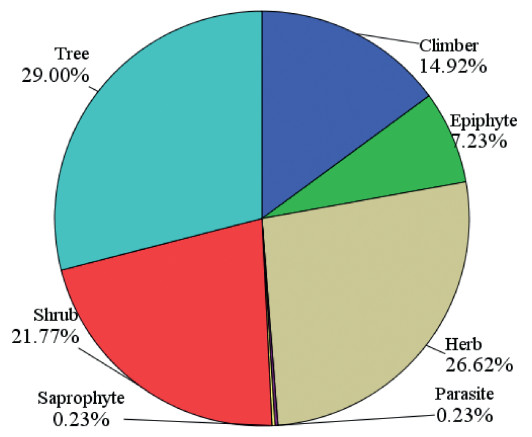
3.3. Species compositionA total of 1300 species belonging to 645 genera and 172 families were recorded from 87 community forests (Table S2). Trees with 377 species were the dominant life form, followed by herbs (346 species), shrubs (283), climbers (194) and epiphytes (94). Parasites and saprophytes together contributed to 0.46% of the total species (Fig. 3). Of the different forest categories, reserve forests had the highest number of species (1190), followed by sacred forests (987 species) and village forests (786 species). The average number of species in reserve, village and sacred forests was 185, 138 and 174, respectively. Sacred forests had the highest proportion of herbs (28.7%), followed by trees (28.2%), shrubs (22.8%), climbers (13.0%), epiphytes (6.9%) and parasites and saprophytes (0.2% each). The reserve forests had the highest proportion of trees (28.6%), followed by herbs (27.4%), shrubs (22.5%), climbers (14.9%), epiphytes (6.1%) and parasites and saprophytes (0.3% each). Similarly, village forests had the highest proportion of herbs (30.4%), followed by shrubs (25.1%), trees (22.9%), climbers (17%), epiphytes (4.2%), saprophytes (0.1%) and parasites (0.3%) (Table 3).
|
| Fig. 3 Distribution of species in different life forms (%) recorded from 87 community forests. |
| Life form | Total number of species | ||
| Reserve forests | Village forests | Sacred forests | |
| Trees | 340 (33–75) | 180 (19–65) | 278 (34–74) |
| Herbs | 326 (35–80) | 239 (22–70) | 283 (30–71) |
| Shrubs | 268 (46–86) | 197 (40–79) | 225 (33–69) |
| Climbers | 177 (10–21) | 134 (5–19) | 128 (9–15) |
| Epiphytes | 73 (5–22) | 33 (5–9) | 68 (12–30) |
| Parasites | 3 (0–3) | 2 (0–2) | 3 (1–3) |
| Saprophytes | 3 (0–3) | 1 (0–1) | 2 (0–2) |
The mean density of trees (≥5 cm dbh) was 891 individuals ha−1 in the reserve forests, 681 and 958 individuals ha−1 in the village- and the sacred-forests, respectively (Table 4). The stand density varied significantly (P < 0.01) between the forest categories but there was no significant difference (P > 0.05) between sacred- and reserve-forests (Table 4). The mean density of shrub and herb in reserve-, village- and sacred-forests ranged from 6729 to 10, 800 and 205, 056 to 264, 060 individual's ha−1, respectively (Table 4). Among ground vegetation, the shrub density varied significantly (P < 0.05) between all the forest categories, but in case of herb density no significant difference (P > 0.05) was observed between reserve- and village-forests (Table 4). The mean stand basal area of different forest categories ranged from 16.25 to 38.10 m2 ha−1. However, the basal area was significantly (P < 0.05) higher in the sacred forests as compared to village- and reserve-forests (Table 4).
| Community forests | Number of sites | Trees | Shrubs | Herbs | Disturbance index | ||||||
| Species richness | Density (ha−1) | Basal area (m2 ha−1) |
Species richness | Density (ha−1) | Species richness | Density (ha−1) | |||||
| Reserve forest | 42 | 62a | 891a | 27.85 | 57 | 9155 | 55 | 247, 733a | 46 | ||
| Village forest | 20 | 41 | 681 | 16.25 | 47a | 10, 800 | 46 | 264, 060a | 61 | ||
| Sacred forest | 25 | 65a | 958a | 38.1 | 46a | 6729 | 48 | 205, 056 | 25 | ||
| ANOVA (F value) | – | 26.71 | 11.13 | 13.24 | 12.79 | 21.60 | 8.88 | 7.05 | 70.12 | ||
During the current study, a total of 400 species were recorded that were rare, endemic and threatened (Table S3). These species were distributed in 110 families and 272 genera. Among the life form, trees were dominant with 144 species, followed by herbs (72 species), shrubs (87), climbers (52), epiphytes (41), parasites and saprophytes (2 each) (Fig. 4). The number of species in different threat and endemic categories are listed in Table 5.

|
| Fig. 4 Proportion of different life forms of RET plant species. |
| Category | No. of species | % of RET species | % of total species |
| Rare | 277 | 69.3 | 21.3 |
| Endemic | 199 | 49.8 | 15.3 |
| Narrowly endemic | 23 | 5.8 | 1.8 |
| Critically endangered | 3 | 0.8 | 0.2 |
| Endangered | 8 | 2.0 | 0.6 |
| Vulnerable | 5 | 1.3 | 0.4 |
| Near threatened | 2 | 0.5 | 0.2 |
The number of RET species varied significantly (P < 0.01) between the different forest categories. Of all the forest categories, reserve forests had the highest number of endemic (184) and narrowly endemic (23) species, followed by sacred forests (152 and 18) and village forests (106 and 12), respectively. The same order was observed for the rare category with reserve forests having 250 species, followed by sacred forests (203) and village forests (126). Similarly, endangered category of species was high in reserve forests (7 species), followed by sacred forests (6) and village forests (4) (Table 6).
| Category | Community forests | ||
| Reserve forest | Village forest | Sacred forest | |
| Endemic | 184 (11–33) | 106 (9–26) | 152 (14–34) |
| Narrowly endemic | 23 (1–8) | 12 (1–6) | 18 (1–6) |
| Rare | 250 (10–36) | 126 (5–28) | 203 (12–39) |
| Critically endangered | 1 (1) | 1 (1) | 3 (1) |
| Endangered | 7 (1–4) | 4 (1–4) | 6 (1–4) |
| Vulnerable | 5 (1–3) | 5 (1–2) | 2 (1–2) |
| Near threatened | 2 (1–2) | 1 (1) | 2 (1–2) |
Of all the community forests, sacred forests had the highest number (3 species) of Critically Endangered species (Saurauia punduana, Ilex khasiana and Vatica lanceifolia), while reserve- and village-forests had one species (I. khasiana) each. Both reserve and village forests had five species (Elaeocarpus prunifolius, E. rugosus, Ixonanthes khasiana, Aglaia perviridis and Allophylus zeylanicus) each of vulnerable category, while sacred forests had only two species (Ixonanthes khasiana and E. prunifolius) of the same (Table S3).
There was an effect of disturbance on the population of RET species. The village forest being more disturbed had lesser number of RET species. Linear regression analysis showed a negative and significant (P < 0.01) relationship between the disturbance and number of rare, endemic and threatened species (Fig. 5).

|
| Fig. 5 Relationship between disturbances and number of rare, endemic and threatened species. |
Of the 87 community forests studied, 56 (64.3%) were recorded to have high (> 160 species), 22 (25.2%) had medium (120–160 species) and 9 (10.3%) forests had low (< 120 species) species richness (Table 7). Among reserve forests, 85.7% showed high species richness and 14.2% forests showed medium species richness, whereas there were no reserve forests having low species richness. Similarly, 5%, 55% and 40% village forests fall under high, medium and low species richness respectively. There were 76%, 20% and 4% sacred forests, which showed high, medium and low species richness, respectively (Table 7).
| Parameter | Type | Category | |||||||
| Low | Medium | High | |||||||
| Number (%) | Area (ha) | Number (%) | Area (ha) | Number (%) | Area (ha) | ||||
| Species richness | RF | 0 | 0 | 6 (14.2) | 1597 | 36 (85.7) | 6363.65 | ||
| VF | 8 (40) | 641.14 | 11 (55) | 1463.5 | 1 (5) | 26.59 | |||
| SF | 1 (4) | 66.9 | 5 (20) | 395 | 19 (76) | 1548.17 | |||
| Irreplaceability level | RF | 0 | 0 | 18 (42.9) | 4409.12 | 24 (57.1) | 3551.52 | ||
| VF | 11 (57) | 1656.03 | 8 (40) | 413.85 | 1 (5) | 61.4 | |||
| SF | 0 | 0 | 8 (32) | 398.55 | 17 (32) | 1611.15 | |||
| Vulnerability level | RF | 2 (4.8) | 1197.73 | 12 (28.6) | 786.54 | 28 (66.7) | 5976.38 | ||
| VF | 11 (55) | 1689 | 5 (25) | 179.4 | 4 (20) | 262.9 | |||
| SF | 1 (04) | 66.52 | 2 (08) | 236.15 | 22 (88) | 1707.03 | |||
| Risk zone | RF | 0 | 0 | 32 (76.1) | 6105.6 | 10 (23.9) | 1855.05 | ||
| VF | 0 | 0 | 2 (10) | 116.45 | 18 (90) | 2014.83 | |||
| SF | 14 (56) | 1666.8 | 10 (40) | 276.38 | 1 (4) | 66.52 | |||
| RF = reserve forest, VF = village forest, SF = sacred forest. | |||||||||
In terms of area, the forests under high, medium and low species richness category covered 7938.41 (65.5%), 3455.5 (28.6%) and 708.04 (5.9%) hectares, respectively. These categories accounted to about 1%, 0.4% and 0.1% of the total geographical area of Khasi Hills, respectively. This also accounts to 0.51%, 0.22% and 0.05% of the total forest cover and 0.35%, 0.15% and 0.03% of the total geographical area of the state, respectively (Table 7).
Village forests contributed highest area (641.14 ha) towards low species richness, followed by sacred forests (66.9 ha), whereas, none of the reserve forests falls under this category. With respect to medium species richness, the highest area was represented by reserve- (1597 ha), followed by village- (1463.5 ha) and sacred-forests (395 ha). The reserve forests had 6363.65 ha area falling under high species richness, followed by sacred forests (1548.17 ha) and village forests (26.59 ha) (Table 7; Fig. 6a).

|
| Fig. 6 (a) Species richness in different types of community forests in Khasi Hills. (b) Irreplaceability level in different types of community forests. (c) Vulnerability level in different types of community forests. (d) Risk level in different types of community forests. (e) Priority conservation areas among community forests in Khasi Hills. (1 = Cherrapunjee, 2 = Mawsynram, 3 = Pynursla-Pongtung, 4 = Nongstoin, 5 = Mairang, 6 = Mawkyrwat, 7 = Lyngiong-Weiloi, 8 = Pariong, 9 = Lynshing, 10 = Upper Shillong, 11 = Smit). |
Of all the forests, high, medium and low irreplaceability levels were shown by 48%, 39% and 13%, respectively (Table 7; Fig. 6b). In case of reserve forests, 57% showed high irreplaceability level and 43% forests showed medium irreplaceability level, whereas there were no forests that represented low irreplaceability level. Similarly, 5%, 40% and 57% village forests showed high, medium and low irreplaceability levels, respectively. There were 68% and 32% sacred forests, which showed high and medium irreplaceability levels respectively, whereas none of the sacred forests had low irreplaceability level (Table 7).
In terms of area, the forests under high, medium and low category covered 5224.07 (43.2%), 5221.52 (43.1%) and 1656.03 (13.7%) hectares, respectively. The categories of high, medium and low represented 0.2%, 0.2% and 0.1% of the total geographical area of the Khasi Hills respectively. This also accounts to 0.33%, 0.33%, 0.11% and 0.65%, 0.65%, 0.21% of the total forest cover and geographical area of the state respectively (Table 7). The area for low irreplaceability level was highest in village forests (1656.03 ha), whereas sacred- and reserve-forests were not represented under this category. For medium levels of irreplaceability, the highest area was represented by reserve forests (4409.12 ha), followed by village forests (413.85 ha) and sacred forests (398.55 ha). Reserve forests contributed highest area (3551.52 ha) for high irreplaceability level, followed by sacred forests (1611.15 ha) and village forests (61.4 ha) (Table 7; Fig. 6b).
3.7. Mapping of vulnerability levelHigh vulnerability level was shown by 62%, medium by 21% and low by 16% community forests (Table 7; Fig. 6c). The categories of high, medium and low represented 0.35%, 0.05% and 0.13% of the total geographical area of Khasi Hills, respectively. This also accounts to 0.51%, 0.08%, 0.19% and 0.99%, 0.15%, 0.37% of the total forest cover and geographical area of the state (Table 7).
Among the reserve forests, 67%, 28% and 5% forests showed high, medium and low vulnerability levels, respectively. Similarly, 20%, 25% and 55% village forest and 88%, 8% and 4% sacred forest showed high, medium and low vulnerability levels, respectively (Table 7).
3.8. Mapping of risk zonesThe disturbance index from all the forests ranged from 2 to 70. Of all the forests categories village forests were highly disturbed with the values ranging from 50 to 70, followed by reserve forests (27–66). Sacred forests showed low levels of disturbances, where the disturbance index ranged from 2 to 70. The highly disturbed (DI = 70) sacred forest was at Upper Shillong. Among all the community forests studied, 44 forests covering an area of 6498.43 ha were at medium risk, followed by high risk (29 forests, 3936.4 ha) and low risk (14 forests, 1666.8 ha) (Table 7; Fig. 6d). The categories of high, medium and low risk represented 0.49%, 0.81% and 0.21% respectively of the total area of Khasi Hills. This also accounted to 0.25%, 0.42% and 0.11% and 0.18%, 0.29%, 0.07% of total forest cover and geographical area of the state, respectively.
Among the reserve forests, majority (76%) were in medium risk level followed by high risk (24%). In case of village forests, majority (90%) were in high risk followed by medium risk (10%) category, whereas there were no forests under low risk levels. Similarly, there were 4%, 40% and 56% sacred forests, which showed high-, medium- and low-risk level, respectively (Table 7). Among the studied forests, the area of low risk was mainly represented by sacred forests (1666.8 ha), whereas the village- and reserve-forests were not denoted under this category. For medium levels of risk, the highest area was represented by reserve- (6105.6 ha), followed by sacred- (276.38 ha) and village-forests (116.45 ha). Village forests contributed to the highest area (2014.83 ha) under high risk zone, followed by reserve- (1855.05 ha) and sacred-forests (66.52 ha) (Table 7, Fig. 6d).
3.9. Community forests for priority conservationIn the present study, it was observed that the community forests that fall under high priority zone accounted for 7661.56 ha (63.3%) area, followed by medium- and low-priority zone. The medium and low priority zone accounted for 2711.32 ha (22.4%) and 1728.81 ha (14.28%), respectively (Fig. 6e). High-, medium- and low-priority zone represents 0.95%, 0.34% and 0.21%, respectively of the total area of Khasi Hills. Similarly, high-, medium- and low-priority zone represents 0.49%, 0.17%, 0.11% and 0.3%, 0.1%, 0.1% of the total forest cover and geographical area of the state, respectively.
The community forests that fall under high priority zone were concentrated in five areas including (1) Cherrapunjee (Sohra, Mawmluh, Mawsmai, Laitryngew, Swer, Rngimawsaw, Laitlyndop, Mawkisyiem, Khrang, Pomshomen, Wahkaliar, Nongthmai, Nongrim, Dympep, Pdengshnong, Maraikaphon), (2) Mawsynram (Mawsynram, Kynshuild, Lawbah, Phlangwanbroi, Laitsohum, Mawrapat, Mawkasain, Phlangmawsyrpat, Mawsawa), (3) Pynursla-Pongtung (Pynursla, Mynrieng, Saitbakon, Ureksew, Rangthaliang, Mawkyrnot, Pongtung), (4) Nongstoin (Nongstoin, Sangriang) and (5) Mairang (Pyndengnongbri, Mawnai, Mairang). Medium priority areas include (7) Lyngiong-Weiloi (Lyngiong, Mawphlang, Tyrsad, Umlangmmar, Weiloi, Mawlynuu, Sawsymper, Jakrem, Pongkung), (8) Pariong (Rngisawlia, Pariong, Nongsynrieh), (6) Mawkyrwat (Mawkyrwat, Nonglang, Nonglynkie, Hilland, Tynnai, Phudjuad, Mawthenriew, Mawten, Mawlangwir, Mawranglang) and (9) Lynshing (Lynshing, Umtong), (10) Upper Shillong and (11) Smit (Jongksha, Smit) region falls under low priority area. Overall, reserve forests contributed highest towards conservation priority area, followed by sacred- and village-forests (Fig. 7).

|
| Fig. 7 Percent contribution of reserve-, village- and sacred-forests for priority conservation. |
The current study focused on plant diversity conservation targets at a landscape level in Khasi Hills and generated a baseline data on floristic diversity of the community forests. By classifying the forests into different categories to meet the livelihood demands, sociocultural ethos and ecological integrity, the local communities are playing an important role in managing these forest resources. The results have showed that these community forests are rich in plant diversity and harbor many rare, endemic and threatened plant species. The rich diversity of plants in the region is due to the confluence of three biogeographic realms namely, Indo-Burma, Indo-Malayan, and Indo-Chinese (Balakrishnan, 1981). The traditional management system has an effect on the structure and composition of these forests as evidenced by a variation in the community characteristics (diversity, density, basal area) among sacred-, reserve- and village-forests. Such variation can be attributed to the fact that the sacred forests being important from socio-cultural and religious services point of view are also the places of worship (Upadhaya, 2016) and thus well protected. Whereas other community forests (reserve and village) play an important role in sustaining the livelihood of local people by providing them with many amenities including timber, fire and fuel wood, raw materials for house construction and crafts, medicine, economy, fodder (Tiwari et al., 1998).
The significant decrease in stand density and basal area of trees in the order of sacred > reserve > village forests clearly showed that the management system affects the forest categories differently. The prevalence of all types of disturbances (extraction-of timber/poles, -fuel wood, -NTFP's, grazing, encroachment of forest land for agriculture, building roads and fire) in village forests (mean DI = 61) makes them highly vulnerable and are in a degraded state. Among the reserve forests, the incidence of disturbances include fuel wood and NTFP's extraction, grazing, encroachment of forest land for agriculture and fire (DI = 46) and these forests are also prone to degradation. Except the sacred forest at Upper Shillong (DI = 70) most of them are well protected owing to the socio-religious beliefs. The disturbances prevalent in the sacred forests include NTFP's collection, building roads and fire.
The overall low species richness in village forests as compared to reserve- and sacred-forests may be attributed to repeated human disturbances including timber and non-timber product extraction, fires and grazing. Disturbance in the form of extraction reduces the densities of naturally occurring plants and also changes the ecological conditions of vegetation particularly of endemic and rare plants that often have localized distribution (Moxham and Turner, 2011; Iralu et al., 2020). This is further evident by a significant negative relationship of disturbance with RET species. Human disturbances cause an immediate decline in plant diversity and leads to the disappearance and extinction of the local species and their subsequent replacement by immigrant species (Lin and Cao, 2009). This is evident by an increase in the proliferation of disturbance tolerant species like Lantana camara, Eupatorium adenophorum, Crassocephalum crepidioides, Ageratum conyzoides and Galinsoga parviflora at the highly disturbed village and reserve forests (Table S2). Even low-intensity disturbances e.g. grazing, browsing, firewood extraction, selective logging, road building and agricultural expansion may strongly affect the forest structure and the ability of the species to regenerate (Upadhaya et al., 2008). High species richness in reserve forests as compared to sacred- and village-forests could be due to intermediate levels of disturbances in these forests. Intermediate levels of disturbances create an environment in which both late-successional and disturbance-tolerant species can coexist, thereby adding to overall species diversity (Connell, 1978). The decrease in diversity along with an increase in disturbance as observed in the present study is similar to that reported from tropical and subtropical forests (Upadhaya et al., 2008; Rasingam and Parthasarathy, 2009; Dutta and Devi, 2013).
The forest resources are under severe threat due to growing human activities and over exploitation, as a result, many of the species are now threatened in the state (Upadhaya et al., 2013). The highest number of species of the family Orchidaceae in rare category depicts their over-exploitation due to ornamental values, unregulated collection and habitat destruction (Purkayastha, 2016). Moreover, many species, particularly in the genera Anoectochilus, Calanthe, Coelogyne, Cymbidium, Dendrobium and Pleione are being extracted from natural habitats for their aesthetic beauty and long-lasting flowers. Among the life forms of threatened species, the high proportion of trees indicates that many plants might have become endangered due to their over-exploitation as raw materials for construction purposes. Selective cutting of straight boles of Acer laevigatum, Alseodaphne khasyana, Betula alnoides, Calophyllum polyanthum, Cinnamomum tamala, Elaeocarpus lancifolius, E. prunifolius, Magnolia insignis, M. lanuginosa, M. punduana, Podocarpus neriifolius, Persea spp., Litsea spp. and Quercus glauca for use as timber and poles by villagers was observed. Large trees of Litsea glutinosa were selectively debarked or felled for their medicinal bark. Over-exploitation is considered as one of the sternest problems all over the world because it causes rapid loss of biodiversity in forest ecosystems (Corlett, 2006). Tree felling often leads to creation of forest openings, destroys the habitats for epiphytes and other shade loving species (Simberlov, 2009).
In the present study, it was observed that about 7661.56 ha area of the community forests deserve urgent conservation attention. Similarly, the study also recorded an area of 1728.81 ha that fall under low conservation potential. These forests harbor many rare, endemic and threatened species as evidenced by the presence of 400 RET species, which largely adds to their conservation value. Thus, community forests which exist on the socio-cultural and religious beliefs play an important role in conservation by proving a safe refuge for the threatened taxa (Haridasan and Rao, 1985; Khan et al., 1997). The RET species recorded in the present study is higher than that reported (29 threatened species and 86 endemic species) from National Parks and Wildlife Sanctuaries of the state (Upadhaya et al., 2013). The high priority area is mostly seeded with many important RET species including Acer laevigatum, Adinandra griffithii, Aquilaria khasiana, Anoectochilus roxburghii, Aristolochia saccata, Carpinus viminea, Ceropegia angustifolia, Cedrela toona, Cleyera grandiflora, Citrus latipes, Elaeocarpus prunifolius, E. rugosus, Engelhardtia spicata, Fissistigma rubiginosum, Fraxinus floribunda, Gnetum gnemon, Ilex embelioides, I. venulosa, I. khasiana, Illicium griffithii, Livistona jenkinsiana, Magnolia insignis, M. lanuginosa, M. oblonga, M. punduana, M. rabaniana, Mangifera sylvatica, Mitrastemon yamamotoi, Monotropa uniflora, Photinia cuspidata, P. neriifolius, Pyrenaria cherrapunjeana.
The easy accessibility and unrestricted extraction from the village forests makes them prone to the wrath of elevated deforestation and pressure by increasing population, thus making it a low conservation priority. This is supported by the fact that large tracts (2014.83 ha) of high risk overlap with village forests. The sacred forests are also suffering from rapid plant species depletion due to erosion in traditional beliefs and the resulted conversion of sacred- to reserve- and village-forests (Mir and Upadhaya, 2017).
The high concentration of RET species and subsequently higher conservation value of reserve- and sacred-forests could be attributed to the favorable habitat, environmental conditions and comparatively fewer disturbances as compared to village forests. The highest species diversity and the presence of species of conservation value are located in the Cherrapunjee-Mawsynram region. This may be attributed to favorable climatic factors such as heavy rainfall (total annual of 9966–11, 000 mm/year) in the area as compared to other parts. High rainfall has been considered as an important factor to elevate plant diversity (Gentry, 1988; Upadhaya, 2015) as also reported from Neotropics (Pitman et al., 2002), rain forests of Borneo (Slik et al., 2003), Western Ghats (Ramesh et al., 2010) and northeast India (Joseph et al., 2012). The low conservation value of the community forests located in Upper Shillong and Smit region could be attributed to anthropogenic disturbances. Levels of human pressures have been considered as an important predictor of RET species abundance and occurrence in a particular area (Sodhi et al., 2010; Mir et al., 2017).
The priority areas identified in the present study overlaps with some of the sites recognized by earlier works for plant diversity conservation (Upadhaya et al., 2013). These include Cherrapunjee, Mawsynram, Pynursla and Pongtung. The study also confirms that future conservation planning must include community forests located at Mairang and Nongstoin areas in addition to the previously described areas, as they also have high conservation value. The present study has a number of advantages as compared to the previous study (Upadhaya et al., 2013), which was too coarse-scaled and had focused only on presence-absence data, used herbarium and secondary literature data and hardly deliver through data on site-specific locations to deploy realistic conservation actions.
The present study at landscape levels brings additional sites onto the conservation agenda for the first time. Strengthening of traditional management is required to conserve the important species that they shelter and to allow for the continuing provision of biodiversity goods and services to people. The priority forest identified for conservation will help to concentrate the protection strategy by conservationists and planners to the demarcated areas. The identification of priority areas has a huge importance to indigenous communities as it would generates avenues like conservation-related employment and income, maintenance of ecosystem services, adaptation to climate change, opportunities for educational and community pride in local nature (Foster et al., 2012). Using our results as a base map, any conservation organization can readily target those areas that supply the best set of objectives, whether it is to conserve habitat or prevent the extinction of a particular plant species. Such landscape study would help in developing effective strategies for conservation planning for the state especially Khasi Hills.
Authors' contributionsThe idea was conceived by KU, the work was executed by AHM; AHM did the analysis with the help of KU and KS. First draft of MS was written by AHM and KS; later all authors worked on it. All authors read and approved the final manuscript.
Declaration of competing interestThe authors declare that they have no competing interests.
AcknowledgementsThe authors are thankful to the local traditional heads and the people for allowing us to work in the forests. The help received from Botanical Survey of India, Eastern Regional Circle, Shillong is also acknowledged. We are also partially thankful for financial support to Ministry of Environment, Forest and Climate Change, Government of India (No.14/25/2011-ERS/RE).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.11.010.
Ahmedullah, M., 2000. Prioritization of endangered plants of India. In, Singh S, Sastry ARK, Mehta R, Uppal V eds., Setting Biodiversity Priorities for India. World Wide Fund for Nature India, New Delhi vol. 2, 442-459.
|
Ambal, R.G.R., Duya, M.V., Cruz, M.A., et al., 2012. Key biodiversity areas in the Philippines: priorities for conservation. J. Threat. Taxa, 4: 2788-2796. DOI:10.11609/JoTT.o2995.2788-96 |
Ando, A., Camm, J., Polasky, S., et al., 1998. Species distribution, land values, and efficient conservation. Science, 279: 2126-2128. DOI:10.1126/science.279.5359.2126 |
Balaguru, B., Britto, S.J., Nagamurugan, N., 2006. Identifying conservation priority zones for effective management of tropical forests in Eastern Ghats of India. Biodivers. Conserv., 15: 1529-1543. DOI:10.1007/s10531-004-6678-1 |
Balakrishnan, N.P., 1981-1983. Flora of Jowai. Vol vols. I-II. Botanical Survey of India. Howrah.
|
Behera, M.D., Roy, P.S., 2010. Assessment and validation of biological richness at landscape level in part of the Himalayas and Indo Burma Hotspots using geospatial modelling approach. J. Indian Soc. Remote Sens., 38: 415-429. DOI:10.1007/s12524-010-0044-4 |
Bottrill, M., Joseph, L.N., 2008. Is conservation triage just smart decision-making?. Trends Ecol. Evol., 23: 649-654. DOI:10.1016/j.tree.2008.07.007 |
Brooks, T.M., Mittermeier, R., A., daFonseca, G.A.B., et al., 2006. Global biodiversity conservation priorities. Science, 313: 58-61. DOI:10.1126/science.1127609 |
Castellanos, J., Jaramillo, V.J., Sanford, R.L., et al., 2001. Slash-and-burn effects on fine root biomass and productivity in a tropical dry forest ecosystem in México. For. Ecol. Manag., 148: 41-50. DOI:10.1016/S0378-1127(00)00523-5 |
Champion, H.G., Seth, S.K., 1968. A Revised Survey of the Forest Types of India. Government of India Press, New Delhi, India.
|
Connell, J.H., 1978. Diversity in tropical rain forests and coral reefs. High diversity of trees and corals is maintained only in a non-equilibrium state. Science, 199: 1302-1310. DOI:10.1126/science.199.4335.1302 |
Corlett, R.T. 2006. Conservation of biodiversity in a highly degraded landscape: problems and prospects. In: Jim, C.Y., Corlett, R.T., (eds. ), Sustainable Management of Protected Areas for Future Generations. Friends of the Country Parks, Hong Kong. pp 77-92.
|
Curnutt, J., Lockwood, J., Luh, H.K., et al., 1994. Hotspots and species diversity. Nature, 367: 326-327. DOI:10.1038/367326a0 |
Dawson, T.P., Jackson, S.T., House, J.I., et al., Beyond predictions, biodiversity conservation in a changing climate. Science. |
Defries, R., 2010. Interactions between protected areas and their surroundings in human-dominated tropical landscapes. Biol. Conserv., 143: 2870-2880. DOI:10.1016/j.biocon.2010.02.010 |
Dobson, A.P., Rodriguez, J.P., Roberts, W.M., et al., 1997. Geographic distribution of endangered species in the United States. Science, 275: 550-553. DOI:10.1126/science.275.5299.550 |
Dutta, G., Devi, A., 2013. Plant diversity, population structure, and regeneration status in disturbed tropical forests in Assam, northeast India. J. For. Res., 24: 715-720. DOI:10.1007/s11676-013-0409-y |
Foster, M.N., Brooks, T.M., Cuttelod, A., et al., 2012. The identification of sites of biodiversity conservation significance: progress with the application of a global standard. J. Threat. Taxa, 4: 2733-2744. DOI:10.11609/JoTT.o3079.2733-44 |
Garg, J.K., Narayan, A., Basu, A., 1988. Monitoring environmental changes over Kudremukh iron ore mining area, India using remote sensing technique. Proceedings of the Indo-British workshop on remote Sensing of Environment in Mining field. ISM. Dhanbad. pp. 41-47.
|
Gentry, A.H., 1988. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard., 75: 1-34. DOI:10.2307/2399464 |
Haridasan, K., Rao, R.R., 1985-1987. Forest Flora of Meghalaya. vol. 2 Volumes. Bishen Singh Mahendra Pal Singh, Dehra Dun, India.
|
Hoekstra, J.M., Boucher, T.M., Ricketts, et al., 2005. Confronting a biome crisis, global disparities of habitat loss and protection. Ecol. Lett., 8: 23-29. |
Iralu, V., Pao, N.T., Upadhaya, K., 2020. An assessment of population structure and regeneration status of Magnolia punduana Hk. f. & Th. (Magnoliaceae) in fragmented forests of northeast India. J. For. Res., 31: 937-943. DOI:10.1007/s11676-019-00930-z |
ISFR., 2019. Indian state of forests report 2019. Volume vol. II. Forest Survey of India (Ministry of Environment Forest and Climate Change) Kaulagarh road, P.O. IPE Dehradun - 248195, Uttarakhand. India. Pp 173-181.
|
IUCN., 2003. Guidelines for Application of IUCN Red List Criteria at Regional Levels, Version 3.0. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge.
|
Joseph, J., 1982. Flora of Nongpoh and Vicinity. Government of Meghalaya, Shillong. pp. 376.
|
Joseph, J., Anitha, K., Srivastava, K., et al., 2012. Rainfall and elevation influence the local-scale distribution of tree community in the southern region of Western Ghats biodiversity Hotspot (India). Int. J. For. Res., 2012: 1-10. DOI:10.1155/2012/576502 |
Kanjilal, V.N., Kanjilal, P.C., Das, A., et al., 1934-1940. Flora of Assam, vol. 5 Volumes. Government Press, Shillong, India.
|
Kardol, P., Wardle, D.A., 2010. How understanding aboveground–belowground linkages can assist restoration ecology. Trends Ecol. Evol., 25: 670-679. DOI:10.1016/j.tree.2010.09.001 |
Kataki, S.K., 1986. Orchids of Meghalaya. Botanical Survey of India, Howrah, India.
|
Khan, M.L., Menon, S., Bawa, K.S., 1997. Effectiveness of the protected area network in biodiversity conservation, a case study of Meghalaya state. Biodivers. Conserv., 6: 853-868. DOI:10.1023/B:BIOC.0000010406.35667.c0 |
Khandel, K.A., Ganguly, S., Bajaj, A., et al., 2012. New records, ethno-pharmacological applications and indigenous uses of Gloriosa superba L. Glory lily practices by tribes of Pachmarhi Biosphere reserve, Madhya Pradesh, Central India. Nat. Sci., 10: 23-48. |
Kier, G., Barthlott, W., 2001. Measuring and mapping endemism and species richness, a new methodological approach and its application on the flora of Africa. Biodivers. Conserv., 10: 1513-1529. DOI:10.1023/A:1011812528849 |
Kushwaha, S.P.S., Nandy, S., 2012. Species diversity and community structure in Sal Shorea robusta forests of two different rainfall regimes in West Bengal, India. Biodivers. Conserv., 21: 1215-1228. DOI:10.1007/s10531-012-0264-8 |
Langhammer, P.F., Bakarr, M.I., Bennun, L.A., et al., 2007. Identification and Gap Analysis of Key Biodiversity Areas, Targets for Comprehensive Protected Area Systems. Gland, Switzerland. 134 p.
|
Lin, L.X., Cao, M., 2009. Edge effects on soil seed banks and understory vegetation in subtropical and tropical forests in Yunnan, SW China. For. Ecol. Manag., 257: 1344-1352. DOI:10.1016/j.foreco.2008.12.004 |
Margules, C.R., Pressey, P.L., 2000. Systematic conservation planning. Nature, 405: 243-253. DOI:10.1038/35012251 |
Margules, C.R., Pressey, P.L., Williams, P.H., 2002. Representing biodiversity, data and procedures for identifying priority areas for conservation. J. Biosci., 27: 309-326. |
Mir, A.H., Iralu, V., Pao, N.T., et al., 2016. Magnolia lanuginosa (Wall.) Figlar & Noot. In West Khasi hills of Meghalaya, northeastern India, re-collection and implications for conservation. J. Threat. Taxa, 8: 8398-8402. DOI:10.11609/jott.2242.8.1.8398-8402 |
Mir, A.H., Upadhaya, K., 2017. Effect of traditional management practices on woody species composition and structure in montane subtropical forests of Meghalaya, Northeast India. J. Mt. Sci., 14: 1500-1512. DOI:10.1007/s11629-016-4145-6 |
Mir, A.H., Upadhaya, K., Choudhury, H., 2014. Diversity of endemic and threatened ethnomedicinal plant species in Meghalaya, North-East India. Int. Res. J. Environ. Sci., 3: 64-78. |
Mir, A.H., Upadhaya, K., Odyuo, N., et al., 2017. Rediscovery of Magnolia rabaniana (Magnoliaceae): a threatened tree species of Meghalaya, northeast India. J. Asia Pac. Biodivers., 10: 127-131. DOI:10.1016/j.japb.2016.10.004 |
Moxham, C., Turner, V., 2011. The effect of fragmentation on the threatened plant community Coastal Moonah Woodland in Victoria, Australia. Urban Ecosyst., 14: 569-583. DOI:10.1007/s11252-011-0171-x |
Myers, N., 1988. Threatened biotas: "hot spots" in tropical forests. Environmentalist, 8: 187-208. |
Myers, N., Mittermeier, R.A., Mittermeier, C.G., et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Natarajan, D., Britto, S.J., Balaguru, B., et al., 2004. Identification of conservation priority sites using remote sensing and GIS - a case study from Chitteri hills, Eastern Ghats, Tamil Nadu. Curr. Sci.: 1316-1323. |
Nayar, M.P., Sastry, A.R.K., 1987-1990. Red Data Book of Indian Plants, vol. 3 Vols. Botanical Survey of India, Howrah Calcutta, India. pp. 905.
|
Oliver, I., Beattie, A.J., 1996. Designing a cost-effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecol. Appl., 6: 594-607. DOI:10.2307/2269394 |
Olson, D.M., Dinerstein, E., 1998. The Global 200: a representation approach to conserving the Earth's most biologically valuable ecoregions. Conserv. Biol., 12: 502-515. DOI:10.1046/j.1523-1739.1998.012003502.x |
Ormsby, A.A., Bhagwat, S.A., 2010. Sacred forests of India, a strong tradition of community-based natural resource management. Environ. Conserv., 373: 320-326. |
Pant, S., Samant, S.S., 2007. Assessment of plant diversity and prioritization of communities for conservation in Mornaula reserve Forest. Appl. Ecol. Environ. Res., 5: 151-166. |
Pao, T., Upadhaya, K., 2017. Effect of fragmentation and anthropogenic disturbances on floristic composition and structure of subtropical broad leaved humid forest in Meghalaya, Northeast India. Appl. Ecol. Environ. Res., 15: 385-407. DOI:10.15666/aeer/1504_385407 |
Pitman, N.C.A., Terborgh, J.W., Silman, M.R., et al., 2002. A comparison of tree species diversity in two upper Amazonian forests. Ecology, 83: 3210-3224. DOI:10.1890/0012-9658(2002)083[3210:ACOTSD]2.0.CO;2 |
Purkayastha, J. 2016. Bioprospecting of Indigenous Bioresources of North-East India. New York, Springer.
|
Ramesh, B.R., Venugopal, P.D., Elissier, R.P., et al., 2010. Mesoscale patterns in the floristic composition of forests in the central Western Ghats of Karnataka, India. Biotropica, 42: 435-443. DOI:10.1111/j.1744-7429.2009.00621.x |
Rana, M.S., Samant, S.S., 2009. Prioritization of habitats and communities for conservation in the Indian Himalayan region: a state-of-the-art approach from Manali Wildlife sanctuary. Curr. Sci., 97: 326-335. |
Rao, R.R., Hajra, P.K., 1986. Floristic diversity of eastern Himalaya– in a conservation perspective. Proc. Indian Acad. Sci. Anim. Sci./Plant Sci. Suppl.: 103-125. |
Rasingam, L., Parthasarathy, N., 2009. Tree species diversity and population structure across major forest formations and disturbance categories in Little Andaman Island, India. Trop. Ecol., 50: 89-102. |
Richards, M., 1996. Stabilising the Amazon Frontier, Technology, Institutions and Policies. Natural Resource Perspectives No. vol. 10, Overseas Development Institute, London. pp 1-8.
|
Rodgers, W.A., 1994. The sacred groves of Meghalaya. Man India, 74: 339-348. |
Roy, P.S., Kushwaha, S.P.S., Roy, A., 2012. Landscape level biodiversity databases in India, status and the scope. Proc. Natl. Acad. Sci. India B Biol. Sci., 82: 261-269. |
Samant, S.S., Joshi, H.C., Arya, S.C., 2002. Studies on the structure.; composition and changes of vegetation in Nanda Devi Biosphere reserve of west Himalaya. In J.K. Sharma, P.S., Easa, C.N., Mohanan, et al., (eds. ) Biosphere Reserves in India and their Management. Kerala Forest Research Institute and Ministry of Environment & Forests, New Delhi.
|
Scott, J.M., Csuti, B., Jacobi, J.D., et al., 1987. Species richness, a geographic approach to protection future biological diversity. Bioscience, 37: 782-788. DOI:10.2307/1310544 |
Shimrah, T., Sarma, K., Varga, O.G., et al., 2019. Quantitative assessment of landscape transformation using earth observation datasets in Shirui Hill of Manipur, India. Remote Sens. Appl.: Soc. Environ., 15: 1-6. |
Simberlov, D., 2009. We can eliminate invasions or live with them. Successful management projects. Biol. Invasions, 11: 149-157. |
Slik, J.W.F., Poulsen, A.D., Ashton, P.S., et al., 2003. A floristic analysis of the lowland dipterocarp forests of Borneo. J. Biogeogr., 30: 1517-1531. |
Sodhi, N.S., Posa, M.R.C., Lee, T.M., et al., 2010. The state and conservation of South-east Asian biodiversity. Biodivers. Conserv., 19: 317-328. DOI:10.1007/s10531-009-9607-5 |
Tiwari, B.K., Barik, S.K., Tripathi, R.S., 1998. Biodiversity value, status, and strategies for conservation of sacred Groves of Meghalaya, India. Ecosys. Health, 4: 20-32. |
Upadhaya, K., 2015. Structure and floristic composition of subtropical broad-leaved humid forest of Cherapunjee in Meghalaya, Northeast India. J. Biodivers. Manage. Forestry. 4, 4. http://dx.doi.org/2327-4417.1000149.
|
Upadhaya, K., 2016. Ecological analysis of sacred groves and its conservation. In: Upadhaya, K., (ed. ) Biodiversity and Environmental Conservation. Discovery Publishing House, New Delhi, India. pp. 149-158.
|
Upadhaya, K., Barik, S.K., Pandey, H.N., et al., 2008. Response of woody species to anthropogenic disturbances in sacred forests of North East India. Int. J. Ecol. Environ. Sci., 34: 245-257. |
Upadhaya, K., Pandey, H.N., Law, P.S., et al., 2003. Tree diversity in sacred groves of the Jaintia hills in Meghalaya, northeast India. Biodivers. Conserv., 12: 583-597. |
Upadhaya, K., Thapa, N., Lakadong, J.N., et al., 2013. Priority areas for conservation in North East India, A case study in Meghalaya based on plant species diversity and endemism. Int. J. Ecol. Environ. Sci., 39: 125-136. |
Ved, D.K., Kinhal, G.A., Ravikumar, K., et al., 2005. Conservation assessment and management prioritization CAMP for the wild medicinal plants of Northeast India. Med. Plant Conserv., 11: 40-44. |
Vetaas, O.R., Salihb, A., Jurasinskic, G., 2012. Vegetation changes in the Red Sea Hills, from mist oasis to arid shrub. Plant Ecol. Divers., 5: 527-539. DOI:10.1080/17550874.2012.749954 |
Walter, K.S., Gillett, H.J., 1998. 1997 IUCN Red List of Threatened Plants. Complied by the World Conservation Union.; Gland, Switzerland and Cambridge, pp. 31.
|
Wang, B., Luo, F., Zhen, X., et al., 2009. Quantitative method for identifying networks of minimum priority sites for protection of rare and endangered plant species in Guangdong, China. Front. Biol. China, 4: 117-123. DOI:10.1007/s11515-008-0086-y |
Williams, P.H., Margules, C.R., Hilbert, D.W., 2002. Data requirements and data sources for biodiversity priority area selection. J. Biosci., 27: 327-338. |



