b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. Institute of Botany, Academy of Sciences of Uzbekistan, Tashkent 100053, Uzbekistan;
d. Key Laboratory of Plant Resources Conservation and Utilization, Jishou University, Jishou 416000, Hunan, China;
e. School of Life Sciences, Yunnan Normal University, Kunming 650500, Yunnan, China
The genus Oreocharis Benth. (Bentham, 1876) (Gesneriaceae) contains rosette plants that have spirally arranged leaves and scapose, axillary inflorescences (Möller et al., 2011b). This genus contains ca. 139 species (125 plus new species recently published) that mainly occur in Northeast India, southern and southwestern China, Vietnam, Thailand, Myanmar (Möller et al., 2011b, 2016, 2017, 2018, 2019; Fu et al., 2019a, 2019b; Pan et al., 2019; Wen et al., 2019; Yang et al., 2019, 2020; Cai et al., 2019, 2020; Chen et al., 2020b; Du et al., 2020; Ling et al., 2020; Qin et al., 2020). In earlier taxonomic studies, the genus Oreocharis was thought to contain about 28 species (Wang et al., 1990, 1998), but after revision based on morphological and molecular evidence it now contains 11 previously recognized genera (i.e., Ancylostemon Craib, Bournea Oliv., Briggsia Craib, Dayaoshania W.T. Wang, Deinocheilos W.T. Wang, Isometrum Craib, Opithandra B.L. Brutt, Paraisometrum W.T. Wang, Thamnocharis W.T. Wang, Tremacron Craib, and Oreocharis) (Möller et al., 2011b). In this expanded genus, vegetative habit and fruit traits are almost identical in all species; nevertheless, there are many variations in flowers, such as the form of corolla (actinomorphic or zygomorphic), corolla color (purple, yellow, orange, or white), length of the lip (longer abaxial, nearly equal or longer adaxial lip), form of the stamen (inserted or exserted, fertile or vestigial, free or fused anthers) and number of stigmas (1 or 2). Hence, floral traits are important for distinguishing Oreocharis species (Wang et al., 1990, 1998; Möller et al., 2011b). A large number of new species within this genus, such as Oreocharis rubrostriata F. Wen & L.E. Yang and Oreocharis ovatilobata Q. Fu & Y.Q. Wang (Yang et al., 2019; Fu et al., 2019b), have also been recognized in recent years as the taxonomic definition of this taxon has been updated.
The Wuling Mountains are located at the junction between Hunan, Chongqing, Hubei, and Guizhou provinces in China. Multiple geographic landforms are found here and the flora is subtropical and temperate (Chen et al., 2003). The Wuling Mountains are very rich in biodiversity, containing a total of 4119 recorded species, belonging to 1005 genera and 201 families (Liao et al., 2002). Moreover, as 133 nature reserves have been created to protect ecosystems and maintain biodiversity (Jiang et al., 2020), numerous new species may yet remain undiscovered.
During a botanical survey in the Wuling Mountains, we discovered a new species of Oreocharis. Flowers of this new species had similar color and morphology as those of Oreocharis benthamii and Oreocharis xiangguiensis. Furthermore, the adaxial lip of this new species was conspicuously long, similar to that of the recently described O. rubrostriata. Similarities in generic characters (fertile stamens, filaments shape, stigma, rosette habit, capsule shape, and dehiscence, etc.) also strongly indicated that the new species should be assigned to Oreocharis. Thus, after a literature review (Pellegrin, 1930; Ohwi, 1965; Wang et al., 1990, 1998; Wu, 1991; Ho, 2000; Li and Wang, 2004; Wei et al., 2010), we compared the morphology of the new species to that of similar species. We then used Maximum Likelihood and Bayesian Inference (BI) analyses to reconstruct the phylogenetic position of the new species. Finally, we used character evolution analysis to determine its uniqueness. Taken together, these three lines of evidence show that we have discovered a new species of Oreocharis.
2. Materials and methods 2.1. Morphological analysesAll the specimens of the new species examined as part of this study came from the Wuling Mountains National Nature Reserve, Hunan, China. Characters were described based on fresh plants and herbaria specimens. Material of the new species was carefully compared morphologically with similar species. Voucher specimens of species used for comparisons with the new species were deposited in the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN). Voucher information of the new species included for analysis are included in Appendix A.
2.2. DNA sequencing and molecular analysesTo reconstruct a phylogenetic tree of Oreocharis species, we sampled a total of 60 species, including the vast majority of those with available sequence data in GenBank. O. rubrostriata, which has a similar corolla to our new species, could not be included. Agalmyla Blume (four species) and Metapetrocosmea peltata were chosen as outgroups based on a previous study (Möller et al., 2011a).
DNA from the new Oreocharis species was extracted using a TaKaRa Extraction Kit following the manufacturer's protocol (DV811A, TaKaRa, Osaka, Japan). Two primers and relevant PCR protocols were selected for DNA regions ITS and trnL-F following Möller et al. (2011b). Contigs were assembled in Sequencher™ v.4.1.2 (Gene Codes Corporation, Ann Arbor, MI, USA). Sequences were aligned using MAFFT v.7.308 (Katoh and Standley, 2013) and the gaps were treated as missing data. jModeltest v.2.1.10 (Darriba et al., 2012) was then used to determine the most suitable models for ITS data, trnL-F data and the combined data set by the Akaike information criterion (AIC). The phylogeny was calculated using the software MrBayes v.3.2.7 (Ronquist and Uelsenbeck, 2003). Two independent runs, each of four Markov chain Monte Carlo (MCMC) chains were run for 20,000,000 generations, sampling every 1000 generations, and a 50% majority rule consensus tree was produced by combining trees of both independent runs after the first 20% of samples were discarded as burn-in. Runs were considered to have converged to stationarity when average standard deviation of split frequencies was stable below 0.01. ML trees were constructed using RAxML v.8.2.10 (Alexandros et al., 2014) with 1000 bootstrap replicates.
2.3. The evolution of morphological charactersThe evolution of morphological characters was studied to determine the patterns of flower evolution in Oreocharis. The following three characters were selected for analysis: 1) Corolla color: [0] purple, [1] yellow, [2] orange, [3] white, [4] red, [5] green; 2) Corolla lip: [0] longer abaxially, [1] nearly equal, [2] longer adaxially; 3) Stamen: [0] inserted, [1] exserted. Character states for the species were derived from specimen observations as well as previous descriptions (Ohwi, 1965; Wang et al., 1990, 1998; Wu, 1991). The matrix of characters used here was coded for 65 species of Gesneriaceae, including sixty of Oreocharis, four Agalmyla, and one species of Metapetrocosmea (Appendix A). Characters were mapped onto a ML tree based on ITS and trnL-F regions. Character state analysis was performed using the software Mesquite v.3.61 (Maddison and Maddison, 2018) with the Likelihood Ancestral States reconstruction method. The Markov k-state 1 (Mk1) parameter model was chosen as the probability model used in this analysis.
3. Results 3.1. MorphologyThe new species is morphologically similar to Oreocharis xiangguiensis in that they both have purple corollas of similar size and leaf veins covered with rust-brown hairs. The new species is also similar to O. rubrostriata as both have a long adaxial lip. However, the leaves, corolla, corolla tube, stamens, and stamen filaments of the new species differ from those of O. rubrostriata (Table 1).
| Character | O.xieyongii | O.xiangguiensis | O.rubrostriata |
| Leaves | Rhombic-lanceolate to elliptic, base sometimes slightly oblique, rounded or cuneate | Oblong elliptic to oblanceolate or narrowly ovate, base oblique, rounded to cuneate | Broadly elliptic to nearly rounded, base sometimes slightly oblique, cordate |
| Corolla | Purple, ca. 1–1.2cm long, bilateral symmetry, tube has a slight dorsal hump at throat, longer adaxial lips | Purple-red, ca. 1.3–1.6cm long, actinomorphic, tube gradually slightly ampliate from base to throat, the lobes equal | Yellow, ca. 1.5cm long, bilateral symmetry, tube not swollen, longer adaxial lips |
| Stamens | Adnate to corolla from base, slightly longer than corolla | Adnate to corolla 1.5–5mm above base, inserted on corolla | Adnate to corolla form base, exserted |
| Filaments | Glabrous | Glabrous | Glandular puberulous |
The aligned combined data matrix used in this analysis includes 1629 characters (ITS: 745; trnL-F: 884). They contained 296 parsimony informative and 489 variable sites. Results of jModeltest show that the best fit model for the ITS data is GTR + I + G, the trnL-F data is GTR + G, and the combined ITS and cpDNA data set is GTR + I + G. Hence, the best-fit model of ITS and trnL-F in MrBayes software was set to GTR + I + G and GTR + G, respectively. The GTRGAMMAI model was used in ML analysis in RAxML software.
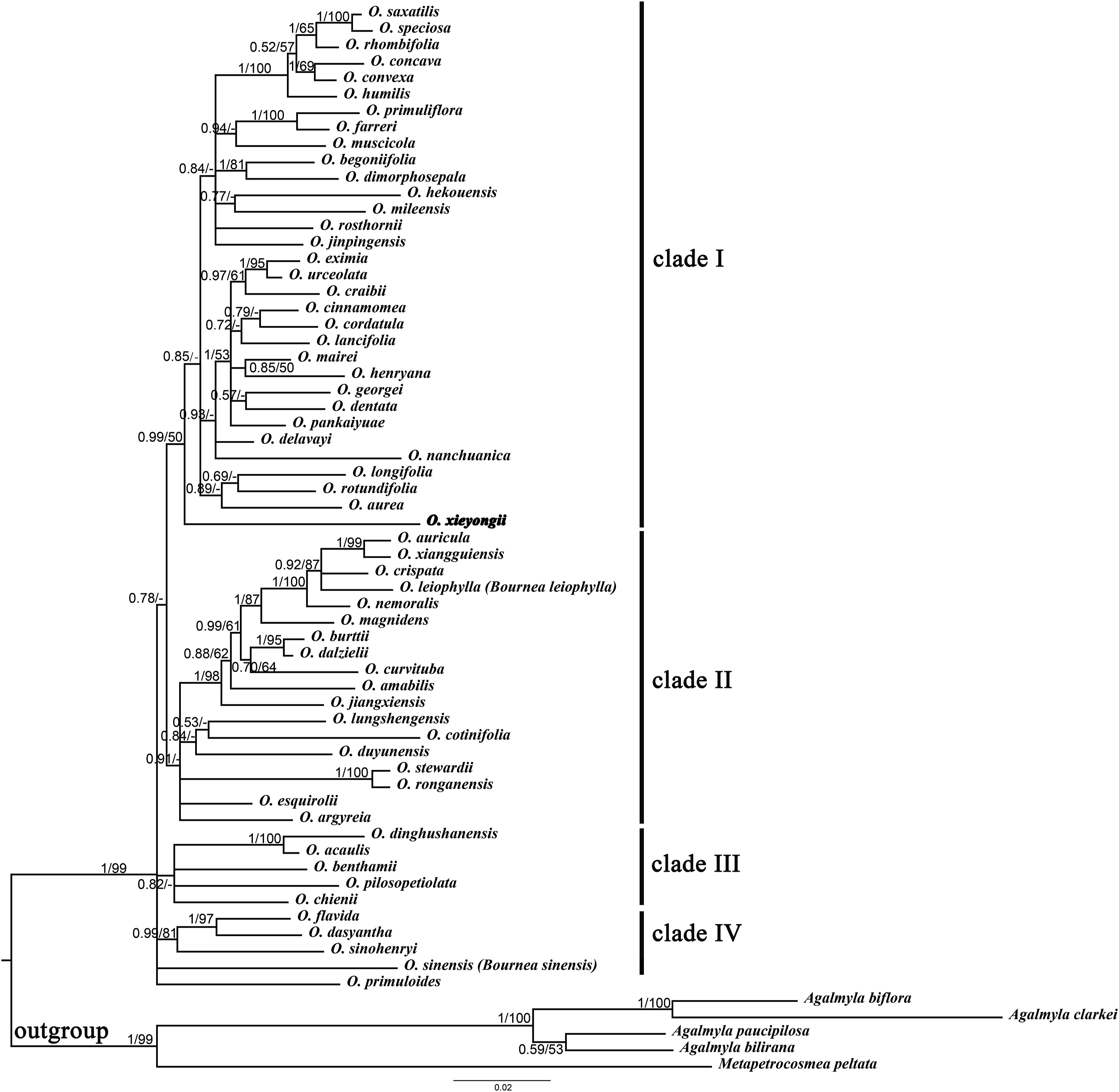
Phylogenetic analysis shows that the ML and BI trees had a similar topology (Figs. S1 and S2). Thus, the branch support values of results of both reconstructions [i.e., posterior probabilities (PP) and bootstrap values (BS)] can be summarized on a Bayesian consensus tree (Fig. 3). The 60 samples of Oreocharis are monophyletic and this hypothesis is strongly supported (PP = 1, BS = 99). Four major clades (clade I, II, III, and IV) are recovered within the genus Oreocharis, including two larger and two smaller ones. However, the relationships of the four clades are not fully resolved. In these relationships, clade I contains 32 species, accounting for more than half of the samples. The new species is nested in clade I with a bootstrap score of 50 and BI score of PP = 0.99. In addition, it represents the earliest diverging lineage in clade I.

|
| Fig. 1 Fig. S1. Phylogeny based on Bayesian Inference analysis of combined nuclear and plastid data. |
|
| Fig. 2 Fig. S2. Phylogeny based on the ML analysis of combined nuclear and plastid data. |
|
| Fig. 3 Bayesian 50% majority-rule consensus tree for Oreocharis inferred from combined trnL-F and ITS sequence data. Bayesian posterior probabilities are shown above branches and ML values are given below; “-”denotes branches with <50% bootstrap support. The new species is shown in bold. |
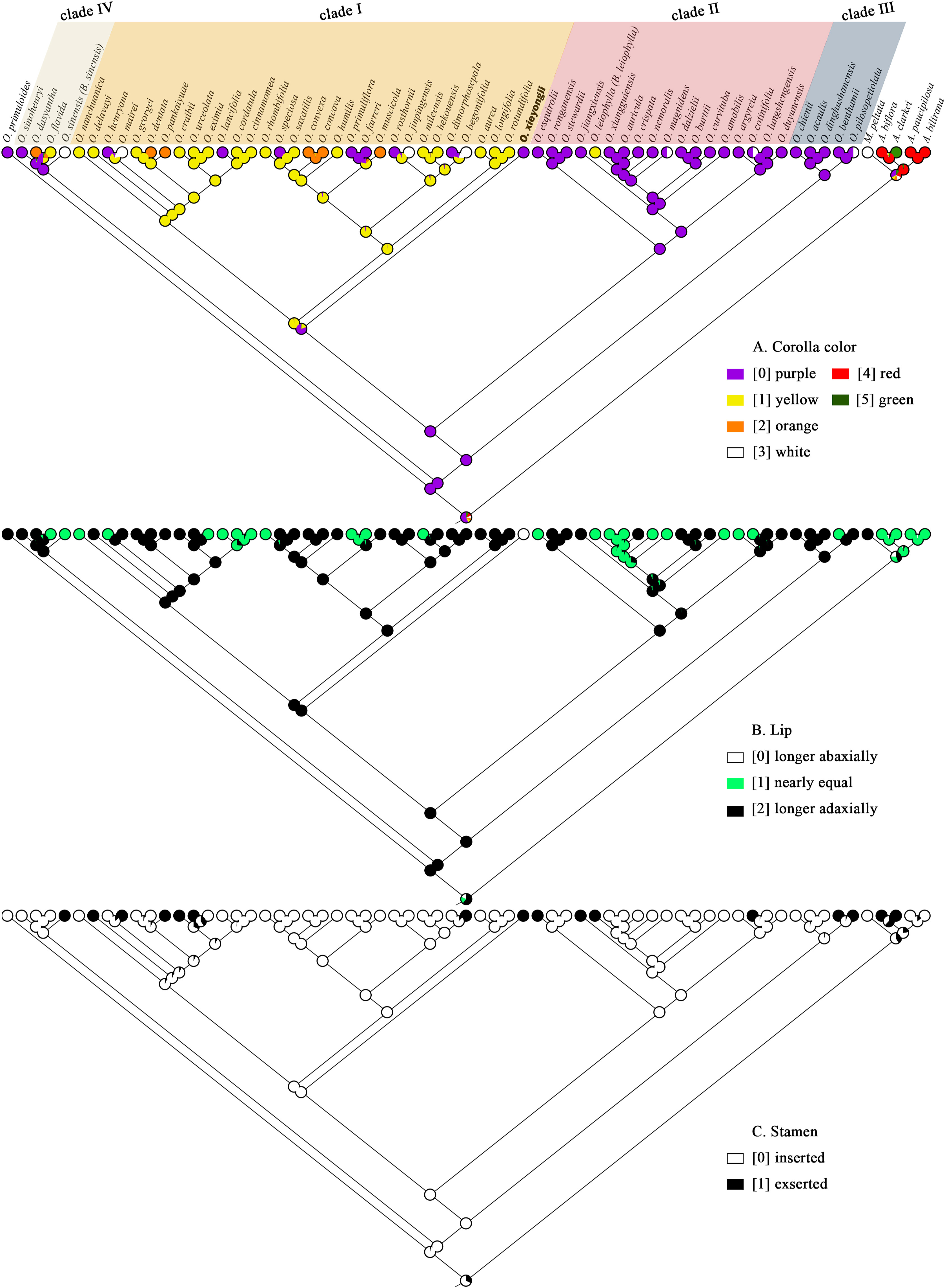
Three morphological characters (corolla color, length of lips, and relation of stamens to corolla) with character reconstruction results that are relevant to the taxonomy of the group were mapped to the ML tree (Fig. 4). Likelihood inferences of character states indicate that all the morphological characters are homoplastic. Purple corolla, longer abaxial lip, and inserted stamens are likely the ancestral state of the last common ancestor of Oreocharis. Our analysis indicates that these three characters have complex evolutionary histories.
|
| Fig. 4 State reconstructions for three morphological characters of the Oreocharis lineage containing 65 species and mapped on the ML tree. The color circles on notes and tip are explained in the legend. A. Corolla color; B. Lip; C. Stamen. |
Oreocharis xieyongii is most similar to O. xiangguiensis, although it can be distinguished from the latter by having a bilaterally symmetrical corolla, an adaxial lip significantly longer than the abaxial lip, and stamens slightly longer than the corolla lobes. The longer adaxial lip is an unusual floral character in Oreocharis. During our examination, we found that several species possess a corolla shape with a longer adaxial lip, such as Oreocharis lancifolia and O. ovatilobata (Wang et al., 1990, 1998; Fu et al., 2019b); however, the difference in the length between the adaxial and abaxial lip is minimal (ca. 1 mm) in O. lancifolia and in O. ovatilobata is almost none. Only the corolla of O. rubrostriata was similar in shape to the new species. However, the yellow corolla of O. rubrostriata is easily distinguished from that of O. xieyongii by its purple corolla. Furthermore, sometimes the positioning of the sepals strongly spread backwards in O. xieyongii, which is a special trait that is also present in Oreocharis duyunensis Z.Y. Li, X.G. Xiang et Z.Y. Guo (Guo et al., 2018).
4.2. Phylogenetic relatednessThe phylogenetic results presented here are congruent with those presented by Möller et al. (2011b), Chen et al. (2014) and Guo et al. (2018). However, a recently published phylogenetic study of Oreocharis based on seven DNA regions indicated that Bournea is a monophyletic group independent of Oreocharis (Chen et al., 2020a), which is not supported by our analysis. Moreover, some phylogenetic relationships differ from our results; for instance, Oreocharis saxatilis is the sister of Oreocharis eximia. These differences are probably caused by the unequal number of matrix information sites between their data and ours. Although Oreocharis comprises a clade with high support, the relationship among clades is not well resolved. Combined morphological data is unable to reveal correlations within this lineage; indeed, Möller et al. (2011b) speculated that this phenomenon is the result of an early and rapid evolutionary radiation.
The new species described here is identified as a member of Oreocharis (Fig. 3). O. xieyongii is nested within clade I and diverged first even though this species is separated from closely related taxa with a high degree of support and very distant from the morphologically similar O. xiangguiensis. The morphology of this new species nevertheless differs from other members of this lineage (clade I) and is characterized by the peculiar possession of a particularly long adaxial corolla lip. Unfortunately, DNA information for another species, O. rubrostriata (also with an obvious long adaxial lip), is not available. The relationship between O. xieyongii and O. rubrostriata needs to be further investigated.
4.3. Character evolutionOur analysis of the evolution of character states is preliminary as it includes only half of all described Oreocharis species. Nevertheless, the character traits of Oreocharis species are largely similar, except for floral traits (Wang et al., 1990, 1998; Möller et al., 2011b). Hence, floral traits (e.g., variation in corolla color, size, shape, and morphology of pistils and stamens) are usually used to distinguish between species within this lineage. Our analysis of the evolution of character states was based on three characters: corolla color, length of lips, and relation of stamens to corolla. Corolla color mainly evolved into yellow and purple forms, although rare orange and white varieties are also seen. However, corolla color is not consistent within clades. For example, O. xieyongii, which has a purple corolla, is placed in a clade with species that have yellow corollas (clade I). This clade also has species with orange and white corollas, and corollas that vary in shade. We speculate that the complex set of corolla colors within the clade may be the result of selection pressure from diverse pollination strategies corresponding to different environments. Floral traits are usually related to pollination mechanisms, e.g., color and shape convey signals perceived by pollinators (Fenster et al., 2004, Rodriguez-Girones and Santamaria, 2004, Rosas-Guerrero et al., 2014). Moreover, birds are known to prefer red and orange flowers, whereas bees are more attracted to purple, blue and yellow flowers (Grant, 1950, Rosas-Guerrero et al., 2014). Oreocharis species, which are mostly distributed in northern Yunnan and southern Sichuan, where the most common pollinators are birds (Ling et al., 2017), have orange flowers.
The new species and 13 additional Oreocharis species have inserted stamens, suggesting that this is the ancestral state of Oreocharis. Although uncommon, exerted stamens seem to have evolved independently many times and occur in multiple clades (clade I, II and III). This character is probably an evolutionary adaptation to pollinator-depauperate environments (Martén-Rodríguez et al., 2015, Rodriguez-Girones and Santamaria, 2004, Tur et al., 2015), as exserted stamens can come into contact with more visitors (Ling et al., 2017). O. xieyongii has evolved a very rare character (long adaxial corolla lip), which is also present in O. rubrostriata (from Guangxi). The specific function of this character and whether it is a synapomorphy of the new species and O. rubrostriata needs to be studied in more detail.
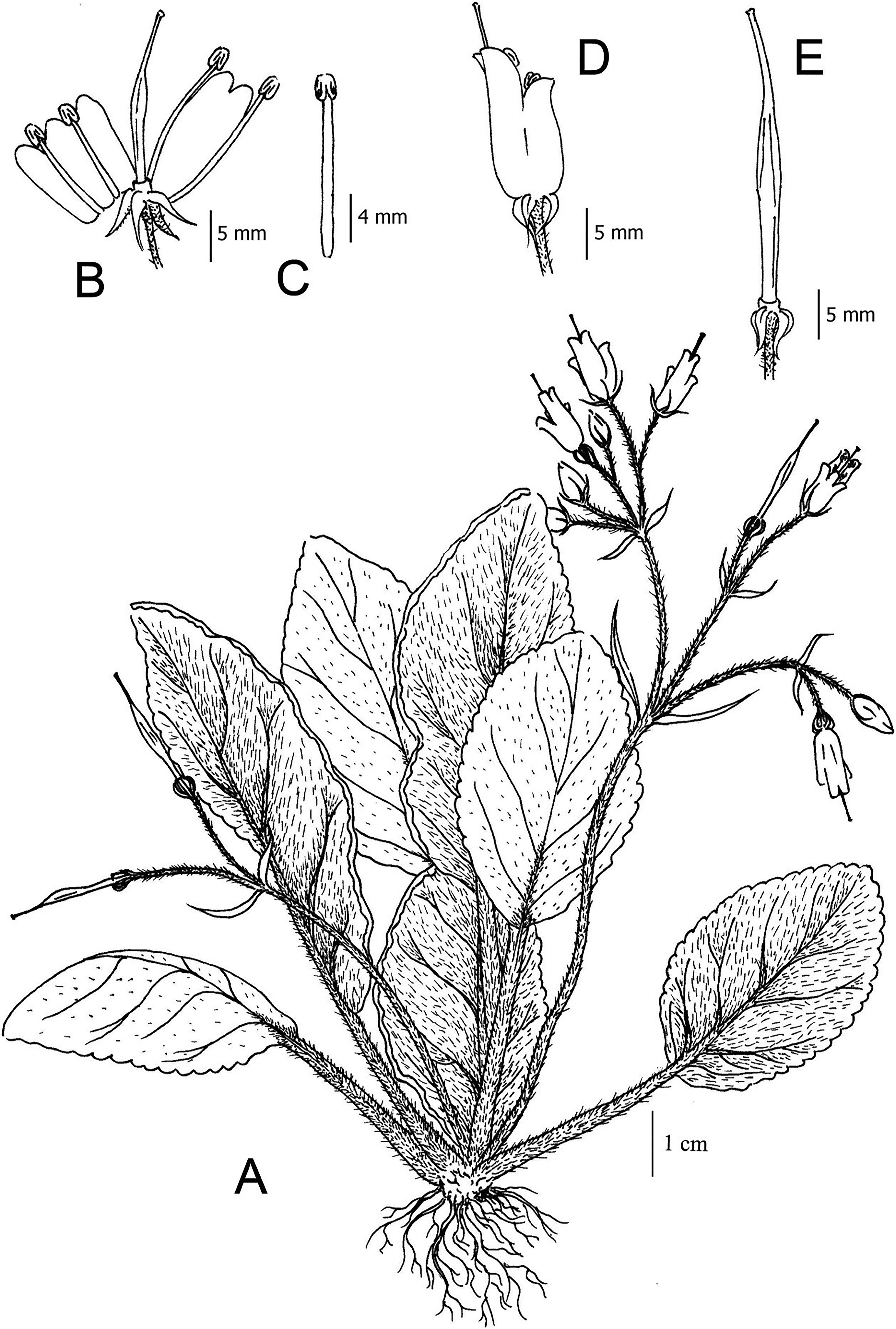
5. Taxonomic treatmentOreocharis xieyongii T. Deng, D.G. Zhang & H. Sun sp. (Fig. 1, Fig. 2).
Diagnosis: —The new species can easily be distinguished from most other species except Oreocharis rubrostriata by the obvious presence of a longer adaxial lip and exserted stamens. The rhombic-lanceolate to elliptic leaf (vs. broadly elliptic to nearly rounded), purple corolla (vs. yellow), absent staminode (vs. 1), and pistil glabrous (vs. puberulous) in the new species differentiates it from O. rubrostriata (Table 1).
Type: —CHINA. Hunan Province: Huayuan County, Huayuan town, Kadi, on the rock of forests, 28°35′44″N, 109°25′15″E, 220 m alt., 3 November 2012, Tao Deng & Daigui Zhang 4015 (holotype: KUN; isotype: KUN, JIU).
Perennial herb with rhizomatous short stem, ca. 2.5 × 1.5 cm. Leaves in basal rosette. Petioles up to 3 cm long, densely rust-brown strigose; leaf blade elliptic or rhombic-lanceolate, 5–8 × 3.5–5 cm, apex obtuse, base sometimes oblique, cuneate or rounded, margin double dentate, crenate with conspicuous rust-brown pubescence, adaxial surface densely to sparsely rust-brown trichomes, abaxial surface villous; lateral veins 4–6 pairs on each side of midrib, rust-brown trichomes along veins. Cymes 3–12-flowered. Peduncles ca. 6 cm long, second hypopodium ca. 2 cm long, pedicel 1–1.5 cm; peduncles and pedicles rust-brown villous, glandular pubescent. Bracts 2, oblong to linear lanceolate, 5–6 × 1.2–1.7 mm, apex obtuse or acuminate. Calyx 5-lobed, lobes ovate-lanceolate or linear-lanceolate, with 3 veins, 4 mm × 1–1.2 mm, apex cuspidate, villous outside and glabrous inside, spread backward. Corolla purple, 1–1.3 cm long, outside glabrous; corolla tube cylindric and has a slight dorsal hump at throat, 8 × 4–5 mm; limb distinctly two-lipped, adaxial lip always bilobed, semicircular, 4–4.5 × 3–4 mm; abaxial lip usually trilobed, sometimes tetralobed, but the number of stamens strongly correlated with corolla abaxial lip lobes (4 stamens - abaxial lip trilobed, 5 stamens - abaxial lip tetralobed, Fig. 1F, G, H), slightly recurved, 2.2–3 × 1.5–2 mm. Stamens usually 4 (sometimes 5), stamens free, mature stamens exserted or equal to corolla, upper stamens ca. 5 mm long, lower stamens ca. 3 mm long, stamens adnate to corolla from base, filaments flattened and glabrescent, staminode absent. Anthers ovate, ca. 1 mm long, locule subparallel, confluent at apex; adnate to corolla 1.5 mm above. Disc ca. 1 mm, margin crisped. Pistil glabrous, higher than corolla, ovary linear-oblong, ca. 3.5 × 0.6 mm, style short, ca. 1.5 mm long, stigma 2-lobed. Capsule glabrous, ca. 3.5 cm long.
Distribution and habitat: — Oreocharis xieyongii was only found in rocky areas within forests in the Wuling Mountains at elevations of ca. 250 m (China, Hunan Province, Huanyuan County) (Fig. 5).

|
| Fig. 5 Map to show the collection point (pentagram) of Oreocharis xieyongii T. Deng, D.G. Zhang & H. Sun. |
Phenology: — Oreocharis xieyongii was observed flowering in September and fruiting between October and November.
Etymology: — The epithet of this new species refers to Mr. Yong Xie who is the president of the famous cosmetics brand “Dr Plant” and has made a significant contribution to public education of biodiversity.
Vernacular name: — Chinese mandarin: Xiè yǒng mǎ líng jù tái (解勇马铃苣苔).
Conservation status: — Oreocharis xieyongii is so far only known from the type locality. The total distribution area of this species is approximately 10 km2 and there is a population size of around 500 or more mature individuals. However, we feel the data are incomplete, and the new species is categorized as ‘Data Deficient’ (DD) according to IUCN criteria (2019).
Author contributionsH.S., T.D., D.G.Z. collected specimens. Z.Y.L., Y.Z.Z. and N.L. performed the morphometrics, molecular analysis. Z.Y.L. and Z.Y. wrote the paper. X.S.Z. drew the picture. H.S., T.D. and K.T. revised the paper.
Declaration of competing interestThe authors no conflicts of interest.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.11.008.
AcknowledgmentsWe are grateful to Michael Möller and Sergey Volis for their comments and linguistic help. This study was supported by grants from the National Natural Science Foundation of China-Yunnan joint fund to support key projects (U1802232), the Major Program of NSFC (31590823), NSFC (32170215), the Youth Innovation Promotion Association CAS (2019382), the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2019HB039), the Second Tibetan Plateau Scientific Expedition and Research (STEP) program (2019QZKK0502) and the CAS “Light of West China” Program.
Alexandros, S., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Bentham, G., 1876. Gesneriaceae. In: Bentham, G., Hooker, J.D. (Eds.), Genera Plantarum 2. Reeve & Co., London, pp. 990-1025
|
Cai, L., Guo, Y., Zhang, R.M., et al., 2019. Oreocharis panzhouensis (Gesneriaceae), a new species from karst regions in Guizhou, China. Phytotaxa, 393: 287-291. DOI:10.11646/phytotaxa.393.3.5 |
Cai, L., Liu, F.P., Yi, X.B., et al., 2020. Oreocharis wumengensis, a new species of Gesneriaceae from northeastern Yunnan, China. PhytoKeys, 157: 113-119. DOI:10.3897/phytokeys.157.33071 |
Chen, G.X., Liu, S.B., Ao, C.Q., et al., 2003. On endemic genera to China of spermatophytic flora from Mt. Wulingshan region. Acta. Bot. Boreali-Occ., 24: 865-871. DOI:10.3321/j.issn:1000-4025.2004.05.020 |
Chen, W.H., Shui, Y.M., Yang, J.B., et al., 2014. Taxonomic status, phylogenetic affinities and genetic diversity of a presumed extinct genus, Paraisometrum W.T. Wang (Gesneriaceae) from the Karst Regions of Southwest China. PLoS One, 9: e107967. DOI:10.1371/journal.pone.0107967 |
Chen, W.H., Zhang, Y.M., Guo, S.W., et al., 2020. Reassessment of Bournea Oliver (Gesneriaceae) based on molecular and palynological evidence. PhytoKeys, 157: 27-41. DOI:10.3897/phytokeys.157.55254 |
Chen, W.H., Zhang, Y.M., He, D.M., et al., 2020. Four new species of Oreocharis (Gesneriaceae) in Yunnan province, China. PhytoKeys, 157: 83-99. DOI:10.3897/phytokeys.157.32284 |
Darriba, D., Taboada, G.L., Doallo, R., et al., 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods, 9: 772. DOI:10.1038/nmeth.2109 |
Du, C., Liao, S., Boufford, D.E., et al., 2020. Twenty years of Chinese vascular plant novelties, 2000 through 2019. Plant Divers., 42: 393-398. |
Fenster, C.B., Armbruster, W.S., Wilson, P., et al., 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst., 35: 375-403. DOI:10.1146/annurev.ecolsys.34.011802.132347 |
Fu, Q., Xia, Y., Guo, Y., et al., 2019. Oreocharis odontopetala, a new species of Gesneriaceae from Guizhou, China. PhytoKeys, 124: 1-9. DOI:10.3897/phytokeys.124.34609 |
Fu, Q., Guo, Y., Huang, R., et al., 2019. Oreocharis ovatilobata (Gesneriaceae), a new species from Guizhou, China. Ann. Bot. Fenn., 56: 259-265. DOI:10.5735/085.056.0411 |
Grant, V., 1950. The flower constancy of bees. Bot. Rev., 16: 379-398. DOI:10.1007/bf02869992 |
Guo, Z.Y., Li, Z.Y., Xiang, X.G., 2018. Oreocharis duyunensis (Gesneriaceae), a new species from Guizhou, China. Nord. J. Bot., 36: e01514. DOI:10.1111/njb.01514 |
Ho, P.H., 2000. Gesneriaceae. In: Ho, P.H. (Ed.) An Illustrated Flora of Vietnam, vol. 3 Ho Chi Minh City Youth Publishing House, Ho Chi Minh City, pp. 12-29
|
IUCN Standards and Petitions Subcommittee, 2019. Guidelines for using the IUCN red list categories and criteria. Ver., 13: 113. |
Jiang, N., Huang, Y., He, J.Y., et al., 2020. Quantity and distribution of nature protected areas in the Wuling Mountains Region. Int. J. Ecol., 9: 159-172. DOI:10.12677/IJE.2020.92020 |
Katoh, K., Standley, D.M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol., 30: 772-780. DOI:10.1093/molbev/mst010 |
Li, Z.Y., Wang, Y.Z., 2004. Plants of Gesneriaceae in China. Henan Science & Technology Publishing House, Zhengzhou, 721 pp
|
Liao, W.B., Ao, C.Q., Liu, W.Q., et al., 2002. Study on character and feature of seed plants flora of Wuling Mountains region. Bull. Bot. Res., 22: 98-120. DOI:10.3969/j.issn.1673-5102.2002.01.024 |
Ling, S.J., Guan, S.P., Wen, F., et al., 2020. Oreocharis jasminina (Gesneriaceae), a new species from mountain tops of Hainan Island, South China. PhytoKeys, 157: 121-135. DOI:10.3897/phytokeys.157.50246 |
Ling, S.J., Meng, Q.W., Tang, L., et al., 2017. Pollination syndromes of Chinese Gesneriaceae: a comparative study between Hainan island and neighboring regions. Bot. Rev., 83: 1-15. DOI:10.1007/s12229-017-9181-6 |
Maddison, W.P., Maddison, D.R., 2018. Mesquite: A modular system for evolutionary analysis, version 3.61. http://mesquiteproject.org/
|
Martén-Rodríguez, Quesada, M., Castro, A.A., Lopezaraiza-Mikel, M., Fenster, C.B., 2015. A comparison of reproductive strategies between island and mainland Caribbean Gesneriaceae. J. Ecol., 103: 1190-1204. DOI:10.1111/1365-2745.12457 |
Möller, M., Forrest, A., Wei, Y.G., et al., 2011. A molecular phylogenetic assessment of the advanced Asiatic and Malesian didymocarpoid Gesneriaceae with focus on non-monophyletic and monotypic genera. Plant Syst. Evol., 292: 223-248. DOI:10.1007/s00606-010-0413-z |
Möller, M., Middleton, D., Nishii, K., et al., 2011. A new delineation for Oreocharis incorporating an additional ten genera of Chinese Gesneriaceae. Phytotaxa, 23: 1-36. DOI:10.11646/phytotaxa.23.1.1 |
Möller, M., Wei, Y.G., Wen, F., et al., 2016. You win some you lose some: updated generic delineations and classification of Gesneriaceae – implications for the family in China. Guihaia, 36: 44-60. DOI:10.11931/guihaia.gxzw201512015 |
Möller, M., Nampy, S., Janeesha, A.P., et al., 2017. The Gesneriaceae of India: consequences of updated generic concepts and new family classification. Rheedea, 27: 23-41. DOI:10.22244/rheedea.2017.27.1.5 |
Möller, M., Atkins, H.J., Bramley, G.L.C., et al., 2018. Two new species of Oreocharis (Gesneriaceae) from northern Vietnam. Edinb. J. Bot., 75: 309-319. DOI:10.1017/s0960428618000148 |
Möller, M., 2019. Species discovery in time: an example from Gesneriaceae in China. Guangxi Sci., 26: 1-16. DOI:10.13656/j.cnki.gxkx.20190307.002 |
Ohwi, J., 1965. Gesneriaceae. In: Meyer, F.G., Walker, E.H. (Eds.) Flora of Japan. Smithsonian Institution, Washington, D.C., pp. 812-813
|
Pan, B., Tang, G.D., Do, T.V., et al., 2019. Oreocharis tetrapterus (Gesneriaceae), a new species from east Guangxi, China. PhytoKeys, 131: 83-89. DOI:10.3897/phytokeys.131.35434 |
Pellegrin, F., 1930. Gesneriaceae. In: Lecomte, H. (Ed.) Flore generale de L’ Indo-Chine, vol. 4. Masson & Cie., Paris, pp. 487-565
|
Qin, W.H., Ding, D.D., Li, Z.L., et al., 2020. Oreocharis flavovirens, a new species of Gesneriaceae from southern Gansu province, China. PhytoKeys, 157: 101-112. DOI:10.3897/phytokeys.157.31732 |
Rodriguez-Girones, M.A., Santamaria, L., 2004. Why are so many bird flowers red?. PLoS Biol., 2: e350. DOI:10.1371/journal.pbio.0020350 |
Ronquist, F., Uelsenbeck, J.P., 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572-1574. DOI:10.1093/bioinformatics/btg180 |
Rosas-Guerrero, Víctor, Aguilar, Ramiro, Martén-Rodríguez, Silvana, et al., 2014. A quantitative review of pollination syndromes: do floral traits predict effective pollinators?. Ecol. Lett., 17: 388-400. DOI:10.1111/ele.12224 |
Tur, C., Olesen, J.M., Traveset, A., 2015. Increasing modularity when downscaling net-works from species to individuals. Oikos, 124: 581-592. DOI:10.1111/oik.01668 |
Wang, W.T., Pan, K.Y., Li, Z.Y., 1990. Gesneriaceae. In: Wang, W.T. (Ed.) Flora Reipublicae Popularis Sinicae, Vol. 69. Science Press, Beijing, pp. 141-271
|
Wang, W.T., Pan, K.Y., Li, Z.Y., et al., 1998. Gesneriaceae. In: Wu, Z.Y., Raven, P.H. (Eds.) Flora of China, vol. 18. Science Press and St. Louis: Missouri Botanical Garden Press. Beijing. pp. 268-272
|
Wei, Y.G., Wen, F., Moller, M., et al., 2010. Gesneriaceae of South China. Guangxi Science and Technology Publishing House, Yanshan, Guilin, Guangxi, 777 pp
|
Wen, F., Li, S., Xin, Z.B., et al., 2019. The updated plant list of Gesneriaceae in China under the new Chinese naming rules. Guangxi Sci., 26: 37-63. DOI:10.13656/j.cnki.gxkx.20190225.002 |
Wu, Z.Y. (1991). Gesneriaceae. Flora Yunnanica, vol. 5. Science Press, Beijing, pp. 512-687
|
Yang, L.E., Cen, H.F., Sun, H., et al., 2019. Oreocharis rubrostriata (Gesneriaceae), a new species from Guangxi, China. Kew Bull., 74: 23. DOI:10.1007/s12225-019-9810-9 |
Yang, L.H., Wen, F., Kong, H.H., et al., 2020. Two new combinations in Oreocharis (Gesneriaceae) based on morphological, molecular and cytological evidence. PhytoKeys, 157: 43-58. DOI:10.3897/phytokeys.157.32609 |



