b. University of Chinese Academy of Sciences, Yuquanlu, 19A, Beijing, 100049, China;
c. National Authority for Preah Vihear, Choam Khsant, 13403, Cambodia;
d. Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, AZ, 85721, USA;
e. South China Limestone Plants Research Centre, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, 510642, China
Symbiotic relationships between plants and animals have always captivated evolutionary biologists (Darwin, 1862; Thompson, 1994). To understand the evolution of specialization, many studies have focused on brood pollination mutualisms between plants and pollinators as model study systems. Such mutualisms include the associations between figs and fig wasps (Moraceae: Ficus L., Hymenoptera: Agaonidae; Janzen, 1979; Herre et al., 2008), yuccas and yucca moths (Asperagaceae: Yucca L., Hesperoyucca (Engelmann) Baker; Lepidoptera: Prodoxidae: Tegeticula Zeller, Parategeticula Davis; Riley, 1892; Pellmyr, 2003; Rentsch and Leebens-Mack, 2014) and leafflower plants and leafflower moths (Phyllanthaceae: Phyllanthus L. s.l., Lepidoptera: Gracillariidae: Epicephala Meyrick; Kato et al., 2003; Kawakita and Kato, 2006; Hembry et al., 2013a, 2018; Luo et al., 2017).
Brood pollination mutualisms are mutualisms in which the plant provides a portion of its developing seeds as nourishment for the pollinator's offspring (larvae) as a reward for pollination services (Hembry and Althoff, 2016; Kawakita and Kato, 2017). It has been reported that leafflower moths (Lepidoptera: Gracillariidae: Epicephala Meyrick) and several host plant species in the family Phyllanthaceae are engaged in obligate pollination mutualisms (OPMs) (Kato et al., 2003; Kawakita and Kato, 2004a; Okamoto et al., 2013). To date, these OPMs have been observed in species from the genera Glochidion J.R. Forst. & G. Forst., Breynia J.R. Forst. & G. Forst., and Phyllanthus (Kato et al., 2003; Kawakita and Kato, 2004a,b; Hembry et al., 2012; Zhang et al., 2012; Okamoto et al., 2013; Kawakita et al., 2015).
Glochidion is a monoecious tree genus in Phyllanthaceae which has tiny apetalous female flowers with extremely specialized styles (Airy Shaw, 1978; Chakrabarty and Gangopadhyay, 1995). This genus comprises over 300 described species that are distributed in the subtropical and tropical Asia–Pacific region, with a few species in temperate Asia (Govaerts et al., 2000; Hoffmann and McPherson, 2003; Webster, 2014; Angiosperm Phylogeny Group, 2016). Species in this genus engage in obligate pollination mutualisms with species-specific, seed-parasitic leafflower moths (Kato et al., 2003; Kawakita et al., 2010). At night, female leafflower moths actively pollinate the flowers, collecting pollen from male flowers using their specialized proboscises and depositing the pollen on the stigmas of female flowers. After pollination, female leafflower moths oviposit in pollinated flowers so that when the flowers develop into fruits, hatched larvae can eat a portion of the developing seeds and leave a subset of seeds intact for plant reproduction (Kato et al., 2003). The pollination behavior of leafflower moths associated with Glochidion shares similarities to the obligate associations between other species of leafflower moth and close relatives of Glochidion in the family Phyllanthaceae (Kawakita and Kato, 2004a,b).
There is the possibility that many more species in Phyllanthaceae engage with OPMs that remain to be described (Kawakita and Kato, 2004a; Cooper and Cooper, 2013). So far, the mutualistic relationship between Glochidion and leafflower moths has been documented from Japan (Kato et al., 2003; Kawakita and Kato, 2006), China (Luo et al., 2017), Australia (Henderson et al., 2020) and French Polynesia (Hembry et al., 2012, 2013a, 2018). All of these locations are around the margins of the global distribution of Glochidion in the Asia–Pacific region. The center of diversity of Glochidion (~200 species; Govaerts et al., 2000) is tropical Southeast Asia and New Guinea, where up to nine species can co-occur at one locality (Hembry et al., 2013a). Although leafflower moths have been reported reared from Glochidion fruits in several Southeast Asian countries (Laos, Vietnam, Myanmar, and Malaysia; Kawakita et al., 2004; Hembry et al., 2013a), basic biological observations of its behavior and host-specificity are lacking from this region where its hosts, and presumably it as well, are most diverse. Consequently, it is not clear if the biology of this interaction as described from temperate and subtropical Asia and Australia, and the Pacific islands, are representative of this association in its center of diversity. Furthermore, even if mutualistic, we have little data on interspecific differences in the ecology of the interactions between different Glochidion and different leafflower moths, particularly with regards to the number of larvae per fruit and the extent of their seed damage, or the presence of parasitic (non-pollinating) species (Kawakita et al., 2015; Li et al., 2015), which has great implications for the stability and diversity of this mutualism. Here, we report the first detailed descriptions of the ecology and species-specificity of the Glochidion-leafflower moth association in a tropical Asian country, Cambodia. Cambodia has thirteen reported species of Glochidion (Cho et al., 2016). Here we report investigations of the pollination biology and interactions with leafflower moths of five Cambodian Glochidion species. Specifically, we aimed to: (a) observe pollination behavior, (b) investigate the cost of mutualism (the fraction of seeds consumed by leafflower moth larvae and number of larvae per fruit) to the host plant, and (c) assess phylogenetic relationships among the moths associated with different Glochidion hosts. Taken together, these three aims allow us to obtain fundamental information about the biology of this interaction: the diversity of Epicephala moths associated with five species of Cambodian Glochidion, whether or not they are mutualistic pollinators of their hosts, and the extent to which the ecology of this interaction varies among different species pairs.
2. Methods 2.1. Research sites and organismsWe studied the pollination biology of five Glochidion species, Glochidion coccineum (Buch.-Ham.) Müll.Arg., Glochidion littorale Blume, Glochidion sp. 1, Glochidion glomerulatum (Miq.) Boerl., and Glochidion rubrum Blume, at six sites across Cambodia (Preah Vihear Heritage Site [PVHS], Phnom Kulen National Park [PKNP], Kirirom National Park [KRNP], Veal Renh [VR], Ream National Park [RNP], and Bokor National Park [BKNP]; Fig. 1; Table 1) during the periods 12th October to 13th November 2019 and 7th January to 28th March 2020.
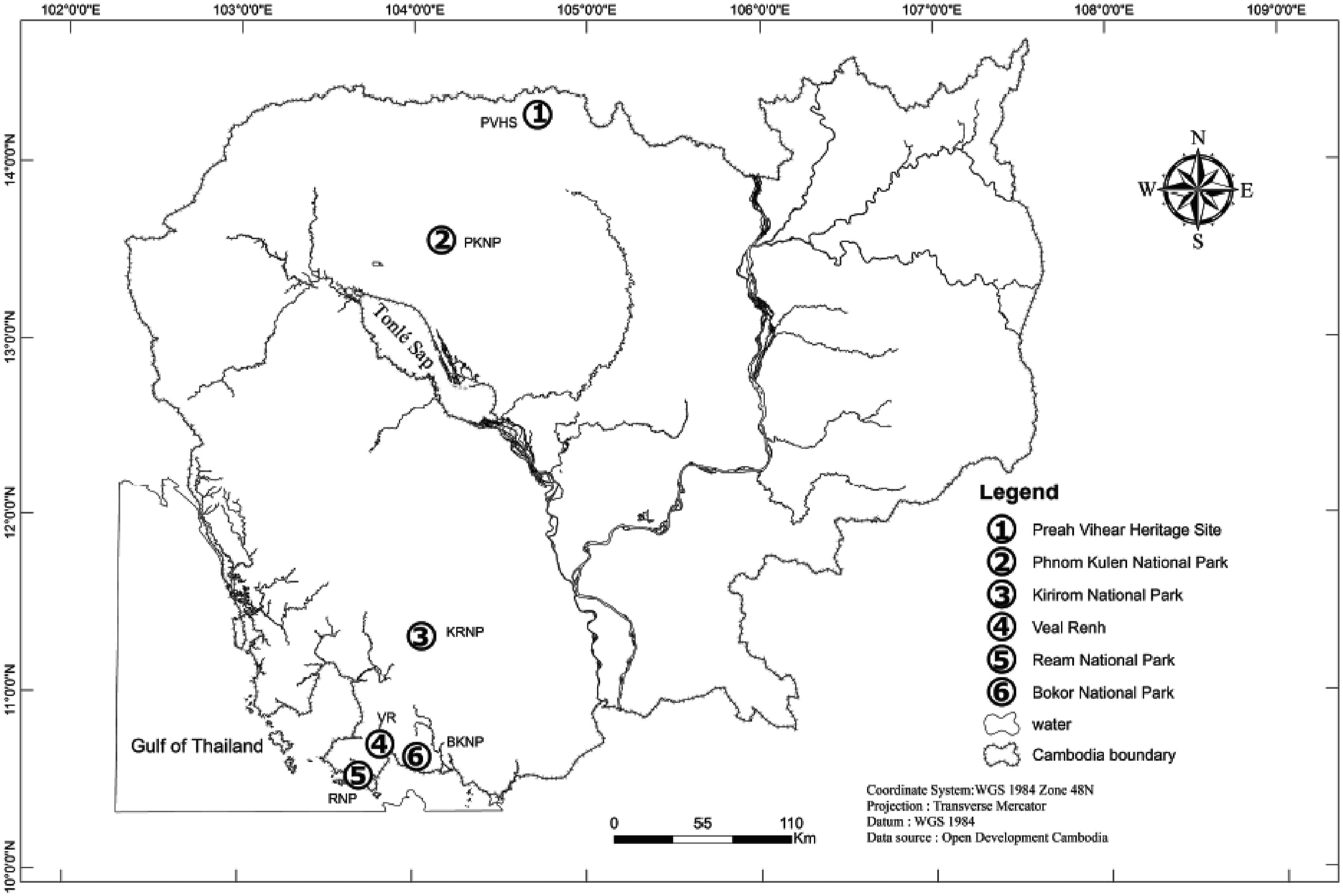
|
| Fig. 1 Map of study sites in Cambodia. The six sites, Preah Vihear Heritage Site (PVHS), Phnom Kulen National Park (PKNP), Kirirom National Park (KRNP), Veal Renh (VR), Ream National Park (RNP), and Bokor National Park (BKNP), are indicated. |
| Glochidion species | Preah Vihear Heritage Site (PVHS) | Phnom Kulen National Park (PKNP) | Kirirom National Park (KRNP) | Veal Renh (VR) | Ream National Park (RNP) | Bokor National Park (BKNP) | ||||||||||||||||||||||||||||
| G.coccineum | ✓ | |||||||||||||||||||||||||||||||||
| G.littorale | ✓ | ✓ | ✓ | |||||||||||||||||||||||||||||||
| G. sp. 1 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||||||||||||||||
| G.glomerulatum | ✓ | |||||||||||||||||||||||||||||||||
| G.rubrum | ✓ | |||||||||||||||||||||||||||||||||
| A check or tick mark (✓) indicates the presence of a species at a site. | ||||||||||||||||||||||||||||||||||
Glochidion coccineum is an evergreen shrub or treelet, usually about 4–10 m high. This species usually grows in disturbed forests, along roadsides or near streams (Fig. 2a). Male flowers are yellow with 6 sepals and 6 stamens (Fig. 2b). The male flowers are aggregated at the proximal ends of branches, whereas female flowers typically are more common along the distal parts of branches and have very short pedicels and 6 sepals (Fig. 2c). The fruit is lobed, and the ovary has 7 to 12 locules (Fig. S1c) (van Welzen, 2007). The flowering period is from February to October, and the fruiting period is from August to January (at our study site). This species was found and collected from Preah Vihear Heritage Site [PVHS] (104°42′54.70″ E, 14°16′44.85″ N) (Fig. 1) where the forest holds a diversity of deciduous plant species representative of tropical deciduous forest ecosystems in the northern and northeastern regions of Cambodia. The fruits were collected to rear Epicephala moths in October 2019. The total of 370 sampled fruits from 15 individual trees were dissected and the pollination behavior observation was made on 13th to 18th January 2020 and on 1st to 5th March 2020.

|
| Fig. 2 Flowers and associated insects of Glochidion coccineum and G. littorale. (a) habitat of G. coccineum; (b) maleflower of G. coccineum; (c) femaleflower of G. coccineum; (d) female Epicephala moth collecting pollen at a male flower of G. coccineum; (e) female Epicephala moth pollinating a female flower of G. coccineum; (f) female Epicephala moth ovipositing into a female flower of G. coccineum; (g) Epicephala larva in a dissected fruit of G. coccineum; (h) Habitat of G. littorale; (i) male flower of G. littorale; (j) female flower of G. littorale; (k) Epicephala moth pollinating female flower of G. littorale; (l) Epicephala moth visiting G. littorale; (m) three Epicephala larvae in a dissected fruit of G. littorale. |
Glochidion littorale is a shrub up to 6 m in height, which grows at low elevations near the coast (Fig. 2h). Male flowers have 6 sepals and 5 or 6 stamens and a pedicel 5.5–6 mm long (Fig. 2i). The fruit is round, apically flattened, and depressed in the center, sometimes also basally flattened (Fig. S1b). The ovary has 10 to 14 locules (van Welzen, 2007). This species was found and collected from three locations: Veal Renh (103°48′59.98″ E, 10°42′34.28″ N), Bokor National Park (104° 1′39.51″ E, 10°38′22.93″ N), and Ream National Park (103°41′40.07″ E, 10°31′57.05″ N) (Fig. 1). These three locations are representative of evergreen forest in southern Cambodia near the ocean with both low- and medium-elevation vegetation (Emerton et al., 2002). The fruits were collected to rear Epicephala moths in November 2019 from Veal Renh, Bokor National Park and Ream National Park. The total of 212 dissected fruits from 9 individuals were sampled from Veal Renh and Ream National Park on 17th to 28th February 2020. Pollination observations were made on 23rd to 28th February 2020 and on 11th to 13th March 2020 at Veal Renh and Ream National Park.
Glochidion sp. 1 is a shrub which is determined to be a distinct species by us and by personal communication with Dr. Peter van Welzen (Naturalis Biodiversity Center, Netherlands). It grows along roadsides and in open forest (Fig. S1a). The male flowers are yellow with 6 sepals and 3 stamens (Fig. 3a). The ovary has 3 locules. The fruit is hairy and slightly lobed (Fig. 3e). This species was found at almost all of our study areas except for Kirirom National Park. The fruits were collected to rear Epicephala moths in October and November 2019. The total of 320 dissected fruits from 10 individual trees were collected from Preah Vihear Heritage Site, Veal Renh and Ream National Park. Pollination observations were made on 17th to 22nd February 2020 and on 8th to 10th March 2020.

|
| Fig. 3 Flowers, fruits and associated insects of Glochidion sp. 1. (a) maleflower; (b) femaleflower; (c) female Epicephala moth pollinating a female flower; (d) female Epicephala moth ovipositing into female flower; (e) fruits; (f) two Epicephala larvae in a dissected fruit. |
Glochidion glomerulatum is a treelet up to 8 m in height. It grows along streams at medium elevation (up to 300 m) at our study site (Fig. S1f). Flowers are pale green with 6 sepals and 3 stamens (Figs. S1g and h). The ovary has 3 locules. Fruits are hairy, slightly lobed, circular, and flattened (Fig. S1i) (van Welzen, 2007). This species was found at Phnom Kulen National Park (104° 9′37.35″ E, 13°34′10.53″ N) (Fig. 1) where there is a combination of evergreen, semi-evergreen, and deciduous dipterocarp forest (Hayes et al., 2013). The fruits were collected to rear Epicephala moths in November 2019 and the total of 25 sampled fruits from 2 individual trees were collected for dissection in January 2020.
Glochidion rubrum is a shrub or treelet up to 5 m in height. It grows along roadsides and forest edges at medium to high elevations (about 500–700 m) at our study site (Fig. S1k). Its male flowers have 6 sepals and 3 stamens (Fig. S1l). Its sepals are strongly ovate to elliptic to obovate. The ovary has 3 locules, and the fruit is glabrous (Fig. S1o). This species was found and collected from Kirirom National Park (104°3′10.56″ E, 11°19′12.87″ N) (Fig. 1). The park comprises lowland evergreen and deciduous forest and some medium-altitude evergreen forest (Emerton et al., 2002). The fruits were collected to rear Epicephala moths in October 2019 and the total of 50 sampled fruits from 2 individual trees were collected on 8th January 2020 for dissection.
2.2. Pollinator observationsPollination behavior was observed, focusing on three species: Glochidion coccineum, G. littorale, and G. sp. 1. We conducted observations of flower visitors. Particular effort was made to focus on any flower visitation by Epicephala moths at night. After they had been observed pollinating female flowers, Epicephala moths were caught to check if pollen grains were attached to their proboscises.
Due to the lack of prior detailed studies of Glochidion species at our study sites, we used photos of Glochidion plants to interview local people in order to locate trees growing in the wild. Then plant phenology was reported to us by local people; we aimed to keep track of flowering time in order to be able to make flower observations at suitable times. Nocturnal flower observations were made during the period 7:00 pm to 11:00 pm using a yellow flashlight to see flower-visiting insects and any pollination behavior. We spent 60 h for nocturnal observations and 20 h for diurnal observations. To detect pollen grains on Epicephala proboscises and record their pollination behavior, we used a PENTAX WG-3 model camera to record both photos and videos.
2.3. Fruit dissectionsTo assess the extent of seed destruction by Epicephala larvae, mature fruits of these five Glochidion species were collected from various individuals and locations for dissection (Table 2). When dissecting, we recorded the number of infested seeds within each mature fruit and noted the cause of seed destruction and the number of Epicephala larvae per each fruit. Seeds which were infested by other insect larvae were discarded and not included in the datasets presented in this study. To avoid a situation in which larvae emerge from the fruits before dissection, we tried to dissect the fruits immediately after they were collected from the field, although we recognize that this may undercount total seed damage by larvae that had not completed development. Our data were then compared with equivalent data from other studies on Phyllanthaceae-Epicephala interactions (Kato et al., 2003; Kawakita and Kato, 2004a,b; Goto et al., 2010; Furukawa et al., 2017; Luo et al., 2017; Finch et al., 2019; Henderson et al., 2020).
| Glochidion species | Number of sampled fruits | Number of infested fruits | Mean number of intact seeds per fruit (SD) | Mean percentage of intact seeds per fruit (SD) | Mean number of infested seeds per fruit (SD) | Mean percentage of infested seeds per fruit (SD) | Mean number of ovules per fruit (SD) | Mean number of sterile/aborted seeds per fruit (SD) | ||||||||||||||||||||||||||||||||||||
| G.coccineum | 370 (N=15) | 328 | 7±3.52 | 58±22.81 | 5±2.6 | ∗42±22.81 | 16±2.45 | 5±2.51 | ||||||||||||||||||||||||||||||||||||
| G.littorale | 212 (N=9) | 192 | 13±5.55 | 55±23.07 | 11±5.75 | ∗45±23.07 | 24±1.79 | 2±1.23 | ||||||||||||||||||||||||||||||||||||
| G. sp. 1 | 320 (N=10) | 244 | 3±1.19 | 52±20.39 | 3±1.23 | ∗48±20.39 | 6 ± 0.0 | 1±0.42 | ||||||||||||||||||||||||||||||||||||
| G.glomerulatum | 25 (N=2) | 18 | 3±0.43 | 54±7.13 | 3±0.43 | 46±7.13 | 6 ± 0.0 | 1 ± 0.0 | ||||||||||||||||||||||||||||||||||||
| G.rubrum | 50 (N=4) | 31 | 3±0.6 | 54±10.3 | 3±0.6 | 46±10.3 | 6 ± 0.0 | 2±0.5 | ||||||||||||||||||||||||||||||||||||
| Asterisk (∗) indicates that totals include data from fruits with one larva and fruits with more than one larva. N indicates the number of tree individuals. | ||||||||||||||||||||||||||||||||||||||||||||
To determine the phylogenetic relationship of the moths associated with different Glochidion hosts, we initially conducted fine-scale sampling of 1–2 larvae or moth individuals per tree, for 4–13 trees per species per site (similar to methods of Hembry et al., 2018). Sites are defined as a circle of area 1000 km2 (radius 18 km), to accommodate dispersal by Epicephala adults or Glochidion seeds, following a previous study (Hembry et al., 2018).
We analyzed nucleotide sequence variation among the larvae and moths reared from fruits of five different Glochidion species from various locations: G. coccineum (N = 13), G. littorale (N = 28), G. sp. 1 (N = 30), G. glomerulatum (N = 5), and G. rubrum (N = 7). Total DNA was extracted from adults and larvae using a standard DNeasy Tissue Kit (Qiagen, Hilden, Germany) protocol. For each larva and adult, we amplified and Sanger-sequenced three loci, mitochondrial cytochrome oxidase I (COI), nuclear elongation factor 1-α (EF1-α) and arginine kinase (ArgK), following protocols from past studies on Epicephala (Hembry et al., 2013a, 2018; Kato et al., 2003; Kawakita et al., 2004; Luo et al., 2017). A fragment of COI, ArgK, and EF1-α were PCR-amplified by using the following primers: for COI, 5′-ATAATTTTTTTTATAGTTATAC-3′ and 3′-GATGGGCTCATACAATAAATCCTA-5' (Kato et al., 2003); for ArgK, 5′-ATTTAGACTCTGGTGTTGG -3′ and 3′-ATGCCGTCGTACATCTCCTT-5’ (Kawakita et al., 2004); for EF1-α, 5′-CCCATTTCKGGCTGGCAYGGAGA-3′ and 3′-GATTTACCRGWACGACGRTC -5' (Kawakita et al., 2004). To this novel dataset (GenBank accession numbers MZ393203–MZ393363, MZ393691–MZ393770), we added a sequence dataset of the same loci for other Epicephala taxa globally from previous studies (Hembry et al., 2013a; Kawakita et al., 2004; Kawakita and Kato, 2009; Luo et al., 2017), ensuring one representative for each Epicephala taxon reared from Glochidion for which published sequence data is available. We used GenBank sequences of the moth Calybites phasianipennella (Hübner) (Gracillariidae: Gracillariinae; a different subfamily than Epicephala) as the outgroup (Kawakita and Kato, 2009). We aligned sequences using MUSCLE (Edgar, 2010) and inferred phylogenetic relationships using maximum likelihood implemented in RAxML-HPC2 (Stamatakis et al., 2006) on the CIPRES server (Miller et al., 2010) setting the model of evolution to GTR + G.
3. Results 3.1. Observations of pollination behaviorFlowers were seldom visited by insects by day, whereas at night a few insects were observed walking past inflorescences. At the beginning of the evening at 7:00 pm, flowers started to release their odor, which attracts Epicephala moths; the odor was noticeable to human observers as a fragrant scent. Although we observed some insects (true bugs, Hemiptera) visiting male flowers and appeared to be eating pollen and tepals, Epicephala moths were the only flower visitors observed pollinating Glochidion flowers. Epicephala moths were observed being active from 8:00 pm to 10:00 pm (although we observed flowers from 7:00 pm to 11:00 pm). Female Epicephala moths first visited male flowers to collect numerous pollen grains (Fig. 2d) using their proboscises. After collecting pollen, Epicephala moths remained stationary on male flowers for a few minutes before flying to a female flower. After detecting a female flower, an Epicephala moth walked around the flower, then started to pollinate it using its specialized proboscis to deposit pollen into the recessed surface of its fused styles (Fig. 2e,k, and 3c). We observed Epicephala pollination behavior on three species of Glochidion: G. littorale (N = 2), G. coccineum (N = 7), and G. sp. 1 (N = 5). For G. coccineum and G. sp. 1, the process of pollination by a female moth took about 2–4 s, whereas one Epicephala moth observed pollinating G. littorale took about 10 min. Subsequently, after pollination behavior was finished, the female Epicephala moth bent its abdomen and inserted its long ovipositor into the narrow stigmatic pit of the female flower and laid eggs (Figs. 2f and 3d). During the day, we observed braconid wasps probing Glochidion fruit with their ovipositors, probably to oviposit in early instar Epicephala larvae (Fig. S1e).
3.2. Seed loss and the cost of mutualismOut of the sampled mature fruits of Glochidion coccineum (N = 370), G. littorale (N = 212), G. sp. 1 (N = 320), G. glomerulatum (N = 25), and G. rubrum (N = 50), 88.6%, 90.56%, 76.25%, 72%, and 62%, respectively, were infested by Epicephala larvae. Epicephala larvae were easily distinguished by their morphology (Figs. 2g, 2m, 3f, and S1p). Interestingly, we often found multiple larvae per fruit in G. coccineum, G. littorale, and G. sp. 1, while G. glomerulatum and G. rubrum had no more than one larva per fruit. 24%, 27%, and 3% of G. coccineum, G. littorale, and G. sp. 1 fruit, respectively, contained two Epicephala larvae, while 7% of G. coccineum fruit and 23% of G. littorale fruit contained three Epicephala larvae (Fig. 4a). The number of seeds consumed per fruit by Epicephala larvae varied across the five Glochidion species, while the number of seeds (Table 2) and the number of larvae per fruit also varied among species (Fig. 4a). Typically, a single larva did not consume all seeds within the fruit, while more than one Epicephala larvae often but not always destroyed all seeds within a fruit (Fig. 4b). The mean percentage of infested seeds consumed by a single larva were 32%, 27%, 49%, 46%, and 46% for G. coccineum, G. littorale, G. sp. 1, G. glomerulatum, and G. rubrum, respectively, whereas the mean percentage of infested seeds consumed by two larvae were 58%, 50%, and 92% for G. coccineum, G. littorale, and G. sp. 1, respectively. The mean percentage of infested seeds consumed by three larvae was 70% for both G. coccineum and G. littorale (Fig. 4b). Furthermore, the mean percentage of seeds consumed by all larvae within a fruit were 43%, 45%, and 48% for G. coccineum, G. littorale, and G. sp. 1, respectively (Table 2).
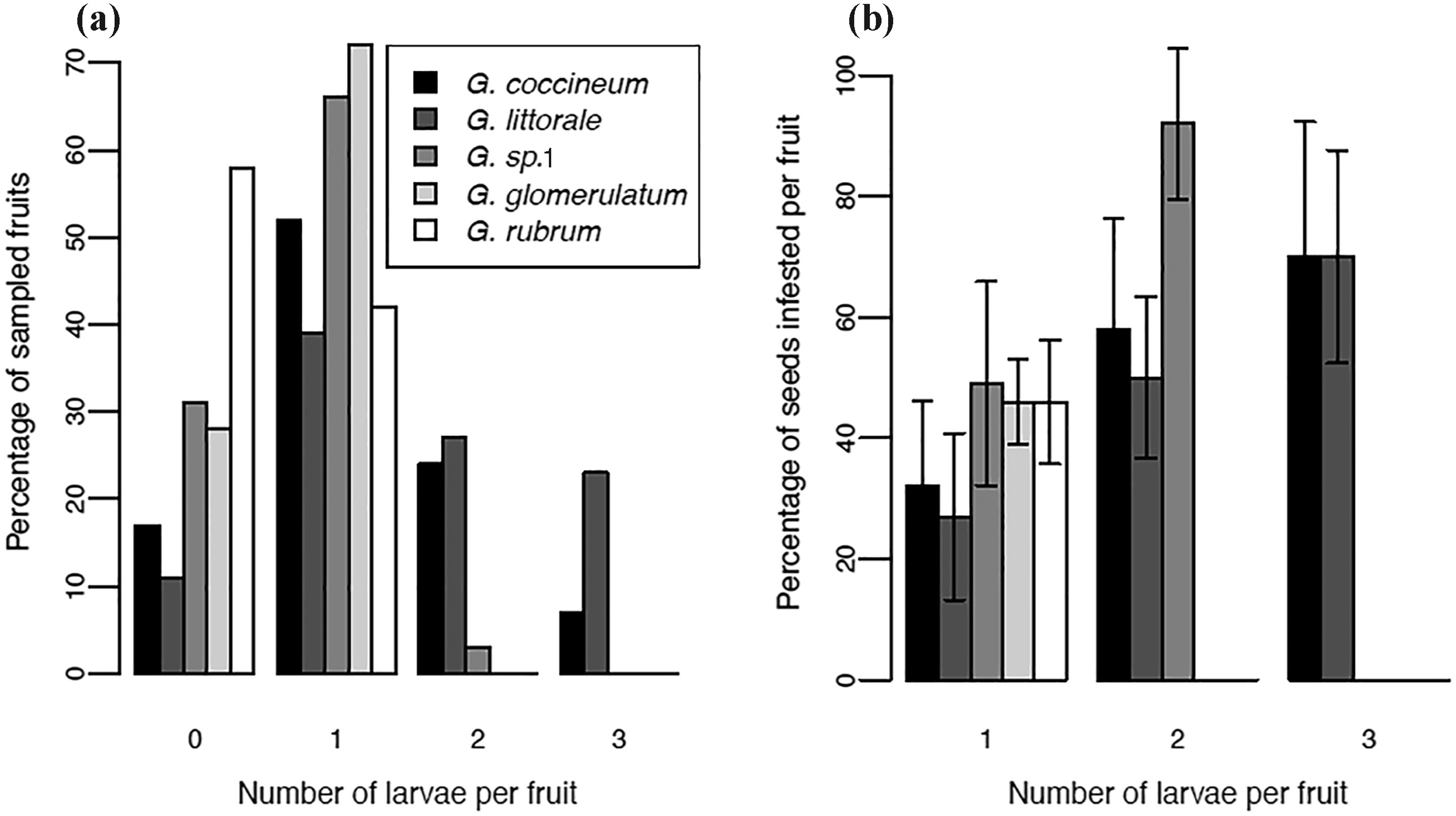
|
| Fig. 4 (a) Percentage of sampled fruits with different numbers of Epicephala larvae in five Glochidion species. (b) Mean percentages of seeds infested by different numbers of Epicephala larvae per fruit, in five Glochidion species. Error bars indicate standard deviations. |
Sanger-sequencing yielded 1775 bp of three combined genes (ArgK, COI, and EF1-α) per larva. Molecular phylogenetic analysis using ML revealed that Epicephala sampled in this study fall into five minimally monophyletic clades with high bootstrap support values (>99), likely indicating five different Epicephala species (here, “taxa”) associated with five different Glochidion species (Fig. 5). Each of these Cambodian Epicephala taxa is uniquely associated with a single species of host Glochidion; many of these Epicephala samples come from localities (PVHS, PKNP, VR, RNP, BKNP) where two Glochidion species co-occur (Table 1). The Epicephala specimens collected from G. littorale group in the phylogeny with a single specimen collected from the same host in Malaysia (Sarawak) in a previous study (Hembry et al., 2013a). Aside from the Epicephala associated with G. littorale, no Cambodian Epicephala specimens form minimally monophyletic clades with specimens collected in previous studies elsewhere in Asia. The Epicephala associated with G. glomerulatum in Cambodia are separated by several strongly supported nodes from an Epicephala specimen collected from the same host in Malaysia (Sarawak). All Epicephala collected in this study fall within the main Glochidion-associated clade of Epicephala; none are sister to the enigmatic and more distantly related E. lanceolaria (the pollinator of Glochidion lanceolarium in southern China; Luo et al., 2017).
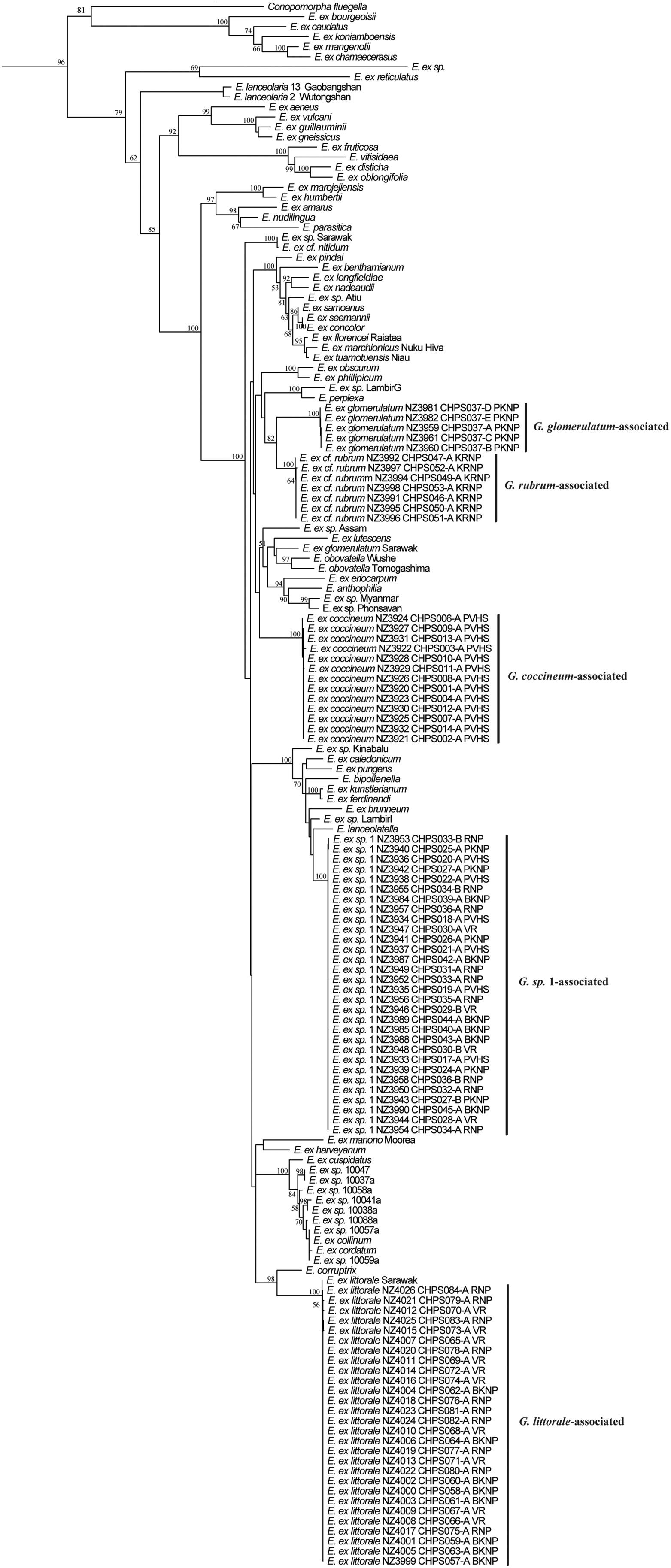
|
| Fig. 5 Phylogenetic hypothesis for Epicephala globally recovered using maximum likelihood (ML) and a sequencealignment of 1775 bp comprising three loci (ArgK, COI, and EF1-α). The five clades comprising Cambodian Epicephala specimens are indicated by the vertical bars and large-font labels indicating hostplants; other Epicephala taxa are from previous studies elsewhere in the world (Kawakita et al., 2004; Kawakita and Kato, 2009; Hembry et al., 2013a, Hembry et al., 2013b; Luo et al., 2017). ML was implemented in RAxML-HPC2 with 1000 bootstrap replicates. Tip labels indicate the species epithet of the moth species (where names are available) or the species epithet of the host plant from which the specimen was reared. Numbers above branches indicate bootstrap values; bootstrap values ≤ 50 are not shown for ease of reading. The outgroup (Calybites phasianipennella) and two non-Epicephala taxa (Stomphastis labyrinthica and Cuphodes diospyrosella) are pruned from this figure. |
Our study revealed that at least three and likely five Cambodian Glochidion species are pollinated by Epicephala moths. At night, Glochidion flowers attract Epicephala moths by releasing their odor (see Okamoto et al., 2007, 2013). After detecting the male flowers, the Epicephala moths actively transport the pollen between the flowers. Female Epicephala used their specialized proboscises to collect pollen and deposit it into the styles of the female flowers, and then laid eggs in the carpels of the female flower. This behavior is similar to that observed in Glochidion in temperate and subtropical Asia (Kato et al., 2003; Kawakita and Kato, 2006; Luo et al., 2017) and the Pacific islands (Hembry et al., 2012), as well as in Phyllanthus (Kawakita and Kato, 2004a) and Breynia (Kawakita and Kato, 2004b; Zhang et al., 2012; Finch et al., 2018). In most cases, we noticed that both pollination and oviposition behaviors were repeated at the same female flower. This constancy might ensure successful pollination and oviposition. Due to the difficulty of pollination behavior observation at night, we still do not know exactly whether moths collect pollen from male flowers and pollinate female flowers on the same tree, or move to another new tree after collecting pollen. Notably, our observation is consistent with those of Kawakita (2010) that a female Epicephala moth visits male flowers only once and successively visits several female flowers to pollinate and oviposit without revisiting male flowers. These five Glochidion species share similar basic pollination ecology and behavior with Glochidion acuminatum Müll.Arg. as described in Kato et al. (2003) and Kawakita (2010). Interestingly, despite the recent discovery of non-pollinating or galling non-mutualistic Epicephala in Asia (Kawakita et al., 2015; Li et al., 2015), we find no evidence that any of the moths discovered here are not mutualistic pollinators. These findings indicate that in the center of diversity of Glochidion in tropical Asia, the pollination ecology of this plant clade is the same in its broad aspects to that reported at the margins of its distribution in temperate and subtropical Asia, Australia, and the Pacific islands. Alongside the specialized, fused stylar morphology seen in of all Glochidion, it constitutes further evidence to suggest that all the ~300 species of Glochidion are pollinated by Epicephala moths (Kato et al., 2003).
4.2. Seed loss and the cost of mutualismA number of studies have measured the extent of seed damage by Epicephala larvae. It is difficult to compare these values directly across studies as they vary in the developmental stage of fruit, time after collection at which seed damage was assessed, the presence or absence of other Lepidoptera or braconid wasps (Hembry et al., 2013b), number of larvae per fruit, whether data are zero-inflated, and whether means or ranges, or standard deviations or standard errors, are reported for the data. Furthermore, some studies do not specify all of these aspects of data collection and analysis. Nevertheless, the ranges of seed infestation per fruit reported here for single Epicephala larvae (~20–70%) (Fig. 4b and Table S1) roughly overlap with those previously reported for other Epicephala associated with Glochidion (~15–80%) (Kato et al., 2003; Goto et al., 2010; Luo et al., 2017; Henderson et al., 2020) and other leafflowers (Tables S1 and S2) (Kawakita and Kato, 2004a,b; Furukawa et al., 2017; Finch et al., 2019). Intriguingly, the mean percentage of intact seeds per fruit is roughly similar across all five species (52–58%), despite variation in the number of total seeds and larvae per fruit (Table 2). Some fruits, however, in this study were found to lack Epicephala larvae (Fig. 4a). This is may be due to egg mortality (perhaps a result of egg predation). Another possibility is that Epicephala moths do not always oviposit in pollinated flowers, as reported in Phyllanthus (Kawakita and Kato, 2004a) and Breynia (Kawakita and Kato, 2004b). Finch et al. (2019) found that 6 of 59 sampled Breynia oblongifolia (Müll.Arg.) Müll.Arg. female flowers had been pollinated but appeared to completely lack Epicephala eggs and any tissue scarring of oviposition on flowers. In this study, although we did not dissect flowers to check for pollen and moth eggs, we suspect that Epicephala moths may not always oviposit in pollinated flowers in Glochidion (see Luo et al., 2017), consistent with observations in Phyllanthus (Kawakita and Kato, 2004a). One intriguing possibility is that a female Epicephala moth may oviposit in only some but not all flowers which it pollinates as a way of concealing larvae from braconid wasp attack, as previously suggested in Breynia (Kawakita and Kato, 2004b) and reported for other plant-herbivore-parasitoid interactions (Thompson, 1986, 1987). To vigorously evaluate these hypotheses, more detailed investigations of oviposition and moth pollination behavior, as well as parasitoid searching strategy, are needed.
In these Glochidion-Epicephala associations studied here, we found that a single Epicephala does not consume all the seeds in a fruit (as previously reported; Kato et al., 2003; Goto et al., 2010; Luo et al., 2017), while the presence of more than one Epicephala larvae can destroy all seeds (Fig. 4b). This suggests that the number of larvae in a fruit also depends on the number of ovules per fruit, as we rarely detect more than one Epicephala larva inside fruits of those species with fewer (e.g., six) ovules (Glochidion sp. 1, G. glomerulatum, and G. rubrum). In the fruits of G. sp. 1, we found that only 3% of fruits had two Epicephala larvae (N = 320 fruits), whereas G. glomerulatum (N = 50 fruits) and G. rubrum (N = 25 fruits) invariably had at most only one Epicephala larva per fruit (Fig. 4a). These findings are similar to past reports that indicate that the number of larvae in a Glochidion fruit usually ranges between zero and two, and is often only one (Kato et al., 2003; Goto et al., 2010; but see G. lanceolarium (Roxburgh) Voigt: Luo et al., 2017). G. coccineum and G. littorale, however, have up to three Epicephala larvae per fruit, indicating that there is considerable variation in the number of seeds and larvae per fruit among Glochidion species, and that seed and larval number may influence each other over the course of evolution, perhaps by an arms race mechanism in which trees increase seed number per fruit to escape seed predation and moths correspondingly lay more eggs per flower to take advantage of the increased number of seeds. Despite this among-species variation, the frequency with which the entire seed set per fruit is eaten by larvae is very low (Fig. 4b). It is unclear that whether these Glochidion species may engage in selective abortion of flowers with high loads of eggs to prevent excessive seed consumption, as reported in yuccas (Pellmyr and Huth, 1994; Richter and Weis, 1995; Wilson and Addicott, 1998; Addicott and Bao, 1999) and G. acuminatum (Goto et al., 2010). Our dissections of G. coccineum did reveal, however, that Epicephala larvae in the fruits of this species appear to be unable to gnaw through the walls of the carpel in which they hatch, suggesting that physical structures in the fruit may limit seed predation in this species, as has been reported in G. lanceolarium (Luo et al., 2017) and Breynia vitis-idaea (Burman) Fischer (Furukawa and Kawakita 2017).
The association between braconid wasps and Glochidion has been previously reported (Hembry et al., 2013b; Kawakita et al., 2015; Henderson et al., 2020). Braconid wasp females probe the fruits and oviposit so that their offspring parasitize and consume moth larvae as their nourishment (Fig. S1e). The presence of braconid wasps may have a significant positive effect on reducing the number of infested seeds caused by Epicephala larvae, although we did not conduct a test of this hypothesis here. Indeed, parasitism rates of Epicephala can be very high: in one survey 60% of developing Epicephala larvae associated with Phyllanthus bourgeoisii Baill. were parasitized by braconid wasps (Kawakita and Kato, 2004a). In contrast, Finch et al. (2019) did not find a significant difference in infested seeds between fruit with braconid wasps and without braconid wasps in B. oblongifolia. This indicates that the damage to the seeds caused by Epicephala larvae may have already occurred before the braconid wasp attacked the Epicephala larvae, or braconid wasp parasitism may not immediately stop or reduce feeding behavior by the parasitized Epicephala larvae. In this study, we found that seeds infested by Epicephala are still found in some fruits from which wasps emerge.
Besides Epicephala and braconid wasps, we also detected another unknown moth species associated with G. coccineum. Interestingly, this undescribed moth species pupates inside empty spaces in the carpels of the fruit of this species (Fig. S1d). In two Glochidion-Epicephala species pairs, G. lanceolarium-E. lanceolaria from China (Luo et al., 2017) and Glochidion ferdinandi (Müll.Arg.) Bailey-Epicephala colymbetella from Australia (Henderson et al., 2020) it has been previously reported that Epicephala moths pupate inside empty sinuses in the carpels of the mature host fruit. In this study, after the larva of this unknown moth destroys all seeds inside the G. coccineum fruit, it creates empty space which may provide a site for itself to pupate in; however, in these spaces the only pupae we found were of this unidentified species of moth. Due to our limited observations, we still don't know exactly why this phenomenon occurs. Regarding this gap, a more detailed study of the associations with this species is needed.
4.3. Phylogenetic relationships of EpicephalaThe multilocus molecular phylogeny showed that Epicephala associated with these five Glochidion species belonged to five clades with high bootstrap support values (>99) (Fig. 5). Each of these Epicephala clades is uniquely associated with a single Glochidion species as its host. G. littorale and G. sp. 1 are each pollinated by their unique Epicephala species across multiple sites in Cambodia. At several of these sites, multiple Glochidion co-occur, and these findings are consistent with the hypothesis that they are each pollinated by a single unique Epicephala species at these locations. Available data thus suggests that across these sampled localities, the relationship between Glochidion and Epicephala may even be one-to-one as has been reported in subtropical Japan using the same loci to assess species-specificity (Kawakita and Kato, 2006) and differing from species-specificity on oceanic islands (Hembry et al., 2018). However, very few Epicephala were sampled from G. glomerulatum (N = 5 moths) and G. rubrum (N = 7 moths), and sampling of additional moths from additional sites for these hosts and G. coccineum is necessary to make a determination about species specificity in this mutualism in Cambodia. Regardless, these results indicate that Glochidion-Epicephala associations in tropical continental regions may be extremely specialized.
5. ConclusionsThe brood pollination mutualism between leafflower trees in the genus Glochidion and leafflower moths in the genus Epicephala has been widely reported from subtropical and temperate Asia as well as from Australia and the Pacific islands. Our study provides the first detailed evidence that in the center of diversity of Glochidion in tropical Asia, this interaction is mutualistic—with female moths actively pollinating host flowers, and moth larvae consuming a subset of the developing seeds—as previously reported at the global margins of its distribution. Furthermore, our study highlights the diversity of this mutualism in the Asian tropics. Although each of the Glochidion species here has a mutualistic relationship with leafflower moths, there is considerable among-species pair variation in the number of seeds and larvae per fruit, and at least one species of Glochidion (G. coccineum) uses physical structures within the fruit to limit seed damage from the larvae of its pollinator, suggesting a range of cost-benefit outcomes in this mutualism. There is also very high species-specificity in this mutualism, with each Glochidion appearing to depend on a single unique pollinating moth species for its reproduction. The mutualism between Glochidion and leafflower moths in Cambodia and elsewhere in tropical Asia provides an example to understand the role of coevolution in promoting species diversification and the range of mechanisms underlying mutualism stability. For a deeper understanding of the evolution of this species-rich and specialized mutualism, we recommend further research on the diversity of Glochidion-leafflower associations in the Asian tropics.
Author contributionsPC and S-XL designed research; PC performed research; GY, S-XL, and DHH conducted species identification; PC and GY formatted and uploaded data to GenBank; PC, S-XL, and DHH analyzed data and wrote manuscript.
Data availability statementThe data that support the findings of this study are openly available in GenBank (https://www.ncbi.nlm.nih.gov/genbank/), accession numbers MZ393203–MZ393363, MZ393691–MZ393770.
Declaration of competing interestThe authors declare no conflicts of interest.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.07.001.
AcknowledgementsWe express our deep thanks to Mr. Khou Eanghourt for his advice and facilitation of fieldwork. We also thank Mr. Nung Mao, Pisal Chheang's family, and many friends for their kind assistance with the field and laboratory work, and Peter van Welzen for discussing the identification of Cambodian Glochidion with us. We also thank the Ministry of Environment (Cambodia), Preah Vihear Heritage Site, Phnom Kulen National Park, Bokor National Park, Kirirom National Park, and Ream National Park for allowing us access to field sites and permission to conduct fieldwork. This project was supported by the National Natural Science Foundation of China (grants no. 31170217 and 31370268 to S.-X. Luo), and the Chinese Academy of Sciences’ “The Belt and Road” Master Fellowship Programme for providing P. Chheang a fully funded master's scholarship.
Addicott, J.F., Bao, T., 1999. Limiting the costs of mutualism: multiple modes of interaction between yuccas and yucca moths. Proc. R. Soc. B, 266: 197-202. DOI:10.1098/rspb.1999.0622 |
Airy Shaw, H.K., 1978. Notes on Malesian and other Asiatic euphorbiaceae. Kew Bull., 33: 25-77. DOI:10.2307/4110102 |
Angiosperm Phylogeny Group, et al., 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc., 181: 1-20. DOI:10.1111/boj.12385 |
Chakrabarty, T., Gangopadhyay, M., 1995. The genus Glochidion (Euphorbiaceae) in the Indian subcontinent. J. Econ. Taxon. Bot., 19: 173-234. |
Cho, S.H., Chhang, P., Kim, Y.D., 2016. A Checklist for the Seed Plants of Cambodia. National Institute of Biological Resources, Ministry of Environment, Incheon
|
Cooper, W., Cooper, W., 2013. Australian Rainforest Fruits. CSIRO Publishing, Melbourne
|
Darwin, C., 1862. On the Various Contrivances by Which British and Foreign Orchids are Fertilised by Insects and On the Good Effects of Intercrossing. J. Murray, London
|
Edgar, R.C., 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26: 2460-2461. DOI:10.1093/bioinformatics/btq461 |
Emerton, L., Seilava, R., Pearith, H., 2002. Bokor, Kirirom, Kep and Ream National Parks, Cambodia: Case studies of economic and development linkages, field study report. In Review of Protected Areas and their Role in the Socio-Economic Development of the Four Countries of the Lower Mekong Region. International Centre for Environmental Management, Brisbane and IUCN Regional Environmental Economics Programme, Karachi. http://cmsdata.iucn.org/downloads/casestudy03ream.pdf
|
Finch, J.T.D., Power, S.A., Welbergen, J.A., et al., 2018. Two's company, three's a crowd: co-occurring pollinators and parasite species in Breynia oblongifolia (Phyllanthaceae). BMC Evol. Biol., 18: 193. DOI:10.1186/s12862-018-1314-y |
Finch, J.T.D., Power, S.A., Welbergen, J.A., et al., 2019. A non-pollinating moth inflicts higher seed predation than two co-pollinators in an obligate pollination mutualism. Ecol. Entomol., 44: 780-791. DOI:10.1111/een.12754 |
Furukawa, S., Kawakita, A., 2017. Limiting the cost of mutualism: the defensive role of elongated gynophore in the leafflower–moth mutualism. Oecologia, 184: 835-846. DOI:10.1007/s00442-017-3910-8 |
Goto, R., Okamoto, T., Kiers, E.T., et al., 2010. Selective flower abortion maintains moth cooperation in a newly discovered pollination mutualism. Ecol. Lett., 13: 321-329. DOI:10.1111/j.1461-0248.2009.01425.x |
Govaerts, R., Frodin, D.G., Radcliffe-Smith, A., 2000. World Checklist and Bibliography of Euphorbiaceae, vol. 3. Royal Botanic Gardens, Kew, London
|
Hayes, B., Mould, A., Khou, E., et al., 2013. A biodiversity assessment of Phnom Kulen National Park with recommendations for management. Unpublished report to Conservation International, Phnom Penh
|
Hembry, D.H., Okamoto, T., Gillespie, R.G., 2012. Repeated colonization of remote islands by specialized mutualists. Biol. Lett., 8: 258-261. DOI:10.1098/rsbl.2011.0771 |
Hembry, D.H., Kawakita, A., Gurr, N.E., et al., 2013. Non-congruent colonizations and diversification in a coevolving pollination mutualism on oceanic islands. Proc. R. Soc. B, 280: 20130361. DOI:10.1098/rspb.2013.0361 |
Hembry, D.H., Okamoto, T., McCormack, G., et al., 2013. Phytophagous insect community assembly through niche conservatism on oceanic islands. J. Biogeogr., 40: 225-235. DOI:10.1111/j.1365-2699.2012.02792.x |
Hembry, D.H., Althoff, D.M., 2016. Diversification and coevolution in brood pollination mutualisms: windows into the role of biotic interactions in generating biological diversity. Am. J. Bot., 103: 1783-1792. DOI:10.3732/ajb.1600056 |
Hembry, D.H., Raimundo, R.L.G., Newman, E.A., et al., 2018. Does biological intimacy shape ecological network structure? A test using a brood pollination mutualism on continental and oceanic islands. J. Anim. Ecol., 87: 1160-1171. DOI:10.1111/1365-2656.12841 |
Henderson, E., Missen, M., Zalucki, J., 2020. To pupate or not to pupate: a case study of an obligate pollination mutualism in Glochidion ferdinandi (Phyllanthaceae) and Epicephala colymbetella (Gracillariidae). Aust. J. Bot., 67: 473-479. |
Herre, E.A., Jandér, K.C., Machado, C.A., 2008. Evolutionary ecology of figs and their associates: recent progress and outstanding puzzles. Annu. Rev. Ecol. Evol. Syst., 39: 439-458. DOI:10.1146/annurev.ecolsys.37.091305.110232 |
Hoffmann, P., McPherson, G., 2003. Transfer of Madagascan Glochidion to Phyllanthus (Euphorbiaceae s.l. or Phyllanthaceae). Novon, 13: 307-310. DOI:10.2307/3393263 |
Janzen, D.H., 1979. How to be a fig. Annu. Rev. Ecol. Syst., 10: 13-51. DOI:10.1146/annurev.es.10.110179.000305 |
Kato, M., Takimura, A., Kawakita, A., 2003. An obligate pollination mutualism and reciprocal diversification in the tree genus Glochidion (Euphorbiaceae). Proc. Natl. Acad. Sci. U.S.A., 100: 5264-5267. DOI:10.1073/pnas.0837153100 |
Kawakita, A., 2010. Evolution of obligate pollination mutualism in the tribe Phyllantheae (Phyllanthaceae). Plant Species Biol., 25: 3-19. DOI:10.1111/j.1442-1984.2009.00266.x |
Kawakita, A., Kato, M., 2004. Evolution of obligate pollination mutualism in new caledonian Phyllanthus (Euphorbiaceae). Am. J. Bot., 91: 410-415. DOI:10.3732/ajb.91.3.410 |
Kawakita, A., Kato, M., 2004. Obligate pollination mutualism in Breynia (Phyllanthaceae): further documentation of pollination mutualism involving Epicephala moths (Gracillariidae). Am. J. Bot., 91: 1319-1325. DOI:10.3732/ajb.91.9.1319 |
Kawakita, A., Kato, M., 2006. Assessment of the diversity and species specificity of the mutualistic association between Glochidion trees and Epicephala moths. Mol. Ecol., 15: 3567-3581. DOI:10.1111/j.1365-294X.2006.03037.x |
Kawakita, A., Kato, M., 2009. Repeated independent evolution of obligate pollination mutualism in the Phyllantheae-Epicephala association. Proc. R. Soc. B, 276: 417-426. DOI:10.1098/rspb.2008.1226 |
Kawakita, A., Kato, M., 2017. Evolution and diversity of obligate pollination mutualisms, in: Kato, M., Kawakita, A. (Eds.), Obligate Pollination Mutualism. Springer, Tokyo, pp. 249-270
|
Kawakita, A., Mochizuki, K., Kato, M., 2015. Reversal of mutualism in a leafflower–leafflower moth association: the possible driving role of a third-party partner. Biol. J. Linn. Soc., 116: 507-518. DOI:10.1111/bij.12633 |
Kawakita, A., Okamoto, T., Goto, R., et al., 2010. Mutualism favours higher host specificity than does antagonism in plant–herbivore interaction. Proc. R. Soc. B, 277: 2765-2774. DOI:10.1098/rspb.2010.0355 |
Kawakita, A., Takimura, A., Terachi, T., et al., 2004. Cospeciation analysis of an obligate pollination mutualism: have Glochidion trees (Euphorbiaceae) and pollinating Epicephala moths (Gracillariidae) diversified in parallel?. Evolution, 58: 2201-2214. |
Li, H., Wang, Z., Hu, B., 2015. Four new species of Epicephala Meyrick, 1880 (Lepidoptera, Gracillariidae) associated with two species of Glochidion (Phyllanthaceae) from Hainan island in China. ZooKeys, 508: 53-67. DOI:10.3897/zookeys.508.9479 |
Luo, S.-X., Yao, G., Wang, Z., et al., 2017. A novel, enigmatic basal leafflower moth lineage pollinating a derived leafflower host illustrates the dynamics of host shifts, partner replacement, and apparent coadaptation in intimate mutualisms. Am. Nat., 189: 422-435. DOI:10.1086/690623 |
Miller, M.A., Pfeiffer, W., Schwartz, T., 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees, in: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA. Institute of Electrical and Electronics Engineers, New York, pp. 1-8
|
Okamoto, T., Kawakita, A., Kato, M., 2007. Interspecific variation of floral scent composition in Glochidion and its association with host-specific pollinating seed parasite (Epicephala). J. Chem. Ecol., 33: 1065-1081. DOI:10.1007/s10886-007-9287-0 |
Okamoto, T., Kawakita, A., Goto, R., et al., 2013. Active pollination favours sexual dimorphism in floral scent. Proc. R. Soc. B, 280: 20132280. DOI:10.1098/rspb.2013.2280 |
Pellmyr, O., Huth, C.J., 1994. Evolutionary stability of mutualism between yuccas and yucca moths. Nature, 372: 257-260. DOI:10.1038/372257a0 |
Pellmyr, O., 2003. Yuccas, yucca moths, and coevolution: a review. Ann. Mo. Bot. Gard., 90: 35-55. DOI:10.2307/3298524 |
Rentsch, J.D., Leebens-Mack, J., 2014. Yucca aloifolia (Asparagaceae) opts out of an obligate pollination mutualism. Am. J. Bot., 101: 2062-2067. DOI:10.3732/ajb.1400351 |
Richter, K.S., Weis, A.E., 1995. Differential abortion in the yucca. Nature, 376: 557-558. |
Riley, C.V., 1892. The yucca moth and yucca pollination. Ann. Mo. Bot. Gard., 3: 99-158. DOI:10.2307/2992075 |
Stamatakis, A., 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688-2690. DOI:10.1093/bioinformatics/btl446 |
Thompson, J.N., 1986. Oviposition behaviour and searching efficiency in a natural population of a braconid parasitoid. J. Anim. Ecol., 55: 351-360. DOI:10.2307/4713 |
Thompson, J.N., 1987. Variance in number of eggs per patch: oviposition behaviour and population dispersion in a seed parasitic moth. Ecol. Entomol., 12: 311-320. DOI:10.1111/j.1365-2311.1987.tb01010.x |
Thompson, J.N., 1994. The Coevolutionary Process. University of Chicago Press, Chicago
|
van Welzen, P.C., 2007. Glochidion, in: Chayamarit, K., van Welzen, P.C. (Eds.), Flora of Thailand, vol. 8 (2). Royal Forest Department, Bangkok, pp. 308-331
|
Webster, G.L., 2014. Euphorbiaceae. in: Kubitzki, K. (Ed.), Flowering Plants. Eudicots. Springer, Berlin, Heidelberg, pp. 51-216
|
Wilson, R.D., Addicott, J.F., 1998. Regulation of mutualism between yuccas and yucca moths: is oviposition behavior responsive to selective abscission of flowers?. Oikos, 81: 109-118. DOI:10.2307/3546473 |
Zhang, J., Wang, S., Li, H., et al., 2012. Diffuse coevolution between two Epicephala species (Gracillariidae) and two Breynia species (Phyllanthaceae). PLoS One, 7: e41657. DOI:10.1371/journal.pone.0041657 |



