b. Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, Yunnan, 650201, China;
c. Gongshan Bureau of the Gaoligongshan National Nature Reserve, Gongshan, Yunnan, 673500, China
Comprising approximately 400 species, Dryopteris Adanson (1763) (Dryopteridaceae) is one of the largest fern genera (PPG, 2016). Dryopteris consists of terrestrial plants distributed worldwide that usually grow in forests, and occasionally in rocky areas of temperate mountain regions, with the highest species diversity occurring in East Asia, especially in the Himalaya and the Hengduan Mountains Area (Fraser-Jenkins, 1986; Wu and Lu, 2000; Wu et al., 2013; Sessa et al., 2015). Currently Dryopteris is divided into four subgenera with about 24 sections (Zhang, 2012; Zhang et al., 2012; Zhang and Zhang, 2012; Wu et al., 2013; Kuo et al., 2016).
Although Dryopteris sect. Nephrocystis H. Itô was widely accepted by Asian pteridologists (Itô, 1935; Fraser-Jenkins, 1986; Wu and Lu, 2000; Wu et al., 2013), it is actually a later synonym of D. sect. Diclisodon (T. Moore) C. Chr (Christensen, 1905; Widén et al., 2001). The type of D. sect. Diclisodon, Diclisodon deparioides T. Moore (= Dryopteris deparioides (T. Moore) Kuntze) belongs to D. sect. Nephrocystis, although the morphology of D. deparioides is special and does not represent the ‘typical’ morphological characteristics of this section. Dryopteris sect. Diclisodon is characterized by typical asymmetrical bases of pinnales, basiscopic pinnule of basal pair of pinnae elongated as pinnatifid, and brown, lanceolate to ovate scales, and is distributed in East Asia and South Asia, and extends to the Pacific Islands. Mainly based on different morphological characters, D. sect. Diclisodon was placed in D. subg. Eudryopteris C. Chr (Itô, 1935), D. subg. Nephrocystis (H. Itô) Fras.-Jenk (Fraser-Jenkins, 1986; Widén et al., 2001), or D. subg. Dryopteris (Wu and Lu, 2000). A previous molecular phylogeny indicated that most members of this section should be placed in D. subg. Dryopteris (Zhang et al., 2012). Fraser-Jenkins (1986) included 14 species in D. sect. Nephrocystis. Darnaedi et al. (1989a) described three new species related to the widespread D. sparsa (D. Don) Kuntze, and recognized two other synonyms of Fraser-Jenkins (1986) as distinct species in this section. Wu et al. (2013) recorded ten species in this section in Flora of China after removing several members of D. sect. Acrorumohra (H. Itô) Li Bing Zhang & H. He (He et al., 2013) based on molecular phylogenetic study (Zhang et al., 2012). A central piece of the taxonomic problem in D. sect. Diclisodon is the relationships among the D. sparsa complex, which has about ten names (Fraser-Jenkins, 1986, 1989; Darnaedi et al., 1989a; Fraser-Jenkins et al., 2018). This may be related to their complicated polyploidy (2n = 82, 2n = 123, 2n = 164, apomictic ‘n’ = 82, and apomictic ‘n’ = 123) (Gibby, 1985; Darnaedi and Iwatsuki, 1987; Imaichi et al., 1987; Darnaedi et al., 1989b; Weng, 1989; Tindale and Roy, 2002; Fraser-Jenkins, 2008; Hori et al., 2021). During our recent fieldwork, we collected many samples of D. sect. Diclisodon in China, which enabled us to address some significant taxonomic issues of this section.
2. Material and methods 2.1. Morphological studyWe checked 332 specimens of Dryopteris sect. Diclisodon deposited in the herbaria of BM, HNNU, IBSC, K, KUN, P, PE, PYU, TI, and TNS, particularly digitalized type specimens at BM, K, P, TI, and TNS. Sixteen related species were chosen from morphological comparisons and previous studies (Fraser-Jenkins, 1986; Darnaedi et al., 1989a; Zhang et al., 2012; Wu et al., 2013) (Table 1). Eleven morphological features of these species were extracted from specimens and species descriptions of previous studies, and particular attentions were paid to the diagnostic morphological characters adopted in Flora of China (Wu et al., 2013).
| Taxon | Characters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rhizome | Frond dimorphism | Color of upper part of stipe and rachis | Basal pinnae of well-developed fronds | Lamina dissection of well-developed fronds except for the basal pinnae | Length of pinnae stalks | Pinnule shape of upper pinnae | Pinnule apices | Appendage on rachis and pinna rachis | Indusia | Sori location | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.angustipalea Darnaedi, M. Kato & K. Iwats. | Erect to ascendent | Monomorphic | Stramineous | Tripinnate | Tripinnate | Longer than 10mm, with distinct stalks | Lanceolate | Obtuse | Glabrous | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.cacaina | Erect | Monomorphic | Castaneous to purplish brown | Tripinnate | Bipinnate | Longer than 10mm, with distinct stalks | Triangular-ovate | Obtuse | Glands | Entire | Close to costule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.deparioides | Erect | Monomorphic | Stramineous | Tripinnate | Bipinnate | Less than 5mm, sessile or subsessile | Lanceolate | Acuminate | Glabrous | Entire | Marginal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.gaoligongensis | Creeping | Monomorphic | Stramineous to castaneous | 4-pinnate | Tripinnate | Longer than 10mm, with distinct stalks | Triangular-ovate | Acuminate | Hairy | Entire | Close to costule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.indonesiana | Erect | Monomorphic | Stramineous | 4-pinnate | Tripinnate | Longer than 10mm, with distinct stalks | Lanceolate | Acuminate | Glabrous | Entire | Close to costule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.maximowiczii | Creeping | Monomorphic | Stramineous | 4-pinnate | Tripinnate | Longer than 10mm, with distinct stalks | Triangular-ovate | Obtuse | Scales | Entire | Submarginal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.melanocarpa | Erect | Monomorphic | Stramineous | Tripinnate | Tripinnate | Longer than 10mm, with distinct stalks | Triangular-ovate | Obtuse | Glands | Entire | Submarginal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.platypus (Kunze) Kuntze | Erect | Slightly dimorphic | Castaneous to purplish brown | Tripinnate | Bipinnate | Less than 5mm, sessile or subsessile | Lanceolate | Obtuse | Glands | Entire | Close to costule or medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.polita | Erect | Monomorphic | Stramineous | Bipinnatifid to tripinnate | Bipinnate | Longer than 10mm, with distinct stalks | Triangular-ovate to Lanceolate | Acuminate to obtuse | Glabrous | Exindusiate | Medial to submarginal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.renchangiana | Creeping | Monomorphic | Castaneous to purplish brown | Bipinnate to tripinnatifid | Bipinnate | Less than 5mm, sessile or subsessile | Lanceolate | Obtuse to Acuminate | Glands | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sabaei (Franch. & Sav.) C. Chr. | Erect | Monomorphic | Stramineous | Tripinnate | Bipinnate | Longer than 10mm, with distinct stalks | Lanceolate | Acuminate | Scales | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sinonepalensis | Erect | Monomorphic | Stramineous | Tripinnate | Tripinnate | Less than 5mm, sessile or subsessile | Lanceolate | Obtuse to Acuminate | Glabrous | Entire | Close to costule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sparsa TYPE a | ? | Monomorphic | Stramineous | Tripinnate | Tripinnate | Longer than 10mm, with distinct stalks | Lanceolate | Acuminate | Glabrous | Entire | Close to costule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sparsa b | Erect to ascendent | Monomorphic, sometimes slightly dimorphic | Stramineous to castaneous | Bipinnatifid to tripinnate | Bipinnate to tripinnate | Longer than 10mm, with distinct stalks | Lanceolate to ovate | Obtuse, acuminate or truncate | Glabrous or with glands | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sparsa var. ryukyuensis | Erect | Monomorphic | Stramineous | Tripinnate | Bipinnate | Longer than 10mm, with distinct stalks | Lanceolate | Obtuse | Glands | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.sparsa subsp. rectipinnula Fraser-Jenk. c | Erect | Monomorphic | Stramineous | Tripinnate | Tripinnate | Longer than 10mm, with distinct stalks | Lanceolate | Obtuse | Glabrous | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.subexaltata d | Erect | Monomorphic | Stramineous | Bipinnatifid to bippinnate | Bipinnate | Less than 5mm, sessile or subsessile | Ovate | Truncate | Glabrous | Irregularly lacerate | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.yakusilvicola | Erect | Monomorphic | Stramineous | Tripinnate | Tripinnate | Longer than 10mm, with distinct stalks | Lanceolate | Acuminate | Glands | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D.yoroii | Erect | Monomorphic | Purplish brown | Tripinnate | Bipinnate to tripinnate | Longer than 10mm, with distinct stalks | Triangular-ovate | Obtuse to Acuminate | Glands or scales | Entire | Medial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| A new species Dryopteris gaoligongensis and two newly recognized species with new names, Dryopteris renchangiana and D. sinonepalensis are showed in Bold. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a Information was extracted from the holotype (from Nepal, BM001066027). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| b Information was extracted from Flora Reipublicae Popularis Sinicae (Wu and Lu, 2000), Flora of China (Wu et al., 2013), Ebihara (2017), and most specimens from China and Japan. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| c Three names were submerged in this species: Dryopteris sinosparsa Ching & Shing, D.viridescens (Baker) Kuntze (Fraser-Jenkins, 1986) and D.rheophila Mitsuta ex Darnaedi, M. Kato & K. Iwats. (Fraser-Jenkins etal., 2018). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| d Including samples identified as Dryopteris hayatae Tagawa. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leaf material was collected from living plants in the field or from herbarium specimens. Total DNA of 12 samples (Table S1) was extracted from 15 mg of silica-gel-dried leaf material using a modified 4 × CTAB DNA extraction method (Doyle and Doyle, 1987). For plastid DNA, library preparation and Illumina sequencing were conducted at the Germplasm Bank of Wild Species, Kunming Institute of Botany (CAS), and BGI Genomics Co., Ltd (Shenzhen, China), respectively. More than 2 Gb of clean data of 150 bp pair-end reads were generated for each sample. Read data were de-novo assembled using GetOrganelle v.1.7.0 (Jin et al., 2020). Connection and annotation were subsequently conducted using Bandage 0.8.1 (Wick et al., 2015) and Geneious 9.1.4 (Kearse et al., 2012) based on the previously published plastome of Dryopteris filix-mas (L.) Schott (LT618774). For the low-copy nuclear gene AK1, primer design and reaction procedures followed Hori et al. (2021). The voucher information and GenBank accession numbers of complete plastomes, plastid regions, and the nuclear gene AK1 used in this study are provided in Tables S1–S3, respectively.
2.2.2. Data matrices and phylogenetic analysesWe acquired 93 sequences of nine chloroplast regions (atpB, psbA-trnH, rbcL, rbcL-accD, rps4-trnS, trnG-trnR, trnL, trnL-F, and trnP-petG) from 32 samples, and ten additional plastomes from GenBank. We extracted nine chloroplast regions from 22 plastomes and constructed a combined plastid matrix of 54 samples. Fourteen AK1 sequences from nine samples were newly obtained in this study and 18 sequences were acquired from GenBank. The plastid and nuclear matrices were aligned using MAFFT v.7.017 (Katoh et al., 2002) and were subsequently corrected in Geneious 9.1.4 (Kearse et al., 2012).
Maximum Likelihood (ML) and Bayesian Inference (BI) analyses were performed to infer the phylogenetic relationships within Dryopteris sect. Diclisodon based on the combined plastid regions and nuclear AK1 gene, respectively. ML analyses were conducted using RAxML 8.2.10 (Stamatakis, 2014). A thorough tree search for the best ML tree was performed, and bootstrap analyses were performed with 1000 replications; bipartition information from the bootstrap trees was drawn on the best ML tree. BI analyses were conducted with MrBayes 3.2.6 (Ronquist et al., 2012), using ten million generations with one tree sampled every 1000 generations; four runs with four chains were performed in parallel. The first 25% of trees were discarded as burn-in. The Markov chain Monte Carlo (MCMC) output was examined to check for convergence and to ensure that all the effective sample size (ESS) values were above 200.
3. Results and discussion 3.1. MorphologyThe diagnostic morphological characters of Dryopteris sect. Diclisodon are mainly related to leaves, e.g., lamina dissection and colors of the upper part of the stipe and rachis. Nevertheless, the creeping rhizome, irregularly lacerated indusia, and marginal sori are also important in distinguishing different species (Table 1).
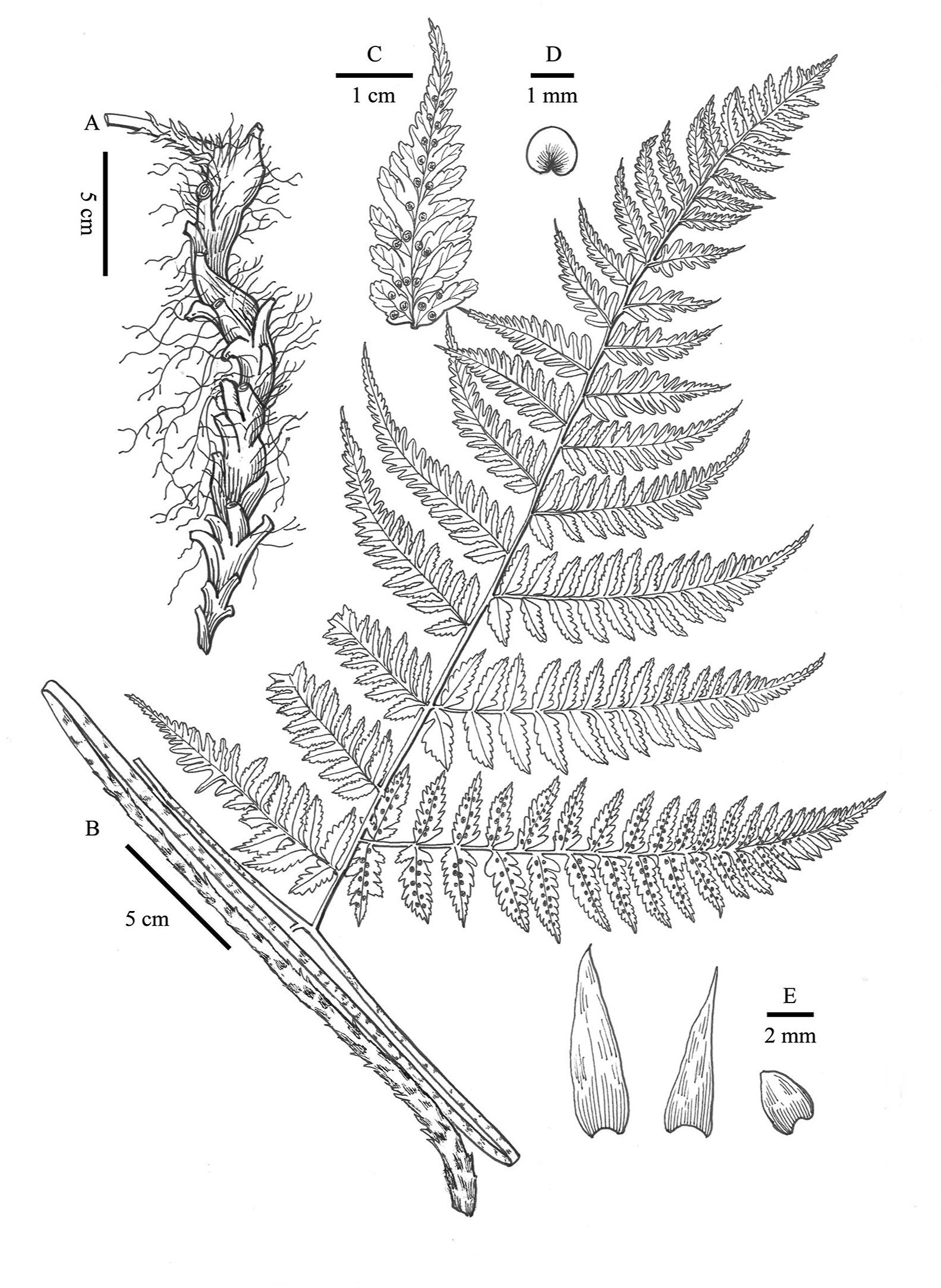
The new species, Dryopteris gaoligongensis Z.Y. Zuo, Jin Mei Lu & D.Z. Li, resembles the Southeast Asian D. indonesiana Darnaedi, M. Kato & K. Iwats (Darnaedi et al., 1989a), but it can be easily distinguished from the latter and other known related species by its creeping rhizome Fig. 1, Fig. 2B; Table 1) and having large 4-pinnate fronds long than 1 m (Fig. 1A). The creeping rhizome was previously only known in D. maximowiczii (Baker) Kuntze in D. sect. Diclisodon. Actually, it can be found in D. gaoligongensis and the newly recognized D. renchangiana Z.Y. Zuo & D.Z. Li (Fig. 3C; Table 1). Compared with D. gaoligongensis, the pinnae of D. renchangiana are usually sessile or subsessile, and the laminae are bipinnate to tripinnatifid at the basal pinnae (Fig. 3A; Table 1). The other species, D. sinonepalensis Z.Y. Zuo & Fraser-Jenkins, is similar to D. yoroii Seriz., but can be easily distinguished from it morphologically. Dryopteris yoroii has monomorphic fronds, distinct pinnae stalks, and usually triangular-ovate pinnule of the upper pinnae (Fig. 4A–D), whereas D. sinonepalensis usually has slightly dimorphic fronds, very short pinnae stalks, and lanceolate pinnule of upper pinnae (Fig. 4F–I). In addition, the scales of the two species are different. The stipe base of D. yoroii is densely clothed with bicolorous (brown with pale edges) spreading scales (Fig. 4C and E), whereas that of D. sinonepalensis is sparsely clothed with concolorous (pale brown) adpressed scales (Fig. 4H and G).

|
| Fig. 1 Dryopteris gaoligongensis Z.Y. Zuo, Jin Mei Lu & D.Z. Li. A. Habitat and well-developed fronds. B. 4-pinnate lower pinnae. C. The bipinnate-pinnatifid fronds in a young plant. D. Rhizome. E. Sori on ultimate pinnules. F. Scales. |
|
| Fig. 2 Illustration of Dryopteris gaoligongensis Z.Y. Zuo, Jin Mei Lu & D.Z. Li. A. Rhizome. B. Stipe and basal pinna. C. Sori on ultimate pinnules. D. Indusia. E. Scales (Drawn by Ling Wang, based on the holotype (Z.Y. Zuo1610)). |

|
| Fig. 3 Dryopteris renchangiana Z.Y. Zuo & D.Z. Li. A. Habitat and well-developed fronds. B. Sori on ultimate pinnules. C. Rhizome. |

|
| Fig. 4 Comparison of Dryopteris yoroii (Holotype: A, B & C; Z.Y. Zuo, 1857: D & E) and D. sinonepalensis (Lectotype: F, G & H; Z.Y. Zuo 1331: I & J). A & F. Overview. B, D, G & I. Sori on ultimate pinnules. C, E, H & J. Scales on stipe base. |
The concatenated matrix of nine plastid regions was 8466 bp in length, with 1692 variable sites and 1093 parsimony informative sites. The phylogenetic analyses based on the concatenated plastid matrix resolved the relationships among clades and subclades in Dryopteris sect. Diclisodon with strong support values (Fig. 5). There was no substantial conflict between the topologies that resulted from BI and ML analyses. Dryopteris polita Rosenst. is not nested in D. sect. Diclisodon, which is congruent with previous studies (Sessa et al., 2012; Zhang et al., 2012). Dryopteris sect. Diclisodon, which excludes D. polita and Acrorumohra group, is strongly supported as a monophyletic group and sister to the Pallidae clade in the plastid phylogeny. Dryopteris sect. Diclisodon is divided into three clades. The Yoroii clade, consisting of only one species D. yoroii, is the sister group to other sampled species of D. sect. Diclisodon. Dryopteris yoroii is only found in alpine habitat above the tree line (alt. 2900–3500 m) of the Himalaya to the Hengduan Mountains, and Taiwan Island, and three samples are included in the present study (Fig. 5). The Sabaei clade consists of three species distributed in Japan and the Korean Peninsula, i.e., D. maximowiczii, D. sabaei (Franch. & Sav.) C. Chr, and D. yakusilvicola Sa. Kurata (Fig. 5), of which D. yakusilvicola has been previously reported as a hybrid species (Darnaedi et al., 1990; Ebihara, 2017; Hori et al., 2021). The Sparsa clade is composed of two subclades (hereafter referred to as Subclade A and Subclade B). Subclade A consists of D. gaoligongensis, D. deparioides, and three samples of D. sparsa from the Himalaya, the Hengduan Mountains, and their adjacent areas (Fig. 5); D. gaoligongensis is sister to all other members of this subclade. Subclade B consists of D. cacaina Tagawa, D. melanocarpa Hayata, D. renchangiana, D. sinonepalensis, D. subexaltata (Christ) C. Chr., and several samples of D. sparsa (including D. sparsa var. ryukyuensis Seriz.). Within subclade B, D. cacaina and D. sinonepalensis together are sister to other species.

|
| Fig. 5 ML phylogeny of Dryopteris sect. Diclisodon based on nine plastid regions. ML bootstrap support (MLBS) values and the posterior probabilities of Bayesian inference (BIPP) are indicated near nodes, with MLBS over the branches and BIPP under the branches. The thickened branch indicates 100% MLBS and 1.00 PP. |
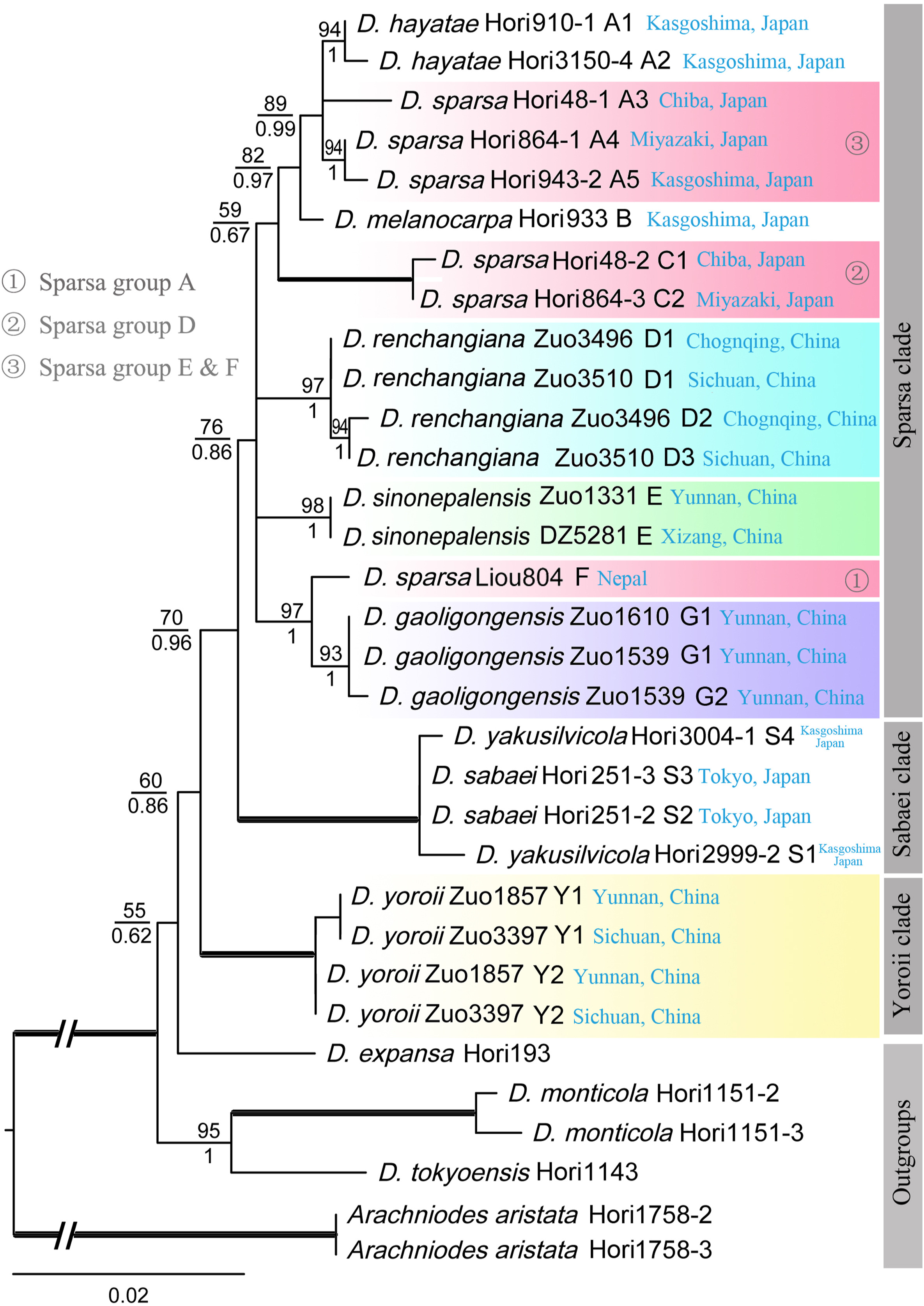
The AK1 matrix was 631 bp in length, with 131 variable sites and 90 parsimony informative sites. The relationships among different clades and subclades have not been well resolved in the AK1 phylogeny, although most terminal nodes are strongly supported (Fig. 6). Three haplotypes in D. renchangiana, two haplotypes in D. gaoligongensis and D. yoroii, and only one haplotype in D. sinonepalensis were detected. The results show that D. yoroii is sister to other species of D. sect. Diclisodon and D. gaoligongensis is sister to the Nepalese D. sparsa (Liou804).
|
| Fig. 6 ML phylogeny of Dryopteris sect. Diclisodon based on nuclear gene AK1. ML bootstrap support (MLBS) values and the posterior probabilities of Bayesian inference (BIPP) are indicated near nodes, with MLBS over the branches and BIPP under the branches. The thickened branch indicates 100% MLBS and 1.00 PP. |
Previous studies showed that Dryopteris sparsa was a polymorphic species complex (Imaichi et al., 1987; Darnaedi et al., 1989a) or possibly a polyphyletic group (Liou, 2010). A recent study also showed that many hybridization events occurred in D. sparsa and related species in Japan (Hori et al., 2021). The samples of previous studies were mainly collected from the Sino-Japanese region (Liou, 2010; Hori et al., 2021). Our study confirmed that D. sparsa from the Himalaya to the Hengduan Mountains, and their adjacent areas, is not monophyletic. All D. sparsa samples can be divided into at least six groups (Sparsa groups A–F in Fig. 5). The AK1 phylogeny also implies that these groups are probably different taxa (Sparsa groups A, D, E & F in Fig. 6). It seems that the speciation of the D. sparsa complex is complicated, given the diversity of ploidy levels of this complex. Further studies are needed to elucidate the relationships among the D. sparsa complex with potentially cryptic species.
4. Taxonomic treatment 4.1. Dryopteris gaoligongensis Z.Y. Zuo, Jin Mei Lu & D.Z. Li, sp. nov. (Figs. 1 and 2). 高黎贡山鳞毛蕨 【gāo lí gòng shān lín máo jué】Diagnosis: Dryopteris gaoligongensis is different from other species in D. sect. Diclisodon by being a large plant with a creeping rhizome. It is similar to some species of D. sect. Marginatae (e.g., D. subimpressa Loyal.) in leaf shape. However, it can be identified as a member of D. sect. Diclisodon by typical asymmetrical bases of pinnales, basiscopic pinnule of basal pair of pinnae elongated as pinnatifid.
Type: CHINA. Yunnan: Baoshan, 25°17′N, 98°46′E, alt. 2338 m, 20 April 2019, Z.Y. Zuo 1610 (Holotype, KUN, in 3 cross-referenced sheets.)
Plants 50–120 cm tall. Rhizome stout, creeping, up to 20 cm long and 3 cm in diameter, densely clothed with brown, ovate-lanceolate, entire scales. Fronds approximate, stipe shorter than lamina, ca. 30–50 cm, brown at base, with spreading ovate-lanceolate, entire, brown scales, glabrous upward. Rachis and costae sparsely bearing fibrillose scales. Lamina broadly deltate-lanceolate to ovate-lanceolate, ca. 40–60 × 30–50 cm, tripinnate to 4-pinnate, base not narrowed, apex acuminate. Pinnae 15–30 pairs, opposite, oblique, stalked up to 5 cm. Pinnae lanceolate, basal pinnae largest, deltate-lanceolate, up to 30 × 15 cm on each side, apex caudate-acuminate. Pinnules 20–30 pairs, opposite at base and alternate upward, lanceolate, base broadly cuneate, usually asymmetrical, apex long acuminate. Basiscopic pinnule at base of basal pinnae longer than acroscopic ones, ca. 5 × 2 cm, base widest, 2-pinnate. Segments oblong, apices obtuse and spinulose, margin shallowly lobed to several serrate. Lamina papery, glossy, veins pinnate, forked, distinct on both surfaces. Sori close to costa on pinnules, with wide sterile belt on both sides of costa distal to sori; indusia orbicular-reniform, entire.
Distribution and habitat: Presently, only known from southern part of the Gaoligong Mountains. Forest, on the ground.
Etymology: The specific epithet “gaoligongensis” refers to its type locality, the Gaoligong Mountains, on the border of Yunnan of China and Myanmar.
Additional specimens examined (Paratypes):
CHINA. Yunnan: Baoshan, 25°17′N, 98°46′E, alt. 2317°m, 17 October 2019, DLJ2019398 (KUN); Tengchong, 25°18′N, 98°46′E, alt. 2367 m, 18 April 2019, Z.Y. Zuo 1539 (KUN).
4.2. Dryopteris renchangiana Z.Y. Zuo & D.Z. Li, nom. nov. (Fig. 3). 仁昌鳞毛蕨【rén chāng lín máo jué】Dryopteris glabrior Ching & Z.Y. Liu in Bull. Bot. Res., Harbin 4(4): 11 (1984), nom. illeg. Type: CHINA. Chongqing: Jinfoshan, 21 July 1983, Z.Y. Liu 4231 (Holotype PE01863796; Isotype IMC0007913). Non Dryopteris glabrior Copel. in Philipp. J. Sci. C, 5(4): 283 (1910). Type: Borneo. August 1908, Brooks C.J. s.n. (Holotype P00630771).
Distribution and habitat: It is currently known in Chongqing (Nanchuan) and Sichuan (Xuyong), China. Montane forest, on wet cliffs or the mouths of caves by streams, at 500–1500 m.
Etymology: We name Dryopteris renchangiana in honor of late Prof. Ren-Chang Ching (1898–1986), for his great contributions to the study of ferns. He proposed the classification system of the families and genera of the Chinese Pteridophytes, and made a revision of the Chinese and Sikkim-Himalayan Dryopteris.
Additional specimens examined:
CHINA. Chongqing: Nanchuan, Z.Y. Liu 840758 (IMC), Z.Y. Zuo 3496 (KUN).
Sichuan: Xuyong, Z.Y. Zuo 3498 & 3510 (KUN).
Notes: Copeland (1910) published Dryopteris glabrior, but it was transferred to Hypodematium glabrius (Copel.) Holttum (Hassler, 2018). Ching and Liu (1984) published D. glabrior Ching & Z.Y. Liu based on a different specimen collected in Chongqing, which was treated as an unresolved species in Flora of China (Wu et al., 2013). The name D. glabrior Ching & Z.Y. Liu is a later homonym and is illegitimate according to Art. 53 of the Shenzhen Code (Turland et al., 2018). We collected the specimens of “D. glabrior Ching & Z.Y. Liu” from its type locality (Chongqing, China) and found one more population of the same species in Sichuan, China. Morphological and molecular phylogenetic studies suggest that “D. glabrior Ching & Z.Y. Liu” is a distinct species, and a new name is needed.
4.3. Dryopteris sinonepalensis Z.Y. Zuo & Fraser-Jenkins, nom. nov. 中尼鳞毛蕨 【zhōng ní lín máo jué】Aspidium nitidulum Wallich in Num. List: no. 392 (1828), nom. nud.;
Aspidium nitidulum Wallich ex Kuhn in Linnaea 36: 117 (1869), nom. illeg.; Non A. nitidulum (C.Presl) Kunze ex Mett. in Pheg. u. Asp. 105, n.249c (1858), Basionym: Nephrodium nitidulum C.Presl in Epimel. Bot. 46 (1851). Type: PHILIPPINES. 1849. Hugh Cuming, s.n. (Type BM001045024 & PRC450276);
Lastrea sparsa var. nitidula Beddome in Suppl. ferns S. Ind, 17, pl. 374 (1876);
Nephrodium sparsum var. nitidulum (Beddome) C.B. Clarke in Trans. Linn. Soc. Lond. II (Bot.) 1: 524 (1880);
Dryopteris sparsa subsp. nitidula (Beddome) C. Chr. in Index flic, 293 (1905);
Dryopteris sparsa var. nitidula (Beddome) Ching in Bull. Fan Mem. Inst. Biol (Bot.) 8: 472 (1938);
Dryopteris nitidula (Wall. ex Kuhn) Mitsuta et S.K. Wu in Vasc. Pl. Hengduan Mount. 1: 131 (1993), nom. illeg. Non Dryopteris nitidula (C.Presl) Kuntze in Revis. Gen. Pl. 2: 813 (1891) (Basionym: N. nitidulum C.Presl).
Type: NEPAL. Nagarjum, 1821, Wallich 392 (Lectotype K001109948) (selected by Fraser-Jenkins, 1989).
Distribution and habitat: Montane forest, on the ground at alt. 2000–3000 m in the Himalaya and the Hengduan Mountains.
Notes: Two specimens collected from Tibet (Xizang) and Yunnan were initially identified as Dryopteris yoroii. However, the characteristics of these two samples were obviously different from those of the holotype of D. yoroii and other specimens from the type locality (Taiwan). After checking and comparing more specimens and records (including the lectotype of D. sparsa subsp. nitidula and the holotype of D. yoroii), we concluded that the two specimens are not truly D. yoroii. Dryopteris nitidula (Wall. ex Kuhn) Mitsuta et S.K. Wu is a later homonym of D. nitidula (C.Presl) Kuntze and is therefore illegitimate. In this case, a new name, D. sinonepalensis is proposed here as the lectotype of the substituted name was collected from Nepal, but widely distributed in Southwest China. The scale color is one of key characters to distinguish D. sinonepalensis and D. yoroii. However, the scales easily fall off and are rarely present on specimens, and we are usually unable to find complete scales on specimens, leading to confusion and misidentification of many specimens of D. sinonepalensis and D. yoroii. The descriptions of D. yoroii in Flora Reipublicae Popularis Sinicae (Wu and Lu, 2000) and Flora of China (Wu et al., 2013) may be partly based on specimens of D. sinonepalensis as they mentioned the fronds are usually dimorphic. The cytotype of one sample (Z.R. Wang & Z.X. Zhang C188) of D. sinonepalensis was reported as sexual diploid (Wang and Zhang, 1981).
Additional specimens examined:
CHINA. Chongqing: Youyang, Z.Y. Liu 6720, 6976 & 6978 (IMC). Sichuan: Chongzhou, W.B. Ju & D.K. Chen AZH00521 (CDBI). Meishan, Z.R. Wang et al. 519 (PE), Z.R. Wang & Z.X. Zhang C188 (PE), K.J. Guan et al. 2695 (PE); Hanyuan, X.X. Kong 3928 & 4081 (PE). Shimian, L.B. Zhang 523 (CDBI). Tibet (Xizang): Mêdog, S.Y. Dong & Z.Y. Zuo DZ5281 (IBSC, KUN). Yunnan: Daguan, W.M. Chu 5255 (PE, PYU); Lijiang, W.M. Chu 23,153 (HGAS, PYU), K.M. Feng 2630 & 9499 (PE), Z.Y. Zuo 3330 (KUN); Mengzi, W. Hancock 20 (PE); Yangbi, Jinshajiang Team 4298 (PE, PYU), Z.Y. Zuo 1331 (KUN); Yingjiang, Z.Y. Zuo 1289 (KUN).
Nepal, Makawanpur: Daman, Fraser-Jenkins 29,111 (FN 5086) (BM).
4.4. Dryopteris yoroii Serizawa, J. Jap. Bot. 46: 20. 1971. 栗柄鳞毛蕨 【lì bǐng lín máo jué】Type: CHINA. Taiwan: between Yushankou and Paiyun cottage, Mt Yushan, 8 August 1968, Reiko Yoroi s.n. (Holotype TNS401323).
Distribution and habitat: Montane forest, growing on wet cliffs or on the ground above the tree line (2900–3500 m) of the Himalaya to the Hengduan Mountains, and Taiwan Island.
Notes: The distribution of Dryopteris yoroii has the highest altitude of all species in D. sect. Diclisodon. The sample of D. yoroii from Yunnan (S.K. Wu et al. 64) was reported as an apomictic diploid (Xiang et al., 2006).
Additional specimens examined:
CHINA. Sichuan: Hanyuan, X.X. Kong 3965 (PE); Meishan, Z.Y. Zuo 3397 (KUN). Taiwan: Hualien, C.M. Kuo 428 (TAI); Nantou, W.T. Liou 552 (TNU). Tibet (Xizang): Mêdog, Z.Y. Zuo 2519 & 2566 (KUN); Yunnan: Gongshan, DLJ2019040 (KUN), DLJ-ET 2240 (PE), S.K. Wu et al. 64 (KUN), Z.Y. Zuo 1857 (KUN).
INDIA. Sikkim: Yakla, C.B.Clarke 9876 (BM).
Key to species of
1a Pinnae caudate-acuminate; indusia irregularly lacerate. D. subexaltata
1b Pinnae acuminate; indusia entire.
2a Rhizome creeping.
3a Basal pinnae bipinnate to tripinnatifid. D. renchangiana
3b Basal pinnae tripinnate to 4-pinnate. D. gaoligongensis
2b Rhizome erect to ascendent.
4a Stipe scales bicolorous: brown with pale edge. D. yoroii
4b Stipe scales concolorous: pale brown or brown.
5a Stipe and rachis brown to castaneous.
6a Fronds slightly dimorphic, pinnae with short stalks. D. sinonepalensis
6b Fronds monomorphic, pinnae with distinct stalks. D. cacaina
5b Stipe mostly stramineous, at least in middle to upper part, only brown in lower part.
7a Basal pinnae bipinnatifid to bipinnate. D. sparsa
7b Basal pinnae bipinnate to tripinnatifid. D. melanocarpa
Dryopteris polita was included in D. sect. Nephrocystis in Flora of China (Wu et al., 2013). A recent molecular phylogenetic study (Zhang et al., 2012) and our phylogeny suggest that it should be excluded from D. sect. Diclisodon.
5. ConclusionIn this study, we proposed three nomenclatural novelties in Dryopteris sect. Diclisodon: a new species, D. gaoligongensis; and two newly recognized species with new names, D. renchangiana and D. sinonepalensis. Our findings lead us to recommend that rhizome morphology be taken into account and more carefully recorded in future specimen collection of Dryopteris. We reconstructed the first phylogeny of D. sect. Diclisodon using combined sequence data of plastid regions and the nuclear AK1 gene. Our phylogeny implies that D. sparsa is a complex group that contains many genotypes. Future research on D. sect. Diclisodon should take an integrative approach that includes both morphological observation and molecular phylogeny based on a greater number of species, especially those from the D. sparsa complex.
Author contributionsZYZ and DZL designed research. ZYZ, TZ, and YX collected the materials. ZYZ, and DXY analyzed data. ZYZ, JML, DZL, as well as TZ, DXY wrote the paper.
Declaration of competing interestThe authors declare no competing interests.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.09.005.
AcknowledgmentsThe study was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDB31000000), the National Natural Science Foundation of China (31970232), and the Technological leading talent project of Yunnan (2017HA014). We also thank Mr. Wei-Ting Liou of National Taiwan University for sample collection, Dr. Fraser-Jenkins C.R., Dr. Li-Bing Zhang, and an anonymous reviewer for their constructive comments and suggestions.
Adanson, M., 1763. Familles naturelles des plantes. Paris: Chez Vincent, Imprimeur-Libraire de M le Comte de Provence
|
Ching, R.C., Liu, Z.Y., 1984. New ferns from Jinfoshan, Nanchuan, Sichuan (III). Bull. Bot. Res., 4: 1-35. |
Christensen, C., 1905. Index Filic., XXI. Hafniae
|
Copeland, E.B., 1910. Additions to the Bornean fern flora. Philipp. J. Sci. C., 5: 283-296. |
Darnaedi, D., Iwatsuki, K., 1987. On the structure and systematic position of the fern Dryopteris yakusilvicola Kurata. Fac. Sci. Univ. Tokyo Sect. III Bot., 14: 121-136. |
Darnaedi, D., Kato, M., Iwatsuki, K., 1989. Five new or ill-defined species related to Dryopteris sparsa (Dryopteridaceae). J. Jpn. Bot., 64: 299-310. |
Darnaedi, D., Kato, M., Iwatsuki, K., 1989. A cytotaxonomic study of Dryopteris sparsa and closely related species (Dryopteridaceae). J. Jpn. Bot., 64: 330-340. |
Darnaedi, D., Kato, M., Iwatsuki, K., 1990. Electrophoretic evidence for the origin of Dryopteris yakusilvicola (Dryopteridaceae). Bot. Mag. (Tokyo)., 103: 1-10. DOI:10.1007/BF02488406 |
Doyle, J.J., Doyle, J.L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull., 19: 11-15. |
Ebihara, A., 2017. The Standard of Ferns and Lycophytes in Japan II. Tokyo. Gakken
|
Fraser-Jenkins, C.R., 1986. A classification of the genus Dryopteris (Pteridophyta: Dryopteridaceae). Bull. Br. Mus. (Nat. Hist.) Bot., 14: 183-218. |
Fraser-Jenkins, C.R., 1989. A monograph of Dryopteris (Pteridophyta: Dryopteridaceae) in the Indian subcontinent. Bull. Br. Mus. (Nat. Hist.) Bot., 18: 323-477. |
Fraser-Jenkins, C.R., 2008. Taxonomic revision of three hundred Indian subcontinental pteridophytes with a revised census-list - a new picture of fern-taxonomy and nomenclature in the Indian subcontinent. Dehra Dun: Bishen Singh Mahendra Pal Singh
|
Fraser-Jenkins, C.R., Gandhi, K.N., Kholia, B.S., et al., 2018. An annotated checklist of Indian Pteridophytes II. Dehra Dun: Bishen Singh Mahendra Pal Singh
|
Gibby, M., 1985. Cytological observations on Indian subcontinental and Chinese Dryopteris and Polystichum (Pteridophyta: Dryopteridaceae). Bull. Brit. Mus. Nat. Hist. (Bot)., 14: 1-42. |
Hassler, M., 2018. World Ferns: Checklist of Ferns and Lycophytes of the World (version Apr 2018). In Roskov, Y., Abucay, L., Orrell, T., et al. (Eds.), Species 2000 & ITIS Catalogue of Life, 2018 Annual Checklist Digital resource at www.catalogueoflife.org/annual-checklist/2018
|
He, H., Zhang, L.B., Barrington, D.S., 2013. Dryopteris sect. Acrorumohra, in: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China, Vol. 2-3 (Pteridophytes). Beijing: Science Press; St. Louis: Missouri Botanical Garden Press, pp. 613-615
|
Hori, K., Ebihara, A., Murakami, N., 2021. Origin of the apogamous Japanese fern Dryopteris yakusilvicola (Dryopteridaceae). Taxon, 70: 16-26. DOI:10.1002/tax.12416 |
Imaichi, R., Darnaedi, D., Kato, M., 1987. Adventitious buds of Dryopteris sparsa complex (Dryopteridaceae): developmental anatomy and systematic implication. Bot. Mag. (Tokyo)., 100: 365-372. DOI:10.1007/BF02488855 |
Itô, H., 1935. Filices Japonenses II. Bot. Mag. (Tokyo)., 49: 432-437. DOI:10.15281/jplantres1887.49.432 |
Jin, J.J., Yu, W.B., Yang, J.B., et al., 2020. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol., 21: 1-31. DOI:10.1117/1.oe.59.1.016110 |
Katoh, K., Misawa, K., Kuma, K., et al., 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res., 30: 3059-3066. DOI:10.1093/nar/gkf436 |
Kearse, M., Moir, R., Wilson, A., et al., 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28: 1647-1649. DOI:10.1093/bioinformatics/bts199 |
Kuo, L.Y., Chang, Y.S., Glowienka, J.M., et al., 2016. A revised framework of Dryopteris subg. Nothoperanema (Dryopteridaceae) inferred from phylogenetic evidence, with descriptions of two new sections. Syst. Bot., 41: 596-605. DOI:10.1600/036364416X692334 |
Liou, W.T., 2010. A taxonomy study of Dryopteris subgenus Nephrocystis (Dryopteridaceae) in Taiwan. Taipei, National Taiwan Normal University
|
PPG, I., 2016. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol., 54: 563-603. DOI:10.1111/jse.12229 |
Ronquist, F., Teslenko, M., van der Mark, P., et al., 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol., 61: 539-542. DOI:10.1093/sysbio/sys029 |
Sessa, E.B., Zhang, L.B., Väre, H., et al., 2015. What we do (and don't) know about ferns: Dryopteris (Dryopteridaceae) as a case study. Syst. Bot., 40: 387-399. DOI:10.1600/036364415X688844 |
Sessa, E.B., Zimmer, E.A., Givnish, T.J., 2012. Unraveling reticulate evolution in north American Dryopteris (Dryopteridaceae). BMC Evol. Biol., 12: 1-24. DOI:10.1186/1471-2148-12-1 |
Stamatakis, A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312-1313. DOI:10.1093/bioinformatics/btu033 |
Tindale, M.D., Roy, S.K., 2002. A cytotaxonomic survey of the Pteridophyta of Australia. Aust. Syst. Bot., 15: 839-937. DOI:10.1071/SB00034 |
10.12705/Code.2018 |
Wang, Z.R., Zhang, Z.X., 1981. Cytological observation on some Chinese ferns. Acta Bot. Sin., 23: 427-433. |
Weng, R.F., 1989. Cytological observation on Dryopteris from East China. In: Proceeds of international symposium on systematic Pteridology, Shing, K.H. and Kramer, K.U. (Eds.), Beijing: China Science and Technology Press, pp. 141-146
|
Wick, R.R., Schultz, M.B., Zobel, J., et al., 2015. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics, 31: 3350-3352. DOI:10.1093/bioinformatics/btv383 |
Widén, C.J., Fraser-Jenkins, C.R., Reichstein, T., et al., 2001. A survey of phenolic compounds in Dryopteris and related fern genera. Part III. Phloroglucinol derivatives in subgenera Erythrovaria and Nephrocystis and related genera (Pteridophyta, Dryopteridaceae). Ann. Bot. Fenn., 38: 99-138. |
Wu, S.G., Lu, S.G., 2000. Dryopteris, In: Wu, C.Y. (Ed.), Flora Reipublicae Popularis Sinicae, Vol. 5(1) (Dryopteridaceae). Beijing: Science Press, pp. 102-220
|
Wu, S.G., Xiang, J.Y., Lu, S.G., et al., 2013. Dryopteris, in: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China, Vol. 2-3 (Pteridophytes). Beijing: Science Press; St. Louis: Missouri Botanical Garden Press, pp. 571-628
|
Xiang, J.Y., Cheng, X., Wu, S.G., 2006. Chromosome numbers of 13 species in the genus Dryopteris (Dryopteridaceae) from Yunnan, China. Acta Phytotaxon. Sin., 44: 304-319. DOI:10.1360/aps040152 |
Zhang, L.B., 2012. Reducing the fern genus Dryopsis to Dryopteris and the systematics and nomenclature of Dryopteris subgenus Erythrovariae section Dryopsis (Dryopteridaceae). Phytotaxa, 71: 17-27. DOI:10.11646/phytotaxa.71.1.4 |
Zhang, L.B., Zhang, L., 2012. The inclusion of Acrophorus, Diacalpe, Nothoperanema, and Peranema in Dryopteris: the molecular phylogeny, systematics, and nomenclature of Dryopteris subg. Nothoperanema (Dryopteridaceae). Taxon, 61: 1199-1216. DOI:10.1002/tax.616003 |
Zhang, L.B., Zhang, L., Dong, S.Y., et al., 2012. Molecular circumscription and major evolutionary lineages of the fern genus Dryopteris (Dryopteridaceae). BMC Evol. Biol., 12: 180. DOI:10.1186/1471-2148-12-180 |



