b. University of Chinese Academy of Sciences, Beijing 100049, China
Plant functional traits are morphological, biochemical, physiological and phenological features that can be quantified or qualitatively described (Díaz et al., 2013). Functional traits, which evolve in response to natural selection, provide crucial information about the ecological strategies of plants, and are, thus, widely used to investigate important ecological issues (Violle et al., 2007; Zanne et al., 2014). Previous studies have explicitly examined the relationship between functional traits and fitness to explore the mechanisms that underlie how species coexist and biodiversity is maintained in communities (Violle et al., 2012; Adler et al., 2014). Community ecologists also incorporate both phylogenetic and functional approaches to reveal the processes that structure community assemblages (Webb et al., 2002; Yang et al., 2014), which are based on the premise that close relatives are more ecologically similar (Losos, 2008). Functional trait analysis requires the quantification of phylogenetic signal, or the tendency of related taxa to appear more ecologically similar. The relationship between phylogenetic relatedness and ecological similarity between species is not always easy to predict (Silvertown et al., 2005; Swenson, 2013). For example, environmental filtering is considered a critical factor that structures communities, especially in stressful environments (Coyle et al., 2014). Extreme environmental conditions have been shown to select for a limited range of traits, filtering out species with unviable traits (Kraft et al., 2015). In these environmental conditions, traits between species are more likely to be convergent (Coyle et al., 2014; Akram et al., 2020), with weak or non-significant phylogenetic signals (Losos, 2008). However, in a fire-stressed Brazilian savanna, significant phylogenetic signals were also detected in leaf traits (Cianciaruso et al., 2012). Furthermore, at more refined scales, substantial phenotypic plasticity (i.e. intraspecific trait variation) may be more important than interspecific variation (Cianciaruso et al., 2012; Dantas et al., 2013), for phenotypic plasticity might interfere the detection of phylogenetic signal (Losos, 2008).
Intraspecific trait variation (ITV) is closely related to local environmental conditions (Cochrane et al., 2015; Umaña et al., 2015). Generally, species tend to exhibit more ITV in complex forests, and less ITV in habitats with relatively homogeneous environmental conditions (Sultan, 2000; Umaña et al., 2015). At local scales, species demonstrate strong adaptive capacity by changing leaf traits in response to micro-environmental gradients, such as variation in light availability associated with vertical gradients (Coopman et al., 2011; Kenzo et al., 2015). Previous studies suggest that, in forest communities, ontogenetic environment and changes in canopy positions lead to strong ITV in tree species. For example, in tropical rain forests, Parashorea chinensis H. Wang, a dominant species, has been observed to shift adaptive strategies throughout development from shade-tolerant to competitive in response to changes in both light and tree height level (Deng et al., 2020). At the top of the canopy, twigs of some tree species respond to restricted water-supply and potential water deficits by producing many vessels with minimal size, which reduces the possibility that the entire conduction system will collapse due to embolisms in single vessels (Kafuti et al., 2020). However, previous studies have been limited by small sample sizes due to the difficulties of accessing the upper parts of trees in forest canopies (Keenan and Niinemets, 2016). In addition, sampling leaves in the lower part of trees (shade leaves) may bias the measurement of plasticity and adaptability of plant traits (Hulshof and Swenson, 2010). Accordingly, leaf sampling at different heights of the canopy is crucial for studies on plant ITV. Furthermore, a nested sampling design can reveal how traits change across scales, thereby clarifying the contribution of intraspecific variation to phenotypic variation in traits (Messier et al., 2010; Siefert et al., 2015).
Savannas differ from closed-canopy forests. Savanna community structure is simplified due to the strong stress of seasonal drought and herbivory. Trees and shrubs in savanna are very short and sparsely scattered over the continuous grass layer, leaving the crowns apart from each other (Walter, 1985). Plants in such habitats have adapted morphologically and physiologically to less moisture, intensive solar irradiance, and strong evapotranspiration (Borchert, 1994; Lüttge, 2008). For instance, specific leaf area (SLA) is lower in savanna trees than in evergreen riparian forest (Hoffmann et al., 2005), and leaf turgor loss point (πtlp), a key trait representing plant drought tolerance, is higher in dry biomes (Bartlett et al., 2012a).
One noteworthy savanna community is located in the dry-hot habitats of southwestern China. Although previous studies have examined the response and adaptability of plants in these communities to harsh environmental conditions at the species-level (Zhu et al., 2009; Zhang et al., 2012), ITV has yet to be investigated. In this study, we ask the following questions: How strong are the phylogenetic signals in leaf traits under such stressful environmental condition? What are the ITV patterns of leaf traits along the vertical stratification of woody species? What are the patterns of trait change across scales in savanna community?
2. Material and methods 2.1. Study siteThe savanna vegetation in SW China mainly occurs in Yunnan Province in the deep valleys of Jinshajiang, Yuanjiang, and Nujiang (below 1000 m), which are predominated by hot-dry climate caused by the foehn effect (Wu et al., 1987; Zhu et al., 2020). The physiognomy of Yunnan savanna vegetation is similar to that in India–Myanmar, sharing some common species with African savannas, such as Woodfordia fruticosa (L.) Kurz and Calotropis gigantea (L.) W.T. Aiton (Zhu et al., 2020). The floras of Yunnan savanna are influenced by the extrusion of the Indochina Plate, the northward movement of the Burma Plate, and river direction (the northwest-southeast in Yuanjiang; north-south in Nujiang), which allow the northward migration of tropical elements. Consequently, these floras are dominated by tropical elements with a large proportion of tropical Asian elements (Zhu et al., 2020).
This study was conducted in a savanna community in Yuanjiang County (23°28′N, 102°10′E), Yunnan Province, southwest China. The Ailao Mountains and Wuliang Mountains in the west obstruct monsoon rainfall from the Indian Ocean, resulting in a hot-dry climate (Wu et al., 1987). Mean annual temperature is 24.7 ℃, with a mean temperature ranging from 16.8 ℃ in the coldest month to 28.7 ℃ in the hottest month. Mean annual rainfall is 732.8 mm, of which more than 85% occurs in the rainy season (May to October). Annual potential evaporation is 1750 mm (based on the data collected by Yuanjiang Savanna Ecological Station, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences). Plant communities growing in this area are exposed to strong seasonality, with little rainfall and high evaporation. As a result, plant communities in these valley areas mainly consist of drought-tolerant shrubs and herbs, over which few trees are scattered (Li and Walker, 1986). Tree and shrub layers range from 2 to 6 m high, and commonly include Lannea coromandelica (Houtt.) Merr., Polyalthia cerasoides (Roxb.) Benth. et Hook. f. ex Bedd., Pistacia weinmanniifolia J. Poisson ex Franchet. The grass layer is dominated by Heteropogon contortus (L.) P. Beauv. ex Roem. et Schult., which grows continuously under the trees and shrubs.
2.2. Collection and measurement of functional traitsWe randomly selected nine dominant woody species (six deciduous and three evergreen) from the savanna community during the dry season (April 2020) (Table 1). For each species, we collected leaves from 30 randomly selected individuals. Leaf samples were collected from six tree species at two heights: the upper layer (3–4 m) and the lower layer (1–2 m). The average height of the three shrub species is 2 m; therefore, leaves were collected from the top of plant. For simple-leaved plants, 10 leaves from each layer of an individual were sampled. For compound-leaved plants, three leaves from each layer of an individual were collected, and every leaflet in a leaf was measured.
| Species | Abbreviation | Leaf type | Life form | Leaf phenology |
| Polyalthia cerasoides | POLYCE | Simple leaf | Tree | Deciduous plant |
| Lannea coromandelica | LANNCO | Compound leaf | Tree | Deciduous plant |
| Diospyros yunnanensis | DIOSYU | Simple leaf | Tree | Deciduous plant |
| Pistacia weinmanniifolia | PISTWE | Compound leaf | Tree | Evergreen plant |
| Tarenna depauperata | TAREDE | Simple leaf | Shrub | Evergreen plant |
| Woodfordia fruticosa | WOODFR | Simple leaf | Shrub | Deciduous plant |
| Vitex negundo | VITENE | Compound leaf | Shrub | Deciduous plant |
| Olea europaea subsp. cuspidata | OLEAEU | Simple leaf | Tree | Evergreen plant |
| Haldina cordifolia | HALDCO | Simple leaf | Tree | Deciduous plant |
We used standard protocols to measure eight key functional traits that reflect plant adaptability to the arid environments (Pérez-Harguindeguy et al., 2013); leaf turgor loss point was measured according to Bartlett et al. (2012b), and leaf chlorophyll content was measured according to Vos and Bom (1993) (Table S1). We collected the environmental data (including air temperature, relative air humidity and photosynthetically active radiation) from the eddy covariance system and from a meteorological station (Fei et al., 2017). Specific leaf area (SLA) and leaf dry matter content (LDMC) reflect fundamental functional trade-offs between rapid material production (high SLA with low LDMC) and efficient nutrient conservation (low SLA with high LDMC) (Poorter and Garnier, 1999). Leaf area (LA) characterizes trade-offs between carbon fixation, water loss and heat dissipation (Wright et al., 2017). Leaf thickness (LT) indicates investment in defense (e.g., preventing intensive irradiance) and photosynthesis (Niinemets, 2001). Leaf chlorophyll content (LCC) symbolizes photosynthetic capacity of plant (Croft et al., 2017). Leaf turgor loss point (πtlp) reflects plant drought tolerance (Bartlett et al., 2012a). Leaf carbon content (Leaf C) indicates nutritional quality and palatability of leaves. Leaf nitrogen content (Leaf N) indicates the photosynthetic capability of leaf (Pérez-Harguindeguy et al., 2013).
2.3. Phylogenetic tree reconstructionWe used the APG Ⅲ taxonomic phylogenetic framework (Phylomatic tree version) to reconstruct the phylogenetic structure of all sampled species at the family level (Zanne et al., 2014); we then used the “ape” package in R 4.0.2 to randomly resolve polytomies, and reconstruct a binary phylogenetic tree (Paradis et al., 2004).
2.4. Data analysisThe phylogenetic signal in eight functional traits for target species was tested using the Brownian motion evolutionary model (Blomberg et al., 2003). Blomberg's K was used to measure the deviation of trait variation from random variation under the assumption of Brownian evolution. A value of K = 1 indicates that the evolution of a given trait corresponds to the Brownian motion evolutionary model; a value of K < 1 indicates a convergent evolutionary pattern of a target trait; and a value of K > 1 indicates a high phylogenetic conservatism of a given trait. The significance of phylogenetic signals were assessed by a randomization test. By comparing the observed value of K to the distribution of K values that were obtained by shuffling the traits across the tips in phylogenetic tree 999 times, a p value was obtained to assess significance of phylogenetic signals. The phylogenetic signal and significance tests were performed using the ‘Picante’ package in R 4.0.2 (Kembel, 2009).
We used the student t-test to test for differences in environmental factors (air temperature, relative air humidity and photosynthetically active radiation) between the two leaf layers (upper layer = 3–4 m vs. lower layer = 1–2 m). We calculated coefficient of variation (CV = sd/mean × 100%) to quantity intraspecific variation for each trait at the individual level and at the layer level. To test for trait differences among species, we calculated the average of all sampled leaves in each individual for each trait, then performed an ANOVA. We used Principal Components Analysis (PCA) to analyze sample similarity based on individual data (all samples were classified as nine species and two leaf phenologies).
Variance decomposition was implemented to evaluate how traits (SLA, LDMC, LA, LT, LCC and πtlp) varied across different scales (leaf phenology, species, individual, layer and leaf). The variance at each scale was transformed on a proportional scale. Leaf phenology was classified as evergreen or deciduous. When analyzing contributions to trait variations, we took into account that the six tree species were divided into two layers, and the three shrub species were sampled at the upper layer. Variance decomposition was performed using ‘varcomp’ function in R 4.0.2 (Messier et al., 2010).
3. Results 3.1. Phylogenetic signal testing of functional traitsValues for Blomberg's K for LA, LCC, Leaf C, Leaf N and πtlp were less than 1, ranging from 0.49 for LCC to 0.87 for πtlp (Table 2); K values of SLA, LDMC and LT were higher than 1 (Table 2). In the plants sampled, no trait exhibited a significant phylogenetic signal, indicating that ecological and phylogenetic similarities were inconsistent (p > 0.05).
| Traits | K value | p value |
| Leaf area (LA) | 0.74 | 0.407 |
| Specific leaf area (SLA) | 1.09 | 0.080 |
| Leaf thickness (LT) | 1.01 | 0.111 |
| Leaf dry matter content (LDMC) | 1.02 | 0.912 |
| Leaf chlorophyll content (LCC) | 0.49 | 0.130 |
| Leaf turgor loss point (πtlp) | 0.87 | 0.200 |
| Leaf carbon content (Leaf C) | 0.66 | 0.447 |
| Leaf nitrogen content (Leaf N) | 0.80 | 0.300 |
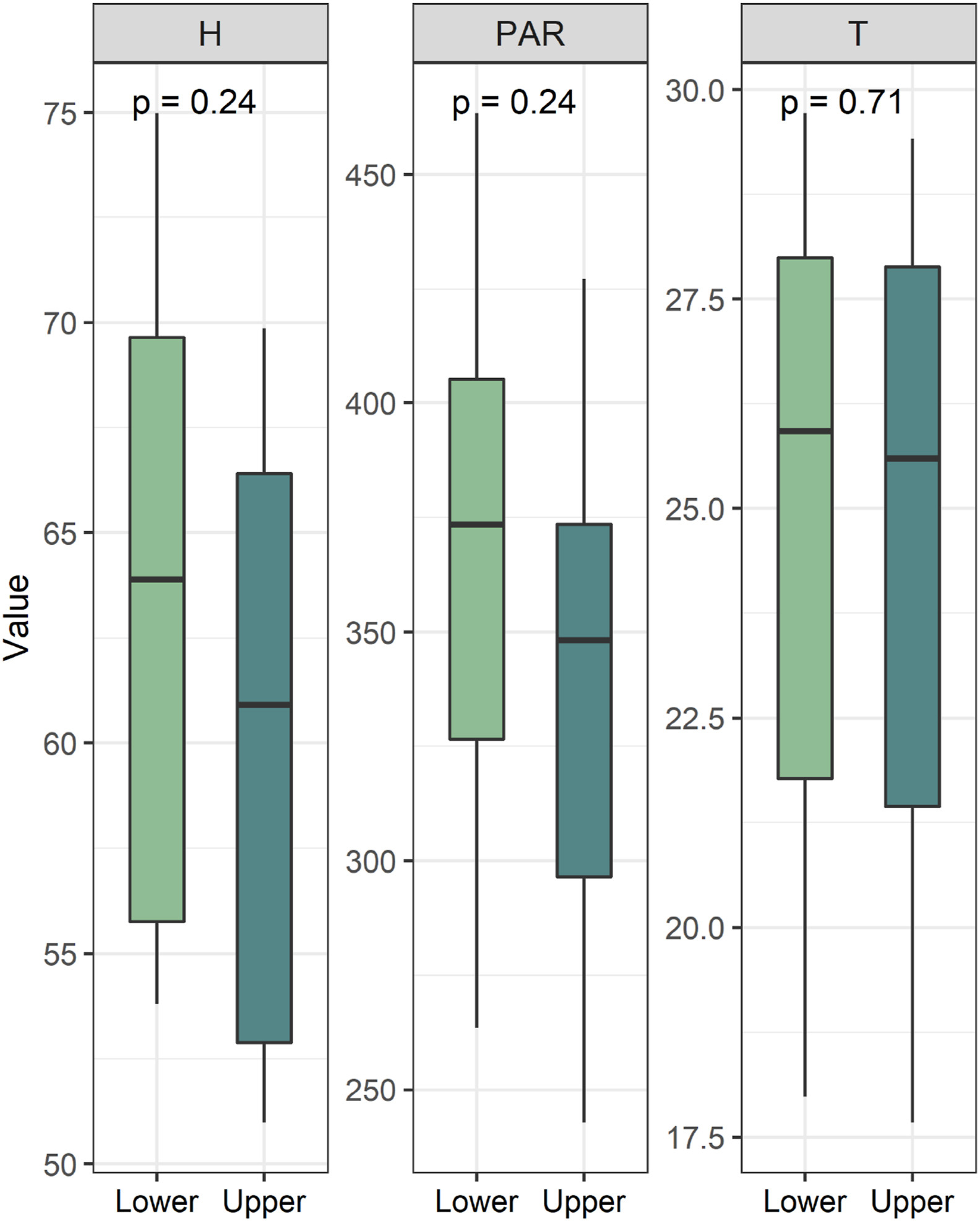
Photosynthetically active radiation, air temperature and relative air humidity were not significantly different between the two canopy layers (p > 0.05) (Fig. 1).
|
| Fig. 1 Comparison of major environmental factors of two canopy layers. H: Relative air humidity (%); PAR: Photosynthetically active radiation (μmol/m2s); T: Air temperature (℃). |
Traits varied intra-specifically and interspecifically at both the individual- and layer-level (Tables 3, S3 and S4). For most species, LA, SLA, LDMC and Leaf N varied more than other traits (Table 3). LA exhibited the highest average intraspecific variation (average CV = 24.83%), whereas Leaf C had the lowest variation (CV = 1.44%). For the tree species, trait variation between the upper and lower leaf levels was low (CV = 0.07%–8.68%, Table S3).
| Intraspecific level | Coefficient of variation CVs | |||||||
| LA | SLA | LT | LDMC | LCC | πtlp | Leaf C | Leaf N | |
| Canopy layer level | 4.52% | 3.49% | 2.80% | 1.40% | 1.25% | 0.85% | – | – |
| Individual level | 24.83% | 14.59% | 10.62% | 12.71% | 9.28% | 9.29% | 1.44% | 11.92% |
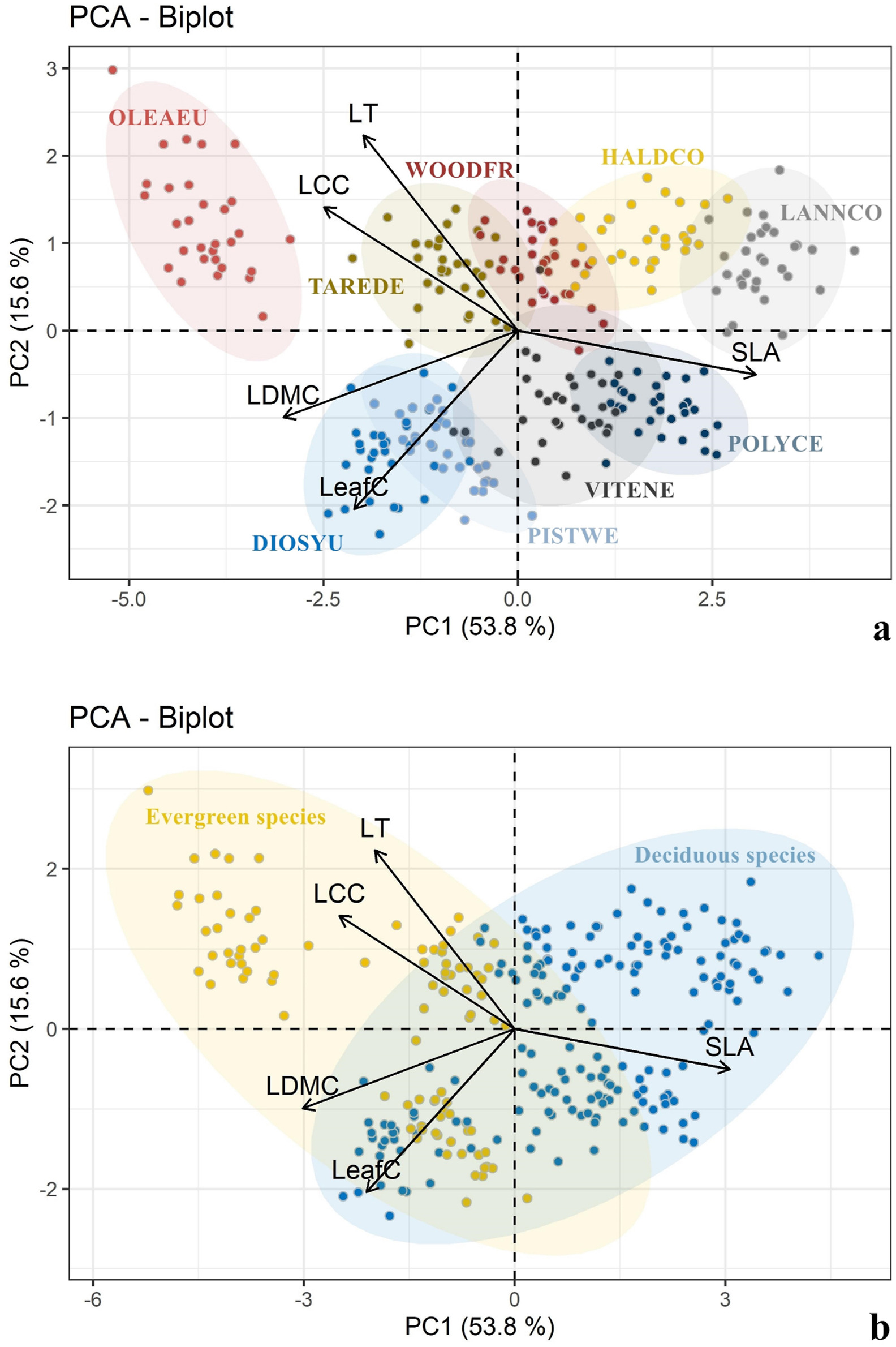
Leaf functional traits differed significantly between species (Fig. 2, Fig. 3a; Table S2). PCA clustered evergreen and deciduous species into different groups. Evergreen species gathered in the left of PC1 axis, reflecting more conservative strategies (Fig. 3b). At the same time, the contribution of each trait to the first two principal component axes was different between evergreen species and deciduous species, but the correlations between traits were basically the same (Figs. S1 and S2).

|
| Fig. 2 ANOVA of plant traits. a–h: Distribution of the trait values (after log 10 transformed) among nine woody species. Abbreviations of the species names refer to Table1. The dotted line indicates the mean value of corresponding trait for all species. “∗” and “ns” represent the difference level of trait between corresponding species and the mean value. ns: no significant difference; ∗: p < 0.05; ∗∗: p < 0.01; ∗∗∗: p < 0.001; ∗∗∗∗: p < 0.0001. |
|
| Fig. 3 Principle component analysis (PCA) based on individual data. (a) PCA of nine woody species. (b) PCA between deciduous species and evergreen species. Small solid circles represent individual samples, while the distance between ellipses represent the similarity of the corresponding species. The top five variables with the highest contribution to the principal components were plotted. Abbreviations of the species names and the leaf phenology (evergreen vs. deciduous) of species refer to Table1. |
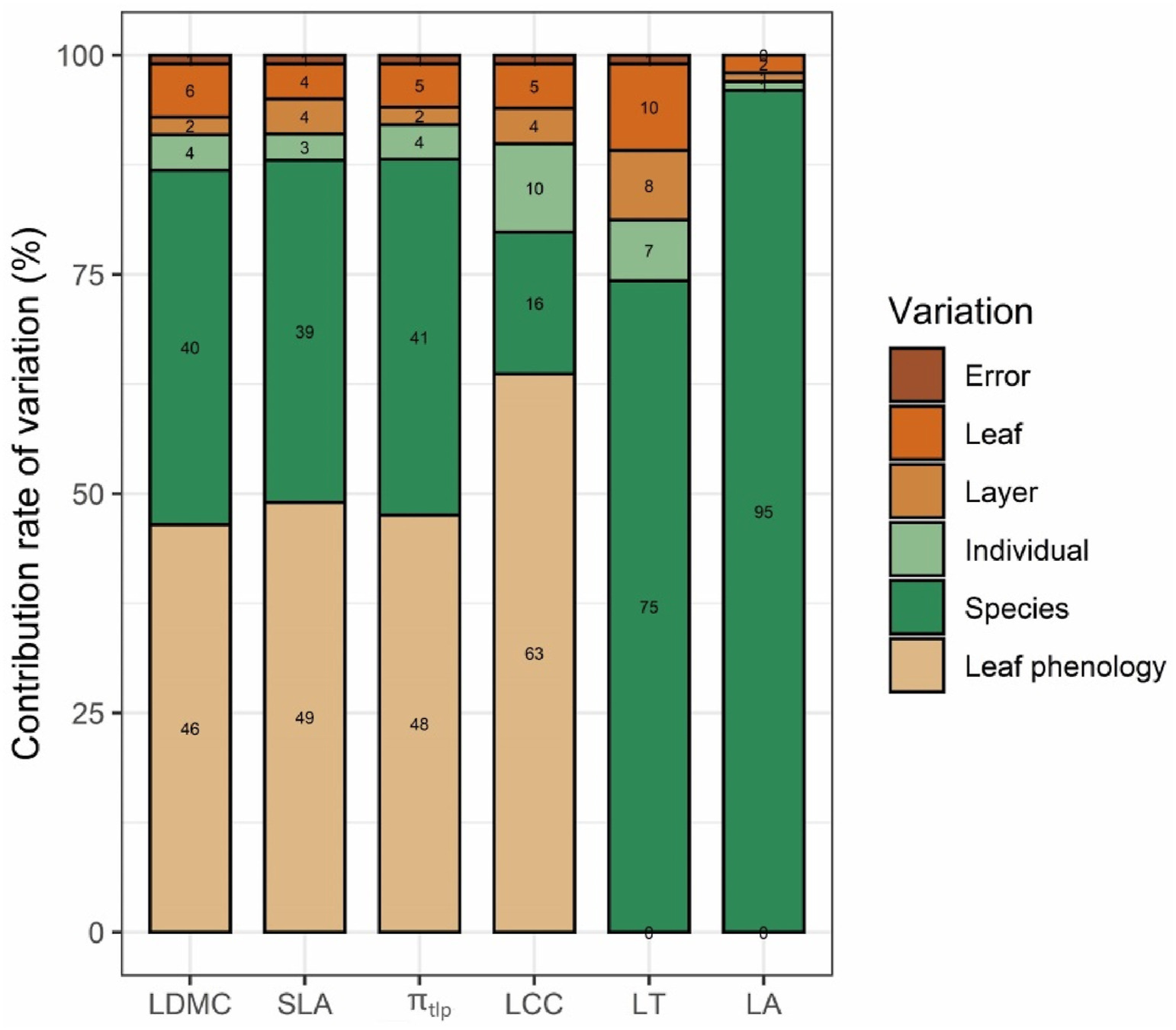
Variation caused by each scale varied among traits (Fig. 4). For example, interspecific variation contributed the majority of total variance in LA (95%) and LT (75%). Interspecific variation and variation in leaf phenology contributed equally scale (around 40%) to total variance of LDMC, SLA and πtlp, with the remaining 20% explained by intraspecific variation (including leaf-, canopy- and individual-scales). For LCC, variation in leaf phenology accounted for 63% of total variance, whereas interspecific variation accounted for only 16% of total variance.
|
| Fig. 4 Variance structure of six leaf functional traits across six scales. LDMC: leaf dry matter content; SLA: specific leaf area; πtlp: leaf turgor loss point; LCC: leaf chlorophyll content; LT: leaf thickness; LA: leaf area. |
In this study, we found that savanna woody species have lower ITV at both the individual- and layer-level. Similar results have been reported from a long-term nutrient manipulation study in a Brazilian savanna, suggesting that nutrient-poor environments lead to conservatism of traits (Scalon et al., 2017). In the dry season, plants are threatened by intense seasonal drought and radiation, and are particularly vulnerable to photoinhibition and even photodamage (Zhang et al., 2012, 2016). In habitats with strong abiotic stresses, where resources are limited, plant phenotype is severely restricted (Valladares et al., 2007). In other words, resources limit the degree of variation in traits (Sultan, 2000; Gotsch et al., 2010). Accordingly, savanna species generally maximize resource utilization for local species (Sultan, 2000) by limiting ITV (e.g., bark thickness, δ13C, De Cássia-Silva et al., 2017).
In a closed forest, significant micro-environmental gradients occur across the canopy (Parker, 1995). For instance, one important factor that determines trait variation is the position (i.e., the height) within an individual plant, because in forests as height increases water availability is increasingly limited (Kafuti et al., 2020). In savannas, however, species are usually short and in an open canopy; thus, variation in leaf functional traits within the canopy are predicted to be much lower than that in closed forests with taller trees. In our study, the two canopy strata were exposed to few differences in air temperature, air relative humidity or photosynthetically active radiation, reflecting the relatively homogeneous environmental conditions within the crown (Fig. 1). These local species, subject to stabilizing selection, are under less pressure to adapt to environmental changes. Consequently, plants living in less heterogeneous habitat show low ITV (Umaña et al., 2015). The low ITV of dominant woody species could probably be explained by specific ecological conditions in the savanna, and both multiple stresses and relatively homogeneous environment are likely to be the driving factors.
4.2. Leaf phenology determines traits syndromesSpecies that coexist within the same habitat often adapt to similar constraints via alternative means, as shown by differences in trait values and trait covariation (Scalon et al., 2017; Fyllas et al., 2020). Similar trait syndromes can achieve approximately equal fitness in the same habitat (Marks and Lechowicz, 2006). We found that in savanna communities leaf functional traits still differed significantly among species (Fig. 2), indicating that various combinations of traits are feasible in infertile and stressful habitats. Furthermore, PCA showed that deciduous species displayed greater resource acquisition ability than evergreen species (Fig. 3b), with different investment in different traits (Figs. S1 and S2). Savannas contain numerous woody species that drop leaves during the dry season (Williams et al., 1989), limiting the period plants are able to photosynthesize. For these plants, larger SLA (Pringle et al., 2011) and higher leaf nitrogen content (Field and Mooney, 1986) allow plants to photosynthesize efficiently. In plants that grow in areas with a high resource supply (e.g., long rainy season), the cost of leaf production is low (Coley et al., 1985). For instance, deciduous species have commonly been observed to have high leaf water content (i.e. lower LDMC) (Powers and Tiffin, 2010). Conversely, plants exposed to multiple stresses throughout year, such as evergreen plants, bear a higher cost of leaf construction and maintenance, and thus produce long-lived schlerophyllous leaves (Williams et al., 1989). These smaller and thicker leaves increase the number of mesophyll cells per leaf, increasing LCC, and consequently reducing the damage caused by intensive light in the dry season (Bosabalidis and Kofidis, 2002). In addition, photosynthesis and transpiration is balanced in leaves with a higher πtlp value, which allows evergreen plants to maintain greater drought-resistance over long periods of water deficiency (Bartlett et al., 2012a; Zhu et al., 2018). Our results suggest that species with similar leaf phenology seem to have similar trait syndromes.
4.3. Intraspecific variation contributes little to trait variationPrevious studies have reported that traits seem to respond independently to different drivers among different biomes (Auger and Shipley, 2012; Siefert et al., 2015). In lowland tropical forests, variance in morphological traits (LMA and LDMC) is derived equally from intraspecific and interspecific variation, indicating that small-scale environmental gradients and genetic variation contribute approximately the same amount to trait variation (Messier et al., 2010). In the savanna community we studied, traits varied differently across scales (leaf phenology, species, individual, layer and leaf). Specifically, intraspecific variation (including leaf-, layer- and individual-scales) contributed less to total trait variance (<30%). For example, the majority of the total variance in LA and LT was related to interspecific differences (>75%), indicating that the major variation in leaf area and leaf thickness is driven by genetic differences (Messier et al., 2017). Our finding that total variance in LA is largely due to interspecific differences (95%) is consistent with results from global-scale studies (Wright et al., 2017; Kleyer et al., 2018). We also found that SLA, LDMC and πtlp had similar patterns of variance across scales, and that interspecific variation and variation in leaf phenology contributed equally to total variance (around 40%). LCC, the least species-specific trait, is greatly sensitive to different leaf phenology, which contributed 63% to phenotypic variation. This conclusion was also supported by the extent to which leaf chlorophyll content contributes to variation in evergreen species and deciduous species (Figs. S1 and S2). Our study revealed that intraspecific variation (including at the leaf-, layer- and individual-scales) contributed little to overall trait variation in a savanna community, whereas leaf phenology and interspecific variation are both important to variation in SLA, LDMC, πtlp and LCC.
5. ConclusionsIn the savanna community studied, no significant phylogenetic signal was detected in leaf traits, although this finding may have been influenced by small sample size. Stressful and infertile conditions of the savanna severely restricted trait variation within species, and the lower and relatively open canopy created smaller heterogeneity in environmental gradients, resulting in the lower ITVs within individuals. However, great differences occurred in traits among coexisting species, revealing that alternative functional designs are feasible even under habitats with great pressures and multiple resources constraints. Finally, intraspecific variation contributed little to total variance in all traits observed in savanna community, whereas interspecific variation and variation in leaf phenology contributed to substantial variation.
Author contributionM.C. and J.Y. conceived the ideas and designed methodology; Q.H.S. summarized the environmental data; L.B.L. collected and analyzed the data, and took charge of the manuscript writing. M.C. and J.Y. contributed critically to manuscript writing. All authors agreed to publish.
Declaration of competing interestWe declare that there is no commercial or associated conflict of interest that could influence the review of the manuscript entitled “Intraspecific trait variation of woody species reduced in a savanna community, southwest China”.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.06.002.
AcknowledgementsThis research was supported by the National Natural Science Foundation of China (31870410 and Dimensions US-China: 32061123003), the Chinese Academy of Sciences Youth Innovation Promotion Association, the West Light Foundation of the Chinese Academy of Sciences and the Ten Thousand Talents Program of Yunnan (YNWR-QNBJ-2018-309). We thank all the staff of Yuanjiang Savanna Ecological Station (YSES), for their dedication and assistance in the performance of field work. We are grateful to Public Technology Service Center XTBG, for providing professional analysis of our specimens. Thanks are also due to Global Change Group for setting up the eddy covariance system in 1-ha monitoring plot at YSES, from which we obtained environmental data.
Adler, P.B., Salguero-Gomez, R., Compagnoni, A., et al., 2014. Functional traits explain variation in plant life history strategies. Proc. Natl. Acad. Sci. U.S.A., 111: 740-745. DOI:10.1073/pnas.1315179111 |
Akram, M.A., Wang, X., Hu, W., et al., 2020. Convergent variations in the leaf traits of desert plants. Plants, 9: 990. DOI:10.3390/plants9080990 |
Auger, S., Shipley, B., 2012. Inter-specific and intra-specific trait variation along short environmental gradients in an old-growth temperate forest. J. Veg. Sci., 24: 419-428. |
Bartlett, M.K., Scoffoni, C., Ardy, R., et al., 2012. Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods Ecol. Evol., 3: 880-888. DOI:10.1111/j.2041-210X.2012.00230.x |
Bartlett, M.K., Scoffoni, C., Sack, L., 2012. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol. Lett., 15: 393-405. DOI:10.1111/j.1461-0248.2012.01751.x |
Blomberg, S.P., Garland, T.J.R., Ives, A.R., 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution, 57: 717-745. DOI:10.1111/j.0014-3820.2003.tb00285.x |
Borchert, R., 1994. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology, 75: 1437-1449. DOI:10.2307/1937467 |
Bosabalidis, A.M., Kofidis, G., 2002. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci., 163: 375-379. DOI:10.1016/S0168-9452(02)00135-8 |
Cianciaruso, M.V., Silva, I.A., Batalha, M.A., et al., 2012. The influence of fire on phylogenetic and functional structure of woody savannas: moving from species to individuals. Perspect. Plant Ecol. Evol. Syst., 14: 205-216. DOI:10.1016/j.ppees.2011.11.004 |
Cochrane, A., Yates, C.J., Hoyle, G.L., et al., 2015. Will among-population variation in seed traits improve the chance of species persistence under climate change?. Global Ecol. Biogeogr., 24: 12-24. DOI:10.1111/geb.12234 |
Coley, P.D., Bryant, J.P., Chapin, F.S., 1985. Resource availability and plant antiherbivore defense. Science, 230: 895-899. DOI:10.1126/science.230.4728.895 |
Coopman, R.E., Briceno, V.F., Corcuera, L.J., et al., 2011. Tree size and light availability increase photochemical instead of non-photochemical capacities of Nothofagus nitida trees growing in an evergreen temperate rain forest. Tree Physiol., 31: 1128-1141. DOI:10.1093/treephys/tpr094 |
Coyle, J.R., Halliday, F.W., Lopez, B.E., et al., 2014. Using trait and phylogenetic diversity to evaluate the generality of the stress-dominance hypothesis in eastern North American tree communities. Ecography, 37: 814-826. DOI:10.1111/ecog.00473 |
Croft, H., Chen, J.M., Luo, X., et al., 2017. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol., 23: 3513-3524. DOI:10.1111/gcb.13599 |
Dantas, V.L., Pausas, J.G., Batalha, M.A., et al., 2013. The role of fire in structuring trait variability in Neotropical savannas. Oecologia, 171: 487-494. DOI:10.1007/s00442-012-2431-8 |
De Cássia-Silva, C., Cianciaruso, M.V., Maracahipes, L., et al., 2017. When the same is not the same: phenotypic variation reveals different plant ecological strategies within species occurring in distinct Neotropical savanna habitats. Plant Ecol., 218: 1221-1231. DOI:10.1007/s11258-017-0765-3 |
Deng, Y., Deng, X.B., Dong, J.L., et al., 2020. Detecting growth phase shifts based on leaf trait variation of a canopy dipterocarp tree species (Parashorea chinensis). Forests, 11: 1145. DOI:10.3390/f11111145 |
Díaz, S., Purvis, A., Cornelissen, J.H.C., et al., 2013. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol., 3: 2958-2975. DOI:10.1002/ece3.601 |
Fei, X.H., Jin, Y.Q., Zhang, Y.P., et al., 2017. Eddy covariance and biometric measurement show that a savanna ecosystem in Southwest China is a carbon sink. Sci. Rep., 7: 41025. DOI:10.1038/srep41025 |
Field, C.B., Mooney, H.A., 1986. The photosynthesis-nitrogen relationship in wild plants. In: Givnish, T.J. (Eds.), On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, pp. 25-55
|
Fyllas, N.M., Michelaki, C., Galanidis, A., et al., 2020. Functional trait variation among and within species and plant functional types in mountainous mediterranean forests. Front. Plant Sci., 11: 10.3389/fpls.2020.00212. DOI:10.3389/fpls.2020.00212 |
Gotsch, S.G., Powers, J.S., Lerdau, M.T., 2010. Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: patterns of intra-specific variation across forests and seasons. Plant Ecol., 211: 133-146. DOI:10.1007/s11258-010-9779-9 |
Hoffmann, W.A., Franco, A.C., Moreira, M.Z., et al., 2005. Specific leaf area explains differences in leaf traits between congeneric savanna and forest trees. Funct. Ecol., 19: 932-940. DOI:10.1111/j.1365-2435.2005.01045.x |
Hulshof, C.M., Swenson, N.G., 2010. Variation in leaf functional trait values within and across individuals and species: an example from a Costa Rican dry forest. Funct. Ecol., 24: 217-223. DOI:10.1111/j.1365-2435.2009.01614.x |
Kafuti, C., Bourland, N., De Mil, T., et al., 2020. Foliar and wood traits covary along a vertical gradient within the crown of long-lived light-demanding species of the Congo basin semi-deciduous forest. Forests, 11: 1-18. |
Keenan, T.F., Niinemets, U., 2016. Global leaf trait estimates biased due to plasticity in the shade. Nat. Plants, 3: 16201. |
Kembel, S.W., 2009. Disentangling niche and neutral influences on community assembly: assessing the performance of community phylogenetic structure tests. Ecol. Lett., 12: 949-960. DOI:10.1111/j.1461-0248.2009.01354.x |
Kenzo, T., Inoue, Y., Yoshimura, M., et al., 2015. Height-related changes in leaf photosynthetic traits in diverse Bornean tropical rain forest trees. Oecologia, 177: 191-202. DOI:10.1007/s00442-014-3126-0 |
Kleyer, M., Trinogga, J., Cebrián-Piqueras, M.A., et al., 2018. Trait correlation network analysis identifies biomass allocation traits and stem specific length as hub traits in herbaceous perennial plants. J. Ecol., 107: 829-842. |
Kraft, N.J., Adler, P.B., Godoy, O., et al., 2015. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol., 29: 592-599. DOI:10.1111/1365-2435.12345 |
Li, X.W., Walker, D., 1986. The plant geography of Yunnan Province, southwest China. J. Biogeogr., 13: 367-397. DOI:10.2307/2844964 |
Losos, J.B., 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett., 11: 995-1007. DOI:10.1111/j.1461-0248.2008.01229.x |
Luttge, U., 2008. Physiological Ecology of Tropical Plants. Springer, Berlin, Germany
|
Marks, C., Lechowicz, M., 2006. Alternative designs and the evolution of functional diversity. Am. Nat., 167: 55-66. DOI:10.1086/498276 |
Messier, J., McGill, B.J., Enquist, B.J., et al., 2017. Trait variation and integration across scales: is the leaf economic spectrum present at local scales?. Ecography, 40: 685-697. DOI:10.1111/ecog.02006 |
Messier, J., McGill, B.J., Lechowicz, M.J., 2010. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett., 13: 838-848. DOI:10.1111/j.1461-0248.2010.01476.x |
Niinemets, Ü., 2001. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82: 453-469. DOI:10.1890/0012-9658(2001)082[0453:GSCCOL]2.0.CO;2 |
Paradis, E., Claude, J., Strimmer, K., 2004. APE: analyses of phylogenetics ang evolution in R language. Bioinformatics, 20: 289-290. DOI:10.1093/bioinformatics/btg412 |
Parker, G.G., 1995. Structure and microclimate of forest canopies. In: Lowman, M.E., Nadkarni, N.M. (Eds.), Forest Canopies. Academic Press, San Diego, pp. 73-106
|
Pérez-Harguindeguy, N., Díaz, S., Garnier, E., et al., 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot., 64: 715-716. |
Poorter, H., Garnier, E., 1999. Ecological significance of inherent variation in relative growth rate and its components. In: Pugnaire, F.I., Valladares, F. (Eds.), Handbook of Functional Plant Ecology. Marcel Dekker, New York, pp. 81-120
|
Powers, J.S., Tiffin, P., 2010. Plant functional type classifications in tropical dry forests in Costa Rica: leaf habit versus taxonomic approaches. Funct. Ecol., 24: 927-936. DOI:10.1111/j.1365-2435.2010.01701.x |
Pringle, E.G., Adams, R.I., Broadbent, E., et al., 2011. Distinct leaf-trait syndromes of evergreen and deciduous trees in a seasonally dry tropical forest. Biotropica, 43: 299-308. DOI:10.1111/j.1744-7429.2010.00697.x |
Scalon, M.C., Haridasan, M., Franco, A.C., 2017. Influence of long-term nutrient manipulation on specific leaf area and leaf nutrient concentrations in savanna woody species of contrasting leaf phenologies. Plant Soil, 421: 233-244. DOI:10.1007/s11104-017-3437-0 |
Siefert, A., Violle, C., Chalmandrier, L., et al., 2015. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett., 18: 1406-1419. DOI:10.1111/ele.12508 |
Silvertown, J., Mcconway, K., Gowing, D., et al., 2005. Absence of phylogenetic signal in the niche structure of meadow plant communities. Proc. Roy. Soc. Lond. B., 273: 39-44. |
Sultan, S.E., 2000. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci., 5: 1360-1385. |
Swenson, N.G., 2013. The assembly of tropical tree communities-the advances and shortcomings of phylogenetic and functional trait analyses. Ecography, 36: 264-276. DOI:10.1111/j.1600-0587.2012.00121.x |
Umaña, M.N., Zhang, C.C., Cao, M., et al., 2015. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecol. Lett., 18: 1329-1337. DOI:10.1111/ele.12527 |
Valladares, F., Gianoli, E., Gómez, J.M., 2007. Ecological limits to plant phenotypic plasticity. New Phytol., 176: 749-763. DOI:10.1111/j.1469-8137.2007.02275.x |
Violle, C., Enquist, B.J., McGill, B.J., et al., 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol., 27: 244-252. DOI:10.1016/j.tree.2011.11.014 |
Violle, C., Navas, M.L., Vile, D., et al., 2007. Let the concept of trait be functional. Oikos, 116: 882-892. DOI:10.1111/j.0030-1299.2007.15559.x |
Vos, J., Bom, M., 1993. Hand-held chlorophyll meter: a promising tool to assess the nitrogen status of potato foliage. Potato Res., 36: 301-308. DOI:10.1007/BF02361796 |
Walter, H. 1985. Vegetation of the Earth (third ed.). Springer-Verlag, Berlin, Germany
|
Webb, C.O., Ackerly, D.D., McPeek, M.A., et al., 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst., 33: 475-505. DOI:10.1146/annurev.ecolsys.33.010802.150448 |
Williams, K., Field, C.B., Mooney, H.A., 1989. Relationships among leaf construction cost, leaf longevity, and light environment in rain-forest plants of the genus piper. Am. Nat., 133: 198-211. DOI:10.1086/284910 |
Wright, I.J., Dong, N., Maire, V., et al., 2017. Global climatic drivers of leaf size. Science, 357: 917-921. DOI:10.1126/science.aal4760 |
Wu, Z.Y., Zhu, Y.C., Jiang, H.Q., 1987. Vegetation of Yunnan. Science Press, Beijing
|
Yang, J., Ci, X.Q., Lu, M.M., et al., 2014. Functional traits of tree species co-vary with environmental niches in two large forest dynamics plots. J. Plant Ecol., 7: 115-125. DOI:10.1093/jpe/rtt070 |
Zanne, A.E., Tank, D.C., Cornwell, W.K., et al., 2014. Three keys to the radiation of angiosperms into freezing environments. Nature, 506: 89-92. DOI:10.1038/nature12872 |
Zhang, J.L., Poorter, L., Hao, G.Y., et al., 2012. Photosynthetic thermotolerance of woody savanna species in China is correlated with leaf life span. Ann. Bot., 110: 1027-1033. DOI:10.1093/aob/mcs172 |
Zhang, S.B., Zhang, J.L., Cao, K.F., 2016. Differences in the photosynthetic efficiency and photorespiration of co-occurring Euphorbiaceae liana and tree in a Chinese savanna. Photosynthetica, 54: 438-445. DOI:10.1007/s11099-016-0188-8 |
Zhu, H., Tan, Y.H., Yan, L.C., et al., 2020. Flora of the savanna-like vegetation in hot dry valleys, southwestern China with implications to their origin and evolution. Bot. Rev., 86: 281-297. DOI:10.1007/s12229-020-09227-x |
Zhu, J.J., Zhang, J.L., Liu, H.C., et al., 2009. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plantarum, 135: 62-72. DOI:10.1111/j.1399-3054.2008.01171.x |
Zhu, S.D., Chen, Y.J., Ye, Q., et al., 2018. Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol., 38: 658-663. DOI:10.1093/treephys/tpy013 |



