b. Department of Land, Air and Water Resources, University of California at Davis, Davis, CA, 95616, USA;
c. School of Biological Sciences, University of Western Australia, Perth, WA, 6009, Australia
About 80–90% of ≥10,000 plant species in terrestrial ecosystems have symbiotic relationships with mycorrhizal fungi to form mycorrhizas, which are beneficial to both the plant and fungi, thereby increasing their chances of surviving (Wang and Qiu, 2006; Smith and Read, 2008; Brundrett, 2009), although only < 1.0% of all classified plant species have been evaluated for their mycorrhizal status (Albornoz et al., 2021). At present six mycorrhizal types are categorized as arbuscular mycorrhiza, arbutoid mycorrhiza, ectomycorrhiza, ericoid mycorrhiza, monotropoid mycorrhiza and orchid mycorrhiza (Smith and Read, 2008). Among them, the first and second common mycorrhizas, also the most economically important mycorrhizas in agricultural and natural ecosystems, are arbuscular mycorrhiza (AM) and ectomycorrhiza, which colonize ~80% and 2% of all tested plant species, respectively (He et al., 2003; Wang and Qiu, 2006; Smith and Read, 2008; Brundrett, 2009). The mycorrhizas improve plant nutrient uptake, growth and yield, while the fungus receives photosynthetically assimilated carbon (C) from the associated mycorrhizal host plant (hereafter host) (Smith and Read, 2008; Bonfante and Genre, 2010; Wagg et al., 2015).
In nature, a plant may associate with multiple fungi and each fungal individual may associate with more than one plant, when mycorrhizal fungi connect individual plants of the same or different species together belowground, (common) mycorrhizal networks (CMNs) formed (Pringle, 2009; van der Heijden et al., 2015). Through a CMN, C, nitrogen (N), and other nutrients can be transferred among individual plants, and plants can also use these networks to communicate the presence of pests and diseases, and to release chemicals that provide plants with a competitive advantage over other non-mycorrhizal plants (He et al., 2003; Hoeksema, 2015; Wagg et al., 2015; Gilbert and Johnson, 2017).
However, there are ~10% of land plants are non-mycorrhizal species (Wang and Qiu, 2006; Smith and Read, 2008; Brundrett, 2009). Moreover, each mycorrhizal fungus has a limited number of host plant species, resulting in a large number of plant species that are non-mycorrhizal host plants for a specific mycorrhizal fungus. Currently if and how non-mycorrhizal or non-host plants are able to involve in CMNs remains unknown, while relevant studies can help to understand plant community establishment and stability in terrestrial ecosystems. Additionally, intercropping and agro-forestry systems become globally popular and thus AM and/or EM mediating host and non-host plant interactions warrant attention. Great progresses on host plants-mycorrhizal fungi interactions and their ecological functions have been made in the past two decades (e.g. He et al., 2003; Simard and Durall, 2004; Smith and Read, 2008; Wagg et al., 2015; Wipf et al., 2019). Regarding non-mycorrhizal or non-host plants, studies are mainly focused on their contribution to understand the mechanisms and evolution of mycorrhizal symbiosis (see reviews Giovannetti and Sbrana, 1998; Cosme et al., 2018). To date, studies on the mycorrhizal host, mycorrhizal fungus and non-mycorrhizal host tripartite relation (hereafter tripartite) have not attracted enough attention, leading to the effects of the tripartite interaction on plant growth and nutrient acquisition and their relevant underlying mechanisms remain largely unknown (Fernandez et al., 2019). Currently, to our knowledge, from the point of view of the interaction between mycorrhizal and non-mycorrhizal plants, only two reviews have discussed the performance and coexistence of mycorrhizal and non-mycorrhizal species under extremes of nutrient availability, particularly under phosphorus deficiency (Lambers and Teste, 2013; Lambers et al., 2018).
In this review, we first define the concepts and scopes of non-mycorrhizal and non-host plants. Next, we summarize experimental data from published studies to discuss (1) if AM or EM hyphae could penetrate or colonize roots of non-host plants; (2) how plant growth and nutrient acquisition has been affected by host-mycorrhizal fungi-non-host tripartite; (3) molecular dialogue between AM hyphae and non-host model plant Arabidopsis thaliana; (4) the induced system resistance (ISR) or induced system susceptibility (ISS) responses when non-host plant roots were colonized by mycorrhizal hyphae. Finally, we discuss future perspectives in this research area. The overarching goal is to review recent progress in mycorrhizas mediated host and non-host plant interaction, and discuss if non-host plants can be involved in CMNs.
2. Non-mycorrhizal or non-host plantsIn contrast to plants that have facultative mycorrhizal associations with fungi, there are some plant species that do not usually form any well-recognized types of mycorrhizas, with roots that are highly resistant to mycorrhizal fungi and normally remain uncolonized, so called non-mycorrhizal (NM) plants (Tester et al., 1987; Brundrett, 1991). Generally, various members in families of Proteaceae, Chenopodiaceae, and Brassicaceae, and some genera in families of Fabaceae and Cactaceae, including several major agricultural crops and weeds are considered to be NM plants (Tester et al., 1987; Lambers and Teste, 2013; Albornoz et al., 2021). Caution should be taken when terming NM plants because there is no any clear line between mycorrhizal and non-mycorrhizal plants, especially when the criteria used to define functionally mycorrhizal colonization evolved with time (for details please see Cosme et al., 2018). For instance, although clear evidences showed that the model plant species Arabidopsis thaliana can be colonized by fungi Piriformospora indica (Peskan-Berghofer et al., 2004; Shahollari et al., 2007; Mandyam et al., 2013; Keim et al., 2014), from the genomic comparisons and mycorrhizal structure analysis, A. thaliana is not a true or typical mycorrhizal plant (Cosme et al., 2018).
Meanwhile, even for mycorrhizal plants, they cannot have symbiotic mycorrhizal relationships with all mycorrhizal fungi. Plants that cannot be colonized by a specified mycorrhizal fungus are considered to be non-host plants, e.g. plants that unable to establish a symbiosis with an AM fungus may be colonized by other AM fungi or EM fungi and then these plants are non-host to a specific AMF (Veiga et al., 2013; Cosme et al., 2018; Fernandez et al., 2019). Non-mycorrhizal plants that unable to form any mycorrhizas are definitely non-host plants, hence the non-host plants hereafter refer to NM plants and plants that could not establish a typical mycorrhizal symbiosis with a specific mycorrhizal fungus.
3. Can mycorrhizal hyphae access to (colonize) non-host roots?At present our understanding of the formation and function of CMNs is based on two key points: 1) multiple host plants are connected by mycorrhizal fungi simultaneously (Babikova et al., 2013; van der Heijden et al., 2015; Song et al., 2019; Alaux et al., 2020); 2) symbiotic fungi utilize carbohydrates from hosts and transport elements along the mycorrhizal networks (Robinson and Fitter, 1999; Barto et al., 2012; Fellbaum et al., 2014). These indicate that the non-host plants are shut out of CMNs. To understand if non-host plants are involved in a CMN, we first searched publications that have paid attention to mycorrhizal hyphae in non-host plants, and a number of studies showed that mycorrhizal hyphae could penetrate roots of non-host plants without forming typical mycorrhizal structures (Table 1), suggesting that roots of non-host plants could be linked to CMNs.
| Mycorrhizal fungi | Host plants | Growth conditions | Non-host plants | Identification methods | The presence of host | References |
| Glomus spp. or Gigaspora margarita (AMF) | Agropyron dasystachyum | Root observation chambers with soil | Salsola kali | Microscope observation of autofluorescence and stained roots | No | Allen etal. (1989) |
| Glomus fasciculatus | Citrus macrophylla or Allium cepa | Pots filled with an autoclaved soil mix of loam-sand (2:1) | Chenopodium album, C. amaranticolor, C. quinoa, Spinacea oleracea, Brassica nigra, Raphanus sativus | Microscope observation of stained roots | Yes | Hirrel etal. (1978) |
| Glomus mosseae | Sorghum vulgare | A double pot system using sterilized P-deficient soil | Brassica oleracea | Microscope observation of stained roots | Yes | Ocampo (1986) |
| Glomus intraradices | Triticum aestivum | Microcosms filled with sterilized mixture of quartz sand with 20% field soil | Stellaria media | Microscope observation of stained roots | Yes | Veiga etal. (2012) |
| Rhizophagus irregularis (previously named Glomus intraradices) | Trifolium pratense or Lolium multiflorum | Microcosms filled with a sterilized mixture of quartz sand with 10% field soil | Arabidopsis thaliana | Microscope observation of stained roots, confocal microscopy of fungal cell walls, Electron microscopy | Yes | Veiga etal. (2013) |
| Rhizophagus irregularis | Medicago truncatula | Microcosm system filled with a river-sand soil mixture (5:12, v/v) | Arabidopsis thaliana | Microscopy observation of stained roots | Yes | Fernandez etal. (2019) |
| Glomus spp. (one or mix of three species) | Helianthus annuus | Microcosms filled with a mixture of autoclaved sand and grassland soil | Amaranthus retroflexus, Chenopodium album, Sinapis arvensis | Microscope observation of stained roots | No | Rinaudo etal. (2010) |
| Cortinarius cinnamomeus (EMF) | Picea abies | Grassland located in the Burren in Western Ireland | Carex flacca, C. pilulifera | Microscope observation of stained roots, RFLP analysis of ITS region of rDNA | Not determined | Harrington etal. (2002) |
| Sebacinales (ectomycorrhizas to ericoid and orchid mycorrhizas) | Not available | A Caribbean and two European sites | 39 different plant species | PCR and sequencing | Not determined | Selosse etal. (2009) |
| Tricholoma matsutake, T. fulvocastaneum | Pinus densiflora | Pots with soil obtained from a Pinusdensiflora forest | Cedrela odorata, Prunus speciosa | Microscope observation of washed roots | No | Murata et al., 2013, Murata et al., 2014 |
| Tuber melanosporum | Not available | Truffle grounds located in Occitanie, southern France | Tuber melanosporum was detected in 89.7% of the 80 non-EM species | PCR, sequencing, metabarcoding | Tuber spp. were not detected in roots out of brûlés | Schneider-Maunoury et al. (2018) |
| Tuber aestivum | Carpinus betulus | A deciduous forest contains brûlés, in Czech Republic | 16 non-EM plant species | qPCR | Root were sampled in brûlés | Gryndler etal. (2014) |
| Tuber melanosporum, T.aestivum | Coryllus avelana and Quercus petrae | Truffle ground of Rollainville, Lorraine, France | 9 of 10 non-EM plant species | Fluorescent in situ hybridization, PCR and ITS sequencing | Root were collected in brûlés | Schneider-Maunoury et al. (2020) |
To date, many studies have shown that AM hyphae could penetrate roots of non-host plants (Ocampo, 1986; Allen et al., 1989; Francis and Read, 1994; Veiga et al., 2012, 2013; Fernandez et al., 2019). For instance, the fungi initially invaded roots and formed arbuscules and peletons, and then the invaded root segment turned brown while the fungus disappeared from the root within two days, when the non-mycorrhizal plant Salsola kali was inoculated by a mixture of Glomus spp. and Gigaspora margarita (Allen et al., 1989). In contrast, penetrations of fungal hyphae to non-mycorrhizal plant roots without arbuscules were observed in a number of studies (Hirrel et al., 1978; Rinaudo et al., 2010; Veiga et al., 2013; Fernandez et al., 2019). In addition, studies have showed that the root mycorrhizal colonization of a non-mycorrhizal plant (Arabidopsis thaliana) by AMF required the concurrent presence of AMF host plants, suggesting that AMF hyphae alone might not be able to colonize roots of a non-host plant (Hirrel et al., 1978; Ocampo, 1986; Veiga et al., 2012, 2013; Fernandez et al., 2019). Only a few studies suggested that a host plant was not necessary (Allen et al., 1989; Rinaudo et al., 2010), indicating that the colonization of non-host roots might be driven by the host plant.
3.2. EM hyphae penetrate non-host plant rootsFor ectomycorrhiza (EM), PCR using specific primers for ribosomal DNA of EM fungi and root fungal communities barcoding suggested that some EM fungi could colonize living roots of non-EM plants (Harrington and Mitchell, 2002; Selosse et al., 2009; Murata et al., 2013; Gryndler et al., 2014; Schneider-Maunoury et al., 2018; Toju and Sato, 2018). These EM fungi may colonize roots of non-EM plants in a loose way, without EM morphology, called endophytism (Rodriguez et al., 2009; Weiß et al., 2016). Endophytism in non-EM plants has been claimed for EM taxa such as Helotiales, Pyronemataceae, Sebacinaceae and Thelephoraceae (Selosse et al., 2009; Weiß et al., 2016; Schneider-Maunoury et al., 2018). The endophytic colonization of Tuber melanosporum hyphae was observed on the apoplast of healthy roots of non-EM plants, providing morphological evidence of endophytism between EM T. melanosporum and >70 non-EM plant species from a total of 80 tested plant species (Schneider-Maunoury et al., 2018, 2020). Additionally, quantified PCR results showed that the housekeeping gene RPS3 and laccase gene that expressed in Tuber aestivum EM root tips and ascocarps were also expressed in 12 of 17 sampled non-EM roots; and the sugar transporter, glucose–methanol–choline oxidoreductase and secreted protein genes were expressed in some non-EM root systems as well, confirming a living status and potential functions (Schneider-Maunoury et al., 2020). Most above-mentioned studies collected non-EM roots near EM roots, e.g. non-EM plants sampled from brûlé (Schneider-Maunoury et al., 2018, 2020), indicating that the colonization of non-EM roots by EM hyphae might have acquired from the hosts. However, studies also showed that the formation of endophytism between EM fungi of Tricholoma matsutake and T. fulvocastaneum, with non-EM but AM plants, Cedrela odorata and Prunus speciosa, did not require the presence of their hosts (Murata et al., 2013, 2014).
Collectively, numerous studies have suggested that NM plants could be connected by host-supported mycorrhizal hyphae as illustrated by Fig. 1, which is a prerequisite for establishing a CMN. Although no formation of typical mycorrhizal structures, colonized mycorrhizal hyphae can be alive and have certain functions within roots of non-host plants.
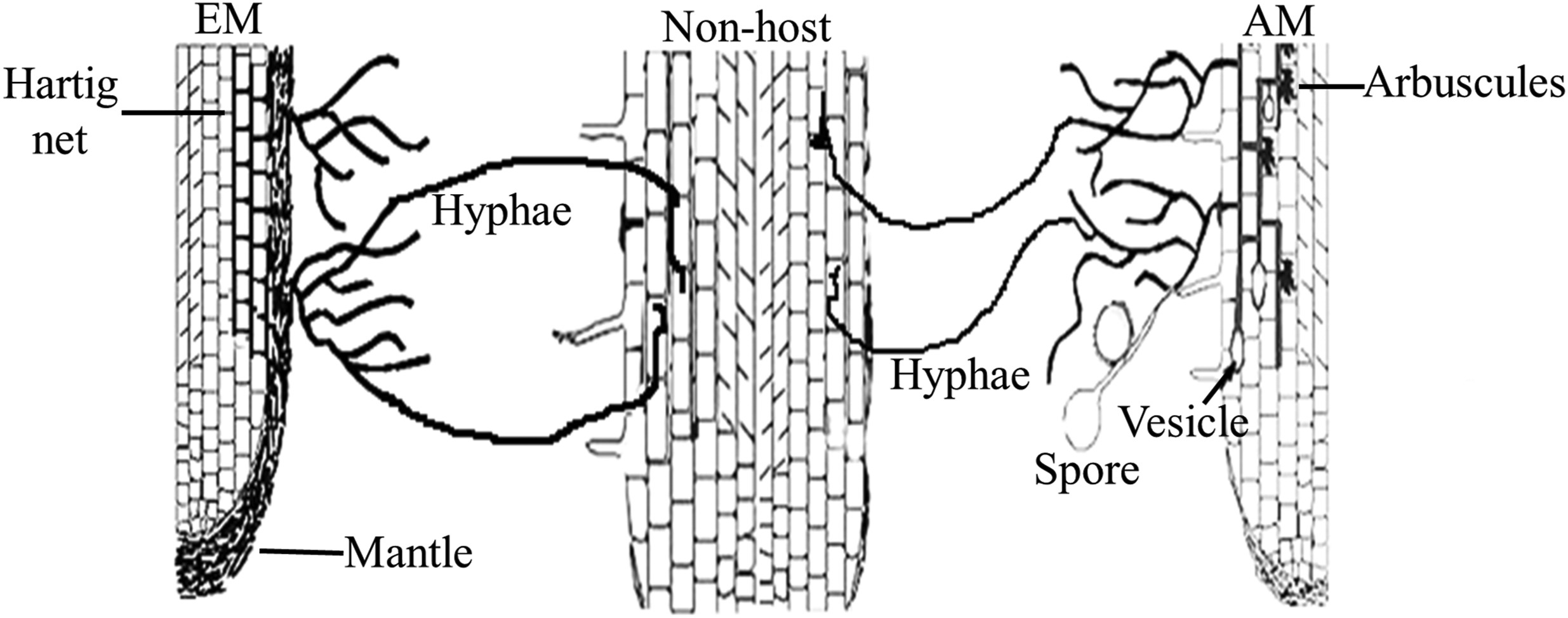
|
| Fig. 1 A conceptual diagram showing the host-supported AM and EM hyphae can penetrate roots of non-host plant. No formation of typical mycorrhizal structures in non-host roots, but Veiga et al. (2013) observed vesicles in Arabidopsis roots colonized by Loliummultiflorum and Trifoliumpratense nursed Rhizophagus hyphae, and Fernandez et al. (2019) observed hyphopodia-like structures on the surface of Arabidopsis roots colonized by Medicago-supported Rhizophagus hyphae. |
It has long been known that AM species have a hyphal-mediated adverse effect on the growth of NM neighbors, especially for Brassicaceae (Lambers and Teste, 2013). Root branching, root-hair development and aboveground biomass production of NM neighbors were inhibited when the fungal hyphae were present while their roots are separated by nylon mesh barrier (Ocampo, 1986; Francis and Read, 1994; Veiga et al., 2013). Such adverse effects of NM species on their mycorrhizal neighbors could result from allelochemicals released through AM roots, however, these had been denied (Vierheilig et al., 1996; Wurst et al., 2010; Lambers and Teste, 2013). Similarly, when NM white lupin (Lupinus albus, belongs to Proteaceae) was grown with AM wheat (Triticum aestivum) under both glasshouse and field conditions where presumably allowed wheat to form mycorrhizas, the biomass and grain yield of white lupin was adversely affected; but when white lupin was grown in pots without mycorrhizal fungi, no adverse effects of wheat on the growth of white lupin were observed (Gardner and Boundy, 1983; Cu et al., 2005). The nutrient acquisition of non-host plants in these two studies was not affected by their mycorrhizal neighbors, neither. Similarly, no significant differences were in the phosphorus, N or C content between A. thaliana colonized by host-supported Rhizophagus irregularis and non-R. irregularis colonized plants (Fernandez et al., 2019). However, when young non-host cabbage grown with AMF-colonized mature sorghum, significant decrease in yields and nutrient (N, P, K, Ca and Mg) concentrations were detected; and similar trends were found in young sorghum when grown with mature sorghum (Ocampo, 1986). Moreover, the N and P uptake of three non-host weed species (Amaranthus retroflexus, Chenopodium album, Sinapis arvensis) were decreased when grown with AMF host sunflower (Rinaudo et al., 2010). In summary, the biomass of non-hosts, rather than their nutrient acquisition, was always adversely affected by host-supported AM colonization (Table 2). Thus, the competition of nutrients between host and non-host plants is unlikely to appropriately explain the growth reduction of non-host plants.
| Host – mycorrhizal fungi – non-host tripartite | Mycorrhizal type | Host plants | Non-host plants | Reference | ||||
| Shoot biomass | Nutrients uptake | Colonization rate | Shoot biomass | Nutrients uptake | Colonization rate | |||
| Quercus ilex - Tuber melanosporum - six arbuscular mycorrhizal plant species | EMF | −16.87% | N (+38.46%) P (ns), C (ns) | Soil EM mycelium biomass +90.48% | On average −23.66% | N (−12.69%) P (−30.23%) C (ns) | ND, and soil AM fungi DNA amounts −83.16% | Taschen etal. (2019) |
| Mature Sorghum vulgare - Glomus mosseae - Young Brassica oleracea | AMF | ns | N (ns), P (ns) K (ns), Ca (ns) Mg (ns) | ns | −64.66% | N (−66.67%) P (−73.68%) K (−68.18%) Ca (−75.76%) Mg (−71.43%) | 3.8±1.2% | Ocampo (1986) |
| Trifolium pratense or Lolium multiflorum - Rhizophagus irregularis - Arabidopsis thaliana | AMF | ns | N (+12.10%) P (+78.57%) | ND | −50.00% | ND | 12.0±2.0% | Veiga etal. (2013) |
| Medicago truncatula - Rhizophagus irregularis - Arabidopsis thaliana | AMF | ns | P (+36.36%) | ND | −50.00% | N (ns), P (ns) C (ns), K (ns) | 5.0±1.0% | Fernandez etal. (2019) |
| Helianthus annuus - Glomus spp. - Amaranthus retroflexus, Chenopodium album, or Sinapis arvensis | AMF | ns | P (+48.00%) N (ns) | ns | On average −47% | P (−41.00%) N (−26.88%) | Ranged from 1.2% to 14.8% | Rinaudo etal. (2010) |
Studies on the effects of EM on the growth of non-host plants are mainly attracted by the phenomenon so-called brûlé, a zone around host plants colonized by some EMs especially the Tuber aestivum, T. indicum complex and T. melanosporum, where the growth of non-EM herbaceous and shrubby plants are inhibited (Streiblova et al., 2012). Mycelia, mycorrhizas, and fruiting bodies of brûlé-forming truffles can produce volatile organic compounds and plant hormones like ethylene and auxin to impair seed germination, root morphogenesis and rhizosphere microflora of non-hosts (Splivallo et al., 2007, 2009; Streiblova et al., 2012). Additionally, a direct endophytic interaction in the roots of non-EM grasses and other weeds particularly Anthoxanthum odoratum and Leontodon faraxacoides might affect non-host plant health as immuno-localization had detected T. melanosporum mycelium in unhealthy root tissues of two brûlé herbs (Plattner and Hall, 1995). Recently, Taschen et al. (2019) found that T. melanosporum colonized Quercus ilex decreased the shoot and root biomass, and leaf N and P concentration of the non-host plants, highlighting the inhibition role of T. melanosporum on the growth of the non-host plants. Similarly, we found that the growth of A. thaliana was dramatically inhibited when planting with T. melanosporum colonized 37-month-old Quercus mongolica seedlings (unpublished data). It should be noted that not all Tuber species (e.g. Tuber magnatum and T. borchii) can form brûlé or similar phenomenon (Pacioni, 1991; Angelini et al., 2015), and very few studies have focused on the interactions between non-brûlé truffle EMs and non-EM hosts (Riccioni et al., 2016). Thus, at the ecosystem level, it is possible that EM hosts have no adverse effects on non-host plants, and further studies culturing non-brûlé EM plants and non-EM plants together would provide more insights.
4.2. Plant growth and nutrient acquisition of hosts affected by the tripartiteStudies have shown that non-mycorrhizal species had various impacts on the growth and nutrients acquisition of their mycorrhizal neighbors. The adverse effects of NM species on their mycorrhizal neighbors or subsequent crops have been reported in the Brassicaceae species (Lambers and Teste, 2013). The presence of a non-host cabbage had no effects on the growth and nutrient acquisition of its mycorrhizal neighbor sorghum was also reported (Ocampo, 1986). Whilst the shoot P content of sunflower was significantly higher (+48%) when grown in mycorrhizal microcosms with weeds than without weeds (Rinaudo et al., 2010). Moreover, when Quercus ilex seedlings associated with Tuber melanosporum grew together with companied non-host plants, the companion plants promoted the development of truffle mycelium and the leaf N content of the host, resulting in positive effects to host plants conferred by non-host neighbors (Taschen et al., 2019). Although the underlying mechanisms had not yet explored, nutrient transfer between host and non-host plants through EM mycelium (or an established mycorrhizal network) could not be excluded. Furthermore, the more of the mycorrhizal hyphae, the more of the nutrients can be absorbed (Smith and Read, 2008). Mycorrhizal hyphae can also modify rhizosphere exudates (Wang and Lambers, 2020; Wang et al., 2021) or hyphae-associated bacterial community (Zhang et al., 2018; Wang et al., 2021), thereby affect plant nutrient uptake.
Taken together, plant growth and nutrient acquisition of non-hosts are generally adversely affected by host-supported mycorrhizal hyphae (Table 2); and the host-supported AM and EM fungi colonization might differentially modulate the nutrient status of non-host plants. While plant growth and nutrient acquisition of host plants could be variously affected, depending on plant species and fungus involved. The mechanisms underlying the effects of tripartite interaction on plant growth and nutrient uptake remain largely unexplored; factors including nutrient transfer, signal communication, mycorrhizal hyphae proliferation, rhizosphere exudates or soil microbiome modification cannot be excluded (Fig. 2). Future studies using multi-omics and new technologies such as nanoscale secondary ion mass spectrometry (NanoSIMS) and cryo-scanning electron microscopy, to trace movement of C, N and P along mycorrhizal hyphae (e.g. Yu et al., 2020) would help to underpin such mechanisms. In addition, the function of mycorrhizas should be re-examined, particularly the non-nutritional benefits of mycorrhizas (Albornoz et al., 2021).
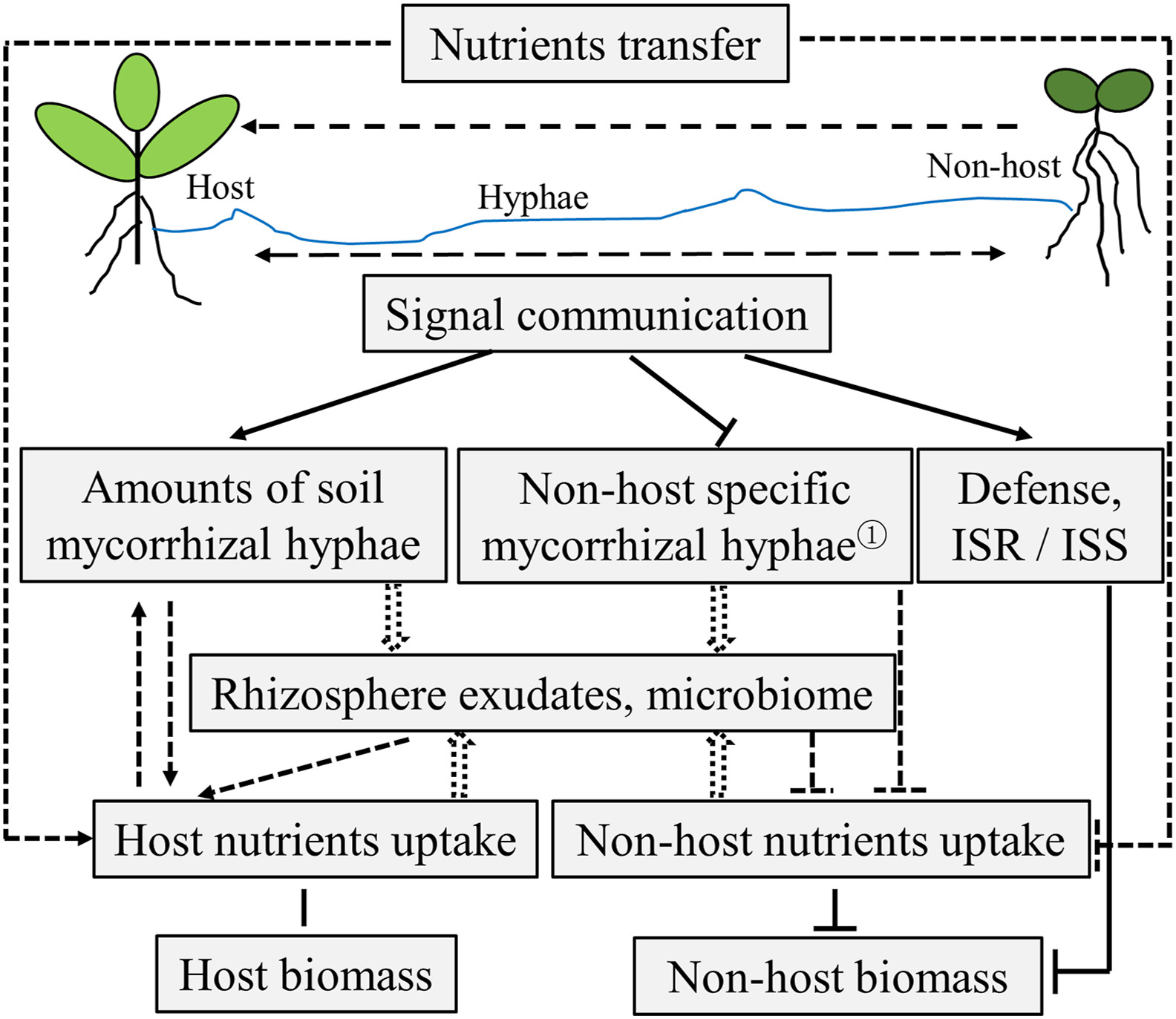
|
| Fig. 2 A conceptual illustration showing how the tripartite of host, mycorrhizalfungi and non-host plant affects nutrientsuptake and biomass of both host and non-host plants. To date, as shown in Table2, experimental data has confirmed that the nutrients uptake and biomass of non-host were most likely adversely affected, while the nutrients uptake of host were improved in many cases. In addition, host-nursed mycorrhizal hyphae accessed to roots of non-host could stimulate ISR (induced system resistance) or ISS (induced system susceptibility) responses at late stage (Fernandez et al., 2019), which may lead to growth–defense tradeoffs. ① The host- EM – AM plant tripartite could increase amounts of soil EM hyphae but reduce amounts of soil AM hyphae (Taschen et al., 2019). Solid lines indicate mechanisms that have been experimental proved; dotted lines indicate possible relationships; arrows indicate stimulation; ‘T’ arrows indicate inhibition; without arrows indicate no effect; hollow arrows indicate change. |
Host plants can recognize AM fungi and trigger early responses by increasing the expression of strigolactone biosynthesis genes CCD7 and CCD8, resulting in the increased production and exudation of strigolactones (Akiyama et al., 2005; Kohlen et al., 2012; López-Ráez et al., 2015). The molecular mechanism responsible for mycorrhiza-non host plant interaction remains poorly understood. Using a hyphal mesh to separate roots of Arabidopsis thaliana from roots of the host species, Veiga et al. (2013) set up the basis for molecular and genetic studies to understand the relative mechanisms. By using this system researchers have found that the detection of AM fungus Rhizophagus irregularis in Arabidopsis induced the expression of CCD7 and CCD8 significantly within 24 h (Fernandez et al., 2019). Generally, the defense-related genes like PR1, MYB51 etc. would be up-regulated at the early stage of interaction between AM fungi and host, and between Arabidopsis and pathogenic or endophytic fungi (García-Garrido and Ocampo, 2002; Berrocal-Lobo and Molina, 2008; Fernandez et al., 2019). However, the expression of these defense genes were not activated in R. irregularis colonized Arabidopsis roots (Fernandez et al., 2019). These results indicated that AM fungi are neither pathogenic nor endophytic fungi to non-host Arabidopsis, and the exposure of Arabidopsis roots to Rhizophagus mycelia starts early AM fungi-host recognition (Fig. 3).
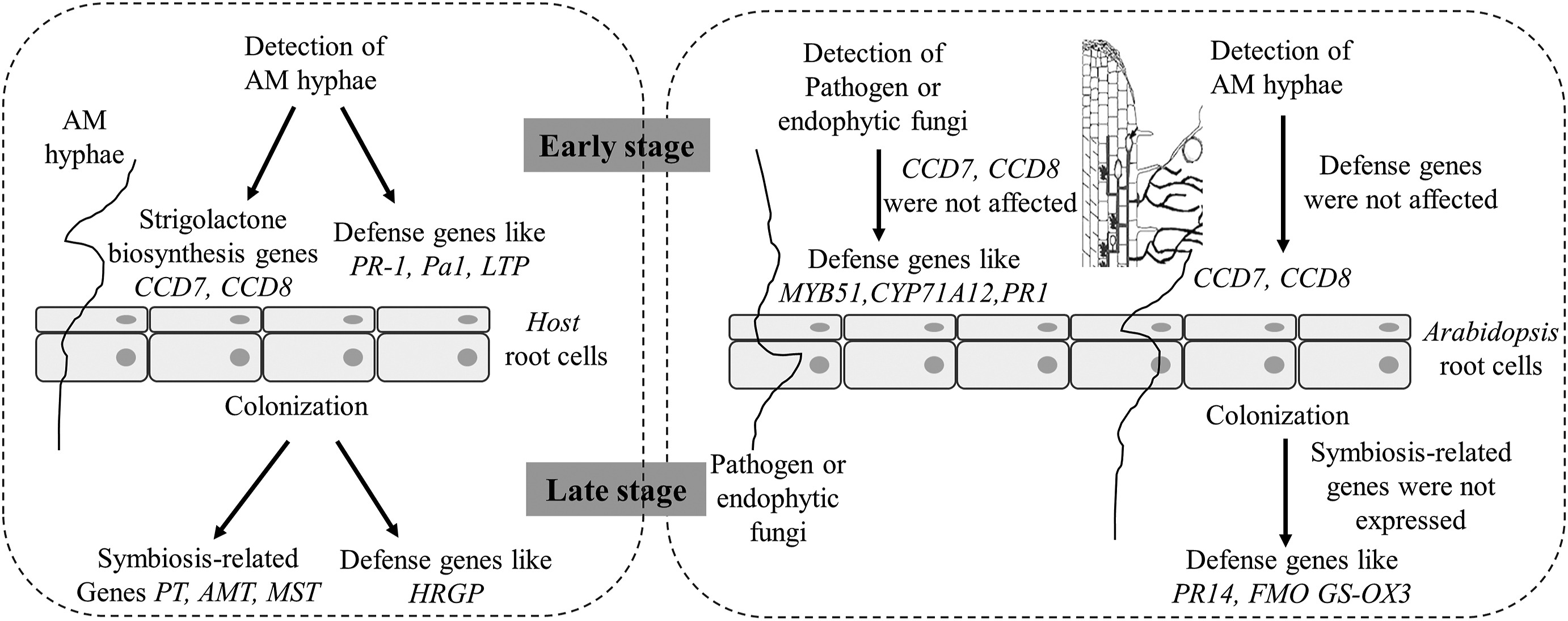
|
| Fig. 3 A conceptual illustration showing current knowledge on molecular dialogues between AM hyphae and non-host plant Arabidopsis, and the differences in comparison with AM-host, and pathogen or endophytic fungi - Arabidopsis interaction. |
In addition, once stable AM symbiosis was established, the expression of well-characterized symbiosis-related genes GintPT, GintAMT2, GintMST2 and GintMST4 would be strongly upregulated, and gene ontology (GO) terms associated with carbohydrate metabolism and nutrient transport should be overrepresented to maintain a functional AM symbiosis (Helber et al., 2011; Pérez-Tienda et al., 2011; Zouari et al., 2014; Fernandez et al., 2019). In contrast, when host-supported Rhizophagus colonizes Arabidopsis roots, the symbiosis-related genes were not expressed in roots; but defense-related GO terms like sulfur compound biosynthesis and metabolism, salicylic acid biosynthesis and systemic acquired resistance, as well as defense-related gene FMO GS-OX3 (flavin-monooxygenase S-oxygenase 3) and pathogenesis-related gene PR-14 were overrepresented (Fernandez et al., 2019).
Collectively, as shown in Fig. 3, the early stage of interaction between non-host Arabidopsis and host-supported AMF showed some processes that were usually observed during the presymbiotic stages of host–AM fungus interactions; but in a later interaction stage the AM fungus activated plant defenses and the fungus was recognized as an unwanted invader (Fernandez et al., 2019). These results suggest that the colonization of non-host plant roots by host-supported AMF may transfer signal compounds from host plants and/or AMF to non-host plants to activate plant defense responses. Consequently, these plant costly defense responses might lead to growth–defense tradeoffs and result in inhibition of non-host plant growth (Stringlis et al., 2018) as described in the Non-host plant growth and nutrient acquisition affected by CMNs section. However, the growth-defense tradeoffs could not explain the growth and nutrient acquisition of host plants impaired by mycorrhizal hyphae connected non-hosts as discussed in the Host growth affected by mycorrhizal hyphae connected non-host neighbors section.
6. Induced system resistance (ISR) or susceptibility (ISS) responsesOne of the main advantages of CMNs is to communicate the presence of pests and diseases among plant individuals connected by mycorrhizal networks (Song et al., 2019). Indeed, Rhizophagus-colonized Arabidopsis displayed a significant decrease in foliar necrotrophic fungus Botrytis cinerea induced disease (Fernandez et al., 2019). Similarly, EM fungus Laccaria bicolor triggered ISR against a foliar pest cabbage looper Trichoplusia ni and ISS against a bacterial pathogen Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Furthermore, heat-killed L. bicolor and chitin were sufficient to trigger these ISR and ISS responses, suggesting that the colonization to non-host plant is not necessary to trigger these responses (Vishwanathan et al., 2020). The different results between AM (Fernandez et al., 2019) and EM fungi (Vishwanathan et al., 2020) indicate that the direction and magnitude of mycorrhizal-mediated non-host plant ISR or ISS responses may be distinct depending on mycorrhizal type. To date, although no direct evidence showed that challenge of host plants could trigger ISR of non-host plants in the tripartite, it is possible that the perceiving signals of pests and diseases can be transferred from a host to a non-host to fight against pathogen infection in non-hosts. Thus, the host, mycorrhizal fungi and non-host tripartite system (see Fernandez et al., 2019; Taschen et al., 2019) could be used to monitor the transmission of warning signals from infected to uninfected plants (Babikova et al., 2013; Alaux et al., 2020).
7. Concluding remarks and future perspectivesExperimental data have directly proved that host-supported mycorrhizal fungi could penetrate or colonize non-host plant roots, without forming typical mycorrhizal structures. The colonization of non-host plant roots by mycorrhizal fungi differs to that with pathogenic or endophytic fungi, since similar early AM fungi-host recognition can be initiated normally, but plant defense responses could be triggered in a late stage. Moreover, the non-host plants are most likely to be adversely affected by host-supported mycorrhizal fungi and the growth of hosts are also variously impaired; the growth-defense tradeoffs alone could not explain these phenomenon sufficiently. AM and EM fungi show some different impacts on plant growth and nutrient acquisition in the tripartite system. These findings result in to a promising conclusion that non-mycorrhizal or non-host plants are also involved in CMNs (Fig. 4), which would offer excite future studies to answer the following questions:

|
| Fig. 4 A conceptual diagram showing potential common mycorrhizal linkages or networks among arbuscualr mycorrhizal (AM), ectomycorrhizal (EM) and/or non-mycorrhizal (NM) plants (Picture drew by Wenjun Xu, Kunming Institute of Botany, Kunming, China), as well as the potential functions of this kind of networks. Ectomycorrhizal (EM) trees or arbuscular mycorrhizal (AM) plants are connected by EM hyphae (red lines) or AM hyphae (blue lines), respectively (note: some trees can have dual EM and AM symbioses); and host supported both AM and EM hyphae may penetrate roots of non-mycorrhizal (NM) plants and/or their non-host plant species. Thus, all the plants could be connected underground via AM and/or EM fungi. |
1. Can mycorrhizal fungi get and/or translocate carbon, nitrogen and other mineral nutrients from non-host plants through hyphal colonization?
2. Do host-supported mycorrhizal hyphae always negatively affect the growth of non-host plants? And for host plants? What's the mechanisms underlying the effects of the tripartite on host and non-host plant growth and nutrient acquisition?
3. How rhizosphere traits such as root exudates and rhizosphere microbiome changes in response to the host, mycorrhizal fungi and non-host plant tripartite?
4. Is there any molecular signal communication within the tripartite, such as microRNAs or peptides/proteins?
5. Can pest or pathogenic organisms induced warning signals transmit from host to non-host plants? And vice versa?
6. Will intercropping of AMF crops and non-AMF crops or EM trees and crops lead to resistance to diseases but reduction of yield?
7. In natural ecosystems, are all plants connected by mycorrhizal fungi belowground? And what is the role of mycorrhizal fungi in shaping plant community?
8. Are there any new techniques, suitable plant species or plant cultural system that can be used to promote the research in this area?
Currently, intercropping is attracting attention due to its ability to produce high yields at lower inputs and to suppress pests and diseases (Li et al., 2020; Tang et al., 2021). Given the fact that a cereal and a legume is by far the most common intercrop combination worldwide (Martin-Guay et al., 2018; Li et al., 2020), mycorrhizal effects on intercropping of a mycorrhizal (e.g. cereal or legume) and a non-mycorrhizal (e.g. canola) plant species with mycorrhizal fungus inoculation warrant attention. Understand the interaction between mycorrhizal and non-host plants will also benefit agro-forestry mixed system and underpin important drivers for plant community establishment under natural conditions. In future, growing non-mycorrhizal Brassicaceae species with mycorrhizal cereal species as their neighbors would be an excellent system to study the interactive impacts and mechanisms between host and non-host plants. Application of new techniques such as SIMS, stable isotope of C and N, and multi-omics (e.g. transcriptome, metabolome and proteome) will facilitate the research progress in this direction.
Author contributionsYW drafted the manuscript. XH and FY contributed to the revision of this manuscript. All authors approved the submission.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgementsThis study was supported by grants from Yunnan High Level Talent Introduction Plan, Kunming Institute of Botany (Y9627111K1) and Natural Sciences Foundation of China (31901204).
Akiyama, K., Matsuzaki, K., Hayashi, H., 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature, 435: 824-827. DOI:10.1038/nature03608 |
Alaux, P.L., Naveau, F., Declerck, S., et al., 2020. Common mycorrhizal network induced JA/ET genes expression in healthy potato plants connected to potato plants infected by Phytophthora infestans. Front. Plant Sci., 11: 602-602. DOI:10.3389/fpls.2020.00602 |
Albornoz, F.E., Dixon, K.W., Lambers, H., 2021. Revisiting mycorrhizal dogmas: are mycorrhizas really functioning as they are widely believed to do?. Soil Ecol. Lett., 3: 73-82. DOI:10.1007/s42832-020-0070-2 |
Allen, M.F., Allen, E.B., Friese, C.F., 1989. Responses of the non-mycotrophic plant Salsola kali to invasion by vesicular–arbuscular mycorrhizal fungi. New Phytol., 111: 45-49. DOI:10.1111/j.1469-8137.1989.tb04216.x |
Angelini, P., Tirillini, B., Properzi, A., et al., 2015. Identification and bioactivity of the growth inhibitors in Tuber spp. methanolic extracts. Plant Biosys. An Int. J. Deal. Aspect. Plant Biol., 149: 1000-1009. DOI:10.1080/11263504.2014.983575 |
Babikova, Z., Gilbert, L., Bruce, T.J.A., et al., 2013. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecol. Lett., 16: 835-843. DOI:10.1111/ele.12115 |
Barto, E.K., Weidenhamer, J.D., Cipollini, D., et al., 2012. Fungal superhighways: do common mycorrhizal networks enhance below ground communication?. Trends Plant Sci., 17: 633-637. DOI:10.1016/j.tplants.2012.06.007 |
Berrocal-Lobo, M., Molina, A., 2008. Arabidopsis defense response against Fusarium oxysporum. Trends Plant Sci., 13: 145-150. DOI:10.1016/j.tplants.2007.12.004 |
Bonfante, P., Genre, A., 2010. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun., 1: 48. DOI:10.1038/ncomms1046 |
Brundrett, M.C., 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil, 320: 37-77. DOI:10.1007/s11104-008-9877-9 |
Brundrett, M.C., 1991. Mycorrhizas in natural ecosystems. Adv. Ecol. Res., 21: 171-313. |
Cosme, M., Fernandez, I., Van der Heijden, M.G.A., et al., 2018. Non-mycorrhizal plants: the exceptions that prove the rule. Trends Plant Sci., 23: 577-587. DOI:10.1016/j.tplants.2018.04.004 |
Cu, S.T.T., Hutson, J., Schuller, K.A., 2005. Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant Soil, 272: 143-151. DOI:10.1007/s11104-004-4336-8 |
Fellbaum, C.R., Mensah, J.A., Cloos, A.J., et al., 2014. Fungal nutrient allocation in common mycorrhizal networks is regulated by the carbon source strength of individual host plants. New Phytol., 203: 646-656. DOI:10.1111/nph.12827 |
Fernandez, I., Cosme, M., Stringlis, I.A., et al., 2019. Molecular dialogue between arbuscular mycorrhizal fungi and the nonhost plant Arabidopsis thaliana switches from initial detection to antagonism. New Phytol., 223: 867-881. DOI:10.1111/nph.15798 |
Francis, R., Read, D.J., 1994. The contributions of mycorrhizal fungi to the determination of plant community structure. Plant Soil, 159: 11-25. DOI:10.1007/BF00000091 |
García-Garrido, J.M., Ocampo, J.A., 2002. Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J. Exp. Bot., 53: 1377-1386. DOI:10.1093/jxb/53.373.1377 |
Gardner, W.K., Boundy, K.A., 1983. The acquisition of phosphorus by Lupinus albus L. IV. The effect of inter-planting wheat and white lupin on the growth and mineral composition of the two species. Plant Soil, 70: 391-402. DOI:10.1007/BF02374894 |
Gilbert, L., Johnson, D., 2017. Plant-plant communication through common mycorrhizal networks, In: Becard, G. (Ed.), Advances in Botanical Research. Academic Press, pp. 83-97
|
Giovannetti, M., Sbrana, C., 1998. Meeting a non-host: the behaviour of AM fungi. Mycorrhiza, 8: 123-130. DOI:10.1007/s005720050224 |
Gryndler, M., Cerna, L., Bukovska, P., et al., 2014. Tuber aestivum association with non-host roots. Mycorrhiza, 24: 603-610. DOI:10.1007/s00572-014-0580-9 |
Harrington, T.J., Mitchell, D.T., 2002. Colonization of root systems of Carex flacca and C. pilulifera by Cortinarius (Dermocybe) cinnamomeus. Mycol. Res., 106: 452-459. DOI:10.1017/S0953756202005713 |
He, X.-H., Critchley, C., Bledsoe, C., 2003. Nitrogen transfer within and between plants through common mycorrhizal networks (CMNs). Crit. Rev. Plant Sci., 22: 531-567. DOI:10.1080/713608315 |
Helber, N., Wippel, K., Sauer, N., et al., 2011. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus spp. is crucial for the symbiotic relationship with plants. Plant Cell, 23: 3812-3823. DOI:10.1105/tpc.111.089813 |
Hirrel, M., Mehravaran, H., Gerdemann, J., 1978. Vesicular-arbuscular mycorrhizae in the Chenopodiaceae and Cruciferae: do they occur?. Can. J. Bot., 56: 2813-2817. DOI:10.1139/b78-336 |
10.1007/978-94-017-7395-9_9 |
Keim, J., Mishra, B., Sharma, R., et al., 2014. Root-associated fungi of Arabidopsis thaliana and Microthlaspi perfoliatum. Fungal Divers., 66: 99-111. DOI:10.1007/s13225-014-0289-2 |
Kohlen, W., Charnikhova, T., Lammers, M., et al., 2012. The tomato carotenoid cleavage dioxygenase8 (SlCCD8) regulates rhizosphere signaling, plant architecture and affects reproductive development through strigolactone biosynthesis. New Phytol., 196: 535-547. DOI:10.1111/j.1469-8137.2012.04265.x |
Lambers, H., Teste, F., 2013. Interactions between arbuscular mycorrhizal and non-mycorrhizal plants: do non-mycorrhizal species at both extremes of nutrient availability play the same game?. Plant Cell Environ., 36: 1911-1915. |
Lambers, H., Albornoz, F., Kotula, L., et al., 2018. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil, 424: 11-33. DOI:10.1007/s11104-017-3427-2 |
Li, C., Hoffland, E., Kuyper, T.W., et al., 2020. Syndromes of production in intercropping impact yield gains. Nat. Plant., 6: 653-660. DOI:10.1038/s41477-020-0680-9 |
López-Ráez, J.A., Fernández, I., García, J.M., et al., 2015. Differential spatio-temporal expression of carotenoid cleavage dioxygenases regulates apocarotenoid fluxes during AM symbiosis. Plant Sci., 230: 59-69. DOI:10.1016/j.plantsci.2014.10.010 |
Mandyam, K.G., Roe, J., Jumpponen, A., 2013. Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol., 117: 250-260. DOI:10.1016/j.funbio.2013.02.001 |
Martin-Guay, M.-O., Paquette, A., Dupras, J., et al., 2018. The new Green Revolution: sustainable intensification of agriculture by intercropping. Sci. Total Environ., 615: 767-772. DOI:10.1016/j.scitotenv.2017.10.024 |
Murata, H., Yamada, A., Maruyama, T., et al., 2013. Root endophyte interaction between ectomycorrhizal basidiomycete Tricholoma matsutake and arbuscular mycorrhizal tree Cedrela odorata, allowing in vitro synthesis of rhizospheric “shiro”. Mycorrhiza, 23: 235-242. DOI:10.1007/s00572-012-0466-7 |
Murata, H., Yamada, A., Yokota, S., et al., 2014. Root endophyte symbiosis in vitro between the ectomycorrhizal basidiomycete Tricholoma matsutake and the arbuscular mycorrhizal plant Prunus speciosa. Mycorrhiza, 24: 315-321. DOI:10.1007/s00572-013-0534-7 |
Ocampo, J.A., 1986. Vesicular-arbuscular mycorrhizal infection of “host” and “non-host” plants: effect on the growth responses of the plants and competition between them. Soil Biol. Biochem., 18: 607-610. DOI:10.1016/0038-0717(86)90083-0 |
Pacioni, G., 1991. Effects of Tuber metabolites on the rhizospheric environment. Mycol. Res., 95: 1355-1358. DOI:10.1016/S0953-7562(09)80384-5 |
Pérez-Tienda, J., Testillano, P.S., Balestrini, R., et al., 2011. GintAMT2, a new member of the ammonium transporter family in the arbuscular mycorrhizal fungus Glomus intraradices. Fungal Genet. Biol., 48: 1044-1055. DOI:10.1016/j.fgb.2011.08.003 |
Peskan-Berghofer, T., Shahollari, B., Giong, P.H., et al., 2004. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol. Plantarum, 122: 465-477. DOI:10.1111/j.1399-3054.2004.00424.x |
Plattner, I., Hall, I.R., 1995. Parasitism of non-host plants by the mycorrhizal fungus Tuber melanosporum. Mycol. Res., 99: 1367-1370. DOI:10.1016/S0953-7562(09)81223-9 |
Pringle, A., 2009. Mycorrhizal networks. Curr. Biol., 19: R838-R839. DOI:10.1016/j.cub.2009.07.003 |
Riccioni, C., Rubini, A., Belfiori, B., Gregori, G., Paolocci, F., 2016. Tuber magnatum: The special one. What makes it so different from the other Tuber spp., In: Zambonelli, A., Iotti, M., Murat, C. (Eds.), True Truffle (Tuber spp.) in the World: Soil Ecology, Systematics and Biochemistry. Springer International Publishing, Cham, pp. 87-103
|
Rinaudo, V., Bàrberi, P., Giovannetti, M., et al., 2010. Mycorrhizal fungi suppress aggressive agricultural weeds. Plant Soil, 333: 7-20. DOI:10.1007/s11104-009-0202-z |
Robinson, D., Fitter, A., 1999. The magnitude and control of carbon transfer between plants linked by a common mycorrhizal network. J. Exp. Bot., 50: 9-13. DOI:10.1093/jxb/50.330.9 |
Rodriguez, R.J., , Arnold, A.E., et al., 2009. Fungal endophytes: diversity and functional roles. New Phytol., 182: 314-330. DOI:10.1111/j.1469-8137.2009.02773.x |
Schneider-Maunoury, L., Deveau, A., Moreno, M., et al., 2020. Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, endophytically colonise roots of non-ectomycorrhizal plants in natural environments. New Phytol., 225: 2542-2556. DOI:10.1111/nph.16321 |
Schneider-Maunoury, L., Leclercq, S., Clément, C., et al., 2018. Is Tuber melanosporum colonizing the roots of herbaceous, non-ectomycorrhizal plants?. Fungal Ecol., 31: 59-68. DOI:10.1016/j.funeco.2017.10.004 |
Selosse, M.-A., Dubois, M.-P., Alvarez, N., 2009. Do Sebacinales commonly associate with plant roots as endophytes?. Mycol. Res., 113: 1062-1069. DOI:10.1016/j.mycres.2009.07.004 |
Shahollari, B., Vadassery, J., Varma, A., et al., 2007. A leucine-rich repeat protein is required for growth promotion and enhanced seed production mediated by the endophytic fungus Piriformospora indica in Arabidopsis thaliana. Plant J., 50: 1-13. DOI:10.1111/j.1365-313X.2007.03028.x |
Simard, S.W., Durall, D.M., 2004. Mycorrhizal networks: a review of their extent, function, and importance. Can. J. Bot., 82: 1140-1165. DOI:10.1139/b04-116 |
Smith, S.E., Read, D.J., 2008. Mycorrhizal symbiosis. Academic press
|
Song, Y., Wang, M., Zeng, R., et al., 2019. Priming and filtering of antiherbivore defences among Nicotiana attenuata plants connected by mycorrhizal networks. Plant Cell Environ., 42: 2945-2961. DOI:10.1111/pce.13626 |
Splivallo, R., Fischer, U., Göbel, C., et al., 2009. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol., 150: 2018-2029. DOI:10.1104/pp.109.141325 |
Splivallo, R., Novero, M., Bertea, C.M., et al., 2007. Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. New Phytol., 175: 417-424. DOI:10.1111/j.1469-8137.2007.02141.x |
Streiblova, E., Gryndlerova, H., Gryndler, M., 2012. Truffle brûlé: an efficient fungal life strategy. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol., 80: 1-8. DOI:10.1111/j.1574-6941.2011.01283.x |
Stringlis, I.A., Proietti, S., Hickman, R., Van Verk, M.C., Zamioudis, C., Pieterse, C.M.J., 2018. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J., 93: 166-180. DOI:10.1111/tpj.13741 |
Tang, X., Zhang, C., Yu, Y., Shen, J., van der Werf, W., Zhang, F., 2021. Intercropping Legumes and Cereals Increases Phosphorus Use Efficiency; a Meta-Analysis. Plant Soil, 460: 89-104. DOI:10.1007/s11104-020-04768-x |
Taschen, E., Sauve, M., Vincent, B., et al., 2019. Insight into the truffle brûlé: tripartite interactions between the black truffle (Tuber melanosporum), holm oak (Quercus ilex) and arbuscular mycorrhizal plants. Plant Soil, 446: 577-594. |
Tester, M., Smith, S., Smith, F., 1987. The phenomenon of "nonmycorrhizal" plants. Can. J. Bot., 65: 419-431. DOI:10.1139/b87-051 |
Toju, H., Sato, H., Root-associated fungi shared between arbuscular mycorrhizal and ectomycorrhizal conifers in a temperate forest. Front. Microbiol.. |
van der Heijden, M.G.A., Martin, F.M., Selosse, M.-A., et al., 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol., 205: 1406-1423. DOI:10.1111/nph.13288 |
Veiga, R.S., Faccio, A., Genre, A., et al., 2013. Arbuscular mycorrhizal fungi reduce growth and infect roots of the non-host plant Arabidopsis thaliana. Plant Cell Environ., 36: 1926-1937. |
Veiga, R.S.L., Howard, K., van der Heijden, M.G.A., 2012. No evidence for allelopathic effects of arbuscular mycorrhizal fungi on the non-host plant Stellaria media. Plant Soil, 360: 319-331. DOI:10.1007/s11104-012-1256-x |
Vierheilig, H., Iseli, B., Alt, M., et al., 1996. Resistance of Urtica dioica to mycorrhizal colonization: a possible involvement of Urtica dioica agglutinin. Plant Soil, 183: 131-136. DOI:10.1007/BF02185572 |
Vishwanathan, K., Zienkiewicz, K., Liu, Y., et al., 2020. Ectomycorrhizal fungi induce systemic resistance against insects on a nonmycorrhizal plant in a CERK1-dependent manner. New Phytol., 228: 728-740. DOI:10.1111/nph.16715 |
Wagg, C., Veiga, R., van der Heijden, M.G.A., 2015. Facilitation and antagonism in mycorrhizal networks, In: Horton, T.R. (Ed.), Mycorrhizal Networks. Springer Netherlands, Dordrecht, pp. 203-226
|
Wang, B., Qiu, Y.L., 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza, 16: 299-363. DOI:10.1007/s00572-005-0033-6 |
Wang, Y., Lambers, H., 2020. Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil, 447: 135-156. DOI:10.1007/s11104-019-03972-8 |
Wang, Y., Ran, W., Lu, B., et al., 2021. Mycorrhization of Quercus mongolica seedlings by Tuber melanosporum alters root carbon exudation and rhizosphere bacterial communities. Plant Soil, 467: 391-403. DOI:10.1007/s11104-021-05112-7 |
Weiß, M., Waller, F., Zuccaro, A., et al., 2016. Sebacinales – one thousand and one interactions with land plants. New Phytol., 211: 20-40. DOI:10.1111/nph.13977 |
Wipf, D., Krajinski, F., van Tuinen, D., et al., 2019. Trading on the arbuscular mycorrhiza market: from arbuscules to common mycorrhizal networks. New Phytol., 223: 1127-1142. DOI:10.1111/nph.15775 |
Wurst, S., Vender, V., Rillig, M., 2010. Testing for allelopathic effects in plant competition: does activated carbon disrupt plant symbioses?. Plant Ecol., 211: 19-26. DOI:10.1007/s11258-010-9767-0 |
Yu, G., Chi, Z.-L., Kappler, A., et al., 2020. Fungal nanophase particles catalyze iron transformation for oxidative stress removal and iron acquisition. Curr. Biol., 30: 2943-2950. DOI:10.3390/rs12182943 |
Zhang, L., Shi, N., Fan, J., et al., 2018. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol., 20: 2639-2651. DOI:10.1111/1462-2920.14289 |
Zouari, I., Salvioli, A., Chialva, M., et al., 2014. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genom., 15: 221. |



