b. University of Chinese Academy of Sciences, Beijing, 100049, China;
c. Flower Research Institute of Yunnan Academy of Agricultural Sciences, Kunming, 650205, Yunnan, China
Members of the genus Paphiopedilum (Orchidaceae) are world-famous ornamental orchids because of their unique flower shape and colors, large flowers, and long flower lifespan. They have been cultivated throughout the world for more than 100 years, but their commercial production is still limited (Averyanov et al., 2003; Liu et al., 2009). One of the important reasons for this is the insufficient understanding of the environmental adaptability of Paphiopedilum species, which limits the development of cultivation techniques. Meanwhile, due to illegal collection and habitat destruction, the number of wild populations and individuals of Paphiopedilum have drastically decreased (Liu et al., 2009). Paphiopedilum species are sensitive to environmental change. For example, the forest exploitation influences the light intensity and temperature in the natural distribution areas of Paphiopedilum (Averyanov et al., 2003). They are a particularly vulnerable group of plants which are amongst the first to disappear during degradation of primary native habitats. All known Paphiopedilum species are listed in Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, 2012), and are strictly protected by this international convention. Both the cultivation and conservation of Paphiopedilum species depend on knowledge of their environmental adaptabilities. However, only a few studies have focused on the adaptations of Paphiopedilum species to low temperature and light intensity (Zhang et al., 2011; Yang et al., 2017), and their environmental requirements and adaptative mechanisms remain unclear.
The genus Paphiopedilum comprises more than 70 species, which usually occur in limestone or mountainous forests of tropical and subtropical zones from Asia to the Pacific Islands (Cribb, 1998; Liu et al., 2009). Previous studies have suggested that Paphiopedilum plants are characterized by drought tolerance derived from their leaf anatomical traits, including fleshy leaves, thick leaves, thick cuticles, bigger adaxial epidermal cells, lower total stomata area and sunken stomata (Guan et al., 2011; Zhang et al., 2011; Yang et al., 2018). Stomatal and vein densities also tend to increase from terrestrial to facultative and epiphytic Paphiopedilum species (Zhang et al., 2012). The lack of guard cell chloroplasts affects the induction of photosynthesis in Paphiopedilum, which may reflect a physiological adaptation to water shortage (Assmann and Zeiger, 1985; Zhang et al., 2011). Therefore, previous studies infer that leaf morphological traits play an important function in reducing the loss of water (Guan et al., 2011; Zhang et al., 2011). However, these species vary largely in their growing environments, leaf morphology and anatomy, flowering period and floral lifespan (Guan et al., 2011; Zhang et al., 2012, 2017). Research on how leaf morphology and anatomy affect environmental adaptations of Paphiopedilum species through controlled experiments is still lacking.
Photosynthesis is a major mechanism for plants to assimilate carbon, and is extremely sensitive to environmental changes, such as light, temperature, and water (Long et al., 2006; Greer and Weedon, 2011; Toscano et al., 2018). Thus, the photosynthetic rate is a crucial trait reflecting a plant's response to environmental changes. Usually, plants have a higher light-saturated photosynthetic rate under optimum temperature conditions (An et al., 2017). Both low and high temperatures can depress the photosynthetic rate and stomatal conductance of plants (Greer and Weston, 2010; Greer and Weedon, 2011; Wu et al., 2014). Low temperature can decrease photosynthetic performance of Phalaenopsis plants (Pollet et al., 2009). Similarly, high temperature reduces relative growth rates by the direct effects of temperature on photosynthesis (Marchin et al., 2014). The photosynthetic rate is depressed, while respiration is promoted when plants are exposed to a temperature which is above the photosynthetic optimum. The vegetative growth is strongly inhibited due to the imbalance between carbon-fixation and consumption (Iersel, 2003). Recently, Yang et al. (2017) found that P. armeniacum and P. micranthum can use cyclic electron flow to protect photosystem I and photosystem II under chilling conditions. However, how the photosynthesis of Paphiopedilum plants respond to temperature changes remains unclear.
Leaf morphology and anatomy play an important role in response to changing environments, because they affect light capture, CO2 diffusion conductance, heat balance, water loss and storage of leaves. Leaf anatomical traits are significantly influenced by temperature. Plants will increase their leaf thicknesses under warming conditions (Soudzilovskaia et al., 2013). Under high temperatures, plants may increase their leaf and adaxial cuticle thickness (Zheng et al., 2013; Zhou et al., 2019; Habermann et al., 2019), but decrease stomatal density (Ferris et al., 1996). Recently, Wu et al. (2018) found that stomatal density of Schima superba significantly decreases under warming conditions, while a non-significant change is observed in Syzygium rehderianum. Warm temperatures in the early spring, when shoots are emerging, appear favored, while high temperatures during a thesis appear detrimental, reducing both vegetative growth and flowering (Bleho et al., 2021). However, how the leaf anatomy of Paphiopedilum plants varies with the change of temperature remains unclear.
Phenotypic plasticity may play an important role in the establishment of plant population in novel environments, either after transplant to a new habitat or as a response to changing environments (Richards et al., 2006; Matesanz et al., 2010). Although the ability to respond to environmental change is often beneficial, phenotypic plasticity, just like any other aspect of the phenotype, cannot automatically be assumed to be adaptive (Richards et al., 2006). It is adaptive plasticity, however, that is of particular importance for ecological and evolutionary studies (Richards et al., 2006). For example, compared with stomatal length, stomatal density is relatively plastic (Richardson et al., 2001) and potentially adaptive to environmental change (Poulos et al., 2007; Sekiya and Yano, 2008). Thus, we speculate that large phenotypic plasticity indices of Paphiopedilum species may help them to tolerate the change of temperature.
In the present study, we investigated the responses of leaf photosynthetic and anatomical traits of P. dianthum and P. micranthum to different environmental temperatures. These two Paphiopedilum species can occur in the same natural habitats, but P. dianthum plants have a greater leaf, cuticle and epidermis thickness, and higher stomata density than P. micranthum (Zhang et al., 2012; Yang et al., 2018). In addition, P. dianthum flowers in autumn, while P. micranthum flowers in spring. This indicates that there may be differences in temperature adaptation between the two species. Thus, our aim was to address how do photosynthetic, anatomical traits and flowering performances of two sympatric species with different leaf anatomies respond to different temperatures. Such information will contribute to the conservation and cultivation of Paphiopedilum species. We speculate that the adaptation of the two Paphiopedilum species to temperature is related to their geographical distribution, and the plasticity of leaf anatomical traits may play an important role in the adaptions of two Paphiopedilum species to growth temperature.
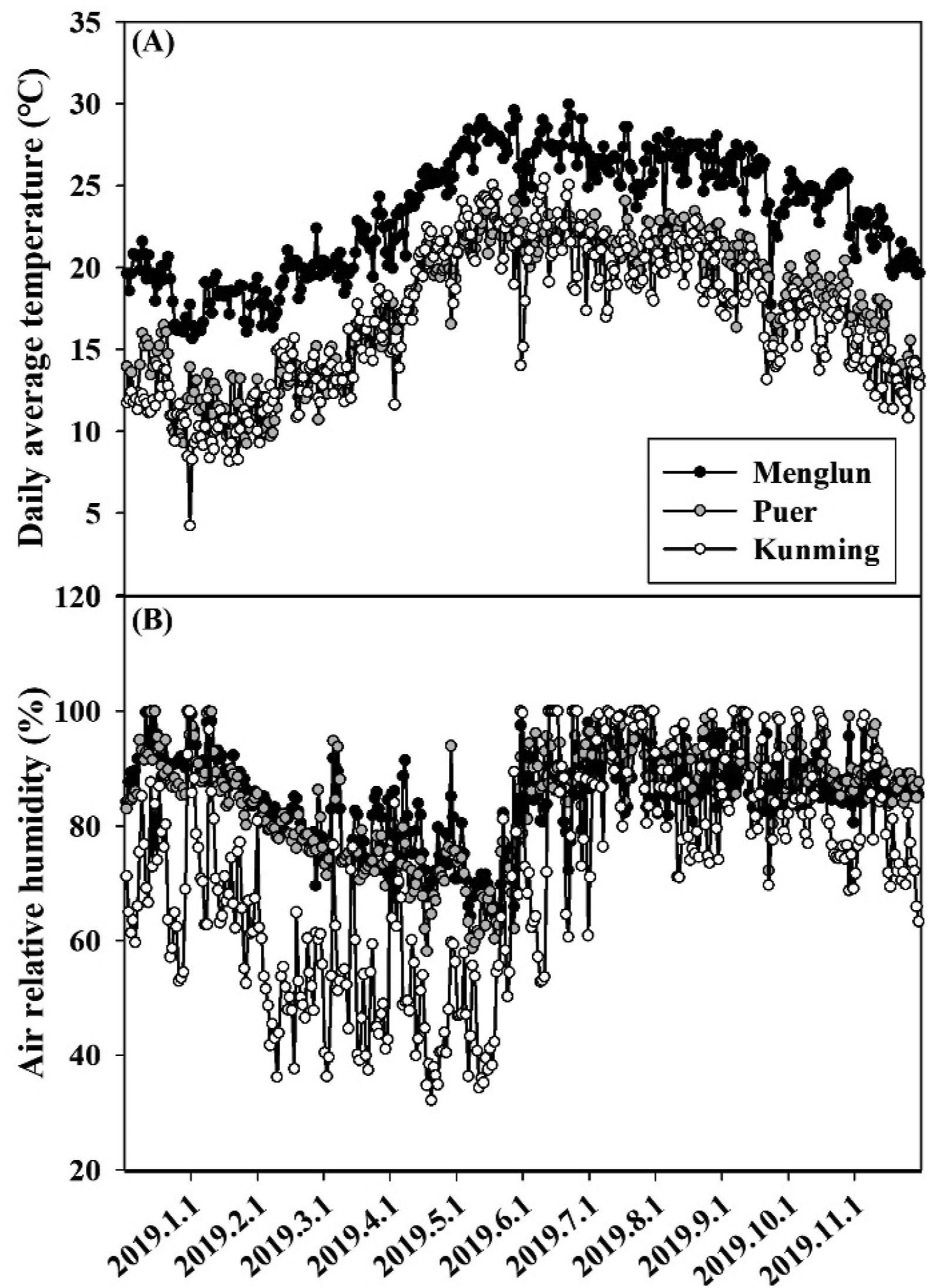
2. Materials and methods 2.1. Study sites and plant materialsThe study was carried out at Menglun, Puer, and Kunming in southwest China (Fig. 1). These three sites locate in tropical, south subtropical, and mid-subtropical zone, respectively. The daily average temperature and air relative humidity was 23.3 ± 0.2 ℃ and 86% at Menglun, 17.7 ± 0.2 ℃ and 81% at Puer, and 16.7 ± 0.2 ℃, and 73% at Kunming, respectively (Fig. 2).

|
| Fig. 1 The study sites (red triangles) and the natural distribution of Paphiopedilum dianthum (green cycles) and P. micranthum (dark pentagons). The map was made with a software of ArcGIS (Version 10.2, Esri, Inc., CA, USA). |
|
| Fig. 2 Change in daily average temperature (A) and air relative humidity (B) at the three study sites. |
In this study, two sympatric species, P. dianthum and P. micranthum, were chosen for the experiments. P. dianthum occurs on rocks in evergreen broad-leaved forests at an elevation of 1000–2250 m. P. micranthum occurs in rocky and bushy places or crevices of rocks in forests in limestone areas at an elevation 400–1700 m (Liu et al., 2009). In November 2018, two-year old artificially propagated plants (300 plants of each species at each site) were placed at three study sites. The plants were planted in porous plastic pots (10 cm × 15 cm) filled with bark and humus (7/3, v/v). We used 70% shade-net to keep the maximum light intensity at approximately 400–600 μmol m−2 s−1. These plants were watered as needed, and fertilized with control release fertilizer (Osmocote, nitrogen: phosphate: potash = 14:14:14, Geldermalsen, Netherlands) about 0.5 g in each pot every month.
2.2. Measurement of photosynthetic gas exchangeAll measurements of leaf gas exchange were performed in an open infrared gas exchange system with an integrated fluorescence chamber (LI-6400-40; Li-Cor, Lincoln, NE, USA) in June and December 2019. Before photosynthetic measurements, the leaf steady-state conditions of P. dianthum and P. micranthum were induced for 20–30 min using a chamber CO2 concentration of 400 μmol mol−1 and a photosynthetic photon flux density (PPFD) of 300 μmol m−2 s−1. Photosynthetic light response curves were tested with light intensities ranging from 1000 to 0 μmol m−2 s−1. The CO2 concentration in the chamber was set to 400 μmol mol−1, and temperature was 25 ℃. Photosynthetic rates were recorded within a steady state by waiting for 3 min at each light intensity. Five mature leaves per species were measured from different individuals. The respiration rate in dark (Rd) and light-saturated photosynthetic rate (Pmax) were obtained during the measurements of light response curves which were fitted with the software of Photosynthesis (LI-COR Application, Lincoln, NE, USA). The stomatal conductance (gs), transpiration rate (Tr), and instantaneous water use efficiency (WUEi) were calculated under saturated light.
2.3. Leaf anatomical and floral traitsIn December 2019, ten leaves were collected from the second leaf of different individuals from the top down per species at each site, which were produced in the new growth environment. Of the ten leaves, five were fixed in FAA (95% ethanol: distilled water: formaldehyde: glacial acetic acid, 10:7:2:1, v/v/v/v) for at least 24 h. The other five leaves were measured with a LI-3000 A leaf portable area meter (Li-Cor, Lincoln, NE, USA), then the samples were oven-dried at 70 ℃ to a constant weight. The leaf dry mass was then weighed and evenly divided into three samples. The leaf dry mass per unit area (LMA) was calculated by the leaf dry mass divided by the leaf area. The leaf carbon content (Cmass), leaf nitrogen content (Nmass), and leaf phosphorus content (Pmass) were measured using an element analyzer (Elementar Analysensysteme GmbH, Vario EL III, Hanau, Germany).
In order to calculate the stomatal density (SD) and stomatal area (As), about 1 cm2 of the middle parts of the leaves were cut, and then immersed into the disinfectant to remove the leaf mesophyll and chlorophyll. The other parts of leaf were dehydrated in an ethanol series and embedded in paraffin for sectioning. Transverse sections were made on a Leica RM2126RT rotary microtome (Leica Inc., Bensheim, Germany). After treatments, five views of each abaxial epidermis were observed and photographed with a Leica DM2500 light microscope (Leica Microscope Vertrieb GmbH, Wetzlar, Germany). To measure the thickness of the leaf (LT), cuticle (CTad and CTab), epidermis (ETad and ETab) and mesophyll (MT), five sections tissue of each leaf were observed and photographed with a light microscope. All the images were analyzed with the ImageJ software package. The flower number per stalk was recorded during anthesis. The peduncle, petal, lip, and dorsal sepal size were measured with a ruler.
2.4. Statistical analysisStatistical analyses were performed with the IBM SPSS 20.0 software package (SPSS Inc., Chicago, IL, USA). A one-factor ANOVA (Tukey's posthoc test) was performed at p = 0.05 significance level to determine the differences in leaf photosynthetic, leaf anatomical, and floral traits among sites after testing for normality and homogeneity of variances. The differences between two parameters were analyzed with t tests of independent samples. The light response curves were fitted with the software of Photosynthesis (LI-COR Application). The phenotypic plasticity index was calculated for each measured variable as the difference between maximum and minimum values divided by the maximum value (Valladares et al., 2000). The map of natural distribution of Paphiopedilum species was performed on the software of ArcGIS 10.2 (Esri, Inc., CA, USA). Graphic images were produced using the Sigma Plot 10.0 package (Systat Software Inc., CA, USA).
3. Results 3.1. Leaf photosynthesis at different sitesThe leaf photosynthetic rates (Pn) of P. dianthum and P. micranthum increased greatly with the increase in PPFDs when the light intensity below 300 μmol m−2 s−1 at the three sites (Fig. 3). There were slightly changes of leaf photosynthetic rates of the two Paphiopedilum species with the increase in PPFDs when the light intensity over 300 μmol m−2 s−1 at the three sites. Compared with Puer and Menglun, the two tested species had a higher Pmax and gs at Kunming (Table 1). For example, the Pmax for P. dianthum at Kunming (5.24 ± 0.29 μmol CO2 m−2 s−1) was significantly higher than at Menglun (3.24 ± 0.32 μmol CO2 m−2 s−1). Similarly, the gs at Kunming (97.0 ± 11.5 mmol H2O m−2 s−1) was also significantly higher than at Menglun (39.4 ± 14.4 mmol H2O m−2 s−1). For P. micranthum, the Pmax (3.00 ± 0.10 μmol CO2 m−2 s−1) and gs (61.8 ± 11.1 mmol H2O m−2 s−1) were significantly higher at Kunming than those at Menglun (1.62 ± 0.04 μmol CO2 m−2 mol−1, 31.1 ± 5.5 mmol H2O m−2 s−1, respectively). However, except for the Pmax being significantly higher at Kunming than at Menglun for P. micranthum, there was no significant difference in gs for the two species between Puer and Menglun. The Rd and Tr of the two species had no significant differences among the three sites, except the Tr value of P. micranthum was significantly higher at Puer than at Menglun. Moreover, the values of Pmax, gs and Tr of the two species were significantly higher in summer than in winter (Table 2).

|
| Fig. 3 Responses of photosynthetic rate (Pn) to photosynthetic photon flux density (PPFD) in Paphiopedilum dianthum (A) and P. micranthum (B) at the three study sites. The light response curve is fitted with Exponential Rise to Maximum (single, two parameters) in Sigma Plot 10 package. Each data point represents mean ± SE for five measurements from individual plants. |
| Species | Traits | Menglun (alt. 570m) | Puer (alt. 1302m) | Kunming (alt. 1990 m) | Plasticity index (%) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P.dianthum | Rd (μmol m−2 s−1) | 0.64±0.24a | 0.68±0.03a | 0.48±0.04a | 29.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmax (μmol CO2 m−2 s−1) | 3.24±0.32b | 4.80±0.32a | 5.24±0.29 a | 38.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| gs (mmol H2O m−2 s−1) | 39.4±14.4b | 61.7±8.0ab | 97.0±11.5 a | 59.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tr (mmol H2O m−2 s−1) | 0.74±0.17a | 0.54±0.10a | 0.71±0.07a | 27.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WUEi (μmol CO2 mmol−1 H2O) | 6.87±4.17a | 7.53±1.05a | 7.04±0.28a | 8.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LT (μm) | 1043.4±19.7c | 1371.8±33.0 a | 1223.1±18.1b | 23.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTad (μm) | 34.57±1.38a | 35.04±1.18a | 31.54±1.71a | 10.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTab (μm) | 24.35±0.89a | 23.27±0.70a | 26.19±1.20a | 11.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETad (μm) | 304.6±16.06b | 510.9±13.5 a | 451.4±16.4a | 40.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETab (μm) | 59.42±1.75a | 54.45±1.42a | 60.70±1.79a | 10.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MT (μm) | 613.9±10.1b | 715.4±23.8 a | 659.4±16.4ab | 14.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD (mm−2) | 36.0±0.4b | 45.3±2.2 a | 39.6±2.1ab | 20.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| As (μm−2) | 1298.6±27.6a | 1463.6±23.2a | 1477.4±29.2a | 12.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LMA (g m−2) | 146.4±8.01a | 165.2±5.5a | 161.1±7.8a | 11.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cmass (%) | 49.38±0.24 a | 48.30±0.38ab | 47.53±0.40b | 3.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nmass (%) | 1.05±0.09a | 0.86±0.10a | 1.11±0.10a | 22.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmass (%) | 1.29±0.19a | 1.06±0.10a | 1.20±0.05a | 17.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P.micranthum | Rd (μmol m−2 s−1) | 0.26±0.04a | 0.33±0.08a | 0.13±0.04a | 60.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmax (μmol CO2 m−2 s−1) | 1.62±0.04c | 2.10±0.05b | 3.00±0.10 a | 46.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| gs (mmol H2O m−2 s−1) | 31.1±5.5b | 46.5±3.3ab | 60.8±11.1 a | 48.8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tr (mmol H2O m−2 s−1) | 0.26±0.05b | 0.46±0.05 a | 0.35±0.04ab | 43.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WUEi (μmol CO2 mmol H2O−1) | 6.87±1.64a | 5.91±1.23a | 7.67±0.74a | 22.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LT (μm) | 949.8±20.0b | 905.2±16.7b | 1041.0±35.9 a | 13.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTad (μm) | 22.05±0.83b | 23.45±1.04ab | 27.24±2.35 a | 19.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTab (μm) | 19.77±0.70a | 18.54±1.02a | 19.24±1.15a | 6.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETad (μm) | 165.0±6.6a | 156.2±5.4a | 164.6±10.1a | 5.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETab (μm) | 68.02±2.69a | 68.87±2.64a | 66.57±2.09a | 3.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MT (μm) | 674.2±16.1b | 644.0±15.3b | 759.42±29.1 a | 15.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD (mm−2) | 16.0±0.6b | 19.2±1.0 a | 17.6±0.6ab | 16.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| As (μm−2) | 2775.0±61.4a | 2318.9±44.0a | 2327.0±45.5a | 16.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LMA (g m−2) | 177.4±10.6a | 170.1±7.6a | 160.4±8.4a | 9.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cmass (%) | 43.86±0.20a | 44.02±0.43a | 43.44±0.19a | 1.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nmass (%) | 0.90±0.07a | 0.77±0.10a | 0.92±0.07a | 16.3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmass (%) | 0.53±0.04a | 0.49±0.05a | 0.54±0.04a | 9.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Different letters indicated significant differences between study sites and the maximum value was bold (P < 0.05, based on ANOVA, followed by Tukey's tests for comparison). Values were means ± SE (n = 5). Rd, respiration rate in dark; Pmax, light-saturated photosynthetic rate; gs, stomatal conductance; Tr, transpiration rate; WUEi, instantaneous water use efficiency; LT, leaf thickness; CTad, adaxial cuticle thickness; CTab, abaxial cuticle thickness; ETad, adaxial epidermis thickness; ETab, abaxial epidermis thickness; MT, mesophyll thickness; SD, stomatal density; As, stomatal apparatus area; LMA, leaf dry mass per unit area; Cmass, leaf carbon concentration; Nmass, leaf nitrogen concentration; Pmass, leaf phosphorus concentration. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Traits | Summer | Winter | P value | |||||||||||||||||||||||||||||||||||||
| P.dianthum | Rd (μmol m−2 s−1) | 0.60±0.06 | 0.23±0.11 | 0.047 | |||||||||||||||||||||||||||||||||||||
| Pmax (μmol CO2 m−2 s−1) | 4.43±0.01 | 0.71±0.22 | 0.005 | ||||||||||||||||||||||||||||||||||||||
| gs (mmol H2O m−2 s−1) | 66.0±16.9 | 8.3±2.9 | 0.028 | ||||||||||||||||||||||||||||||||||||||
| Tr (mmol H2O m−2 s−1) | 0.66±0.06 | 0.13±0.07 | 0.004 | ||||||||||||||||||||||||||||||||||||||
| WUEi (μmol CO2 mmol H2O−1) | 7.15±0.20 | 9.50±3.93 | 0.611 | ||||||||||||||||||||||||||||||||||||||
| P.micranthum | Rd (μmol m−2 s−1) | 0.24±0.06 | 0.23±0.14 | 0.943 | |||||||||||||||||||||||||||||||||||||
| Pmax (μmol CO2 m−2 s−1) | 2.24±0.40 | 0.81±0.40 | 0.066 | ||||||||||||||||||||||||||||||||||||||
| gs (mmol H2O m−2 s−1) | 46.0±8.7 | 14.0±5.0 | 0.033 | ||||||||||||||||||||||||||||||||||||||
| Tr (mmol H2O m−2 s−1) | 0.36±0.06 | 0.13±0.02 | 0.021 | ||||||||||||||||||||||||||||||||||||||
| WUEi (μmol CO2 mmol H2O−1) | 6.82±0.51 | 8.46±5.37 | 0.789 | ||||||||||||||||||||||||||||||||||||||
| Values were means ± SE (n = 3). Analysis of difference based on t tests of independent samples and the maximum value was bold. The abbreviations are the same as Table 1. | |||||||||||||||||||||||||||||||||||||||||
There were significant differences in many leaf anatomical and chemical traits for P. dianthum and P. micranthum among the three sites (Table 1). For P. dianthum, the values of LT, ETad, and MT were significantly higher at Puer than those at Kunming and Menglun. The SD was larger at Puer than at Menglun. The Cmass was significantly higher at Menglun than at Kunming and Puer. However, no significant difference was found in the CTad, CTab, ETab, As, LMA, Nmass, and Pmass in P. dianthum among the three sites. For P. micranthum, the LT, CTad, and MT were significantly thicker at Kunming than at Puer and Menglun. The change trend in the SD among the three sites was similar to that of P. dianthum. However, the values of CTab, ETad, ETab, As, LMA, Cmass, Nmass, and Pmass in P. micranthum was not significantly different among the three sites. The plasticity indices of LT, CTab, ETad, ETab, SD, LMA, Cmass, Nmass, and Pmass in P. dianthum among the three sites were higher than those of P. micranthum. Nevertheless, except for the ETab and As, the CTad, CTab, SD, Cmass, Pmass, Rd, Pmax and Tr of P. dianthum were significantly higher than those of P. micranthum (Table 3).
| Traits | P.dianthum | P.micranthum | P value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LT (μm) | 1212.8±94.9 | 965.3±40.0 | 0.074 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTad (μm) | 33.72±1.10 | 24.25±1.55 | 0.008 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTab (μm) | 24.60±0.85 | 19.18±0.36 | 0.004 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETad (μm) | 422.3±61.3 | 161.9±2.9 | 0.051 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ETab (μm) | 58.19±1.91 | 67.82±0.67 | 0.009 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MT (μm) | 662.9±29.4 | 692.6±34.6 | 0.549 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SD (mm−2) | 40.30±2.71 | 17.60±0.92 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| As (μm−2) | 1413.2±57.4 | 2473.6±150.7 | 0.003 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LMA (g m−2) | 157.6±5.7 | 169.3±4.9 | 0.195 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cmass (%) | 48.40±0.54 | 43.77±0.17 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nmass (%) | 1.015±0.08 | 0.86±0.05 | 0.182 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmass (%) | 1.18±0.07 | 0.52±0.02 | 0.001 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rd (μmol m−2 s−1) | 0.60±0.06 | 0.24±0.06 | 0.013 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pmax (μmol CO2 m−2 s−1) | 4.43±0.61 | 2.24±0.40 | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| gs (mmol H2O m−2 s−1) | 66.0±16.9 | 46.0±8.7 | 0.351 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tr (mmol H2O m−2 s−1) | 0.66±0.06 | 0.36±0.06 | 0.023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| WUEi (μmol CO2 mmol H2O−1) | 7.15±0.20 | 6.82±0.51 | 0.578 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Values were means ± SE (n = 3). Analysis of difference based on t tests of independent samples and the maximum value was bold. The abbreviations are the same as Table 1. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The flowering performance of P. dianthum was significantly different at the three sites (Table 4). The flower number was significantly fewer at Menglun than at Puer and Kunming. The scape length was significantly larger at Menglun than at Puer and Kunming, but the petal length, lip length and dorsal sepal length showed significant opposite trends. However, the floral buds of P. micranthum were aborted before blossoming at Menglun, while the plants at Puer and Kunming flowered.
| Traits | Study sites | ||||||||||||||||||
| Menglun | Puer | Kunming | |||||||||||||||||
| Number of flowers per scape | 1.5±0.0b | 2.7±0.1 a | 2.3±0.1a | ||||||||||||||||
| Scape length (cm) | 35.02±0.96 a | 27.89±0.90b | 28.09±0.86b | ||||||||||||||||
| Petal length (cm) | 9.21±0.39b | 11.69±0.17a | 11.80±0.16 a | ||||||||||||||||
| Lip length (cm) | 4.54±0.10b | 4.84±0.04 a | 4.83±0.04a | ||||||||||||||||
| Dorsal sepal length (cm) | 4.53±0.13b | 5.11±0.07a | 5.29±0.05 a | ||||||||||||||||
| Values were means ± SE (n = 5). Different letters indicated significant differences between study sites and the maximum value was bold (P < 0.05, based on ANOVA, followed by Tukey's tests for comparison). | |||||||||||||||||||
We found in this study that the photosynthetic rate, leaf thickness and stomatal density of the two Paphiopedilum species showed the same changing trend in response to temperature. Warming significantly reduced photosynthetic rate, leaf thickness and stomatal density, and inhibited the flower performances of the two Paphiopedilum species. Compared with P. dianthum, P. micranthum showed less resistance to elevated temperature in flower performances.
In this study, the photosynthetic traits of P. dianthum and P. micranthum showed significant differences under various environmental temperatures. Among the three study sites, the Pmax of the two Paphiopedilum species was the lowest at Menglun, where the annual average temperature and air relative humidity were the highest (Fig. 2, Table 1). Compared with that at Kunming, the Pmax of P. dianthum was decreased by 38% at Menglun, while the Pmax of P. micranthum was decreased by 46%. Contrary to our result, an increased temperature increases the Pmax of Dryas Octopetala var. asiatica and Rhododendron confertissimum (Zhou et al., 2019). Such inconsistency may be caused by differences in the optimum temperature requirement of different species. Both P. dianthum and P. micranthum are distributed in limestone areas above 1000 m a.s.l. in subtropical Asia (Cribb, 1998), where the temperature is not too high. However, Menglun (alt. 570 m) locates at the northern edge of the tropics has high air relative humidity and temperature. The summer temperature at Menglun may exceed the annual average temperature (about 16 ℃) of natural distribution area of the two Paphiopedilum species. Thus, the Pmax at Menglun may be depressed by high temperatures. Previous studies have suggested that the gs and Rubisco activity of plants decrease under high temperature conditions (Greer and Weston, 2010; Greer and Weedon, 2011; Wise et al., 2004). Compared with P. dianthum, the Pmax of P. micranthum at Menglun was reduced by a greater proportion. These results showed that the two Paphiopedilum species had weak tolerance to high temperature at Menglun, and P. micranthum was less tolerant to high temperature than P. dianthum. The reason why P. micranthum was less tolerant to high temperature than P. dianthum was that the former was more geographically distributed in the North (Fig. 1). In addition, the photosynthetic rates of the two Paphiopedilum species in winter were significantly lower than that in summer. This might be related to the seasonal variation in the vigor of Paphiopedilum plants which was affected by seasonal climate. Thus, growth temperature was a key factor affecting the photosynthetic performances of two Paphiopedilum species.
The changes in leaf anatomical traits at the three sites were species-specific. For P. dianthum, the values of LT, ETad, MT, and SD were significantly higher at Puer than those at Menglun, but were not significantly different between Puer and Kunming. This indicated that leaf anatomical traits of P. dianthum were significantly affected by temperature. Previous studies have suggested that LT is significantly reduced under warming environments (Jin et al., 2011; Yang et al., 2011; Zheng et al., 2013; Habermann et al., 2019; Zhou et al., 2019). In this study, the change of LT of P. dianthum was 23.9% which was mainly due to the change of ETad and MT. Thus, high temperature might decrease LT, mainly via reducing the ETad and MT of P. dianthum. A high temperature is usually accompanied by high water loss. To reduce water loss via stomata, plants usually regulate the number or area of stomata (Xu and Zhou, 2008; Cruz et al., 2019). Here, the SD in two Paphiopedilum species reduced significantly under warmer conditions. This is consistent with the result from a study on S. superba (Wu et al., 2018). Thus, the low photosynthetic rate in P. dianthum at Menglun was related to the reduced SD. For P. micranthum, the values of LT, CTad, and MT were significantly higher at Kunming than those at Menglun. This is consistent with previous results (Bacelar et al., 2004; Cassola et al., 2019). The increase in cuticle thickness is a response of plant development to water deficits (Chen et al., 2020). Plants may increase the CTad to prevent water loss under conditions of high transpiration demand (Bacelar et al., 2004). There were no significant differences in the values of CTab, ETad, ETab, and As among the three sites. The LT and SD of the two Paphiopedilum species are important traits in response to the changing growth temperatures. Thus, the adjustments of LT and SD of Paphiopedilum species may play essential roles in plant responses to different environmental temperatures. In addition, some leaf anatomical traits of P. dianthum were significantly higher than those of P. micranthum, such as the thickness of cuticles and stomatal densities. Thus, these variations in leaf anatomical traits may help P. dianthum to adapt to environmental change better than P. micranthum.
The value of Cmass in the two species was higher at Menglun than at Kunming, but there were no significant differences in Nmass, Pmass, and LMA among the three sites. Compared with P. dianthum, the values of Nmass and Pmass were higher than those in P. micranthum, while the value of LMA was opposite in the two species. Previous studies have also suggested that the plants with higher LMA have lower values of Nmass and Pmass (Guan et al., 2011), and LMA shows a significant increase in drought environments (Wang et al., 2011; Toscano et al., 2018). This indicated that the two Paphiopedilum species were not stressed by water availability in summer. However, the two species become dormant during winter, and no new leaves are produced. Thus, further research is still required to illustrate the response of Paphiopedilum species to water status.
The Pmax is influenced by leaf anatomical and physiological traits. Leaf photosynthesis depends on the concentration of CO2 which arrives at the carboxylation site (Muir et al., 2014). Stomata, intercellular airspaces, cells, plasma membrane, cytosol, and chloroplast envelopes and stroma are the major barriers that limit atmospheric CO2 arrival at the carboxylation site (Flexas et al., 2012; Muir et al., 2014). A previous study has found that there is a significantly positive correlation between photosynthetic rate and gs (Gago et al., 2016). The gs is determined by SD, stomatal size and whether the stomata are open (Franks and Beerling, 2009; De Boer et al., 2016). In our study, the change trend of Pmax was same with gs, SD, LT, CTab, ETad, but opposite with ETab and As among the three sites (Table 1). This is similar to previous studies which found that the Pmax increases with SD (Xu and Zhou, 2008; Jin et al., 2011; Yang et al., 2018). There was no significant difference in the As among the three sites, which further proved that the two species may not be stressed by drought (Xu and Zhou, 2008). In addition, the increase in ETab may decrease the mesophyll conductance, which is positively correlated with the photosynthetic rate (Grassi and Magnani, 2005). Thus, leaf anatomy plays an important role in regulating photosynthesis of two Paphiopedilum species in different growth environments.
Temperature has a vital influence on flowering traits, the flowering of Doritaenopsis ‘Newberry Parfait’ was completely suppressed under high-temperature (Newton and Runkle, 2009). However, high temperature can increase the inflorescence size of Phalaenopsis hybrid (Lee and Lin, 1984), and leads to the reduction of flower number and size of tepals in Phalaenopsis hybrid and Tipularia discolor (Newton and Runkle, 2009; Marchin et al., 2014). In our study, the flowering performance of the two Paphiopedilum species were significantly different at the three sites. The number of flowers of P. dianthum was significantly fewer, and no normal flowers developed on the P. micranthum plants at Menglun. High temperature significantly decreased the length of petal, dorsal sepal, and lip of P. dianthum (Table 4). We inferred that there was a trade-off between inflorescence size and tepals size of Paphiopedilum species under high temperature conditions. These results not only indicated that high temperature inhibited the flowering performances of the two Paphiopedilum species, but also revealed that P. dianthum might be tolerant to high temperature better than P. micranthum.
Plant plasticity has been recognized as a vital aspect of how plants develop, function and evolve in their environments (Sultan, 2000). High phenotypic plasticity is not only associated with wide geographical distribution of a species, but it also demonstrates the ability of plants to adapt to new environments (Sultan, 2000; Velikova et al., 2020). Moreover, greater phenotypic plasticity would enable a plant to respond more rapidly to an adverse environment in a short time, which shows that these species may accelerate the process of adaptation in new environments (Ghalambor et al., 2007; McLean et al., 2014). In our study, compared with P. micranthum, higher plasticity indexes of P. dianthum were found in LT, CTad, CTab, ETad, ETab, SD, LMA, Cmass, Nmass, and Pmass. In particular, the plasticity indices of LT, ETab, and SD of P. dianthum were larger than those of P. micranthum. Similar to our hypothesis, these results indicated that the stronger adaptability of P. dianthum than P. micranthum was related to the plasticity of leaf traits under the tested environments.
In conclusion, we focused on the leaf photosynthetic, anatomical, and flowering responses of P. dianthum and P. micranthum to different environmental temperatures in southwest China. The photosynthetic rate and flowering performance of the two species were strongly affected by temperature. However, compared with P. micranthum, the photosynthetic rate of P. dianthum was less sensitive to high temperature. The leaf thickness, mesophyll thickness, and stomatal density were lower at Menglun than those at Puer and Kunming. Furthermore, a larger plasticity of leaf anatomical traits makes P. dianthum more adaptable to the tested environments. This study indicates that P. dianthum plants can more effectively regulate their leaf structure to respond to various environmental temperatures than P. micranthum. The findings will contribute to the conservation and utilization of Paphiopedilum species.
Author contributionsS.B.Z. and J.Q.F. designed the study; J.Q.F. carried out the experiments; J.Q.F. and S.B.Z analyzed the data; J.Q.F., J.H.W. and S.B.Z. wrote and revised this manuscript.
Declaration of competing interestThe authors declare no competing financial interest.
AcknowledgementsThis work was financially supported by the National Natural Science Foundation of China (31970361), the Applied Basic Research Plan of Yunnan Province (2018FA016), the Science and Technology Plan of Yunnan (2018BB010), and the project for Construction of International Flower Technology Innovation Center and Achievement Industrialization (2019ZG006), and the Project for Innovation Team of Yunnan Province. Thanks to Dr. John A Meadows for proofreading and editing. Thanks to Mr. Jianbo Yang for helping to make the map of species distribution.
An, H.R., Kim, Y.J., Kwon, O.K., et al., 2017. High temperature promotes growth and flowering in Sophrolaeliocattleya. Hortic. Environ. Biotechnol., 58: 268-273. DOI:10.1007/s13580-017-0181-6 |
Assmann, S.M., Zeiger, E., 1985. Stomatal responses to CO2 in Paphiopedilum and Phragmipedium–role of the guard cell chloroplast. Plant Physiol., 77: 461-464. DOI:10.1104/pp.77.2.461 |
Averyanov, L., Cribb, P., Ke Loc, P., et al., 2003. Slipper orchids of Vietnam. Royal Botanical Gardens, Kew
|
Bacelar, E.A., Correia, C.M., Pereira, J.M.M., et al., 2004. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol., 24: 233-239. DOI:10.1093/treephys/24.2.233 |
Bleho, B.I., Borkowsky, C.L., Grantham, M.A., et al., 2021. A 20 y analysis of weather and management effects on a small white lady’s-slipper (Cypripedium candidum) population in Manitoba. Am. Midl. Nat., 185: 32-48. |
Cassola, F., Silva, M.H.R., Borghi, A.A., et al., 2019. Morphoanatomical characteristics, chemical profiles, and antioxidant activity of three species of Justicia L. (Acanthaceae) under different growth conditions. Ind. Crop. Prod., 131: 257-265. DOI:10.1016/j.indcrop.2019.01.053 |
Chen, M.J., Zhu, X.F., Zhang, Y., et al., 2020. Drought stress modify cuticle of tender tea leaf and mature leaf for transpiration barrier enhancement through common and distinct modes. Sci. Rep., 10: 6696. DOI:10.1038/s41598-020-63683-4 |
CITES, 2012. Convention on international trade in endangered species of wild fauna and flora, Appendices I, II and III. http://www.cites.org
|
Cribb, P., 1998. The Genus Paphiopedilum (2nd edn). Natural History Publications, Kota Kinabalu (Borneo) in association with Royal Botanic Gardens, Kew, UK
|
Cruz, Y.D.C., Scarpa, A.L.M., Pereira, M.P., et al., 2019. Growth of Typha domingensis as related to leaf physiological and anatomical modifications under drought conditions. Acta Physiol. Plant., 41: 64. DOI:10.1007/s11738-019-2858-1 |
De Boer, H.J., Price, C.A., Wagner-Cremer, F., et al., 2016. Optimal allocation of leaf epidermal area for gas exchange. New Phytol., 210: 1219-1228. DOI:10.1111/nph.13929 |
Ferris, R., Nijs, I., Behaeghe, T., et al., 1996. Elevated CO2 and temperature have different effects on leaf anatomy of perennial ryegrass in spring and summer. Ann. Bot., 78: 489-497. DOI:10.1006/anbo.1996.0146 |
Flexas, J., Barbour, M.M., Brendel, O., et al., 2012. Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci., 193: 70-84. |
Franks, P.J., Beerling, D.J., 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. U. S. A., 106: 10343-10347. DOI:10.1073/pnas.0904209106 |
Gago, J., Daloso, D.M., Figueroa, C.M., et al., 2016. Relationships of leaf net photosynthesis, stomatal conductance, and mesophyll conductance to primary metabolism: a multispecies meta-analysis approach. Plant Physiol., 171: 265-279. DOI:10.1104/pp.15.01660 |
Ghalambor, C.K., Mckay, J.K., Carroll, S.P., et al., 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol., 21: 394-407. DOI:10.1111/j.1365-2435.2007.01283.x |
Grassi, G., Magnani, F., 2005. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ., 28: 834-849. DOI:10.1111/j.1365-3040.2005.01333.x |
Greer, D.H., Weston, C., 2010. Heat stress affects flowering, berry growth, sugar accumulation and photosynthesis of Vitis vinifera cv. Semillon grapevines grown in a controlled environment. Funct. Plant Biol., 37: 206-214. DOI:10.1071/FP09209 |
Greer, D.H., Weedon, M.M., 2011. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant Cell Environ., 35: 1050-1064. |
Guan, Z.J., Zhang, S.B., Guan, K.Y., et al., 2011. Leaf anatomical structures of Paphiopedilum and Cypripedium and their adaptive significance. J. Plant Res., 124: 289-298. DOI:10.1007/s10265-010-0372-z |
Habermann, E., San Martin, J.A.B., Contin, D.R., et al., 2019. Increasing atmospheric CO2 and canopy temperature induces anatomical and physiological changes in leaves of the C4 forage species Panicum maximum. PLoS One, 14: e0212506. DOI:10.1371/journal.pone.0212506 |
Iersel, M.W.V., 2003. Short-term temperature change affects the carbon exchange characteristics and growth of four bedding plant species. J. Am. Soc. Hortic. Sci., 128: 100-106. |
Jin, B., Wang, L., Wang, J., et al., 2011. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biol., 11: 35. DOI:10.1186/1471-2229-11-35 |
Lee, N., Lin, G.M., 1984. Effect of temperature on growth and flowering of Phalaenopsis white hybrid. J. Chin. Soc. Hortic. Sci., 30: 223-231. |
Liu, Z.J., Chen, S.C., Chen, L.J., et al., 2009. The genus Paphiopedilum in China. Beijing: Science Press, 4-12
|
Long, S.P., Zhu, X.G., Naidu, S.L., et al., 2006. Can improvement in photosynthesis increase crop yields?. Plant Cell Environ., 29: 315-330. DOI:10.1111/j.1365-3040.2005.01493.x |
Marchin, R.M., Dunn, R.R., Hoffmann, W.A., 2014. Are winter-active species vulnerable to climate warming? A case study with the wintergreen terrestrial orchid, Tipularia discolor. Oecologia, 176: 1161-1172. DOI:10.1007/s00442-014-3074-8 |
Matesanz, S., Gianoli, E., Valladares, F., 2010. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci., 1206: 35-55. DOI:10.1111/j.1749-6632.2010.05704.x |
McLean, E.H., Prober, S.M., Stock, W.D., et al., 2014. Plasticity of functional traits varies clinally along a rainfall gradient in Eucalyptus tricarpa. Plant Cell Environ., 37: 1440-1451. DOI:10.1111/pce.12251 |
Muir, C.D., Hangarter, R.P., Moyle, L.C., et al., 2014. Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant Cell Environ., 37: 1415-1426. DOI:10.1111/pce.12245 |
Newton, L.A., Runkle, E.S., 2009. High-temperature inhibition of flowering of Phalaenopsis and Doritaenopsis orchid. Hortscience, 44: 1271-1276. DOI:10.21273/hortsci.44.5.1271 |
Pollet, B., Steppe, K., Vanlabeke, M.C., et al., 2009. Diurnal cycle of chlorophyll fluorescence in Phalaenopsis. Photosynthetica, 7: 309-312. DOI:10.1007/s11099-009-0048-x |
Poulos, H.M., Goodale, U.M., Berlyn, G.P., 2007. Drought response of two Mexican oak species, Quercus laceyi and Q. sideroxyla (Fagaceae), in relation to elevational position. Am. J. Bot., 94: 809-818. DOI:10.3732/ajb.94.5.809 |
Richardson, A.D., Ashton, P.M.S., Berlyn, G.P., et al., 2001. Within-crown foliar plasticity of western hemlock, Tsuga heterophylla, in relation to stand age. Ann. Bot., 88: 1007-1015. DOI:10.1006/anbo.2001.1538 |
Richards, C.L., Bossdorf, O., Muth, N.Z., et al., 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett., 9: 981-993. DOI:10.1111/j.1461-0248.2006.00950.x |
Sekiya, N., Yano, K., 2008. Stomatal density of cowpea correlates with carbon isotope discrimination in different phosphorus, water and CO2 environments. New Phytol., 179: 799-807. DOI:10.1111/j.1469-8137.2008.02518.x |
Soudzilovskaia, N.A., Elumeeva, T.G., Onipchenko, V.G., et al., 2013. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl. Acad. Sci. U. S. A., 110: 18180-18184. DOI:10.1073/pnas.1310700110 |
Sultan, S.E., 2000. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci., 5: 537-542. DOI:10.1016/S1360-1385(00)01797-0 |
Toscano, S., Ferrante, A., Tribulato, A., et al., 2018. Leaf physiological and anatomical responses of Lantana and Ligustrum species under different water availability. Plant Physiol. Biochem., 127: 380-392. DOI:10.1016/j.plaphy.2018.04.008 |
Valladares, F., Martinez-Ferri, E., Balaguer, L., et al., 2000. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource-use strategy?. New Phytol., 148: 79-91. DOI:10.1046/j.1469-8137.2000.00737.x |
Velikova, V., Arena, C., Izzo, L.G., et al., 2020. Functional and structural leaf plasticity determine photosynthetic performances during drought stress and recovery in two Platanus orientalis populations from contrasting habitats. Int. J. Mol. Sci., 21: 3912. DOI:10.3390/ijms21113912 |
Wang, R.Z., Huang, W.W., Chen, L., et al., 2011. Anatomical and physiological plasticity in Leymus chinensis (Poaceae) along large-scale longitudinal gradient in northeast China. PLoS One, 6: e26209. DOI:10.1371/journal.pone.0026209 |
Wise, R.R., Olson, A.J., Schrader, S.M., et al., 2004. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ., 27: 717-724. DOI:10.1111/j.1365-3040.2004.01171.x |
Wu, G.L., Liu, H., Hua, L., et al., 2018. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Front. Plant Sci., 9: 467. DOI:10.3389/fpls.2018.00467 |
Wu, G.Y., Hui, J.A., Wang, Z.H., et al., 2014. Photosynthetic characteristics of four wild Dendrobium species in China. Hortscience, 49: 1023-1027. DOI:10.21273/hortsci.49.8.1023 |
Xu, Z.Z., Zhou, G.S., 2008. Response of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot., 59: 3317-3325. DOI:10.1093/jxb/ern185 |
Yang, Y., Wang, G.X., Klanderud, K., et al., 2011. Responses in leaf functional traits and resource allocation of a dominant alpine sedge (Kobresia pygmaea) to climate warming in the Qinghai-Tibetan Plateau permafrost region. Plant Soil, 349: 377-387. DOI:10.1007/s11104-011-0891-y |
Yang, Y.J., Chang, W., Huang, W., et al., 2017. The effects of chilling-light stress on photosystem I and II in three Paphiopedilum species. Bot. Stud., 58: 53. DOI:10.1186/s40529-017-0208-4 |
Yang, Z.H., Huang, W., Yang, Q.Y., et al., 2018. Anatomical and diffusional determinants inside leaves explain the difference in photosynthetic capacity between Cypripedium and Paphiopedilum,. Orchidaceae. Photosynth. Res., 136: 315-328. DOI:10.1007/s11120-017-0466-8 |
Zhang, F.P., Yang, Y.J., Yang, Q.Y., et al., 2017. Floral mass per area and water maintenance traits are correlated with floral longevity in Paphiopedilum (Orchidaceae). Front. Plant Sci., 8: 501. |
Zhang, S.B., Guan, Z.J., Chang, W., et al., 2011. Slow photosynthetic induction and low photosynthesis in Paphiopedilum armeniacum are related to its lack of guard cell chloroplast and peculiar stomatal anatomy. Physiol. Plantarum, 142: 118-127. DOI:10.1111/j.1399-3054.2011.01448.x |
Zhang, S.B., Guan, Z.J., Sun, M., et al., 2012. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS One, 7: e40080. DOI:10.1371/journal.pone.0040080 |
Zheng, Y.P., Xu, M., Shen, R.C., et al., 2013. Effects of artificial warming on the structural, physiological, and biochemical changes of maize (Zea mays L.) leaves in northern China. Acta Physiol. Plant., 35: 2891-2904. DOI:10.1007/s11738-013-1320-z |
Zhou, Y.M., Deng, J.F., Tai, Z.J., et al., 2019. Leaf anatomy, morphology and photosynthesis of three tundra shrubs after 7-year experimental warming on Changbai Mountain. Plants, 8: 271. DOI:10.3390/plants8080271 |



