b. Department of Medicinal Plants and Cultivation, College of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang, 550025, China;
c. Botanischer Garten und Botanisches Museum Berlin, Freie Universität Berlin, Königin-Luise-Str. 6–8, 14195, Berlin, Germany;
d. CAS State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou, 510275, China;
e. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201, China
During the course of field work in 2017, we discovered a single population of yellow-flowered chasmophytic Asteraceae plants of the tribe Cichorieae in the mountains of the Mojiang Hani Autonomous County in SW Yunnan (Fig. 1). No other populations of these plants have yet been identified. Our attempt to identify the plants as a known species of the tribe in China failed; moreover, it became evident that the combination of morphological features of these plants does not match any of the known genera of the tribe.

|
| Fig. 1 Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng – (A) the cliff; (B) secondary trailing roots; (C) plants growing in the rock face. Photographs from type population by Zhi-jian Yin in April, 2018. |
Morphological analysis indicated that the newly discovered plants had an affinity to plants in the subtribe Crepidinae. This is the largest subtribe in the Cichorieae, with 21 genera and >360 species (the Taraxacum microspecies not counted), and it is distributed across most of the northern hemisphere with a centre of diversity in E Asia (Kilian et al., 2009a; 2009b). Our knowledge of the Crepidinae has increased considerably during the last decade and an up-to-date classification with the relevant references is provided by Kilian et al. (2009b). However, given the paucity of diagnostic morphological features and frequent homoplasies in the tribe Cichorieae (Kilian et al., 2009a), no reliable systematic placement of the Mojiang population appeared possible based on morphology alone.
Consequently, the aims of our study were to infer the systematic position of the Mojiang population from molecular phylogenetic and cytological analyses, to characterize it by morphological comparison with the closer allies and to draw the necessary taxonomic conclusions.
2. Material and methods 2.1. Plant materialThe study was based on live plants observed and photo-documented in the field during 2017 and 2018 as well as on the herbarium collection made on these occasions. For morphological comparison we consulted specimens in the herbaria of Berlin (B), Kunming (KUN) and Bejing (PE).
2.2. Morphological studiesFor scanning electron microscopic (SEM) study, achenes and pollen were mounted on SEM stubs with double-sided sticky tape, coated with 20 nm Pt–Pd using a Cressington 108 Auto sputter-coater and examined using a ZEISS SIGMA 300.
2.3. Karyological studyRoot-tips were obtained by germinating achenes (collected from the type population by Zhi-jian Yin s.n on 23 Jun 2020) on wet filter paper in Petri dishes at approximately 20–27 ℃. For chromosome counting, root-tips were pre-treated with 0.1% colchicine for at least 2.5 h, fixed in Carnoy I (glacial acetic acid–absolute ethanol = 1:3), then stained and squashed in carbol fuchsin.
2.4. Sampling, DNA extraction, amplification, sequencing and phylogenetic analysisMolecular phylogenetic analyses were carried out using four individuals of the possible new species from its only known population. DNA was extracted, and the nuclear ribosomal internal transcribed spacer (nrITS) region and three plastid DNA markers (trnL-F, psbA-trnH, matK) were amplified and sequenced as described by Wang et al. (2020). Sequences were aligned into somewhat extended versions of the Crepidinae backbone matrices for nrITS and plastid DNA used by Wang et al. (2020) and the same indel coding and partitioning was applied. The GenBank accession numbers of the newly generated sequences are given in Table 1; those of the published sequences included in the matrices follow the taxon name in the phylogenetic trees (Fig. 2, Fig. 3). Phylogenetic relationships were inferred with maximum parsimony (MP), maximum likelihood (ML) and Bayesian inference (BI) on the high-performance computing system of the Freie Universität Berlin (Bennett et al., 2020), using the same software as described by Wang et al. (2020): PRAP v.2.0 (Müller, 2004) and PAUP v.4.0b10 (Swofford, 2003) for MP, ModelTest-NG (Darriba et al., 2019) and RAxML-NG 0.9.0 (Kozlov et al., 2019) for ML, and MrBayes (Ronquist et al., 2012) for BI, SeqState (Müller, 2005) for indel coding, and TreeGraph v.2 (Stöver and Müller, 2010) for displaying the trees with statistical node support, and also applying the same parameters.
| Marker | Taxon name | Specimen | Locality | GenBank acc. no. |
| ITS | Mojiangia oreophila | Yin Zhi-Jian & Zhao Ming-Xu YZJ 0515 (KUN) | China, Yunnan Province, Puer Municipiality, Mojiang County, Xinfu Town | Mojiangia 1: MW790611 Mojiangia 2&4: MW790612 |
| trnL-F | MW984537 | |||
| psbA-trnH | MW984539 | |||
| matK | MW984538 |

|
| Fig. 2 Majority consensus phylogram of the Crepidinae from Bayesian analysis (support values: first line: maximum parsimony jackknife, second line: Bayesian posterior probability/maximum likelihood bootstrap) based on the nrITS region. |

|
| Fig. 3 Majority consensus phylogram of the Crepidinae from Bayesian analysis (support values: first line: maximum parsimony jackknife, second line: Bayesian posterior probability/maximum likelihood bootstrap) based on plastid DNA trnL-F, psbA-trnH, matK sequences. |
The nrITS sequences of three individuals investigated deviate only in one case (sample Mojiangia 1), which is the result of a few low-quality stretches of that single sequence and not of actual genetic differences. The aligned nrITS region had a length of 695 characters; together with the coded indels the matrix included a total of 807 characters, of which 378 were parsimony-informative. The MP analysis resulted in 827 most parsimonious trees (L = 1946, CI = 0.433, RI = 0.697, RC = 0.301, HI = 0.567), largely congruent in topology with the trees of the BI and ML analyses. Fig. 2 shows the BI majority consensus phylogram with the BI posterior probabilities (PP) and ML bootstrap (BS) support values (bootstrapping converged after 700 replicates with a cut-off set to 3%) below the branches and the MP jackknife (JK) support values above the branches.
The plastid DNA sequences of three individuals investigated were identical (i.e., only one accession number in Table 1). The aligned concatenated plastid DNA markers had a length of 2302 characters; together with the coded indels the matrix included a total of 2395 characters, of which 272 were parsimony-informative. The MP analysis resulted in 56 most parsimonious trees (L = 759, CI = 0.775, RI = 0.793, RC = 0.615, HI = 0.225), largely congruent in topology with the trees of the BI and ML analyses. Fig. 3 shows the BI majority consensus phylogram with the BI posterior probabilities (PP) and ML bootstrap (BS) support values (bootstrapping converged after 1300 replicates with a cut-off set to 3%) below the branches and the MP jackknife (JK) support values above the branches.
The Mojiang population is resolved in the nrITS phylogeny (Fig. 2) as member of a polytomy in the Crepidinae, which otherwise includes the large E Asian-North American Soroseris-Dubyaea-Nabalus clade, the Central to E Asia centred Ixeris-Taraxacum clade, the chiefly Irano-Turanian Heteracia-Garhadiolus clade and the Central Asian monogeneric Acanthocephalus clade. The genera of the last two clades are small or monospecific and show derived features, such as annuality, pronounced heterocarpy mostly combined with atelechoric adaptations and, as far as is known, a low basic chromosome number of x = 3 to 5 (Kilian et al., 2009b+); Acanthocephalus benthamianus Regel has recently also been reported for China (Ya et al., 2018).
In the plastid DNA phylogeny (Fig. 3), of which all deeper nodes lack statistical support, the Mojiang population is nested in the well-supported Faberia clade, which solely includes the Chinese endemic genus Faberia, and is resolved as sister to F. cavaleriei H. Lév., but this latter relationship is not supported statistically.
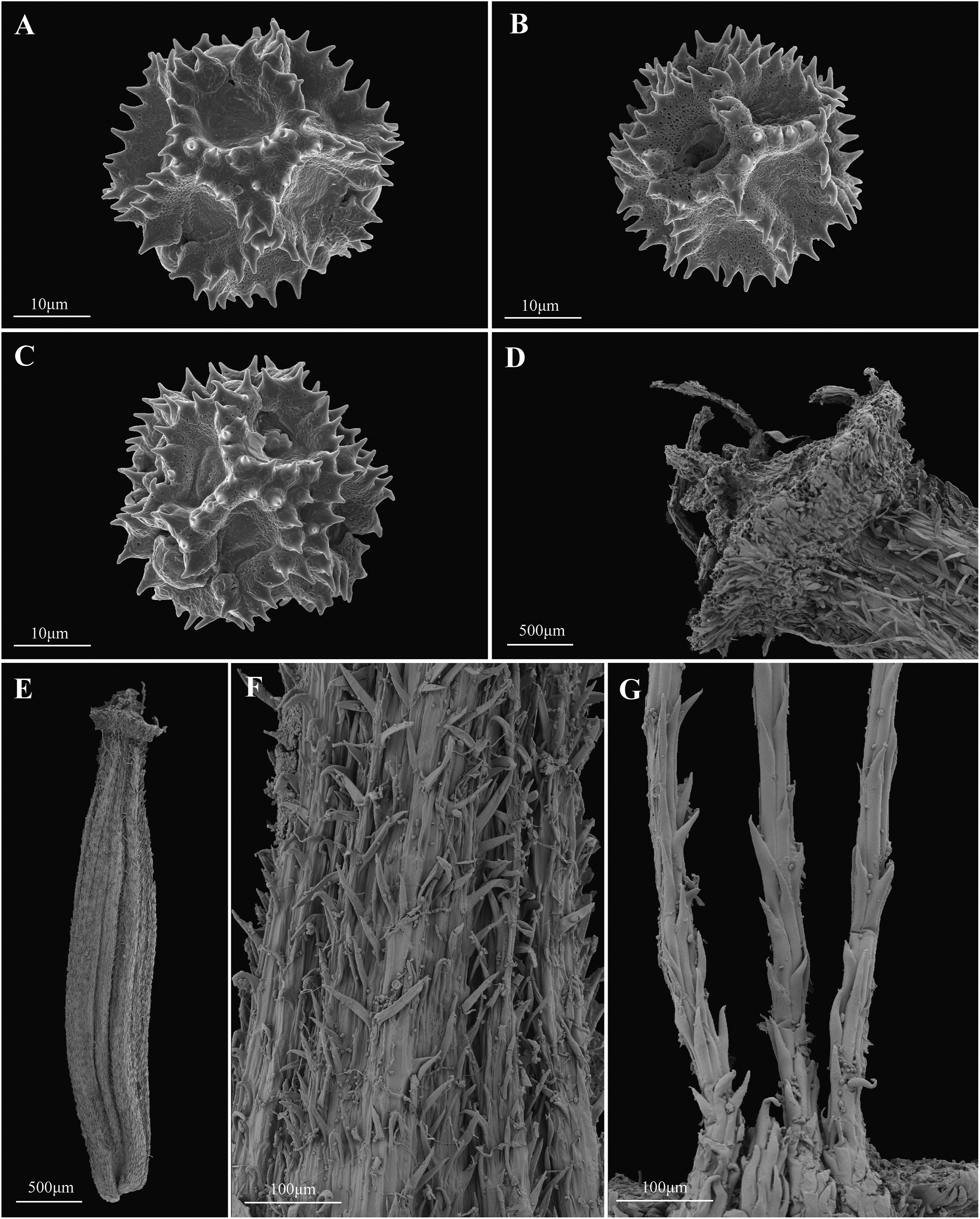
3.2. Morphological comparisonThe combination of morphological features present in the Mojiang population (Table 2) — in particular densely long-papillose homomorphic achenes with 5 main ribs each accompanied by 2 secondary ribs (Fig. 4, Fig. 5E–F), coarse brownish pappus bristles (Fig. 4, Fig. 5G), moderately many-flowered capitula (Fig. 4A+C), a small (7 mm long) involucre with numerous (9–12) outer phyllaries 1/3 to 1/2 as long as the inner ones (Fig. 4D), and the perennial rosette herb growth (Fig. 1, Fig. 4A) — is unique in the subtribe Crepidinae. Achenes with 15 ribs, of which 5 are stronger, is the state considered plesiomorphic for the tribe Cichorieae, and in the Crepidinae it is much less frequent than a reduction or an increase in the number of secondary ribs often combined with an equalling in the shape of main and secondary ribs (Stebbins, 1940; Kilian et al., 2009a). Achenes with a ribbing pattern like the Mojiang population are also present in Sonchella, in many species of Crepidiastrum, Dubyaea and Youngia; achenes with only 10 ribs, by fusion of the adjacent secondary ribs, are a synapomorphy of the Askellia-Ixeris-Ixeridium clade, and progressive reduction towards 10 ribs is also found in the Youngia-Crepidiastrum clade; achenes with no secondary ribs are found in Syncalathium. Increases in the number of secondary ribs are present in Crepis, Nabalus. Sinoseris, Soroseris and Taraxacum. Densely long-papillose achenes are particularly rare in the Crepidinae; coarse pappus bristles are rather common only in the Dubyaea-Nabalus-Soroseris-Syncalathium clade and rarely in Crepis, but are then usually white; numerous and sizable outer phyllaries are present in some genera but in others their number and size are conspicuously small. Faberia, in the clade in which the Mojiang population is nested in the plastid DNA phylogeny, also has numerous and sizable outer phyllaries (in single species), coarse and brownish pappus bristles, but never long-papillose achenes and always cyanic instead of yellow flowers. The pollen, which is of the most widespread Cichorium type (Blackmore, 1986), is tricolpate in the Mojiang population (Fig. 5A–C), but always tetracolpate in Faberia (Liu et al., 2013, Liu et al., 2018).
| Features of Mojiang population | Presence in Crepidinae | Presence in Faberia |
| Perennial rosette herb | In all genera except Acanthocephalus, Garhadiolus, Heteracia, Heteroderis and Lagoseriopsis | + |
| Leaf axiles brown-woolly | Rare, e.g. in Nabalus | + |
| Synflorescence corymbiform with up to 90 capitula | In many genera | Corymbiform synflorescences only sparsely branched |
| Capitula with 18–26 flowers | In many genera | + |
| Involucre small (7mm long) | In many genera | Involucre≥10mm |
| Involucre differentiated into inner and outer phyllary series | In all genera except Syncalathium (there uniseriate) | + |
| Outer phyllaries 9–12, up to 1/3 to 1/2 as long as inner | Numerous outer phyllaries rather rare, chiefly in Crepis, Dubyaea, Hololeion, Nabalus s.l., Taraxacum | + |
| Inner phyllaries 11–13 | More than 8 only in Crepis , Dubyaea, Hololeion, Nabalus s.l., Soroseris | + |
| Corolla yellow | In all genera | Always cyanic |
| Achenes with 15 ribs (5 main ribs and each with 2 secondary ribs) | In a minority of genera, e.g. Crepidiastrum, Dubyaea, Sonchella and Youngia | + |
| Achene surface densely long-papillose | Only in Heteroderis, Lagoseriopsis and apically in Sinoseris | Always glabrous |
| Pappus bristles coarse, pale brown | Frequently coarse in the Dubyaea-Nabalus-Soroseris-Syncalathium clade and p.p. in Crepis, but at the same time brownish only in Dubyaea and Hololeion | + |
| Pollen of the Cichorium type, tricolpate | In all genera | +, but always tetracolpate |
| Polar area of pollen small with 1–3 isolated central echinae | In several genera such as Crepidiastrum, Crepis, Hololeion, Youngia | + p.p. |
|
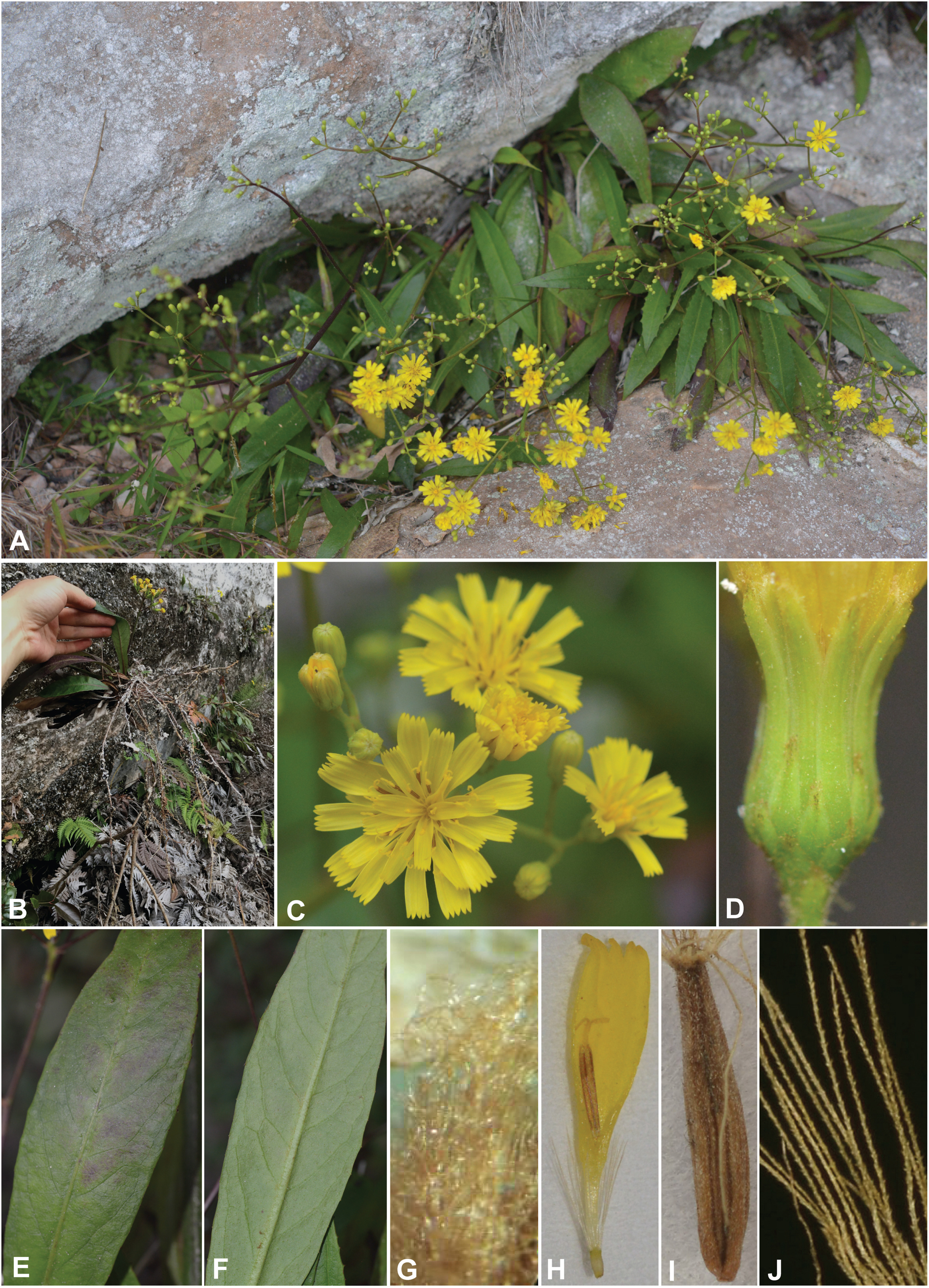
| Fig. 4 Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng – (A) plants in situ; (B) roots; (C) capitulum; (D) involucre; (E) upper face of leaves; (F) lower face of leaves; (G) brown-woolly indumentum of stem base; (H) floret; (I) achene; (J) bristles of pappus. All photographs from type population by Zhi-jian Yin in April, 2018, except I and J from the same population by Ming-xu Zhao in July, 2018. |
|
| Fig. 5 Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng – A–C: pollen. (A) polar view; (B) equatorial view; (C) paraporal view. D–G: achene. (D) pappus disk; (E) the overall view of achene; (F) apex of corpus; (G) bristles of pappus. Pollen photographs from material of type collection; achene photographs from achenes collected on 7 August, 2019 from the type population (Zhi-jian Yin s.n., KUN). |
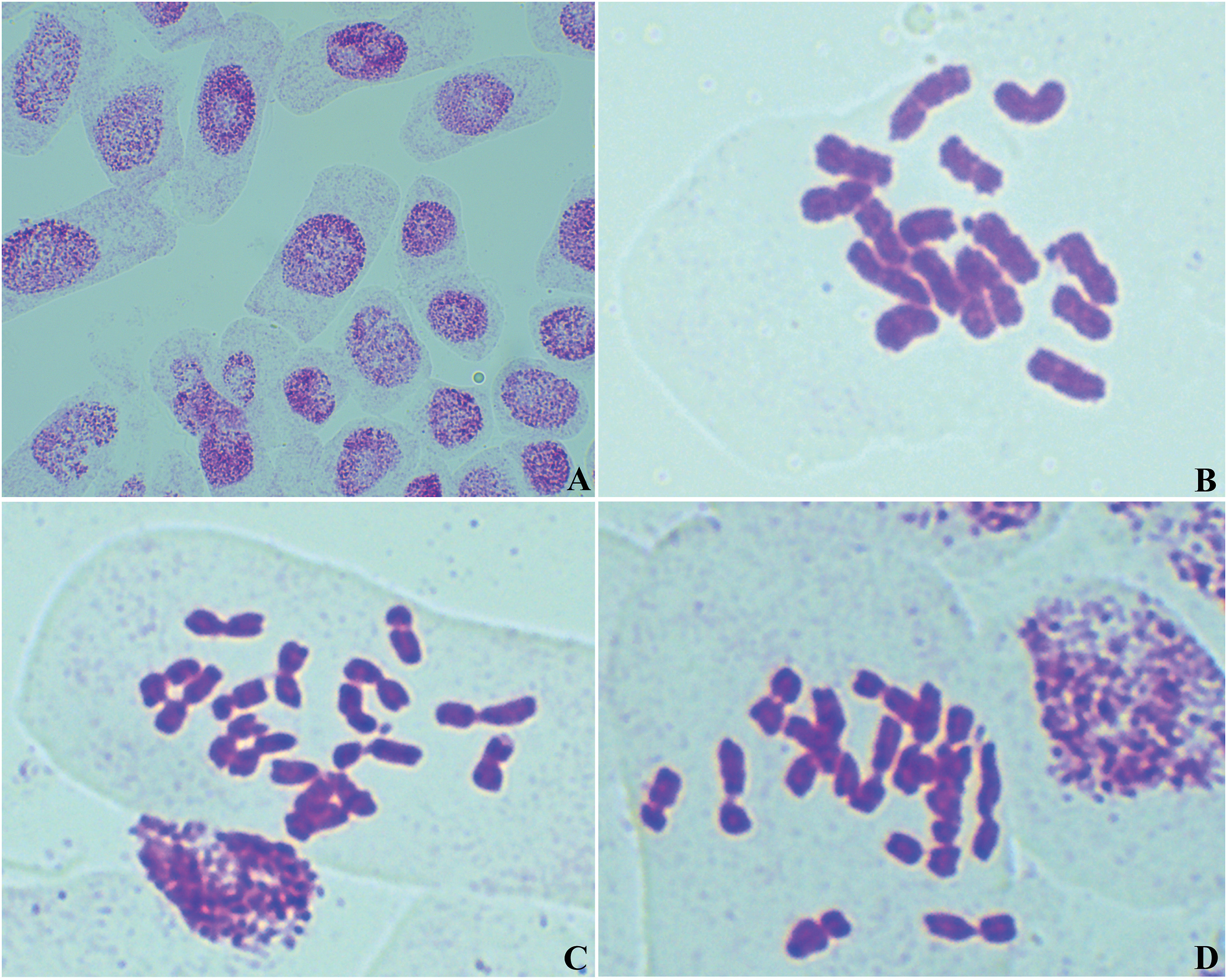
Root tip mitoses revealed a chromosome complement of 2n = 16 (Fig. 6), of which one pair has satellite chromosomes.
|
| Fig. 6 Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng – (A) interphase nucleus; B–D: metaphase plates of root tip mitoses showing 2n = 16. (B) mitotic metaphase of sample 1; (C) one mitotic metaphase cell of sample 2; (D) another mitotic metaphase cell of sample 2. Preparations from germinating achene samples of the type population. |
Both the molecular phylogenetic and morphological findings support the Mojiang population to represent a lineage of its own that is consistently resolved as a member of the Crepidinae in both the plastid and nrITS trees. Classification of the plants as a new monotypic genus thus appears appropriate.
Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng, gen. et sp. nov.
Holotype: China, Yunnan Province, Puer Municipality, Mojiang County, Xinfu Town, 23°39′22.64″N, 101°15′3.38″E, 1400 m, in soil on cliffs or rocks, 4 Apr 2017, Yin Zhi-Jian et Zhao Ming-Xu YZJ 0515 (KUN, see Fig. 7; isotypes: B, PE).

|
| Fig. 7 Mojiangia oreophila Ze H. Wang, N. Kilian et H. Peng – holotype at KUN. |
Further material: Type population, 7 August 2019, Zhi-jian Yin s.n. (KUN, achenes only); type population 23 Jun 2020, Zhi-jian Yin s.n. (KUN, achenes only).
Descriptio generico-specifica/generic-specific description (Shenzhen code art. 38.5; Turland et al., 2018): Perennial rosette herb to c. 50 cm high, with sparsely branched woody caudex and usually few to several, often densely spaced leaf rosettes; rosette shoots with the marcescent remains of older leaves below the actual leaf rosette, and further below covered with the scars of former leaf bases and brown-woolly indumentum (Fig. 4G); with taproot usually rooted in rock face crevices or holes, funiform secondary roots sometimes trailing clung to the rock surface (Fig. 1B). Rosette leaves narrowly elliptic to narrowly spathulate, 6–30 × 1–3.5 cm, apically acute to acuminate, basally attenuate, margin (almost) entire to, mostly shallowly, sinuate-dentate, sometimes revolute, lamina papery to thickly papery on drying, fresh to dark green and sometimes purplish with age on upper face, paler green on lower face, glabrous on upper face and sparsely hairy on lower face especially along the midrib, brown-woolly in leaf axils. Flowering stems usually one per leaf rosette, erect, sparsely to richly branched from base or more frequently in distal half or third, leafless except for linear-lanceolate bracts subtending the branches, purplish and with brownish indumentum more densely in the axils; remnants of withered flowering stems from previous years frequently present in a leaf rosette. Synflorescence of a flowering stem corymbiform, of up to 90 capitula; peduncles mostly 0.5–1 cm long, somewhat brown-woolly. Capitula with 18–26 florets. Involucre narrowly cylindric to narrowly campanulate, c. 7 mm long, clearly differentiated into outer and inner series of phyllaries; outer phyllaries mostly 9–12, ± imbricate, triangular to linear-lanceolate, acute, the outermost 1–1.5 × 0.6–0.8 mm, the longest of the outer phyllaries 1/3–1/2 as long as the inner ones; inner phyllaries 11–13, linear-lanceolate and similar in length, green and with narrow scarious margin, towards apex sparsely brown-woolly on outer face. Receptacle epaleate and glabrous. Florets with bright yellow corolla, tube 2.1–2.3 mm long, ligule broadly elliptical to obovate, 6.4–6.7 mm long and up to c. 2 mm wide; anther tube yellow, fertile part 2.6–2.8 mm long, apical appendages rounded, c. 0.3 mm long, basal appendages c. 0.5 mm long; pollen echinolophate, tricolpate, of the Cichorium type (sensu Blackmore 1986) with moderately extensive polar areas with only one or a few spines in addition to those bordering the adjacent lacuna, and with moderately narrow interlacunar gaps (Fig. 5A–C); style and style arms yellow. Achenes 2.7–3 mm long, columnar to subfusiform with largest diameter in lower third and stronger attenuate toward the truncate apex than towards the somewhat oblique base, brown, densely covered with antrorse, subulate, laterally flattened papillae to c. 60 μm (Fig. 5F); main ribs 5, very prominent, each ± differentiated, except for the very basal portion, into triplets with a secondary rib on either side. Pappus c. 3.5 mm long, persistent, pale brownish, with one series of rather coarse bristles similar in length and diameter, at base of c. 12–16 (or more?) rows of cells in cross section.
4.1. Habitat and ecologyThe species occurs in secondary sub-humid evergreen broad-leaved forest at an elevation of about 1400 m near the summit of a mountain. It grows in vertical sandstone cliffs, rooting in crevices, holes, niches or steps of the rock face (Fig. 1). If holes or crevices are not deep enough, secondary roots are clung to the rock surface and trailing. Flowering March to May, fruiting May to August.
4.2. DistributionOnly known from the type population near Xinfu in the Mojiang County, SW Yunnan, China.
4.3. Threat statusThe only known population of M. oreophila consists of about 60 individuals in a tiny area of 300–400 m2. The very small extent of occurrence and low individual number of the species suggests it is very threatened. If further field work in the area does not detect other populations, the species must be considered as Endangered (EN) because the estimated number of individuals is lower than 250 (IUCN Standards and Petitions Subcommittee, 2019). The habitat and its environment are part of the Mojiang National Forest Park, Yunnan, which was established in 2017 and thus guarantees some protection to the Mojiangia population. Its location near the foot of a cliff face of the summit area could, however, be extremely threatened if this summit cliff face should be used to establish a scenic spot for park visitors.
5. DiscussionMorphological comparison shows that all diagnostically relevant features of Mojiangia are present in other genera of the subtribe Crepidinae, at least in some portion of their species, and – as in a good number of other genera – only their combination in Mojiangia is unique in the subtribe. This applies also to the pollen features. The Cichorium pollen type present in Mojiangia is the most common in the entire tribe Cichorieae (Blackmore, 1986) and its subtypes occur scattered across several subtribes with no correlation to their systematics (Blackmore, 1984; Blackmore and Persson, 1996; Wang et al., 2009). The Mojiangia pollen is of the subtype with a small polar area and only 1–4 isolated central echinae, the occurrence of which is also inconsistent with generic boundaries, e.g., in the cases of Youngia and Sinoseris, where the subtype with an extended polar area is also present (Peng et al., 2013; Wang et al., 2020).
In contrast, Mojiangia shares the majority of its diagnostically relevant features with Faberia, a genus of nine species placed in the subtribe Lactucinae (Wang et al., 2013; Kilian et al., 2017, 2009b+). This even holds true for the pollen, because apart from it being tetracolpate instead of tricolpate, a portion of its species (Faberia pinnatifida Ying Liu, Yousheng Chen et Boufford, F. nanchuanensis C. Shih, F. sinensis Hemsl.; Liu et al., 2018) have a similarly small polar area as Mojiangia, whereas the others have a moderately larger one. Faberia has been shown to be of intersubtribal hybrid origin, with the maternal ancestor of the Crepidinae and the parental ancestor of the Lactucinae (Liu et al., 2013, Liu et al., 2018; Wang et al., 2013, Wang et al., 2014; Kilian et al., 2017). In the context of Faberia, where reticulate evolution was accompanied by genome duplication, leading to a basic number of 2n = 34, it is notable that tetracolpate pollen is considered a deviation from the usually triradiate pollen correlated with polyploidy (Woodhouse, 1935).
Such morphological resemblance agrees well with the remarkable finding that Mojiangia is nested in the plastid DNA tree in the Faberia clade, although resolved in the nrITS phylogeny in a clade of its own. This finding may have either of the following causes: chloroplast capture (Tsitrone et al., 2003; Lee-Yaw et al., 2018) from a Faberia species, or maternal ancestry of the intersubtribal hybridogenous Faberia. In the first case, hybridisation and introgression may have occurred between ancestors of the Mojiang population and a Faberia species, in the course of which the chloroplasts of the invading Faberia ancestor were captured by the Mojiangia ancestor and after some time had replaced the resident Mojiangia ancestor chloroplast. Chloroplast capture is known from many plant taxa (Tsitrone et al., 2003) and may be a frequent cause of cytonuclear discordance; thus, the different phylogenetic patterns between nuclear and organellar genomes (Lee-Yaw et al., 2018). The strong genetic similarity of the plastid markers of Mojiangia and a single Faberia species (F. cavaleriei) makes alternative explanations, such as convergent evolution or incomplete lineage sorting, highly unlikely.
Only one other cause provides a plausible alternative explanation. Given that Faberia has evolved by reticulate evolution involving a paternal Lactucinae and a maternal Crepidinae ancestor (compare Liu et al., 2013; Wang et al., 2013; Kilian et al., 2017), the ancestor of the Mojiang population may have been the maternal Crepidinae ancestor of Faberia. This hypothesis is the more parsimonious one, because it assumes only one case of cytonuclear discordance, namely in Faberia, as a result of its known ancient hybridisation, whereas none in Mojiangia, because it contributed the plastid genome as hitherto unknown maternal ancestor of Faberia. The chromosome number 2n = 16, thus a basic number of x = 8, of Mojiangia agrees with this explanation. The basic number of x = 8 is common in the large Soroseris-Dubyaea-Nabalus clade and several other genera, and is the highest in the Crepidinae, in which other progressive reductions down to x = 3 have occurred. All Faberia species have a basic chromosome number of x = 17 as the result of hybrid formation of an x = 9 and x = 8 genome, and because the basic number of x = 9 is unknown in the Crepidinae, it is assumed that the x = 8 genome comes from the maternal Crepidinae ancestor (Liu et al., 2012, 2013; Liu and Ren, 2014; Liu and Yang, 2014).
Author contributionsY.Z.J. and M.X.Z. discovered the wild population, collected the specimens and have taken the photos of it. Ze-Huan Wang prepared the DNA samples and performed the sequencing. Y.L. counted the chromosomes. N.K., Z.H.W. and H.P. elaborated the taxonomy. N.K. performed the molecular phylogenetic analyses and wrote the manuscript in discussion with and with substantial contributions from Z.H.W. All authors read and commented on the manuscript.
Declaration of competing interestThis article does not involve conflicts of interest.
AcknowledgementsThe authors are grateful to the staff of KUN and the Institute of Cultivation and Processing of Chinese Medicinal Materials for research facilities. The use of high-performance computing resources at the Scientific Computing Service of the Freie Universität Berlin is also gratefully acknowledged. This study was supported by the National Natural Science Foundation of China (grant no. 31500168) and specific funds for the Fourth National Survey on Chinese Materia Medica Resources (GZY-KJS-2018-004). Finally, we would like to thank the Editors and two anonymous reviewers for their valuable comments on earlier versions of this paper.
Bennett, L., Melchers, B., Proppe, B., 2020. Curta: a general-purpose high-performance computer at ZEDAT, Freie Universitat Berlin. Refubium, Dokumente FU. Available from: https://doi.org/10.17169/refubium-26754 (accessed 15 March 2021)
|
Blackmore, S., 1984. The Northwest European pollen flora 32. Compositae – Lactuceae. Rev. Paleobot. Palyno., 42: 45-85. DOI:10.1016/0034-6667(84)90062-9 |
Blackmore, S., 1986. The identification and taxonomic significance of lophate pollen in the Compositae. Can. J. Bot., 64: 3101-3112. DOI:10.1139/b86-409 |
Blackmore, S., Persson, V., 1996. Palynology and systematics of the Crepidinae (Compositae: Lactuceae), in: Hind, D.J.N., Beenthe, H.J. (Eds.), Proceedings of the International Compositae Conference, Kew, 1994, vol. 1, Compositae: Systematics. Royal Botanic Gardens, Kew, pp. 111-122
|
Darriba, D., Posada, D., Kozlov, A.M., et al., 2019. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol., 37: 291-294. |
IUCN Standards and Petitions Subcommittee, 2019. Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Available from: http://www.iucnredlist.org/documents/RedListGuidelines. pdf (accessed 30 May 2020)
|
Kilian N., Gemeinholzer, B., Lack, H.W., 2009a. Tribe Cichorieae, in: Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J. (Eds.), Systematics, Evolution, and Biogeography of the Compositae. IAPT, Vienna, pp. 343-383
|
Kilian, N., Hand, R., Raab-Straube, E. von, (general ed.) 2009b+. Cichorieae Systematics Portal. Available from: http://cichorieae.e-taxonomy.net/portal/. (accessed 30 May 2020)
|
Kilian, N., Sennikov, A., Wang, Z.H., et al., 2017. Sub-Paratethyan origin and Middle to Late Miocene principal diversification of the Lactucinae (Cichorieae, Compositae) inferred from molecular phylogenetics, divergence-dating and biogeographic analysis. Taxon, 66: 675-703. DOI:10.12705/663.9 |
Kozlov, A.M., Darriba, D., Flouri, T., et al., 2019. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics, 35: 4453-4455. DOI:10.1093/bioinformatics/btz305 |
Lee-Yaw, J.A., Grassa, C.J., Joly, S., et al., 2018. An evaluation of alternative explanations for widespread cytonuclear discordance in annual sunflowers (Helianthus). New Phytol.: 10.1111/nph.15386. DOI:10.1111/nph.15386 |
Liu, Y., Chen, Y.S., Boufford, D.E., 2018. Faberia pinnatifida (Asteraceae; Cichorieae), a new species from Sichuan, China. Syst. Bot., 43: 316-324. DOI:10.1600/036364418X697049 |
Liu, Y., Chen, Y.S., Yang, Q.E., 2013. Generic status, circumscription, and allopolyploid origin of Faberia (Asteraceae: Cichorieae) as revealed by ITS and chloroplast DNA sequence data. Taxon, 62: 1235-1247. DOI:10.12705/626.14 |
Liu, Y., Ren, C., 2014. Cytotaxonomy of Prenanthes faberi (Compositae – Cichorieae). Nord. J. Bot., Le, 32: 115-118. |
Liu, Y., Deng, T., Yang, Q.E., 2012. Karyology of the genus Faberia (Cichorieae – Asteraceae) and its systematic implications. Nord. J. Bot., 30: 365-371. DOI:10.1111/j.1756-1051.2011.01305.x |
Liu, Y., Yang, Q.E., 2014. Cytotaxonomy of Dubyaea glaucescens (Compositae – Cichorieae). Nord. J. Bot., 32: 871-874. DOI:10.1111/njb.00448 |
Müller, K., 2004. PRAP — computation of Bremer support for large data sets. Mol. Phylogenet. Evol., 31: 780-782. DOI:10.1016/j.ympev.2003.12.006 |
Müller, K., 2005. SeqState: primer design and sequence statistics for phylogenetic DNA datasets. Appl. Bioinf., 4: 65-69. DOI:10.2165/00822942-200504010-00008 |
Peng, Y.L., Gao, X.F., Peng, L., 2013. Pollen morphology of Youngia and six related genera (Asteraceae: Cichorieae) and its systematic significance. Phytotaxa, 139: 39-62. DOI:10.11646/phytotaxa.139.1.2 |
Ronquist, F., Teslenko, M., Van der Mark, P., et al., 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol., 61: 539-542. DOI:10.1093/sysbio/sys029 |
Stebbins, G.L., 1940. Studies in Cichorieae: Dubyaea and Soroseris. Endemics of the Sino-Himalayan region. Mem. Torrey Bot. Club, 19: 1-76. |
Stöver, B.C., Müller, K., 2010. TreeGraph 2: combining and visualizing evidence from different phylogenetic analyses. BMC Bioinf., 11: 7. DOI:10.1186/1471-2105-11-7 |
Swofford, D.L., 2003. PAUP∗: Phylogenetic Analysis Using Parsimony (∗and other methods), version 4.0b 10. Sinauer Associates Inc. Publishers, Sunderland
|
Tsitrone, A., Kirkpatrick, M., Levin, D.A., 2003. A model for chloroplast capture. Evolution, 57: 1776-1782. DOI:10.1111/j.0014-3820.2003.tb00585.x |
Turland, N.J., Wiersema, J.H., Barrie, F.R., et al., International code of nomenclature for algae, fungi and plants (Shenzhen code) adopted by the Nineteenth International Botanical congress Shenzen, China, July 2017. Regnum Veg.. |
Wang, Z.H., Peng, H., Kilian, N., 2013. Molecular phylogeny of the Lactuca alliance (Cichorieae subtribe Lactucinae, Asteraceae) with focus on their Chinese centre of diversity detects potential events of reticulation and chloroplast capture. PloS One, 8: e82692. DOI:10.1371/journal.pone.0082692 |
Wang, H., Wortley, A.H., Blackmore, S., 2009. Pollen morphology of Crepidinae and Lactucinae (Asteraceae: Cichorieae) and its systematic significance. Grana, 48: 160-178. DOI:10.1080/00173130902931209 |
Wang, Z.H., Kilian, N., Chen, Y.P., et al., 2020. Sinoseris (Crepidinae, Cichorieae, Asteraceae), a new genus of three species endemic to China, one of them new to science. Willdenowia, 50: 91-110. DOI:10.3372/wi.50.50109 |
Wang, G.Y., Meng, Y., Deng, T., et al., 2014. Molecular phylogeny of Faberia (Asteraceae: Cichorieae) based on nuclear and chloroplast sequences. Phytotaxa, 167: 223-234. DOI:10.11646/phytotaxa.167.3.1 |
Woodhouse, R.P., 1935. Pollen Grains, Their Structure, Identification and Significance in Science and Medicine. McGraw-Hill, New York
|
Ya, J.D., Cai, J., Zhang, Q.R., 2018. Two genera and five species newly recorded in China. Turk. J. Bot., 42: 239-245. DOI:10.3906/bot-1705-36 |



