Understanding the mechanisms that form and maintain biodiversity at regional scales has long been a major focus of macroecology and biogeography (Grierson et al., 2011; Ma, 2017; Patino et al., 2017). Researchers have suggested that biodiversity is mediated by both contemporary environmental factors, such as climate and habitat heterogeneity (Kerr and Packer, 1997; Brown et al., 2004; Currie et al., 2004; Wang et al., 2009a), and historical processes, such as speciation, extinction, and dispersal (Zobel, 1997; Ricklefs, 2005; Mittelbach et al., 2007). Despite the widely acknowledged importance of contemporary environments and historical processes, to date, no theory has integrated the relative influences of these factors on biodiversity patterns (Hawkins and Porter, 2003; Svenning and Skov, 2005, 2007; Montoya et al., 2007; Wang et al., 2012). In addition, it is a challenge to distinguish the effects of historical processes from those of contemporary environments because of collinearity.
For over a century, ecologists have proposed hypotheses to explain how biodiversity is maintained. Today, three theories are widely accepted: niche theory (Silvertown, 2004), neutral theory (Hubbell, 2005), and contemporary coexistence theory (HilleRisLambers et al., 2012; Chu et al., 2017). Niche theory, which can be used to explore ecological and evolutionary processes at regional scales (Mittelbach and Schemske, 2015; Bañares-de-Dios et al., 2020), argues that species have different niches, and that habitat filtering and competitive exclusion are the major underlying factors that maintain biodiversity. Neutral theory acknowledges that different species within an ecological community can have equivalent ecological functions, and posits that the maintenance of biodiversity are random (Hubbell, 2001). The contemporary coexistence theory attempts to integrate niche theory and neutral theory; however, it has yet to be used to quantify biodiversity at regional scales.
Community phylogenetic structure has been shown to be influenced simultaneously by the abiotic environment, contemporary biotic interactions, and evolutionary history (Webb et al., 2002; Kraft et al., 2007). The analysis of community phylogenetic structure can help identify which ecological and evolutionary processes regulate biodiversity at different scales. For example, phylogenetically clustered communities are thought to be structured by evolutionary processes such as rapid in situ speciation, niche conservatism, and dispersal limitation (Lu et al., 2018). In contrast, phylogenetically overdispersed communities are thought to be structured by niche evolution, convergent evolution, and colonization (Allen and Gillooly, 2006). Ecological processes can also explain how species are assembled (Webb et al., 2002; Kraft et al., 2007). For example, habitat filtering, which refers to the selection of certain traits of species in a community (Wiens and Graham, 2005), can lead to phylogenetic clustering, whereas competitive exclusion might result in phylogenetic dispersion (Burns and Strauss, 2011). Identifying the evolutionary and ecological mechanisms that underlie both the formation and maintenance of biodiversity at different scales has been made easier today by rapid advances in molecular biology, phylogeography, and phylogenetics (Faith, 1992; Webb et al., 2002).
Mountains are topographically complex regions that affect biodiversity and neighboring lowland ecosystem processes by facilitating biotic interchange, influencing regional climate and nutrient runoff (Rahbek et al., 2019a). Mountains reportedly influence global terrestrial biodiversity disproportionately, particularly in the tropics, where they host hotspots with extraordinary levels of richness. In the arctic and temperate regions, however, mountain regions host few endemic species and typically have low levels of species diversity, which barely exceed those of the adjacent lowlands (Rahbek et al., 2019b). Consequently, they serve as natural laboratories for studying the mechanisms that govern biodiversity at different scales.
The high mountains in China are distributed mainly on the Qinghai-Tibet Plateau (QTP) and in adjacent regions (Wang et al., 2004). The QTP refers to the plateau proper, which is the highest and most expansive plateau on the globe, occupying an area of 2.5 million km2 with an average elevation of over 4000 m (Zhang et al., 2002). Extensive research on the QTP has yielded data sets that may provide insight into the maintenance of biodiversity in the region (Favre et al., 2015). Several studies have determined the geographical distribution of species in the QTP (Wu, 2008; APGIV, 2016); however, the complex environment of the region suggests that species richness may vary considerably (Tang et al., 2006; Yang et al., 2013). Since the climatic fluctuations and glaciations of the Quaternary, the QTP has experienced four major glacial events (Shi et al., 1998; Zhang et al., 2002; Yi et al., 2005; Owen et al., 2008; Owen and Dortch, 2014). These geological processes have driven radiation and species diversification in various groups of plants (Wen et al., 2014). However, climatic fluctuations and glaciations have also led to mass extinction events in some areas of the QTP. Previous studies indicate that the QTP harbors ~10,000 vascular plants species (Wu, 2008; APGIV, 2016), of which ~20% are endemic to the region (Wu, 2008; Yan et al., 2013; Yu et al., 2018b); the southern regions have especially high levels of species richness (Mao et al., 2013).
Methodological advances in phylogeography have identified the evolutionary histories and underlying adaptations of plants in the QTP (Liu et al., 2014), including those of Saussurea DC. (Wang et al., 2009b), Rheum L. (Sun et al., 2012), Gentiana L. (Favre et al., 2016), Rhodiola L. (Zhang et al., 2014), Saxifraga L. (Ebersbach et al., 2016), Syncalathium Lipsch (Zhang et al., 2011), and others (Qiu et al., 2011; Liu et al., 2017). In addition, numerous researchers have integrated principles from different disciplines, including taxonomy, phylogeny, ecology, biogeography, phylogeography, and paleontology to provide insight into the regionalization of floristic assemblages (Li et al., 2018b). Previous studies have reported that biodiversity on the QTP has been largely formed and maintained through rapid speciation and habitat filtering, and that the phylogenetic structure of most communities of vascular plants on the QTP is clustered (Yan et al., 2013). Data sets from different regions have facilitated our understanding of plant diversity on the QTP. For example, an analysis of the community phylogenetic structure of alpine plants in the Hengduan Mountains using the net related index (NRI) and net nearest taxon index (NTI) revealed that the phylogenetic relationships of the taxa are overdispersed at low elevations and clustered at high elevations (Li et al., 2014, 2017). In addition, recent studies have shown that the main phylogeographic patterns of seed plant species are contraction/recolonization, platform refugia/local expansion, and microrefugia in the Tibeto-Himalayan region (Muellner-Riehl, 2019).
Most previous studies have focused on the entire QTP, whereas little research has been conducted on the independent physical geographic subunits in the region. The Kunlun Mountains are an independent physical geographical subunit with plant distribution data and a relatively clear geographic range; however, the Kunlun Mountains are not classified as a biodiversity hotspot (Su, 1998; Zheng, 1999; Pan, 2000; Zachos and Habel, 2011; Wu, 2012–2015; Sun et al., 2015). According to the phytogeographical regions of the Chinese flora, the Kunlun Mountains are in the transition zone between the Tethyan region and the QTP (Ye et al., 2019, 2020). In this study, we used data sets to explore the current patterns of plant diversity in the Kunlun Mountains region. Specifically, we aimed to 1) clarify how the species pool on the Kunlun Mountains emerged, 2) reveal patterns in the phylogenetic structure of seed plants in the region, and 3) explore the factors that drive plant diversity at a regional scale. An improved understanding of the plant diversity patterns among different regions will help develop better biodiversity conservation strategies.
2. Materials and methods 2.1. Study areaThe Kunlun Mountains, located in northwestern China, are an independent physical geographic subunit of the QTP. The mountains span three provincial administrative units: Qinghai, Tibet (Xizang), and Xinjiang. Geographically, they border the Pamirs Plateau to the west, southeast Qinghai to the east, the Qaidam and Tarim Basins to the north, and northwest Tibet Autonomous Region to the south. The Kunlun Mountain range is oriented east-west, located between 34°N−40°N and 75°E−100°E, with an average elevation > 4000 m. The range extends for a total length of ~2500 km and width of 130–200 km. The mountain range is narrower in the west than in the east and covers a total area > 500,000 km2 (Zheng, 1999; Wu, 2012–2015, Fig. 1).
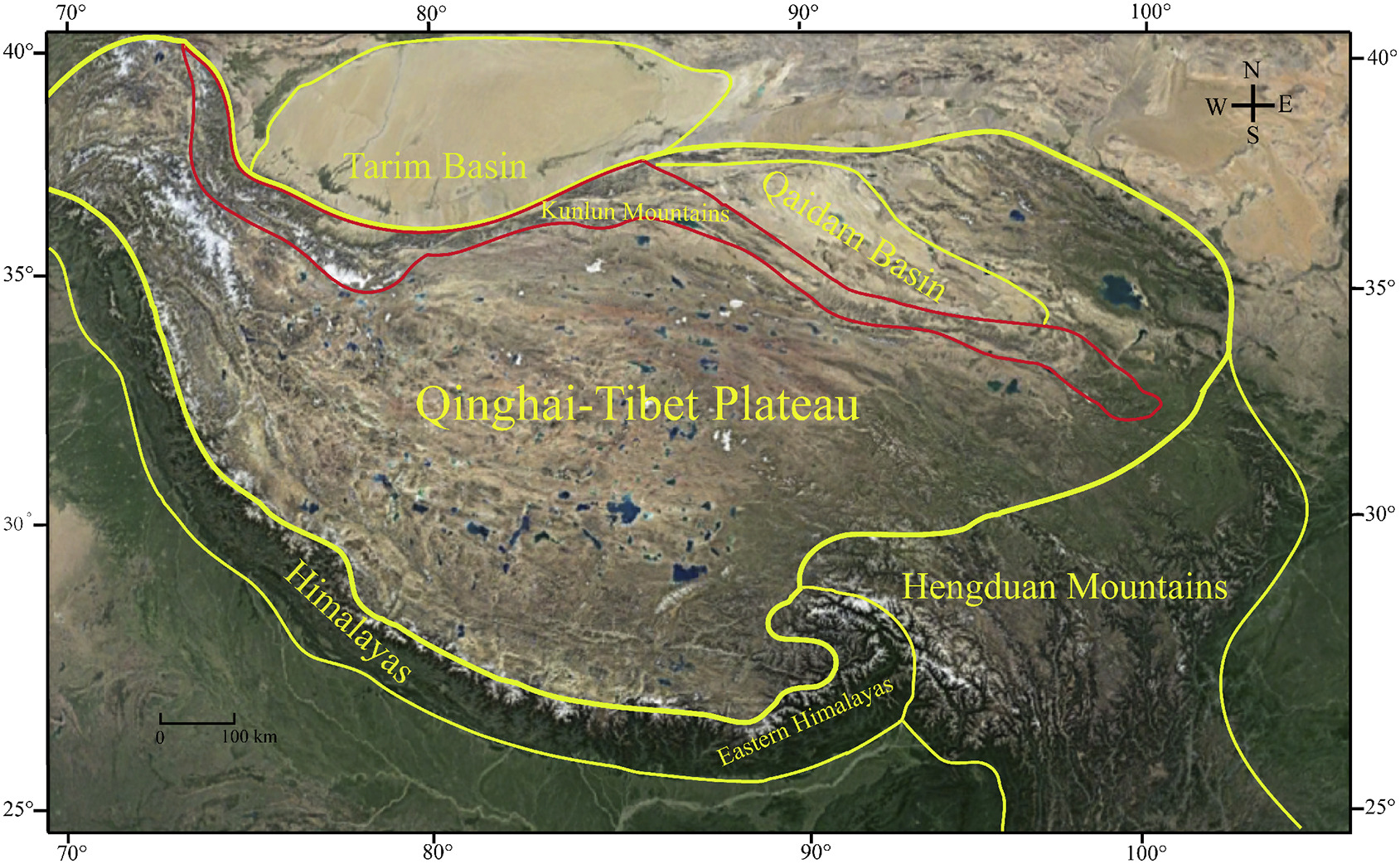
|
| Fig. 1 Geographical location of the Kunlun Mountains (outlined in red), China. |
The elevation of the mountain range increases from the east to the west, and ranges between 3000 m and 7719 m. The area has an annual precipitation that varies from ~100 to 500 mm and an average annual temperature < 0 ℃. Annual precipitation in the mountain range decreases markedly from east to west. The climate on the slopes of the mountain range varies greatly and the steep climate gradient results in dramatic changes in vegetation cover. From east to west, the vegetation types are alpine scrub, alpine meadow, and alpine steppe. In addition, there are a few coniferous forests in the east and west of the Kunlun Mountains (Zheng, 1999; Wu, 2012–2015).
The formation of the Kunlun Mountains coincided with the Himalayan uplift. The Qinghai-Tibet Plateau consists of multiple physical geographic subunits, each having its own geological history and uplift (Sun and Zheng, 1998; Spicer et al., 2003, 2020; Renner, 2016; Deng et al., 2017). However, it is clear that the QTP experienced strong climatic fluctuations and glaciations during the Quaternary (Owen et al., 2008; Owen and Dortch, 2014; Renner, 2016). The Kunlun Mountains also experienced these processes, and the current ecosystems of the Kunlun Mountains were formed following climatic fluctuations and glaciations of the Quaternary (Owen and Dortch, 2014; Renner, 2016).
To characterize plant diversity in the Kunlun Mountains, we divided the study area into 28 county-level geographical units according to the county area and vegetation type (Appendix S1: Table A1). Four counties are part of Tibet (Xizang), while 12 counties each belong to Qinghai and Xinjiang. Geographically, the Kunlun Mountains are divided into three regions: east, west, and central. The western regions consist of six counties; the central regions consist of 14 counties, with six counties on the southern slope and eight counties on the northern slope; and the eastern regions consist of eight counties (Fig. 2; Table 1).
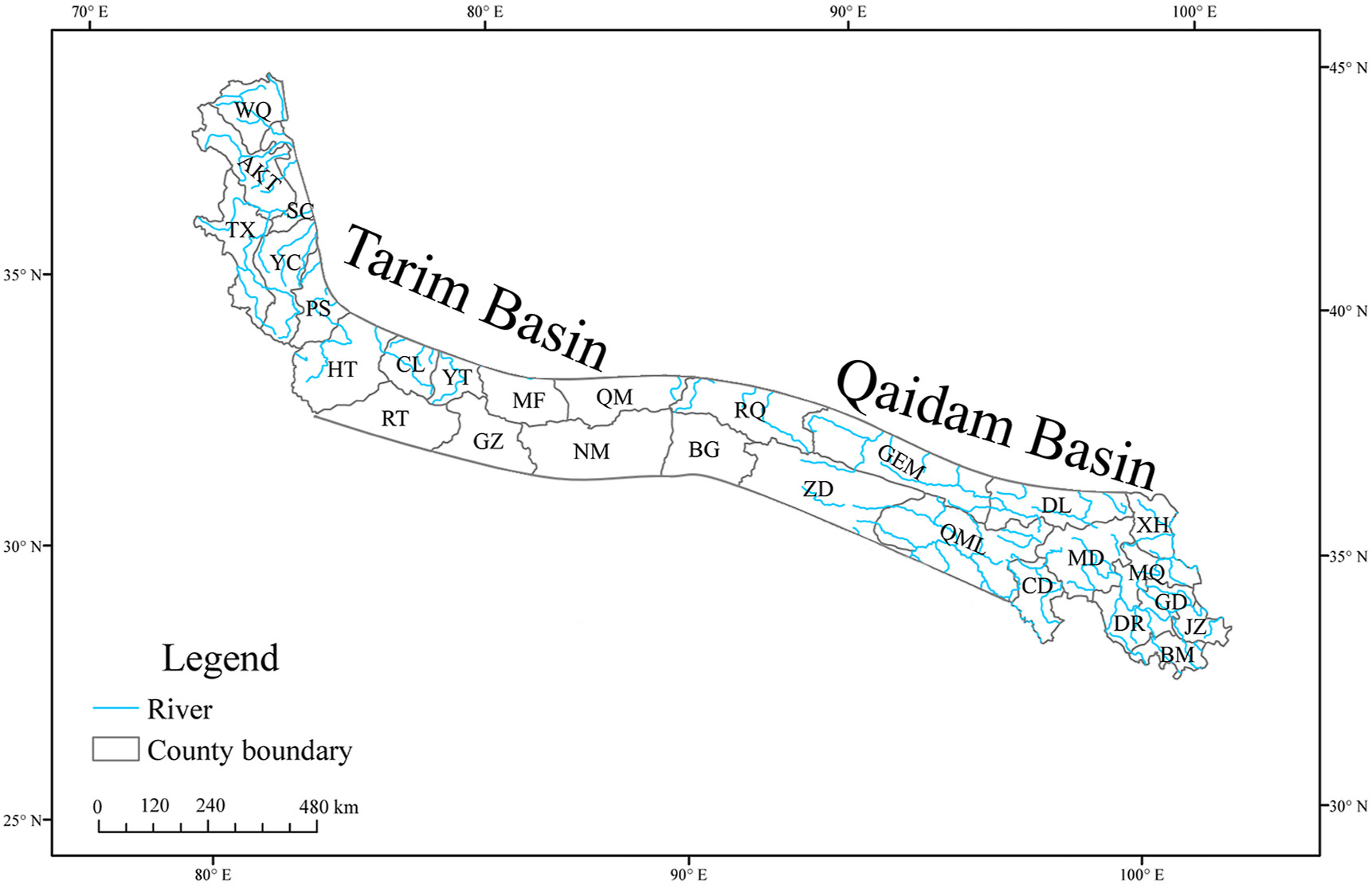
|
| Fig. 2 The county-level geographical units of the Kunlun Mountains, China. |
| East Kunlun Mountains (EK) | North slope of Central Kunlun Mountains (NCK) | ||||||
| Counties | Herbaceous plants | Woody plants | Species richness | Counties | Herbaceous plants | Woody plants | Species richness |
| Banma (BM) | 292 | 99 | 391 | Dulan (DL) | 207 | 36 | 243 |
| Jiuzhi (JZ) | 487 | 49 | 536 | Geermu (GRM) | 249 | 32 | 281 |
| Dari (DR) | 273 | 15 | 288 | Ruoqiang (RQ) | 266 | 28 | 294 |
| Gande (GD) | 142 | 11 | 153 | Qiemo (QM) | 141 | 16 | 157 |
| Chenduo (CD) | 457 | 33 | 490 | Minfeng (MF) | 61 | 8 | 69 |
| Maduo (MD) | 451 | 20 | 471 | Yutian (YT) | 105 | 15 | 120 |
| Maqin (MQ) | 683 | 66 | 749 | Cele (CL) | 184 | 14 | 198 |
| Xinghai (XH) | 664 | 68 | 731 | Hetian (HT) | 169 | 8 | 177 |
| South slope of Central Kunlun Mountains (SCK) | West Kunlun Mountains (WK) | ||||||
| Counties | Herbaceous plants | Woody plants | Species richness | Counties | Herbaceous plants | Woody plants | Species richness |
| Qumalai (QML) | 338 | 22 | 360 | Pishan (PS) | 157 | 16 | 173 |
| Zhiduo (ZD) | 156 | 6 | 162 | Yecheng (YC) | 340 | 48 | 388 |
| Bange (BG) | 123 | 6 | 129 | Shache (SC) | 98 | 17 | 115 |
| Nima (NM) | 106 | 3 | 109 | Taxian (TX) | 442 | 50 | 492 |
| Gaize (GZ) | 102 | 12 | 114 | Aketao (AKT) | 295 | 45 | 340 |
| Ritu (RT) | 239 | 24 | 263 | Wuqia (WQ) | 289 | 29 | 318 |
We used previously published data to compile a comprehensive checklist of seed plant species of the Kunlun Mountains. To analyze the spatial distribution patterns of these plants, each species was assigned a county-level geographical unit based on species distribution data. Basic distribution data were obtained from Flora Kunlunica, published in four volumes by Wu and his colleagues (Wu, 2012–2015), Flora of Xinjiang (Shen, 1993–2011), Flora of Qinghai (Liu, 1996–1999), Flora of Tibet Autonomous Region (Wu, 1983–1987), The Vascular Plants and Their Eco-geographical Distribution of the Qinghai-Tibet Plateau (Wu, 2008) and the National Specimen Information Infrastructure (http://nsii.org.cn/2017/home.php). Based on these sources and using the order of families from the Angiosperm Phylogeny Group IV (APGIV, 2016), the genera were classified into families according to A Dictionary of the Families and Genera of Chinese Vascular Plants (Li et al., 2018a). All the names were standardized following the Catalogue of Life Checklist (http://www.catalogueoflife.org/annual-checklist/2019/), and The Plant List (http://www.theplantlist.org). Names that differed according to these sources were standardized following The Plant List. Species that were not native to the Kunlun Mountains were excluded, and the infraspecific taxa were preserved.
2.3. Floristic similarityTo determine how the Kunlun Mountains flora was formed, we compared species from the Kunlun Mountains flora to those of nearby biodiversity centers. Three biodiversity hotspots exist around the Kunlun Mountains, including the Mountains of Central Asia, the Eastern Himalayas, and the Mountains of Southwest China, which are located to the north, south, and southeast of the Kunlun Mountains, respectively (Myers et al., 2000; Zachos and Habel, 2011). In addition, the Xinjiang region is the exclusive passage for species dispersal from the Mountains of Central Asia to the Kunlun Mountains. Therefore, the species on the Kunlun Mountains in Xinjiang are indirectly representative of shared taxa between the Kunlun Mountains flora and the Mountains of Central Asia. The Hengduan Mountains harbor one of the richest temperate floras worldwide, with ~12,000 vascular plants, which are representative of the Mountains of Southwest China (Boufford, 2014). In addition, the alpine flora of the Hengduan Mountains is the largest source of species dispersal for the Himalaya (Ding et al., 2020). More importantly, the Hengduan Mountains are the largest refugia for species from the glacial age (Liu et al., 2017), a diversity hotspot of Chinese endemic seed flora (Huang et al., 2016), and a center for species diversification for many extant plants (Wen et al., 2014; Chen et al., 2018). Numerous studies have indicated that the Hengduan Mountains have acted as an independent biogeographic source following climatic fluctuations and glaciations of the Quaternary (Liu et al., 2017; Xing and Ree, 2017; Muellner-Riehl, 2019; Ding et al., 2020). Therefore, we evaluated the species-level, genus-level, and family-level similarities between the floras of the Kunlun and Hengduan mountains. Floristic similarity (FS) was calculated as follows:
where SR represents the number of shared taxa between samples from the Kunlun Mountains flora and the Hengduan Mountains flora, taxonomic richness (TR) represents the number of taxa in the Kunlun Mountains flora sample (taxa represent families, genera, or species), and FS is the floristic similarity between samples of the Kunlun Mountains flora and the Hengduan Mountains flora.
2.4. Phylogenetic diversity and structureWe calculated the species level phylogenetic diversity, NRI, and NTI in each county.
Phylogenetic diversity has been used to assess the conservation value of different communities in the region (Faith, 1992; Faith et al., 2004). We called phylogenetic diversity as the sum of the phylogenetic length of the communities in each sample based on the approach developed by Faith (1992). We also statistically tested the phylogenetic diversity in different regions of the Kunlun Mountains using ANOVA and Tukey's honestly significant difference (HSD).
NRI and NTI were calculated to analyze community phylogenetic structure (clustering or overdispersion), and to examine possible ecological and evolutionary processes within communities (Webb et al., 2002). NRI was based on the mean phylogenetic distance (MPD), which is an estimate of the average phylogenetic relatedness between all possible pairs of taxa within a sample. NRI primarily reflects the structure in the deeper parts of a phylogeny. NTI was based on the mean nearest taxon distance (MNTD), which is an estimate of the mean phylogenetic relatedness between each pair of taxa in a sample and its nearest relative in a phylogeny. NTI reflects the structure in shallower parts of a phylogeny (Webb et al., 2002; Miller et al., 2017). At the community level, positive NRI and NTI values indicate phylogenetic clustering, whereas negative values indicate phylogenetic dispersion. The NRI and NTI values were calculated as follows:
where, MPDobserved and MNTDobserved represent the observed MPD and MNTD values; MPDrandom and MNTDrandom represent the mean values of the expected MPD and MNTD of the randomized assemblages (n = 999); s.d. (MPDrandom) and s.d. (MNTDrandom) represent the standard deviations of the MPDrandom and MNTDrandom values for the randomized assemblages. The null distributions of MPD and MNTD were created by randomly selecting the observed number of taxa in each sample 999 times, with all taxa in the phylogeny serving as the sampling pool.
To construct a phylogenetic tree, we used the stored tree data in Zanne et al. (2014) with Phylomatic v. 4.2 (http://phylodiversity.net/phylomatic/). Phylomatic standardizes the species names according to The Plant List (Qian and Jin, 2016). Therefore, the phylogenetic tree obtained using this method also depends on standardized species names and the Angiosperm Phylogeny Group IV. The ecological indexes were calculated using R v.3.3.3 (R Core Team, 2017) and picante packages (Kembel et al., 2010).
3. Results 3.1. Richness of taxaA total of 1,911 seed plants (including subspecies and varieties) belonging to 397 genera, 75 families, and 32 orders have been recorded on the Kunlun Mountains. Gymnosperms account for only 26 of these seed plants, which belong five genera, three families, and three orders. The remaining seed plants are all angiosperms. Plant species richness in the Kunlun Mountains is approximately one sixth of that in the Hengduan Mountains.
The seed plants of the Kunlun Mountains comprise 226 woody species and 1,685 herbaceous species, accounting for 11.83% and 88.17% of the total species, respectively (Table 1). Specifically, the woody species are represented by 22 trees, 197 shrubs, and seven lianas; and the herbaceous species are represented by nine herbaceous climbers, 224 annual herbs, and 1,452 perennial herbs. There are 570 species endemic to China, accounting for 29.83% of the total, including 81 woody species and 489 herbaceous species. The seed plants of the Kunlun Mountains are characterized by 39 woody genera, 347 herbaceous genera, and 11 genera that consist of both woody and herbaceous species. Overall, seven of these genera (all herbaceous genera) are endemic to China, of which six are only distributed in the eastern regions of the Kunlun Mountains. Approximately one third of the plant species are limited to 15 genera, which contain more than 20 species per genus. There are 155 genera and each genus contains only one species (Appendix 1).
According to provincial administrative units, there are 1,396, 941, and 315 seed plants in Qinghai, Xinjiang, and Tibet (Xizang), respectively. At the genus-level, the number of genera is 350, 261, and 144, respectively. In addition, the biodiversity of the Kunlun Mountains varies spatially, with the eastern regions of the Kunlun Mountains showing higher biodiversity than the western and central regions (Fig. 5c). There are similar patterns of genera richness in the Kunlun Mountains, where genera richness is higher in the southeastern regions. The distribution patterns of seed plants indicate that species and genera are mainly distributed in eastern regions, especially the southeastern regions (Fig. 5c; Appendix S1: Table A2).
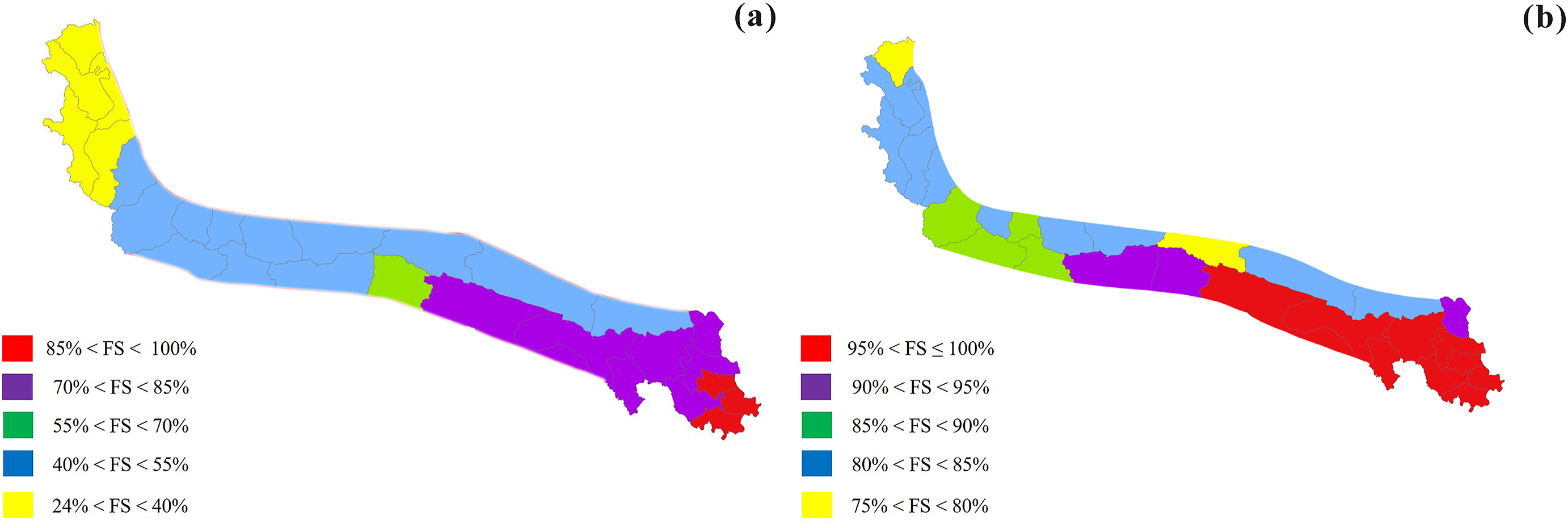
|
| Fig. 3 Floristic similarity (FS) between the Kunlun Mountains flora and the Hengduan Mountains flora. a-b, (a) species-level, (b) genus-level. |
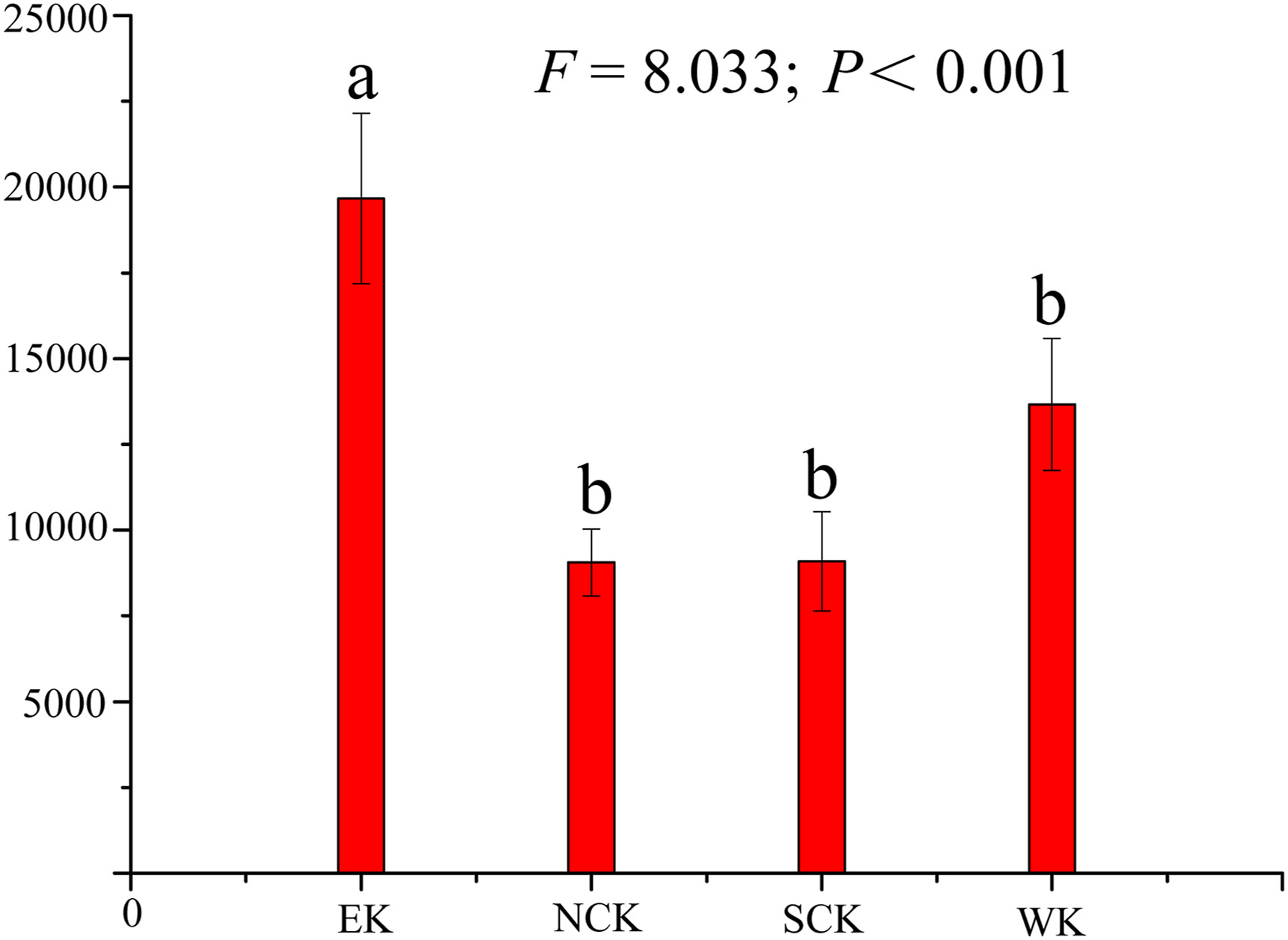
|
| Fig. 4 Phylogenetic diversity across four regions of the Kunlun Mountains. Significant differences were determined using Tukey's honestly significant difference (HSD) test after one-way ANOVA. P < 0.001, indicated by dissimilar letters. |
|
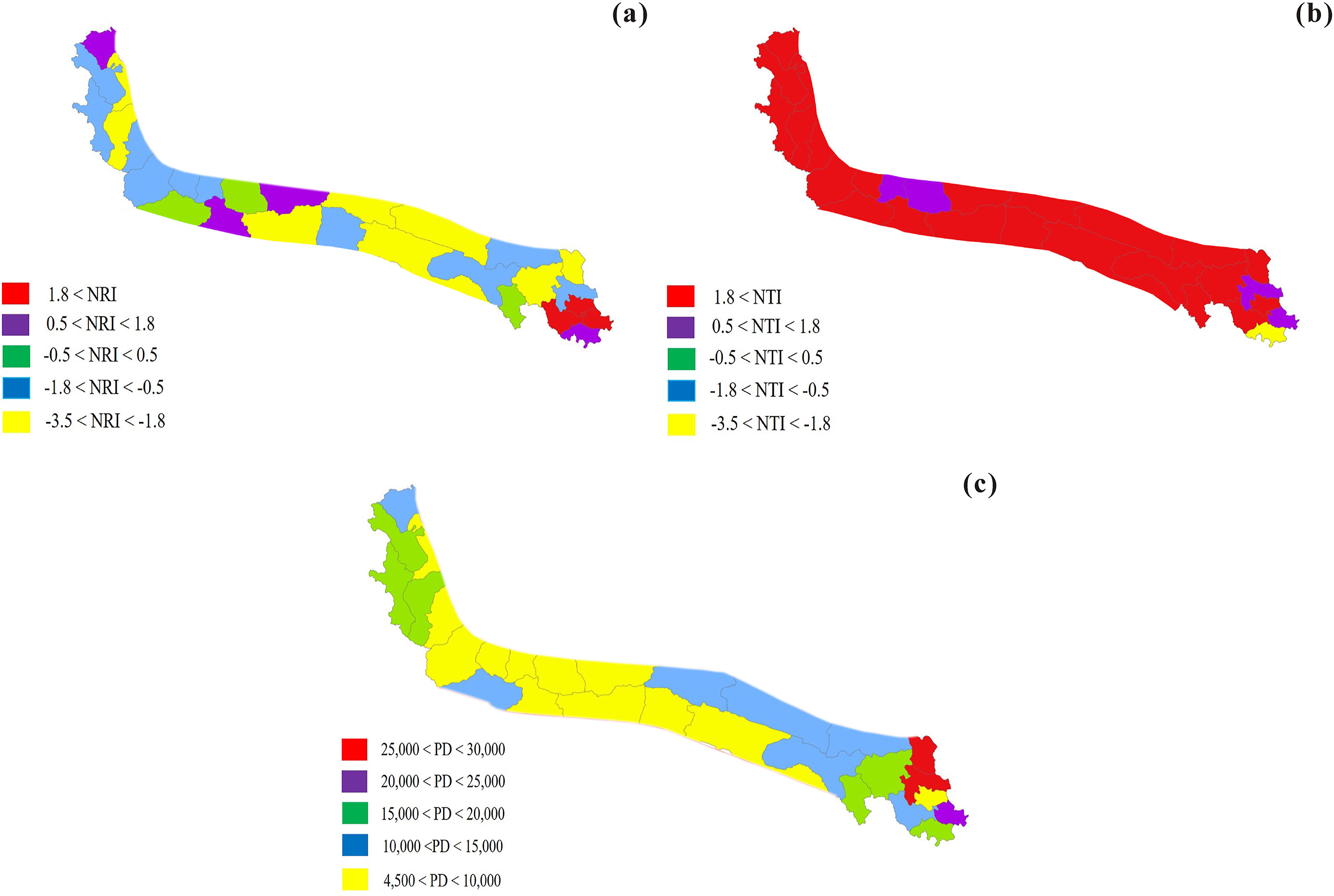
| Fig. 5 Patterns of net relatedness index (NRI), nearest taxon index (NTI), and phylogenetic diversity (PD) at the county-level geographical units of the Kunlun Mountains. a-c, (a) NRI, (b) NTI, and (c) PD. |
The shared taxa belong to 941 species (261 genera) between the Kunlun Mountains and Xinjiang, whereas 1,069 species (332 genera) in the Kunlun Mountains are also distributed in the Hengduan Mountains. At the species level, floristic similarity between the Kunlun Mountains flora and the Hengduan Mountain flora is 55.94%; at the genus-level, floristic similarity is 83.63%. Family-level floristic estimates had little value for assessing patterns of biodiversity. For example, only one family (Nitrariaceae), which is only distributed in 10 counties of the study area, were not found in the Hengduan Mountains. The floristic similarity between woody species of the Kunlun and Hengduan mountains is 57.52% (genera FS = 82.05%), and the floristic similarity between herbaceous species of these regions is 55.67% (genera FS = 83.86%). According to the data of endemic taxa, the floristic similarity for Chinese endemic species in these mountains is 70%; furthermore, Chinese endemic genera in the Kunlun Mountains were also found in the Hengduan Mountains. The floristic similarity of genera with both woody and herbaceous species in the Kunlun and Hengduan mountains is 72.73%.
The maximum floristic similarity of species in the 28 county-level geographical units is 93.86% (Banma) and the minimum is 24.53% (Wuqia). The floristic similarity is higher than 70% in the eastern regions, 40%–60% in the central regions, and less than 40% in the western regions of the Kunlun Mountains (Fig. 3a). The species-level floristic similarity of the counties decreased from east to west, and increased from north to south (Fig. 3a). At the genus-level, 26 counties have floristic similarity estimates higher than 80%, and two of the 26 counties have floristic estimates of 100% (Banma and Gande). The minimum genus floristic similarity is 76.61% (Ruoqiang), while the floristic similarity of Wuqia is 78.75% (Fig. 3b). Regarding similarity of taxonomic composition, distinct patterns exist between the Kunlun Mountains flora and Xinjiang; the similarity of taxonomic composition of the counties decreased from west to east and increased from south to north. Similar patterns were observed for genus-level similarity.
3.3. Phylogenetic diversity and structureTaxonomic richness and phylogenetic diversity were higher at both ends of the ranges of the Kunlun Mountains. The lowest taxonomic richness and phylogenetic diversity values were observed in Minfeng (Fig. 5c), and the values in the eastern area were observably higher than those in the central and western areas (Fig. 4).
The phylogenetic structure can be revealed by the NRI and NTI. However, they were calculated using an angiosperm phylogenetic tree. In addition, the gymnosperm phylogenetic tree could not support further calculation of these indexes (Appendix S1: Fig. A 3). Negative NRIs in 20 county-level communities indicated phylogenetic dispersion. Conversely, positive NRIs in the eight other county-level communities indicated phylogenetic clustering. Significant differences were detected between eight negative NRIs (P < 0.05) and three positive NRIs (P < 0.05), while the NRIs of the remaining 17 counties were not significantly different. In addition, the NTIs of 27 county-level communities were positive, of which 23 NTIs were statistically significant (P < 0.05). Only the community in Banma had a statistically significant negative NTI (P < 0.05) (Fig. 5a and b).
In the western regions of the Kunlun Mountains, five NRIs were negative, while one NRI was positive. However, significant differences were observed in two negative NRIs (P < 0.05). The six counties had positive NTI values. All NTIs showed significant differences (P < 0.05). In the southern slopes of the central regions, one county had positive NRI values, while the remaining NRIs were negative, and significant differences were observed in two negative NRIs (P < 0.05). All NTIs were positive and showed significant differences (P < 0.05). In the northern slopes of the central regions, two counties had positive NRI values, while six NRIs were negative. The two negative NRIs showed significant differences (P < 0.05). Eight NTIs were positive, and six of them showed significant differences (P < 0.05). In the eastern regions of the Kunlun Mountains, the NRI and NTI of two counties were positive, and the two indexes showed statistical significance (P < 0.05). The results suggested that the communities in these two counties were phylogenetically clustered. In addition, two counties also had positive NRI values, while the remaining NRIs were negative. Significant differences were observed in two negative NRIs and three positive NRIs (P < 0.05). Seven NTIs were positive, and five of these showed significant differences (P < 0.05). One NTI was negative and showed significant differences (P < 0.05).
Overall, both the NRI and NTI values were positive in the seven counties. Positive NRI values and negative NTI values were observed in Banma. The other 20 counties had positive NTI values and negative NRI values. Inconsistent trends were observed between the NRIs and NTIs in 21 counties, most of which bordered the Qaidam and Tarim Basins (Fig. 5a, b).
4. Discussion 4.1. Formation of the Kunlun Mountains floraWe observed that species distribution differed between the Kunlun Mountains and the Hengduan Mountains: 55.94% of the species on the Kunlun Mountains also inhabited the Hengduan Mountains, and the floristic similarity at the genus level was higher than 80%. Similar patterns were observed in the Chinese endemic taxa, where 70% of the Chinese endemic species in the Kunlun Mountains were also distributed in the Hengduan Mountains; all the Chinese endemic genera in the Kunlun Mountains were found in the Hengduan Mountains. Furthermore, although the Kunlun Mountains flora and Xinjiang have also similar taxonomic compositions, at both genus and species levels the floristic similarity between the Kunlun Mountains flora and Xinjiang was smaller than that between the Kunlun Mountains flora and the Hengduan Mountains flora. Therefore, our study indicates that mass species dispersal occurred between the Kunlun Mountains flora and the Hengduan Mountains flora, or that species in these floras might have dispersed from the same species pool.
Some studies have suggested that the diversity hotspots of Chinese endemic seed flora are located in the Qinling Mountains and further south, or in the Hengduan Mountains and further east in China (Huang et al., 2016). The hotspots of endemic woody seed plants in China show similar patterns (Huang et al., 2012). Approximately 20% of the total species are endemic to the QTP (Wu, 2008; Yan et al., 2013; Yu et al., 2018b), and 32.4% of the total species are endemic to the Hengduan Mountains (Zhang et al., 2009). The Kunlun Mountains have not been identified as a hotspot of Chinese endemic seed flora or a center of diversification for extant plants (Huang et al., 2012, 2016). However, 29.8% of the total species on the Kunlun Mountains are seed plant species endemic to China. The proportion of Chinese endemic species on the Kunlun Mountains is higher than that on the QTP, and closer to that on the Hengduan Mountains. Consequently, these Chinese endemic species on the Kunlun Mountains may have dispersed from other areas that acted as speciation centers, such as the Hengduan Mountains.
Since the Quaternary, climate fluctuations characterizing by glacial and interglacial periods prevailed on the QTP (Sun and Zheng, 1998; Zheng, 1999; Deng et al., 2017, 2019). The Kunlun Mountains have also experienced dramatic climatic fluctuations (Deng et al., 2019) and numerous glacial events (Su, 1998; Owen et al., 2008; Owen and Dortch, 2014; Renner, 2016), including the Largest Glaciation (1.2–0.6 Ma) and the Last Glacial Maximum (Shi et al., 1997; Liu et al., 2014). In the Last Glacial Maximum, the Kunlun Mountains were mostly covered by a unified ice sheet (Shi et al., 1997; Su, 1998; Owen et al., 2008; Owen and Dortch, 2014). These numerous glaciations have led to mass extinction events on the Kunlun Mountains (Liu et al., 2014). Recent studies have shown that the main phylogeographic patterns of seed plant species are contraction/recolonization, platform refugia/local expansion, and microrefugia in the Tibeto-Himalayan region (Muellner-Riehl, 2019). However, other studies indicate that there are no Chinese endemic species in the platform refugia and microrefugia, and few species were harbored in these refugia (López-Pujol et al., 2011; Muellner-Riehl, 2019). In addition, the latest study revealed that there are no platform refugia on the Kunlun Mountains (Yu et al., 2018a). Several plant molecular phylogeography studies have reported that the QTP was recolonized by most extant plants during the postglacial period, and the primary direction of dispersal for several species was from east to west or from south to north during the interglacial periods in the QTP and adjacent areas (Wiens and Donoghue, 2004; Qiu et al., 2011; Yu and Zhang, 2013; Yan and Tang, 2019). In this study, species-level floristic similarity between the Kunlun Mountains flora and the Hengduan Mountains flora decreased across 28 county-level geographical units the further the counties were located from the Hengduan Mountains (Fig. 3a). Consequently, after these glacial events, the extant plants might have dispersed from southern or eastern refugia adjacent to the Kunlun Mountains.
The Hengduan Mountains are located to the southeast of the Kunlun Mountains, and have been confirmed as the largest refugia for species from the glacial periods (Liu et al., 2017), a center of species diversification for many extant plants (Wen et al., 2014; Chen et al., 2018), a hotspot for Chinese endemic seed flora (Huang et al., 2016), and a biogeographic source after climatic fluctuations and glaciations of the Quaternary (Liu et al., 2017; Xing and Ree, 2017; Muellner-Riehl, 2019; Ding et al., 2020). In addition, the Kunlun Mountains, Tethyan region, the Mountains of Central Asia, and the alpine flora share similarly dry and cold climates (Zheng, 1999; Liu et al., 2017). These colonizing species, being well adapted to drought stress and cold conditions, may have dispersed from the Tethyan regions, the alpine flora of the Hengduan Mountains, the alpine flora of Eastern Himalayas, the Mountains of Central Asia, nearby platform refugia, undiscovered microrefugia, or other regions.
Therefore, the formation of the Kunlun Mountains flora may have occurred by species colonization after climatic fluctuations and glaciations of the Quaternary. The Hengduan Mountains might actually be the largest source of species colonization of the Kunlun Mountains after the Quaternary, especially in the eastern regions of the Kunlun Mountains.
4.2. Patterns of phylogenetic structure on the Kunlun MountainsEcologists have indicated that abiotic environment, contemporary biotic interactions, and evolutionary history simultaneously contribute to community phylogenetic structure at different scales (Webb et al., 2002; Kraft et al., 2007). Previous studies have shown that abiotic determinism tends to increase with spatial scale, while biotic determinism tends to decrease with spatial scale. Abiotic determinism is more important than biotic interactions in biodiversity maintenance at regional scales (Charles et al., 2010; Cardillo, 2011; Niu et al., 2011; Villalobos et al., 2013; Yang et al., 2014). Therefore, it is likely that the abiotic environment and the formation of species pool significantly influence community phylogenetic structure at county-level geographical units.
In the Kunlun Mountains, although NRI and NTI values were consistent in seven counties, values were inconsistent in 21 counties (Fig. 5a, b). NRI values for 20 counties indicates that community phylogenetic structure was dispersed. In addition, NRI values of eight counties (Yecheng, Shache, Zhiduo, Nima, Geermu, Ruoqiang, Maduo, and Xinghai) showed significant differences (P < 0.05). Positive NRI values in eight counties indicated that community phylogenetic structure was clustered. Of these, NRI values in three counties (Jiuzhi, Dari, and Gande) showed significant differences (P < 0.05) (Fig. 5a).
NTI values revealed that the community phylogenetic structure in 27 counties was clustered and indicated that the NTI values of 23 counties showed significant differences (P < 0.05) (Fig. 5b). Positive NTI values in four counties (Jiuzhi, Maqin, Yutian, and Minfeng) did not show significant differences (Fig. 5b). A negative NTI value in one county (Banma) showed significant differences (P < 0.05) (Fig. 5b).
The potential sources of species that colonized the Kunlun Mountains may be revealed by the geographical location and floristic similarity of counties in our study area (Fig. 2, Fig. 3). In the western regions of the Kunlun Mountains, the primary potential sources of colonizing species include Tethyan regions and the Mountains of Central Asia. Species may also have colonized the western region of the Kunlun Mountains through dispersion of the alpine flora of Hengduan Mountains or an undiscovered microrefugia. The primary potential sources of species that colonized the central regions of the Kunlun Mountains may be the Tethyan regions, the Mountains of Central Asia, and the alpine flora of the Hengduan Mountains. In addition, nearby platform refugia may serve as potential sources of species, particularly the southern slopes of the central regions. The primary potential sources of the species pool of the eastern regions of Kunlun Mountains include the alpine flora of the Hengduan Mountains, the forest flora of the Hengduan Mountains, and nearby platform refugia. In addition, species may have also dispersed from the Tethyan regions, the Mountains of Central Asia, or an undiscovered microrefugia. The varied phytogeographical regions that serve as potential sources of species pool of the Kunlun Mountains suggest that the Kunlun Mountains flora included complex lineages (Ye et al., 2019, 2020).
NRI and NTI primarily reflect community structure in the deeper and shallower parts of a phylogeny, respectively. When NRI and NTI were closer to 0, the community assembly was explained by neutral theory. In contrast, niche theory reveals the community assembly. In addition, the evolutionary history of taxa has an impact on community structure, particularly on NRI (Webb et al., 2002). The complex sources we have identified for the species colonization of the Kunlun Mountains was likely a deeper evolutionary process that affected the phylogenetic community structure after glacial events. We speculate that complex sources of species colonization have led to irregular NRIs, as the NRIs of 17 counties were not significantly different. However, the complex sources of species colonization had little effect on NTIs, 24 of which were statistically significant (P < 0.05). NTI is more appropriate for exploring the community assembly at regional scales.
Overall, these potential sources of the Kunlun Mountains species pool indicate that the Kunlun Mountains flora included complex lineages. NTIs indicate that habitat filtering structured these community assemblies. We speculate that community structure trends of 17 counties were inconsistent because the phylogenetic relationships of the taxa were distant at the genus-level, while the phylogenetic relationships of the species were closer in each genus. Consistent community structure trends (e.g., Wuqia, Gande, and Dari) likely arose from phylogenetic relationships of taxa that were closer at both genus-level and species-level. We speculate that the phylogenetic relationships of the taxa of six counties (Bange, Gaize, Ritu, Qiemo, Minfeng, and Chenduo) were moderate at the genus-level, and that the phylogenetic relationships of the species were closer in each genus. For Banma and Jiuzhi, we assume that the proportions of families and genera, which contained only one genus or one species, were high. In addition, the phylogenetic relationships of the taxa were closer at both genus and family-level. Therefore, species from the alpine flora and the forest flora of the Hengduan Mountains together may explain the community phylogenetic structure in Banma and Jiuzhi (Li et al., 2014, 2017).
5. ConclusionsTaxonomic richness and phylogenetic diversity in the eastern Kunlun Mountains were notably higher than those in the central and western areas, while no significant differences were observed within the eastern area. In addition, genera richness was higher in the southeastern regions. Thus, taxonomic richness, phylogenetic diversity, and genera richness suggest that the eastern regions of the Kunlun Mountains should be a center for biodiversity conservation, particularly the southeastern regions. However, in the entire Qinghai-Tibet Plateau, the conservation value on the Kunlun Mountains flora is relatively lower than that on the southeastern part of the QTP (Mao et al., 2013; Yan et al., 2013).
Research on how species diversity on the QTP responds to climate indicate a clear dependence on biotypes. The diversity of woody plants has been shown to have stronger climatic associations than that of herbaceous plants. Specifically, the diversity of woody plants has been shown to be jointly regulated by energy and water availability, whereas diversity of herbaceous plants is predominantly regulated by water availability (Yan et al., 2013). The dominant vegetation types of the Kunlun Mountains consist of herbaceous plants, with a few coniferous forests in the east and west. Furthermore, annual precipitation decreases notably from east to west (Zheng, 1999; Wu, 2012–2015), and there are abundant rivers in the east and west (Fig. 2). We inferred that species diversity in the Kunlun Mountains is predominantly regulated by water availability.
We also found that the formation of the Kunlun Mountains flora was constructed by species colonization, and the complex sources of species colonization have also revealed that the Kunlun Mountains flora included complex lineages. NTI values indicated that habitat filtering structured the assembly of these communities. In conclusion, the current plant diversity of the Kunlun Mountains was likely formed by both species colonization and habitat filtering. Furthermore, habitat filtering may play an important role in ecological processes, particularly in water availability.
Authors contributionsWBD collected basic data, organized data, posed scientific question and wrote manuscripts; PJ calculated the species level phylogenetic diversity (PD), net relatedness index (NRI), and nearest taxon index (NTI) in each county; this article was guided by GZD.
Declaration of competing interestThere are no conflicts of interest to declare.
AcknowledgmentsWe thank the generations of Chinese botanists who have conducted extensive research on the plants in the study region. This study was supported by Key Program of National Natural Science Foundation China (No. 41671038), National Key Research and Development Program of China (2017YFC0504801).
Appendix A Supplementary dataThe following is the Supplementary data to this article:
Allen, A.P., Gillooly, J.F., 2006. Assessing latitudinal gradients in speciation rates and biodiversity at the global scale. Ecol. Lett., 9: 947-954. DOI:10.1111/j.1461-0248.2006.00946.x |
APGIV, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc., 181: 1-20. DOI:10.1111/boj.12385 |
Bañares-de-Dios, G., Macía, M.J., Cerda, I.G.L., et al., 2020. Linking patterns and processes of tree community assembly across spatial scales in tropical montane forests. Ecology, 101: e03058. |
Boufford, D.E., 2014. Biodiversity hotspot: China's hengduan mountains. Arnoldia, 72: 24-35. |
Brown, J.H., Gillooly, J.F., Allen, A.P., et al., 2004. Toward a metabolic theory of ecology. Ecology, 85: 1771-1789. DOI:10.1890/03-9000 |
Burns, J.H., Strauss, S.Y., 2011. More closely related species are more ecologically similar in an experimental test. Proc. Natl. Acad. Sci. U.S.A., 108: 5302-5307. DOI:10.1073/pnas.1013003108 |
Cardillo, M., 2011. Phylogenetic structure of mammal assemblages at large geographical scales: linking phylogenetic community ecology with macroecology. Phil. Trans. Biol. Sci., 366: 2545-2553. DOI:10.1098/rstb.2011.0021 |
Charles, G.W., Halina, M., Lehman, C., et al., 2010. Phylogenetic community structure in Minnesota oak savanna is influenced by spatial extent and environmental variation. Ecography, 33: 565-577. |
Chen, Y.S., Deng, T., Zhou, Z., et al., 2018. Is the East Asian flora ancient or not?. Natl. Sci. Rev., 6: 1-13. |
Chu, C.J., Wang, Y.S., Liu, Y., et al., 2017. Advances in species coexistence theory. Biodivers. Sci., 25: 345-354. DOI:10.17520/biods.2017034 |
Currie, D.J., Mittelbach, G.G., Cornell, H.V., et al., 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett., 7: 1121-1134. DOI:10.1111/j.1461-0248.2004.00671.x |
Deng, T., Wu, F.X., Wang, S.Q., et al., 2019. Significant shift in the terrestrial ecosystem at the Paleogene/Neogene boundary in the Tibetan Plateau. Chin. Sci. Bull., 64: 2894-2906. DOI:10.1360/TB-2019-0053 |
Deng, T., Zhang, J.W., Meng, Y., et al., 2017. Role of the Qinghai-Tibetan Plateau uplift in the Northern Hemisphere disjunction: evidence from two herbaceous genera of Rubiaceae. Sci. Rep., 7: 13411. DOI:10.1038/s41598-017-13543-5 |
Ding, W.N., Ree, R.H., Spicer, R.A., et al., 2020. Ancient orogenic and monsoon-driven assembly of the world's richest temperate alpine flora. Science, 369: 578-581. DOI:10.1126/science.abb4484 |
Ebersbach, J., Muellner-Riehl, A.N., Michalak, I., et al., 2016. In and out of the Qinghai-Tibet Plateau: divergence time estimation and historical biogeography of the large arctic-alpine genus Saxifraga L. J. Biogeogr., 44: 900-910. |
Faith, D.P., 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv., 61: 1-10. DOI:10.1016/0006-3207(92)91201-3 |
Faith, D.P., Reid, C.A.M., Hunter, J., 2004. Integrating phylogenetic diversity, complementarity, and endemism for conservation assessment. Conserv. Biol., 18: 255-261. DOI:10.1111/j.1523-1739.2004.00330.x |
Favre, A., Michalak, I., Chen, C.H., et al., 2016. Out-of-Tibet: the spatio-temporal evolution of Gentiana (Gentianaceae). J. Biogeogr., 43: 1967-1978. DOI:10.1111/jbi.12840 |
Favre, A., Päckert, M., Pauls, S.U., et al., 2015. The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol. Rev., 90: 236-253. DOI:10.1111/brv.12107 |
Grierson, C.S., Barnes, S.R., Chase, M.W., et al., 2011. One hundred important questions facing plant science research. New Phytol., 192: 6-12. DOI:10.1111/j.1469-8137.2011.03859.x |
Hawkins, B.A., Porter, E.E., 2003. Relative influence of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Global Ecol. Biogeogr., 12: 475-481. DOI:10.1046/j.1466-822X.2003.00060.x |
HilleRisLambers, J., Adler, P.B., Harpole, W.S., et al., 2012. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst., 43: 227-248. DOI:10.1146/annurev-ecolsys-110411-160411 |
Huang, J.H., Chen, B., Liu, C.R., et al., 2012. Identifying hotspots of endemic woody seed plant diversity in China. Divers. Distrib., 18: 673-688. DOI:10.1111/j.1472-4642.2011.00845.x |
Huang, J.H., Huang, J.H., Liu, C.R., et al., 2016. Diversity hotspots and conservation gaps for the Chinese endemic seed flora. Biol. Conserv., 198: 104-112. DOI:10.1016/j.biocon.2016.04.007 |
Hubbell, S.P. (Eds.), 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press. Princeton and Oxford
|
Hubbell, S.P., 2005. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol., 19: 166-172. DOI:10.1111/j.0269-8463.2005.00965.x |
Kraft, N.J.B., Cornwell, W.K., Webb, C.O., et al., 2007. Trait evolution, community assembly, and the phylogenetic structure of ecological communities. Am. Nat., 170: 271-283. DOI:10.1086/519400 |
Kembel, S.W., Cowan, P.D., Helmus, M.R., et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics, 26: 1463-1464. DOI:10.1093/bioinformatics/btq166 |
Kerr, J.T., Packer, L., 1997. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature, 385: 252-254. DOI:10.1038/385252a0 |
Li, D.Z., Chen, Z.D., Wang, H., et al. (Eds.), 2018a. A Dictionary of the Families and Genera of Chinese Vascular Plants. Science Press, Beijing
|
Li, R., Qian, L.S., Sun, H., 2018. Current progress and future prospects in phylofloristics. Plant Divers., 40: 141-146. DOI:10.1016/j.pld.2018.07.003 |
Li, X.H., Zhu, X.X., Niu, Y., et al., 2014. Phylogenetic clustering and overdispersion for alpine plants along elevational gradient in the Hengduan Mountains region, southwest China. J. Systemat. Evol., 52: 280-288. DOI:10.1111/jse.12027 |
Li, X.H., Sun, H., 2017. Phylogenetic pattern of alpine plants along latitude and longitude in Hengduan Mountains region. Plant Divers., 39: 37-43. DOI:10.1016/j.pld.2016.11.007 |
Liu, J., Luo, Y.H., Li, D.Z., et al., 2017. Evolution and maintenance mechanisms of plant diversity in the Qinghai-Tibet Plateau and adjacent regions: retrospect and prospect. Biodivers. Sci., 25: 163-174. |
Liu, J.Q., Duan, Y.W., Hao, G., et al., 2014. Evolutionary history and underlying adaptation of alpine plants on the Qinghai-Tibet Plateau. J. Systemat. Evol., 52: 241-249. DOI:10.1111/jse.12094 |
Liu, S.W. (Eds.), 1996-1999. Flora of Qinghai vol vols. 1-5. Qinghai People's Publishing House, Xining
|
López-Pujol, J.L., Zhang, F.M., Sun, H.Q., et al., 2011. Centres of plant endemism in China: places for survival or for speciation?. J. Biogeogr., 38: 1267-1280. DOI:10.1111/j.1365-2699.2011.02504.x |
Lu, L.M., Mao, L.F., Yang, T., et al., 2018. Evolutionary history of the angiosperm flora of China. Nature, 554: 234-238. DOI:10.1038/nature25485 |
Ma, K.P., 2017. Frontiers in biodiversity science: insular biogeography, community assembly and application of big data. Biodivers. Sci., 25: 343-344. DOI:10.17520/biods.2017137 |
Mao, L.F., Chen, S.B., Zhang, J.L., et al., 2013. Vascular plant diversity on the roof of the world: spatial patterns and environmental determinants. J. Systemat. Evol., 51: 371-381. DOI:10.1111/j.1759-6831.2012.00240.x |
Miller, E.T., Farine, D.R., TrisosE, C.H., 2017. Phylogenetic community structure metrics and null models: a review with new methods and software. Ecography, 40: 461-477. DOI:10.1111/ecog.02070 |
Mittelbach, G.G., Schemske, D.W., 2015. Ecological and evolutionary perspectives on community assembly. Trends Ecol. Evol., 30: 241-247. DOI:10.1016/j.tree.2015.02.008 |
Mittelbach, G.G., Schemske, D.W., Cornell, H.V., et al., 2007. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett., 10: 315-331. DOI:10.1111/j.1461-0248.2007.01020.x |
Montoya, D., Rodríguez, M.A., Zavala, M.A., et al., 2007. Contemporary richness of holarctic trees and the historical pattern of glacial retreat. Ecography, 30: 173-182. DOI:10.1111/j.0906-7590.2007.04873.x |
Muellner-Riehl, A.N., 2019. Mountains as evolutionary arenas: patterns, emerging approaches, paradigm shifts, and their implications for plant phylogeographic research in the Tibeto-Himalayan region. Front. Plant Sci., 10: 195. DOI:10.3389/fpls.2019.00195 |
Myers, N., Mittermeier, R.A., Mittermeier, C.G., et al., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Niu, H.Y., Wang, Z.F., Lian, J.Y., et al., 2011. New progress in community assembly: community phylogenetic structure combining evolution and ecology. Biodivers. Sci., 19: 275-283. DOI:10.3724/SP.J.1003.2011.09275 |
Owen, L.A., Caffee, M.W., Finkel, R.C., et al., 2008. Quaternary glaciation of the Himalayan-Tibetan orogen. J. Quat. Sci., 23: 513-531. DOI:10.1002/jqs.1203 |
Owen, L.A., Dortch, J.M., 2014. Nature and timing of Quaternary glaciation in the Himalayan-Tibetan orogen. Quat. Sci. Rev., 88: 14-54. DOI:10.1016/j.quascirev.2013.11.016 |
Pan, Y.S. (Eds.), 2000. Geological Formation and Evolution of the Karakorum-Kunlun Mountains. Science Press, Beijing
|
Patino, J., Whittaker, R.J., Borges, P.A.V., et al., 2017. A roadmap for island biology: 50 fundamental questions after 50 years of the theory of island biogeography. J. Biogeogr., 44: 963-983. DOI:10.1111/jbi.12986 |
Qian, H., Jin, Y., 2016. An updated megaphylogeny of plants, a tool for generating plant phylogenies and an analysis of phylogenetic community structure. J. Plant Ecol., 9: 233-239. DOI:10.1093/jpe/rtv047 |
Qiu, Y.X., Fu, C.X., Comes, H.P., 2011. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world's most diverse temperate flora. Mol. Phylogenet. Evol., 59: 225-244. DOI:10.1016/j.ympev.2011.01.012 |
R Core Team., 2017. R: a Language and Environment for Statistical Computing. R foundation for Statistical Computing, Vienna, Austria: Retrieved from https://www.R-project.org/
|
Rahbek, C., Borregaard, M.K., Antonelli, A., et al., 2019. Building mountain biodiversity: geological and evolutionary processes. Science, 365: 1114-1119. DOI:10.1126/science.aax0151 |
Rahbek, C., Borregaard, M.K., Colwell, R.K., et al., 2019. Humboldt's enigma: what causes global patterns of mountain biodiversity?. Science, 365: 1108-1113. DOI:10.1126/science.aax0149 |
Renner, S.S., 2016. Available data point to a 4-km-high Tibetan Plateau by 40 Ma, but 100 molecular-clock papers have linked supposed recent uplift to young node ages. J. Biogeogr., 43: 1479-1487. DOI:10.1111/jbi.12755 |
Ricklefs, R.E., 2005. Historical and ecological dimensions of global patterns in plant diversity. Biol. Skr., 55: 583-603. |
Shen, G.M. (Eds.), 1993-2011. Flora of Xinjiang vol vols. 1-6. Xinjiang Renmin Publishing House, Urumqi
|
Shi, Y.F., Li, J.J., Li, B.Y. (Eds.), 1998. Uplift and Environmental Changes of Qinghai-Xizang (Tibetan) in the Late Cenozoic. Guangdong Science and Technology Press. Guangzhou
|
Shi, Y.F., Zheng, B.X., Yao, T.D., 1997. Glaciers and environments during the largest glacial maximum (LGM) on the Tibetan plateau. J. Glaciol. Geocryol., 19: 97-113. |
Silvertown, J., 2004. Plant coexistence and the niche. Trends Ecol. Evol., 19: 605-611. DOI:10.1016/j.tree.2004.09.003 |
Spicer, R.A., Harris, N.B.W., Widdowson, M., et al., 2003. Constant elevation of southern Tibet over the past 15 million years. Nature, 421: 622-624. DOI:10.1038/nature01356 |
Spicer, R.A., Su, T., Valdes, P.J., et al., 2020. Why ‘the uplift of the Tibetan Plateau’ is a myth?. Natl. Sci. Rev., 8: nwaa091. |
Su, Z. (Eds.), 1998. Glaciers of the Karakorum-Kunlun Mountains. Science Press, Beijing
|
Sun, B., Wang, Y.F., Li, C.S., et al., 2015. Early Miocene elevation in northern Tibet estimated by palaeobotanical evidence. Sci. Rep., 5: 10379. DOI:10.1038/srep10379 |
Sun, H.L., Zheng, D. (Eds.), 1998. Formation, Evolution and Development of the Qinghai-Xizang (Tibetan) Plateau. Guangdong Science and Technology Press. Guangzhou
|
Sun, Y.S., Wang, A.L., Wan, D.S., et al., 2012. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Mol. Phylogenet. Evol., 63: 150-158. DOI:10.1016/j.ympev.2012.01.002 |
Svenning, J.C., Skov, F., 2005. The relative roles of environment and history as controls of tree species composition and richness in Europe. J. Biogeogr., 32: 1019-1033. DOI:10.1111/j.1365-2699.2005.01219.x |
Svenning, J.C., Skov, F., 2007. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecol. Biogeogr., 16: 234-245. DOI:10.1111/j.1466-8238.2006.00280.x |
Tang, Z.Y., Wang, Z.H., Zheng, C.Y., et al., 2006. Biodiversity in China's mountains. Front. Ecol. Environ., 4: 347-352. DOI:10.1890/1540-9295(2006)004[0347:BICM]2.0.CO;2 |
Villalobos, F., Rangel, T.F., Diniz-Filho, J.A.F., 2013. Phylogenetic fields of species: cross-species patterns of phylogenetic structure and geographical coexistence. P. Roy. Soc. B-Biol. Sci., 280: 20122570. DOI:10.1098/rspb.2012.2570 |
Wang, Z.H., Brown, J.H., Tang, Z.Y., et al., 2009. Temperature dependence, spatial scale, and tree species diversity in eastern Asia and North America. Proc. Natl. Acad. Sci. U.S.A., 106: 13388-13392. DOI:10.1073/pnas.0905030106 |
Wang, Z.H., Fang, J.Y., Tang, Z.Y., et al., 2012. Relative role of contemporary environment versus history in shaping diversity patterns of China's woody plants. Ecography, 34: 1-10. |
Wang, X.P., Wang, Z.H., Fang, J.Y., 2004. Mountain ranges and peaks in China. Biodivers. Sci., 12: 206-212. DOI:10.17520/biods.2004025 |
Wang, Y.J., Susanna, A., Von Raab-Straube, E., et al., 2009. Island-like radiation of Saussurea (asteraceae: cardueae) triggered by uplifts of the Qinghai-Tibetan plateau. Bot. J. Linn. Soc., 97: 893-903. DOI:10.1111/j.1095-8312.2009.01225.x |
Webb, C.O., Ackerly, D.D., McPeek, M.A., et al., 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst., 33: 475-505. DOI:10.1146/annurev.ecolsys.33.010802.150448 |
Wen, J., Zhang, J.Q., Nie, Z.L., et al., 2014. Evolutionary diversifications of plants on the Qinghai-Tibet Plateau. Front. Genet., 5: 1-16. |
Wiens, J.J., Donoghue, M.J., 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol., 19: 639-644. DOI:10.1016/j.tree.2004.09.011 |
Wiens, J.J., Graham, C.H., 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst., 36: 519-539. DOI:10.1146/annurev.ecolsys.36.102803.095431 |
Wu, Y.H. (Eds.), 2012-2015. Flora Kunlunica vol vols. 1-4. Chongqing Publishing Group: Chongqing Publishing House, Chongqing
|
Wu, Y.H. (Eds.), 2008. The Vascular Plants and Their Eco-Geographical Distribution of the Qinghai-Tibet Plateau. Science Press, Beijing
|
Wu, Z.Y. (Eds.), 1983-1987. Flora of Tibet Autonomous Region vol vols. 1-4. Science Press, Beijing
|
Xing, Y.W., Ree, R.H., 2017. Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A., 114: E3444-E3451. |
Yan, Y.J., Tang, Z.Y., 2019. Protecting endemic seed plants on the Tibetan Plateau under future climate change: migration matters. J. Plant Ecol., 12: 962-971. DOI:10.1093/jpe/rtz032 |
Yan, Y.J., Yang, X., Tang, Z.Y., 2013. Patterns of species diversity and phylogenetic structure of vascular plants on the Qinghai-Tibetan Plateau. Ecol. Evol., 3: 4584-4595. DOI:10.1002/ece3.847 |
Yang, J., Zhang, G.C., Ci, X.Q., et al., 2014. Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Funct. Ecol., 28: 520-529. DOI:10.1111/1365-2435.12176 |
Yang, W.J., Ma, K.P., Kreft, H., 2013. Geographical sampling bias in a large distributional database and its effects on species richness-environment models. J. Biogeogr., 40: 1415-1426. DOI:10.1111/jbi.12108 |
Ye, J.F., Liu, Y., Chen, Z.D., 2020. Dramatic impact of metric choice on biogeographical regionalization. Plant Divers., 42: 67-73. DOI:10.1016/j.pld.2019.12.003 |
Ye, J.F., Lu, L.M., Liu, B., et al., 2019. Phylogenetic delineation of regional biota: a case study of the Chinese flora. Mol. Phylogenet. Evol., 135: 222-229. DOI:10.1016/j.ympev.2019.03.011 |
Yi, C.L., Cui, Z.J., Xiong, H.G., 2005. Numerical periods of quaternary glaciations in China. Quat. Sci., 25: 609-619. |
Yu, H.B., Favre, A., Sui, X.H., et al., 2018. Mapping the genetic patterns of plants in the region of the Qinghai-Tibet Plateau: implications for conservation strategies. Divers. Distrib., 25: 310-324. |
Yu, H.B., Zhang, Y.L., 2013. Advances in phylogeography of alpine plants in the Tibetan Plateau and adjacent regions. Acta Bot. Boreali Occident. Sin., 33: 1268-1278. |
Yu, H.B., Zhang, Y.L., Liu, L., et al., 2018. Floristic characteristics and diversity patterns of seed plants endemic to the Tibetan Plateau. Biodivers. Sci., 26: 130-137. DOI:10.3390/sym10050130 |
Zachos, F.E., Habel, J.C. (Eds.), 2011. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. Springer, Berlin Heidelberg
|
Zanne, A.E., Tank, D.C., William, K., et al., 2014. Three keys to the radiation of angiosperms into freezing environments. Nature, 506: 89-92. DOI:10.1038/nature12872 |
Zhang, D.C., Boufford, D.E., Ree, R.H., et al., 2009. The 29°N latitudinal line: an important division in the Hengduan Mountains, a biodiversity hotspot in southwest China. Nord. J. Bot., 27: 405-412. DOI:10.1111/j.1756-1051.2008.00235.x |
Zhang, J.Q., Meng, S.Y., Allen, G.A., et al., 2014. Rapid radiation and dispersal out of the Qinghai-Tibetan Plateau of an alpine plant lineage Rhodiola (Crassulaceae). Mol. Phylogenet. Evol., 77: 147-158. DOI:10.1145/2590296.2590323 |
Zhang, J.W., Nie, Z.L., Wen, J., et al., 2011. Molecular phylogeny and biogeography of three closely related genera, Soroseris, Stebbinsia, and Syncalathium (Asteraceae, Cichorieae), endemic to the Tibetan Plateau, SW China. Taxon, 60: 15-26. DOI:10.1002/tax.601003 |
Zhang, Y.L., Li, B.Y., Zheng, D., 2002. A discussion on the boundary and area of the Tibetan Plateau in China. Geogr. Res., 21: 1-8. |
Zheng, D. (Eds.), 1999. Physical Geography of the Karakorum-Kunlun Mountains. Science Press, Beijing
|
Zobel, M., 1997. The relative role of species pools in determining plant species richness: an alternative explanation of species coexistence?. Trends Ecol. Evol., 12: 266-269. DOI:10.1016/S0169-5347(97)01096-3 |



