b. University of Chinese Academy of Science, Beijing 100049, China
The plant-specific NAC (NAM, ATAF1, 2, and CUC2) transcription factors (TFs) family is one of the largest plant TF families. The NAC members have been reported to participate in diverse biological processes, including plant development (Vroemen et al., 2003; Yamaguchi et al., 2010), stress responses (Nakashima et al., 2007, 2012; Huang et al., 2015; Yan et al., 2017) and leaf senescence (Guo and Gan 2006; Chen et al., 2011; Fan et al., 2015; Li et al., 2018; Ren et al., 2018). NAC-LIKE, ACTIVATED BY AP3/PI (NAP), a gene encoding NAC transcription factor, is found to regulate leaf senescence in Arabidopsis thaliana. Leaf senescence is delayed in the nap mutants and this phenotype can be restored to wild type (WT) by the intact NAP (Guo and Gan 2006). NAP can directly bind to the promoter of SAG113 which mediated stomatal movement and water loss during leaf senescence and it also interact with the promoter of abscisic acid (ABA) synthetic gene AAO3 to activate their expression during leaf senescence (Zhang and Gan 2012; Yang et al., 2014).
Plant defensins are small cysteine-rich peptides of 45—54 amino acids. They have been identified in many plant species as part of innate immune system induced when attacked by pathogens (Anderson 2005). It has been reported that most defensins are against fungi and several have the antibacterial activity (Lacerda et al., 2014; Kovalchuk et al., 2019). Plant defensins can permeabilize the fungal membrane and disrupt the Ca2+ and K+ gradient and thus preventing fungal growth (Thevissen et al., 2003). NaD1, a defensin produced in the flower of Nicotiana alata, can bind to the fungal cell walls, permeate the fungal membranes, and then enter the cytoplasm to interact with intracellular targets (van der Weerden et al. 2008, 2010). The expression of defensins are associated with phytohormones. GbD, a novel defensin similar to NaD1 isolated from Ginkgo biloba, can be elicited by methyl jasmonate (MeJA) treatment (Shen et al., 2005). PDF1.2A, a well-known defensin existing in Arabidopsis thaliana, can be regulated by the transcription factor ANAC032 by modulating JA and SA signaling in response to Pseudomonas syringae infection (Allu et al., 2016). SAmediated suppression of PDF1.2 and synergistic induction of PDF1.2 by JA/ethylene are modulated by the interaction of ORA59 and EIN3 (He et al., 2017).
The tobacco pathotype of Alternaria alternata is a necrotrophic fungus causing brown spot disease on Nicotiana leaves and this disease is usually observed on senescent leaves near the soil (LaMondia 2001). The wild tobacco Nicotiana attenuata is a model plant species used widely for studies of interaction with herbivore and A. alternata (Yamaguchi et al., 2010; Sun et al., 2014b; Song et al., 2019). After attacked by A. alternata, the N. attenuata plants activate an array of intricate defense mechanisms, including production of phytohormones ABA, JA and ethylene, and phytoalexins scopoletin, scopolin and capsidiol (Sun et al. 2014a, 2014b, 2017; Song et al., 2019). Interestingly, mature leaves of N. attenuata get bigger lesion compared with young leaves after A. alternata inoculation (Sun et al., 2014b), which is consistent with the findings in the field that the brown spot diseases are often found in senescent leaves. However, it is unclear how leave development stage is linked to disease resistance.
During transcriptome analysis, we isolated a NAC transcription factor NaNAC29 highly expressed after Alternaria alternata inoculation. The NaNAC29 possesses similar tertiary structure to NAP in Arabidopsis thaliana. However, whether NaNAC29 functions in leaf senescence and whether it is involved in plant resistance to A. alternata are unknown. In this study, we investigated the role of NaNAC29 in plant resistance to A. alternata and leaf senescence in NaNAC29-silenced Nicotiana attenuata plants and nap mutant of A. thaliana in detail.
2. Materials and methods 2.1. Plant and fungal materialsSeeds of the 31st generation of an inbred line of Nicotiana attenuata were used as wild type (WT) genotype. Stably transformed plant of JA-deficient (irAOC) N. attenuata was generated previously (Kallenbach et al., 2012). Seed germination and plant growth was conducted as described by Krügel et al. (Krügel T 2002).
The Arabidopsis thaliana ecotype Columbia-0 (Col-0) and T-DNA insertion mutant nap (Salk_005010) were obtained from ABRC (https://abrc.osu.edu/). They were grown in the chamber with the conditions of 16 h light/8 h dark, 22 ℃ and 60% relative humidity.
Alternaria alternata was grown and inoculated into leaves as described by Sun et al., (2014b).
2.2. Subcellular localization analysis of NaNAC29To determine the subcellular localization of NaNAC29, the NaNAC29 without stop codon was amplified by primers NaNAC29 pM999-F and NaNAC29 pM999-R (Table S1) and inserted into the pM999 vector (kindly provided by Dr. Shen guojing) with eGFP (enhanced green fluorescent protein) under the control of the CaMV 35S promoter. The above construct was transformed to Nicotiana attenuata protoplast and the protoplast isolation followed a protocol established for Arabidopsis thaliana with little modifications (Song et al., 2019).
2.3. Real-time PCR assayTotal RNA of leaves was extracted with TRI reagent (Invitrogen). First-strand cDNA was synthesized with 1 μg total RNA by using a PrimeScript II first strand cDNA synthesis kit (Thermo Scientific). Real-time PCR was performed as described by Sun et al., (2014a) with iTaq Universal SYBR Green Supermix (Biorad). The gene expression was normalized by the internal control NaActin II, whose expression was not altered in leaf samples inoculated with Alternaria alternata or treated with MeJA (Xu et al., 2018). All primers used in this study were listed in Table S1. The expression levels were presented as the mean ± SE of four biological samples.
2.4. Generation of VIGS plantsA 272 bp fragment of the NaNAC29 cDNA sequence amplified by primers NaNAC29 VIGS-F and NaNAC29 VIGS-R (Table S1) was inserted into pTV00 (Frank et al., 2001) in reverse orientation. The completed plasmid was transferred into Agrobacterium tumefaciens GV3101 and mixed with pBINTRA strain, and then they were inoculated into Nicotiana attenuata leaves generating NaNAC29-silenced plants (virus-induced gene silencing, VIGS NaNAC29). The Agrobacterium tumefaciens-mediated transformation procedure was performed as described previously (Saedler and Baldwin 2004). The empty vector-inoculated plants (EV plants) were used as control. Meanwhile, the N. attenuata phytoene desaturase (NaPDS) was also silenced to monitor the progress of VIGS (Saedler and Baldwin 2004). Around 30 plants were inoculated with each construct and usually 16 plants exhibiting efficient silencing were used for each experiment. All VIGS experiments were repeated twice.
A 221 bp NaDLP1 cDNA fragment amplified by primers NaDLP1 VIGS-F and NaDLP1 VIGS-R (Supplementary Table S1) was inserted into pTV00 via BamHI and HindIII. The VIGS NaDLP1 plants were generated as above.
2.5. Generation of NaNAC29-silenced stable transformation plants in Nicotiana attenuataA 283 bp NaNAC29 cDNA fragment amplified by primers NaNAC29 RNAi-F and NaNAC29 RNAi-R (Table S1) was inserted into pRESC8 vector after the 35S promoter in inverted repeats (von Dahl et al., 2007). Then the complete construct was transformed into Agrobacterium tumefaciens LBA4404 and the stable transformation plants were acquired according to Krügel et al. (Krügel T 2002). The positive seedlings with single insertion were obtained through Hygromycin (35 mg L-1) screening. The T3 generation homozygotes of NaNAC29-RNAi plants (193#) exhibiting efficient silencing were used for the further experiments. The wild type (WT) plants were used as control.
2.6. Ov-NaNAC29 of plasmid construction and plant transformation in Arabidopsis thalianaFor over-expression of NaNAC29 in Arabidopsis thaliana, the full length of NaNAC29 was amplified by primers NaNAC29 OV-F and NaNAC29 OV-R (Table S1) from the leaves of Nicotiana attenuata and inserted into pCAMBIA1301 vector after the 35S promoter via Nco I and Spe I. The complete construct 35S: NaNAC29 was transferred into GV3101 Agrobacterium strains and transformed to A. thaliana Col-0 and nap mutants through floral dip, respectively. The positive seedlings were generated through Hygromycin (35 mg L-1) screening and homozygotes of transformation plants exhibiting efficient over-expression were used for the further experiments.
2.7. Dark and MeJA treatmentFor dark-induced leaf senescence assay, the fifth or sixth rosette leaves were detached from 4-week-old plants and placed into Petri dishes containing 15 mL of water, and then the detached leaves of Arabidopsis thaliana were covered with aluminum foil for 4 days and 5 days of dark treatment for Nicotiana attenuata.
For treating Nicotiana attenuata with methyl jasmonate (MeJA), the stock solution was diluted to 1 mM MeJA (www.sigmaaldrich. com) with distilled water. The MeJA (1 mM) was sprayed directly on the fifth or sixth rosette leaves of 4-week-old plants and covered with a plastic bag. The treated plants were harvested after 1 h and 3 h and the plants sprayed with distilled water were used as control. The detail method was performed as Zhen Xu et al. (2018).
2.8. Measurement of chlorophyll fluorescence and chlorophyll contentChlorophyll fluorescence was measured using Pulse Amplitude Modulation (PAM) Chlorophyll Fluorometer. The maximum quantum yield of photosystem II (PS II) Fv/Fm was recorded during a saturating photon pulse (4000 μmol m-2 s -1) after the plants were under dark treatment for 20 min.
0.1 g fresh leaves used for chlorophyll extraction were grounded into powder in liquid nitrogen. 1 mL of 80% acetone (v/v) was added to the powder with vortex for 10 min. The supernatants were acquired after centrifuged at 5000×g for 10 min and their absorption values were determined at 646 and 663 nm by using a spectrophotometer (Tecan). Chlorophyll contents were quantified as previously described (Ren et al., 2018; Li et al., 2019).
3. Results 3.1. Identification and subcellular localization of NaNAC29During transcriptome analysis (Song et al., 2019), we isolated a NAC transcription factor 29 gene (NaNAC29; GenBank accession no. XM_019411386.1), whose expression was highly induced by Alternaria alternata in Nicotiana attenuata leaves at 1 day post inoculation (dpi). The elicitation of NaNAC29 by the fungus was verified by real-time PCR and the result showed that the relative NaNAC29 transcripts was induced by 52-fold at 1 dpi and 130-fold at 3 dpi in sourceesink transition leaves of wild-type plants (WT, Fig. 1 a). In addition, the NaNAC29 protein exhibited nuclear localization in the protoplast of N. attenuata when its eGFP (enhanced green fluorescent protein) fused protein was driven by a 35S promoter (Fig. 1 b). The sequence analysis suggested the NaNAC29 shared highest amino acid sequence similarity to NAP in A. thaliana with 60% identity (Supplemental Fig. 1 a) and the predicted tertiary structure of NaNAC29 also showed high similarity to NAP (Supplemental Fig. 1 b). The data above suggested that NaNAC29, a homolog of NAP in A. thaliana, was highly induced by A. alternata and encoding a nuclear localized protein in N. attenuata.
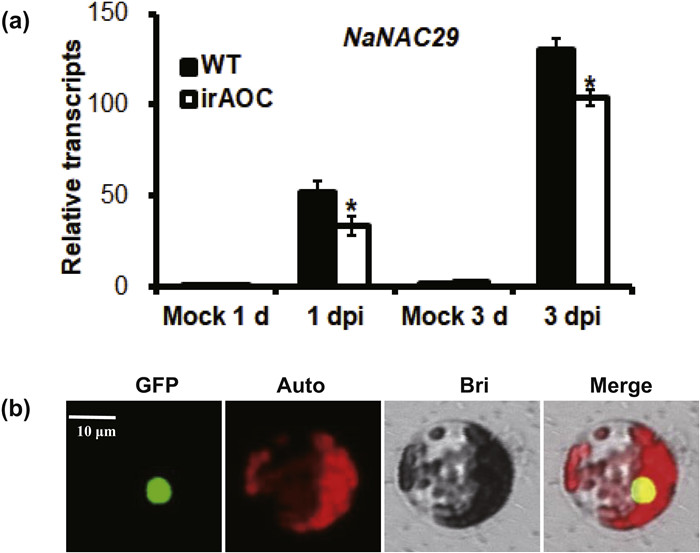
|
| Fig. 1 The relative expression of NaNAC29 induced by Alternaria alternata and subcellular localization of NaNAC29. (a) The relative transcripts of NaNAC29 were measured by realtime PCR in wild type (WT) and NaAOC deficient plants (irAOC) treated with mock or with A. alternata inoculation at 1 and 3 days post inoculation (dpi). (b) The NaNAC29-eGFP fusion protein was transiently expressed in Nicotiana attenuata protoplasts and the images were acquired by confocal microscopy. The asterisks indicate the level of significant difference between the WT and irAOC plants (Student's t-test: *P < 0.05). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. Scare bar = 20 μm. |
It has been reported Nicotiana attenuata plants activate JA signaling pathway after Alternaria alternata infection (Sun et al. 2014b, 2017). To illustrate whether the NaNAC29 expression was induced by A. alternata through JA pathway, we performed quantitative real-time PCR in N. attenuata leaves inoculated with the fungus at 1 and 3 dpi. NaNAC29 expression was highly induced at 1 dpi and attained even higher levels at 3 dpi. Compared with WT plants, the A. alternata-induced levels of NaNAC29 transcripts were decreased slightly in JA-deficient irAOC plants (Fig. 1 a). However, when the plants were treated by 1 mM MeJA at 1 h and 3 h, there was no change in the mRNA levels of NaNAC29 compared with control (Supplemental Fig. 2), suggesting that JA signaling pathway played a minor role in A. alternata-induced expression of NaNAC29.
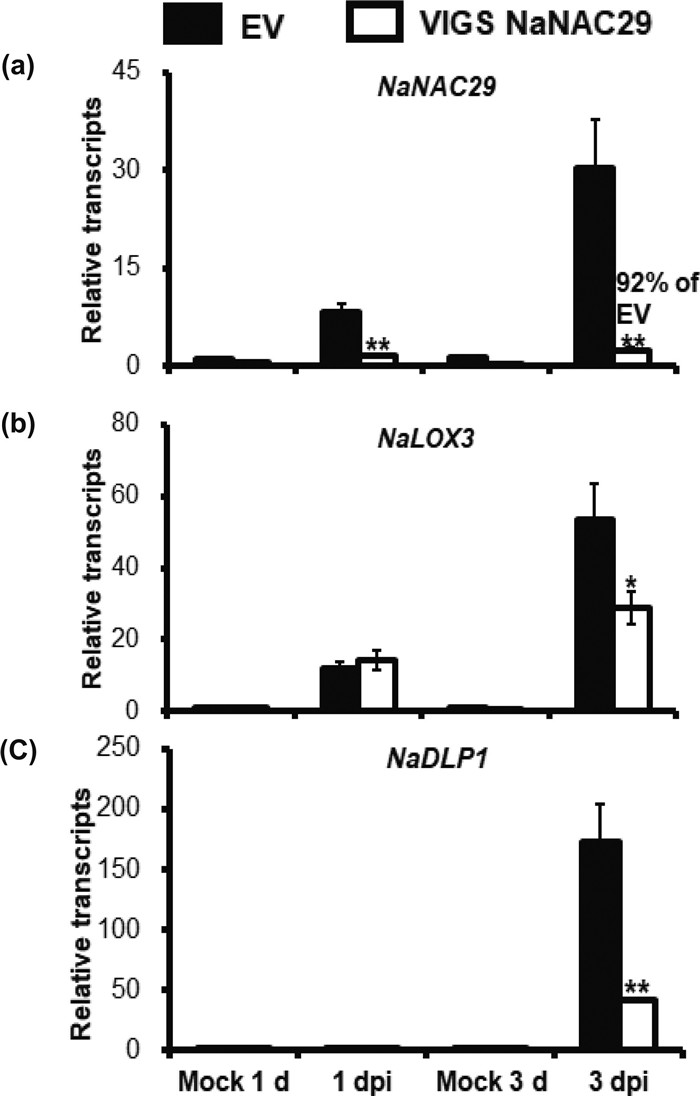
|
| Fig. 2 The relative transcripts of different genes induced by Alternaria alternata at 1dpi and 3 dpi.(a) The relative expression of NaNAC29, (b) NaLOX3, (c) NaDLP1 were detected in EV and VIGS NaNAC29 plants at 1 and 3 dpi. The asterisks indicate the level of significant difference between the EV and VIGS NaNAC29 plants. (Student's t-test: * *P < 0.01, *P < 0.05). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. |
To further evaluate the role of NaNAC29 in resistance against Alternaria alternata in Nicotiana attenuata, the NaNAC29 was silenced via VIGS. The VIGS NaNAC29 plants showed a 92% reduction in NaNAC29 transcripts compared with EV plants at 3 dpi (Fig. 2 a). However, there was no difference in lesion diameter between the VIGS NaNAC29 plants and EV (Supplemental Fig. 3).
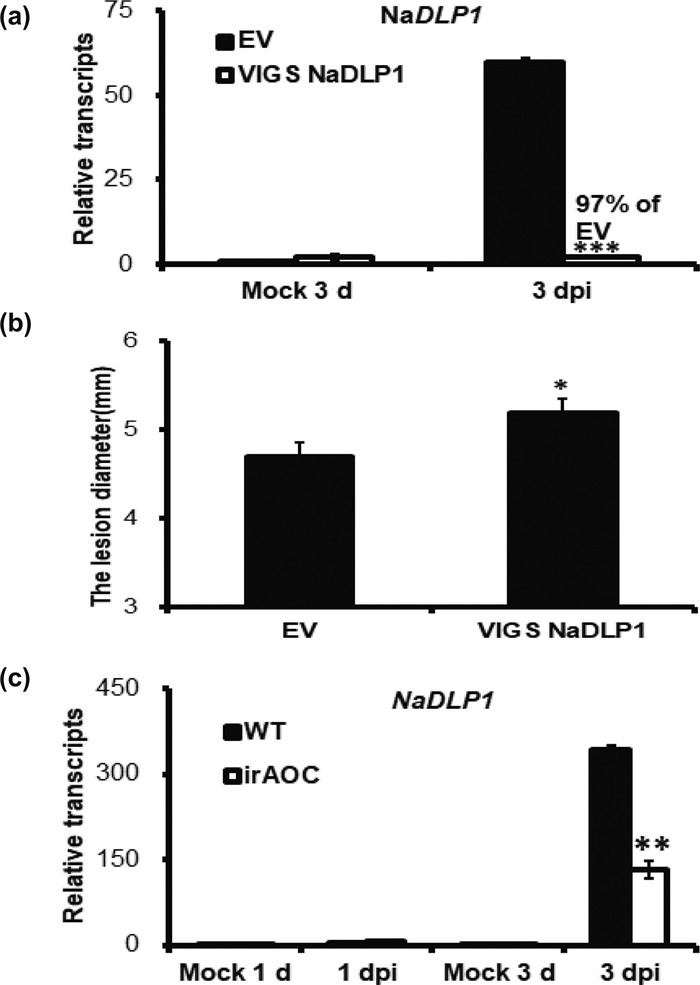
|
| Fig. 3 The role of NaDLP1 in the defense responses to Alternaria alternata infection. (a) The NaDLP1 expression was detected in EV and VIGS NaDLP1 plants at Mock 3 d and 3 dpi (b) The lesion diameter were measured in EV and VIGS NaDLP1 plants at 4 dpi. (c) The relative expression of NaDLP1 in WT and irAOC plants at 1 and 3 dpi. The asterisks indicate the level of significant difference between the EV and VIGS NaDLP1 plants or the WT and irAOC plants. (Student's t-test: ***P < 0.001, * *P < 0.01, *P < 0.05). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. |
Next, we tested the JA biosynthesis enzyme gene NaLOX3 and it showed slightly decreased transcripts in VIGS NaNAC29 plants compared with EV at 3 dpi (Fig. 2 b). The defensin-like protein 1 (NaDLP1, GenBank accession no. XM_019410997.1), a putative gene encoding defensin-like protein, was induced highly by Alternaria alternata at 3 dpi in EV plants, but it decreased by about 76% in VIGS NaNAC29 plants compared with EV (Fig. 2 c). This was also proved in the NaNAC29-RNAi plants (the T3 generation homozygotes of NaNAC29-silenced stable transformation plants #193 with a 44% reduction of NaNAC29 transcripts) which had 48% reduction of NaDLP1 transcripts when compared with WT at 3 dpi (Supplemental Fig. 4 a, b). However, the yeast-one hybrid experiment result showed no interaction between the putative NaNAC29 transcription factor and the promoter of NaDLP1.

|
| Fig. 4 The relative transcripts of NaNAC29 and physiological analysis during leaf senescence in Nicotiana attenuata. (a) N. attenuata leaves at five development stages (YL, young leaves half the size of fully expanded leaves, ES, leaves at early senescence, with < 50% leaf area yellowing, LS, leaves at late senescence, with >50% leaf area yellowing, CL1, the first cauline leaves from bottom to top, CL3, the third cauline leaves from bottom to top). (b) Relative expression of NaNAC29. (c) Relative Fv/Fm value. (d) ChlorophyII content at various development stages. (e—f) The NaNAC29 expression in the different parts of early senescent leaves. Tukey test: the letters a, b and c represented difference with significance (p < 0.05) and A, B and C (p < 0.01). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. |
The data above indicated that NaNAC29 participated in the defense response to Alternaria alternata by regulating the expression of NaLOX3 and NaDLP1.
3.3. NaDLP1, a defensin protein gene required for plant resistance to Alternaria alternata, is regulated by NaNAC29 independent of JA pathwayAfter the NaDLP1 was silenced by 97%, there was a slight but significantly enlargement in lesion diameter in VIGS NaDLP1 plants (Fig. 3 a, b), which indicated that NaDLP1 played a positive role in defense against Alternaria alternata.
The Alternaria alternata-induced expression of NaDLP1 was not only regulated by NaNAC29, but also by JA signaling pathway. The mRNA levels of NaDLP1 were highly induced by A. alternata at 3 dpi, but they were decreased by 61% in irAOC plants (Fig. 3 c).
To investigate whether MeJA can restore NaDLP1 expression in NaNAC29-silenced plants, we applied MeJA (1 mM) on sourceesink transition leaves of #193 and WT plants. The NaDLP1 transcripts were both induced significantly by MeJA in WT and NaNAC29-silenced plants, but its level was lower in #193 than in WT plants (Supplemental Fig. 4 b), suggesting that NaNAC29 and JA signaling functioned in a separated way in regulating NaDLP1 expression.
3.4. NaNAC29 expression is associated with senescence in Nicotiana attenuata leavesTo investigate whether NaNAC29 was involved in leaf senescence, we harvested young leaves (YL), leaves at early senescence (ES), leaves at late senescence (LS), the first cauline leaves (CL1) and the third cauline leaves (CL3) (Fig. 4 a). Low levels of NaNAC29 transcripts were detected in relative young leaves including YL, CL1 and CL3 leaves; however, NaNAC29 transcripts were increased significantly in ES leaves. The highest NaNAC29 transcript was detected in LS leaves (Fig. 4 b) and the LS leaves showed the lowest levels of chlorophyll content and Fv/Fm value, both of which were often used as leaf senescence indexes (Fig. 4 c, d). In addition, consistent with the results above, the higher levels of NaNAC29 transcripts were detected in the senescent yellow tip (Tip) than in other relative young parts of the ES leaves (Fig. 4 e, f). These results indicated that NaNAC29 expression levels were associated with leaf senescence.
3.5. Delayed-senescence phenotype in NaNAC29-silenced leaves under darkIt has long been known that dark is an inducer of leaf senescence for the detached leaves (Weaver and Amasino 2001). The NaNAC29 transcripts were significantly induced after dark treatment for 3 days in the detached leaves of Nicotiana attenuata (Fig. 5 a). To investigate the role of NaNAC29 in dark-induced senescence, we generated NaNAC29-silenced plants by virus-induced gene silencing (VIGS NaNAC29). As expected, the VIGS NaNAC29 leaves with a reduction of NaNAC29 transcript by 89% showed delayed senescence phenotype compared with leaves at the same position in EV (transformed with empty vector) plants under dark treatment for 5 days (Fig. 5 b, c). Consistently, the VIGS NaNAC29 leaves had higher Fv/Fm value compared with EV plants (Fig. 5 d). The VIGS NaNAC29 leaves also had the decreased expression levels of Protein phosphatase 2C 37-like (NaPP2C 37-LIKE, GenBank accession no. XM_019384432.1), a homolog of SAG113 in A. thaliana, which was usually used as a senescence marker (Fig. 5 e). Thus, NaNAC29 played a positive role in dark-induced leaf senescence in N. attenuata.
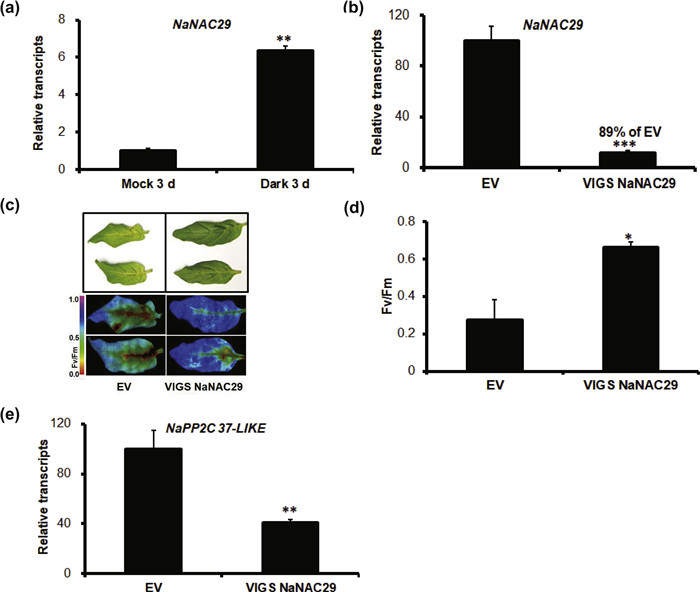
|
| Fig. 5 Role of NaNAC29 in the leaf senescence induced by dark treatment. (a) The relative transcripts of NaNAC29 was measured by real-time PCR in four replicate Nicotiana attenuata leaves under dark treatment for 3 days. (b, d, e) The relative expression of NaNAC29 and NaPP2C 37-LIKE and Fv/Fm value were detected in the detached leaves of EV and VIGS NaNAC29 plants under dark treatment for 5 days. (c) The phenotypes of EV and VIGS NaNAC29 plants following dark treatment for 5 days. The asterisks indicate the level of significant difference between the EV and VIGS NaNAC29 plants (Student's t-test: ***P < 0.001, * *P < 0.01, *P < 0.05). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. |
To confirm that NaNAC29 was the functional homolog of NAP in Arabidopsis thaliana, we over-expressed NaNAC29 in nap mutant plants (nap + NaNAC29) and Col-0 (Col + NaNAC29) and harvested the fifth rosette leaves of each genotype. As expected, the levels of NAP transcripts were very low in nap and nap + NaNAC29 plants (Supplemental Fig. 5 a) and the NaNAC29 levels were not detectable in Col-0 and nap mutant plants but were highly expressed in nap + NaNAC29 and Col + NaNAC29 plants (Supplemental Fig. 5 b). The detached leaves of transgenic plants with NaNAC29 exhibited yellowing phenotype following dark treatment for 5 days (Fig. 6 a). Consistently, the leaf senescence indexes chlorophyll content and Fv/Fm value were investigated. Compared with Col-0, the highest chlorophyll content was detected in nap and the lowest in Col + NaNAC29. However, there was no difference between Col0 and nap + NaNAC29 in chlorophyll contents (Fig. 6 b). Accordingly, the Fv/Fm values also suggested the over-expression of NaNAC29 complemented the senescence phenotype of nap mutants (Fig. 6 c), which was further supported by the observation of yellowing leaves in 7-week-old Col + NaNAC29 and nap + NaNAC29 plants but not in Col-0 and nap mutants (Fig. 6 d). All the data above suggested that NaNAC29 promoted leaf senescence in A. thaliana and it could complement the delayed leaf senescence phenotype of nap mutants.

|
| Fig. 6 NaNAC29 promoted leaf senescence in Arabidopsis thaliana. (a) The leaves phenotypes of different genotypes of Arabidopsis thaliana following dark treatment for 4 days. (b) (c) ChlorophyII content and Fv/Fm values were detected under dark treatment for 4 days in different plants. (d) Phenotypes of 7-week-old different plants under natural conditions. The asterisks indicate the level of significant difference between the Col-0 and other genotype plants (Student's t-test: ***P < 0.001, *P < 0.05). Error bars indicate the ±SE for four replicates. All experiments are repeated at least twice. |
Homologous genes of NAP have been investigated in various plant species, and found that many of them are involved in leaf senescence, plant development and abiotic stress responses (Chen et al., 2011; Fan et al., 2015; Kou et al., 2016; Ren et al., 2018; Li et al., 2004; Zhang and Gan 2012; Seok et al., 2016). In this study, we demonstrated that NaNAC29, a NAP homolog in Nicotiana attenuata, was involved in the defense response to Alternaria alternata by affecting NaDLP1 expression and it was a positive regulator of leaf senescence.
Plant defensins played a crucial role in the plant innate immune system with anti-fungi activity (Lacerda et al., 2014; Sathoff and Samac 2019). In this study, we found that a defensin gene NaDLP1 was highly induced at 3 days post inoculation and played an important role in defending against Alternaria alternata in Nicotiana attenuata plants (Fig. 3).
Several reports showed that plant defensins could be regulated by the phytohormones JA, SA and ethylene pathway (Shen et al., 2005; Allu et al., 2016; He et al., 2017). For example, the plant defensin PDF1.2 in Arabidopsis was a defense marker gene and it was suppressed by SA and activated by coaction of JA and ethylene through the coordination node ORA59 which encoded a transcription factor binding to the promoter of PDF1.2 to regulate its expression (Ndamukong et al., 2007; Pre et al., 2008; He et al., 2017). Similar to PDF1.2, A. alternata-elicited NaDLP1 transcripts were decreased by 61% in JA-deficient irAOC plants (Fig. 3 c). Interestingly, we found that A. alternata-induced NaDLP1 transcripts were decreased by 76.2% in NaNAC29-silenced plants (Fig. 2 c). Because JA biosynthesis enzyme gene NaLOX3 showed a significant decrease in VIGS NaNAC29 plants (Fig. 2 b), we needed to verify whether the NaDLP1 expression regulated by NaNAC29 was through JA pathway. NaDLP1 expression could be induced to higher levels in both WT and NaNAC29-silenced plants 193# at 3 dpi when treated with lanolin paste of MeJA, but the induction levels in NaNAC29-silenced plants 193# were only 50.6% of those of WT (Supplemental Fig. 4 b), indicating that MeJA could not complement the induction levels of NaDLP1 in NaNAC29-silenced plants to the levels in WT plants, and thus the regulation of NaDLP1 by NaNAC29 was not dependent on JA pathway.
Several reports indicated the functional roles of NAP in plant development and abiotic stress responses via ethylene and ABA pathway (Li et al., 2004; Greco et al., 2012; Zhang and Gan 2012), while JA had no significant effect on NAP expression levels (Guo and Gan 2006). In this study, we found that the expression levels of A. alternata-induced NaNAC29 decreased slightly in JA-deficient irAOC plants at both 1 and 3 dpi, and MeJA treatments had no influence on NaNAC29 expression (Fig. 1 a; Supplemental Fig. 2), indicating that JA pathway affected A. alternata-induced NaNAC29 expression to a small extent. In addition, A. alternata-induced transcripts of NaLOX3, a JA biosynthetic gene, was also significantly reduced in VIGS NaNAC29 plants, suggesting that silencing NaNAC29 also affected JA biosynthesis.
It has been reported the NAP homologous genes in many species are involved in the leaf senescence (Guo and Gan 2006; Chen et al., 2011; Fan et al., 2015; Ren et al., 2018). Our data also supported that NaNAC29 was a positive regulator of leaf senescence in Nicotiana attenuata. The NaNAC29 had similar tertiary structure to NAP with 60% amino acid identity, and its transcripts were expressed in very low level in young leaves but highly in mature and senescent leaves (Fig. 4). Silencing NaNAC29 delayed leaf senescence in N. attenuata leaves (Fig. 5). Importantly, ectopic expression of NaNAC29 in A. thaliana promoted leaf senescence and complemented nap phenotype (Fig. 6).
When Nicotiana attenuata leaves were inoculated with A. alternata, bigger lesion was developed on older leaves (Sun et al., 2014a), which suggested that the resistance against the fungus was decreased while leaves became senescent. We speculated that silencing NaNAC29 would delay leaf senescence thereby enhance plant resistance to A. alternata. Meanwhile, A. alternata-elicited expression of a defensin like protein NaDLP1 was dramatically reduced in VIGS NaNAC29 plants. Therefore, similar lesion size was developed in VIGS NaNAC29 and EV plants.
Based on the results above, we proposed a working model of NaNAC29 in the defense responses of Nicotiana attenuata to A. alternata (Fig. 7). When the N. attenuata plants were infected by A. alternata, plants activated two separate pathways, JA and NaNAC29, to up-regulate the expression of NaDLP1, a gene encoding the defensin protein to defend against the fungus. In addition, a slight crosstalk between JA pathway and NaNAC29 occurred and NaNAC29 promoted leaf senescence which might attenuate plant resistance to the fungus.

|
| Fig. 7 The working model of NaNAC29 in the defense responses of Nicotiana attenuata to Alternaria alternata inoculation. A. alternata induces NaNAC29 transcripts and JA signaling pathway and there exists a slight crosstalk between NaNAC29 and JA pathway. Silencing NaNAC29 and NaAOC reduces NaDLP1 defense to A. alternata infection. In the other way, NaNAC29 might weakens the defense responses through accelerating the leaf senescence. |
JW and LM conceived the project, designed the experiments and prepared the manuscript. LM conducted experiments and was responsible for data processing. RL participated in the RNA extraction. LYM generated NaNAC29-RNAi plants. NS and ZX provided cDNA samples applied with MeJA. All authors read and approved the manuscript.
Declaration of competing interestThe authors declare that they have no conflict of interest.
AcknowledgementsWe thank Biological Technology Open Platform of Kunming Institute of Botany, the Chinese Academy of Sciences for greenhouse services. This project is supported by CAS "Light of West China" Program and NSFC grant (No. 31700231) to LM.
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.11.003.
Allu A.D., Brotman Y., Xue G.P., et al, 2016. Transcription factor ANAC032 modulates JA/SA signalling in response to Pseudomonas syringae infection. EMBO Rep, 17: 1578-1589. DOI:10.15252/embr.201642197 |
Anderson FTLaMA, 2005. Defensins e components of the innate immune system in plants. Curr. Protein Pept. Sci, 6: 85-101. DOI:10.2174/1389203053027575 |
Chen Y., Qiu K., Kuai B., et al, 2011. Identification of an NAP-like transcription factor BeNAC1 regulating leaf senescence in bamboo (Bambusa emeiensis'Viridiflavus'). Physiol. Plantarum, 142: 361-371. DOI:10.1111/j.1399-3054.2011.01472.x |
Fan K., Bibi N., Gan S., et al, 2015. A novel NAP member GhNAP is involved in leaf senescence in Gossypium hirsutum. J. Exp. Bot, 66: 4669-4682. DOI:10.1093/jxb/erv240 |
Frank R., Am M-H, Dc B, 2001. echnical advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J, 25: 237-245. |
Greco M., Chiappetta A., Bruno L., et al, 2012. Arabidopsis ATNAP regulates fruit senescence. J. Exp. Bot, 63: 695-709. DOI:10.1093/jxb/err313 |
Guo Y., Gan S., 2006. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J, 46: 601-612. DOI:10.1111/j.1365-313X.2006.02723.x |
He X., Jiang J., Wang C.Q., et al, 2017. ORA59 and EIN3 interaction couples jasmonate-ethylene synergistic action to antagonistic salicylic acid regulation of PDF expression. J. Integr. Plant Biol, 59: 275-287. DOI:10.1111/jipb.12524 |
Huang Q., Wang Y., Li B., et al, 2015. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol, 15: 268. DOI:10.1186/s12870-015-0644-9 |
Kallenbach M., Bonaventure G., Gilardoni P.A., et al, 2012. Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proc. Natl. Acad. Sci. U.S.A, 109: E1548-E1557. DOI:10.1073/pnas.1200363109 |
Kou X., Liu C., Han L., et al, 2016. NAC transcription factors play an important role in ethylene biosynthesis, reception and signaling of tomato fruit ripening. Mol.Genet. Genom, 291(3): 1205-1217. DOI:10.1007/s00438-016-1177-0 |
Kovalchuk N., Wu W., Bazanova N., et al, 2019. Wheat wounding-responsive HDZip IV transcription factor GL7 is predominantly expressed in grain and activates genes encoding defensins. Plant Mol. Biol, 101: 41-61. DOI:10.1007/s11103-019-00889-9 |
Lacerda A.F., Vasconcelos E.A., Pelegrini P.B., et al, 2014. Antifungal defensins and their role in plant defense. Front. Microbiol, 5: 116. |
LaMondia J.A., 2001. Outbreak of brown spot of tobacco caused by Alternaria alternata in Connecticut and Massachusetts. Plant Dis, 85: 230. |
Li W., Li X., Chao J., et al, 2018. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front. Plant Sci, 9: 1900. DOI:10.3389/fpls.2018.01900 |
Li Y., Sorefan K., Hemmann G., Bevan M.W., 2004. Arabidopsis NAP and PIR regulate actin-based cell morphogenesis and multiple developmental processes. Plant Physiol, 136: 3616-3627. DOI:10.1104/pp.104.053173 |
Li S., Zhang J., Liu H., et al, 2019. Dodder-transmitted mobile signals prime host plants for enhanced salt tolerance. J. Exp. Bot.. |
Nakashima K., Takasaki H., Mizoi J., et al, 2012. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta, 1819: 97-103. DOI:10.1016/j.bbagrm.2011.10.005 |
Nakashima K., Tran L.S., Van Nguyen D., et al, 2007. Functional analysis of a NACtype transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J, 51(4): 617-630. DOI:10.1111/j.1365-313X.2007.03168.x |
Ndamukong I., Abdallat A.A., Thurow C., et al, 2007. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1. 2 transcription. Plant J, 50(1): 128-139. DOI:10.1111/j.1365-313X.2007.03039.x |
Pre M., Atallah M., Champion A., et al, 2008. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol, 147(3): 1347-1357. DOI:10.1104/pp.108.117523 |
Ren T., Wang J., Zhao M., et al, 2018. Involvement of NAC transcription factor SiNAC1 in a positive feedback loop via ABA biosynthesis and leaf senescence in foxtail millet. Planta, 247(1): 53-68. DOI:10.1007/s00425-017-2770-0 |
Saedler R., Baldwin I.T., 2004. Virus-induced gene silencing of jasmonate-induced direct defences, nicotine and trypsin proteinase-inhibitors in Nicotiana attenuata. J. Exp. Bot, 55: 151-157. |
Sathoff A.E., Samac D.A., 2019. Antibacterial activity of plant defensins. Mol. Plant Microbe Interact, 32(5): 507-514. DOI:10.1094/MPMI-08-18-0229-CR |
Seok H.-Y., Woo D.-H., Nguyen L.V., et al, 2016. Arabidopsis AtNAP functions as a negative regulator via repression of AREB1 in salt stress response. Planta, 245(2): 329-341. |
Shen G., Pang Y., Wu W., et al, 2005. Molecular cloning, characterization and expression of a novel jasmonate-dependent defensin gene from Ginkgo biloba. J. Plant Physiol, 162(10): 1160-1168. DOI:10.1016/j.jplph.2005.01.019 |
Song N., Ma L., Wang W., et al, 2019. An ERF2-like transcription factor regulates production of the defense sesquiterpene capsidiol upon Alternaria alternata infection. J. Exp. Bot, 70(20): 5895-5908. DOI:10.1093/jxb/erz327 |
Sun H., Hu X., Ma J., Hettenhausen C., Wang L., Sun G., Wu J., Wu J., 2014a. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol, 63: 1070-1077. DOI:10.1111/ppa.12181 |
Sun H., Song N., Ma L., et al, 2017. Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol, 66: 277-284. DOI:10.1111/ppa.12568 |
Sun H., Wang L., Zhang B., Ma J., Hettenhausen C., Cao G., Sun G., Wu J., Wu J., 2014b. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot, 65(15): 4305-4315. DOI:10.1093/jxb/eru203 |
Thevissen K., Ferket K.K., Francois I.E., et al, 2003. Interactions of antifungal plant defensins with fungal membrane components. Peptides, 24(11): 1705-1712. DOI:10.1016/j.peptides.2003.09.014 |
van der Weerden N.L., Hancock R.E., Anderson M.A., 2010. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J. Biol. Chem, 285(48): 37513-37520. DOI:10.1074/jbc.M110.134882 |
van der Weerden N.L., Lay F.T., Anderson M.A., 2008. The plant defensin, NaD1, enters the cytoplasm of Fusarium oxysporum hyphae. J. Biol. Chem, 283(21): 14445-14452. DOI:10.1074/jbc.M709867200 |
von Dahl C.C., Winz R.A., Halitschke R., et al, 2007. Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J, 51(2): 293-307. DOI:10.1111/j.1365-313X.2007.03142.x |
Vroemen C.W., Mordhorst A.P., Albrecht C., et al, 2003. The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. Plant Cell, 15(7): 1563-1577. DOI:10.1105/tpc.012203 |
Weaver L.M., Amasino R.M., 2001. Senescence is induced in individually darkened Arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiol, 127(3): 876-886. DOI:10.1104/pp.010312 |
Xu Z., Song N., Ma L., et al, 2018. NaPDR1 and NaPDR1-like are essential for the resistance of Nicotiana attenuata against fungal pathogen Alternaria alternata. Plant Divers, 40: 68-73. DOI:10.1016/j.pld.2018.01.001 |
Yamaguchi M., Ohtani M., Mitsuda N., et al, 2010. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell, 1105(22): 1249-1263. |
Yan H., Zhang A., Ye Y., et al, 2017. Genome-wide survey of switchgrass NACs family provides new insights into motif and structure arrangements and reveals stress-related and tissue-specific NACs. Sci. Rep, 7: 3056. DOI:10.1038/s41598-017-03435-z |
Yang J., Worley E., Udvardi M., 2014. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell, 26: 4862-4874. DOI:10.1105/tpc.114.133769 |
Zhang K., Gan S.S., 2012. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol, 158: 961-969. DOI:10.1104/pp.111.190876 |



