b. Gongju National University of Education, 27, Ungjin-ro, Gongju-si, Chungcheongnam-do 32553, South Korea;
c. CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China;
d. University of the Chinese Academy of Sciences, Beijing 100049, China;
e. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan 650201, China;
f. Department of Botany, Smithsonian Institution, Washington, DC 20013, USA
The importance of Bering land bridge (BLB) as a corridor for floristic exchange between Asia and North America has been repeatedly emphasized in phytogeographical studies (Sanmartín, 2001; Tiffney and Manchester, 2001; Donoghue and Smith, 2004; Jia et al., 2015, 2018; Wen et al., 2016; Liu et al., 2017; Jiang et al., 2019; Tian et al., 2021). However, one major limitation of the BLB as a corridor for plant migration is its high latitudinal position (Tiffney and Manchester, 2001). From its formation in the Cretaceous to its disruption in the Pliocene, the BLB has been consistently situated within or near the Arctic Circle (Sanmartín, 2001). Therefore, the BLB was possibly inaccessible to mesothermal plants, which are restricted to more southern latitudes today. This raises important questions as to the efficacy of the BLB as a universal exchange route for mesothermal plants between Asia and North America. In order for a mesothermal lineage to have crossed the BLB, it would have needed to be ecologically capable of becoming established at high latitudes within Asia and/or North America at least at certain point(s) in geologic time. Theoretically, mesothermal plants could have grown at such high latitudes in warm intervals of the Cenozoic (Harris et al., 2016; Westerhold et al., 2020). However, this idea requires testing from the fossil record, which provides unequivocal data on the past distributions of plants.
The middle Miocene is recognized as a pronounced warm interval in the Neogene, during which many major ecological shifts occurred around the globe (Mosbrugger et al., 2005; Holbourn et al., 2015; Zheng et al., 2019; Westerhold et al., 2020). In Asia, paleoclimatic reconstructions show significant warming and a shallow equator-to-pole latitudinal temperature gradient (Deng et al., 2004; You et al., 2009; Zhao et al., 2017). In recent years, many fossil sites of middle Miocene age have been studied from eastern and northeastern Asia. Some of these are nicely summarized by Japanese, Russian, Korean, and Chinese authors (Nikitin, 1976, 2007; Shi and Li, 2010; Jacques et al., 2015; Zheng et al., 2019). Studies of these sites document evidence of mesothermal and microthermal plants growing together in the arctic region (Nikitin, 1976, 2007), as well as some megathermal plants expanding north to the Fotan Basin, Fujian province, southern China, during the Mid-Miocene Climatic Optimum (Shi and Li, 2010; Jacques et al., 2015; Zheng et al., 2019). However, given the broad area and high botanical diversity of eastern Asia, current fossil evidence is insufficient for understanding, in detail, broader patterns of latitudinal migration across numerous plant groups (Zheng et al., 2019). New discoveries of plant fossils from the middle Miocene of Asia are crucial for improving our understanding of phytogeographic patterns during this geologically significant period. Here we present fruits of Malvaceae from the middle Miocene of South Korea, representing important data for examining these broader biogeographic questions.
Malvaceae Juss., as recently circumscribed, comprises nine subfamilies (Grewioideae, Byttnerioideae, Sterculioideae, Tilioideae, Dombeyoideae, Brownlowioideae, Helicteroideae, Malvoideae, and Bombacoideae), mainly distributed in the tropics with some members extending into subtropical and temperate regions (Bayer et al., 1999; APG IV, 2016). Firmiana Marsili (Sterculioideae) and Tilia L. (Tilioideae) are both woody genera in the Malvaceae, widely cultivated as ornamentals (Kostermans, 1957; Tang et al., 2007a, 2007b; Abdullah et al., 2019). Firmiana contains 16 species, disjunctly distributed in Asia and eastern Africa (Tang et al., 2007a; Abdullah et al., 2019), while Tilia includes about 24 species, widely distributed in the Northern Hemisphere (Tang et al., 2007b; Pigott, 2012). Relationships within these genera remain poorly known due to frequent hybridization and polyploidization, processes that greatly complicate the resolution of species relationships (Cai et al., 2015; Abdullah et al., 2019). Reliable fossil records of Firmiana are relatively scarce; the genus has been documented from the middle Miocene and Pliocene of China (Sun, 1999; Xie et al., 2014). In contrast, Tilia has an excellent fossil record in the Northern Hemisphere from the early Eocene to Miocene (Spitzlberger, 1984; Manchester, 1994). However, important gaps remain in the Tilia fossil record. For example, fossils with T. endochrysea-type infructescence morphology (i.e., Tilia Type B in Manchester, 1994) have never been documented in Asia, although the sole living species with this type, T. endochrysea, is restricted to Asia. In general, the geographic distributions of these two malvaceous genera in the middle Miocene of Asia remain poorly understood.
Recently, fossil representatives of Malvaceae based on wellpreserved fruit valves of Firmiana and an infructescence of Tilia were uncovered from the middle Miocene of South Korea. These fossils expand the known records of Firmiana and Tilia, and provide the first fossil record of the Tilia Type B in Asia. Considering previously described fossils of Firmiana and Tilia, we discuss the significance of these new discoveries for understanding the evolutionary and biogeographic histories of these two genera. In light of previously described middle Miocene plant fossils from Asia, we also discuss the implications of these new fossils for understanding the northern extent of mesothermal plants in Asia during this period, and whether the BLB would have been accessible and ecologically suitable for mesothermal plant migration at this time.
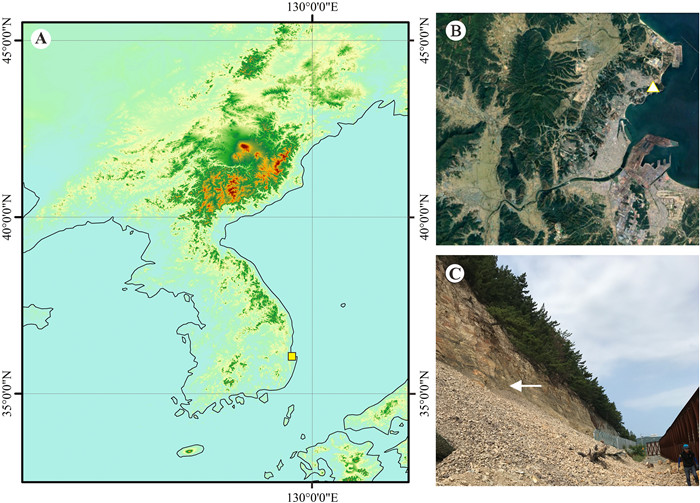
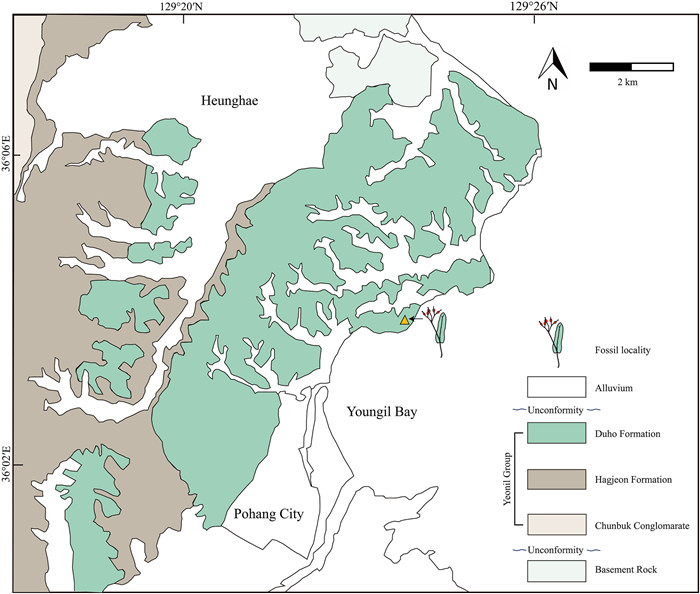
2. Materials and methods 2.1. Geological settingThe new fossil fruits were collected from northeastern Pohang Basin, South Korea (Fig. 1). Cenozoic sediments in the Pohang Basin are composed of the lower Yangbuk Group and the upper Yeonil Group (Yun, 1986; Yun et al., 1997) (Fig. 2). The Yeonil Group is further divided into the Chunbuk conglomerate, the Hagjeon Formation, and the Duho Formation in ascending order (Yun, 1986; Yun et al., 1997) (Fig. 2). The sediments from which the new fossil material was obtained belong to the Duho Formation. They are lithologically characterized by yellowish to dark grey cyclic deposits of siltstone and mudstone. Although the Duho Formation has not yet been radiometrically dated, studies based on dinoflagellate cysts and paleomagnetic stratigraphy have inferred ages of 14-12 Millions of year ago (Ma) and 14.5-11.5 Ma respectively (Kim et al., 1993; Yun et al., 1997), coinciding with the middle Miocene.
|
| Fig. 1 The fossil site where the fossils described in this study were collected in Pohang Basin, South Korea. A, the position of the fossil site (yellow square) in Asia; B, the Pohang Basin and the fossil site (white triangle); C, the outcrop and the fossil bearing layers (white arrow). |
|
| Fig. 2 Geological map of the Pohang Basin. Modified from Jung and Lee (2009). |
Previous paleobotanical studies from the Duho Formation reported plant taxa, including Betulaceae Gray, Malvaceae, Oleaceae Hoffmannsegg et Link, Ulmaceae Mirbel, and Pinaceae Lindley (Chun, 1982, 2004; Jung and Lee, 2009) (See Table S1). However, the fossils described here have not been previously documented in the Duho Formation.
2.2. Morphological studyThree Firmiana fruit valves, one Tilia endochrysea-type bractinfructescence, and one T. endochrysea-type bract were collected. These fossils were first photographed using a digital camera (Nikon D700, Kanagawa, Japan). Then they were moved to a stereo microscope (Leica S8APO, Wetzlar, Germany) to observe fine-scale details. The morphology of living species was studied from specimens kept in the Herbarium of the Institute of Botany, Chinese Academy of Sciences (PE), and the Herbarium of the Kunming Institute of Botany, Chinese Academy of Sciences (KUN). The figures were made using CorelDraw 2018 (Corel, Canada) and Photoshop CS6 (Adobe Systems Incorporated, United States).
2.3. Fossil records, temperature preferences, and distributional mapsFossil records were collected from published literature and the Cenozoic Angiosperm Database (Xing et al., 2016). The temperature preferences of the taxa examined were demarcated mainly based on Jiménez-Moreno (2006) and Li et al. (2015). They include megathermal plants (living in areas with a tropical climate), mesothermal plants (living in areas with a subtropical to warmtemperate climate), and microthermal plants (living in areas with a cool-temperate to boreal climate).
The modern coordinates of the fossil records were converted into paleo-coordinates at 15 Ma using the method introduced in GPlates (http://www.gplates.org/). Modern and paleo-maps were downloaded from Natural Earth (http://www.naturalearthdata.com/) and GPlates (http://www.gplates.org), respectively. The coordinates were then plotted on the modern map and paleo-map, respectively, using ArcGIS 10.1 (ESRI, United States).
3. SystematicsFamily: Malvaceace Juss.
Genus: Firmiana Marsili
Species: Firmiana sinomiocenica Hu et Chaney
1940 Firmiana sinomiocenica Hu et Chaney, pages 67-68, plate 44, Figs. 3 and 4
|
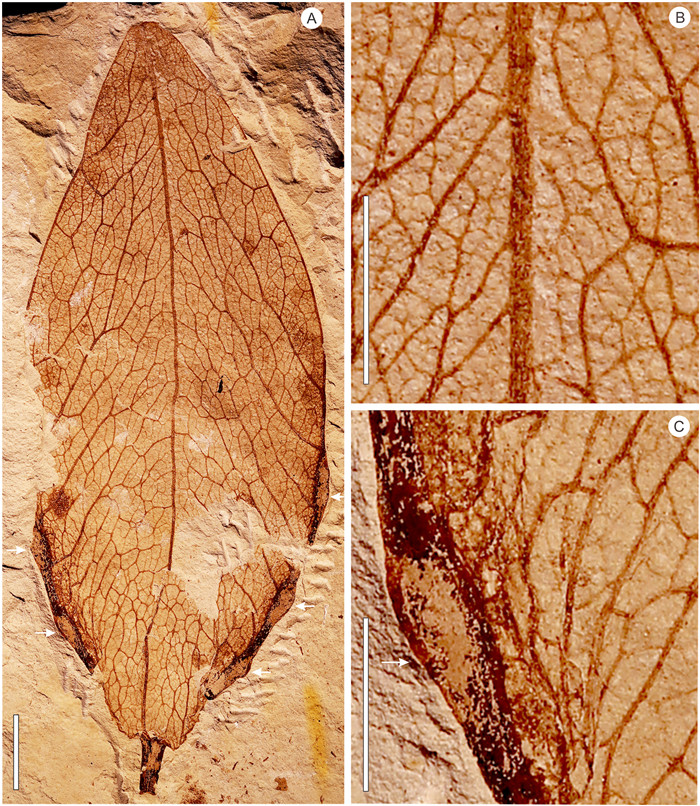
| Fig. 3 Fruit valve of Fimiana sinomiocenica and its details. A, gross morphology of fruit valve showing a stout pedicle, pinnate venation, attachment scars of the seeds, and a round apex; B, enlargement showing the reticulate venation; C, enlargement showing attachment scar. A—C, GNUE3322585. Scale bar = 5 mm. |
|
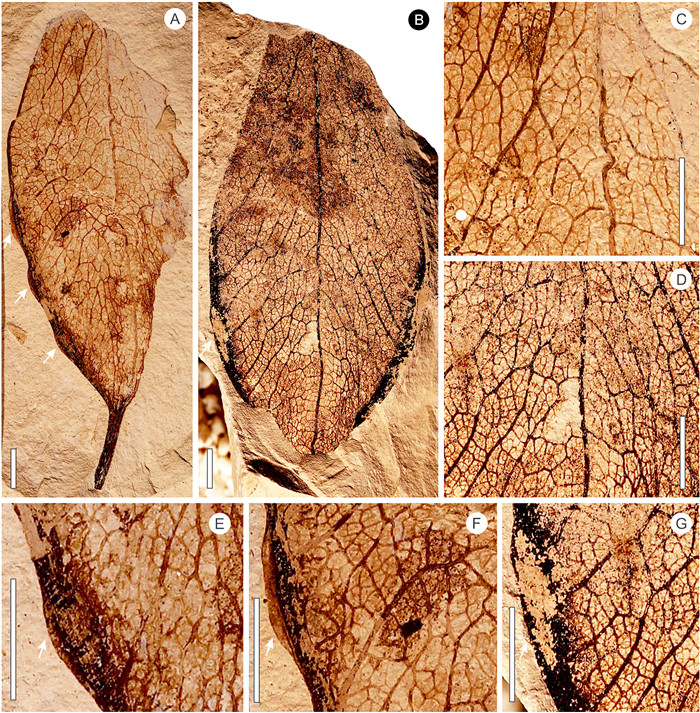
| Fig. 4 Fruit valves of Fimiana sinomiocenica and their details. A, B, gross morphology of fruit valves showing the pedicle, pinnate venation, and attachment scars of the seeds; C, D, enlargement showing reticulate venation; E—G, enlargement showing the attachment scars of seeds. A, C, E, F, GNUE3322587; B, D, G, GNUE3322584. Scale bar = 5 mm. |
Specimens: GNUE3322584 (Fig. 4B); GNUE3322585 (Fig. 3A); GNUE3322587 (Fig. 4A)
Repository: All fossil specimens are stored in the Paleontology Laboratory of Gongju National University of Education.
Locality: Pohang Basin, southeastern South Korea (Fig. 1).
Age: The middle Miocene
Description: Fruit valve foliaceous, obovate to ovate, 105.5 mm long, and 23.7-42.4 mm wide, borne on a stout stalk that is 7.4-11.1 mm in length (Figs. 3 and 4). Proximal end acute to convex (Figs. 3A, 4A-B), distal end constricted and rounded (Fig. 3A). Ventral suture thick, split to form the left and right margins of the valve (Figs. 3A, 4A-C). Venation pinnate (Figs. 3A, 4A and 4B). Secondary veins arising from the ventral suture at an angle of 77°—147° (Fig. 3A, C, 4E-G), finally reaching the dorsal suture (Figs. 3B, 4C-D), thinning in the course or ramifying near the dorsal suture (Figs. 3A, 4C-D). Tertiary veins mixed percurrent, straight or forming chevrons (Figs. 3B, 4C-D). Quaternary veins irregular reticulate (Figs. 3B, 4C-G). Seeds positioned on each side of the split ventral sutures (valve margin) at the proximal end of the valve, with one to three seeds per side, as revealed by attachment scars on the fossil material (Figs. 3C, 4E-G).
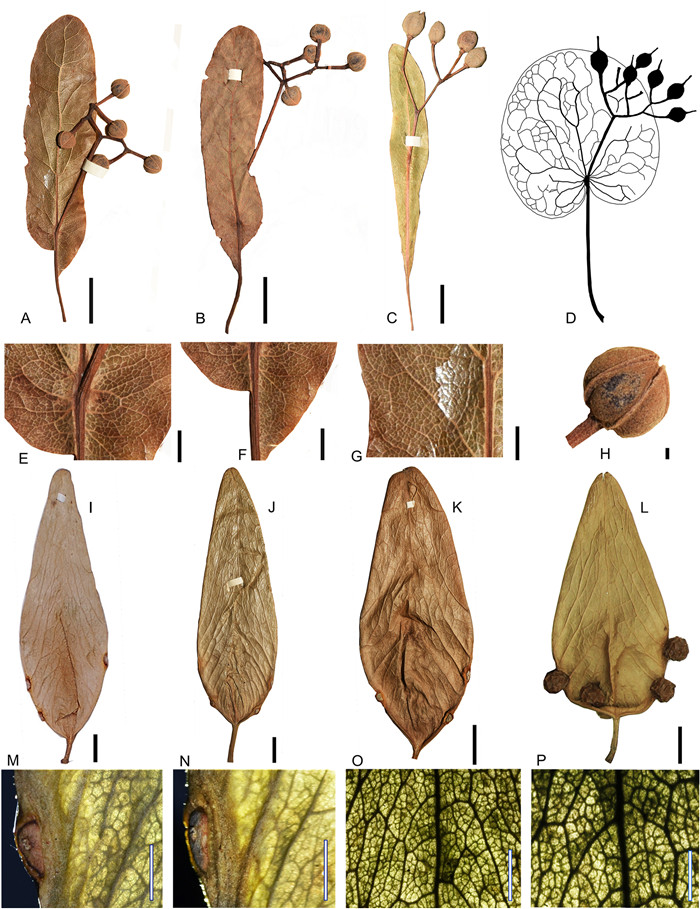
Comparisons: The new fruit valve fossils (Figs. 3 and 4) are characterized by an elliptical outline, pinnate venation, secondary veins arising from the ventral suture and obliquely reaching the dorsal suture, and the attachment scars of the seeds situated on the ventral suture. These characters are identical with those of mature fruits of Firmiana, which dehisce along the ventral suture to form membrane valves (Fig. 6I-P). Therefore, the new fruit fossils are unequivocally assigned to Firmiana.
Firmiana contains about 16 extant species. The fruit valves of the different species of living Firmiana cannot be readily distinguished from each other. The shape of proximal end of the fruit valves varies from broad ovoid to inverse wedge-shaped even in the same species (Tang et al., 2007a) (Fig. 6I-L). Their ranges of size, seed number, and indumentum type usually overlap among different species (Tang et al., 2007a). For example, the fruit valve of F. simplex (L.) W. Wight is 60-110 mm long, 15-35 mm wide, 2-4 seeded, and puberulent or nearly glabrous, while that of F. danxiaensis H.H. Hsue et H.S. Kiu is 80-100 mm long, 25-30 mm wide, 2-3 seeded, and subglabrous (personal observations; Tang et al., 2007a). Compared with living Firmiana species, the new fruit valve fossils do not completely fall into the morphological range of any of the living species. For example, the length of the new fruit valve fossils (105 mm) falls within the range of F. simplex (60-110 mm), but their width range (23.7-42.4 mm) is different from that of F. simplex (15-35 mm). The variation in seed number (3-5) for the new fruit valve fossils is consistent with that of F. hainanensis Kosterm. (3-5), but its length (about 105.5 mm) is larger than that of F. hainanensis (about 70 mm). So far, there are two known fossil species of Firmiana: F. sinomiocenica Hu et Chaney and F. yunnanensis Xie et Manchester (Hu and Chaney, 1940; Xie et al., 2014). The former has a strong and somewhat straight dorsal suture while that of the latter is much weaker and with a zigzag appearance (Xie et al., 2014). The new fruit valve fossils have a stronger and straighter dorsal suture, and thus differ from F. yunnanensis. Their gross morphology, including the dorsal suture, is similar to F. sinomiocenica. Although the former is usually larger than the latter, this difference might simply be an artifact of limited available fossil specimens of F. sinomiocenica. Thus, here we adopt a broad circumscription and assign the new fossil material to F. sinomiocenica.
Genus: Tilia L.
Species: Tilia asiatica L.B. Jia et G.S. Nam sp. nov.
Specific diagnosis: Bract leaf-like, elongate to oblong; venation pinnate; midvein stout, thinning toward the apex (Fig. 5A and D). Secondary veins joining the midvein in a decurrent or deflected way, variable in course, often forming loops near the margin (Fig. 5A and D). Tertiary and quaternary veins irregular reticulate (Fig. 5C). Peduncle diverging from the very base of the bract, branching to form cymes (Fig. 5A). Fruit globose with longitudinal ribs (Fig. 5B).

|
| Fig. 5 Bract-infructescence of Tilia asiatica and its details. A, gross view of the bract-infructescence (holotype); B, enlargement of the globose fruit, showing latitudinal ribs; C, enlargement of the bract, showing the reticulate quaternary venation. A—C, GNUE3222154; D, GNUE3322622. Scale bar = 5 mm. |
Holotype: GNUE3322154 (Fig. 5A)
Paratype: GNUE3322622 (Fig. 5D)
Repository: All fossil specimens are stored at the Paleontology Laboratory of Gongju National University of Education.
Type locality: Pohang Basin, southeastern South Korea (Fig. 1).
Age: The middle Miocene
Etymology: The specific epithet "asiatica" means of Asia and refers to the status of the species as the first fossil record in Asia with affinity to the Tilia endochrysea lineage.
Description: Bract leaf-like, elongate to oblong, apex not preserved, at least 68.7 mm in length, 12.9 mm in width (Fig. 5A and D). Base of the bract nearly symmetrical (Fig. 5D) or asymmetrical (Fig. 5A). Venation pinnate (Fig. 5A and D). Midvein stout, thinning toward the apex (Fig. 5A and D). Secondary veins joining the midvein in a decurrent or deflected way, variable in course, often forming loops near the margin (Fig. 5A and D). Tertiary and quaternary veins irregularly reticulate (Fig. 5A and D). Peduncle diverging from the very base of the leaf-like bract, extending 60.2 mm before branching to form the cymes (Fig. 5A). Fruit globose, 7.5 mm in diameter, with longitudinal ribs (Fig. 5B).
Comparisons: The new bract-infructescence (Fig. 5) is characterized by a large, leaf-like bract with pinnate venation, a peduncle emerging from the bract to form cymes, and globose fruits having longitudinal ribs on the surface. These characters are typical of the bract-infructescence of Tilia (Fig. 6A-H). Based on the position of the peduncle on the bract, and the shape and venation of the bract, bract-inflorescences of Tilia are classified into three types: Types A, B, and C (Manchester, 1994). The bract of Types A and B is fused only at its base to the peduncle, which is different from the usual condition (Type C) seen in most extant Tilia species, in which the bract is fused to the peduncle along 1/4 to 1/2 of its lower length (Manchester, 1994). The difference between Types A and B is that the former has an orbicular bract with palmate venation as seen in North American Tilia circulara (Manchester, 1994) and European Tilia brassicoides (Kvačcek and Walther, 2004), while the latter has an oblong bract with pinnate venation (Manchester, 1994). The new species described here features a bract with pinnate venation and only the base of the bract fused with the peduncle (Fig. 5A and D), consistent with Type B. At present, T. endochrysea, restricted to the middle latitudes of China, is the only species known to possess Type B bracts (Tang et al., 2007b; Pigott, 2012). The morphology of the fossils described here falls within the range of variation of the extant species, T. endochrysea. However, in the absence of other characters from organs such as flowers and leaves, it is difficult to confidently determine whether these Miocene fossil materials correspond to this extant species. Thus, we place the new fossils in an extinct species, but highlight their affinities (and possibly conspeficity) with T. endochrysea. Fossils of Tilia Type B have been documented from several localities in Europe and North America, and they have been named as Tilia pedunculata Chaney, Tilia longebracteata Andrae, and Tilia ovoidea Givulescu (Hall and Swain, 1971; Spitzlberger, 1984; Manchester, 1994). However, a more comprehensive examination of these specimens suggests that they may represent a single species with variable morphology (Manchester, 1994). Features such as surface shape and texture of the fruits may aid in distinguishing distinct species, but these characters are often not available due to limited preservation (Manchester, 1994). Manchester (1994) adopted an approach that applies different names to fossils from different continents, namely, T. pedunculata for Type B fossils from North America and T. longebracteata for these from Europe. Here we follow this approach and erect a new species, T. asiatica for type B fossils from Asia based on the new infructescence fossil.
|
| Fig. 6 Infructescence of Tilia and fruit valves of Firmiana for comparisons. A, B, T. endochrysea Hand.-Mazz. (Tilia Type B); C, T. mongolica Maxim. (Tilia Type C); D, T. circularis (Chaney) Manchester (Tilia Type A), based on Manchester (1994), Fig. 1; E-H, Details of T. endochrysea; I—L, F. simplex (L.) W. Wight; M-P, Details of F. simplex (L.) W. Wight. Scale bar = 10 mm (A-C, I-L), 5 mm (M, N), 1 mm (E-H), 500 μm (O, P). |
Fossil leaves, comparable to Firmiana, have been reported from the Miocene to Pliocene of Asia (Writing Group of Cenozoic plants from China, 1978; Sun, 1999; Xie et al., 2014). The earliest reliable fossil record of Firmiana is based on fruit valves and leaves from the early to middle Miocene of Shanwang Basin, eastern China (Writing Group of Cenozoic plants from China, 1978; Xie et al., 2014). The other known reliable fossils are from the Pliocene of Lincang Basin, southwestern China in the form of fruit valves (Xie et al., 2014). The discovery of F. sinomiocenica from the middle Miocene of South Korea adds a geographically significant new reliable fossil record of Firmiana. Although Firmiana is disjunctly distributed in Asia and eastern Africa at the present (Abdullah et al., 2019), fossils of the genus have never been reported from Africa.
Fossils of Tilia are well-represented in the Cenozoic of the Northern Hemisphere by pollen, leaves, and bracts, sometimes with the attached inflorescence or infructescence (Hall and Swain, 1971; Spitzlberger, 1984; Manchester, 1994; Givulescu, 1997; Kvaček and Walther, 2004; Pigott, 2012). Pollen grains comparable to those of Tilia can be traced back to the early Eocene of Europe (Mai, 1961; Dejax et al., 2001), and leaves attributed to those of Tilia can be traced back to the middle Eocene of western North America (Wolfe and Wehr, 1987). The earliest reliable fossil record of Tilia based on bract-infructescences is from the late Eocene of Nevada, western North America (Manchester, 1994).
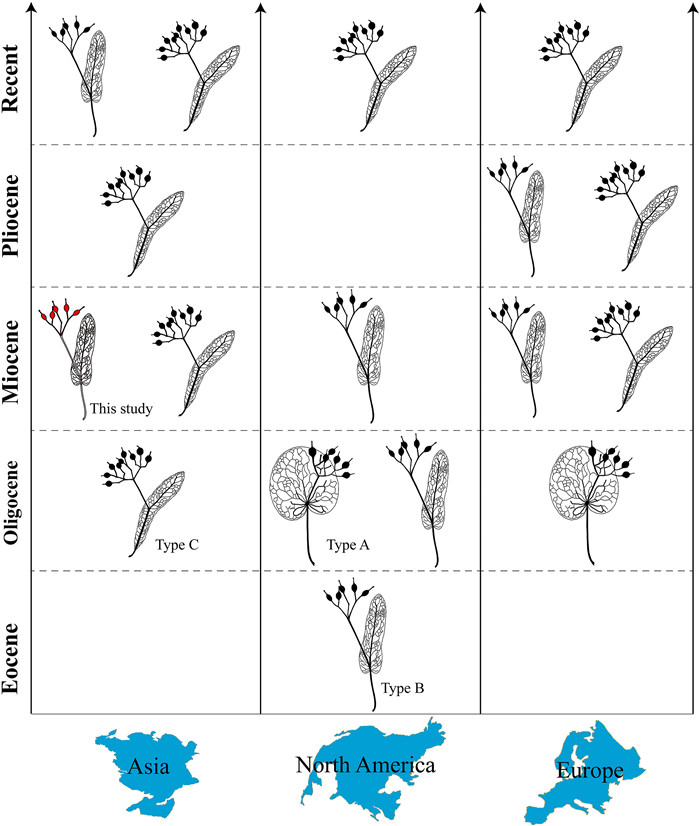
As mentioned above, there are three morphological groupings of bract-inflorescences in Tilia, Types A, B, and C, based on bract morphology (Manchester, 1994) (Fig. 7). Fossil records of Type A have been documented only from the Oligocene of Europe and the early Oligocene of North America (Manchester, 1994; Kvaček and Walther, 2004) (Fig. 7). Fossil records of Type B have been reported from the late Eocene to Miocene of North America and the Miocene to Pliocene of Europe (Hall and Swain, 1971; Manchester, 1994; Givulescu, 1997) (Fig. 7). Until the present paper, fossils of Type B have never been documented from Asia, which has the only extant species with this bract configuration, T. endochrysea. Type C, which is widely distributed in the Northern Hemisphere today, has been found in the Oligocene to Pliocene of Asia, and the Pliocene of Europe, but it has not been documented from the North American fossil record (Spitzlberger, 1984; Manchester, 1994) (Fig. 7). Our discovery of T. asiatica is the first report of a Type B Tilia bracts in the Asian fossil record, providing evidence that this bract configuration was established in Asia at least by the middle Miocene.
|
| Fig. 7 The occurrence of Tilia infructescence morphologies (Types A, B, and C) in the Cenozoic of the Northern Hemisphere. The Type B with red fruits indicates the discovery of this study, which documents the first occurrence of this type in Asia. The morphology of Tilia infructescence is modified from Fig. 1 in Manchester (1994). |
The earliest reliable fossil record of Type B Tilia bracts is from the late Eocene of North America (Manchester, 1994), well before the Miocene occurrences of this type in Asia and Europe. This may indicate that Type B originated in North America, and subsequently dispersed to Asia and Europe. Given that previous molecular studies have failed to resolve the phylogeography of Tilia due to frequent hybridization and polyploidization (McCarthy, 2012; Phuekvilai, 2014; Cai et al., 2015), our finding may provide new insight on the phytogeography of this genus, underscoring the value of paleobotanical evidence for understanding the biogeographic histories of evolutionarily complicated lineages.
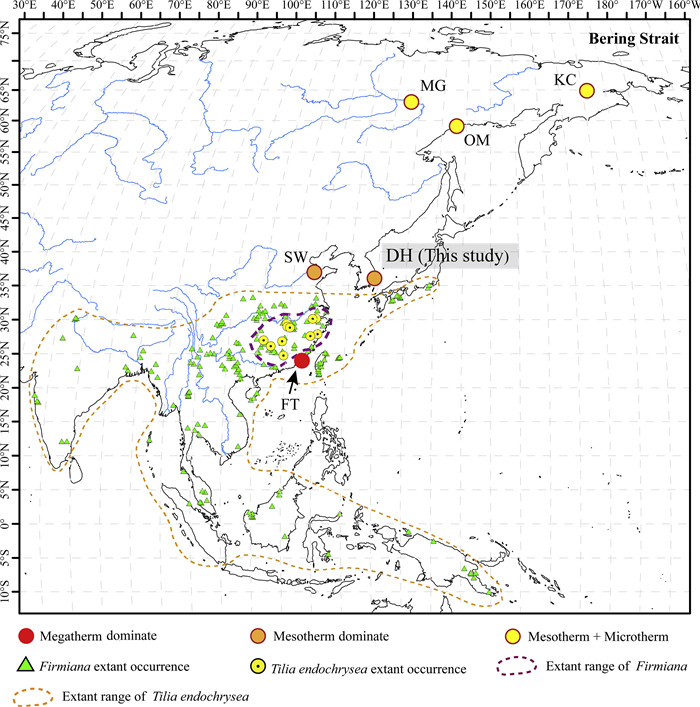
4.2. Significance of a more northerly distribution of mesothermal plants in the middle MioceneThe middle Miocene is the most recent prolonged warming interval of Earth's history (Mosbrugger et al., 2005; Holbourn et al., 2015; Zheng et al., 2019; Westerhold et al., 2020). The distributions of many plants likely shifted into higher latitudes during this period (Utescher et al., 2011; Popova et al., 2017; Zheng et al., 2019). As previously mentioned, Firmiana and T. endochrysea are two lineages of mesothermal plants. Firmiana is now distributed in tropical to subtropical Asia, while T. endochrysea is distributed in subtropical Asia (Tang et al., 2007a, 2007b) (Fig. 8). The discovery of F. sinomiocenica and T. asiatica, in Pohang Basin, South Korea, indicates that Firmiana and the Tilia Type B lineage previously inhabited much more northerly regions in Asia during the middle Miocene (Fig. 8). Our study, therefore, provides a reference for the northern extent of the mesothermal lineages in Asia during this warm episode.
|
| Fig. 8 The extant distribution of Firmiana and Tilia endochrysea in Asia, and the middle Miocene fossil sites from eastern and southeastern Asia. DH, the Duho assemblage, South Korea; FT, the Fotan assemblage, southern China; SW, the Shanwang assemblage, eastern China; OM, the Omolon Mouth assemblage, Far East of Russia; MG, the Mamontova Gora assemblage, Russia; KC, the Kannachan assemblage, Russia. The extant distribution of Firmiana is based on Kostermans (1957) and Tang et al. (2007a), while that of T. endochrysea is based on Tang et al. (2007b) and Pigott (2012). The extant occurrence data of the two genera are from the Global Biodiversity Information Facility (GBIF), i.e., GBIF. org (4 ecember 2019) GBIF Occurrence Download https://doi.org/10.15468/dl.mvpqwf and GBIF.org (8 November 2019) GBIF Occurrence Download https://doi.org/10.15468/dl.dwp6ku. |
Similar more northerly distributions during the middle Miocene have been documented in other plant groups. Fossil evidence shows that mesothermal plants such as Evodia Gaertn., Mallotus Lour., Sapindus L., Stachyurus Siebold et Zucc., and Tetrastigma (Miq.) Planch., which are currently mainly distributed south of central China in Asia, were distributed farther north in the Shanwang Basin, Shandong Province, central China in the late early to middle Miocene (17-15 Ma) (Sun, 1999; Yang et al., 2007) (Figs. 8 and 9; Table S1). Other mesothermal elements such as Glyptostrobus Endl., Metasequoia Hu et W. C. Cheng, Juglans L., and Pterocarya Kunth, which are currently mainly distributed in subtropical to temperate Asia, previously occurred in the Far East of Russia in the middle Miocene (Nikitin, 1976, 2007) (Figs. 8 and 9; Table S1). Additionally, megathermal plants, indicative of tropical rainforest, such as Calophyllum L., Dipterocarpus C.F. Gaertn., Flacourtia Comm. ex L'Hér., and Shorea Roxb. ex C.F. Gaertn., were previously established in Fotan Basin, Fujian province, China, in the Mid-Miocene Optimum (Shi et Li, 2010; Jacques et al., 2015; Zheng et al., 2019) (Figs. 8 and 9; Table S1), about two degrees north of the present distribution of tropical rain forests.
4.3. Efficacy of the Bering land bridge for mesothermal plants in the middle Miocene of AsiaGeological inferences indicate that the BLB was situated exclusively at high latitudes, from around 75° or 69°N in the Paleogene according to different estimates, moving southward to about 65°N at present (Wen et al., 2016). Therefore, in order for a plant species to have crossed the BLB in the middle Miocene, it must first have been able to inhabit the ecological territory adjacent to the BLB at that time. Documenting the northern extent of different plant groups relative to the BLB in the middle Miocene will therefore provide important data for assessing the feasibility of particular lineages utilizing the BLB as migratory route during this period.
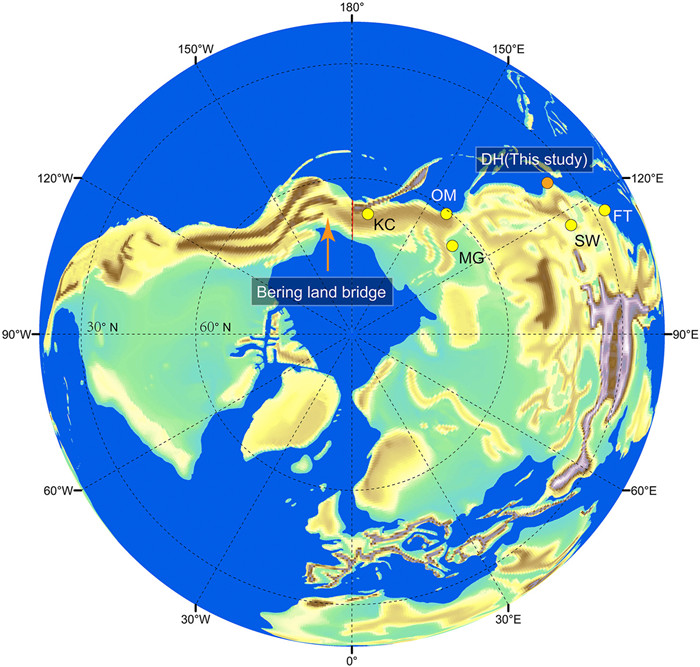
The northernmost fossil occurrences of the two malvaceous lineages described here are from the Shanwang Basin (36.9°N) and the Pohang Basin (36.1°N), respectively (Table S1). However, these occurrences are geographically remote from the BLB, occurring ca. 26° to the south. This may suggest that these two lineages (Firmiana and Tilia endochrysea) were unable to reach and traverse the BLB during the middle Miocene. However, it is worth noting that absence of a fossil is no proof that a taxon did not exist in a particular location. Alternatively, future research might recover fossils from more northerly regions, which would perhaps serve as evidence in favor of BLB migration. Fossils of some other mesothermal plants (such as Glyptostrobus Endl., Azolla Lam., Broussonetia L'Hér. ex Vent., Cephalanthus L., Pilea Lindl., and Pterocarya Kunth), and microthermal plants (such as Abies Miller, Picea A. Dietrich, Alnus Miller, Betula L., Carpinus L., Rubus L., Potentilla L. and Ranunculus L.) have been discovered from the Far East of Russia (64.8°N—67.3°N) in the middle Miocene (Figs. 8 and 9; Table S1). This indicates that, during the middle Miocene, the flora surrounding the BLB was likely dominated by a mixture of mesothermal and microthermal plants. Therefore, it is reasonable to hypothesize that these particular lineages were able to utilize the BLB as migratory route during this period.
|
| Fig. 9 The position of middle Miocene assemblages from eastern and northeastern Asia on a paleo-map at 15 Ma. DH, the Duho assemblage, South Korea; FT, the Fotan assemblage, southern China; SW, the Shanwang assemblage, eastern China; OM, the Omolon Mouth assemblage, Far East of Russia; MG, the Mamontova Gora assemblage, Russia; KC, the Kannachan assemblage, Russia. |
Not surprisingly, megathermal plants were distributed even farther to the south, with a northern limit at ca. 25.9°N during the Mid-Miocene Optimum based on available fossil evidence (Figs. 8 and 9; Table S1). Thus, tropical elements would not have been able to colonize the regions near the BLB, let alone cross the BLB.
However, it is worth noting that the middle Miocene lasted about 4.3 million years. Although the interval is regarded as generally warm, climate oscillations did apparently occur (Mosbrugger et al., 2005; Holbourn et al., 2015; Bouchal et al., 2018; Westerhold et al., 2020). During oscillations, plants may have tracked their climatic envelope by moving south or north at different points of the middle Miocene. Thus, more extensive fossil exploration and collection in Asia will be necessary to document in greater detail plant distribution throughout this time, which will better inform our understanding of the role of the BLB in Holarctic plant migration throughout the later Cenozoic.
Author contributionsL.J., G.N., Y.H, and Z.Z. planned and designed the research; L.J., and G.N. conducted the research; L.J., T.S., and S.L wrote the manuscript.
Declaration of competing interestWe have no competing interest.
AcknowledgementsWe are grateful to the Herbarium of the Institute of Botany (PE) and the Herbarium of the Kunming Institute of Botany (KUN), Chinese Academy of Sciences (CAS) for providing access to comparative specimens. This work was supported by the National Natural Science Foundation of China (No. 31900194), the Foundation of the State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (No. 183112), the Yunnan Province Natural Science Foundation (No. 2019FB061), the "Light of West China" Program, CAS, and the Youth Innovation Promotion Association, CAS (No. 2017439).
Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2020.12.006.
Abdullah, Shahzadi I., Mehmood F., et al, 2019. Comparative analyses of chloroplast genomes among three Firmiana species: identification of mutational hotspots and phylogenetic relationship with other species of Malvaceae. Plant Gene 19, 100199. |
APG IV, 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc, 181: 1-20. DOI:10.1111/boj.12385 |
Bayer C., Fay M.F., Bruijn A.Y.D., et al, 1999. Support for an expanded family concept of Malvaceae within a recircumscribed order Malvales: a combined analysis of plastid atpB and rbcL DNA sequences. Bot. J. Linn. Soc, 129: 267-303. |
Bouchal J.M., Güner T.H., Denk T., 2018. Middle Miocene climate of southwestern Anatolia from multiple botanical proxies. Clim. Past, 14: 1427-1440. DOI:10.5194/cp-14-1427-2018 |
Cai J., Ma P.F., Li H.T., et al, 2015. Complete plastid genome sequencing of four Tilia species (Malvaceae): a comparative analysis and phylogenetic implications. PloS One, 10: e0142705. DOI:10.1371/journal.pone.0142705 |
Chun H.Y., 1982. Plant fossils from the Tertiary Pohang sedimentary basin, Korea. Korea Inst. Energ. Resour, 14: 7-23. |
Chun H.Y., 2004. Taxonomic and morphological diversity of the Miocene Pohang flora. Geol. Korea. Spec. Publ, 2: 25-38. |
Dejax J., De Franceschi D., Lugardon B., et al, 2001. Le contenu cellulaire du pollen fossilis#233; dans l'ambre préservé à l'état organique. Inst. Earth Sci. Pl. Sci. ser, 332: 339-344. |
Deng T., Wang X.M., Wang X.J., et al, 2004. Cenozoic stratigraphic sequence of the Linxia Basin in Gansu, China and its evidence from mammal fossils. Vertb. PalA, 42: 45-66. |
Donoghue M.J., Smith S.A., 2004. Patterns in the assembly of temperate forests around the Northern Hemisphere. Philos. T. R. Soc. B, 359: 1633-1644. DOI:10.1098/rstb.2004.1538 |
Givulescu R., 1997. Eine Übersicht über die fossilen Tilia-Hochblätter Rumäniens und einige Bemerkungen über die Nomenklatur der europäischen TiliaHochblätter. Feddes Repert, 108: 143-149. DOI:10.1002/fedr.19971080303 |
Hall J.W., Swain A.M., 1971. Pedunculate bracts of Tilia from the tertiary of western United States. B. Torrey Bot. Club, 98: 95-100. DOI:10.2307/2483773 |
Harris A., Walker C., Dee J.R., et al, 2016. Latitudinal trends in genus richness of vascular plants in the Eocene and Oligocene of North America. Plant Divers, 38: 133-141. DOI:10.1016/j.pld.2016.06.002 |
Holbourn A., Kuhnt W., Kochhann K.G.D., et al, 2015. Global perturbation of the carboncycle at the onsetof the Miocene Climatic Optimum. Geology, 43: 123-126. DOI:10.1130/G36317.1 |
Hu H.H., Chaney R.W., 1940. A Miocene Flora from Shantung Province, China.. Washington: Carnegie Institution of Washington Publication.
|
Jacques F.M.B., Shi G., Su T., et al, 2015. A tropical forest of the middle Miocene of Fujian (SE China) reveals Sino-Indian biogeographic affinities. Rev. Palaeobot. Palynol, 216: 76-91. DOI:10.1016/j.revpalbo.2015.02.001 |
Jia L.B., Manchester S.R., Su T., et al, 2015. First occurrence of Cedrelospermum(Ulmaceae) in Asia and its biogeographic implications. J. Plant Res, 128: 747-761. DOI:10.1007/s10265-015-0739-2 |
Jia L.B., Su T., Huang Y.J., et al, 2018. First fossil record of Cedrelospermum(Ulmaceae) from the QinghaieTibetan plateau: implications for morphological evolution and biogeography. J. Systemat. Evol, 57: 94-104. |
Jiang D., Klaus S., Zhang Y.-P., et al, 2019. Asymmetric biotic interchange across the bering land bridge between eurasia and north America. Natl. Sci. Rew. |
Jiménez-Moreno G., 2006. Progressive substitution of a subtropical forest for a temperate one during the middle Miocene climate cooling in Central Europe according to palynological data from cores Tengelic-2 and Hidas-53 (Pannonian Basin Hungary). Rev. Palaeobot. Palynol, 142: 1-14. DOI:10.1016/j.revpalbo.2006.05.004 |
Jung S.-H., Lee S.-J., 2009. Fossil-winged fruits of Fraxinus (Oleaceae) and Liriodendron (Magnoliaceae) from the Duho Formation, Pohang Basin, Korea. Acta Geol. Sin, 83: 845-852. DOI:10.1111/j.1755-6724.2009.00113.x |
Kim K.H., Doh S., Hwang C., et al, 1993. Paleomagnetic study of the Yeonil group in Pohang Basin. J. Korean Inst. Min. Geol, 26: 507-518. |
Kostermans A.J.G.H., 1957. The genus Firmiana Marsili (Sterculiaceae). Reinwartia, 4: 281-310. |
Kvaček Z., Walther H., 2004. Oligocene flora of Bechlejovice at Děčín from the neovolcanic area of the Ceské Středohoří mountains Czech republic. Acta. Muse. Nat. Prague, Nat. Hist, 60: 9-60. |
Li S.F., Mao L.M., Spicer R.A., et al, 2015. Late Miocene vegetation dynamics under monsoonal climate in southwestern China. Palaeogeogr. Palaeoclimatol. Palaeoecol, 425: 14-40. DOI:10.1016/j.palaeo.2015.02.030 |
Liu P., Wen J., Yi T., 2017. Evolution of biogeographic disjunction between eastern Asia and North America in Chamaecyparis: insights from ecological niche models. Plant Divers, 39: 111-116. DOI:10.1016/j.pld.2017.04.001 |
Mai D.H., 1961. Über eine fossile Tiliaceen-Blüte und tilioid Pollen aus dem deutschen Tertiär. Geologie, 10: 54-93. |
Manchester S.R., 1994. Inflorescence bracts of fossil and extant Tilia in North America, Europe, and Asia: patterns of morphologic divergence and biogeographic history. Am. J. Bot, 81: 1176-1185. DOI:10.1002/j.1537-2197.1994.tb15612.x |
McCarthy D., 2012. Systematics and Phylogeography of the Genus Tilia in North America. Chicago, Illinois.. Chicago: Illinois.
|
Mosbrugger V., Utescher T., Dilcher D.L., 2005. Cenozoic continental climatic evolution in central Europe. Proc. Natl. Acad. Sci. U.S.A, 102: 14964-14969. DOI:10.1073/pnas.0505267102 |
Nikitin, V.P., 1976. Flora of Mamontova Gora (Seeds and Fruits). Miocene Strata of Mamontova Gora (Stratigraphy and Fossil Flora). Nauka, Moscow, pp. 131-194.
|
Nikitin V.P., 2007. Paleogene and Neogene strata in northeastern Asia: paleocarpological background. Russ. Geol. Geophys, 48: 675-682. DOI:10.1016/j.rgg.2006.06.002 |
Phuekvilai, P., 2014. Relicts, refugia and reticulation: a study of population history, hybrids and phylogeny in the long-lived flowering tree genus Tilia. Newcastle University, Newcastle, p. 172.
|
Pigott D., 2012. Lime-trees and Basswoods: A Biological Monograph of the Genus Tilia.. New York: Cambridge University Press.
|
Popova S., Utescher T., Gromyko D.V., et al, 2017. Cenozoic vegetation gradients in the mid- and higher latitudes of Central Eurasia and climatic implications.. Palaeogeogr. Palaeoclimatol. Palaeoecol., 467: 69-82. DOI:10.1016/j.palaeo.2016.09.016 |
Sanmartín I., 2001. Patterns of animal dispersal vicariance and diversification in the Holarctic. Biol. J. Linn. Soc, 73: 345-390. DOI:10.1006/bijl.2001.0542 |
Shi G.L., Li H.M., 2010. A fossil fruit wing of Dipterocarpus from the middle Miocene of Fujian, China and its palaeoclimatic significance. Rev. Palaeobot. Palynol, 162: 599-606. DOI:10.1016/j.revpalbo.2010.08.001 |
Spitzlberger G., 1984. Eine urtümliche Lindenart der Tertiärzeit (Tilia atavia nov. spec.) von Goldern bei Landshut (Niederbayern). Nat. Zeits. Nied, 30: 133-171. |
Sun B., 1999. Fossil Plants from Shanwang Flora. Jinan: Shandong Science and Technology Press.
|
Tang, Y., Gilbert, M.G., Dorr, L.J., 2007a. Sterculiaceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds. ), Flora of China. Science Press and Missouri Botanical Garden Press, Beijing and St. Louis, pp. 302-330.
|
Tang, Y., Gilbert, M.G., Dorr, L.J., 2007b. Tiliaceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds. ), Flora of China. Science Press and Missouri Botanical Garden Press, Beijing and St. Louis, pp. 240-263.
|
Tian Y.-M., Huang J., Su T., et al, 2021. Early Oligocene Itea (Iteaceae) leaves from east Asia and their biogeographic implications. Plant Divers, 43: 142-151. DOI:10.1016/j.pld.2020.09.006 |
Tiffney B.H., Manchester S.R., 2001. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci, 162: S3-S17. DOI:10.1086/323880 |
Utescher T., Bruch A.A., Micheels A., et al, 2011. Cenozoic climate gradients in Eurasiada palaeo-perspective on future climate change?. Palaeogeogr. Palaeoclimatol. Palaeoecol, 304: 351-358. DOI:10.1016/j.palaeo.2010.09.031 |
Wen J., Nie Z.-L., Ickert-Bond S.M., 2016. Intercontinental disjunctions between eastern Asia and western North America in vascular plants highlight the biogeographic importance of the Bering land bridge from late Cretaceous to Neogene. J. Systemat. Evol,, 54: 469-490. DOI:10.1111/jse.12222 |
Westerhold T., Marwan N., Drury A.J., et al, 2020. An astronomically dated record of Earth's climate and its predictability over the last 66 million years. Science, 369: 1383-1387. DOI:10.1126/science.aba6853 |
Wolfe J.A., Wehr W., 1987. Middle Eocene dicotyledonous plants from Republic, northeastern Washington. U.S. Geol. Surv. B, 1597: 1-25. |
Writing Group of Cenozoic plants from China, 1978. Cenozic Plants from China. Beijing: Science Press.
|
Xie S.P., Manchester S.R., Liu K.N., et al, 2014. Firmiana (Malvaceae: Sterculioideae) fruits from the Upper Miocene of Yunnan, Southwest China. Geobios, 47: 271-279. DOI:10.1016/j.geobios.2014.03.005 |
Xing Y., Gandolfo M.A., Onstein R.E., et al, 2016. Testing the biases in the rich Cenozoic angiosperm macrofossil record. Int. J. Plant Sci, 177: 371-388. DOI:10.1086/685388 |
Yang J., Wang Y.F., Spicer R.A., et al, 2007. Climatic reconstruction at the Miocene Shanwang Basin, China, using leaf margin analysis, CLAMP, coexistence approach, and overlapping distribution analysis. Am. J. Bot, 94: 59-608. |
You Y., Huber M., Müller R.D., et al, 2009. Simulation of the Middle Miocene Climate Optimum. Geophys. Res. Lett, 36: L04702. |
Yun H., 1986. Emended stratigraphy of the Miocene formations in the Pohang Basin, Part I. J. Paleontol. Soc. Korea, 2: 54-69. |
Yun H., Yi S., Byun H., 1997. Tertiary system of Korea. Spec. Publ. Paleontol. Soci. Korea, 3: 1-30. DOI:10.1017/S1089332600000176 |
Zhao H., Sun Y., Qiang X., 2017. Iron oxide characteristics of mid-Miocene Red Clay deposits on the western Chinese Loess Plateau and their paleoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol, 468: 162-172. DOI:10.1016/j.palaeo.2016.12.008 |
Zheng D., Shi G., Hemming S.R., et al, 2019. Age constraints on a Neogene tropical rainforest in China and its relation to the Middle Miocene Climatic Optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol, 518: 82-88. DOI:10.1016/j.palaeo.2019.01.019 |



