b. Center of Conservation Biology, Core Botanical Gardens, Chinese Academy of Sciences, Mengla, 666303, China;
c. School of Environmental & Natural Sciences, Griffith University, Nathan QLD, 4111, Brisbane, Australia;
d. Department of Environment and Science, Queensland Herbarium, Toowong, Australia
Elevational gradients, which encompass suites of changing environmental conditions (Körner, 2007), are ideal for assessing how species respond to habitat gradients and predicting the effects of climate change on species diversity (Colwell et al., 2008; Rabasa et al., 2013). Tree species richness on mountains declines with increasing elevation (Stevens, 1992; Malizia et al., 2020), although intermediate peaks of species-density/species richness may be observed along the cline (Lomolino, 2001; Tito et al., 2020). For instance, research in an Ecuadorian montane rain forest reported that tree species richness, leaf area index, and tree basal area increment were highest in valleys along an elevational gradient (Homeier et al., 2010). Many factors have been found to affect elevational patterns of species diversity, including climatic conditions (e.g., temperature, precipitation and humidity) (Bhattarai and Vetaas, 2003; Crimmins et al., 2011; Homeier et al., 2010; Liu et al., 2007; Shen et al., 2012; Tito et al., 2020), topography (Martinez-Camilo et al., 2018), and soil properties (Carpenter, 2005; Salas-Morales and Meave, 2012; Irl et al., 2015; Cuesta et al., 2017). Temperature and water conditions are the major factors that determine plant species distributions on a large scale (Box, 1995), but their relative contributions vary across climatic regions (Pausas and Austin, 2001; McCain and Grytnes, 2010). Previous research has shown that patterns of elevational diversity vary between tropical and temperate mountains in Asia (Ohsawa, 1995). These differences between climatic zones were explained by differences in temperature; however, soil moisture was not examined in these studies, even though it has been associated with tree species abundance, e.g., in a lowland dipterocarp forest in Malaysia (Marryanna et al., 2012). A further study in an Indian forest reserve reported that tree species richness and diversity indices are strongly correlated with soil moisture and organic carbon content (Sarvade et al., 2016). Overall, these results suggest that the factors driving tree species distribution on mountains vary with latitude, topography and other variables. Accordingly, mountain tree species diversity at particular elevations may respond to climate change differently due to habitat variation along elevational gradients (Corlett and Westcott, 2013; Song et al., 2016a, b). For these reasons, mountains are natural laboratories in which to examine issues related to climate change (Tito et al., 2020).
Most earlier studies, however, have focused on adult trees, with seedlings or juveniles receiving less attention (Lenoir et al., 2009). Because tree seedlings are quite sensitive to their abiotic environment and play a key role in forest regeneration (Bace et al., 2012), it is very important to determine where they occur along elevational gradients and how they respond to environmental factors (Teketay 1997; Li et al., 2010; Bace et al., 2012; Song et al., 2016b). Changes in the distribution of seedlings driven by climate change, for instance, could help predict future forest assemblage structure (Laidlaw et al., 2011; Dang et al., 2013).
A positive spatial relationship between the abundance of adult tree species and their seedlings is expected since recruitment is largely governed by abundance of reproductive adults (Comita et al., 2007). Forest canopy composition, however, does not always match abundance of seedlings in the understory (Hubbell et al., 1999). For example, the seedlings of most tree species on Barro Colorado Island in Panama were present in less than 1% of seedling plots, suggesting strong recruitment limitations (Comita et al., 2007). Many of the most dominant canopy species were rarely represented as seedlings in the understory in Mediterranean (Pérez-Ramos and Marañón, 2012) and subtropical forests (Gong et al., 2011). Overall, how these differences vary along environmental gradients remains poorly studied (Song et al., 2016b).
Here, we investigate species composition of adult trees and seedlings across three elevational gradients that represent typical tropical, subtropical and subalpine forests in Yunnan Province. We address the following questions:
(1) What are the elevational differences in species diversity and composition between adult trees and seedlings within the elevational gradients in the three forest types?
(2) How does the composition of tree species respond to variation in major environmental factors (e.g., air temperature and soil moisture) along with elevational change?
Previous research has posited that montane elevation gradients of species-density result from the combined effects of four features: topographical elevational gradients, climatological elevational gradients, geographical isolation, and feedback among zonal communities (Lomolino, 2001). Thus, we hypothesized that species diversity will decrease with increasing elevation for both seedling and adult tree assemblages in all three elevational transects. Similarly, across all forest types, the dissimilarity of species composition for both adult trees and seedlings communities should increase with increasing differences in elevation. Species composition between seedlings and adult trees should show large variation and species similarity between adult trees and seedlings should increase with elevation. We also expect that soil moisture and air temperature will be the main factors that drive the elevational species distributions in this region of monsoonal climates, but the relative contributions of each may vary across climatic zones.
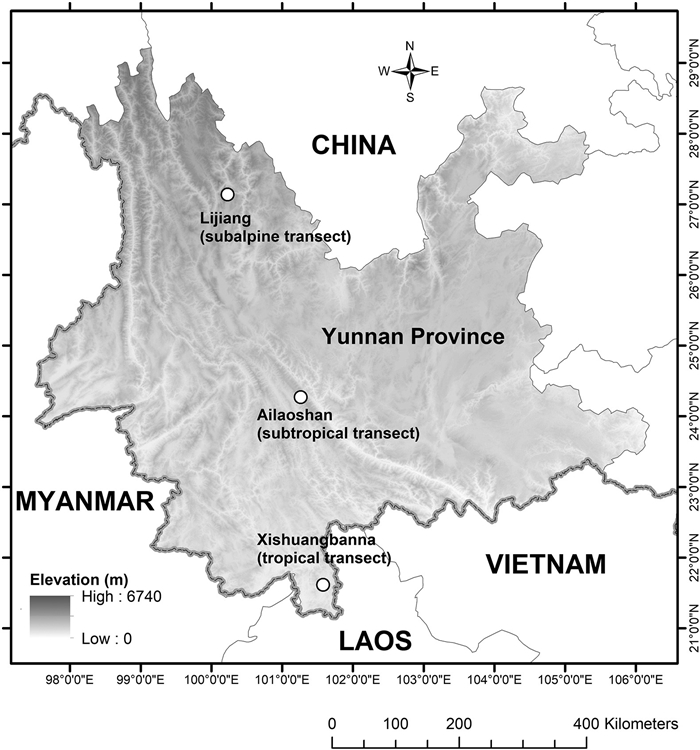
2. Materials and methods 2.1. Study siteYunnan Province in southern China lies in the transitional region between the Hengduan Mountains and Asian tropics. It is mostly an uplifted, high elevation region with a terraced topography stretching from the northwest (maximum elevation at 6740 m) to the south (minimum at 76 m). The whole of Yunnan Province experiences a monsoonal climate, which leads to distinct rainy seasons (May–October) and dry seasons (November–April). Three major climatic zones are recognized in accordance with the three basal terrains: subalpine (ca. 3000 m), subtropical (ca. 2000 m) and tropical (ca. 600–800 m) (Zhu, 2012; Li et al., 2015, 2016b). Correspondingly, the vegetation in this province also comprises three regional types: montane coniferous forest on the Qinghai–Tibet Plateau; subtropical evergreen broad-leaved forest; and tropical forest (Li and Walker, 1986; Wu et al., 1987). In 2011–2012, we established four elevational belts in each transect established at the Xishuangbanna National Nature Reserve (tropical climate: 800 m, 1000 m, 1200 m and 1400 m), Ailaoshan National Nature Reserve (subtropical climate: 2000 m, 2200 m, 2400 m and 2600 m), and Yulong Snow Mountain Nature Reserve (subalpine climate: 3200 m, 3400 m, 3600 m and 3800 m) (Fig. 1; details of each location and elevational transect are provided in Supporting Information Appendix S1). These elevational transects represent typical tropical (Xishuangbanna), subtropical (Ailaoshan) and subalpine (Lijiang) forests in this province. Within each elevational belt, we set up five 20 m × 20 m replicate plots that were spaced approximately 200 m from each other to maximize the independence of samples. Plots were established in areas without obvious signs of anthropogenic disturbances or major canopy gaps that would have confounded the observed distribution of trees and seedlings in these sites.
|
| Fig. 1 Locations for the three elevational transects established in tropical (Xishuangbanna: 800 m, 1000 m, 1200 m and 1400 m), subtropical (Ailaoshan: 2000 m, 2200 m, 2400 m and 2600 m) and subalpine (Lijiang: 3200 m, 3400 m, 3600 m and 3800 m) forests, Yunnan Province, southwestern China. The DEM map was generated using ArcGIS 10.1 (www.esri.com). |
To survey seedlings, five 1 m × 1 m seedling quadrats were established at the four corners and center of each 20 m × 20 m plot. In total, we surveyed 20 plots and 100 seedling quadrats in each elevational transect.
2.2. Data collectionWithin each plot, all trees with a diameter at breast height (DBH) ≥ 5 cm (hereafter referred to as adult trees; Song et al., 2016b) were measured, tagged and identified in 2011 and 2012. Within each of the seedling quadrats, all stems less than 1 cm of stem diameter (measured near the ground; hereafter referred to as seedlings; Song et al., 2016a) were measured, tagged and identified. Seedlings were surveyed in 2015 at the end of the rainy season (October). For adult trees, we recorded 2602 individuals of 239 species; for seedlings, we recorded 3668 individuals of 215 species (excluding 27 individuals that could not be identified to species) (details of species list in Tables S1–S6). A more detailed description of the tropical transect is inSong et al. (2016b).
Soil moisture was measured at the end of dry season (April 2015) and the rainy season (October 2015) on the same dates when the seedlings were surveyed (following several days without rainfall, so that the soil moisture data would not be affected by occasional rainfall), using a conductivity probe (Theta probe MPM–160B, ICT International Proprietary Limited, Armidale, Australia), which is widely used to measure typical soil moisture in forest and agricultural systems (Swella et al., 2015; Song et al., 2017). In each seedling quadrat, we measured soil moisture at a depth of 5 cm at five randomly selected points and calculated the average soil moisture in each quadrat. We recorded hourly air temperatures from January to December, 2015 using a thermo–logger (DS1923 Hygrochron iButton, Maxim, CA, USA). These data were published earlier in Song et al. (2016a) and Li et al., 2016a respectively, but we present them again here for the sake of providing the soil moisture variation during our seedling sampling. Temperature loggers (iButtons) were placed in PVC tubes (with slots for ventilation) to avoid direct solar radiation, and one logger was placed at 1.3 m (breast height on a tree trunk) in each plot, so that we had five replicates for each elevation in total. We assumed that these data would represent the ambient temperature in the understory where the seedling quadrats were located, although a few of loggers were lost during our observation period. We calculated the monthly mean air temperature for each elevational belt based on data collected by the data loggers maintained until the end of December 2015, when temperature monitoring was terminated. For each plot, elevation was determined using a GPS device (GARMIN GPSMAP 60CSX, Garmin Corporation, USA). Slope and aspect were determined using an HRB DQY–1 geologic compass (Harbin Optical Instrument Factory, China).
2.3. Data analysisThe data collected from each of the five seedling quadrats in each plot were aggregated before analysis. We calculated the relative abundance (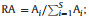
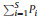
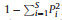
Kruskal–Wallis tests were also used to examine the elevational differences in soil moisture in dry and rainy seasons for each elevational transect. Paired sample t-tests were used to assess differences in soil moisture between rainy and dry seasons in each elevational belt.
Differences in community composition of adult trees and seedlings (using the data from the rainy season) among elevational belts in each transect were examined using non-metric multi-dimensional scaling (NMDS) ordination with 10 random restarts (Minchin, 1987), based on the Bray–Curtis index (Bray and Curtis, 1957). One depauperate seedling sample from 2000 m, with less than five individuals, appeared as an outlier, which resulted in clustering of all other samples in the ordination. We therefore removed this outlier from the subsequent ordination analysis. Permutational multivariate analysis of variance (PERMANOVA) was used by comparing the observed similarity with a null model generated randomly 999 times to test if the species composition of adult trees and seedlings was significantly different from one another across the four elevational belts in each transect (Anderson, 2001; McArdle and Anderson, 2001). We tested the associations between environmental variables and species composition using the envfit function in the "vegan" R package. We fitted the regression of environmental factors and the ordination, then used 999 permutations to test for significance. Six environmental characters served as the potential explanatory variables: (1) elevation, (2) dry season soil moisture, (3) rainy season soil moisture, (4) minimum monthly temperature, (5) slope and (6) aspect (sine-transformed). To assess the sensitivity of tree species to elevation, we also calculated the proportion of species that occurred only in a single elevational belt.
All analyses were performed using the R packages agricolae (De Mendiburu, 2017) and vegan (Oksanen et al., 2018) in R 3.5.0 (R Core Team, 2018).
3. Results 3.1. Elevational and seasonal variation of soil moisture and air temperatureSignificant differences in soil moisture were found across the elevational belts and between seasons within each transect. As expected, soil moisture was significantly lower in the dry season than in the rainy season, but followed the same trends as the rainy season across all elevational belts (Fig. 2). In the tropical transect, soil moisture was the highest at the lowest elevational belt (800 m) in both the rainy and dry seasons. In the subtropical transect, soil moisture was the highest at 2200 m. In the subalpine transect, soil moisture increased with elevation (Fig. 2).

|
| Fig. 2 Soil moisture (per unit volume) in dry (April 2015) and rainy (October 2015) seasons at each of the four elevational belts in each elevational transect. Error bars correspond to standard errors (□= Dry season, ■= Rainy season). Lowercase letters indicate differences between elevations in the dry season. Capital letters indicate differences between elevations in the rainy season. ∗∗ indicates significant difference between seasons at each elevation (P < 0.01). |
Mean monthly air temperature showed distinct seasonal variation in the three transects, with higher air temperature in the rainy season (May to October) than in the dry season (November to April) (Fig. 3). In the tropical transect, air temperatures from January through March were higher at 1000 m, 1200 m and 1400 m than at 800 m, but this trend was reversed from April through December. In contrast, in the subtropical and subalpine transects, air temperature generally decreased with elevation.
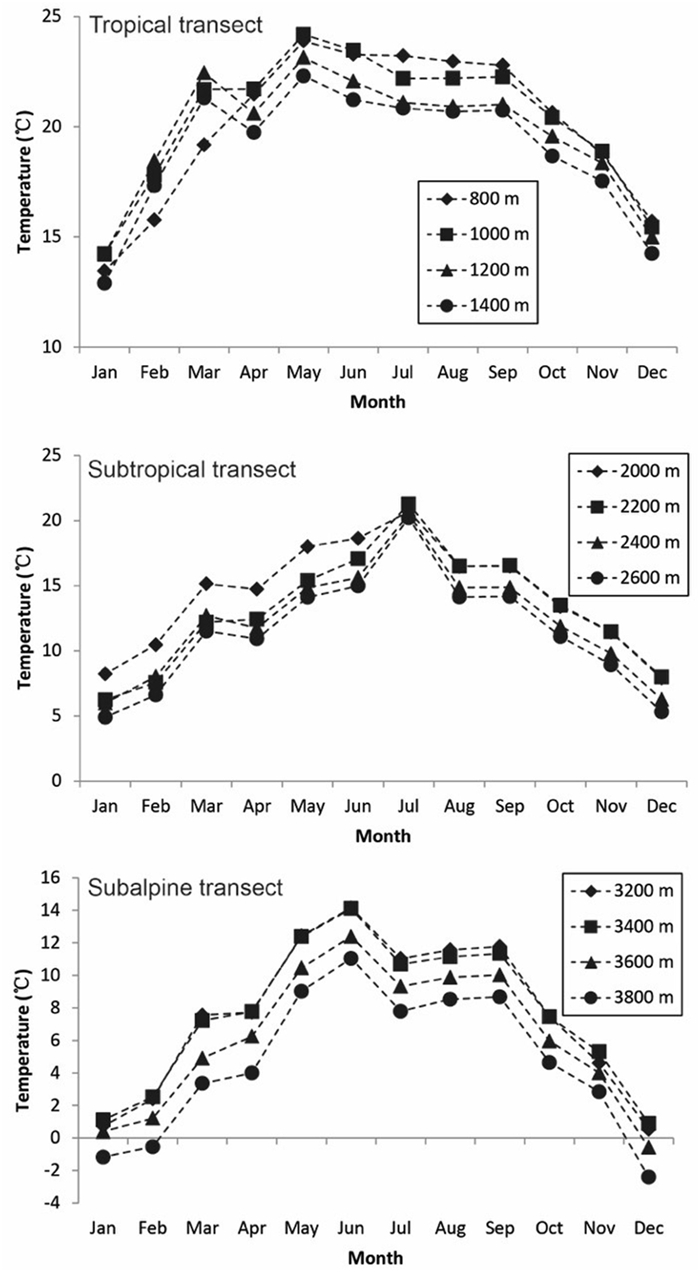
|
| Fig. 3 Mean monthly air temperature in 2015 at the four elevational belts in the three elevational transects. |
In the tropical and subalpine forests, species richness, Shannon index and the Simpson index for both adult trees and seedlings decreased significantly as elevation increased, whereas in the subtropical forests measures of diversity were not correlated with elevation (Table S7 and Fig. 4). Although there was an apparent increase in seedling species richness species in the subtropical transect, it was not statistically significant.
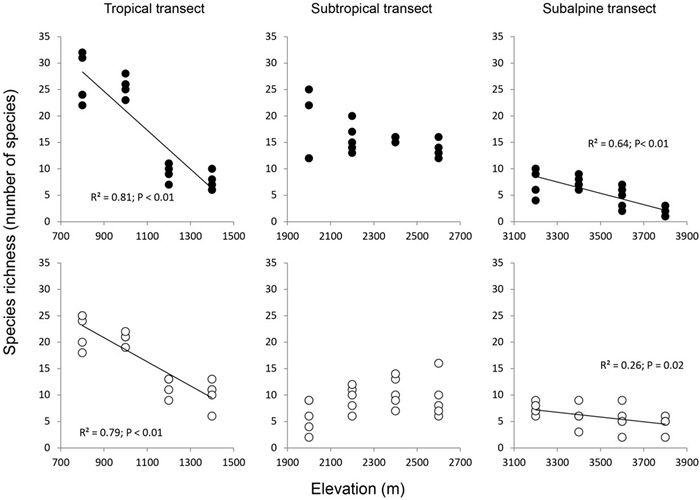
|
| Fig. 4 Species richness of adult trees (closed circles) and seedlings (open circles) at each elevation in three elevational transects. Lines indicate significant correlations with elevation. |
NMDS ordination and PERMANOVA analysis revealed clear and significant (P < 0.01) elevational differences in species composition for both adult trees and seedlings in all transects (Fig. 5). In the tropical transect, ordinations showed a notable separation between the 'low' elevations (800 and 1000 m) and the 'high' elevations (1200 and 1400 m). In the subtropical transect, although there was no significant difference in species diversity (Table S7), species composition was more similar between the two higher elevations (2400 m and 2600 m) than other paired elevations for both adult trees and seedlings (Fig. 5c and d). For both adult trees and seedlings in the subalpine transect, species composition showed continuous change from low to high elevations, with the number of species decreasing with elevation (Tables S5 and S6).
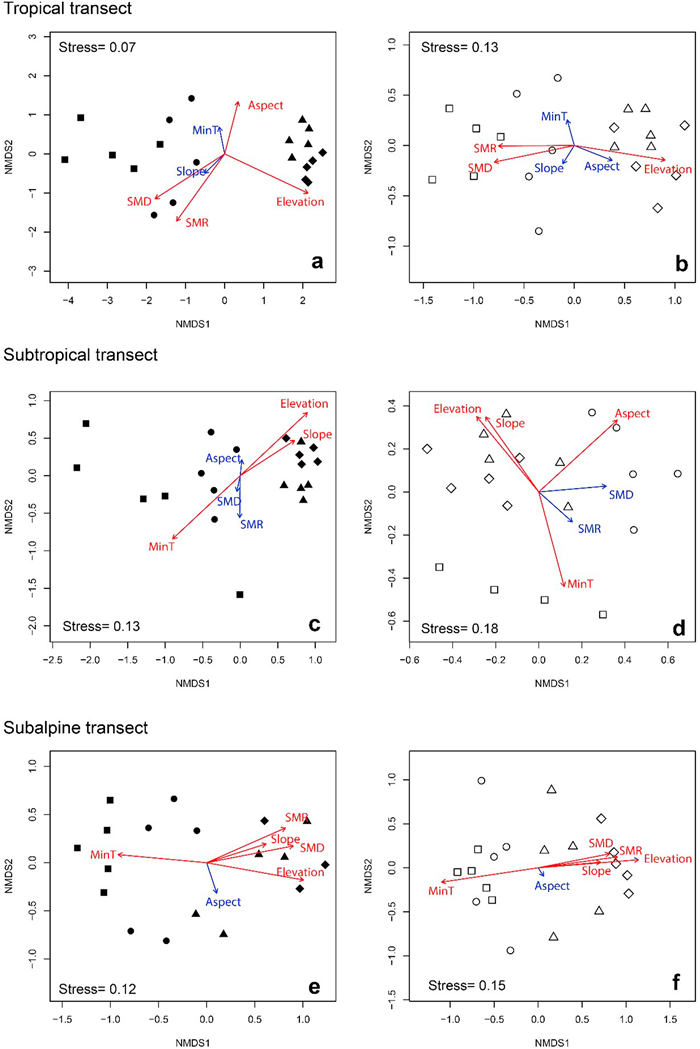
|
| Fig. 5 NMDS ordinations based of Bray–Curtis index similarity matrices of adult (left) and seedling (right) assemblages across the three elevational transects. Different elevational belts are represented by the following symbols: tropical transect, ■= 800 m, ●= 1000 m, ▲= 1200 m, ♦= 1400 m; subtropical transect, ■= 2000 m, ●= 2200 m, ▲= 2400 m, ♦= 2600 m; subalpine transect, ■= 3200 m, ●= 3400 m, ▲= 3600 m, ♦= 3800 m. The symbols are the same for both adults and seedlings. Environmental variables include elevation, slope, aspect, soil moisture in the dry season (SMD), soil moisture in the rainy season (SMR), and minimum monthly temperature (MinT). |
Elevation was one of the main environmental variables for explaining the variation of species composition for adult trees and for seedlings (Fig. 5). For the tropical transect, the variation in species composition of both adult trees and seedlings was better explained by soil moisture (Fig. 5a and b). In the subtropical transect, minimum monthly temperature was better than other environmental variables in explaining the variation of species composition of both adult trees and seedlings (Fig. 5c and d). In the subalpine transect, minimum monthly temperature and soil moisture both showed significant effects in explaining the elevational variation of species composition of adult trees and seedlings (Fig. 5e and f).
3.3. Differences in species distribution patterns between adults and seedlingsIn the tropical transect, the Bray–Curtis similarity between adult trees and seedlings decreased with elevation. In the subtropical transect, the similarity did not show significant correlation with elevation. For the subalpine transect, the similarity between adult trees and seedlings increased with elevation (Fig. 6).

|
| Fig. 6 The similarity in species composition (Bray–Curtis index) between adult trees and seedlings along elevations for each of the three transects. Lines indicate significant correlations between composition of adult trees and seedlings. |
We found that dominant adult tree species were not necessarily as common as seedlings at some elevations (Table 1). In the tropical and subtropical forests, many species of Fagaceae were abundant as adults but very rare as seedlings (e.g., Lithocarpus truncatus (King ex J.D. Hooker) Rehder et E. H. Wilson at 1200 m and 1400 m, Claoxylon khasianum Hook. F at 2000 m, Lithocarpus hancei (Bentham) Rehd. at 2200 m, Lithocarpus xylocarpus (Kurz) Markgraf at 2400 m and Castanopsis wattii (King ex J.D. Hooker) A. Camus at 2600 m). In subalpine forests, Abies georgei Orr was abundant as adult trees across elevations, but seedlings were rare or absent at a low elevation (3400 m) (Table 1). In contrast, Abies forrestii C.C. Rogers had a high number of seedlings at the 3600 m, even though at this elevation its adult trees were not abundant. Nevertheless, some species were very abundant at both the adult and seedling stage (e.g., Pittosporopsis kerrii Craib at 800 m and A. georgei at 3600 m and 3800 m).
| Tropical transect | |||||||||||
| 800m | 1000m | 1200m | 1400m | ||||||||
| Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | ||||
| Garcinia cowa | 3.4 | 1.5 | |||||||||
| Mezzettiopsis creaghii | 6.8 | 2.3 | |||||||||
| Parashorea chinensis | 14.4 | 8 | |||||||||
| Pittosporopsis kerrii | 16.4 | 19 | 8.8 | 4.7 | |||||||
| Baccaurea ramiflora | 4.8 | 0.8 | 4.4 | 0 | |||||||
| Metadina trichotoma | 5.1 | 0 | |||||||||
| Actinodaphne henryi | 4.8 | 1.6 | |||||||||
| Aporusa yunnanensis | 11.8 | 2.8 | 22.5 | 4.0 | |||||||
| Castanopsis echidnocarpa | 18.4 | 64.9 | 8.4 | 19.7 | |||||||
| Castanopsis mekongensis | 10.7 | 0 | 36.4 | 0.5 | |||||||
| Lithocarpus truncatus | 13.9 | 0 | 25.2 | 0 | |||||||
| Wendlandia tinctoria | 8.6 | 0 | |||||||||
| Schima argentea | 10.7 | 0 | |||||||||
| Lithocarpus vestitus | 6.4 | 0 | |||||||||
| Subtropical transect | |||||||||||
| 2000m | 2200m | 2400m | 2600m | ||||||||
| Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | ||||
| Ficus henryi | 3.1 | 0 | |||||||||
| Melia toosendan | 6.1 | 0 | |||||||||
| Lithocarpus truncatus | 6.6 | 5.4 | |||||||||
| Claoxylon khasianum | 22.4 | 0 | |||||||||
| Manglietia insignis | 10.7 | 0 | 7.3 | 0 | |||||||
| Lithocarpus hancei | 12.6 | 0.7 | |||||||||
| Camellia assamica | 11.5 | 8.1 | |||||||||
| Michelia floribunda | 6.9 | 0 | |||||||||
| Rhododendron leptothrium | 6.9 | 0 | 18.6 | 0 | 10 | 0 | |||||
| Lithocarpus xylocarpus | 18 | 0.7 | |||||||||
| Eurya quinquelocularis | 13.6 | 0 | |||||||||
| Eurya paratetragonoclada | 7.3 | 0 | |||||||||
| Vaccinium duclouxii | 5.7 | 2.1 | |||||||||
| Castanopsis wattii | 25.8 | 0 | |||||||||
| Camellia forrestii | 10 | 4.7 | |||||||||
| Eriobotrya bengalensis | 7.9 | 7.9 | |||||||||
| Schima noronhae | 5.8 | 0 | |||||||||
| Subalpine transect | |||||||||||
| 3200m | 3400m | 3600m | 3800m | ||||||||
| Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | ||||
| Rhododendron yunnanense | 8.1 | 0.4 | |||||||||
| Acanthopanax evodiaefolius | 20.1 | 0 | |||||||||
| Sorbus rufopilosa | 17.4 | 0 | 10.5 | 0 | |||||||
| Abies forrestii | 32.2 | 2.7 | 7 | 49.2 | 3.6 | 0 | |||||
| Quercus pannosa | 5.4 | 3.5 | 19.2 | 1.6 | 4.2 | 0.5 | |||||
| Rhododendron siderophyllum | 29.1 | 0 | |||||||||
| Abies georgei | 16.9 | 0 | 69.7 | 82.2 | 95.3 | 79.6 | |||||
| Rhododendron vernicosum | 6.7 | 0.5 | |||||||||
| Larix potaninii var. macrocarpa | 6.7 | 0 | |||||||||
| Lonicera tangutica | 2.3 | 0 | |||||||||
| Salix delavayana | 1.6 | 0 | |||||||||
| Acer forrestii | 0.8 | 0 | |||||||||
The proportions of seedling and adult tree species that were restricted to only one elevation decreased from the tropical (adult tree, 72.97%; seedling, 66.44%) to subtropical (adult tree, 55.84%; seedling, 54.72%) to subalpine (adult tree, 47.06%; seedling, 40.00%) transects (Table 2). Only a small proportion of species was distributed at three or more elevational belts, and the proportion increased from tropical (adult tree, 2.70%; seedling, 4.03%) to subtropical (adult tree, 23.38%; seedling, 18.87%) to subalpine (adult tree, 23.53%; seedling, 26.67%) transects.
| Tropical transect | Subtropical transect | Subalpine transect | ||||||
| Adults | Seedlings | Adults | Seedlings | Adults | Seedlings | |||
| Elevational belt 1 | 72.97% | 66.44% | 55.84% | 54.72% | 47.06% | 40.00% | ||
| Elevational belt 2 | 24.32% | 29.53% | 20.78% | 26.42% | 29.41% | 33.33% | ||
| Elevational belt 3 | 2.03% | 3.36% | 14.29% | 15.09% | 23.53% | 20.00% | ||
| Elevational belt 4 | 0.68% | 0.67% | 9.09% | 3.77% | 0.00% | 6.67% | ||
Our study suggests that the elevational distribution of tree species is significantly affected by soil moisture at lower elevations and by air temperature at higher elevations. Typically, the distribution of plant species tracks changes in water availability along elevational gradients (Crimmins et al., 2011). Studies in the Himalayas (Bhattarai and Vetaas, 2006) and Hengduan Mountains (Liu et al., 2007) also suggest that water availability is a key factor affecting patterns of species richness of woody plants. The dominant role of soil moisture regimes in defining plant diversity distribution has been highlighted by a few studies in tropical forests (Givnish, 1999; Engelbrecht et al., 2007; Bonetti et al., 2017). Previous studies have reported that the spatial distributions of tree species in seasonal tropical forests, especially in the dry season, are largely shaped by water availability (Engelbrecht et al., 2007; Comita and Engelbrecht, 2009; Esquivel-Muelbert et al., 2017). In the tropical forests of Hainan Island, rainfall is a limiting factor for plant dynamics across large-scale elevational gradients (Ali et al., 2019), especially for tree seedlings in the understory, which are more sensitive to water availability in the dry season than in the rainy season (Comita and Engelbrecht, 2009). In the tropical seasonal rain forest surveyed in this study, on the other hand, fog drip contributes around 5% of the annual precipitation, with 86% of fog drip occurring in the dry season. Fog drip to the forest floor accounts for 33–49% of the total precipitation in the dry season, providing significant amounts of moisture during the dry months (Liu et al., 2004, 2008).
In our tropical transect, species diversity decreased during the dry season in parallel with decreasing soil moisture across elevations (Fig. 2). The ordinations also showed that species compositions for both adult trees and seedlings were closely related to soil moisture in the tropical transect (Figs. 5a and 5b). Air temperatures from January through March, however, were much lower at 800 m than at high elevations (1000, 1200 and 1400 m) (Fig. 3). This so-called temperature inversion in mountainous areas has been observed in other studies (Jiang, 1981; Liu et al., 2016). The inconsistent temperature pattern through the year in the tropical transect may be the reason that temperature does not appear to be a strong driver of patterns of elevational diversity (Fig. 3).
For subtropical and subalpine transects, NMDS ordinations indicated that species distribution of both adult tress and seedlings was most strongly affected by air temperature. Previous studies in these regions have implied that tree species at higher elevations prefer cool and wet habitats (Wu et al., 1987), whereas those at lower elevations are more drought tolerant (Jiang, 1980). According to Li et al., 2016a, Li et al., 2016b the climatic variable that overwhelmingly controls the distribution of temperate coniferous forests (i.e., subalpine coniferous forest in the present study) in Yunnan is the minimum temperature of the coldest month.
Besides climate, many other abiotic factors have been reported to affect tree species composition, such as topography and soil characters (Carpenter, 2005; Irl et al., 2015; Martinez-Camilo et al., 2018). In mountain ecosystems of central Europe, elevation and valley depth have been shown to be the most important predictors of tree species distribution. Geomorphometric variability has been found to influence patterns of species occurrence and biomass, contributing to higher species diversity (Dyderski and Pawlik, 2020). Consistent with these findings, the elevational species composition in the subalpine transect was significantly affected by slope and aspect, in addition to the minimum monthly temperature (Fig. 5).
4.2. Species diversity patterns along elevationsTree species richness, the Shannon index and the Simpson index all decreased significantly with increasing elevation in tropical and subalpine forests for both adult trees and seedlings (Table S7 and Fig. 4). A similar trend has been reported in studies in other tropical forests in Malesia (Van Steenis, 1984), the central Bolivian Andes (Kessler, 2000), in Mt. Kilimanjaro (Hemp, 2006), the Cordillera Central (Martin et al., 2011), and in Sulawesi (Brambach et al., 2017). The elevational diversity patterns we observed for the subtropical transect differed from those of subtropical forests in eastern Nepal, which showed hump-shaped patterns for shrub and tree species richness along a more extensive and continuous elevational gradient (100–4300 m) (Bhattarai and Vetaas, 2003). Hump-shaped species diversity patterns have been found in other subalpine regions in Yunnan, such as in Yulong Snow Mountain (2900–4200 m; Feng et al., 2006), Meili Snow Mountain (2100–5100 m; Feng, 2013) and Haba Snow Mountain (1600–4300 m; Tao et al., 2011). These studies all showed that tree species diversity was highest between 3300 and 3800 m. However, those studies also pointed out that the lower tree species richness at low elevations could be a consequence of human disturbance (Feng et al., 2006, 2013; Tao et al., 2011). These findings suggest that our subalpine transect shows a more natural biological pattern than those of previous studies.
4.3. Inconsistent abundance of adult trees and their seedlingsThe composition and relative abundance of adult tree species differed from that of seedling species in the three transects (Table 2 and Fig. 6). This discrepancy between adult tree and seedling species may have several explanations. For instance, the understory environmental conditions (e.g., light availability and soil properties) may limit seedling establishment of canopy species (Yavitt and Wright, 2008; Rueger et al., 2009; Myster, 2012). Strong ecological filtering or competition may occur during the transition from seedlings to adults (Calcagno et al., 2006; Baldeck et al., 2013; Wright et al., 2014), which affects the demographic dynamics of seedling recruitment, and hence the instability in seedling composition over time. Conspecific negative density dependence (Janzen, 1970; Connell, 1971; Comita et al., 2014) and mast seeding (Pearse et al., 2016) may also be responsible for the absence of conspecific seedlings in the forest understory.
We found that adult tree and seedling composition was similar in transects with high moisture (Fig. 4, Fig. 6). Soil moisture has been shown to play a key role in the seedling establishment of tree species (Comita and Engelbrecht, 2009; Gong et al., 2011). As a result, the relatively high soil moisture at certain elevations may allow more seedlings of tree species to survive in the understory, while the relatively low soil moisture at other elevations may limit the recruitment of many tree species.
The different composition of adult trees and seedlings, on the other hand, may also be an indicator of future changes in forest vegetation type (Pérez-Ramos and Marañón, 2012). Although the composition of canopy tree species may remain stable for an extended period, the seedlings in the understory may already exhibit changes in response to climate change or other human disturbances (Lenoir et al., 2009; Lloret et al., 2009). Dang et al. (2013) suggested that low recruitment rates of a subalpine fir species (Abies fargesii) at low and middle elevations is due to the impacts of climatic warming in central China. In the subalpine transects of our study, the relative abundance of the seedlings of A. georgei was lower at low elevations than at high elevations (Table 1). This could be partially attributed to relatively higher air temperature at lower elevations in Yulong Mountain (Xin et al., 2013).
4.4. Implications for climate changeIn the present study, species composition showed considerable variability across elevations in all transects (Fig. 5), and most tree species were restricted to a narrow zone at lower elevations (Table 2). Southwestern China is predicted to be drier and hotter in the future (Fan et al., 2011; Fan and Thomas, 2013); therefore, those species unable to shift their elevational ranges to remain in suitable climates face the greatest risk of extinction (Corlett and Westcott, 2013). Moreover, compared with the subtropical and subalpine transects, species in the tropical transect had a greater number of species restricted to a narrow zone and might thus face a larger proportion of biodiversity loss (Table 2).
In the tropical transect, our results suggest that the low soil moisture at high elevations in the late dry season, rather than air temperature, may limit the upward migration of low-elevation tree species. Species at low elevations are likely to be more drought sensitive than those at high elevations (Song et al., 2016a). Accordingly, under the local dry-hot trend scenario (He and Zhang, 2005; Fan et al., 2011; Fan and Thomas, 2013), while drought-sensitive tree species at low elevations may have "retracted" elevational distributions or may become locally extinct, drought-tolerant species at high elevations may expand their distribution to lower elevations (Ledo et al., 2009; Crimmins et al., 2011). In the future, the tropical seasonal rainforest at low elevations may be replaced by mountain evergreen broad-leaved forest originating from higher elevations (Song et al., 2016a).
In subalpine forests, local climate warming and drought may favor the hot- and drought-tolerant tree species at lower elevations, expanding their distributions to high elevations. At high elevations, the distribution of tree species that do not tolerate dry and hot environmental conditions may progressively shrink to higher elevations. In our study, Abies forrestii trees are very abundant at 3200 m, but at 3400 m A. forrestii seedlings are most abundant. In contrast, A. georgei trees outnumber A. forrestii trees at 3400 but no seedlings were found at that elevation, which suggests that A. forrestii is moving upward and potentially replacing A. georgei at lower elevations. This suggests that subalpine species in general will move upward in response to climate change and alpine species may face local extinction. Thus, species at high elevation may be confronted with a high extinction risk due to habitat loss and upward expansion of low elevation species as a consequence of local climate warming.
5. ConclusionIn this study, we found that the relative effects of air temperature and soil moisture on elevational distribution of adult trees and their seedlings varied across tropical to subtropical to subalpine zones. Soil moisture has the largest impact on tropical species distribution, whereas air temperature plays the biggest role in determining subalpine species distribution. Our results suggest that when assessing the impact of climate change on tropical forests, we should not only consider increases in temperature, but more importantly, increases in drought conditions.
、Author's contributionsX.S., R.L.K. and M.C. designed the study, X.S. performed analyses, X.S., J.L., A.N., M.J.L., Y.T., Z.S. and W.Z. collected data, X.S, M.C. and J.Y. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
、Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled "Different environmental factors drive tree species diversity along elevation gradients in three climatic zones in Yunnan, southern China".
、AcknowledgementsThis research was supported by the National Natural Science Foundation of China (31800353 and 32061123003), the West Light Foundation of the Chinese Academy of Sciences, the Applied Fundamental Research Foundation of Yunnan Province (2019FB038, 2014GA003 and 2013FB079), the National Key Basic Research Program of China (2014CB954100) and the Queensland–Chinese Academy of Sciences Biotechnology Fund (GJHZ1130). We are grateful for the field assistance from Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies (XSTRES), Ailaoshan Station for Subtropical Forest Ecosystem Studies (ASSTRES), Lijiang Forest Ecosystem Research Station and the Queensland Herbarium, Australia.
、Appendix A. Supplementary dataSupplementary data to this article can be found online at https://doi.org/10.1016/j.pld.2021.04.006.
Ali, A., Lin, S.L., He, J.K., et al, 2019. Climatic water availability is the main limiting factor of biotic attributes across large–scale elevational gradients in tropical forests. Sci. Total Environ., 647: 1211-1221. DOI:10.1016/j.scitotenv.2018.08.072 |
Anderson, M.J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecol., 26: 32-46. |
Bace, R., Svoboda, M., Pouska, V., et al, 2012. Natural regeneration in Central–European subalpine spruce forests: which logs are suitable for seedling recruitment?. For. Ecol. Manag., 266: 254-262. DOI:10.1016/j.foreco.2011.11.025 |
Baldeck, C.A., Harms, K.E., Yavitt, J.B., et al, 2013. Habitat filtering across tree life stages in tropical forest communities. Proc. R. Soc. B, 280: 20130548. DOI:10.1098/rspb.2013.0548 |
Bhattarai, K.R., Vetaas, O.R., 2003. Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Global Ecol. Biogeogr., 12: 327-340. DOI:10.1046/j.1466-822X.2003.00044.x |
Bhattarai, K.R., Vetaas, O.R., 2006. Can Rapoport's rule explain tree species richness along the Himalayan elevation gradient, Nepal?. Divers. Distr., 12: 373-378. DOI:10.1111/j.1366-9516.2006.00244.x |
Bonetti, S., Feng, X., Porporato, A., 2017. Ecohydrological controls on plant diversity in tropical South America. Ecohydrology, 10: e1853. |
Box, E., 1995. Factors determining distribution of tree species and plant functional types. Vegetatio, 121: 101-116. DOI:10.1007/BF00044676 |
Brambach, F., Leuschner, C., Tjoa, A., et al, 2017. Diversity, endemism, and composition of tropical mountain forest communities in Sulawesi, Indonesia, in relation to elevation and soil properties. Perspect. Plant Ecol. Evol. Syst., 27: 68-79. DOI:10.1016/j.ppees.2017.06.003 |
Bray, J.R., Curtis, J.T., 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr., 27: 325-349. DOI:10.2307/1942268 |
Calcagno, V., Mouquet, N., Jarne, P., et al, 2006. Coexistence in a metacommunity: the competition–colonization trade–off is not dead. Ecol. Lett., 9: 897-907. DOI:10.1111/j.1461-0248.2006.00930.x |
Carpenter, C., 2005. The environmental control of plant species density on a Himalayan elevation gradient. J. Biogeogr., 32: 999-1018. DOI:10.1111/j.1365-2699.2005.01249.x |
Chao, A., Chiu, C.H., , Jost, L., 2014. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Ann. Rev. Ecol. Evol. Syst., 45: 297-324. DOI:10.1146/annurev-ecolsys-120213-091540 |
Colwell, R.K., Brehm, G., Cardelus, C.L., et al, 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science, 322: 258-261. DOI:10.1126/science.1162547 |
Comita, L.S., Aguilar, S., Perez, R., et al, 2007. Patterns of woody plant species abundance and diversity in the seedling layer of a tropical forest. J. Veg. Sci., 18: 163-174. DOI:10.1658/1100-9233(2007)18[163:POWPSA]2.0.CO;2 |
Comita, L.S., Engelbrecht, B.M.J., 2009. Seasonal and spatial variation in water availability drive habitat associations in a tropical forest. Ecology, 90: 2755-2765. DOI:10.1890/08-1482.1 |
Comita, L.S., Queenborough, S.A., Murphy, S.J., et al, 2014. Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol., 102: 845-856. DOI:10.1111/1365-2745.12232 |
Connell, J., 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees, dynamics of population. In: Den Boer, P.J., Gradwell, G.R. (Eds.), Dynamics of Populations. Centre for Agricultural Publishing and Documentation Wageningen, The Netherlands, pp. 298e312.
|
Conover, W.J., 1999. Practical nonparametric statistics. third ed. New York: Wiley: pp. 250-257.
|
Corlett, R.T., Westcott, D.A., 2013. Will plant movements keep up with climate change?. Trends Ecol. Evol., 28: 482-488. DOI:10.1016/j.tree.2013.04.003 |
Crimmins, S.M., Dobrowski, S.Z., Greenberg, J.A., et al, 2011. Changes in climatic water balance drive downhill shifts in plant species' optimum elevations. Science, 331: 324-327. DOI:10.1126/science.1199040 |
Cuesta, F., Muriel, P., Llambí, L.D., et al, 2017. Latitudinal and altitudinal patterns of plant community diversity on mountain summits across the tropical Andes. Ecography, 40: 1381-1394. DOI:10.1111/ecog.02567 |
Dang, H.S., Zhang, K.R., Zhang, Y.J., et al, 2013. Regeneration dynamics of subalpine fir (Abies fargesii) forest across the altitudinal range in the Shennongjia Mountains, central China. J. Plant Ecol., 6: 36-47. DOI:10.1093/jpe/rts030 |
De Mendiburu, F., 2017. Agricolae: Statistical Procedures for Agricultural Research. R package, pp. 2-8 version 1. https://CRAN.R-fproject.org/package=agricolae.
|
Dyderski, M.K., Pawlik, L., 2020. Spatial distribution of tree species in mountain national parks depends on geomorphology and climate. For. Ecol. Manag., 474: 118366. DOI:10.1016/j.foreco.2020.118366 |
Engelbrecht, B.M.J., Comita, L.S., Condit, R., et al, 2007. Drought sensitivity shapes species distribution patterns in tropical forests. Nature, 447: 80-82. DOI:10.1038/nature05747 |
Esquivel-Muelbert, A., Baker, T.R., Dexter, K.G., et al, 2017. Seasonal drought limits tree species across the Neotropics. Ecography, 40: 618-629. DOI:10.1111/ecog.01904 |
Fan, Z.X., Braeuning, A., Thomas, A., et al, 2011. Spatial and temporal temperature trends on the Yunnan plateau (southwest China) during 1961–2004. Int. J. Climatol., 31: 2078-2090. DOI:10.1002/joc.2214 |
Fan, Z.X., Thomas, A., 2013. Spatiotemporal variability of reference evapotranspiration and its contributing climatic factors in Yunnan Province, SW China, 1961–2004. Climatic Change, 116: 309-325. DOI:10.1007/s10584-012-0479-4 |
Feng, X., 2013. Altitudinal Pattern of Plant Species Richness in Meili Snow Mountain and a Test of Rapopert's Rule. Master’s Thesis, Yunnan University (In Chinese with English abstract).
|
Feng, J., Wang, X., Xu, C., et al, 2006. Altitudinal patterns of plant species diversity and community structure on Yulong Mountains, Yunnan, China. J. Mount. Sci., 24: 110-116. |
Givnish, T.J., 1999. On the causes of gradients in tropical tree diversity. J. Ecol., 87: 193-210. DOI:10.1046/j.1365-2745.1999.00333.x |
Gong, H., Yang, G., Lu, Z., et al, 2011. Composition and spatio–temporal distribution of tree seedlings in an evergreen broad–leaved forest in the Ailao Mountains, Yunnan. Biodivers. Sci., 19: 151-157. DOI:10.3724/SP.J.1003.2011.07010 |
Greig-Smith, P., 1983. Quantitative Plant Ecology. 3rd edition. Oxford: Blackwell Scientific Publications.
|
He, Y., Zhang, Y., 2005. Climate change from 1960 to 2000 in the Lancang river valley, China. Mt. Res. Dev., 25: 341-348. DOI:10.1360/03ys0295 |
Hemp, A., 2006. Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol., 184: 27-42. DOI:10.1007/s11258-005-9049-4 |
Homeier, J., Breckle, S.W., Günter, S., et al, 2010. Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich Ecuadorian montane rain forest. Biotropica, 42: 140-148. DOI:10.1111/j.1744-7429.2009.00547.x |
Hubbell, S.P., Foster, R.B., O'Brien, S.T., et al, 1999. Light–gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science, 283: 554-557. DOI:10.1126/science.283.5401.554 |
Irl, S.D.H., Harter, D.E.V., Steinbauer, M.J., et al, 2015. Climate vs. topography – spatial patterns of plant species diversity and endemism on a high-elevation island. J. Ecol., 103: 1621-1633. DOI:10.1111/1365-2745.12463 |
Janzen, D.H., 1970. Herbivores and the number of tree species in tropical forests. Am. Nat., 104: 501-528. DOI:10.1086/282687 |
Jiang, A., 1981. Temperature inversion and vegetation inversion in Xishuangbanna, southwestern Yunnan, People's Republic of China. Mt. Res. Dev., 1: 275-280. DOI:10.2307/3673065 |
Jiang, H., 1980. Distributional features and zonal regularity of vegetation in Yunnan. Acta Bot. Yunnanica, 2: 26-32. |
Kessler, M., 2000. Elevational gradients in species richness and endemism of selected plant groups in the central Bolivian Andes. Plant Ecol., 149: 181-193. DOI:10.1023/A:1026500710274 |
Körner, C., 2007. The use of 'altitude' in ecological research. Trends Ecol. Evol., 22: 569-574. DOI:10.1016/j.tree.2007.09.006 |
Laidlaw, M., McDonald, W., Hunter, R.J., et al, 2011. The potential impacts of climate change on Australian subtropical rainforest. Aust. J. Bot., 59: 440-449. DOI:10.1071/BT10319 |
Ledo, A., Montes, F., Condes, S., 2009. Species dynamics in a montane cloud forest: identifying factors involved in changes in tree diversity and functional characteristics. For. Ecol. Manag., 258: S75-S84. DOI:10.1016/j.foreco.2009.07.055 |
Lenoir, J., Gegout, J.C., Pierrat, J.C., et al, 2009. Differences between tree species seedling and adult altitudinal distribution in mountain forests during the recent warm period 1986–2006. Ecography, 32: 765-777. DOI:10.1111/j.1600-0587.2009.05791.x |
Li, J.Q., Song, X.Y., Cao, M., 2016. Response of tree seedlings to altitudinal gradient and its seasonal variation in ailao mountain and Yulong mountain, Yunnan province, China. Chin. J. Appl. Ecol., 27: 3403-3412. |
Li, R., Kraft, N.J.B., Yang, J., et al, 2015. A phylogenetically informed delineation of floristic regions within a biodiversity hotspot in Yunnan, China. Sci. Rep., 5: 9396. DOI:10.1038/srep09396 |
Li, W., Peng, M., Higa, M., et al, 2016. Effects of climate change on potential habitats of the cold temperate coniferous forest in Yunnan province, southwestern China. J. Mt. Sci., 13: 1411-1422. DOI:10.1007/s11629-016-3846-1 |
Li, X.S., Liu, W.Y., Tang, C.Q., 2010. The role of the soil seed and seedling bank in the regeneration of diverse plant communities in the subtropical Ailao Mountains, Southwest China. Ecol. Res., 25: 1171-1182. DOI:10.1007/s11284-010-0742-y |
Li, X.W., Walker, D., 1986. The plant geography of Yunnan Province, southwest China. J. Biogeogr., 13: 367-397. DOI:10.2307/2844964 |
Liu, M., Ye, J., Wen, B., 2016. Seed germination and seedling survival of Baccaurea ramiflora and Saprosma ternata in Xishuangbanna tropical rainforests under habitat heterogeneity. Acta Bot. Boreali Occident. Sin., 36: 1654-1661. |
Liu, W.J., Meng, F.R., Zhang, Y.P., et al, 2004. Water input from fog drip in the tropical seasonal rainforest of Xishuangbanna, Southwest China. J. Trop. Ecol., 20: 517-524. DOI:10.1017/S0266467404001890 |
Liu, W.J., Wang, P.Y., Liu, W.Y., et al, 2008. The importance of radiation fog in the tropical rain forest of Xishuangbanna, SW China. Hydrol. Res., 39: 79-87. DOI:10.2166/nh.2008.031 |
Liu, Y., Zhang, Y., He, D., et al, 2007. Climatic control of plant species richness along elevation gradients in the longitudinal range–gorge region. Chin. Sci. Bull., 52: 50-58. DOI:10.1007/s11434-007-7006-4 |
Lloret, F., Penuelas, J., Prieto, P., et al, 2009. Plant community changes induced by experimental climate change: seedling and adult species composition. Perspect. Plant Ecol. Evol. Systemat., 11: 53-63. DOI:10.1016/j.ppees.2008.09.001 |
Lomolino, M.V., 2001. Elevation gradients of species–density: historical and prospective views Global Ecol. Biogeogr., 10: 3-13. DOI:10.1046/j.1466-822x.2001.00229.x |
Magurran, A.E., 1988. Ecological diversity and its measurement. Springer.
|
Malizia, A., Blundo, C., Carilla, J., et al, 2020. Elevation and latitude drives structure and tree species composition in Andean forests: results from a large–scale plot network. PLoS One, 15: e0231553. DOI:10.1371/journal.pone.0231553 |
Marryanna, L., Rahman, A.K., Aisah, S.S., et al, 2012. Association between soil moisture gradient and tree distribution in lowland dipterocarp forest at Pasoh, Malaysia. Malaysian J. Soil Study, 16: 23-42. |
Martin, P.H., Fahey, T.J., Sherman, R.E., 2011. Vegetation zonation in a neotropical montane forest: environment, disturbance and ecotones. Biotropica, 43: 533-543. DOI:10.1111/j.1744-7429.2010.00735.x |
Martinez-Camilo, R., Gonzalez-Espinosa, M., Ramirez-Marcial, N., et al, 2018. Tropical tree species diversity in a mountain system in southern Mexico: local and regional patterns and determinant factors. Biotropica, 50: 499-509. DOI:10.1111/btp.12535 |
McArdle, B.H., Anderson, M.J., 2001. Fitting multivariate models to community data: a comment on distance–based redundancy analysis. Ecology, 82: 290-297. DOI:10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2 |
McCain, C.M., Grytnes, J.A., 2010. Elevational gradients in species richness. In: Encyclopedia of Life Sciences (ELS). John Wiley, Sons, Ltd, Chichester.
|
Minchin, P.R., 1987. An evaluation of the relative robustness of techniques for ecological ordination. In: Prentice, I.C., van der Maarel, E. (Eds.), Theory and Models in Vegetation Science. Springer, Germany, pp. 89e107.
|
Myster, R.W., 2012. Spatial and temporal heterogeneity of light and soil water along a terra firme transect in Amazonian Ecuador: effects on tree seedling survivorship, growth, and allocation. Can. J. Res., 42: 203-206. DOI:10.1139/x11-168 |
Oksanen, J., et al., 2018. Vegan: Community Ecology Package. R package version 2.5-2. https://CRAN.R-project.org/package=vegan.
|
Ohsawa, M., 1995. Latitudinal comparison of altitudinal changes in forest structure, leaf–type, and species richness in humid monsoon Asia. Vegetatio, 121: 3-10. DOI:10.1007/BF00044667 |
Pausas, J.G., Austin, M.P., 2001. Patterns of plant species richness in relation to different environments: an appraisal. J. Veg. Sci., 12: 153-166. DOI:10.2307/3236601 |
Pearse, I.S., Koenig, W.D., Kelly, D., 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol., 212: 546-562. DOI:10.1111/nph.14114 |
Perez–Ramos, I.M., Maranon, T., 2012. Community–level seedling dynamics in Mediterranean forests: uncoupling between the canopy and the seedling layers. J. Veg. Sci., 23: 526-540. DOI:10.1111/j.1654-1103.2011.01365.x |
Rabasa, S.G., Granda, E., Benavides, R., et al, 2013. Disparity in elevational shifts of European trees in response to recent climate warming. Global Change Biol., 19: 2490-2499. DOI:10.1111/gcb.12220 |
R Core Team, 2018. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
|
Rueger, N., Huth, A., Hubbell, S.P., et al, 2009. Response of recruitment to light availability across a tropical lowland rain forest community. J. Ecol., 97: 1360-1368. DOI:10.1111/j.1365-2745.2009.01552.x |
Salas-Morales, S.H., Meave, J.A., 2012. Elevational patterns in the vascular flora of a highly diverse region in southern Mexico. Plant Ecol., 213: 1209-1220. DOI:10.1007/s11258-012-0077-6 |
Sarvade, S., Gupta, B., Singh, M., 2016. Composition, diversity and distribution of tree species in response to changing soil properties with increasing distance from water source – a case study of Gobind Sager Reservoir in India. J. Mt. Sci., 13: 522-533. DOI:10.1007/s11629-015-3493-y |
Shen, Z., Fei, S., Feng, J., et al, 2012. Geographical patterns of community–based tree species richness in Chinese mountain forests: the effects of contemporary climate and regional history. Ecography, 35: 1134-1146. DOI:10.1111/j.1600-0587.2012.00049.x |
Song, X., Hogan, J.A., Brown, C., et al, 2017. Snow damage to the canopy facilitates alien weed invasion in a subtropical montane primary forest in southwestern China. For. Ecol. Manag., 391: 275-282. DOI:10.1016/j.foreco.2017.02.031 |
Song, X., Li, J., Zhang, W., et al, 2016. Variant responses of tree seedling to seasonal drought stress along an elevational transect in tropical montane forests. Sci. Rep., 6: 36438. DOI:10.1038/srep36438 |
Song, X., Nakamura, A., Sun, Z., et al, 2016. Elevational distribution of adult trees and seedlings in a tropical montane transect, Southwest China. Mt. Res. Dev., 36: 342-354. DOI:10.1659/MRD-JOURNAL-D-15-00109.1 |
Stevens, G.C., 1992. The elevational gradient in altitudinal range: an extension of Rapoport's latitudinal rule to altitude. Am. Nat., 140: 893-911. DOI:10.1086/285447 |
Swella, G.B., Ward, P.R., Siddique, K.H.M., et al, 2015. Combinations of tall standing and horizontal residue affect soil water dynamics in rainfed conservation agriculture systems. Soil Till. Res., 147: 30-38. DOI:10.1016/j.still.2014.11.004 |
Tao, J., Zang, R., Yu, C., 2011. Altitudinal pattern of plant communities and species diversity in the Bahaxueshan Mountains, Yunnan, China. Sci. Silvae Sin., 41: 1-6. |
Teketay, D., 1997. Seedling populations and regeneration of woody species in dry Afromontane forests of Ethiopia. For. Ecol. Manag., 98: 149-165. DOI:10.1016/S0378-1127(97)00078-9 |
Tito, R., Vasconcelos, H.L., Feeley, K.J., 2020. Mountain ecosystems as natural laboratories for climate change experiments. Front. For. Glob. Chang., 19: 2490-2499. |
Van Steenis, C.G.G.J., 1984. Floristic altitudinal zones in Malesia. Bot. J. Linn. Soc., 89: 289-292. DOI:10.1111/j.1095-8339.1984.tb02560.x |
Wright, A., Schnitzer, S.A., Reich, P.B., 2014. Living close to your neighbors: the importance of both competition and facilitation in plant communities. Ecology, 95: 2213-2223. DOI:10.1890/13-1855.1 |
Wu, Z., Zhu, Y., Jiang, H., 1987. The Vegetation of Yunnan. Beijing: Science Press.
|
Xin, H., He, Y., Zhang, T., et al, 2013. The features of climate variation and glacier response in Mt. Yulong, Southeastern Tibetan Plateau. Adv. Earth Sci., 28: 1257-1268. |
Yavitt, J.B., Wright, S.J., 2008. Seedling growth responses to water and nutrient augmentation in the understorey of a lowland moist forest, Panama. J. Trop. Ecol., 24: 19-26. DOI:10.1017/S0266467407004713 |
Zhu, H., 2012. Biogeographical divergence of the flora of Yunnan, southwestern China initiated by the uplift of himalaya and extrusion of Indochina block. PLoS One, 7: e45601. DOI:10.1371/journal.pone.0045601 |



