b. Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Lanhei Road 132, Heilongtan, Kunming, Yunnan, 650201, China;
c. State Key Laboratory of Systematic and Evolutionary Botany & Herbarium (PE), Institute of Botany, Chinese Academy of Sciences, Beijing, 100093, China;
d. Southwest Forestry University, Kunming, 650224, China;
e. China Wild Dali Nature Education and Research Center, Dali, Yunnan, 671000, China;
f. Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan, 666303, China
Gastrodieae (Epidendroideae, Orchidaceae) has been characterized primarily on the basis of their obligate mycoheterotrophic metabolism, in which mature plants rely on the presence of endophytic mycorrhizae in the subterranean portions of the plant body to supply their organic carbon needs. This lifestyle is specialized within Orchidaceae, and a number of recent studies have indicated that some phylogenetically unrelated holomycotrophs have acquired similar vegetative and floral morphologies (Molvray et al., 2000). Their morphological similarities have led to assumptions of a close evolutionary relationship, which is in some instances unjustified (Pridgeon et al., 2005). Based on early molecular work using the nuclear 18S ribosomal gene, it appears that tribe Gastrodieae sensu Dressler (1993) is a heterogeneous assemblage of at least three distinct groups that have been afforded tribal rank (Molvray et al., 2000). Meanwhile, Molvray et al. (2000) found a monophyletic 'core' Gastrodieae that consist of Auxopus Schlechter, Didymoplexis Griif, Gastrodia R. Brown, Uleiorchis Hoehne, Didymoplesiella Garay and Neoclemensia Carr. However, at recent phylogenetic study reduced Neoclemensia to synonymy with Gastrodia (Wood et al., 2011). Hence, these five genera constitute tribe Gastrodieae as delimited in these treatments, but the placement of this tribe within the subfamily Epidendroideae is still uncertain (Pridgeon et al., 2005; Chase et al., 2015).
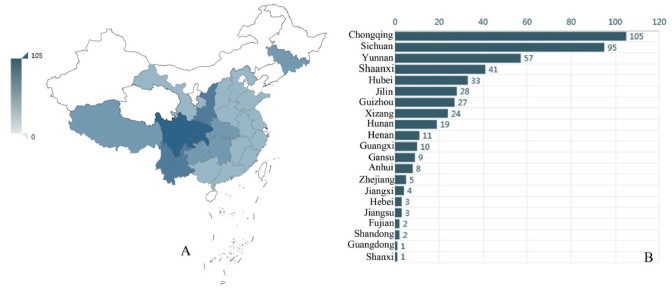
Gastrodia (Epidendroideae, Gastrodieae) is from the Greek gaster, stomach, and -odes, denoting likeness. Sepals and petals in Gastrodia are joined to form a conspicuous floral tube distended basally to accommodate the column foot. The presence of a floral tube and mentum give the flower a stomach-like appearance (Pridgeon et al., 2005). It is a mycoheterotrophic genus, characterized by a fleshy tuber or coralloid underground stem, the absence of leaves, the union of sepals and petals and two mealy pollinia with/without caudicles (Dressler, 1993; Seidenfaden and Wood, 1995; Leou, 2000; Chung and Hsu, 2006). It is composed of approximately 92 species, widespread from northeast India through the eastern Himalayas and southern China to Japan and eastern Siberia, southwards to Malaysia and Australia, eastwards to the Pacific Islands as far as Samoa and westwards to Madagascar, Mascarene Islands and tropical Africa (Pridgeon et al., 2005; Chen et al., 2009; Cribb et al., 2010; Tan et al., 2012; Chase et al., 2015; Hsu et al., 2016; Jin and Kyaw, 2017; Aung and Jin, 2018). There are about 30 species (15 endemic) of Gastrodia in China, mainly distributed in southern China, including Fujian, Hainan, Yunnan, Sichuan, and Taiwan (Chen et al., 2009; Hsu et al., 2012; Tan et al., 2012) (Fig. 1), except Gastrodia elata Bl., which is widespread into northeast China and even Siberia (Chen et al., 2009).
|
| Fig. 1 A and B. The specimen of Gastrodia collected from different provinces in China (Cited from: http://www.cvh.ac.cn/spms/statistic.php?taxonName=Gastrodia). |
Gastrodia species are used as both medicine and food across the Asia Pacific. For example, G. elata has been listed as a medicinal herb in Chinese pharmacopoeias as far back as 100 CE (Lu, 1985). Known as tian ma in Chinese, G. elata is used in traditional Chinese medicine to treat a suite of neurological symptoms, including dizziness and neuralgia. In New Zealand, the rhizomes of Gastrodia cunninghamii are regarded as a delicacy by the Maoris (Harris, 1997), who roast the rhizomes in embers.
In terms of vegetative and floral morphology, Gastrodia is similar to Uleiorchis Hoehne. Both genera have sepals and petals that are almost completely joined into a five-lobed floral tube, a simple labellum, and column with an abbreviated column foot. However, Uleiorchis is distinguished from Gastrodia by having a membranous perianth, a labellum disc that lack basal calli, a V-shaped stigma that is borne some distance above the base of the column, and a receptive surface that is densely papillose throughout (Pridgeon et al., 2005). An infrageneric classification forGastrodia was proposed by Schlechter (1911) and three sections were proposed based on the extent of fusion between the labellum and perianth tube, as well as overall shape of the floral tube.Gastrodia sect. Strogadia Schltr. is the smallest of three sections, and the labellum is closely appressed to the perianth tube, whereas in the other two sections the labellum is free from the perianth tube; G. sect. Gastrodia Schltr. has a floral tube that is either cylindrical or apically narrowed, whereas G. sect. Codonathus Schltr. is characterized by a perianth tube distinctly widened toward the apex (Schlechter, 1911).
During our field investigations in northeastern and southeastern Yunnan, China, in August and September in 2019 and 2020, one unusual species of Gastrodia was discovered. After a comprehensive literature and herbarium reviewing, we identified it as a new species belonging to Gastrodia sect. Strogadia.
2. Materials and methodsMorphological observations of the new species were based on living plants (five individuals) and dried herbarium specimens (kept in the herbaria of HFTC, KUN and HITBC). All morphological characters were measured using vernier calipers. Fresh material and specimens were identified by checking Flora of China (Chen et al., 2009) and recently published literature (Li and Liu, 2007; Hu et al., 2014).
Total genomic DNA was extracted from fresh plant material using a modified CTAB method (Li et al., 2013). DNA libraries for sequencing were prepared according to the manufacturer's protocol. Paired-end sequencing (100-bp read lengths) was performed on an Illumina HiSeq 2500 platform at Majorbio in Beijing, which generated 5 GB of raw data. We assembled the plastome according to previously described methods (Feng et al., 2016; Li et al., 2020a, Li et al., 2020b). The filtered paired reads were mapped to the plastome ofG. elata (GenBank accession number: MF163256) using Geneious v.10.1.2. The plastome was assembled de novo using VELVET (Zerbino and Birney 2008) with Kmer values from 37 to 45. We merged contigs from consensus sequences into scaffolds using Geneious with default parameters. Scaffolds were extended by mapping reads and repeated to obtain the draft plastomeusing Geneious. IR boundaries were confirmed by BLAST and the reverse IR region was manually added to complete the plastome. The completed plastome was annotated using PGA (Qu et al., 2019).
3. Results 3.1. Plastome of Gastrodia longistylaThe plastome of Gastrodia longistyla is 30464 bp in length with GC content approximately 24.8% (GenBank accession number: MW879162), which is very similar to that of G. elata (GenBank accession number: MF163256) (Fig. 2) (Table 1). The plastome contains 18 protein-coding genes, five transfer RNA and three ribosomal RNA genes. Several genes and typical plastome regions appear to have been either lost or pseudogenized in G. longistyla. The G. longistyla plastome does not contain some housekeeping genes, such as matK, rpl16, or all photosynthesis genes. In addition, the G. longistyla plastome lacks an IR region. This indicates that plastomes of Gastrodia are in last stage of plastome degradation (see Barrett and Davis, 2012).

|
| Fig. 2 Plastome of Gastrodia longistyla and G. elata. |
| Species | Length of chloroplast genome (bp) | GC content (%) | Number of genes | ||
| Protein coding genes | tRNA genes | rRNA genes | |||
| Gastrodia longistyla | 30, 464 | 24.8 | 18 | 5 | 3 |
| Gastrodia elata | 35, 304 | 26.8 | 20 | 5 | 3 |
Gastrodia longistyla Q. Liu, J.D. Ya & X.H. Jin, sp. nov. (Figs. 3 and 4). 长柱天麻

|
| Fig. 3 Gastrodia longistyla Q. Liu, J.D. Ya & X.H. Jin, sp. nov. A. Habitat. B. Plant in the field. C. Population in Zhanyi County, Yunnan Province (type location) (Photographed by Q. Liu). |
|
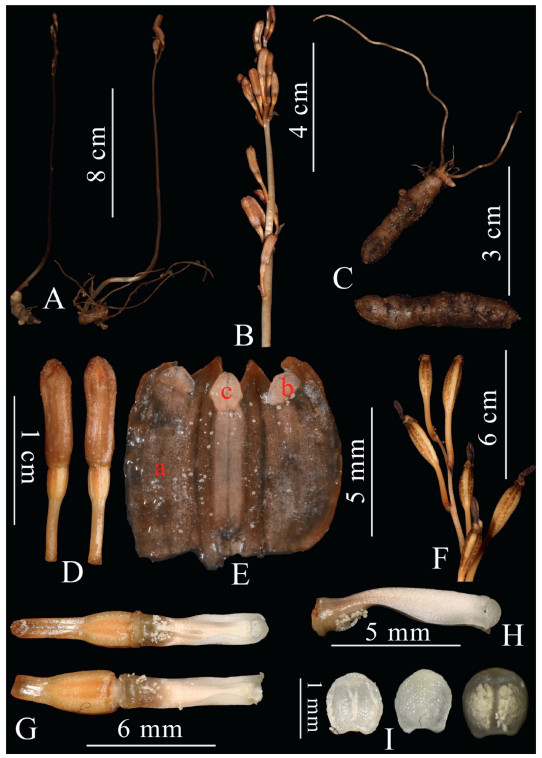
| Fig. 4 Gastrodia longistyla Q. Liu, J.D. Ya & X.H. Jin, sp. nov. A. Plant. B. Inflorescence. C. Rhizome. D. Flowers. E. Sepals and petals united forming a tube (a. sepal; b. petal; c. epichile). F. Fruits. G. Front view of column with pollen massulae (showing different length of appendage at base of stigma). H. Lateral view of column. I. Anther cap (Photographed by Q. Liu). |
Type. CHINA. Yunnan Province: Zhanyi County, Haifeng Natural Reserve, pine forest, 2200-2350 m in elevation, 30 September 2019, Q. Liu 738 (Holotype, HFTC!)
Diagnosis. Gastrodia longistyla is similar to Gastrodia peichatieniana S.S. Ying, but it can be easily distinguished from the latter by having a rhombic epichile, long column (6.0-7.5 mm long), and one long and needle-shaped appendage at base of the stigma.
Description. Mycoheterotrophic plants. Rhizome tuberous, cylindrical, 2.0-3.8 cm long, 9.0-11.0 mm thick, greyish brown, covered by a hairy membranous surface near the base, with several fleshy roots 4.5-6.0 cm. Peduncle erect and slender, glabrous, 16-45 cm long, 3.5-5.0 mm in diam., gray brownish, distantly noded and sheathed. Floral rachis laxly 3-15-flowered, 3.2-9.0 cm long, ca. 3 mm in diameter; floral bracts lanceolate-ovate, acute at apex, brownish, 4.0-5.5 × 1.2-2.0 mm. Pedicle and ovary 7.5-8.0 mm long. Flowers erect, urceolate, barely opening, brownish; sepals and petals united forming a tube, outer surface smooth, apical lobes verrucose and with crisped margins; sepals similar, dorsal sepal slightly wider than the lateral sepals, 9.0-10.0 mm long, connate with petals for 4/5 of their length, free apical portions ovate-triangular, slightly incurved, margin undulate; petals and labellum fused with sepal tube, whitish color; petals transverse oblong, obtuse, ca. 1.0 × 1.0 mm; epichile rhombic, obtuse, entire, ca. 2.0 × 1.5 mm; Column erect, 6.0-7.5 mm long, 1.2-1.5 mm wide, narrowly winged column wings dilated toward the apex, orbicular-triangular; rostellum not apparent; stigma ventral, located at the base of column, slightly concave and contained within column wings; one long and needle-shaped appendage at base of stigma, ca. 1.8-3.2 mm long; anther cap hemispheric, ca. 1.0 × 1.0 mm; pollinia 2, loosely contained within the anther, mealy and degenerating into individual massulae; column foot inconspicuous. Capsule ellipsoid, 1.8-2.0 cm long, 0.5-0.6 cm in diameter.
Etymology. The specific epithet refers to the conspicuously long column of flower.
Distribution and habitat. Gastrodia longistyla is a terrestrial mycoheterotrophic species that grows in pine forest and coniferous and broadleaf mixed forests at elevations from 1931 to 2500 m, which are dominated by Pinus yunnanensis Franch., Quercus franchetii Skan, Lithocarpus mairei (Schottky) Rehder, Lithocarpus variolosus (Franch.) Chun, and associated with other orchids, such as Epipactis helleborine (L.) Crantz, Cheirostylis pingbianensis K.Y. Lang, Stigmatodactylus sikokianus Maxim. ex Makino, Chamaegestrodia inverta (W.W. Sm.) Seidenfaden.
Phenology. Gastrodia longistyla was observed flowering and fruiting in its habitat from September to October.
Pollination implication. Flowers of Gastrodia longistyla barely open, and pollen massulae were observed on the stigma when flowers were dissected (Fig. 4G). These observations indicate that this new species is probably self-pollinating.
Additional specimens examined. CHINA. Yunnan Province, Kunming City, Anning Distinct, Xiyang town, pine forest, 2100-2300 m in elevation, 19 September 2019, Q. Liu 721 (HFTC); Dali City, Wanqiao Town, pine forest, 2350 m in elevation, 8 September 2019, J.D. Ya & H.L. Zhen 19CS18605 (KUN); Dali City, Wanqiao Town, pine forest, 2500 m in elevation, 11 October 2019, J.D. Ya 19CS18607 (KUN); Shiping County, Longpeng Town, Sanjia Village, mixed coniferous and broadleaf forests, 1931 m in elevation, 2 October 2019, J.W. Li 5096 (HITBC); Lincang City, Linxiang District, Boshang Town, mixed coniferous and broadleaf forests, 2271 m in elevation, 5 October 2020, J.W. Li 6222 (HITBC).
Conservation status. Gastrodia longistyla grows in the pine forest and mixed coniferous and broadleaf forests of nature reserves in Yunnan Province, southwest China. At least five populations, consisting of over 3000 mature individuals, have been discovered to date. The habitat of the new species is common in northeast and southeast Yunnan Province, suggesting that more populations and individuals were expected to be discovered in the adjacent forests in this region. However, local people collect these plants for medicine, and we note that habitats were seriously disturbed. Therefore, we evaluate it as vulnerable (VU) according to the guidelines of the IUCN Red List Categories and Criteria (IUCN Standards and Petitions Subcommittee, 2020).
3.3. NoteThe genus Gastrodia is divided into three sections, and Gastrodia sect. Strogadia is the smallest of three sections. In this section, plants have a labellum that is closely appressed to the perianth tube, whereas in the other two sections the labellum is free from the perianth tube.G. longistyla is similar to G. peichatieniana and both belong to the Gastrodia sect. Strogadia. However, G. longistyla can be easily distinguished from the latter by having brownish flowers, perianth tube with apical lobes and entire margins, a rhombic epichile, long column (6.0-7.5 mm in length), and one long and needle-shaped appendage (1.8-3.2 mm) at base of the stigma (vs. pale beige or whitish flowers, perianth tube with apical lobes and crisped margins, an ovate-orbicular epichile, short column (4-4.8 mm long), and no appendage on the base of column in G. peichatieniana) (Hu et al., 2014).
Gastrodia longistyla mainly grows in pine forest or mixed coniferous and broadleaf forests at 1931-2500 m in elevation; whereas, G. peichatieniana grows in broadleaved ever-green mountain forest at 850-1500 m in elevation (Chen et al., 2009; Hu et al., 2014).
4. Discussion 4.1. Taxonomic study for genus of GastrodiaTo date, the genus Gastrodia is composed of approximately 92 species, and has yet to be monographed. Many taxa remain poorly understood, and those that have been described have been given regional treatments. Consequently, synonymy for many species is unclear (Pridgeon et al., 2005). Therefore, a systematic phylogenetic study of Gastrodia is warranted.
4.2. Conservation implication of Gastrodia in ChinaTo determine the conservation implications of a newGastrodia species in China, we reviewed relevant literature, including the Threatened species list of China's higher plants (Qin et al., 2017), IUCN Standards and Petitions Subcommittee, and published papers. Only eleven Gastrodia species are listed as threatened, two of which (Gastrodia appendiculata and Gastrodia wuyishanensis) are designated as Critically Endangered (CR), three as Endangered (EN), and six as Vulnerable (VU) (Table 2).
| Species | Threatened species list of China's higher plants | IUCN | Assessment in published paper | Citation |
| Gastrodia augusta | EN | EN | ||
| Gastrodia appendiculata | CR | CR | ||
| Gastrodia autumnalis | VU | VU | ||
| Gastrodia confusa | VU | VU | ||
| Gastrodia gracilis | VU | VU | ||
| Gastrodia javanica | VU | VU | ||
| Gastrodia longitubularis | / | / | EN | Meng etal. (2007) |
| Gastrodia longistyla | / | / | VU | Present study |
| Gastrodia menghaiensis | EN | EN | ||
| Gastrodia tuberculata | VU | VU | ||
| Gastrodia wuyishanensis | / | / | CR | Li and Liu (2007) |
| Note: EN, endangered; CR, critically endangered; VU, vulnerable. | ||||
Although most species of Gastrodia are not threatened according to the literature, the current situation was not optimistic. For example, G. elata, the species within the genus with the widest distribution species, has been designated as not threatened. However, its rhizomes are widely used in traditional Chinese medicine to treat rheumatism, epilepsy, and paralysis (Pei and Yang, 2018). Despite the feasibility of cultivating this species, it is overexploited in the field because of deep-rooted perceptions in China that wild plants are better than cultivated plants. In addition, Gastrodia species are sensitive to their habitats, which in recent years have been severely disturbed by rapid economic development. Regardless, the conservation status of these species has not been updated recently.
The current conservation status of Gastrodia may be inaccurate. Thus, we recommend that the conservation status of all species of Gastrodia be re-evaluated on the basis of recent field investigations and specimen information. This re-assessment of Gastrodia will allow conservationists to take effective measures to conserve wild Gastrodia resources and promote their sustainable utilization in the future.
Author contributionsQL found it in the field investigation and write the manuscript, JDY provide the information of other distributed locations and co-write the manuscript. XHJ and BYS analyzed the molecular data, XHJ revise and give the important suggestions for manuscript, others authors give the help in the field investigation. All authors read and approved the manuscript.
Declaration of competing interestNone declared.
AcknowledgementsThis work was financially supported by National Forestry and Grassland Administration, China (No. 2019073018), and Doctoral Program of Yunnan Forestry Technological College (KY (ZD) 201905) to Q. Liu. We are grateful for staff of Haifeng Natural Reserve in Zhanyi County for his kind help in the field work.
Aung Y.L., Jin X.H., 2018. Gastrodia kachinesis (Orchidaceae), a new species from Myanmar. PhytoKeys, 94: 23-29. DOI:10.3897/phytokeys.94.21348 |
Barrett C.F., Davis J.I., 2012. The plastid genome of the mycoheterotrophic Corallorhiza striata (Orchidaceae) is in the relatively early stages of degradation. Am. J. Bot, 99: 1513-1523. DOI:10.3732/ajb.1200256 |
Chase M.W., Cameron K.M., Freudenstein J.V., et al, 2015. An updated classification of Orchidaceae. Bot. J. Linn. Soc, 177: 151-174. DOI:10.1111/boj.12234 |
Chen, S.C., Gale, S.W., Cribb, P.J., 2009. Gastrodia. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds. ), Flora of China, vol. 25. Science Press, Beijing, pp. 201-205.
|
Chung S.W., Hsu T.C., 2006. Gastrodia shimizuana, a new record of Gastrodia(Orchidaceae) in Taiwan. Taiwania, 51: 50-52. |
Cribb P., Fischer E., Killmann D., 2010. A revision of Gastrodia (Orchidaceae: Epidendroideae, Gastrodieae) in tropical Africa. Kew Bull, 65: 315-321. DOI:10.1007/s12225-010-9193-4 |
Dressler R.L., 1993. Phylogeny and Classification of the Orchid Family. Oregon: Dioscorides Press.
|
Feng Y.L., Wicke S., Li J.W., et al, 2016. Lineage-specific reductions of plastid genomes in an orchid tribe with partially and fully mycoheterotrophic species. Genome Biol. Evol, 8: 2164-2175. DOI:10.1093/gbe/evw144 |
Harris G., 1997. Huperei d the black orchid. N. Z. Garden J, 2: 7-9. |
Hsu T.C., Chung S.W., Kuo C.M., 2012. Supplements to the orchid flora of Taiwan(ⅵ). Taiwania, 57: 271-277. |
Hsu T.C., Fanerii M., Yang T.Y.A., et al, 2016. Gastrodia isabelensis and G. solomonensis (Gastrodieae, Epidendroideae, Orchidaceae): two new species representing a new generic record in the Solomon Islands. Phytotaxa, 270: 137-145. DOI:10.11646/phytotaxa.270.2.6 |
Hu A.Q., Gale S.W., Kumar P., et al, 2014. Taxonomic notes on Didymoplexiella siamensis and Gastrodia peichatieniana, two fully mycoheterotrophic orchids new to the flora of Hong Kong. Ann. Bot. Fenn, 51: 177-184. DOI:10.5735/085.053.0106 |
IUCN Standards and Petitions Subcommittee, 2020. Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee.
|
Jin X.H., Kyaw M., 2017. Gastrodia putaoensis sp. nov. (Orchidaceae, Epidendroideae) from north Myanma. Nord. J. Bot, 35: 730-732. DOI:10.1111/njb.01581 |
Leou, C.S., 2000. Gastrodia. In: Su, H.J. (Ed. ), Orchidaceae. Flora of Taiwan, second ed., vol. 5. Editorial Committee of the Flora of Taiwan, Dept Bot., NTU, Taipei, -Taiwan, pp. 890-896.
|
Li J.L., Wang S., Yu J., et al, 2013. A modified CTAB protocol for plant DNA extraction. Chin. Bull. Bot, 48: 72-78. DOI:10.3724/SP.J.1259.2013.00072 |
Li Z.H., Jiang Y., Ma X., et al, 2020a. Plastid genome evolution in the subtribe Calypsoinae (Epidendroideae, Orchidaceae). Genome Biol. Evol, 12: 867-870. DOI:10.1093/gbe/evaa091 |
Li Z.H., Ma X., Wen Y., et al, 2020b. Plastome of the mycoheterotrophic eudicot Exacum paucisquama (Gentianaceae) exhibits extensive gene loss and a highly expanded inverted repeat region. PeerJ, 8: e9157. DOI:10.7717/peerj.9157 |
Li D.M., Liu C.D., 2007. Gastrodia wuyishanensis, a new species of Orchidaceae from Fujian, China. Novon, 17: 354-356. DOI:10.3417/1055-3177(2007)17[354:GWANSO]2.0.CO;2 |
Lu G.W., 1985. Studies on Gastrodia elata tuber and its active components. Chin.Tradit. Herb. Drugs, 16: 40-41. |
Meng Q.W., Song X.Q., Luo Y.B., 2007. A new species of Gastrodia (Orchidaceae) from Hainan Island, China and its conservation status. Nord. J. Bot, 25: 23-26. DOI:10.1111/j.0107-055X.2007.00067_17.x |
Molvray, M., Kores, P.J., Chase, M.W., 2000. Polyphyly of mycoheterotrophic orchids and functional influences on floral and molecular characters. In: Wilson, K.L., Morrison, D.A. (Eds. ), Monocots: Systematics and Evolution. CSIro, Melbourne, pp. 441-448.
|
Pei S.J., Yang Z.W., 2018. Orchids and its uses in Chinese medicine and health care products. Med. Res. Innov, 2: 1-3. |
Pridgeon, A.M., Cribb, P.J., Chase, M.W., et al., 2005. Genera Orchidacearum 4, Epidendroideae (Part One). Oxford University Press, Oxford, p. 444.
|
Qin H.N., Yang Y., Dong S.Y., et al, 2017. Threatened species list of China's higher plants. Biodivers. Sci, 25: 696-744. DOI:10.17520/biods.2017144 |
Qu X.J., Moore M.J., Li D.Z., et al, 2019. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Method, 15: 50. DOI:10.1186/s.13007.-01.9-0435-7 |
Schlechter R., 1911. Die Polychondreae (Neottiinae Pfitz. ) undihre systematische Einteilung. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr, 45: 375-410. |
Seidenfaden G., Wood J.J., 1995. The Orchids of Peninsular Malaysia and Singapore. Olsen & Olsen, Fredensborg, Denmark. |
Tan Y.H., Hsu T.C., Pan B., et al, 2012. Gastrodia albidoides (Orchidaceae: Epidendroideae), a new species from yunnan, China. Phytotax, 66: 38-42. DOI:10.11646/phytotaxa.66.1.6 |
Wood, J.J., Beaman, T.E., Lamb, A., et al., 2011. The Orchids of Mount Kinabalu, vol. 2. Natural History Publications with Royal Botanic Gardens, Kota Kinabalu (Kew).
|
Zerbino D.R., Birney E., 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res, 18: 821-829. DOI:10.1101/gr.074492.107 |



