b. University of Chinese Academy of Sciences, Beijing 100049, China
Floral color change is a common phenomenon in angiosperms that is observed in at least 456 species, 253 genera, 78 families, and 33 orders (Weiss and Lamont, 1997). Color change does not refer to darkening or fading during floral senescence, but to hue alterations of fully turgid flowers (Weiss, 1995). Floral color change can occur in whole flowers or be localized to parts of the petal. Weiss (1995) divided floral color change into 10 types: whole flower, center of flower, corolla tube, nectary/hypanth, nectar guide/banner petal spot, eye/corona, selected petals, petal appendages, androecium and gynoecium. Examples of color change include the transition from yellow to red in petals of Lantana camara (Darwin, 1877; Weiss, 1991) and in the inside part of the lower corolla of Weigela middendorffiana (Ida and Kudo, 2003, 2010); from white to pink in both petals and stamens of Tibouchina pulchra (Pereira et al., 2011); from green to red in hypanthium together with the spreading sepals in Fuchsia excorticat (Delph and Lively, 1989); and from lilac to white and turquoise in petals of Desmodium setigerum (Willmer et al., 2009).
Many studies have demonstrated that color-changed flowers increase floral displays to attract pollinators, or act as long-distance honest signals for pollinators while minimizing visitors to nonproductive flowers (Lamont, 1985; Larson and Barrett, 1999; Makino and Ohashi, 2017; Oberrath and Böhning-Gaese, 1999; Ohashi et al., 2015; Weiss, 1995). Thus, flower color change is beneficial for both plants and pollinators. Pollinators quickly obtain rewards (pollen or nectar) by floral color, then avoid repeated visits and improve visiting efficiency (Gori, 1989; Lunau, 1996), which may increase flower pollination and reduce the residence time of pollinators at the same inflorescence, reducing or avoiding geitonogamy (Jones and Cruzan, 1999; Ida and Kudo, 2003, 2010; Sun et al., 2005).
Various biological and ecological factors have been identified to induce floral color changes in different plant species. Previous studies have demonstrated significant relationship between flower color and pollination (Ida and Kudo, 2010; Oberrath and Böhning-Gaese, 1999; Sun et al., 2005; Weiss, 1991). Artificial removal of pollinia or ethrel treatment induced labial color change from white to reddish brown in Cymbidium floribundum (Sugahara et al., 2010). In addition, pollen deposition on the stigma of T. pulchra and Tibouchina sellowiana (Pereira et al., 2011), and Lupinus pilosus (Ne'eman and Nesher, 1995; Nuttman and Willmer, 2003) also accelerated floral color change. However, other studies do not support these findings (Ida and Kudo, 2003, 2010; Sun et al., 2005; Kudo et al., 2007). In other cases, flower color change was not associated with pollinators but simply related to flower growth (Cruzan et al., 1988; Delph and Lively, 1989; Lamont and Collins, 1988; Sun et al., 2005), associated with responses to herbivores or fungi (Frey, 2004), or affected by temperature (Larson and Barrett, 1999), light (Farzad et al., 2002), and water level (Tang and Huang, 2010).
The physiological mechanisms underlying variation in flower color is the result of a complex interaction among anthocyanins, co-pigments, and the pH of vacuoles (Asen et al., 1975; Weiss, 1995). Anthocyanins are important secondary metabolites produced by the flavonoid biosynthetic pathway and contribute to the red, blue, and purple color of many flowers and fruits (Winkel-Shirley, 2001; Ogata et al., 2005). The anthocyanin biosynthetic pathway is controlled by environmental stimuli, such as light and temperature, or internal stimuli such as plant hormones, secondary metabolites, and nutrients (Mol et al., 1996). Changes in pH value can also affect anthocyanin pigments up to a certain extent, further altering red, purple, or blue colors in turgid flowers (Weiss, 1995; Yashida et al., 1995, 2003). These works strongly emphasize the importance of studying the mechanisms underlying flower color change for a better and more comprehensive understanding of flower color evolution.
Quisqualis indica (Combretaceae) is a perennial liana distributed in tropical Asia (primarily in southwest China) and typically grows in rain forests, low woods, riversides, and roadsides. It is also widely cultivated and often naturalized in tropical Asia, whereas in China, it is grown as an ornamental plant because of its beautiful flowers and strong fragrance at blooming, and also medicinally, since its seeds are used to treat intestinal parasites (Chen and Nicholas, 2007). Notably, Q. indica flowers change color from white to pink to red in all five petals. Eisikowitch and Rotem (1987) described the flower orientation and color change in this species and their potential role in attracting pollinators in Israel under cultivation conditions. However, the factors that affect these changes remain unknown. Therefore, based on the recognized knowledge gaps, we aim to address the following questions in the present study: (1) How does pH vary at different floral color stages in Q. indica? (2) Does the deposition of pollen grains on the stigma affect floral color change? and (3) Do light, temperature and ethylene influence floral color change?
2. Materials and methods 2.1. Plant species and study siteThis study was conducted in Yunnan Province, southwest China, where Q. indica blooms from April through mid-May, whereas sporadic inflorescences are produced until early June. The experiments were mainly conducted on the liana collections of Xishuangbanna Tropical Botanical Garden (21°45ʹN, 101°02ʹE; 580 m above sea level). Qindica plants have been cultivated at this site for many years, and some plants have already naturalized themselves into the adjacent limestone forest. During the flowering season, one inflorescence has 1–8 flowers that synchronously bloom each day. A single flower lasts for 2 days, whereas color-changed flowers are retained on their inflorescence for 4–6 days.
Based on field observations, flowers are white at anthesis, which occurs at approximately 19:00–20:00 (Fig. 1a). Their color changes to pink the following morning (Fig. 1b), and finally becomes red in the afternoon (Fig. 1c). This color transition was divided into six stages based on floral color: stage Ⅰ (white flowers at anthesis), stage Ⅱ (white flowers in the morning), stage Ⅲ (pink flowers at noon), stage Ⅳ (red flowers in the second afternoon), stage Ⅴ (red flowers in the third afternoon), and stage Ⅵ (red flowers in the fourth afternoon).
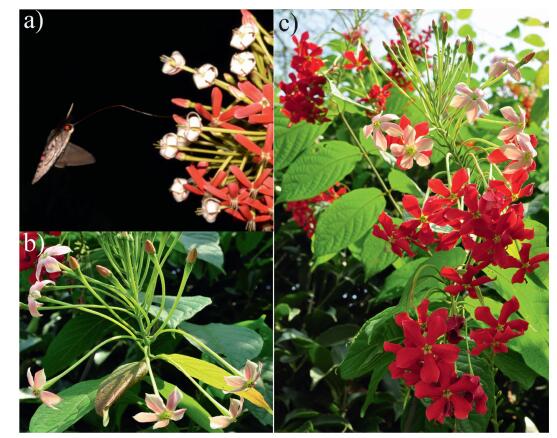
|
| Fig. 1 Flower color stages in Quisqualis indica. Petals are white at anthesis, which occurs at approximately 19:00–20:00 (a), their color changes to pink the following morning (b), and finally becomes red in the afternoon (c). |
In order to study the association of petal color with pH values, we measured pH at different color stages using petals of different inflorescences from five different plots, using plants from roadside collections, liana collections, medicinal plant collections, wild vegetable collections, and semi-natural limestone forest collections. One gram of bulked petal tissue was ground and mixed with 8 ml ultrapure water, and the pH was measured immediately after filtration using a PHS-3C pH meter (Shanghai Yoke Instruments, China) (Zhao et al., 2013). Ten replicates were maintained for each pH measurement, and the pH readings were repeated in triplicate. Mean values were calculated to construct pH profiles for each petal color stage. To understand the difference of pH among petal colors, we performed multivariate analysis of variance (MANOVA) to identify pairwise differences using SPSS Statistics 21 (IBM, Armonk, NY, USA).
2.3. Pollination treatmentsTo test whether pollen deposition on the stigma induces petal color change in Q. indica, 10 inflorescences were randomly selected from different plants in each plot, and the flowers were emasculated before anther dehiscence. All inflorescences were bagged until anthesis to avoid any contact with pollinators. The treatments were as follows: (1) non-pollination, (2) flowers that cross-pollinated at 20:00 when the flowers just opened, and (3) flowers that cross-pollinated at 8:00 the following morning. The petal color was observed and recorded every 2 h in each treatment, and color reflectance of each petal was measured in triplicate by a spectrophotometer (JAZ, Ocean Optics, Oxford, UK).
2.4. Light treatmentsTo determine light mediated induction of petal color change in Q. indica, we set five different light treatments: (1) inflorescences were maintained under natural conditions (control, N + N), (2) inflorescences were covered with transparent white polyester mesh bags which allow light to pass through but prevent insect visitation during budding (8:00–20:00 before anthesis) and flowering period (after 20:00) (W + W), (3) inflorescences were covered with white polyester mesh bags during the bud period and black cotton bags (∼100% light shading in sunlight) during the flowering period, to give light stimulations before anthesis but block sunlight later (W + B), (4) inflorescences were covered with black cotton bags during the bud period and white polyester mesh bags during the flowering period, to block sunlight before anthesis but give sunlight later (B + W), and (5) inflorescences were covered with black cotton bags during both budding and flowering period (B + B). Since after anthesis the petal color remains white overnight, we recorded the color change every 2 h from 7:00 in the following morning to 17:00 in the afternoon. The petal color reflectance was determined by a spectrophotometer. This experiment was conducted in two plots using three inflorescences in each plot for each treatment. One petal was randomly selected from each inflorescence, and each measurement was repeated in triplicate.
2.5. Temperature treatmentsMultiple inflorescences were randomly collected from different individuals, and 2–3 inflorescences were placed in individual bottles filled with sucrose solution (6%). Our previous experiments indicated that with adequate water supply and high humidity, the detached flowers (without leaves) display the same rate of petal color change as those on the mother plants (Zhang et al., unpublished data). To further determine the influence of temperature on petal color change in Q. indica, we performed the same light treatments as described above using five temperature gradients (15, 20, 25, 30, and 35 ℃) in growth chambers under dark and light conditions (white light, 200 μmol m−2s−1 from 7:30–20:00; photoperiod = 12.5 h). The growth chamber conditions were similar to the natural daily temperature range of 17.4–38 ℃ during the blooming period. As temperature changed with illumination intensity, we could not distinguish the individual effects of these two factors, and thus, we also conducted the following treatments in growth chambers to distinguish the light effects from the above experiment: (1) inflorescences in the dark at constant temperature of 20 ℃ and (2) inflorescences in the dark with temperature variations similar to natural conditions. All open flowers were removed, and many buds in different developmental stages were left on the inflorescences. Inflorescences on the mother plants and detached inflorescences under natural conditions were used as controls. We used at least nine inflorescences in each treatment. The petal color was observed and recorded every 2 h. We used a light reflectance of 540 nm under natural conditions to present different color stages for analysis, and we used Univariate Analysis of Variance to test the effect of temperature and light treatments with SPSS Statistics 21 (IBM, Armonk, NK, USA).
2.6. Ethylene treatmentSilver thiosulphate (STS), which blocks the action of ethylene, was used to determine whether ethylene mediates color change in Q. indica. STS solution was prepared by pouring 8 mM AgNO3 into an equal volume of 32 mM Na2S2O3 (Reid et al., 1989). All open flowers were removed from nine control and nine experimental inflorescences from three plots. The leaves and buds of the experimental inflorescences were thoroughly sprayed with STS the evening before anthesis, following Farzad et al. (2002), and then covered with plastic bags. All control and experimental inflorescences were maintained pairwise under the same conditions. Flower petals on all the inflorescences were examined every 2 h from 7:00 in the second morning after STS application until 2 d after petals already changed color to red, because both pollen viability and stigma receptivity declined quickly at day 3 in the red floral stage. We used the light reflectance of 540 nm under natural conditions to present different color stages for analysis. To assess the differences of petal color change rate between control and experimental inflorescences, we performed the Mann–Whitney U test to identify pairwise differences using SPSS Statistics 21 (IBM, Armonk, NY, USA).
3. Results 3.1. pH analysisQ. indica petal pH values decreased gradually from stage Ⅰ to stage Ⅲ, increased slightly at stage Ⅳ, peaking at 5.09 ± 0.04 (mean ± se, n = 9), and then dropped to the lowest value of 4.89 ± 0.02 (n = 9) at stage Ⅵ (Fig. 2). The pH values differed significantly between stage Ⅰ and Ⅲ (P1 = 0.004), stage Ⅰ and Ⅵ (P2 = 0.001), stage Ⅲ and Ⅳ (P3 = 0.004) and stage Ⅳ and Ⅵ (, P4 = 0.001). Otherwise, we observed no significant differences among stage Ⅰ, stage Ⅱ, stage Ⅳ and stage Ⅴ. According to MANOVA analysis, pH changes had no significant effects on petal color change (P5 = 0.672), irrespective of location or petal stage (P6 = 0.512).

|
| Fig. 2 Changes in the pH value at different petal color stages of Quisqualis indica. Different letters indicate significant differences at P < 0.05. |
Q. indica flowers in the non-pollination treatment changed their color at the same rate as those under natural conditions. In addition, the flowers in the cross-pollination treatment at either 20:00 or the next morning at 8:00 had the same color change rate as the flowers in the non-pollination treatment. It is evident from the recorded data that the on-time and delayed pollination had no significant effect on the rate of color change.
3.3. Light treatmentsQ. indica flowers in the W + W treatment changed their color from white to red at the same rate as the control (N + N). The flowers in the W + B treatment had the same color change rate as the control prior to 15:00 and then slowed down. The flowers in the B + W treatment had a slower color change rate prior to 13:00 compared with the control, but the rate increased slightly after 13:00. At 17:00, the flower color in the W + B and B + W treatments was slightly red. However, the flowers in the B + B treatment remained white throughout the experimental period (Fig. 3).
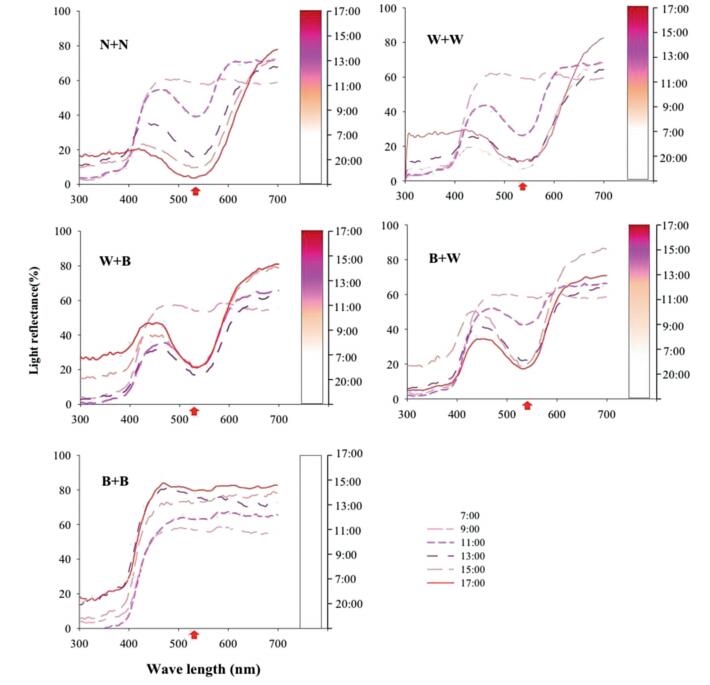
|
| Fig. 3 Spectral reflectance (%, graph) and petal color changes (column) of Quisqualis indica petals under different light conditions. N + N, natural conditions; W + W, covered with white bag during the bud and flowering period; W + B, covered with white bag during the bud period and black bag during the flowering period; B + W, covered with black bag during the bud period and white bag during the flowering period; B + B, covered with black bag during the bud and flowering period. Red arrow indicate the key point that light reflectance is the most different from each treatment. |
Furthermore, on the third day at 10:00 AM, the flower color in the control, W + W and B + W treatments changed to dark red; however, the flower color in the W + B treatment did not change and remained slightly red. The flowers in the B + B treatment were still white after 4–5 days and the color did not change until flower shedding. These results were also confirmed by the spectrophotometry data, which showed a significant change in wavelength (from 500 to 600 nm) when the color changed from white to pink and then to red (Fig. 3).
3.4. Temperature treatmentsThe rate of petal color change in Q. indica did not change at 20–30 ℃ even under four different light treatments, but flowers did not open when exposed to constant temperature at 15 ℃ or 35 ℃. Moreover, the flowers in the B + B treatment remained white throughout the experimental period regardless of temperature. Univariate Analysis Variance showed that temperature had no significant effect on petal color change, whereas light did; in addition, the interaction between light and temperature did not have a significant effect on petal color change (Table 1). However, when we placed the inflorescences in the dark for 1 d before anthesis, the flower petals remained white, regardless of temperature.
| Time | Treatment | df | F | P | R2 |
| 20:00 | Temperature | 2 | – | – | – |
| Light | 3 | – | – | ||
| Tem × Light | 6 | – | – | ||
| 07:00 | Temperature | 2 | 5.19 | 0.007 | 0.354 |
| Light | 3 | 10.384 | 0.000* | ||
| Tem × Light | 6 | 1.835 | 0.100 | ||
| 09:00 | Temperature | 2 | 1.266 | 0.287 | 0.754 |
| Light | 3 | 96.436 | 0.000* | ||
| Tem × Light | 6 | 0.417 | 0.866 | ||
| 11:00 | Temperature | 2 | 1.784 | 0.173 | 0.791 |
| Light | 3 | 119.159 | 0.000* | ||
| Tem × Light | 6 | 0.314 | 0.928 | ||
| 13:00 | Temperature | 2 | 1.437 | 0.243 | 0.561 |
| Light | 3 | 39.471 | 0.000* | ||
| Tem × Light | 6 | 0.260 | 0.954 | ||
| 15:00 | Temperature | 2 | 2.871 | 0.062 | 0.732 |
| Light | 3 | 81.967 | 0.000* | ||
| Tem × Light | 6 | 1.808 | 0.106 | ||
| 17:00 | Temperature | 2 | 0.922 | 0.401 | 0.797 |
| Light | 3 | 120.349 | 0.000* | ||
| Tem × Light | 6 | 0.904 | 0.495 | ||
| Temperature treatments: 20 ℃, 25 ℃, 30 ℃; Light treatments: B + W: covered with a black bag in the bud period and a white bag in the flowering period; W + W: covered with a white bag in both bud and flowering periods; W + B: covered with a white bag in the bud period and a black bag in the flowering period. N + N: flowers under natural condition used as control. Statistical analysis was showed in the table using Univariate Analysis of Variance with SPSS Statistics 21. *Significant difference between light treatment. | |||||
The rate of color change did not differ between flowers sprayed with STS and untreated flowers (all P > 0.05) (Table 2). In the nine plants treated with STS, all 30 new opened flowers changed color almost the same as all 25 new flowers on the nine control plants; only 5 out of the 35 treated flowers from two plants showed color change slower than the control flowers at 7:00 to 9:00 the next morning.
| Time | Control | STS Treatment | P | |||||
| Mean | SE | N | Mean | SE | N | |||
| 20:00a | 58.26 | 0 | 9 | 58.26 | 0 | 9 | 1 | |
| 07:00b | 58.26 | 0 | 9 | 58.26 | 0 | 9 | 1 | |
| 09:00b | 54.07 | 2.77 | 9 | 56.17 | 2.10 | 9 | 0.539 | |
| 11:00b | 43.59 | 2.77 | 9 | 41.49 | 2.10 | 9 | 0.539 | |
| 13:00b | 13.10 | 1.22 | 9 | 13.87 | 1.16 | 9 | 0.638 | |
| 15:00b | 9.24 | 0 | 9 | 9.24 | 0 | 9 | 1 | |
| 17:00b | 5.61 | 0.69 | 9 | 6.65 | 0.82 | 9 | 0.331 | |
| 07:00c | 4.57 | 0 | 9 | 5.61 | 0.69 | 9 | 0.145 | |
| 09:00c | 4.57 | 0 | 9 | 5.61 | 0.69 | 9 | 0.145 | |
| 11:00c | 4.57 | 0 | 9 | 4.57 | 0 | 9 | 1 | |
| P value was showed in the table using Mann–Whitney U test with SPSS Statistics 21. a The day blossoming. b One day after blossoming. c Two days after blossoming. | ||||||||
Floral color change can be influenced by environmental factors such as temperature and light. In Rhexia virginica, temperature affects the rate of color change; on warm days, filaments are dark red and fully recurved by late afternoon, whereas on cool days, they often remain pale reddish and are only slightly recurved by the following morning (Larson and Barrett, 1999). As evident from earlier reports, light is required for anthocyanin production in many plants, including viola (Farzad et al., 2002), petunia (Koes et al., 1989), corn (Procissi et al., 1997), eggplant (Toguri et al., 1993), mustard (Beggs et al., 1987), lisianthus (Griesbach, 1992), and ivy (Murray et al., 1994). In Q indica, the factor that induces petal color change is light rather than temperature, as evidenced by the lack of color change in petals maintained in the dark, regardless of temperature conditions. While both buds and flowers are sensitive to light, buds have higher sensitivity towards light than flowers; but flower development is greatly influenced by extremely low or high temperatures. Light-induced anthocyanin gene transcription is common; petal color change in response to light in Q. indica implies that anthocyanin biosynthesis is regulated, probably at the level of transcription (Dooner et al., 1991; Farzad et al., 2002). However, further comprehensive studies are required to identify the underlying biochemical mechanism of flower color change.
4.2. Effect of chemical factors on petal color changepH values in flower petals mostly fluctuate in the range of 2.5–7.5, and are usually higher in blue petals than in red petals (Stewart et al., 1975). For example, pH changes can cause Aechmea(Bromeliaceae) flowers to turn from pink to blue, Pulmonaria (Boraginaceae) flowers from red to blue, Oxypetalum (Asclepiadaceae) flowers from blue to pink (Weiss, 1995), and extremely high vacuolar pH values of Ipomoea tricolor cv. heavenly blue (Convolvulaceae) cause blue petal color variation (Yashida et al., 1995). However, slight pH variations (0.26 pH units) that occur during the floral color change in Viola cornuta and other malvidin-containing flowers do not cause any alteration in the shade or intensity of floral color (Farzad et al., 2002; Griesbach, 1992; Stewart et al., 1975). In angiosperms where flowers turn blue, pH changes accounted for floral color changes in only 1.2% of genera (3/241) (Weiss, 1995). Our results show that in Q. indica pH values at stages Ⅰ, Ⅲ and Ⅵ, as well as Ⅳ and Ⅵ, differed significantly; however, a 0.197 change in pH over floral ontogeny did not influence floral color change, in agreement with other studies (Farzad et al., 2002; Griesbach, 1992). Interestingly, at stage Ⅳ, the pH value of red flowers exposed to sunlight increased slightly to approximately the same value as opening white flowers, suggesting that floral color change is not affected by pH changes. These changes in pH may be related to photosynthesis; however, the mechanism underlying photosynthetic pH changes requires additional investigation.
Ethylene is involved in many physiological responses, including petal color change (O'Neill et al., 1993; Woltering et al., 1995). In some plants ethylene contribution to anthocyanin production has been demonstrated by observing the effect of exogenous ethylene and the ethylene inhibitors STS and 2, 5 norbornadiene (NBD) on floral color in Lupinus, Cymbidium, and Cattleya (Stead and Reid, 1990; Woltering and Somhorst, 1990; Strauss and Arditti, 1982). In our study, flowers sprayed with STS did not change floral color at different rates than untreated flowers, suggesting that color change is not mediated by ethylene. These results are in agreement with an earlier report by Farzad et al. (2002).
4.3. Effect of ecological factors on floral color changeMany studies have suggested that pollinators are agents of directional selection of floral color (e.g., Campbell et al., 1997; Emms and Arnold, 2000; Schemske and Bradshaw, 1999; Stanton et al., 1989; Streisfeld and Kohn, 2005). Petal color change has been interpreted as a warning mechanism for bees to avoid old flowers (Endress, 1994), increasing foraging efficiency of pollinators and pollen transfer (Larson and Barrett, 1999; Niesenbaum et al., 1999). In plants where petal color change is induced or accelerated by pollination, the presence of color-changed flowers indicates the end of reproductive assignments (pollen output or input) and is a reliable signal to the pollinators to invest their energy in other flowers (Farzad et al., 2002; Nuttman and Willmer, 2003; Pereira et al., 2011; Sugahara et al., 2010). We found that neither pollination nor pollination timing affected the rate of floral color change in Q. indica flowers. According to pollination syndrome framework (Fenster et al., 2004), Q. indica flowers should be pollinated by long-tongued moths. In a previous study, we found that Q. indica flowers first pollinated by moths at night changed color from white to pink to red upon exposure to sunlight; this, in turn, attracted novel butterfly pollinators, revealing that petal color change is associated with a shift in pollinator (Yan et al., 2016).
In conclusion, the primary factor that induces petal color change in Q. indica is light, rather than pH, ethylene, temperature or pollen on the stigma. Buds and petals are both sensitive to light, but the responsiveness of buds is slightly greater than that of flowers. However, the final petal color was not expressed if only the buds were exposed to light, which suggests that light during the flowering period might induce the final transcription of regulatory factors, and also explains the reduction in the rate of color change in the absence of light. Further studies are needed to identify the probable relationships between the different color stages and light irradiation in order to gain insights into the underlying biochemical mechanism of flower color change, while phylogenetic analyses of pollinators and florivores may help to better understand the underlying evolutionary mechanism.
AcknowledgementsThis work was funded by a grant from the National Nature Science Foundation of China (31670393 and 31170406) that was awarded to L. Zhang. We thank Mr. Xinxing He, Mr. Zhiran Wang, Mr. Jianmin Gan, and Mr. Qiaoshun Li from the Xishuangbanna Tropical Botanical Garden for their assistance in field experiments, Dr. Jin Zhao assisted with some data analysis. We would like to thank Dr. Sandhya Mishra for English language editing.
Asen S., Stewart R.N., Norris K.H., 1975. Anthocyanin, flavonol copigments, and pH responsible for larkspur flower color. Phytochemistry, 14: 2677-2682. DOI:10.1016/0031-9422(75)85249-6 |
Beggs C.J., Kuhn K., Bocker R., et al, 1987. Phytochrome induced flavonoid biosynthesis in mustard (Sinapis alba L.) cotyledons. Enzymic control and differential regulation of anthocyanin and quercetin formation. Planta, 172: 121-126. DOI:10.1007/BF00403037 |
Campbell D.R., Waser N.M., Melendez-Ackerman E.J., 1997. Analyzing pollinatormediated selection in a plant hybrid zone:hummingbird visitation patterns on three spatial scales. Am. Nat, 149: 295-315. DOI:10.1086/285991 |
Chen, J., Nicholas, J. T., 2007. Flora China, vol. 13, pp. 315-316.
|
Cruzan M.B., Neal P.R., Willson M.F., 1988. Floral display in Phyla incisa:consequences for male and female reproductive success. Evolution, 42: 505-515. |
Darwin C., 1877. Fritz Müller on flowers and insects. Nature, 17: 78-79. DOI:10.1038/017078b0 |
Delph L.F., Lively C.M., 1989. The evolution of floral color change:pollinator attraction versus physiological constraints in Fuchsia excorticat. Evolution, 43: 1252-1262. DOI:10.1111/evo.1989.43.issue-6 |
Dooner H.K., Robbins T., Jorgensen R., 1991. Genetic and developmental control of anthocyanin biosynthesis. Annu. Rev. Genet, 25: 173-199. DOI:10.1146/annurev.ge.25.120191.001133 |
Eisikowitch D., Rotem R., 1987. Flower orientation and color change in Quisqualis indica and their possible role in pollinator partitioning. Bot. Gaz, 148: 175-179. DOI:10.1086/337645 |
Emms S.K., Arnold M.L., 2000. Site to site differences in pollinator visitation patterns in a Louisiana iris hybrid zone. Oikos, 91: 568-578. DOI:10.1034/j.1600-0706.2000.910319.x |
Endress P.K., 1994. Floral structure and evolution of primitive angiosperms e recent advances. Plant Syst. Evol, 192: 79-97. DOI:10.1007/BF00985910 |
Farzad M., Griesbach R., Weiss M.R., 2002. Floral color change in Viola cornuta L.(Violaceae):a model system to study regulation of anthocyanin production. Plant Sci, 162: 225-231. DOI:10.1016/S0168-9452(01)00557-X |
Fenster C.B., Armbruster W.S., Wilson P., et al, 2004. Pollination syndromes and flora specialization. Annu. Rev. Ecol. Evol. Systemat, 35: 375-403. DOI:10.1146/annurev.ecolsys.34.011802.132347 |
Frey F.M., 2004. Opposing natural selection form herbivores and pathogens may maintain floral-color variation in Claytonia virginica (Portulacaceae). Evolution, 58: 2426-2437. DOI:10.1111/evo.2004.58.issue-11 |
Gori D.F., 1989. Floral color change in Lupinus argenteus (Fabaceae):why should plant advertise the location of unrewarding flowers to pollinators?. Evolution, 43: 870-881. DOI:10.1111/evo.1989.43.issue-4 |
Griesbach R.J., 1992. Correlation of pH and light intensity on flower color in potted Eustoma grandiflorum Grise. Hortscience, 27: 817-818. |
Ida T.Y., Kudo G., 2003. Floral color change in Weigela middendorffiana (Caprifoliaceae):reduction of geitonogamous pollination by bumble bees. Am. J. Bot, 90: 1751-1757. DOI:10.3732/ajb.90.12.1751 |
Ida T.Y., Kudo G., 2010. Modification of bumblebee behavior by floral color change and implications for pollen transfer in Weigela middendorffiana. Evol. Ecol, 24: 671-684. DOI:10.1007/s10682-009-9324-2 |
Jones C.E., Cruzan M.B., 1999. Floral morphological changes and reproductive success in deer weed (Lotus scoparius, Fabaceae). Am. J. Bot, 86: 273-277. DOI:10.2307/2656943 |
Koes R.E., Spelt C.E., Mol J.N.M., 1989. The chalcone synthase multigene family of Petunia hybrida (V30):differential, light-regulated expression during flower development and UV light induction. Plant Mol. Biol, 12: 213-225. DOI:10.1007/BF00020506 |
Kudo G., Ishii H.S., Hirabayashi Y., et al, 2007. A test of the effect of floral color change on pollination effectiveness using artificial inflorescences visited by bumblebees. Oecologia, 154: 119-128. DOI:10.1007/s00442-007-0820-1 |
Lamont B., 1985. The significance of flower colour change in eight co-occurring shrub species. Bot. J. Linn. Soc, 90: 145-155. DOI:10.1111/boj.1985.90.issue-2 |
Lamont B., Collins B.G., 1988. Flower colour change in Banksia ilicifolia:a signal for pollinators. Austral Ecol, 13(2): 129-135. DOI:10.1111/aec.1988.13.issue-2 |
Larson B.M.H., Barrett S.C.H., 1999. The pollination ecology of buzz-pollinated Rhexia virginica (Melastomataceae). Am. J. Bot, 86: 502-511. DOI:10.2307/2656811 |
Lunau K., 1996. Unidirectionality of floral colour changes. Plant Syst. Evol, 200: 125-140. DOI:10.1007/BF00984753 |
Makino T.T., Ohashi K., 2017. Honest signals to maintain a long-lasting relationship:floral colour change prevents plant-level avoidance by experienced pollinators. Funct. Ecol, 31: 831-837. DOI:10.1111/1365-2435.12802 |
Mol J., Jenkins G., Schafer E., et al, 1996. Signal perception, transduction, and gene expression involved in anthocyanin biosynthesis. Crit. Rev. Plant Sci, 15: 525-557. DOI:10.1080/07352689609382369 |
Murray J.R., Smith A.G., Hackett W.P., 1994. Differential dihydroflavonol reductase transcription and anthocyanin pigmentation in the juvenile and mature phases of ivy (Hedera helix L.). Planta, 194: 102-109. |
Ne'eman G., Nesher R., 1995. Pollination ecology and the significance of floral color change in Lupinus pilosus L. (Fabaceae). Isr. J. Plant Sci, 43: 135-145. DOI:10.1080/07929978.1995.10676599 |
Niesenbaum R.A., Patselas M.G., Weiner S.D., 1999. Does flower color change in Aster vimineus cue pollinators? . Am. Midl. Nat., 141: 59-68. DOI:10.1674/0003-0031(1999)141[0059:DFCCIA]2.0.CO;2 |
Nuttman C., Willmer P., 2003. How does insect visitation trigger floral colour change? Ecol. Entomol, 28: 467-474. |
Oberrath R., Bohning-Gaese K., 1999. Floral color change and the attraction of insect pollinators in lungwort (Pulmonaria collina). Oecologia, 121: 383-391. DOI:10.1007/s004420050943 |
Ogata J., Kanno Y., Itoh Y., et al, 2005. Plant biochemistry:anthocyanin biosynthesis in roses. Nature, 435: 757-758. DOI:10.1038/nature435757a |
Ohashi K., Makino T.T., Arikawa K., 2015. Floral colour change in the eyes of pollinators:testing possible constraints and correlated evolution. Funct. Ecol, 29: 1144-1155. DOI:10.1111/fec.2015.29.issue-9 |
O'Neill S.D., Nadeau J.A., Zhang X.S., et al, 1993. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell, 5: 419-432. DOI:10.1105/tpc.5.4.419 |
Pereira A.C., da Silva J.B., Goldenberg R., et al, 2011. Flower color change accelerated by bee pollination in Tibouchina (Melastomataceae). Flora, 206: 491-497. DOI:10.1016/j.flora.2011.01.004 |
Procissi A., Dolfini S., Ronchi A., et al, 1997. Light-dependent spatial and temporal expression of pigment regulatory genes in developing maize seeds. Plant Cell, 9: 1547-1551. DOI:10.1105/tpc.9.9.1547 |
Reid M.S., Evans R.Y., Dodge L.L., et al, 1989. Ethylene and silver thiosulphate influence opening of cut rose flowers. J. Am. Soc. Hortic. Sci, 114: 436-440. |
Schemske D.W., Bradshaw H.D., 1999. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc. Natl. Acad. Sci. USA, 96: 11910-11915. DOI:10.1073/pnas.96.21.11910 |
Stanton M.L., Snow A.A., Handel S.N., et al, 1989. The impact of a flower-color polymorphism on mating patterns in experimental populations of wild radish(Raphanus raphanistrum L.). Evolution, 43: 335-346. DOI:10.1111/evo.1989.43.issue-2 |
Stead A.D., Reid M.S., 1990. The effect of pollination and ethylene on the color change of the banner spot of Lupinus albifrons (Bentham) flowers. Ann. Bot, 66: 655-663. DOI:10.1093/oxfordjournals.aob.a088080 |
Stewart R.N., Norris K.H., Asen S., 1975. Micro spectrophotometric measurement of pH and pH effect on color of petal epidermal cells. Phytochemistry, 14: 937-942. DOI:10.1016/0031-9422(75)85162-4 |
Strauss M.S., Arditti J., 1982. Post-pollination phenomena in orchid flowers:X. Transport and fate of auxin. Bot. Gaz, 143: 286-293. DOI:10.1086/337302 |
Streisfeld M.A., Kohn J.R., 2005. Contrasting patterns of floral and molecular variation across a cline in Mimulus aurantiacua. Evolution, 59: 2548-2559. DOI:10.1111/evo.2005.59.issue-12 |
Sugahara M., Minamoto T., Fuchikawa, et al, 2010. Apis Cerana Japonica discriminates between floral color phases of the Oriental orchid, Cymbidium floribundum. Zool. Sci, 27: 901-906. DOI:10.2108/zsj.27.901 |
Sun S.G., Liao K., Xia J., et al, 2005. Floral colour change in Pedicularis monbeigiana(Orobanchaceae). Plant Syst. Evol, 255: 77-85. DOI:10.1007/s00606-005-0348-y |
Tang X.X., Huang S.Q., 2010. Fluctuating selection by water level on gynoecium colour polymorphism in an aquatic plant. Ann. Bot, 106: 843-848. DOI:10.1093/aob/mcq172 |
Toguri T., Umemoto N., Kobayashi O., et al, 1993. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues and identification of an inducible p-450 cDNA. Plant Mol. Biol, 23: 933-946. DOI:10.1007/BF00021810 |
Weiss M., Lamont B., 1997. Floral color change and insect pollination:a dynamic relationship. Isr. J. Plant Sci, 45: 185-199. DOI:10.1080/07929978.1997.10676683 |
Weiss M.R., 1991. Floral color change as cues for pollinator. Nature, 351: 227-229. |
Weiss M.R., 1995. Floral color change:a widespread functional convergence. Am. J. Bot, 86: 167-185. |
Willmer P., Stanley D.A., Steijven K., et al, 2009. Bidirectional flower color and shape changes allow a second opportunity for pollination. Curr. Biol, 19: 919-923. DOI:10.1016/j.cub.2009.03.070 |
Winkel-Shirley B., 2001. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol, 126: 485-493. |
Woltering E.J., Somhorst D., 1990. Regulation of anthocyanin synthesis in Cymbidium flowers:effects of emasculation and ethylene. J. Plant Physiol, 136: 295-299. DOI:10.1016/S0176-1617(11)80052-0 |
Woltering E.J., Somhorst D., van der Meer P., 1995. The role of ethylene in interorgan signaling during flower senescence. Plant Physiol, 109: 1219-1225. DOI:10.1104/pp.109.4.1219 |
Yan J., Wang G., Sui Y., et al, 2016. Pollinator responses to floral colour change, nectar, and scent promote reproductive fitness in Quisqualis indica (Combretaceae). Sci. Rep, 6: 24408. DOI:10.1038/srep24408 |
Yashida K., Kondo T., Okazaki, et al, 1995. Cause of blue petal colour. Nature, 373: 291. DOI:10.1038/373291a0 |
Yashida K., Toyama-Kato Y., Kameda K., et al, 2003. Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol, 44: 262-268. DOI:10.1093/pcp/pcg033 |
Zhao D.Q., Hao Z.J., Wang J., et al, 2013. Effects of pH in irrigation water on plant growth and flower quality in herbaceous peony (Paeonia lactiflora Pall.). Sci. Hortic. eAmsterdam, 154: 45-53. DOI:10.1016/j.scienta.2013.02.023 |



