b. China Germplasm Bank of Wild Species, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China;
c. University of Chinese Academy of Sciences, Beijing, China;
d. College of Plant Protection, Yunnan Agricultural University, Kunming, China
Heavy metal (HM) pollution is a global environmental problem. Plants play significant roles in the relationship between soil HMs and public health. On the one hand, plants can accumulate HMs from soil; these HMs can be transferred to animals and humans through food chains (Tauqeer et al., 2016), and thus poison these organisms. HM soil contents that exceed environmental, food and human safety standards have become common. Phytoremediation technology is an economic, green, and highly efficient method developed to manage soil HM pollution based on plant adsorption and accumulation of HMs (Ehsan et al., 2014). Specifically, HM hyperaccumulators are ideal tools for phytoremediation (Kramer, 2005). However, although more HM hyperaccumulators have been discovered worldwide (Xiao et al., 2013), many of these plants grow slowly and feature small biomasses, which significantly limit their remediation efficiency (Xiao et al., 2013). In view of these problems, research has focused on various methods to either inhibit or improve HM absorption capacity of plants (Che et al., 2016; Pan et al., 2016; Morikawa, 2017). The former is used to directly reduce the exposure of animals and humans to toxic soil HMs, whereas the latter is useful for promoting phytoremediation efficiency of HM pollution. Modifying Cd accumulation of plants requires understanding the physiological, biochemical, and molecular mechanisms underlying HM tolerance and absorption in plants (Wei et al., 2006).
Cadmium (Cd) contamination has become the most prominent HM pollution in soils due to its high toxicity, wide distribution, and strong mobility (Moreno-Caselles et al., 2000). Solving the problem of Cd pollution is relatively urgent and difficult. Compared with other HMs, especially nickel (Ni), the number of Cd hyperaccumulators remains low (He, 2013). Unlike most HMs, Cd and lead are non-essential elements for plants; however, reported biochemical and molecular mechanisms underlying plant response to Cd are nearly similar to those of other HMs (Choppala et al., 2014; Song et al., 2017). Only limited information is required to establish whether plants use unique strategies or mechanisms to tolerate, absorb, and translocate these HMs (Li et al., 2016). This plant can accumulate over 100 mg Cd kg-1 dry weight (DW) in leaves with minimal damage (Li et al., 2016). Considering the potential role of turnips in the phytoremediation of Cd-polluted soils, in this paper we ask how turnips respond to Cd and protect themselves from the toxicity of this metal at both the physiological and biochemical level. To answer this question, we determined changes in antioxidant systems, phytochelatins (PCs), and free amino acids under different Cd treatment concentrations. This study can improve our understanding regarding biochemical mechanisms underlying high Cd accumulation in turnips. Meanwhile, results may help in exploring possible means of reducing Cd pollution risk in edible turnips or increase Cd accumulation in plants for phytoremediation.
2. Material and methods 2.1. Plant material and Cd treatmentsThe present study used turnip landrace "KTRG-B54" from Ninglang County in China. Seeds were surface-sterilized by soaking in 1% NaClO solution for 5 min and fully rinsed using deionized water. After sterilizing, seeds were germinated on sterile Murashige and Skoog (MS) solid medium (pH = 6.0) with 0, 10, and 30 μmol L-1 CdCl2. Eighteen culture bottles (250 mL) were prepared, and each bottle was filled with 50 mL of MS medium and sown with 20 seeds. Bottles were placed under constant temperature (24 ± 1 ℃) and photoperiod (12 h light/12 h dark cycle). After 10 days of growth, leaves of plants in the same bottles were harvested. Fresh leaves were frozen in liquid nitrogen and immediately stored at -80 ℃.
2.2. Determination of Cd concentration in leavesSamples were dried at 80 ℃ for 48 h and subsequently used to measure Cd concentrations according to a previously reported method (Li et al., 2016) using an inductively coupled plasma mass spectrometer (Thermo Fisher Scientific, US).
2.3. Antioxidant enzyme activity assayAntioxidant enzymes were extracted using previously described methods (Liu et al., 2010; Wang et al., 2010; Li et al., 2014b). Activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) were measured spectrophotometrically by monitoring changes in absorbance values at 560, 470, 240, 290, 265, and 340 nm, respectively (Liu et al., 2010; Wang et al., 2010; Li et al., 2014a). Enzyme activities were presented as nmol or μmol min-1 mg-1 protein.
2.4. Glutathione S-transferase (GST) activity assayGST activity was assayed based on a reported method (Liu et al., 2010). Briefly, 0.5 g samples were homogenized in 2.5 mL of 200 mmol L-1 Tris–HCl extraction solution (3% polyvinylpyrrolidone, 0.1 mmol L-1 ethylenediaminetetraacetic acid (EDTA), pH = 7.8) and subsequently centrifuged at 10, 000×g for 30 min. Supernatants were reacted with 50 mmol L-1phosphate-buffered saline (1 mmol L-1 1-Chloro-2, 4-dinitrochlorobenzene, pH = 7.4), and changes in absorbance value were measured at 340 nm.
2.5. Detection of superoxide anion (O2) and hydrogen peroxide (H2O2) accumulationIn situ detection of O2 and H2O2 was performed according to a previously reported method (Li et al., 2014b). The amount of O2 and H2O2 were detected by using nitro-blue tetrazolium solution (10-2 M) and diaminobenzidine solution (1 mg mL-1), respectively. Three cotyledons and leaves from different plants of each sample were separately vacuum-infiltrated in 10 mL solutions for 2 h and subsequently decolored in boiling ethanol (95%) for 10 min. Results included one representative cotyledon and leaf for each sample.
2.6. Malondialdehyde (MDA) content measurementMDA content was measured as previously described (Li et al., 2014a). Approximately 0.2 g of fresh leaves were homogenized in 10 mL of 10% trichloroacetic acid (TCA) and then centrifuged at 10, 000×g for 10 min. Afterward, 2 mL of 0.6% thiobarbituric acid in 10% TCA were added to 2 mL of supernatant. The mixture was subsequently heated in boiling water for 15 min and then quickly cooled in an ice bath. After centrifugation at 10, 000×g for 10 min, absorbance of supernatant was determined at 532, 600, and 450 nm. MDA concentration was expressed as nmol g-1 fresh weight (FW).
2.7. Glutathione (GSH) and PC content measurementGSH content was measured using a previously reported method with some modifications (Ellman, 1959; Zhang et al., 2001). Approximately 0.2 g of fresh leaves were homogenized with 3 mL 5% metaphosphoric acid solution and subsequently centrifuged for 10 min (12, 000×g) to obtain 3 mL of supernatant. Aliquots of the sample solution (0.2 mL) were mixed with 150 mmol L-1 NaH2PO4 and added with 5, 5′-Dithiobis-(2-nitrobenzoic acid) (DTNB) (73.5 mg dissolved in 30 mL 0.1 M phosphate buffer, pH 6.8). The mixture was kept at 30 ℃ for 5 min, and absorbance was determined at 412 nm.
PC content was represented as difference in content of acid-soluble thiol and GSH content (Song et al., 2011). For acid-soluble thiol analysis, 0.5 g of fresh leaves were homogenized with 5 mL 0.02 M EDTA and centrifuged for 10 min (12, 000×g at 4 ℃). Supernatant was analyzed as follows (Zhang et al., 2004): 1.5 mL of supernatant was mixed with 1.5 mL of 10% TCA and then centrifuged for 10 min (12, 000×g at 4 ℃). Then, supernatant (2 mL) was mixed with 0.4 M Tris buffer solution (4 mL, pH = 8.9) and 0.01 M DTNB (0.1 mL). Absorbance of the mixture was measured at 412 nm. Concentration of acid-soluble thiol was calculated using an extinction coefficient of 13, 100 (Zhang et al., 2004).
2.8. Determination of free amino acidsSamples were treated as follows. Approximately 2–5 g samples were vortex-mixed with 5 mL of 0.02 M HCl for 5 min. Mixtures were extracted by ultrasound for 5 min and then placed in the dark for 2 h. Mixtures were then centrifuged at 4000×g for 10 min. Briefly, 1 mL of 6% sulfosalicylic acid was added to 1 mL of supernatant, and the resulting mixtures were vortexed for 1 min and placed in the dark for 1 h. Mixtures were centrifuged at 15, 000×g for 15 min. Afterward, 500 μl of supernatant was mixed with 250 μl 1 M triethylamine acetonitrile solution and 250 μl of 0.1 M phenyl isothiocyanate acetonitrile solution and was subsequently left under room temperature for 1 h. The mixture was added with 2 mL n-hexane and was allowed to stand for 10 min after intense shaking. The resulting solution was filtered with 0.22 μm aqueous filter membrane and analyzed using high-performance liquid chromatography (Thermo Fisher U3000).
C18 chromatographic columns (4.6 mm × 250 mm, 5 μm) were used, and column temperature was 40 ℃ during experiments. Mobile phase A was 0.1 M sodium acetate solution:acetonitrile = 93:7; mobile phase B was acetonitrile:water = 8:2. Excitation wavelength for fluorescence detection measured 254 nm. Table 1 presents the gradient elution program.
| Time (min) | Flow velocity (mL min-1) | Mobile phase A (%) | Mobile phase B (%) |
| 0 | 1 | 100 | 0 |
| 14 | 1 | 85 | 15 |
| 29 | 1 | 66 | 34 |
| 30 | 1 | 0 | 100 |
| 37 | 1 | 0 | 100 |
| 37.1 | 1 | 100 | 0 |
| 45 | 100 | 0 |
Statistical analyses were performed using SPSS version 18.0. One-way ANOVA was used to analyze significant differences among multiple samples at 0.05 levels.
3. Results 3.1. Plant morphological changes and Cd accumulationWe recorded the morphological changes of turnip seedlings and measured Cd concentrations in turnip roots and leaves to reflect turnip seedling tolerance to Cd. Turnip seedlings were slightly damaged by the addition of 10 and 30 μmol L-1 Cd2+ in culture medium (Fig. 1A). Neither growth of stem nor root lengths of turnip seedlings were significantly inhibited by Cd treatment compared with control plants (Fig. 1B). However, turnip seedlings accumulated high Cd concentrations in both roots and leaves. Cd concentrations in turnip roots and leaves totaled 191.78 and 99.28 mg kg-1 DW, respectively, when treated with 10 μmol L-1 Cd (Fig. 1C). When treated with 30 μmol L-1 Cd, Cd concentrations in turnip roots and leaves reached 625.77 and 248.65 mg kg-1 DW, respectively (Fig. 1C).
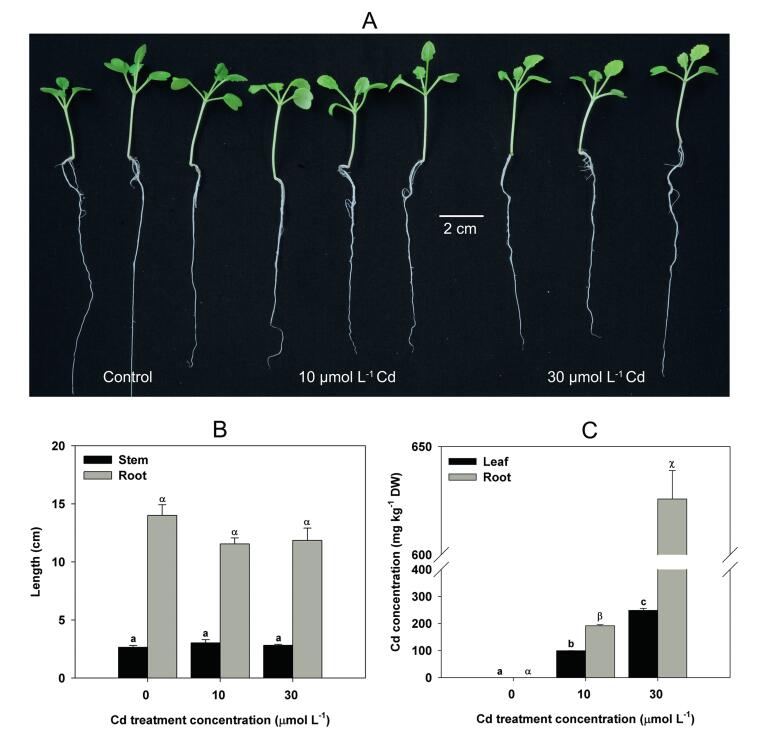
|
| Fig. 1 Plant morphological changes and Cd concentrations in turnip seedlings under different Cd treatment concentrations. (A) Morphology of turnip seedlings. (B) Stem and root length of turnip seedlings. (C) Cd concentrations in leaves and roots of turnip seedlings. Data are represented as means ± SE. Bars labeled with different letters (a–c or α–χ) are significantly different (n = 3, P < 0.05) (B and C). |
In order to explore the potential physiological and biochemical mechanisms of Cd detoxification in turnip seedlings, we first examined changes in the antioxidant system. The activities of different antioxidant enzymes in turnip leaves changed in response to increasing Cd treatment concentrations (Fig. 2). SOD activity was markedly induced by Cd treatment but showed no significant differences between the two Cd treatment concentrations (Fig. 2A). A similar pattern of change was observed for CAT, DHAR, and GR activities (Fig. 2C, E, and F). By contrast, POD activity increased when Cd treatment concentration increased (Fig. 2B), whereas APX activity increased in response to the 10 μmol L-1 Cd treatment but returned to control levels when treated with 30 μmol L-1 Cd (Fig. 2D).
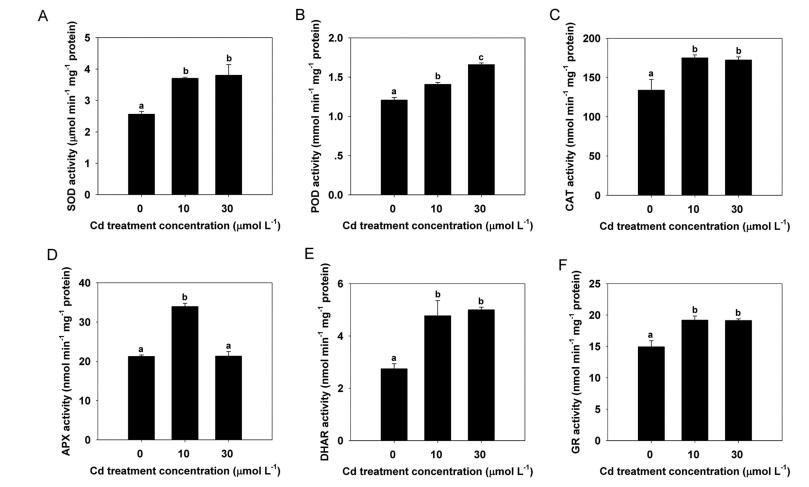
|
| Fig. 2 Changes in antioxidant enzyme activities in turnip leaves under different Cd treatment concentrations. (A) SOD activity. (B) POD activity. (C) CAT activity. (D) APX activity. (E) DHAR activity. (F) GR activity. Data are represented as means ± SE. Bars labeled with different letters are significantly different (n = 3, P < 0.05) (A–F). |
We then detected ROS accumulation to verify the roles of antioxidant enzymes. As shown in Fig. 3, the degree of staining reflected amounts of O2 (blue black) and H2O2 (tan) accumulation. When Cd treatment concentrations increased, O2 gradually accumulated to a certain degree in both leaves and cotyledons (Fig. 3A). However, smaller amounts of H2O2 were observed in turnip leaves and cotyledons treated with Cd compared with those in control conditions (Fig. 3B).
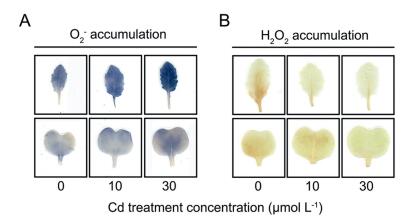
|
| Fig. 3 O2 (A) and H2O2 (B) accumulation in turnip leaves and cotyledons under different Cd treatment concentrations. |
As GSH and GST have played multiple roles in response to HM stresses in other plants (Wilce and Parker, 1994; Jiang et al., 2013), we also measured their contents in the present study. GSH content increased noticeably in response to increased Cd concentrations (Fig. 4A). GST activity was markedly induced by Cd treatment, although no significant differences were noted between the two Cd treatment concentrations (Fig. 4B).
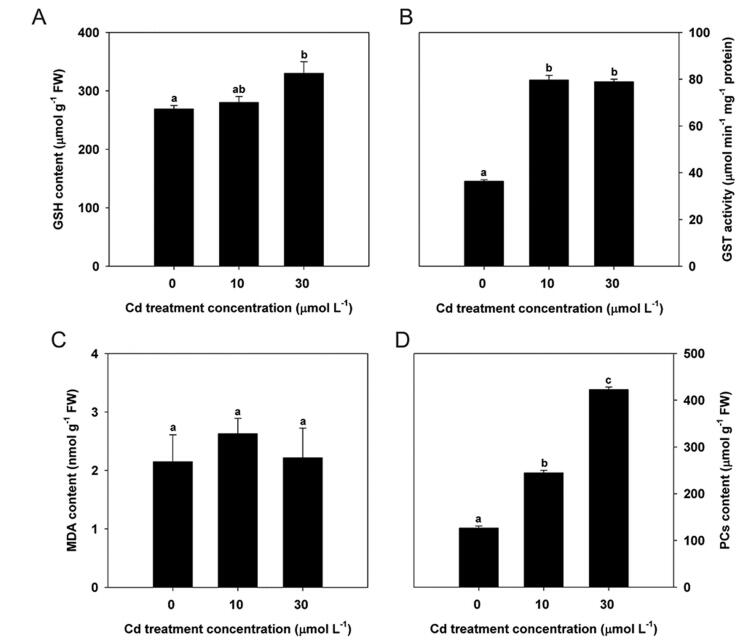
|
| Fig. 4 Changes in GSH content (A), GST activity (B), MDA content (C), and PC content (D) in turnip leaves under different Cd treatment concentrations. Data are represented as means ± SE. Bars labeled with different letters are significantly different (n = 3, P < 0.05) (A–D). |
MDA content was measured to reflect the oxidation stress degree caused by Cd. Interestingly, we found MDA content in turnip leaves was not significantly changed among different Cd treatment concentrations (Fig. 4C).
PCs are usually induced to chelate HMs in plants (Grill et al., 1985; Kneer and Zenk, 1992), so they were also measured in the present study. Compared to controls, PC content in turnip seedlings increased by 93.26% and 233.96% when incubated in Cd concentrations of 10 and 30 μmol L-1, respectively (Fig. 4D).
3.6. Changes in free amino acid contentIn addition, common free amino acid contents were measured to explore their roles in response to Cd stress. In the present study, 16 free amino acids, excluding cysteine, were detected in turnip leaves (Table 2). Contents of most of these amino acids were increased by Cd treatment and showed differing patterns of change (Table 2). Contents of aspartic acid and serine significantly increased under 10 μmol L-1 Cd treatment concentration but were reduced to a certain extent under 30 μmol L-1 Cd treatment concentration (Table 2). Content of aspartic acid remained higher than the control level, whereas serine content returned to control level (Table 2). Increasing Cd treatment concentrations gradually increased contents of glutamate, histidine, threonine, alanine, proline, tyrosine, valine, methionine, and phenylalanine. However, significant differences were not observed until Cd concentration reached 30 μmol L-1 (Table 2). Glycine content was significantly induced by Cd treatment but showed no significant differences between the two Cd treatment concentrations (Table 2). Contents of arginine, leucine, and lysine significantly increased when treated with increasing Cd concentrations, whereas isoleucine content showed no visible difference among different Cd treatment concentrations (Table 2).
| Amino acids | Cd treatment concentrations (μmol L-1) | ||
| 0 | 10 | 30 | |
| Aspartic acid | 0.247a | 0.380c | 0.297b |
| Glutamate | 0.270a | 0.283a | 0.323b |
| Serine | 0.177a | 0.283b | 0.193a |
| Glycine | 0.123a | 0.143b | 0.143b |
| Histidine | 0.093a | 0.080a | 0.130b |
| Arginine | 1.133a | 1.263b | 1.420c |
| Threonine | 0.040a | 0.040a | 0.057b |
| Alanine | 0.057a | 0.060a | 0.077b |
| Proline | 0.030a | 0.033ab | 0.043b |
| Tyrosine | 0.040a | 0.040a | 0.070b |
| Valine | 0.057a | 0.063ab | 0.073b |
| Methionine | 0.027a | 0.030a | 0.040b |
| Isoleucine | 0.030a | 0.030a | 0.037a |
| Leucine | 0.060a | 0.070b | 0.103c |
| Phenylalanine | 0.060a | 0.067a | 0.093b |
| Lysine | 0.153a | 0.173b | 0.220c |
| Cystine | ND | ND | ND |
In the present study, turnip seedlings accumulated high amounts of Cd when treated with relatively low Cd concentrations, further revealing the strong capacity of turnips to absorb Cd (Li et al., 2016). Results for both root and stem lengths indicated that turnip growth was not toxified by such high Cd concentrations in plants. The results were inconsistent with a previous report that the biomass of this turnip landrace was significantly inhibited under 50 mg Cd kg-1 dry soil (Li et al., 2016). The major reason might be attributed to the negative effects of high soil Cd concentration on soil fertility (Zheng and Shang, 2006) rather than the high Cd concentration in turnips. Therefore, understanding physiological and biochemical mechanisms underlying turnip response to Cd stress bears significance. Based on reported biochemical response processes (Fediuc and Erdei, 2002; Shekhawat et al., 2010; Nahar et al., 2016), we investigated activities of different important enzymes and compound contents and analyzed their possible functions during turnip tolerance and absorption of Cd. Ultimately, we determined that the antioxidant system, osmotic adjustment, chelating effects, and amino acid synthesis are involved in turnip response to Cd accumulation.
4.1. Effects on the antioxidant systemSimilar to other abiotic stresses, Cd-stressed plants usually display an imbalance between generation and elimination of ROS (Anjum et al., 2016; Hasanuzzaman et al., 2017). When oxidative injury under stress occurs, large amounts of ROS, including O2, H2O2 and ·OH, are produced and thus cause membrane system damage and cell injury (Sreenivasulu et al., 2000; Song et al., 2016). Plant mitochondria evolved mechanisms to minimize ROS production and to regulate ROS homeostasis (Song et al., 2016). Antioxidant systems, including enzymatic ROS-interactors (e.g., SOD, POD, and CAT), and non-enzymatic antioxidants, such as GSH, ascorbic acid (AsA), and tocopherols, play key roles in controlling excessive ROS accumulation (Moller, 2001). Through dismutation, SOD can reduce O2 to peroxide and H2O2, which can be further scavenged by POD and CAT, respectively. In the present study, we discovered that activities of SOD, POD, and CAT were significantly induced by Cd, indicating important roles for these enzymes in regulating ROS metabolism. Another way to eliminate H2O2 is through the AsA–GSH cycle (May et al., 1998), in which APX, GR, and monodehydroascorbate reductase serve as key enzymes (Li et al., 2010). The AsA–GSH cycle can regulate the redox environment by modulating GSH/glutathione disulfide (GSSG) and AsA/dehydroascorbate (DHA) interconversion (Ogawa, 2005), which includes several metabolic reactions (Secenji et al., 2010). APX serves as the first enzyme to directly degrade H2O2 into water, whereas AsA acts as an electron donor (Tao et al., 1998; Song et al., 2016). DHAR reduces DHA using electrons generated by GSH to provide electrons to AsA (Song et al., 2016). DHA is produced from monodehydroascorbate, whereas DHAR oxidizes GSH into GSSG, and GR subsequently reduces GSSG to GSH (Raseetha et al., 2013). The AsA–GSH cycle participates in the Cd response of plants (Tarhan and Kavakcioglu, 2016). Fang et al. (2004) reported that activities of APX, GR, and DHAR and contents of GSH and AsA significantly increased in pak choi under high-concentration Cd treatments. Our results also showed that activities of APX, DHAR, and GR, and GSH content in turnip were markedly increased by Cd treatment, indicating that the AsA–GSH cycle was efficiently maintained to cope with Cd stress. GSTs, a common category of enzymes, perform various functions in plants (Jiang et al., 2013). GSTs can catalyze a combination of GSH with toxic xenobiotics and oxidation products to improve metabolism, regionalization, isolation, or removal of these substances (Wilce and Parker, 1994; Jiang et al., 2013). In the present study, we observed a significant increase in GST activity under Cd treatment similar to that in previous studies (Kostaropoulos et al., 2005; Hu et al., 2009). Changes in activity of GST were relatively consistent with changes in GSH content. Hence, our results indicate that the GST-GSH system plays an important role in Cd tolerance in turnips. Some studies considered that free proline and PCs, whose contents were significantly increased by Cd treatment in our study, may also serve as antioxidants in cells (Smirnoff and Cumbes, 1989; Alia et al., 2001; Song et al., 2011).
In summary, our results indicate that antioxidant systems may significantly contribute to an increase in Cd tolerance in turnips. For confirmation, we further determined changes in the amounts of two representative ROS components, O2 and H2O2, which showed slight variations. These findings revealed that turnips can utilize antioxidant systems to regulate ROS homeostasis during Cd stress. Correspondingly, the content of MDA, a lipid peroxidation product that reflects the degree of oxidative stress, remained at stable levels.
4.2. Effects of osmotic adjustmentA change in osmotic potential is one of the main reactions in plant cells subjected to drought, extreme temperatures, and salt or HM stresses (Chen and Jiang, 2010; Li et al., 2014b, 2015). Plants undergo osmotic adjustment to reduce osmotic potential and to prevent physiological dehydration (Li, 1994). Maintaining water balance in cells or tissues, stabilizing the structure of biological macromolecules, and maintaining the integrity of membrane structure bear significance in protecting cells from injury (Yang et al., 2015). A stable osmotic environment is the basic requirement for the normal physiological function of cells. Thus, osmotic adjustment capacity often correlates with tolerance of plants to various stresses; this tolerance is usually reflected in the contents of osmotic adjustment substances, such as proline and betaine (Yang et al., 2015). Proline adjusts osmotic potential in plants during Cd stress (Sharma and Dietz, 2006; Li, 2010; Li et al., 2015); accumulation of proline relies on the degree of the water deficit induced by Cd stress in plants. In our study, proline was accumulated in plant seedlings in response to increased Cd concentrations, indicating that proline plays a significant role in osmotic adjustment during Cd stress. Li (2010) mentioned that aspartic acid can also accumulate to maintain cell osmotic pressure; however, evidence remains insufficient to support this conclusion. Accumulations of these water-soluble amino acids may help adjust osmotic potential in cells under Cd stress (Yang et al., 2015).
4.3. Functions of chelating effectChelating effect refers to chelation of free HM ions in plants by various organic molecules, including metallothionein, PCs, some organic acids, or amino acids, into stable complexes to reduce the toxicity of HMs (Cai et al., 2003). PCs are low molecular proteins enriched with –SH and are widespread in plants (Salt et al., 1995). Although PC content is usually maintained at relatively low levels, it rapidly increases when plants are stressed by HMs. Using an isotope tracer technique, Kneer and Zenk (1992) observed that the content of PC–Cd complexes in Cd-tolerant plants increased by 10–1000 folds under Cd treatment. Grill et al. (1985) estimated that 90% of Cd in plants can be bound by PCs, and at least 60% of Cd absorbed in the roots are chelated into Cd–PC complexes. PCs can also reduce HM toxicity to plants in vitro. Srivastava et al. (2004) reported that Arabidopsis mutants poisoned by 0.6 M Cd showed weaker symptoms when PCs were added to solution. PCs mainly consist of cysteine, glutamate, and glycine (Cai et al., 2003) and are synthesized from GSH (Grill et al., 1989), which can also be directly combined with Cd and transport Cd into vacuoles or non-protoplasts (Belcastro et al., 2009). In the present study, the contents of glutamate, glycine, GSH, and PCs were increased greatly by Cd treatment, indicating that synthesis of GSH and PCs was stimulated to chelate Cd2+ in turnip leaves.
Histidine is another important chelating agent in some plants, especially in Ni hyperaccumulators (Assuncao et al., 2003). Histidine takes part in Ni transport in the Ni hyperaccumulator Alyssum lesbiacum. Free histidine content increased substantially when A. lesbiacum plants were treated with Ni; exogenous histidine can significantly promote Ni tolerance and translocation in Ni non-hyperaccumulator A. montanum (Kramer et al., 1996). Similar results were observed in B. juncea (Kerkeb and Kramer, 2003). Histidine also participates in zinc (Zn) transport in plants (Kozhevnikova et al., 2014). Salt et al. (1999) discovered Zn–His complexes in Zn hyperaccumulator Thlaspi caerulescens. However, few studies have reported the involvement of histidine in chelating Cd. Li (2010) discovered that changes in histidine content were moderately correlated with Cd stress in Kandelia candelseedlings. In the present study, histidine content in turnip leaves significantly increased under high Cd treatment concentration, indicating that histidine may exercise similar chelating effects in Ni or Zn hyperaccumulators. However, further studies must be conducted to determine the precise functions of histidine. Proline and aspartic acid also act as metal-chelating agents according to some studies (Bottari and Festa, 1996; Sharma et al., 1998). Li (2010) suggested that Cd may chelate with aspartic acid to form Cd–Asp complexes in K. candel seedlings. In our study, proline and aspartic acid contents both increased in response to Cd treatments, indicating that they may contribute to the Cd response by acting as metal-chelating agents.
4.4. Effects of amino acid synthesisAmino acids are necessary nutrients for plant growth and synthetic materials of many biological macromolecules. In plants, changes in amino acids reflect the condition of nitrogen metabolism in plants; nitrogen metabolism is a primary plant reaction under HM stress (Sharma and Dietz, 2006). Synthesis of amino acids and nitrogen metabolism under HM stresses are complex processes and closely correlate with plant response to stresses (Li, 2010). Previous studies reported increased contents of various amino acids in plants under Cd stress (Sharma and Dietz, 2006; Li, 2010), and almost all detected free amino acids were significantly increased by Cd treatment in turnips. This observation may be attributed to three aspects. First, some amino acids follow the same synthetic pathway (Li, 2010); hence, their contents consistently increase with increasing content of synthetic substrate. Second, some amino acids, such as proline and histidine, are induced to perform special functions in tolerating and transporting Cd. As mentioned above, proline plays an important role in osmotic adjustment and can serve as a chelant and antioxidant, whereas histidine mainly exhibits chelating effects (Li, 2010). However, although many amino acids are closely related to Cd or other HM treatments in plants (Sharma and Dietz, 2006; Li, 2010), their precise function and mode of action remain poorly understood. Most of these amino acids may be induced to synthesize biological macromolecules, especially proteins. In addition to enzymes or proteins mentioned in this paper, many proteins play important roles in tolerating and accumulating HMs. These proteins mainly fall into two categories: resistance proteins and transport proteins. The former comprises members of heat shock proteins (Gulli et al., 2005; Li et al., 2015), late embryogenesis abundant proteins (Gao et al., 2012), and other protein families (Li et al., 2015); whereas the latter includes typical protein families, such as ATP-binding cassette transporters, cation efflux proteins, H+/cation exchangers, and Zrt/Irt-like protein (Zhang et al., 2013). Many proteomic analyses have been conducted to investigate these proteins and explore novel proteins involved in Cd response (Repetto et al., 2003; D'alessandro et al., 2013; Li et al., 2015); thus, proteomics can be used to study mechanisms underlying Cd tolerance and accumulation in turnips. Some amino acids are also substrates for synthesis of many chemicals. For example, phenylalanine serves as a substrate for many phenolic compounds, which pose resistance effects on various biotic and abiotic stresses, including HM stresses (Ye et al., 2006). Thus, metabolomic analysis is also method potential approach for future explorations of the mechanisms underlying turnip tolerance to Cd stress; this method has already been applied to other plants (D'alessandro et al., 2013; Luo et al., 2014, 2015).
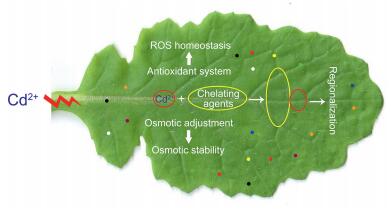
5. ConclusionIn the present study, we compared physiological and biochemical changes in turnip leaves treated with two Cd concentrations versus to controls. We observed that antioxidant system, including the GSH–AsA cycle, was significantly enhanced, whereas ROS accumulation and oxidation remained almost stable under Cd treatment. The contents of PCs and many amino acids increased, indicating that they may play a role in coping with Cd stress. Based on changes in enzyme activity and compounds content, we conclude that turnips initiate a series of response processes when Cd is incorporated in its leaves. First, antioxidant systems, which maintain ROS homeostasis and osmotic adjustment, are excited to retain stability of cell osmotic potential. These responses significantly contribute to protecting cells and tissues from injury. Cd is chelated into stable complexes to reduce its toxicity. Cd may be transported to vacuoles or non-protoplasts for isolation. Amino acid synthesis directly and indirectly plays key roles during these processes. A proposed model was used to reflect potential physiological and biochemical mechanisms underlying turnip response to Cd (Fig. 5). However, the response of amino acids to Cd stress suggests that proteomics and metabolomics are necessary to explore the abundant and accurate material basis for Cd tolerance and accumulation in turnips.
|
| Fig. 5 Schematic illustration of a proposed model for potential physiological and biochemical processes underlying turnip response to Cd stress. Colored dots indicate various free amino acids. |
This work was financially supported by the Western Youth Project B of the "Light of West China" Program of Chinese Academy of Sciences (Y7260411W1) and the National Natural Science Foundation of China (31590823).
Alia Mohanty P., Matysik J., 2001. Effect of proline on the production of singlet oxygen. Amino Acids, 21: 195-200. DOI:10.1007/s007260170026 |
Anjum S.A., Tanveer M., Hussain S., Shahzad B., Ashraf U., Fahad S., Hassan W., Jan S., Khan I., Saleem M.F., Bajwa A.A., Wang L.C., Mahmood A., Samad R.A., Tung S.A., 2016. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res, 23: 11864-11875. DOI:10.1007/s11356-016-6382-1 |
Assuncao A.G.L., Schat H., Aarts M.G.M., 2003. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol, 159: 351-360. DOI:10.1046/j.1469-8137.2003.00820.x |
Belcastro M., Marino T., Russo N., Toscano M., 2009. The role of glutathione in cadmium ion detoxification:coordination modes and binding properties-a density functional study. J. Inorg. Biochem, 103: 50-57. DOI:10.1016/j.jinorgbio.2008.09.002 |
Bottari E., Festa M.R., 1996. Asparagine as a ligand for cadmium (Ⅱ), lead (Ⅱ) and zinc (Ⅱ). Chem. Speciat. Bioavailab, 8: 75-83. DOI:10.1080/09542299.1996.11083272 |
Cai B.S., Lei M., Chen T.B., Zhang G.P., Chen Y., 2003. Phytochelatins and their roles in phyto-tolerance to heavy metals:a review. Acta Ecol. Sin, 23: 2125-2132. |
Che J., Yamaji N., Shao J.F., Ma J.F., Shen R.F., 2016. Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J. Exp. Bot, 67: 1535-1544. DOI:10.1093/jxb/erv545 |
Chen H., Jiang J.G., 2010. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev, 18: 309-319. DOI:10.1139/A10-014 |
Choppala G., Saifullah Bolan, N ., Bibi S., Iqbal M., Rengel Z., Kunhikrishnan A., Ashwath N., Ok Y.S., 2014. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit. Rev. Plant Sci, 33: 374-391. DOI:10.1080/07352689.2014.903747 |
D'alessandro A., Taamalli M., Gevi F., Timperio A.M., Zolla L., Ghnaya T., 2013. Cadmium stress responses in Brassica juncea:hints from proteomics and metabolomics. J. Proteome Res, 12: 4979-4997. DOI:10.1021/pr400793e |
Ehsan S., Ali S., Noureen S., Mahmood K., Farid M., Ishaque W., Shakoor M.B., Rizwan M., 2014. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol. Environ. Saf, 106: 164-172. DOI:10.1016/j.ecoenv.2014.03.007 |
Ellman G.L., 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys, 82: 70-77. DOI:10.1016/0003-9861(59)90090-6 |
Fang X.Z., Zhu Z.J., Sun G.W., 2004. Effects of different concentrations of cadmium on growth and antioxidant system in Brassica campestris ssp. Chinese. J. Agro Environ. Sci, 23: 877-880. |
Fediuc E., Erdei L., 2002. Physiological and biochemical aspects of cadmium toxicity and protective mechanisms induced in Phragmites australis and Typha latifolia. J. Plant Physiol, 159: 265-271. DOI:10.1078/0176-1617-00639 |
Gao C.Q., Wang C., Zheng L., Wang L.Q., Wang Y.C., 2012. A LEA gene regulates cadmium tolerance by mediating physiological responses. Int. J. Mol. Sci, 13: 5468-5481. DOI:10.3390/ijms13055468 |
Grill E., Winnacker E.L., Zenk M.H., 1985. Phytochelatins-the principal heavymetal complexing peptides of higher-plants. Science, 230: 674-676. DOI:10.1126/science.230.4726.674 |
Grill E., Loffler S., Winnacker E.L., Zenk M.H., 1989. Phytochelatins, the heavymetal-binding peptides of plants, are synthesized from glutathione by a specific gamma-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. U.S.A, 86: 6838-6842. DOI:10.1073/pnas.86.18.6838 |
Gulli M., Rampino P., Lupotto E., Marmiroli N., Perrotta C., 2005. The effect of heat stress and cadmium ions on the expression of a small hsp gene in barley and maize. J. Cereal. Sci, 42: 25-31. DOI:10.1016/j.jcs.2005.01.006 |
Hasanuzzaman M., Nahar K., Gill S.S., Alharby H.F., Razafindrabe B.H.N., Fujita M., 2017. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.:an intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci, 8: 115. DOI:10.3389/fpls.2017.00115 |
He Q.X., 2013. Research progress of screening cadmium hyperaccumulators. Environ. Protect. Circular Econ, 33: 46-49. |
Hu Y.L., Zhang C.H., Ju T., Ge Y., 2009. Differential responses of GSH and GST in two rice cultivars under Cd stress. J. Agro-Environ. Sci, 28: 305-310. |
Jiang D.L., Cai H., Duanmu H.Z., Zhu Y.M., 2013. Genome-wide filter, classification and expression analysis of GST gene family in soybean. Mol. Plant Breed, 11: 465-475. |
Kerkeb L., Kramer U., 2003. The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiol, 131: 716-724. DOI:10.1104/pp102.010686 |
Kneer R., Zenk M.H., 1992. Phytochelatins protect plant enzymes from heavy-metal poisoning. Phytochemistry, 31: 2663-2667. DOI:10.1016/0031-9422(92)83607-Z |
Kostaropoulos I., Kalmanti D., Theodoropoulou B., Loumbourdis N., 2005. Effects of exposure to a mixture of cadmium and chromium on detoxification enzyme(GST, P450-MO) activities in the frog Rana ridibunda. Ecotoxicology, 14: 439-447. DOI:10.1007/s10646-004-1349-2 |
Kozhevnikova A.D., Seregin I.V., Erlikh N.T., Shevyreva T.A., Andreev I.M., Verweij R., Schat H., 2014. Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens. New Phytol, 203: 508-519. DOI:10.1111/nph.12816 |
Kramer U., 2005. Phytoremediation:novel approaches to cleaning up polluted soils. Curr. Opin. Biotechnol, 16: 133-141. DOI:10.1016/j.copbio.2005.02.006 |
Kramer U., Cotterhowells J.D., Charnock J.M., Baker A.J.M., Smith J.A.C., 1996. Free histidine as a metal chelator in plants that accumulate nickel. Nature, 379: 635-638. DOI:10.1038/379635a0 |
Li Y., 1994. The relationship between osmotic adjustment and other physiological processes in plants and its application in crop improvement. Plant Physiol. Commun, 30: 377-385. |
Li, W., 2010. Effect of Cd on Nutrient Elements and Free Amino Acids of Kandelia candel Seedlings. Master's thesis. Sun Yat-sen University, Guangzhou.
|
Li Y.L., Liu Y.F., Zhang J.G., 2010. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. China, 4: 84-90. DOI:10.1007/s11703-009-0089-8 |
Li X., Yang Y.Q., Ma L., Sun X.D., Yang S.H., Kong X.X., Hu X.Y., Yang Y.P., 2014a. Comparative proteomics analyses of Kobresia pygmaea adaptation to environment along an elevational gradient on the central Tibetan Plateau. PLoS One, 9: e98410. DOI:10.1371/journal.pone.0098410 |
Li X., Yang Y.Q., Sun X.D., Lin H.M., Chen J.H., Ren J., Hu X.Y., Yang Y.P., 2014b. Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS One, 9: e107605. DOI:10.1371/journal.pone.0107605 |
Li X., Zhou Y.L., Yang Y.Q., Yang S.H., Sun X.D., Yang Y.P., 2015. Physiological and proteomics analyses reveal the mechanism of Eichhornia crassipes tolerance to high-concentration cadmium stress compared with Pistia stratiotes. PLoS One, 10: e0124304. DOI:10.1371/journal.pone.0124304 |
Li X., Zhang X.M., Yang Y., Li B.Q., Wu Y.S., Sun H., Yang Y.P., 2016. Cadmium accumulation characteristics in turnip landraces from China and assessment of their phytoremediation potential for contaminated soils. Front. Plant Sci, 7: 1862. DOI:10.3389/fpls.2016.01862 |
Liu J.X., Wang A.X., Li B.P., 2010. Effects of exogenous nitric oxide donor on the ascorbate-glutathione in Peganum multisectum seedling leaves under NaCl stress. Bull. Bot. Res, 30: 37-41. |
Luo Q., Sun L.N., Hu X.M., Zhou R.R., 2014. The variation of root exudates from the hyperaccumulator Sedum alfredii under cadmium stress:metabonomics analysis. PLoS One, 9: e115581. DOI:10.1371/journal.pone.0115581 |
Luo Q., Sun L.N., Hu X.M., 2015. Metabonomics study on root exudates of cadmium hyperaccumulator Sedum alfredii. Chin. J. Anal. Chem, 43: 7-11. DOI:10.1016/S1872-2040(15)60795-2 |
May M.J., Vernoux T., Leaver C., Van Montagu M., Inze D., 1998. Glutathione homeostasis in plants:implications for environmental sensing and plant development. J. Exp. Bot, 49: 649-667. |
Moller I.M., 2001. Plant mitochondria and oxidative stress:electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol, 52: 561-591. DOI:10.1146/annurev.arplant.52.1.561 |
Moreno-Caselles J., Moral R., Perez-Espinosa A., Perez-Murcia M.D., 2000. Cadmium accumulation and distribution in cucumber plant. J. Plant Nutr, 23: 243-250. DOI:10.1080/01904160009382011 |
Morikawa C.K., 2017. Reducing cadmium accumulation in fresh pepper fruits by grafting. Hortic. J, 86: 45-51. DOI:10.2503/hortj.MI-136 |
Nahar K., Rahman M., Hasanuzzaman M., Alam M.M., Rahman A., Suzuki T., Fujita M., 2016. Physiological and biochemical mechanisms of spermineinduced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res, 23: 21206-21218. DOI:10.1007/s11356-016-7295-8 |
Ogawa K., 2005. Glutathione-associated regulation of plant growth and stress responses. Antioxidants Redox Signal, 7: 973-981. DOI:10.1089/ars.2005.7.973 |
Pan F.S., Meng Q., Wang Q., Luo S., Chen B., Khan K.Y., Yang X.E., Feng Y., 2016. Endophytic bacterium Sphingomonas SaMR12 promotes cadmium accumulation by increasing glutathione biosynthesis in Sedum alfredii Hance. Chemosphere, 154: 358-366. DOI:10.1016/j.chemosphere.2016.03.120 |
Raseetha S., Leong S.Y., Burritt D.J., Oey I., 2013. Understanding the degradation of ascorbic acid and glutathione in relation to the levels of oxidative stress biomarkers in broccoli (Brassica oleracea L. italica cv. Bellstar) during storage and mechanical processing. Food Chem, 138: 1360-1369. DOI:10.1016/j.foodchem.2012.09.126 |
Repetto O., Bestel-Corre G., Dumas-Gaudot E., Berta G., Gianinazzi-Pearson V., Gianinazzi S., 2003. Targeted proteomics to identify cadmium-induced protein modifications in Glomus mosseae-inoculated pea roots. New Phytol, 157: 555-567. DOI:10.1046/j.1469-8137.2003.00682.x |
Salt D.E., Prince R.C., Pickering I.J., Raskin I., 1995. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol, 109: 1427-1433. DOI:10.1104/pp.109.4.1427 |
Salt D.E., Prince R.C., Baker A.J.M., Raskin I., Pickering I.J., 1999. Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using X-ray absorption spectroscopy. Environ. Sci. Technol, 33: 713-717. DOI:10.1021/es980825x |
Secenji M., Hideg E., Bebes A., Gyorgyey J., 2010. Transcriptional differences in gene families of the ascorbate-glutathione cycle in wheat during mild water deficit. Plant Cell Rep, 29: 37-50. DOI:10.1007/s00299-009-0796-x |
Sharma S.S., Dietz K.J., 2006. The significance of amino acids and amino acidderived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot, 57: 711-726. DOI:10.1093/jxb/erj073 |
Sharma S.S., Schat H., Vooijs R., 1998. In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry, 49: 1531-1535. DOI:10.1016/S0031-9422(98)00282-9 |
Shekhawat G.S., Verma K., Jana S., Singh K., Teotia P., Prasad A., 2010. In vitro biochemical evaluation of cadmium tolerance mechanism in callus and seedlings of Brassica juncea. Protoplasma, 239: 31-38. DOI:10.1007/s00709-009-0079-y |
Smirnoff N., Cumbes Q.J., 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry, 28: 1057-1060. DOI:10.1016/0031-9422(89)80182-7 |
Song X.D., Lv J.Y., Di L.J., Liu X.T., Ye Q.F., 2011. Effects of chromium stress on the content of phytochelatins and antioxidative characteristics in Brassica chinensis L. J. Agro-Environ. Sci, 30: 843-848. |
Song L.L., Wang J.H., Shafi M., Liu Y., Wang J., Wu J.S., Wu A.M., 2016. Hypobaric treatment effects on chilling injury, mitochondrial dysfunction, and the ascorbate-glutathione (AsA-GSH) cycle in postharvest peach fruit. J. Agric. Food Chem, 64: 4665-4674. DOI:10.1021/acs.jafc.6b00623 |
Song Y., Jin L., Wang X.J., 2017. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediation, 19: 133-141. DOI:10.1080/15226514.2016.1207598 |
Sreenivasulu N., Grimm B., Wobus U., Weschke W., 2000. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plantarum, 109: 435-442. DOI:10.1034/j.1399-3054.2000.100410.x |
Srivastava S., Tripathi R.D., Dwivedi U.N., 2004. Synthesis of phytochelatins and modulation of antioxidants in response to cadmium stress in Cuscuta reflexa -an angiospermic parasite. J. Plant Physiol, 161: 665-674. DOI:10.1078/0176-1617-01274 |
Tao D.L., Oquist G., Wingsle G., 1998. Active oxygen scavengers during cold acclimation of Scots pine seedlings in relation to freezing tolerance. Cryobiology, 37: 38-45. DOI:10.1006/cryo.1998.2096 |
Tarhan L., Kavakcioglu B., 2016. Glutathione metabolism in Urtica dioica in response to cadmium based oxidative stress. Biol. Plantarum, 60: 163-172. DOI:10.1007/s10535-015-0570-6 |
Tauqeer H.M., Ali S., Rizwan M., Ali Q., Saeed R., Iftikhar U., Ahmad R., Farid M., Abbasi G.H., 2016. Phytoremediation of heavy metals by Alternanthera bettzickiana:growth and physiological response. Ecotoxicol. Environ. Saf, 126: 138-146. DOI:10.1016/j.ecoenv.2015.12.031 |
Wang L.N., Yang L.M., Yang F.J., Li X.G., Song Y.P., Wang X.F., Hu X.Y., 2010. Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J. Plant Physiol, 167: 1298-1306. DOI:10.1016/j.jplph.2010.04.007 |
Wei S.H., Zhou Q.X., Koval P.V., 2006. Flowering stage characteristics of cadmium hyperaccumulator Solanum nigrum L. and their significance to phytoremediation. Sci. Total Environ, 369: 441-446. DOI:10.1016/j.scitotenv.2006.06.014 |
Wilce M.C.J., Parker M.W., 1994. Structure and function of glutathione S-transferases. Bba-Protein. Struct. Mater, 1205: 1-18. DOI:10.1016/0167-4838(94)90086-8 |
Xiao C.W., Luo X.Y., Tian Y., Lu X.Y., 2013. Research progress of bioremediation of heavy metal cadmium pollution. Chem. Bioeng, 30: 1-4. |
Yang D.G., Liu Y.X., Zhang Q., Jiang Z.Z., Song B.G., 2015. Progress on crops osmotic adjustment and genetic engineering of osmotic stress resistance. Crops, 1: 6-13. |
Ye S.F., Zhou Y.H., Sun Y., Zou L.Y., Yu J.Q., 2006. Cinnamic acid causes oxidative stress in cucumber roots, and promotes incidence of Fusarium wilt. Environ. Exp. Bot, 56: 255-262. DOI:10.1016/j.envexpbot.2005.02.010 |
Zhang B.J., Zhang X.X., Luo L.G., 2013. The major gene families related to cadmium absorption and transportation in plants. Genom. Appl. Biol, 32: 127-134. |
Zhang Z.S., Li R.Q., Wang J.B., 2001. Effects of oxalate treatment on the membrane permeability and calcium distribution in pepper leaves under heat stress. Acta Phytophys. Sin, 27: 109-113. |
Zhang W., Cai Y., Downum K.R., Ma L.Q., 2004. Thiol synthesis and arsenic hyperaccumulation in Pteris vittata (Chinese brake fern). Environ. Pollut, 131: 337-345. DOI:10.1016/j.envpol.2004.03.010 |
Zheng S.Y., Shang X.F., 2006. Research progress of cadmium pollution in soil. Anhui Agri. Sci. Bull, 12: 43-44. |



