b. University of Chinese Academy of Sciences, Beijing 100049, China;
c. Systematics and Biodiversity Unit, Central Department of Botany, Tribhuvan University, Kirtipur, Kathmandu, Nepal;
d. Jade Consult Private Limited, Kabilmarg, Thapathali, Kathmandu, POB 746, Nepal;
e. World Agroforestry Centre East and Central Asia, 132 Lanhei Rd, Heilongtan, Kunming, China
The importance of carbon sequestration increases when there is a sufficient number of trees to absorb the emitted carbon (McCarl and Callaway, 1993). Absorption of carbon is only possible when nitrogen binds phytomass with assimilated carbon during photosynthesis. Fixation of atmospheric nitrogen is essential so that it is readily available to trees (Galloway et al., 2004). The genus Alnus, belong to the birch family host species from the symbiotic endophytic genus Frankia (Khan et al., 2007). The symbiont Frankia uses carbohydrates from alder trees to convert atmospheric N2 into reactive nitrogen, a nitrate form directly available to plants growing in the soil (Myrold and Huss-Dannel, 1994). Alnus species host nitrogen-fixing bacteria; therefore, they are ecologically important in nitrogen fixation. Alnus are pioneer species that establish in eroded and exposed soils, which in the course of time improve land health. Alders are vigorous and fast-growing, even in acidic soil and damaged sites such as burned areas and mining sites, further adding to their importance as the species of choice in forest restoration programs.
Alnus spp., specifically A. nepalensis, have commonly been used in traditional agroforestry systems as shade, fodder, fuelwood and timber. Traditional agroforestry systems are unique in hilly regions of Nepal and adjoining Himalayan countries (Sharma et al., 2007). Previous research has revealed that this species is an excellent choice for soil restoration and sloping land management. Alnus plantation with cardamom (Sharma et al., 2002), tea (Mortimer et al., 2015), and mandarin oranges (Chhetri and Gauchan, 2008) represent a few examples of the importance of Alnus in the agroforestry system. Such mixed plantation is beneficial to commercial crops.
Agroforestry systems are examples of the ecological aid and economic viability for people compared to rainfed agriculture (Sharma et al., 2007). Therefore, it is crucial to discover suitable areas for important tree species such as Alnus for agroforestry planning (Ranjitkar et al., 2016). Understanding and identifying potential growing areas will provide insight for the intercropping of commercial plant species that are essential for the livelihood of smallholder farmers. Alder species are planted throughout the hilly region and distributed throughout the Himalayas. It is essential to accurately discern how climatic factors determine the distribution probability of different Alnus species to recommend right species at the appropriate bioclimatic zone. Also, bioclimatic zone identification can identify suitable spaces where these species can be grown alternative to each other. In this study, we aim to describe the habitat suitability of the alder using species distribution modelling (SDM) methods. Specifically, we determine the best model for distribution probability and habitat suitability of focal species. We use that model to identify the climatic variables critical for occurrence of Alnus species. Finally, we determine the areas for plantations of two Alnus spp. in Nepal for appropriate agroforestry practice.
2. Materials and methods 2.1. Focal speciesTwo species of Alnus are reported in Nepal: Alnus nepalensis D. Don and Alnus nitida(Spach) Endl. A. nepalensis is the eastern Himalayan component distributed from northwest India through the Himalayas to Guangxi, Guizhou, Sichuan in China in the east, to Thailand, Vietnam, and Laos in the south (Shaw et al., 2014a). A. nitida is a native to the western Himalayas distributed from Afghanistan and Pakistan to northwestern India and western Nepal (Shaw et al., 2014b). In Nepal, A. nitida is somewhat rare and still requires careful investigation in the western part of the country. The species is known to occur in pure forest stands in the upper Karnali river valley in western Nepal (Fig. 1). It grows well in moist sandy soils, whereas A. nepalensis grows throughout hilly regions, in a wide range of soil including freshly exposed landslide, as well as degraded and disturbed sites. Encroachment, forest degradation, over-exploitation for fuel-wood and timber collection are known threats to A. nepalensis trees growing in the Nepal. Biological threats to A. nepalensis include the stem borers Batocera spp., an aphid Eutfichosiphym alnifoliae pest of economic importance, and a parasitic plant infestation (O'Neill and Rana, 2016). Biological threats have yet to be identified for A. nitida; however, forest degradation and habitat fragmentation are known to threaten the species.

|
| Fig. 1 Himalayan alder records from different parts of Nepal. Vertical lines represent tentative division of eastern (east to 86.3°), central and western (west to 83°) Nepal based on phytogeography (Stearn, 1960). |
We used herbarium specimen data (from 1968 to 2012) and field surveys (Appendix A4) to compile the location of occurrence for the focal species. Specifically, herbarium specimen data were gathered directly at the National Herbarium and Plant Laboratory (KATH) and Tribhuvan University Central Herbarium (TUCH) (Appendix A1 and A5). In addition, specimen data were gathered online from University of Tokyo (TI) (Appendix A2) and the Royal Botanical Garden at Edinburgh (RBGE) (Appendix A3 and A6). Present geographical locations of the species were confirmed through "ground truthing" in several places, where rapid field surveys, communication with community forest members, forest officers and residents were useful. All the geo-referenced recorded points were used to check spatial autocorrelation of the species distribution using binary presence/absence data. We selected random background points within an area of 50 km radius from the point of occurrence for each focal species in order to examine Moran's I for spatial autocorrelation in Arc GIS 10.2.1. Presence points were spread to 10-min spatial grid to obtain least spatial autocorrelation (Ranjitkar et al., 2014a), which reduces A. nepalensis occurrence to 67 and A. nitida to 12 (Appendix B1). We use these treated points for further analysis in the selection of predictive variables and the calibration of the model.
2.3. Environmental and bioclimatic dataTo model the potential distribution of Himalayan alders, we first collected 26 predictive variables (21 bioclimatic, four topographic and LULC). Out of 26 variables, we selected significant variables for modelling, considering their contribution and importance in the model calibration, collinearity among variables, Jackknife analysis and biological significance for the focal species. Among the two highly correlated variables (Pearson correlation coefficient, r > 0.80) (Appendix C1), one was chosen for its biological relevance to the species and ease of interpretation for inclusion in the model (Kumar and Stohlgren, 2009; Rana et al., 2017). Finally, a subset of the least correlated variables was selected (Table 1) as predictor variables.
| Category | Name of variables | Source |
| Years | Current as baseline and 2050 for future | |
| Scenarios | Ensemble of downscaled CIMP5 ESM Models under 4 different RCPs | Taylor et al., 2012 |
| Area | Nepal (1, 47, 181 km2) | |
| Spatial resolution | 1 km (~30 arc-sec) | |
| Climate | Bio3 = Isothermality | www.worldclim.org/bioclim |
| Bio4 = Temperature Seasonality | Hijmans et al., 2005 | |
| Bio6 = Min Temperature of Coldest Month | ||
| Bio9 = Mean Temperature of Driest Quarter | ||
| Bio15 = Precipitation Seasonality | ||
| Bio17 = Precipitation of Driest Quarter | ||
| Bio18 = Precipitation of Warmest Quarter | ||
| Topographic | Slope | www.eros.usgs.gov |
| Aspects (Northness) | Calculated from DEM | |
| Aspects (Eastness) | ||
| t_rgh = Terrain roughness | ||
| LULC | LULC = Landuse Landcover | www.diva-gis.org/gdata (GLC 2000) |
The bioclimatic dataset available from the Worldclim (www.worldclim.org/bioclim; Hijmans et al., 2005) was used for current (1950–2000) predictions. At the same time, future (2050) bioclimatic variables were derived from the results of 19 Earth System Models (ESM) provided by the Coupled Model Inter-comparison Project-Phase5 (CIMP5; Taylor et al., 2012). Slope, aspects, and terrain roughness derived from Aster Digital Elevation Model (DEM) (www.eros.usgs.gov) and Landuse-landcover (LULC) (Roy et al., 2003) were also included in the model (Appendix C2). All the predictor layers are at a spatial resolution of 30 arc-seconds.
2.4. Modeling approachWe used MaxEnt 3.3.3k (Maximum Entropy) for modelling purposes. MaxEnt uses only presence data in combination with environmental data for the whole study area to derive a model and predict suitable conditions or ecological niches (Phillips et al., 2004, 2006; Phillips and Dudik, 2008). Altogether 50 replicates of the model with the least-correlated set of variables were run, setting MaxEnt to select 75% of occurrence localities randomly for calibrating, and the remaining 25% for evaluating at each run. The bootstrap method was used with maximum iteration = 5000, convergence threshold = 0.0001, maximum background points = 10, 000, replication 50 and default features.
The current suitable niche was forecasted using the significant models among the four models using least correlated variables. The four models used bioclimatic variables, bioclimatic with topographic, bioclimatic with LULC and bioclimatic with topographic and LULC.
2.5. Future projectionThe potential future distribution probability and habitat suitability of focal species was forecasted using an ensemble scenario of climate change projection derived from the results of 19 Earth System Models (ESM) provided by the Coupled Model Inter-comparison Project-Phase5 (CIMP5; Taylor et al., 2012). There are four representative concentration pathways (RCP; Vuuren et al., 2011) within each of the 19 ESM, ranging from RCP 2.6 (aggressive mitigation/lowest emissions) to RCP 8.5 (higher emission scenario). All models within each RCP were ensemble using the class with the majority of occurrence within any particular grid cell as the class for location (Zomer et al., 2014; Ranjitkar et al., 2016). For the modelling process, LULC layer was considered as a stable, as the projected for the future is not available (Ranjitkar et al., 2016). It is necessary to measure the similarity between the new climatic conditions and those in the calibration to make a projection for places and time not sampled in calibration data (Elith et al., 2010). The new predicted environment is an extrapolation. The extent of extrapolation in the future projection was examined using multivariate environmental similarity surface (MESS) (Elith and Leathwick, 2009; Elith et al., 2010).
2.6. Spatial analysisWe determined spatial changes in the habitat suitability of focal species by transforming the probability of occurrences from models into presence-absence maps. The resulting raster layers were reclassified as '1' for the presence and '0' for absence using binary maps based on a threshold. The logistic output in Maxent modelling with ten percentile presence was a threshold value in this work. The threshold represents the cut-off point above which climate is favourable for the focal species. We reclassified the MaxEnt output rasters into three classes of habitat suitability: low suitability (10–50% probability of occurrence), medium suitability (50–75% probability of occurrence) and high suitability (> 75% probability of occurrence). We differentiated all the future predictions into stable, retracted and expanded areas, comparing them to current predictions. All the spatial analysis was performed in ArcMap 10.2.1 (ESRI) and the extension Spatial Analysis (ESRI).
2.7. Statistical test of significance of modelThe evaluation of the accuracies of the prediction models generated through MaxEnt was carried out selecting the threshold-independent Receiver Operating Characteristics (ROC) Area Under Curve (AUC) method (Liu et al., 2005; Phillips et al., 2008). The AUC values from the 50 subsets produced by each model were statistically tested to determine if they were significantly better than random AUC (0.5). The AUC averages were calculated and compared with different models.
We examined the mean AUCs of each model against the random model (AUC = 0.5), and other individual models generated. Based on the normality test, a one-tailed T-test was used to test for the significance of the average AUC values against a random model. Analysis of Variance (ANOVA) was conducted to check any significant difference between the means of the AUC. We selected the best model based on the pair-wise comparison with the random model and remaining models.
We formulated two hypotheses:
1) Testing the hypothesis that the models produced for each species are significantly better than a random model, i.e. H0: AUC (train) and AUC (test) = 0.5; H1: AUC (train) and AUC (test) > 0.5.
2) Testing the hypothesis to compare the means of the models, i.e., H0: AUC1 = AUC2; H1: AUC1 ≠ AUC2 (where, AUC1 = models with climatic variables and AUC2 = model that include topographic variables)
3. ResultsThe spatial autocorrelation performed using Moran's I for two species of Alnus in the form of binary prediction indicates that the points are randomly distributed. The Moran's I value for both species are less than 1 (−0.00521 for A. nitida; 0.022107 for A. nepalensis; P > 0.05) indicating negligible spatial autocorrelation. The Shapiro–Wilk test revealed a P-value greater than 0.05 except for the Test AUC1 (0.008) and Test AUC4 (1.281 e−6) of A. nitida (Appendix C3), indicating the training AUC for both the species distributed normally.
3.1. Threshold independent evaluation of the modelsWe found, the distribution probability and habitat suitability of A. nepalensis and A. nitida can be predicted using bioclimatic variables with topographic variables and LULC to achieve both test and training AUC that are significantly better than a random model. Thus, the null hypothesis H0 was rejected and the alternative hypothesis H1 accepted. The P-values calculated on the average of training and test AUC for all four models were significantly better than the random model (P < 0.00001, one sample T-test, 95% CI). The training AUC and training gain were highest for Model4 for both species (Table 2). Thus, Model4 (see Tables 2 and 3) was selected as the best model defining distribution probability and habitat suitability of Himalayan alders.
| Species | Model | Training AUC | Test AUC | Training gain | Test gain | P-values of Average AUC |
| Alnus nepalensis | 1 | 0.83 | 0.767 | 0.613 | 0.515 | < 2.2e−16 |
| 2 | 0.868 | 0.78 | 0.78 | 0.543 | < 2.2e−16 | |
| 3 | 0.84 | 0.773 | 0.679 | 0.536 | < 2.2e−16 | |
| 4 | 0.889 | 0.772 | 0.959 | 0.469 | < 2.2e−16 | |
| Alnus nitida | 1 | 0.879 | 0.852 | 0.707 | 0.847 | < 2.2e−16 |
| 2 | 0.908 | 0.842 | 0.84 | 0.83 | < 2.2e−16 | |
| 3 | 0.869 | 0.827 | 0.668 | 0.754 | < 2.2e−16 | |
| 4 | 0.946 | 0.868 | 1.197 | 1.146 | < 2.2e−16 |
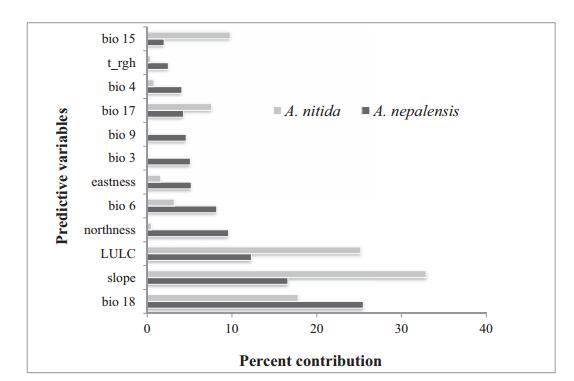
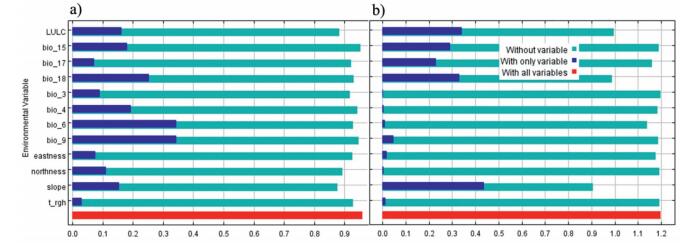
Jackknife analysis showed that the environmental variables with the highest gain, when used in isolation, are bio9 and bio6 for A. nepalensis and slope for A. nitida, which therefore retains the most useful information. For both species, the environmental variable that decreases the model predictability gain when omitted is the slope, which therefore appears to have the most information that is not present in the other variables (Appendix B2). The percent contribution (Fig. 2) estimated that bio18 and slope contributed highly in the model that determines suitable habitat for both Alnus species in the model. Similarly, LULC was an equally important limiting factor for both species in the model.
|
| Fig. 2 Percent contribution for each variable calculated from model 4 produced for both the focal species (t_rgh: terrain roughness; LULC: Land Use Land Cover). |
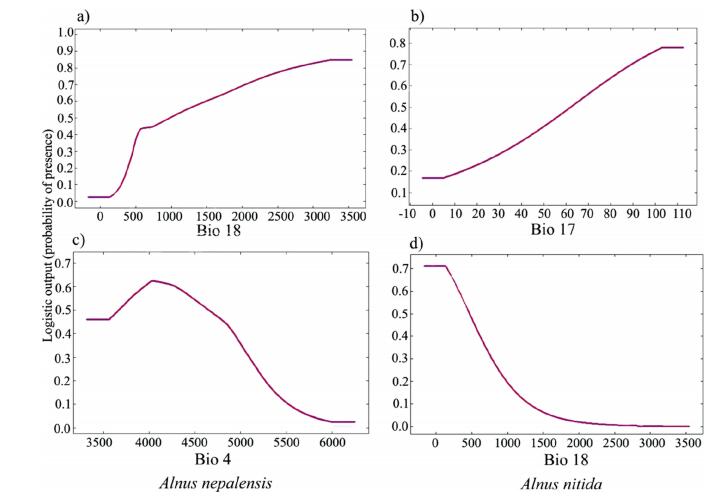
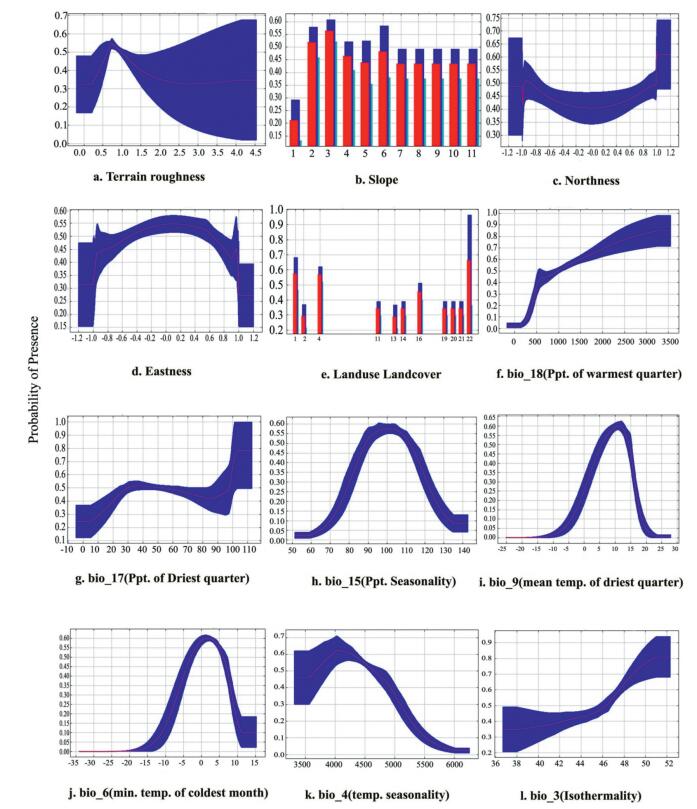
The response curves (Appendix B3) showed that the probability of A. nepalensis occurrence increases with the increase in bio18 (optimum between 500 and 1000 mm) (Fig. 3a) as well as bio3 with 47% temperature variability. Bio4 either larger or smaller than optimum value 4000 decreases the presence probability (Fig. 3c), whereas bio6, bio9, and bio15 have the optimum value 2.5 ℃, 11 ℃ and 100 respective units showing the hump-shaped curve. LULC seems to have fluctuation in the probability of presence.
|
| Fig. 3 Variable response curves for the top two predictors for Alnus nepalensis (a, c) and Alnus nitida (b, d). (Precipitation (Bio17 and Bio18) is expressed in mm and temperature seasonality (Bio4) in standard deviation*100). |
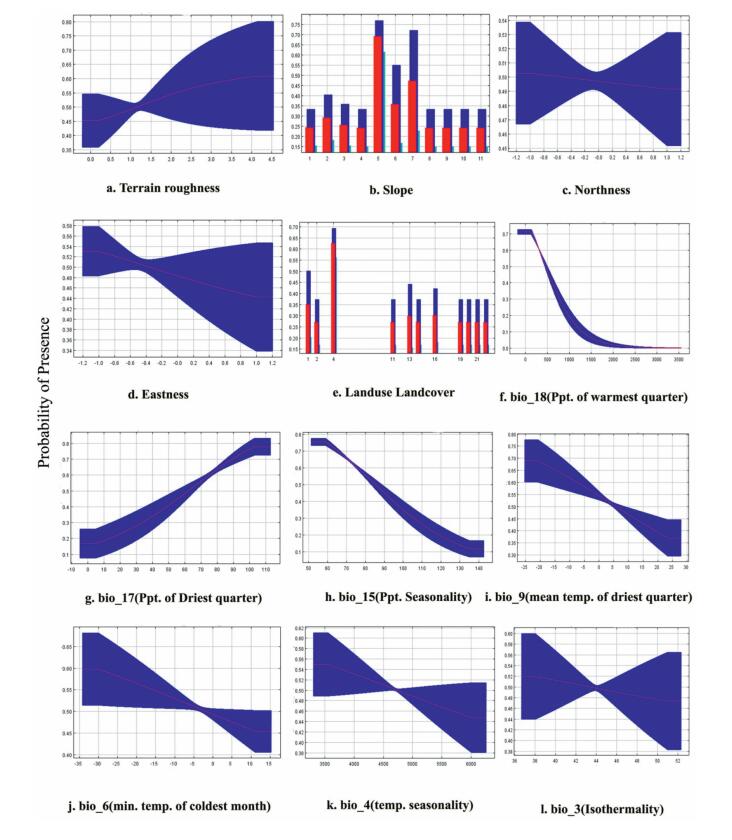
In the case of A. nitida (Appendix B4), the habitat suitability increases with an increase in bio17 with rainfall of at least 80 mm (Fig. 3b) as well as terrain roughness with an optimum value about 1. For the remaining variables, like bio18 (Fig. 3d), bio3, bio4, bio6, bio9, bio15, eastness, and northness, habitat suitability decreases with an increase in their corresponding value. Slope around 23–27° was most suitable for this species. Similarly, habitat suitability increased in the needle-leaved evergreen forest, with closed to open tree cover.
3.3. Comparison of the means of the modelsANOVA results showed a significant difference in the means of AUC of all four models for A. nepalensis (F-value = 19.03, P < 0.05) and A. nitida (F-value = 12.3522, P < 0.05). Further, a pairwise comparison of the average values of the models (Table 3) showed the training AUC for all pairs of means of models produced for A. nepalensis were significantly different (P < 0.05) from each other. In the case of A. nitida, the pair of the average of model 1 and model 3 did not show any significance for training AUC. Similarly, test AUC for all pairs of the average of models were also insignificant.
| Species | Pair-wise mean comparison of the models | ||||||
| 1, 2 | 1, 3 | 1, 4 | 2, 3 | 2, 4 | 3, 4 | ||
| Alnus nepalensis | AUCtr | < 2.2e−16 | 0.00972 | < 2.2e−16 | 1.34e−10 | 2.031e−07 | < 2.2e−16 |
| AUCts | 0.1036 | 0.4834 | 0.5887 | 0.4298 | 0.3767 | 0.9033 | |
| Alnus nitida | AUCtr | 0.0001582 | 0.2648 | < 2.2e−16 | 6.10e−06 | 7.638e−10 | 2.662e−16 |
| AUCts | 0.5357 | 0.09713 | 0.4209 | 0.3558 | 0.2317 | 0.05571 | |
| Note: AUCtr= training AUC and AUCts= test AUC. | |||||||
Contrary to the training AUC, a comparison of the means of the test AUC did not show any significant difference for all model combinations for Alnus species. Therefore, based on the pairwise comparison of the means of the models, it can be concluded that all four models have the same predictive power regarding the test AUC for Alnus species (the test means were not significantly different from each other).
Gains produced from MaxEnt modelling have also been used in determining the best performing model (Yost et al., 2008). Gains describe how well the model fits the training and test dataset available. The average gains for all four models for both species of Alnus showed that model 4 achieved the highest average training gains. The AUC for A. nepalensis was 0.889 and for A. nitida 0.946 in model 4 (Table 2).
3.4. Current suitabilityThe model predicts clear climatic space for two species. East to 83.5° longitude was highly suitable for A. nepalensis, although habitat suitability extended toward the west as well, whereas A. nitida was confined west to 84° longitude. The climatic spaces for both species were along the river valleys. The suitable area for A. nepalensis was predicted to occupy 24% of the total area of Nepal in the baseline climatic scenario (Table 4). The central region of Nepal contains the largest and most continuous area for the species, whereas the western region has a dispersed distribution pattern. Furthermore, out of the total distribution probability and habitat suitable area, only 0.87% has high suitability and is mostly distributed in the central/eastern/upper hill regions; in contrast, 91.58% of the low suitability areas are distributed in the western/lower hill region (Fig. 4a, Table 4). Several isolated patches of varying but relatively small size occur mostly in the west and to some extent in the Siwalik range (Fig. 4a; c). Our model predicts the Terai and High Mountains as unsuitable areas for A. nepalensis.
| Suitability | Low (10%–50%) | Medium (50%−75%) | High (> 75%) | |||||
| km2 | % | km2 | % | km2 | % | |||
| Alnus nepalensis | ||||||||
| Current | 32936 | 91.58 | 2713.7 | 7.55 | 313.41 | 0.87 | ||
| RCP 2.6 | 22081 | 91.74 | 1720 | 7.15 | 268.96 | 1.12 | ||
| RCP 4.5 | 21498 | 92.7 | 1520.3 | 6.56 | 172.52 | 0.74 | ||
| RCP 6.0 | 20208 | 91.62 | 1632.6 | 7.4 | 215.47 | 0.98 | ||
| RCP 8.5 | 19699 | 91.95 | 1533.9 | 7.16 | 189.85 | 0.89 | ||
| Alnus nitida | ||||||||
| Current | 3748.8 | 88.4 | 423.4 | 9.98 | 68.56 | 1.62 | ||
| RCP 2.6 | 9005.9 | 94.01 | 505.52 | 5.28 | 68.56 | 0.72 | ||
| RCP 4.5 | 5619.5 | 88.29 | 614.76 | 9.66 | 130.33 | 2.05 | ||
| RCP 6.0 | 3399.3 | 91.45 | 266.7 | 7.17 | 51.23 | 1.38 | ||
| RCP 8.5 | 7624.2 | 90.88 | 672.77 | 8.02 | 92.67 | 1.1 | ||

|
| Fig. 4 Predicted potential distribution of a) Alnus nepalensis and b) Alnus nitida under current bioclimatic conditions and c) combined habitat suitability of focal species. |
Mountain of Western and High Mountain in central Nepal were climatically suitable for A. nitida(Fig. 4b; c). The total suitable area covers 0.03% of the total modelled area (Table 4). The western region was highly suitable for A. nitida, covering an area of 68.56 km2 (1.62%), whereas areas of low suitability were confined to 3748.82 km2 (88.40%) of the total suitable area (Table 4). The climatically suitable areas were predicted to be more within the extent of the Himalayas in the hilly districts in the Karnali and Dhaulagiri (Fig. 4b; c). The lower high mountains of central region are potentially a novel location for A. nitida, as there were no official reports or collection of the species from the area. The model also predicts some ecotones in the eastern hilly region as new places that lack the actual distribution of this species.
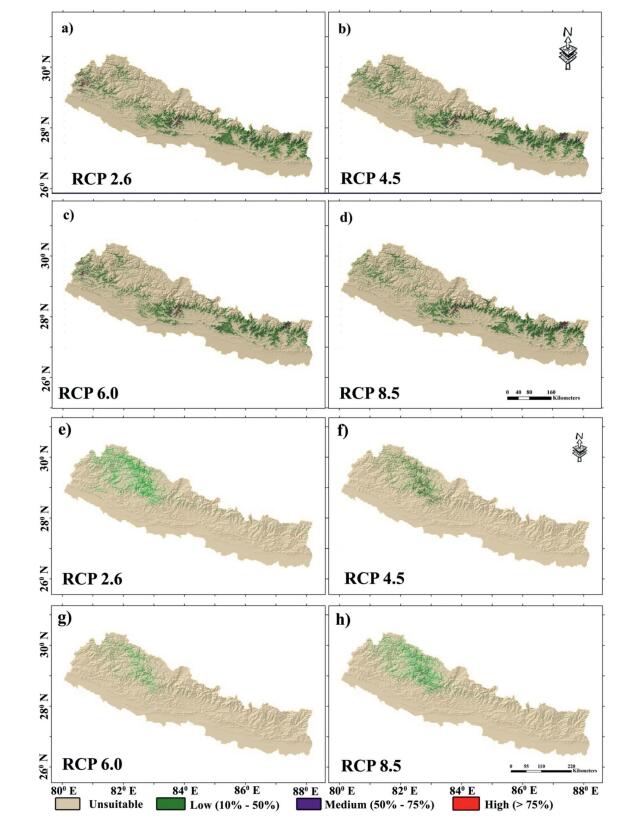
3.5. Future potentialsThe model indicates that current habitat suitability for Alnus species will change greatly in the future (Fig. 5). The shifts in the distribution probability and habitat suitability vary among two species. A. nepalensis was predicted to move towards the northwest in the high mountains under all four RCPs (Fig. 5a–d). Under an ensemble scenario of climate change, A. nepalensisgained maximum suitable area in RCP 2.6 (about 24, 070 km2) and declined to the lowest of about 21, 423 km2 in RCP 8.5 (Table 4). The decline of habitat suitability of A. nepalensis was predicted to be confined mostly to central Nepal compared to eastern and western Nepal. The high and medium suitable areas were predicted to remain in the eastern and central regions, whereas a small fraction of medium and high suitability areas was projected towards the western region under RCP 2.6 and 6.0 (Fig. 5a–d). The habitat suitability of A. nitida was projected to likely increase (0.07% of the total area) by 2050 toward southeast under RCP 2.6 (Fig. 5e, Table 4). The projected suitability decreased under RCP 6.0 by 0.03% of the total suitability area (Fig. 5g, Table 4), whereas under other RCPs, the projected suitable area increased. The habitat suitability of A. nitida was predicted to occur more towards northwest districts of Nepal (Fig. 5e–h).
|
| Fig. 5 Predicted future distribution of Alnus nepalensis (a-d) and Alnus nitida (e-h) in future climate scenarios of CIMP5 derived from 19 ESM under different RCPs. |
According to the model, only 13.59% and 2.52% of the total area of Nepal under RCP 2.6 can be considered stable through 2050 for A. nepalensis and A. nitida respectively (Table 5). By 2050, our model (RCP 8.5) projected more reduction (13.77%), more expansion (3.83%) and less stability by 10.66% in the total area of Nepal compared to the current situation for A. nepalensis (Table 5, Fig. 6a–d). A. nitida was projected to show more expansion (3.97%) under RCP 2.6, reduction (1.17%) under RCP 6.0 and stability (2.52%) under RCP 2.6 (Table 5, Fig. 6e–h).
| Reduction | Expansion | Stable | ||||||
| km2 | %a | km2 | %a | km2 | %a | |||
| Alnus nepalensis | ||||||||
| Current RCP 2.6 | 15964.88 | 10.85 | 4011.75 | 2.73 | 19996.97 | 13.59 | ||
| Current RCP 4.5 | 17447.53 | 11.85 | 4593.36 | 3.12 | 18514.31 | 12.58 | ||
| Current RCP 6.0 | 17621.56 | 11.97 | 3668.21 | 2.49 | 18338.78 | 12.46 | ||
| Current RCP 8.5 | 20268.18 | 13.77 | 5630.76 | 3.83 | 15693.66 | 10.66 | ||
| Alnus nitida | ||||||||
| Current RCP 2.6 | 531.13 | 0.36 | 5837.19 | 3.97 | 3708.89 | 2.52 | ||
| Current RCP 4.5 | 1233.28 | 0.84 | 3349.53 | 2.28 | 3006.74 | 2.04 | ||
| Current RCP 6.0 | 1725.24 | 1.17 | 1194.11 | 0.81 | 2515.54 | 1.71 | ||
| Current RCP 8.5 | 907.82 | 0.62 | 5037.1 | 3.42 | 3332.95 | 2.26 | ||
| a Percentage of reduction, expansion and stable area is calculated out of the total area of Nepal i.e., 147181 km2. | ||||||||

|
| Fig. 6 Maps of the potentially stable, retracted and expanded areas with respect to the current distribution of focal species (a-d: Alnus nepalensis; e-h: Alnus nitida) under different RCPs (The future bioclimatic variables were derived from the results of 19 Earth System Models (ESM) provided by CIMP5). |
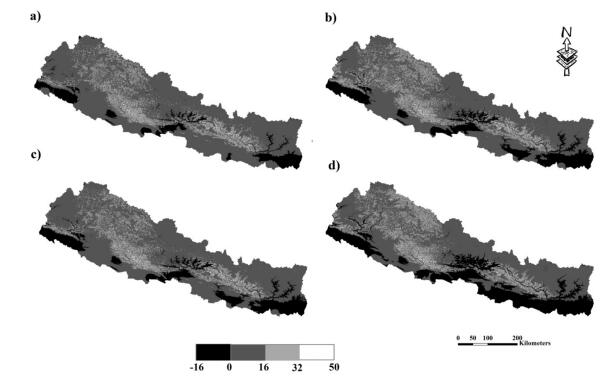
MESS analysis (Fig. 7a–d) indicated some of the extrapolated regions along the western and central hilly region and in the Siwalik range will experience novel climates by 2050. The greater negative MESS values for the Terai region indicated points outside the training range, whereas positive MESS value in the hilly area indicated locations within the training range. The extrapolation is mostly along the river system.
|
| Fig. 7 MESS analysis indicating novel environment across different RCPs: (a) RCP 2.6, (b) RCP 4.5, (c) RCP 6.0 and (d) RCP 8.5. |
In this study, we used a species distribution modelling (SDM) technique not just to understand the species environmental requirements but also to understand aspects of biogeography of Alnus species in Nepal. SDM is widely used in regions where there are least known occurrences of species (Elith et al., 2006; Guisan et al., 2006; Elith and Leathwick, 2009; Ranjitkar et al., 2014a; Zhang et al., 2012). The uncertainties and limitations in SDM techniques lead to under-or over-predicting species distribution (Barry and Elith, 2006; Ranjitkar et al., 2014a), and certainly create obstacles to generating accurate models. While gathering presence only data, spatial autocorrelation could have an impact on SDM (Ranjitkar et al., 2014a). The spatially correlated points lead to predicting the same habitat. Multiple observations within a confined geographic area could spatially correlate, as exemplified by A. nitida in Western Nepal. More importantly, when selecting background points within the region of interest for geographically restricted species, spatial autocorrelation should be prevented, which is properly addressed in the present work.
Physiological tolerance to climatic factors can influence distributions of plants (Woodward, 1992). Precipitation of the warmest quarter (for A. nepalensis) and precipitation of the driest quarter (for A. nitida) separates climatic spaces for Himalayan Alders. A. nepalensis found in the wetter part of Himalayas may require more rainfall than A. nitida found in the drier western Himalayas. Therefore, rainfall during the warmer and drier parts of the year are the important determining factors for distribution probability and habitat suitability of A. nepalensis and A. nitida respectively (Rana, 2014). This is the first study to investigate the relative importance of precipitation and temperature in order to determine Alnus species niches in Nepal. Areas that receive precipitation (during warmest quarter of the year) between 400 and 900 mm are good for occurrence of Himalayan alders. Sakalli (2013) predicted the global distribution of Alnus species was limited to areas of 500–1100 mm annual precipitation and temperatures between 8 and 15 ℃.
The habitat suitability might not necessarily be an indication of species occurrence, as they are limited by edaphic and biogeographic factors (Ranjitkar et al., 2014b). The actual distribution is prone to limitation by seed dispersal mechanism, colonization capacity and LULC change (Sexton et al., 2009; Ranjitkar et al., 2014b). Extensive land use conversion in Nepal is one of the major reasons for the destruction of natural forests that impact the distribution of the least-explored and narrowly-distributed species like A. nitida. Fragmented habitat due to land conversion leads to a clustered distribution of this species, indicating the need to conserve the natural habitat. Mountainous topography and a river valley clearly divide the potential geographic region of Alnus species, possibly limiting seed dispersal and colonization. Climate change might limit or expand habitat suitability for the species.
Future projections indicate significant changes by 2050 in the habitat suitability of both Alnusspecies. The geographical origin of the genus Alnus and the role of climate in shaping their distribution is uncertain, but our model revealed expansions and reductions in suitability in response to climate. In addition to climate, high mountains (> 3000 m asl) in the Northern aspect and increasing elevation prevent A. nepalensis seed dispersal and establishment, whereas an elevational shift of A. nitida might lead to the summit trap phenomena (Salick et al., 2009). The cold-adapted A. nitida, stressed by climate warming, migrate upwards until they reach the highest elevations, where they compete with other species. Sakalli (2017) predicted a northward shift of Alnus spp., and Ranjitkar et al. (2016) projected a contraction in the lower limit of A. nepalensis, both of which provide further support for our results.
The stable area predicted in our model for both species by 2050 can be designated areas for conservation of the species and as a source of germplasm, whereas new suitable areas can be designated zones for promoting an alder tree-based agroforestry system (Rana, 2014). The model may be essential for adaptive responses and preventive measures for the sustainable management of agroforestry tree species in the future. Agroforestry systems help farmers adapt to climate change by mitigating its consequences through carbon sequestration (Luedeling et al., 2014). Agroforestry systems are more complicated than monoculture situations. They consist of annual and perennial plants, which are often integrated with livestock (Luedeling et al., 2014). With respect to Nepal, these multipurpose agroforestry tree species are beneficial for large cardamom, which is an important perennial cash crop. Cardamom requires shade, therefore, intercropped under various types of trees is beneficial. A. nepalensis is the chief shade-providing trees used in large cardamom-based traditional agroforestry systems in the region (Sharma et al., 1998). However, the shifting trends in habitat suitability of A. nepalensismight cause depletion in the existing agroforestry system in Nepal. Insects defoliate most of the seedlings; and recently O'Neill and Rana (2016) reported parasitic (Loranthaceae group) infection of an A. nepalensis population in the hilly regions in central Nepal. Such infestation has not previously been documented in Nepal. Therefore, according to projections from our model, A. nepalensis populations in the stable region should be the focus of management. Also, A. nitida can act as a suitable substitute shade-and nitrogen-fixing tree for an intercropping Alder–Cardamom, Alder–Tea system, particularly in central Nepal where the climatic suitability for A. nitida is projected by 2050 in our model. It is the most suitable farm forestry tree in the high valley and mountains because of its frost hardiness, fast growth and nitrogen-fixing properties (Sheikh, 1993). A. nitida can be easily reproduced from seed and by vegetative means. It has no known insect or pest problems.
The Department of Forestry, the government of Nepal, have compared the growth rate of A. nitida with that of A. nepalensis in eastern Nepal (based on our personal communication). They reported 90% of seedlings of A. nitida survive attaining a mean height of 87 cm after two years. The growth performance of A. nitida closely matches or even exceeds that of A. nepalensis. According to our model, A. nepalensis will decline in the future, whereas A. nitida will gain suitability toward the north-east. Therefore, A. nitida could be a potential substitute for A. nepalensis at some high elevation places where parasite problems are reported. We recommend checking compatibility of this species with cardamom and tea, and establishing this rare species as a nitrogen-fixing shade tree for agroforestry systems in the future.
5. ConclusionOur rigorous statistical model was successful in delineating the niche of focal species. Probably, A. nepalensis found in wetter parts of the Himalayas require more rainfall than A. nitida found in the drier western Himalayas. Therefore, rainfall during warmer parts and drier parts of the year are determining factors for habitat suitability for A. nepalensis and A. nitidarespectively. Thus, wide variation in the species is due to precipitation, where A. nitida receives much less rainfall throughout the year (optimum 70 mm).
The uniqueness of this study is describing the suitable growing areas for Himalayan alders. Thus, the maps generated from the model should be considered as baseline information for the focal species. The most accurate predictive map for each species was used to identify potentially suitable niches as the core area. The results can be valuable inputs for prioritization of the areas for protecting the natural population and revitalizing the alder-based agroforestry system.
AcknowledgementsThe author likes to thank RBGE Edinburg database, the National Herbarium and Plant Laboratories (KATH), Godawari and Tribhuvan University Central Herbarium (TUCH), Kirtipur, Kathmandu, Nepal for facilitating with herbarium study as well as to Ms. Banu Oja and entire Cornell Nepal Study Programme family for partial financial support. We take this opportunity to acknowledge CGIAR research programs on 'Forests, Trees and Agroforestry' (CRP6.2) program and Center for Mountain Ecosystem Studies to be a part of research on species distribution in Asian Highlands from where value added climatic data, species database were made available for the study.
Appendix A. Details on herbarium specimen examined housed at different herbariaAppendix A1. List of herbarium specimen examined at National Herbarium and Plant Laboratories (KATH) and Tribhuvan University Central Herbarium (TUCH) of Alnus nepalensis D. Don (list consists of information on location, elevation, collection date, name of collectors and collection number, and information of each specimen is separeted by semi-colon)
Western Nepal: Ratikhola (Ratanaugla), 2070 m, 13 Dec 1983, T .K. Bhattacharya & P.P. Kurmi 5740 (KATH); Baitadi, Satbanj, 1900 m, 21 Sept 1981, I . Sharma, R. Joshi, R. Uprety and I. Pandey 616 (KATH); Bajhang, Gandaki near Talkot, 1550 m, 21 July 1976, H . Tabata, K.R. Rajbhandary & K. Tsuchiya 1434 (TUCH); Bajura, Bajatoli and Dhamakne, 2030 m, 18 July 1976, H . Tabata, K.R. Rajbhandary & K. Tsuchiya 2584 (TUCH); Bajura, Birseni, 1570 m, 19 Aug 1976, H . Tabata, K.R. Rajbhandary & K. Tsuchiya 2663 (KATH); Dadeldhura, Dadeldhuralekh, 1580 m, 18 Dec 1986, C .M. Joshi and N. Avasti 40 (KATH); Dailekh, Dailekh, 2100 m, 1 Aug 1972, M .S. Bist and D.P Joshi 131 (TUCH); Dailekh, Gairai, 900 m, 25 Feb 1991, N .P. Manandhar 349-91 (KATH); Dailekh, Khursanibari, 1220 m, 16 Aug 1982, U .R. Poudyal and R.N. Shukla 4101 (KATH); Dailekh, Lohari, 790 m, 28 Feb 1991, N .P. Manandhar 556-91 (KATH); Doti, Jhigadana, 2400 m, 20 Aug 1990, N .K. Bhattarai 90/790 (KATH); Jajarkot, Dhunlakot, 2100 m, 13 Aug 1979, K .R. Rajbhandary & B. Roy 4606 (KATH); Kalikot, Dillikot, 1850 m, 7 July 1979, K .R. Rajbhandary & B. Roy 3005 (KATH); Pyuthan, Khalanga, 1200 m, 10 March 1991, P .P. Kurni, KB 0089 (KATH); Salyan, Falabang, 1370 m, 8 May 1984, T .K. Bhattacharya & K.G. Malla 4238 (KATH); Salyan, Kapurkot, 1550 m, 20 June 1992, P .P. Kurni KB176 (KATH); Salyan, Mokhola, 1220 m, 23 Aug 1983, P .P. Kurmi 5665 (KATH)
Central Nepal:Baglung, Jaidi, 1670 m, 29 Oct 2012, S . Sharma 78 (KATH); Chitwan, Near Paidi Bas Choki, 390 m, 5 May 1976, R .G. Troth 780 (TUCH); Dolakha, Malephu to Torikhet, 950 m, 12 July 1977, K .R. Rajbhandary and B. Roy 1237 (KATH); Dolakha, Sunkhani, 1520 m, 10 May 2011, B .K. Oliya 89 (KATH); Gorkha, Chilaune, 1470 m, 10 May 1987, N .P. Manandhar and C.P. Katel 11570 (KATH); Gorkha, Sarjungkhola, 1550 m, 26 July 1994, L . Joshi 9495158 (KATH); Gorkha, Sarjungkhola, 1600 m, 26 July 1994, L . Joshi 9495151 (KATH); Gorkha, Sarjungkhola, 1650 m, 26 July 1994, L . Joshi 9495168 (KATH); Kaski, Dhampus, 1350 m, 21 May 1992, N .P. Manandhar and S.K. Acharya 39–92 (KATH); Kaski, Rote pani, 1000 m, 24 June 1986, N .P. Manandhar and C.P. Katel 10900 (KATH); Kaski, Tamage-banjan, 1730–2035, 9 Aug 1999, Expedition by the herbal garden Kanazawa university Japan 9961182 (KATH); Kathmandu, Dakshinkali, 1530 m, 10 April 1997, S .S. Shrestha 167 (KATH); Kathmandu, Gokarna, 1340 m, 2 Nov 1966, P . Pradhan & R. Thapa 6542 (KATH); Kathmandu, Kirtipur, 1350 m, 12 May 2003, M .K.D. Shrestha 9 (KATH); Kathmandu, Kirtipur, 400 m, 28 Oct 1985, H . Van, I. S. Cotter & J. Metz N184 (KATH); Kathmandu, Suryabinayak, 1370 m, 17 April 1991, xx (KATH); Kavrepalanchowk, Dapcha, 1740 m, 16 Nov 1988, N .P. Manandhar 12043 (KATH); Kavrepalanchowk, Khardarpati, 1550 m, 19 Nov 1988, N .P. Manandhar 12139 (KATH); Lalitpur, Godawari, 1300 m, 22 June 2001, R .K. Khadka 90 (KATH); Lalitpur, Godawari, 1520 m, 13 Sept 2001, N . Joshi 425 (KATH); Makwanpur, Old road from Hetauda to Ktm via Bhimphedi, 800 m, 29 Nov 2004, C .A. Pendry, K.K. Shrestha, S. Dahal, A. Giri, A.G. Miller, N. Pandey, M.R. Pullan, L.R. Shakya, S. Shrestha, M. Siwakoti DNEP2A216 (KATH); Manang, Thonche, 1980 m, 12 Aug 1983, N .P. Manandhar 9733 (KATH); Nuwakot, Between Grang and Thari east side of Trishuli river, 1900 m, 16 Sept 1966, D .H. Nicolson 2363 (KATH); Ramechhap, Kyama-patkare, 2200–2600 m, 4 Aug 1985, H . Ohba, T. Kikuchi, M. Wakabayashi, M. Suzuki, N. Kurosaki, K.R. Rajbhandari and S.K. Wu 8571275 (KATH); Ramechhap, Shivalaya-Khasrubus-Deorali, 1000–2700 m, 5 July 1985, H . Ohba, T. Kikuchi, M. Wakabayashi, M. Suzuki, N. Kurosaki, K.R. Rajbhandari and S.K. Wu 8540144 (KATH); Sindhupalanchowk, Kakani, 1730 m, 22 May 1993, N .P. Manandhar 47–93 (KATH).
Eastern Nepal: Near Nigale, 1600 m, 29 August 1971, P .R. Shakya & M. Ohsawa 1050 (KATH); Phokte-namdo, 1160–1370 m, 1 April 1958, S . G. RaoRolle 13596 (KATH); Dhankuta, Hile-pakhribas, 1740 m, 2 July 1988, M .N. Subedi 219 (KATH); Dhankuta, Hile-Pakhribas-Golikharka, 1770 m, 2 July 1988, M . Suzuki, N. Naruhashi, S. Kurosaki, Y. Kadota, M.N. Subedi, M. Minaki, S. Noshiro, H. Ikeda 8840007 (KATH); Dhankuta, Pakhribas, 1490 m, 15 July 1995, C .M. Joshi 12190 (KATH); Dhankuta, Patle, 1670 m, 30 July 1976, N .P. Manandhar 57 (KATH); Khotang, Khanidanda-Sherakhola-Diktel-mixakhola-Dorpa Churi Danda, 1050–1670 m, 29 Oct 1995, M . Mikage, T. Kajita, F. Kiuchi, N. Kondo, P. Lacoul, M. Suzuki and K. Yonekura 9555078 (KATH); Phidim, Lalikhaira, 2000 m, 18 Nov 1978, P . Pradhan, N.P. Manandhar and N. Acharya 1055 (KATH); Sankhuwasabha, Chyangrima-chhokm KHOL-camp site, 2280 m, 21 Aug 1998, S . Noshiro, N. Acharya, K. Kobayashi, Y. Omori, K. Shinozaki and H. Tsukaya 9840114 (KATH); Taplejung, Dumhan, 1 Nov 1963, H . Hara, H. Kanai, H. Kurosawa, G. Murata, M. Togashi and T. Tuyama 6303371 (KATH).
| Collection date | Locality | District | Altitude (m) | Collection no. | Collector(s) |
| 8/17/1997 | Bhotebas–Gogane–Chichila–Kaptane | Sankhuwasawa | 9755094 | S. Noshiro et al. | |
| 2/12/2003 | Palung–Daman, Nepal | 1056 | T. Watanabe et al. | ||
| 10/29/1995 | KhaniDanda–SheraKhalle–Diktel–MiyaKhola–DorpaChuriDanda | Khotang | 1450 | 9555078 | M. Mikage et al. |
| 9/21/1996 | RituKharka–Lulang–Lumsung–below Lumsung | Myagdi | 2180 | 9686196 | M. Mikage et al. |
| 9/1/1996 | Ratnechour–Beni | Baglung | 830 | 9686008 | M. Mikage et al. |
| 1/19/1996 | Sauraha–Padampur–Bhawanipur–HardaKhola | Chitawan | 9611796 | M. Mikage et al. | |
| 9/2/1995 | Junbesi–Tagtor – a pass–Lanjura Pass–Goyom–Dakchu–Sete–Kensa | Solukhumbu | 9596597 | F. Miyamoto et al. | |
| 8/29/1971 | near Nigale | 1600 | 1050 | M. Ohsawa & P.R. Shakya | |
| 10/23/1971 | above Nhorbu Gaon | 2100 | 997 | M. Ohsawa & P.R. Shakya | |
| 5/5/1976 | Royal Chitwan National Park | Chitawan | 390 | R.G. Troth | |
| 9/23/2007 | Simigaun (2030 m)–Tamakoshi bridge (1430 m)–Lamabagar (1970 m)–Gungurkhola (1300 m) | Dolakha | 2110 | 20740712 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 925 | 20740003 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 980 | 20740151 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 980 | 20740150 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 995 | 20740149 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 960 | 20740148 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 960 | 20740147 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 925 | 20740146 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 20740145 | S. Noshiro et al. | |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 925 | 20740144 | S. Noshiro et al. |
| 9/13/2007 | Dolakha (1750 m)–Nagdaha (890 m)–Malephu (940 m)–Gumukhola (920 m) | Dolakha | 925 | 20740143 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1580 | 20740163 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1580 | 20740164 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1580 | 20740165 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1580 | 20740166 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1670 | 20740167 | S. Noshiro et al. |
| 9/15/2007 | Jagat–Phedi–Buthu–Simigaun | Dolakha | 1970 | 20740168 | S. Noshiro et al. |
| 9/22/2007 | Dongang–Kelche–Suchek–Simigaun | Dolakha | 2845 | 20740698 | S. Noshiro et al. |
| 9/16/2007 | Simigaun (2030 m)–Suchek (kharka) (2490 m)–Kelche (2810 m)–Dongang (2780 m) | Dolakha | 2825 | 20740381 | S. Noshiro et al. |
| 9/16/2007 | Simigaun (2030 m)–Suchek (kharka) (2490 m)–Kelche (2810 m)–Dongang (2780 m) | Dolakha | 2810 | 20740383 | S. Noshiro et al. |
| 9/16/2007 | Simigaun (2030 m)–Suchek (kharka) (2490 m)–Kelche (2810 m)–Dongang (2780 m) | Dolakha | 2810 | 20740384 | S. Noshiro et al. |
| 8/9/1999 | Tamage-Banjan | Kaski | 1950 | 9961182 | M. Mikage et al. |
| 8/1/1977 | Shemma–Yakuwa–Lamobagar | Sankhuwasawa | 770299 | H. Ohashi et al. | |
| 8/2/1977 | Lamobagar–Gola–above Shinbun | Sankhuwasawa | 771940 | H. Ohashi et al. | |
| 8/3/1977 | Above Shinbun–HatiaGola | Sankhuwasawa | 771970 | H. Ohashi et al. | |
| 8/5/1977 | HatiaGola–Digedanra–TaramBhanjyang–Honkon | Sankhuwasawa | 773483 | H. Ohashi et al. | |
| 9/3/1977 | Sindua–Hile–Dhandkuta–TekuNala | Dhankuta | 775560 | H. Ohashi et al. | |
| 9/20/1963 | Sundarijal Waterfall | Kathmandu | 1600 | 6303366 | H. Hara et al. |
| 9/22/1963 | Gokarna Forest | Kathmandu | 6303367 | H. Hara et al. | |
| 10/18/1963 | Dhankuta | Dhankuta | 1200 | 6303368 | H. Hara et al. |
| 10/19/1963 | Dhankuta | Dhankuta | 1200 | 6303369 | H. Hara et al. |
| 10/29/1963 | Minchin Dhap–MulPokhari | Sankhuwasawa | 6303370 | H. Hara et al. | |
| 11/1/1963 | Dumhan–Taplejung | Taplejung | 6303371 | H. Kanai et al. | |
| 11/10/1963 | Helok–BaroyaKhimty | Taplejung | 6303372 | H. Hara et al. | |
| 7/7/1983 | Suiket–Pathana | Kaski | 8340051 | H. Ohba et al. | |
| 7/5/1985 | Shivalaya–Khasrubus–Deorali | Ramechhap | 8540144 | H. Ohba et al. | |
| 8/4/1985 | Kyama–Patkare | Ramechhap | 8571275 | H. Ohba et al. | |
| 7/2/1988 | Hile (Hille)–Pakhribas–GholiKharka (Dholikharka) | Dhankuta | 1770 | 8840007 | M. Suzuki et al. |
| 7/2/1988 | Hile (Hille)–Pakhribas–GholiKharka (Dholikharka) | Dhankuta | 1830 | 8860007 | M. Suzuki et al. |
| 8/18/1988 | Pokhara–Bagar–Hyangja–Suikhet–Majhbhatti–NaudandaPhedi (NaudharaPhedi) | Kaski | 1160 | 8860430 | M. Suzuki et al. |
| 9/13/1988 | Forest Office–Chhap–Shiwapuri Summit | Kathmandu | 1620 | 8861045 | M. Suzuki et al. |
| 7/23/1990 | Hile–Pakhribas–GholiKharka | Dhankuta | 1800 | 9040023 | M. Minaki et al. |
| 8/13/1991 | Baidep–Arun River–Num | Sankhuwasawa | 9153461 | H. Ohba et al. | |
| 5/11/1992 | Mitlung–Thuma–Khokling | Taplejung | 790 | 9261005 | M. Suzuki et al. |
| 5/14/1992 | Papung–Dongen–Sewaden | Taplejung | 2260 | 9261019 | M. Suzuki et al. |
| 5/24/1992 | RamsyangPati–Jongim–WolangchungGola | Taplejung | 2710 | 9261067 | M. Suzuki et al. |
| 6/5/1992 | Amjilasa–bend–YanjolarchaKhola–Gyabla | Taplejung | 2370 | 9261174 | M. Suzuki et al. |
| 6/6/1992 | Gyabla–Nag PokariKhola–Fale–Ghunsa | Taplejung | 2740 | 9261189 | M. Suzuki et al. |
| 6/14/1992 | Yamphudin-SigreDanda | Taplejung | 1900 | 9261278 | M. Suzuki et al. |
| 8/21/1998 | Chyangrima–ChhokamKhola–Camp Site | Sankhuwasawa | 2280 | 9840114 | S. Noshiro et al. |
| 9/23/1963 | Balaju | Kathmandu | H. Hara et al. | ||
| 7/26/2003 | Godawari | Lalitpur | 1570 | T. Watanabe & K.J. Malla | |
| 11/24/2003 | Sagakgaon | Gorkha | T. Watanabe & K.J. Malla |
| Barcode | Collection no. | Collection date | Collector(s) |
| E00240540 | 4441 | 9/16/1954 | Stainton, J.D.A., W. Sykes and J. Williams |
| E00240656 | 1960 | 10/30/1954 | Zimmermann, A. |
| E00293603 | 60/00 | 4/8/2000 | W.R.Sykes |
| E00248246 | A216 | 11/29/2004 | Second Darwin Nepal Fieldwork Training Expedition |
| E00223626 | 9686196 | 9/21/1996 | Botanical Expedition to West Nepal 1996 |
| E00240549 | 1868 | 10/27/1954 | Zimmermann A. |
| E00074569 | 9153461 | 8/13/1991 | East Nepal Expedition to Koshi Zone (1991) |
| E00156584 | 9611796 | 1/19/1996 | Botanical Expedition to Central Nepal, Chitwan (1996) |
| E00240539 | 3760 | 3/31/1952 | Polunin, O., W. Sykes and J. Williams |
| E00240545 | 1868 | 10/27/1954 | Zimmermann, A. |
| E00083102 | 9686196 | 9/21/1996 | Mikage, M., R. Hirano, A. Takahashi and K. Yonekura |
| E00649206 | 280/99 | 4/27/1999 | Sykes, W. R. |
| E00083140 | 9040023 | 7/23/1990 | Botanical Expedition to Himalaya (1990) |
| E00250164 | BY11 | 9/11/2005 | Third Darwin Nepal Fieldwork Training Expedition |
| Locality | District | Altitude (m) |
| Aghor, Namtar-3 | Makwanpur | 1850 |
| Bahundanda | Gorkha | 1690 |
| Chandragiri | Kathmandu | 2050 |
| Chitre | Parbat | 2020 |
| Dapcha | Kavre | 1490 |
| Dubichaur | Gulmi | 1470 |
| Godam | Kathmandu | 1690 |
| Gurba-3, Hastichaur | Gulmi | 980 |
| Harpukot | Gulmi | 1120 |
| Kalikot | Kalikot | 2970 |
| Godawari | Lalitpur | 2230 |
| Mahadevthan | Kathmandu | 1580 |
| Maldi-1, Hastichaur | Gulmi | 1050 |
| Naubise | Dhading | 1270 |
| Nayagaun, Daman-5 | Makwanpur | 2140 |
| Panchase | Parbat | 2830 |
| Phidim | Phidim | 1600 |
| Phulchoki | Lalitpur | 1760 |
| Satghumti, Thakre-8 | Dhading | 1090 |
| Thankot | Kathmandu | 1950 |
Appendix A5. List of herbarium specimen examined and deposited at National Herbarium and Plant Laboratories (KATH) of Alnus nitida (Spach) Endl (list consists of information on location, elevation, collection date, name of collectors and collection number, and information of each specimen is separeted by semi-colon)
Western Nepal: Bajhang, Dhuli, 2120 m, 25 July 1976, H . Tabata, K.R. Rajbhandari and K. Tsuchiya 1586 (KATH); Bajhang, Gangadi near Talkot, 1550 m, 21 July 1976, H . Tabata, K.R. Rajbhandari and K. Tsuchiya 1434 (KATH); Darchula, Makari gad – Khandeswori, 2090 m, P.R. Shakya, H.K. Adhikari and H.N. Subedi 7906 (KATH); Humla, Deoli (Darma)-Lohachaur, 2400 m, 28 July 1979, P .R. Shakya, M.N. Subedi and R. Uprety 8686 (KATH); Humla, Kulli gad, 2400 m, 16 June 2008, K .R. Rajbhandari and B. Roy 4068 (KATH); Humla, Rimigaon, 2450 m, 27 July 1979, K .R. Rajbhandari and B. Roy 4014 (KATH); Humla, South of Lali, 1980 m, 16 Aug 1985, C .A. Pendry, S. Baral, S. Noshiro, S. Rajbhandari, P.P. Kurmi, B. Dell, B. Adhikari JRS B146 (KATH); Humla, Way down from Simikot, 2800 m, 17 July 1968, S .B. Malla 14256 (KATH); Mugu, Gamgadhi, 2200 m, 24 July 1979, K . Shinozaki s.n. (KATH); Mugu, Gamgadhi-pina, 2100 m, 22 Aug 1985, P .R. Shakya, M.N. Subedi and R. Uprety 8773 (KATH); Mugu, Lamagad, 1800 m, 20 July 1979, K .R. Rajbhandari and B. Roy 3814 (KATH); Mugu, Pina, 2250 m, 13 Oct 1975, N .P. Manandhar and D.P. Joshi 6962 (KATH); Mugu, Pinna, 2400 m, 20 July 1979, T .B. Shrestha and N.P. Manandhar 214 (KATH); Mugu, Thyarigaon, 2350 m, 9 Aug 1981, K .R. Rajbhandari and B. Roy 3646 (KATH); Mugu, Thyarigaon, 2400 m, 16 Sept 1995, K .R. Rajbhandari and B. Roy 3645 (KATH).
| Barcode | Collection no. | Collection date | Collector(s) | Cite as:(Stable URL) |
| E00240657 | 1960 | 10/30/1954 | Zimmermann, A. | http://data.rbge.org.uk/herb/E00240657 |
| E00240654 | 5243 | Polunin, O., Sykes, W. & Williams, J. | http://data.rbge.org.uk/herb/E00240654 | |
| E00397283 | B146 | 6/16/2008 | Flora of Nepal Collecting Trip (2008) | http://data.rbge.org.uk/herb/E00397283 |
|
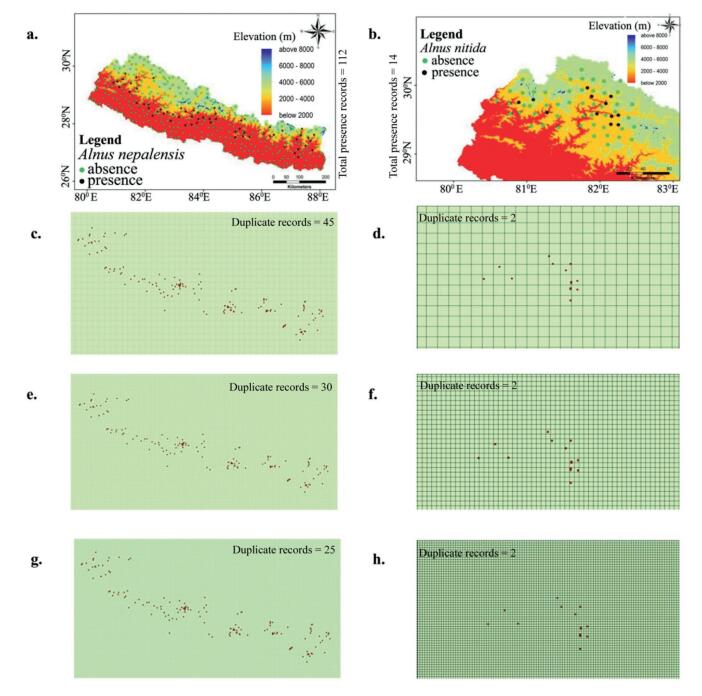
| Appendix B1 Occurrence and background points used for bootstraping and modelling of (a) A. nepalensis and (b) A. nitida; occurrence point location checked in spatial grid of different resolution (c) and (d) 10 min, (e) and (f) 4 min, (g) and (h) 2 min for A. nepalensis and A. nitida respectively. (Note: Total number of species presence records within 10 min grids was used for modelling process) |
|
| Appendix B2 Results of Jackknife evaluations of relative importance of predictor bioclimatic variables for (a) Alnus nepalensis and (b) Alnus nitida in MaxEnt model. |
|
| Appendix B3 Response curves of the predictor variables showing logistic output (probability of presence) for Alnus nepalensis. (Note: slope in degree, temperature in ℃, precipitation in mm, terrain roughness in ratio) |
|
| Appendix B4 Response curves of the predictor variables showing logistic output (probability of presence) for Alnus nitida. (Note: slope in degree, temperature in ℃, precipitation in mm, terrain roughness in ratio) |
| Pearson Correlations Coefficient (r) | |||||||||||||||||||||
| Variables | bio_1 | bio_2 | bio_3 | bio_4 | bio_5 | bio_6 | bio_7 | bio_8 | bio_9 | bio_10 | bio_11 | bio_12 | bio_13 | bio_14 | bio_15 | bio_16 | bio_17 | bio_18 | bio_19 | ai_ann | pet_ann |
| bio_1 | 1 | ||||||||||||||||||||
| bio_2 | 0.333** | 1 | |||||||||||||||||||
| bio_3 | 0.003 | 0.324** | 1 | ||||||||||||||||||
| bio_4 | 0.231 | 0.551** | −0.586** | 1 | |||||||||||||||||
| bio_5 | 0.959** | 0.459** | −0.143 | 0.460** | 1 | ||||||||||||||||
| bio_6 | 0.980** | 0.192 | −0.014 | 0.121 | 0.927** | 1 | |||||||||||||||
| bio_7 | 0.342** | 0.773** | −0.342** | 0.936** | 0.564** | 0.214 | 1 | ||||||||||||||
| bio_8 | 0.997** | 0.364** | 0.003 | 0.263* | 0.960** | 0.966** | 0.373** | 1 | |||||||||||||
| bio_9 | 0.988** | 0.278* | 0.013 | 0.175 | 0.938** | 0.982** | 0.280* | 0.981** | 1 | ||||||||||||
| bio_10 | 0.997** | 0.370** | −0.039 | 0.302* | 0.975** | 0.970** | 0.407** | 0.997** | 0.981** | 1 | |||||||||||
| bio_11 | 0.996** | 0.288* | 0.059 | 0.141 | 0.934** | 0.987** | 0.260* | 0.990** | 0.989** | 0.986** | 1 | ||||||||||
| bio_12 | 0.402** | −0.119 | 0.235 | −0.294* | 0.253* | 0.417** | −0.261* | 0.396** | 0.418** | 0.362** | 0.430** | 1 | |||||||||
| bio_13 | 0.381** | −0.061 | 0.204 | −0.218 | 0.247* | 0.374** | −0.179 | 0.381** | 0.389** | 0.346** | 0.401** | 0.982** | 1 | ||||||||
| bio_14 | 0.016 | −0.252* | −0.253* | 0.035 | 0.041 | 0.087 | −0.085 | 0.011 | 0.017 | 0.026 | 0.018 | −0.003 | −0.107 | 1 | |||||||
| bio_15 | 0.258* | 0.217 | 0.114 | 0.086 | 0.189 | 0.146 | 0.17 | 0.283* | 0.219 | 0.246* | 0.243* | 0.498** | 0.628** | −0.502** | 1 | ||||||
| bio_16 | 0.396** | −0.061 | 0.225 | −0.238 | 0.257* | 0.392** | −0.194 | 0.393** | 0.405** | 0.358** | 0.417** | 0.989** | 0.997** | −0.108 | 0.605** | 1 | |||||
| bio_17 | −0.07 | −0.195 | −0.621** | 0.374** | 0.093 | 0.009 | 0.222 | −0.086 | −0.061 | −0.033 | −0.099 | −0.275* | −0.343** | 0.733** | −0.614** | −0.344** | 1 | ||||
| bio_18 | 0.222 | −0.159 | 0.410** | −0.469** | 0.041 | 0.238 | −0.419** | 0.216 | 0.244* | 0.173 | 0.263* | 0.944** | 0.936** | −0.135 | 0.510** | 0.943** | −0.448** | 1 | |||
| bio_19 | −0.162 | −0.106 | −0.644** | 0.476** | 0.04 | −0.099 | 0.321** | −0.176 | −0.147 | −0.117 | −0.202 | −0.374** | −0.420** | 0.579** | −0.613** | −0.424** | 0.961** | −0.528** | 1 | ||
| ai_ann | 0.032 | −0.407** | 0.221 | −0.505** | −0.144 | 0.074 | −0.538** | 0.025 | 0.057 | −0.016 | 0.074 | 0.901** | 0.882** | 0.054 | 0.414** | 0.881** | −0.252* | 0.918** | −0.358** | 1 | |
| pet_ann | 0.967** | 0.538** | 0.055 | 0.362** | 0.975** | 0.922** | 0.512** | 0.970** | 0.946** | 0.976** | 0.951** | 0.302* | 0.298* | −0.017 | 0.248* | 0.309* | −0.05 | 0.123 | −0.108 | −0.096 | 1 |
| *Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed). | |||||||||||||||||||||
| GLC 2000 LULC Classification of Nepal | |||
| S.N. | GLC 2000 code | LULC type | Description |
| 1 | 1 | Tree cover, broadleaved evergreen, closed to open (> 15%) | The main layer consists of broadleaved evergreen closed to open trees. The crown cover is between 100 and 15%. The height is in the range of > 30–3 m but may be further defined into smaller range. |
| 2 | 2 | Tree Cover, broadleaved deciduous, closed (> 40%) | The main layer consists of broadleaved deciduous closed to open trees. The crown cover is between 100 and 15%. The height is in the range of > 30–3 m but may be further defined into smaller range. |
| 3 | 4 | Tree cover, needleleaved evergreen, closed to open (> 15%) | The main layer consists of needleleaved evergreen closed to open trees. The crown cover is between 100 and 15%. The height is in the range of > 30–3 m but may be further defined into smaller range. |
| 4 | 11 | Shrubcover, closed to open (> 15%), evergreen (broadleaved or needle-leaved) | The main layer consists of broadleaved/needleleaved evergreen closed to open thicket. The crown cover is between 100 and 15%. The height is in the range of 5–0.3 m but may be further defined into smaller range. |
| 5 | 12 | Shrubcover, closed to open (> 15%), deciduous (broadleaved) | The main layer consists of broadleaved deciduous closed to open thicket. The crown cover is between 100 and 15%. The height is in the range of 5–0.3 m but may be further defined into smaller range. |
| 6 | 13 | Herbaceous cover, closed to open (> 15%) | The main layer consists of closed to open herbaceous vegetation. The crown cover is between 100 and 15%. The height is in the range of 3–0.03 m but may be further defined into smaller range. |
| 7 | 14 | Sparse Herbaceous or sparse Shrub cover | The main layer consists of sparse herbaceous vegetation. The crown cover is between (20–10) and 1%. The sparseness of the vegetation may be further specified. The main layer consists of sparse shrubs. |
| 8 | 16 | Cropland (upland crops or inundated/flooded crops as e.g. rice) | Primarily vegetated areas containing more than four percent vegetation during at least two months a year. The environment is influenced by the edaphic substratum. The vegetative cover is characterized by the removal of the (semi) natural vegetation and replacement with a vegetative cover resulting from human activities. This cover is artificial and requires maintenance. It is grown with the intention to be managed and/or (partly) harvested at the end of the growing season. Before or after harvest there may be a period without vegetative cover. The environment is significantly influenced by the presence of water over extensive period of time, i.e. water is present for more than three months a year and when water is present less than three months a year, it is present 75 percent of the flooding time. |
| 9 | 19 | Bare Areas | Primarily non-vegetated areas containing less than four percent vegetation during at least 10 months a year. The environment is influenced by the edaphic substratum. The cover is natural. Included are areas like bare rock and sands. |
| 10 | 20 | Water Bodies (natural or artificial) | The land cover consists of artificial water bodies. A further specification can be made in flowing or standing water. The land cover consists of natural water bodies. |
| 11 | 21 | Snow or Ice (natural or artificial) | The land cover consists of artificial snow. The land cover consists of artificial ice. A further specification can be made in moving or stationary ice. The landcover consists of snow and ice. |
| 12 | 22 | Urban Areas | The land cover consists of built up area(s). |
| Shapiro–Wilk Statistics (P-value) | |||||
| Species | Model1 | Model2 | Model3 | Model4 | |
| Alnus nitida | Training AUC | 0.565 | 0.073 | 0.936 | 0.094 |
| Test AUC | 0.008 | 0.053 | 0.614 | 1.28e−06 | |
| Alnus nepalensis | Training AUC | 0.262 | 0.335 | 0.333 | 0.722 |
| Test AUC | 0.488 | 0.483 | 0.389 | 0.248 | |
Barry S., Elith J., 2006. Error and uncertainty in habitat models. J. Appl. Ecol, 43(3): 413-423. DOI:10.1111/j.1365-2664.2006.01136.x |
Chhetri R.B., Gauchan D.P., 2008. Traditional knowledge on wood processing of Uttis in Panauti of Kavrepalanchok district, Nepal. Indian J. Tradit. Knowl, 7(1): 112-115. |
Elith J., Graham C.H., Anderson R.P., Dudik M., Ferrier S., Guisan A., Hijmann R.J., Huettmann F., Leathwick J.R., Lehmann A., Li J., Lohmann L.G., Loiselle B.A., Manion G., Moritz C., Nakamura M., Nakazawa Y., Overton J.M., Peterson A.T., Phillips S.J., Richardson K., Scachetti-Pereira R., Schapire R.E., Soberon J., Williams S., Wisz M.S., Zimmermann N.E., 2006. Novel methods improve prediction of species distributions from occurrence data. Ecography, 29: 129-151. DOI:10.1111/j.2006.0906-7590.04596.x |
Elith J., Kearney M., Phillips S., 2010. The art of modelling range-shifting species. Methods Ecol. Evol, 1: 330-342. DOI:10.1111/j.2041-210X.2010.00036.x |
Elith J., Leathwick J.R., 2009. Species distribution models:ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst, 40: 677-697. DOI:10.1146/annurev.ecolsys.110308.1201.59 |
Galloway J.N., Dentener F.J., Capone D.G., Boyer E.W., Howarth R.W., Seitzinger S.P., Asner G.P., Cleveland C., Green P., Holland E., Karl D.M., Michaels A.F., Porter J.H., Townsend A., Vorosmarty C., 2004. Nitrogen cycles:past, present, and future. Biogeochemistry, 70: 153-226. DOI:10.1007/s10533-004-0370-0 |
Guisan A., Lehmann A., Ferrier S., Austin M., Overton J.M.C., Aspinall R., Hastie T., 2006. Making better biogeographical predictions of species' distributions. J. Appl. Ecol, 43(6): 386-392. DOI:10.1111/j.1365-2664.2006.01164.x |
Hijmans R.J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A., 2005. Very high resolution interpolated climate surface for global land areas. Int. J. Climatol, 25: 1965-1978. DOI:10.1002/joc.1276 |
Khan A., Myrold D.D., Misra A.K., 2007. Distribution of Frankia genotypes occupying Alnus nepalensis nodules with respect to altitude and soil characteristics in the Sikkim Himalayas. Physiol. Plant, 130: 364-371. DOI:10.1111/j.1399-3054.2006.00872.x |
Kumar S., Stohlgren T.J., 2009. Maxent modelling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ, 1(4): 94-98. |
Liu C.R., Berry P.M., Dawson T.P., Pearson R.G., 2005. Selecting thresholds of occurrence in the prediction of species distributions. Ecography, 28: 385-393. DOI:10.1111/j.0906-7590.2005.03957.x |
Luedeling E., Kindt R., Huth N.I., Koenig K., 2014. Agroforestry systems in a changing climate e challenges in projecting future performance. Curr. Opin. Environ. Sustain, 6: 1-7. DOI:10.1016/j.cosust.2013.07.013 |
McCarl, B. A., Callaway, J. M., 1993. Carbon sequestration through tree planting on agricultural lands. In: International Symposium Soil Processes and Management Systems.
|
Mortimer P.E., Gui H., Xu J., Zhang C., Barrios E., Hyde K.D., 2015. Alder trees enhance crop productivity and soil microbial biomass in tea plantations. Appl. Soil Ecol, 96: 25-32. DOI:10.1016/j.apsoil.2015.05.012 |
Myrold D.D., Huss-Dannel K., 1994. Population dynamics of alder-infective Frankia in a forest soil with and without host trees. Soil Biol. Biochem, 26: 533-540. DOI:10.1016/0038-0717(94)90239-9 |
O'Neill A.R., Rana S.K., 2016. An ethnobotanical analysis of parasitic plants (Parijibi) in the Nepal Himalaya. J. Ethnobiol. Ethnomed, 12: 14. DOI:10.1186/s13002-016-0086-y |
Phillips B., Chipperfield J.D., Kearney M.R., 2008. The toad ahead:challenges of modelling the range and spread of an invasive species. Wildl. Res, 35: 222-234. DOI:10.1071/WR07101 |
Phillips S.J., Dudik M., 2008. Modelling of species distributions with Maxent:new extensions and a comprehensive evaluation. Ecography, 31: 161-175. DOI:10.1111/j.0906-7590.2008.5203.x |
Phillips S.J., Anderson R.P., Schapire R.E., 2006. Maximum entropy modelling of species geographic distributions. Ecol. Model, 190: 231-259. DOI:10.1016/j.ecolmodel.2005.03.026 |
Phillips, S. J., Dudik, M., Schapire, R. E., 2004. A maximum entropy approach to species distribution modelling. In: Proceedings of the 21st International Conference on Machine Learning. ACM Press, New York, pp. 655-662.
|
Rana S.K., Rana H.K., Ghimire S.K., Shrestha K.K., Ranjitkar S., 2017. Predicting the impact of climate change on the distribution of two threatened Himalayan medicinal plants of Liliaceae in Nepal. J. Mt. Sci, 14(3): 558-570. DOI:10.1007/s11629-015-3822-1 |
Rana, S. K., 2014. Species Distribution and Ecological Niche Modelling of Alnus Species in Nepal (M. Sc. dissertation). Central Department of Botany, Institute of Science and Technology, Tribhuvan University, Kathmandu, Nepal.
|
Ranjitkar S., Sujakhu N.M., Lu Y., Wang Q., Wang M., He J., Mortimer P.E., Xu J., Kindt R., Zomer R.J., 2016. Climate modelling for agroforestry species selection in Yunnan Province, China. Environ. Model. Softw, 75: 263-272. DOI:10.1016/j.envsoft.2015.10.027 |
Ranjitkar S., Xu J., Shrestha K.K., Kindt R., 2014a. Ensemble forecast of climate suitability for the trans-Himalayan Nyctaginaceae species. Ecol. Model, 282: 18-24. DOI:10.1016/j.ecolmodel.2014.03.003 |
Ranjitkar S., Kindt R., Sujakhu N.M., Hart R., Guo W., Yang X., Shrestha K.K., Xu J., Luedeling E., 2014b. Separation of the bioclimatic spaces of Himalayan tree Rhododendron species predicted by ensemble suitability models. Glob. Ecol. Conserv, 1: 2-12. DOI:10.1016/j.gecco.2014.07.001 |
Roy, P. S., Agrawal, S., Joshi, P., Shukla, Y., 2003. The Land Cover Map for Southern Asia for the Year 2000. GLC2000 Database, European Commission Joint Research Centre. http://www-gem.jrc.it/glc2000.
|
Sakalli A., 2017. Simulation of potential distribution and migration of Alnus spp. under climate change. Appl. Ecol. Environ. Res, 15(4): 1039-1070. DOI:10.15666/aeer/1504_10391070 |
Sakalli A., 2013. A simple model for predicting the global distribution of the N2 fixing host genus Alnus Mill.:impacts of climate change on the global distribution in 2100. Biogeosci. Discuss, 10: 13049-13095. DOI:10.5194/bgd-10-13049-2013 |
Salick J., Zhendong F., Byg A., 2009. Eastern Himalayan alpine plant ecology, Tibetan ethnobotany, and climate change. Glob. Environ. Change, 19: 147-155. DOI:10.1016/j.gloenvcha.2009.01.008 |
Sexton J.P., McIntyre P.J., Angert A.L., Rice K.J., 2009. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst, 40: 415-436. DOI:10.1146/annurev.ecolsys.110308.120317 |
Sharma E., Sharma R., Pradhan M., 1998. Ecology of Himalayan Alder (Alnus nepalensis D. Don). PINSA, 1: 59-78. |
Sharma G., Sharma E., Sharma R., Singh K.K., 2002. Performance of an age series of Alnus-Cardamom plantations in the Sikkim Himalaya:productivity, energetics and efficiencies. Ann. Bot, 89: 261-272. DOI:10.1093/aob/mcf035 |
Sharma R., Xu J., Sharma G., 2007. Traditional agroforestry in the eastern Himalayan region:land management system supporting ecosystem services. Trop. Ecol, 48(2): 1-12. |
Shaw, K., Roy, S., Wilson, B., 2014a. Alnus nepalensis. The IUCN Red List of Threatened Species 2014: e. T194649A2355690. https://doi.org/10.2305/IUCN.UK. 2014-3. RLTS. T194649A2355690. en (Downloaded on 08. 11. 17. ).
|
Shaw, K., Roy, S., Wilson, B., 2014b. Alnus nitida. The IUCN Red List of Threatened Species 2014: e. T194659A2356455. https://doi.org/10.2305/IUCN.UK. 2014-3. RLTS. T194659A2356455. en (Downloaded on 08. 11. 17. ).
|
Sheikh M.I., 1993. Trees of Pakistan. Forest/Additional Secretary. MINFA, Pakistan, p, 34. |
Stearn W.T., 1960. Allium and Milula in the central and eastern Himalaya. Bull. Br. Mus. Nat. Hist. Bot, 2: 161-191. |
Taylor K.E., Stouffer R.J., Meehl G.A., 2012. An overview of CIMP5 and the experiment design. Bull. Am. Meteorol. Soc, 93: 485-498. DOI:10.1175/BAMS-D-11-00094.1 |
Vuuren D.P., Edmonds J., Kainuma M., Riahi K., Thomson A., Hibbard K., Hurtt G.C., Kram T., Krey V., Lamarque J.F., Masui T., Meinshausen M., Namicenovic N., Smith S.J., Rose S.K., 2011. The representative concentration pathways:an overview. Clim. Change, 109: 5-31. DOI:10.1007/s10584-011-0148-z |
Woodward F.I., 1992. Predicting plant response to global environmental change. New Phytol, 122: 239-251. DOI:10.1111/j.1469-8137.1992.tb04228.x |
Yost A.C., Peterson S.L., Gregg M., Miller R., 2008. Predictive modelling and mapping sage grouse (Centrocercus urophasianus) nesting habitat using maximum entrophy and a long-term dataset from Southern Oregon. Ecol. Inform, 3: 375-386. DOI:10.1016/j.ecoinf.2008.08.004 |
Zhang M.G., Zhou Z.K., Chen W.Y., Slik J.W.F., Cannon C.H., Raes N., 2012. Using species distribution modelling to improve conservation and land use planning of Yunnan, China. Biol. Conserv, 153: 257-264. DOI:10.1016/j.biocon.2012.04.023 |
Zomer R.J., Trabucco A., Wang M., Lang R., Chen H., Metzger M.J., Smajgl A., Beckschafer P., Xu J., 2014. Environmental stratification to model climate change impacts on biodiversity and rubber production in Xishungbanna, Yunan, China. Biol. Conserv, 170: 264-273. DOI:10.1016/j.biocon.2013.11.028 |



