It is now generally accepted that the primary conservation strategy is the one that focuses on populations in their natural habitats, i.e. in situ conservation. However, the importance of conservation of plants outside their natural environment, either as seeds or adult plants, ex situ conservation, has also long been recognized (Cugnac, 1953) and became established in the mid 20th century. It was also the mainstay of the plant genetic resource movement. Ex situ has many features making it useful for conservation. For example, storing seeds in seed banks has advantages: the ability to collect seeds in large number with usually little impact on natural habitats; long-term storage at low temperatures after desiccation at low maintenance costs; and immunity from habitat destruction, grazing, predation and infestation (Ashton, 1987; Roberts, 1991; Schoen and Brown, 2001; Guerrant et al., 2014). Living collections in botanical gardens and arboreta are particularly important for preserving critically endangered species or species extinct in wild, which cannot be conserved through seed-or tissue-banking protocols.
The need to integrate ex situ and in situ approaches was recognized more than four decades ago (Falk, 1987, 1990; Heywood, 1993; Maxted et al., 1997; Maunder et al., 2001). Many botanic gardens and arboreta have been trying to integrate their collections into in situ conservation programs (Griffith et al., 2011, 2015; Cibrian-Jaramillo et al., 2013; Richardson and Saddler, 2015) and a growing number of conservation programs have been utilizing material maintained and/or propagated in botanic gardens (Wendelberger et al., 2008; Noël et al., 2011; Fotinos et al., 2015; Menges et al., 2016; Fenu et al., 2016).
However, ex situ collections have numerous limitations that restrict their utility for conservation. Seeds cannot be stored indefinitely and must be regenerated. Because collecting and regenerating seed samples is costly, the population samples in seed banks are very commonly small and often from fewer than 50 individuals in the wild. The small sample sizes of ex situ collections and the need for regeneration inevitably lead to loss of genetic variation and a rapid increase in the level of inbreeding in regenerated collections (Schoen and Brown, 2001). On the other hand, usage of seed banks is problematic for species in which seeds have a very slow rate of germination, or, alternatively, are recalcitrant, or germinate immediately and cannot be stored. These species can be preserved ex situ only in living collections. Unfortunately, living collections often suffer from poor genetic representation of species, lack of information on accession sampling locality and mislabeling (Hurka, 1994), and are extremely vulnerable to random genetic drift, artificial selection and mutation accumulation. Physical proximity of plants leads to high risk of infestation by pathogens and, if they have different origins, may result in undesirable spontaneous hybridization. To prevent risk of hybridization and production of maladapted offspring, sampled individuals must be maintained separately or through controlled breeding and pedigree design, which introduces other problems for ex situ collections, such as space limitations and high maintenance costs. Most of the above problems apply to both seed and living collections but are much more serious for the latter.
Thus, on the one hand, in situ conservation requires strengthening via ex situ methods, which would be complementary to in situ and mutually reinforcing (Falk, 1990). But, on the other hand, existing ex situ approaches have serious limitations, requiring the introduction of new, more appropriate, flexible and lower cost approaches. Several new approaches that can be called "intermediate" between in situ and ex situ, and bridge them in some way, have been proposed over the last two decades. In these approaches, material collected in natural populations is planted and maintained outside the original location, however, the purpose, degree of intervention and criteria for choosing a planting site differ.
2. Inter situs-offsite planting for reintroduction/restorationThe inter situs (between sites) approach (usually termed incorrectly and ungrammatically "inter situ", Heywood, 2014) was originally proposed as an off-site collection maintained within the natural habitat (Husband and Campbell, 2004). This extremely general description allowed different interpretations, but current use of this term appears to be limited to the interpretation of Burney and Burney (2007) as "the establishment of species by reintroduction to locations outside the current range but within the recent past range of the species" (Burney and Burney, 2007). According to Burney and Burney, this approach should be applied to a location with some degree of environmental degradation. The inter situs actions include intensive horticultural and agricultural management, invasive species control and protection, with gradual withdrawal of care for the reintroduced plants. The goal of this approach is that the reintroduced species complex eventually becomes a natural ecosystem, approaching, as close as possible, one that existed in that area in the past.
As noted previously (Volis and Blecher, 2010), two important advantages of this strategy are that it can be applied to lands of low economic value such as abandoned agricultural lands, and that it allows simultaneous reintroduction of a large number of species, provided they are within their recent past range. Actually, the proposed strategy is an ecological restoration performed within an explicit conservation context. Such a combination of reintroduction of threatened species with simultaneous restoration of degraded habitat deserves much credit, and should be more commonly adopted and applied worldwide.
3. Quasi in situ-offsite planting for germplasm preservation and propagationThe quasi in situ approach (Volis and Blecher, 2010), in contrast to the inter situs approach, addresses the key ex situ issue of long-term storage of species genetic diversity. To overcome the space and logistic limitations of garden living collections, in this approach the living collections of required capacity are created in sites having legal protection status and natural or semi-natural conditions, which can include even the least valuable and, to some degree, degraded parts of archaeological, memorial, cultural and other types of protected areas, as well as buffer zones in nature reserves. Site selection explicitly takes into account an issue of local adaptation, i.e. it assumes that the structure of species genetic variation is related to the environmental conditions, and therefore requires a stratified design with a lower level (populations) nested within a higher one (eco-regions or habitats) both for sampling and establishing collection sites. This means that for a species whose range covers different habitats (e.g. soil types, regions of different aridity, vegetation communities), several geographically isolated populations are sampled in each habitat and there is a close environmental match of ex situ locations with locations of sampled natural populations. Accessions are planted separately at a distance from each other allowing their subsequent identification. Knowledge of plant identity for plants maintained in a collection can be important for identification of best performing genotypes, maintaining collection genetic variation and controlled pollination, if necessary.
Plants grown in this collection can be expected to be locally adapted and genetically different. As all plants at a particular ex situ site originate from the same environment, there will be no maladapted genes to participate in recombination and segregation, and cross-pollination of these plants should not lead to breakdown of co-adapted gene complexes or dilution of local adaptation.
The above described features make quasi in situ well suited to the purpose of long-term preservation of species' genetic variation. On the other hand, this strategy can serve a real bridge between ex situ and in situ conservation because the offspring of cross-pollination occurring in the collection can be used for in situ actions such as reinforcement, reintroduction or habitat restoration. In other words, the propagules produced at quasi in situ sites can be useful for inter situs conservation. Therefore, these two conservation approaches, the inter situs, focusing on reintroduction/restoration, and quasi in situ, focusing on preservation and propagating plant material, can be viewed as complementary.
4. Integration of inter situs and quasi in situA need to widen the scope of conservation approaches and improved their integration was recognized by Donald Falk in the late 80s. In Falk's view, there is a need to develop an array of conservation methods which are complementary and mutually reinforcing (Falk, 1987, 1990). In view of the new realities of worldwide environmental degradation, new conservation approaches are especially needed. As noted by Heywood (2016) the current trajectories of species loss and habitat degradation will continue unless some innovative approaches are adopted.
Several attempts to reconcile conservation practices with the new challenges of worldwide habitat destruction or degradation have been made. All these concepts/ideas emphasized restoration of degraded habitats as crucial for conserving biodiversity in the 21st century (Dobson et al., 1997; Young, 2000; Hobbs et al., 2009).
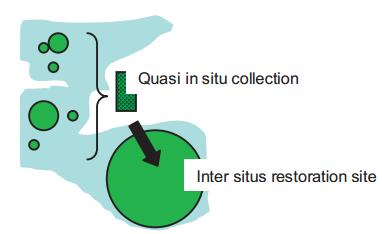
Both inter situs and quasi in situ are related to habitat restoration, either directly (inter situs) or indirectly (quasi in situ) (Fig. 1). Complementarily of their goals, and the wide range of environmental settings where they can be applied, call for their integration. In an attempt to provide a general platform for solving numerous conservation challenges facing the rapidly changing world, I recently introduced a concept called conservation-oriented restoration (Volis, 2016a, b) that implicitly includes inter situs and quasi in situ as necessary components. This concept is based on two major principles.
|
| Fig. 1 A proposed integration of inter situs and quasi in situ strategies. Threatened species collected in remaining populations (in green) of a degraded and/or fragmented habitat (in grey) are planted in quasi in situ collections, and the latter are used to propagate material to be introduced to inter situs restoration sites. |
The first principle is that there is no alternative to active management of populations of threatened species to prevent their extinction. Virtually all ecosystems, including those preserved in nature reserves, have undergone anthropogenic disturbance that have disrupted previously existing species interactions and ecological processes, and reduced population sizes of many species below the viability threshold. The existing individuals indicate that the survival of the species at a specific location is possible in principle, but only if the factors contributing to population decline, and especially those preventing natural regeneration, are identified and eliminated. The latter, in many cases, is impossible without active interventions (e.g. introduction of facilitating germination and establishment plants, thinning of competing species or reintroduction of interacting animals). For this reason, restoration (when necessary) of the regeneration niche must be a precondition for any reintroduction.
The second principle is the wide-scale plant introduction of threatened species, not only within but also outside their known species historical range. Traditionally, introduction outside historic ranges has been discouraged (Hoegh-Guldberg et al., 2008), but this view is now challenged by the climate change effects on species ranges and the rapid disappearance of natural habitats (Vitt et al., 2010, 2016; Thomas, 2011; Butterfield et al., 2016). For endangered species without undisturbed reference habitats (e.g. Tang et al., 2011; Gratzfeld et al., 2015; Xia et al., 2016; Wang et al., 2017) introduction into multiple suitable habitats both inside and outside their known range seems to have no alternative.
Based on these two principles, I have proposed the introduction of multiple threatened species into multiple locations, accompanied by active interventions to these locations such as thinning of competing vegetation or liberation cutting (Volis, 2016a). A set of recommendations is provided for identification of suitable multi-species assemblages, planting design and addressing inter-specific interactions (Volis, 2016b).
The key elements of the concept follow.
1. Assisted colonization of threatened species into as many locations that suit species requirements as possible. When historical knowledge is available, the priority areas for colonization are those within the species past range and having climatic conditions suitable at present and in the expected future.
2. Wide usage of the partly degraded environments for assisted colonization but with the highest priority to the least degraded areas that have extant populations of the threatened species.
3. Targeting alternative states as reference ecosystems in restoration of the degraded habitats through adaptive learning, as a replicated-over-space experiment. As a result, optimal, i.e. leading to the highest restoration success, reference conditions can be determined.
4. Establishing conservation seed banks-storage of threatened and rare species designed to store large number of seeds per species rather than just large number of species, with the stored seeds fully available for in situ actions.
5. Obtaining sufficient number of outplants through seed production in living quasi in situ collections, and then seedling/sapling production in specialized nurseries.
6. Planting design based on experimentation with species assemblage composition and replicating these experimental assemblages over space.
7. Re-establishment of the integrity of disrupted interactions crucial for ecosystem functioning (seed dispersal, pollination, nutrient cycling and food webs) via introduction or control of a suite of interacting species such as soil biota, herbivores, seed predators, frugivorous vertebrates or even top predators.
8. Legal status of the restored site preventing unauthorized anthropogenic disturbance but permitting pre-and post-planting management interventions.
A role for quasi in situ collections in this concept is to provide long-term preservation of species genetic diversity and production of seeds needed for inter situs restoration (Fig. 1).
AcknowledgmentNo grant supported this study. I am grateful to Vernon Heywood for valuable comments on an early version of the manuscript.
Ashton, P. S., 1987. Biological considerations in in situ vs ex situ plant conservation. In: Bramwell, D., et al. (Eds. ), Botanic Gardens and World Conservation Strategy. Academic Press, London, pp. 117-130.
|
Burney D.A., Burney L.P., 2007. Paleoecology and "inter-situ" restoration on Kauai, Hawaii. Front. Ecol. Environ, 5: 483-490. DOI:10.1890/070051 |
Butterfield B.J., et al, 2016. Prestoration:using species in restoration that will persist now and into the future. Restor. Ecol.. |
Cibrian-Jaramillo A., et al, 2013. What is the conservation value of a plant in a botanic garden? Using indicators to improve management of ex situ collections. Bot. Rev, 79: 559-577. DOI:10.1007/s12229-013-9120-0 |
Cugnac A.d., 1953. Le role des jardines botaniques pour la conservation des especes menacees de disparition ou d'alteration. Ann. Biol, 29: 361-367. |
Dobson A.P., et al, 1997. Hopes for the future:restoration ecology and conservation biology. Science, 277: 515-522. DOI:10.1126/science.277.5325.515 |
Falk D.A., 1987. Integrated conservation strategies for endangered plants. Nat. Areas J, 7: 118-123. |
Falk D.A., 1990. Integrated strategies for conserving plant genetic diversity. Ann. Mo. Bot. Gard, 77: 38-47. DOI:10.2307/2399623 |
Fenu G., et al, 2016. The role of fencing in the success of threatened plant species translocation. Plant Ecol, 217: 207-217. DOI:10.1007/s11258-015-0517-1 |
Fotinos T.D., et al, 2015. Genetic evaluation of a reintroduction of Sargent's Cherry Palm, Pseudophoenix sargentii. J. Torrey Bot. Soc, 142: 51-62. DOI:10.3159/TORREY-D-14-00004.1 |
Gratzfeld J., et al, 2015. Whither rare relict trees in climate of rapid change?. BGjournal, 12: 21-25. |
Griffith M.P., et al, 2015. Can a botanic garden cycad collection capture the genetic diversity in a wild population?. Int. J. Plant Sci, 176: 1-10. DOI:10.1086/678466 |
Griffith P., et al, 2011. Palm conservation at a botanic garden:a case study of the Keys Thatch Palm. Palms, 55: 93-101. |
Guerrant E.O.J., et al, 2014. Sampling for effective ex situ plant conservation. Int. J. Plant Sci, 175: 11-20. DOI:10.1086/674131 |
Heywood V.H., 1993. The role of botanic gardens and arboreta in the ex-situ conservation of wild plants. Opera Bot, 121: 309-312. |
Heywood V.H., 2014. An overview of in situ conservation of plant species in the Mediterranean. Flora Mediterr, 24: 5-24. DOI:10.7320/flmedit01.001 |
Heywood V.H., 2016. In situ conservation of plant species-an unattainable goal?. Isr.J. Plant Sci, 63: 211-231. |
Hobbs R.J., et al, 2009. Novel ecosystems:implications for conservation and restoration. Trends Ecol. Evol, 24: 599-605. DOI:10.1016/j.tree.2009.05.012 |
Hoegh-Guldberg O., et al, 2008. Assisted colonization and rapid climate change. Science, 321: 345-346. DOI:10.1126/science.1157897 |
Hurka, H., 1994. Conservation genetics and the role of botanical gardens. In: Sandlund, O. T., et al. (Eds. ), Conservation Genetics. Birkhauser Verlag, Basel, pp. 371-380.
|
Husband, B. C., Campbell, L. G., 2004. Population responses to novel environments: implications for ex situ plant conservation. In: Guerrant, E. O. J., et al. (Eds. ), Ex Situ Plant Conservation: Supporting Species Survival in the Wild. Island Press, Washington, pp. 231-266.
|
Maunder M., et al, 2001. The effectiveness of botanic garden collections in supporting plant conservation:a European case study. Biodivers. Conserv, 10: 383-401. DOI:10.1023/A:1016666526878 |
Maxted, N., et al., 1997. Complementary conservation strategies. In: Maxted, N., et al. (Eds. ), Plant Genetic Conservation, the In Situ Approach. Chapman and Hall, London.
|
Menges E.S., et al, 2016. Adaptive introductions:how multiple experiments and comparisons to wild populations provide insights into requirements for longterm introduction success of an endangered shrub. Plant Divers, 38: 238-246. DOI:10.1016/j.pld.2016.09.004 |
Noël F., et al, 2011. Establishment success of 25 rare wetland species introduced € into restored habitats is best predicted by ecological distance to source habitats. Biol. Conserv, 144: 602-609. DOI:10.1016/j.biocon.2010.11.001 |
Richardson M., Saddler S., 2015. Conservation role for a new arboretum in Canberra, Australia. BGjournal, 12: 12-14. |
Roberts E.H., 1991. Genetic conservation in seed banks. Biol. J. Linn. Soc, 43: 23-29. DOI:10.1111/bij.1991.43.issue-1 |
Schoen D.J., Brown A.D.H., 2001. The conservation of wild plant species in seed banks. BioScience, 51: 960-966. DOI:10.1641/0006-3568(2001)051[0960:TCOWPS]2.0.CO;2 |
Tang C.Q., et al, 2011. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv, 144: 279-289. DOI:10.1016/j.biocon.2010.09.003 |
Thomas C.D., 2011. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol, 26: 216-221. DOI:10.1016/j.tree.2011.02.006 |
Vitt P., et al, 2016. Assisted migration as a climate change adaptation strategy:lessons from restoration and plant reintroductions. Isr. J. Plant Sci, 63: 250-261. |
Vitt P., et al, 2010. Assisted migration of plants:Changes in latitudes, changes in attitudes. Biol. Conserv, 143: 18-27. DOI:10.1016/j.biocon.2009.08.015 |
Volis S., 2016a. Conservation meets restoration-rescuing threatened plant species by restoring their environments and restoring environments using threatened plant species. Isr. J. Plant Sci, 63: 262-275. |
Volis S., 2016b. Conservation-oriented restoration-how to make it a success?. Isr. J. Plant Sci, 63: 276-296. |
Volis S., Blecher M., 2010. Quasi in situ-a bridge between ex situ and in situ conservation of plants. Biodivers. Conserv, 19: 2441-2454. DOI:10.1007/s10531-010-9849-2 |
Wang B., et al, 2017. Floral characteristics and pollination ecology of Manglietia ventii (Magnoliaceae), a plant species with extremely small populations (PSESP) endemic to South Yunnan of China. Plant Divers, 39: 52-59. DOI:10.1016/j.pld.2017.01.001 |
Wendelberger K.S., et al, 2008. Rescue and restoration:experimental translocation of Amorpha herbacea Walter var.crenulata (Rybd.) Isley into a novel urban habitat. Restor. Ecol, 16: 542-552. DOI:10.1111/rec.2008.16.issue-4 |
Xia K., et al, 2016. Conservation and fruit biology of Sichou oak (Quercus sichourensis, Fagaceae)-a critically endangered species in China. Plant Divers, 38: 233-237. DOI:10.1016/j.pld.2016.07.001 |
Young T.P., 2000. Restoration ecology and conservation biology. Biol. Conserv, 92: 73-83. DOI:10.1016/S0006-3207(99)00057-9 |



