b. University of Chinese Academy of Sciences, 100049, Beijing, China;
c. Griffith School of Environment and Environmental Futures Research Institute, Griffith University, Nathan QLD 4111, Brisbane, Australia
The soil seed bank consists of seeds present on or in the soil, and it contributes to vegetation succession when dormancy-breaking and germination requirements of the seeds are met (Bakker et al., 1996; Funes et al., 2001; Erfanzadeh et al., 2013), especially in those ecosystems experiencing frequent disturbances (Davies and Waite, 1998; Li et al., 2004; Lin et al., 2006; Milberg, 1995; Willems and Bik, 1998).
The soil seed bank changes in seed density and species composition with vegetation succession (Cao et al., 1996, 2000a, b; Funes et al., 2003; Perera, 2005; Erfanzadeh et al., 2010; Tang et al., 1999). Ortega et al. (1997) found that both the richness and density of seeds in the soil seed bank decreased with elevation in mountain grasslands. Jalili et al. (2003) observed that seed density decreased with elevation in Iran, because the harsh environment at higher elevations reduced seed production and promoted vegetative reproduction of plants. Alternatively, Funes et al. (2003) showed that soil seed bank richness and density increase with elevation in Argentina as a result of two processes: the relatively warm conditions at the lower elevations which enhance seed predation, while the cold climate at the high elevations may favor the formation of persistent seed banks. Soil seed banks also had high variability in seed density and species composition among different forest types. In a study on the effects of elevational change (1500 m–3500 m) on soil seed banks in the Taibai Mountains of northern China, the number of species decreased with elevation, although seed density peaked at mid-elevation (2600 m) (Zhang and Fang, 2004a). Similar trends of species richness and seed density were also observed in soil seed banks of Picea schrenkiana forests in the Tianshan Mountains (1450–2750 m) in northwest China (Li et al., 2012). In the tropical forests of Xishuangbanna in southwest China, soil seed density ranged from 4585 seeds/m2 (seasonal rain forest) to 65, 665 seeds/m2 (4-year-old secondary forest) in the top 10 cm of soil, while the number of species ranged from 50 to 59 (Cao et al., 2000a, b). Li et al. (2010) found that the soil seed density was 6160-22, 760 seeds/m2, with 29–62 species, in subtropical forests of Ailao Mountain, Yunnan, but only 185.5–1065.6 seeds/m2 were reported in subalpine coniferous forests (Yin and Liu, 2004, 2005).
The similarity between soil seed banks and standing vegetation provides insight into the response of a community to disturbance (Hopfensperger, 2007). Many species in standing vegetation do not occur in soil seed banks, suggesting little similarity (Amiaud and Touzard, 2004; Esmailzadeh et al., 2011). Similarity was also lower in forest ecosystems than in grasslands and wetlands (Hopfensperger, 2007). Tang et al. (1999) found more seeds of common species in both the soil seed bank and standing vegetation at the initial stages of forest succession, but such a similarity in species decreased with succession. In five shrub communities in the Strandveld Succulent Karoo of South Africa, Sørensen indices between standing vegetation and soil seed bank averaged 47.9%, showing a relatively high similarity (Villiers et al., 2003). In subarctic plant communities in the early phase of regeneration in Finland, however, very low similarity occurred between the soil seed bank, seedlings emerging in the field, and standing vegetation (Welling et al., 2015). In the Tianshan Mountains (1750–2750 m) of northwest China, the Jaccard Index between the soil seed bank and standing vegetation decreased with elevation (Zhou et al., 2013). However, it has not been determined how soil seed banks respond to disturbance at different elevations.
Based on the study of soil seed banks in evergreen broad-leaved forests in Yunnan Province, southwest China, a new species group, i.e. nonconstituent species that occur in a natural landscape but are not native to it, has been recognized (Lin et al., 2006). In fragmented forests or disturbed forests, nonconstituent species may become established in habitats that differ from closed forests or their successional communities. Nonconstituent species are mostly exotics when considered in terms of both geographical and ecosystem scales and include exotic species or weeds from neighboring farmland. These species therefore tend to share some similar ecological traits, such as wind dispersal of seeds, small seed size, long life-span of seeds, and abundant seed production (Baker, 1974). This ecological similarity among nonconstituent species results in similar responses to anthropogenic disturbance; Thus, nonconstituent species in soil seed banks could serve as an ecosystem indicator of anthropogenic disturbance in forest ecosystems (Lin et al., 2006).
Although previous studies reported the distribution of forest soil seed banks in some mountains, these studies were conducted only in a single climatic zone and did not include elevational gradients in a continuous geographical gradient of different climatic zones. Yunnan Province is in the southwest of China, on the southeastern extension of the Himalayas, and it has tropical rain forest, subtropical evergreen broad-leaved forest and subalpine coniferous forest (Wu et al., 1987). However, the variation in the elevational distribution of soil seed banks in each climatic zone has received little attention. Therefore, the present study examines elevational changes of soil seed banks in tropical, subtropical and subalpine climatic zones of Yunnan Province. We aim to (1) determine how the size (seed density), species composition and richness of soil seed banks respond to variation in elevation in the three climatic zones; (2) analyze the variations in similarity between the soil seed bank and standing vegetation; and (3) explore the changes in nonconstituent species in soil seeds banks in these forests. We hypothesized that: 1) the size (seed density or abundance) and species richness of soil seed banks decrease with elevation in all three climatic zones; 2) similarity between soil seed bank and standing vegetation increases with elevation, because the species pool becomes smaller due to the harsher environment at high elevations; and 3) both seed abundance and species number of nonconstituent species decrease with elevation, as a consequence of reduced human activities in montane areas.
2. Materials and methods 2.1. Study areaThis study was carried out in Yunnan Province, southwest China. Study sites were in tropical (Xishuangbanna – southwest Yunnan), subtropical (Ailao Mountains – central Yunnan) and subalpine (Lijiang – northwest Yunnan) zones (Fig. 1). Also, the sites represent geographical and climatic gradients of latitude, elevation, temperature and precipitation from south to north in this province (Table 1). In each vegetation zone, an elevational transect comprised of four points (elevations) at 200-m intervals from each other, was established. Five plots (20 m × 20 m) at each elevation were set up, and all the trees with DBH ≥ 5 cm in each plot were measured and identified to species.
|
| Fig. 1 Study sites in Yunnan Province, southwest China. |
| Site | Elevation (m) | Latitude | Longitude | Forest type | Annual mean temperature (℃) | Annual mean precipitation (mm) | Dominant tree species | Mean DBH of trees (mm) | Tree density (trees ha 1) | Intensity of disturbance |
| Xishuangbanna | 800 | 21º36'785''N | 101º34'799''E | Tropical seasonal rain forest | 21.5 (Mengla County, ele. 632 m)a | 1520.5 (Mengla County, ele. 632 m)a | Parashorea chinensis, Pittosporopsis kerrii | 135.8 | 1525 | + |
| 1000 | 21º37'153''N | 101º34'425''E | Tropical montane rain forest | Actinodaphne henryi, Pittosporopsis kerrii | 44.0 | 1650 | + | |||
| 1200 | 21º35'643''N | 101º33'620''E | Tropical montane evergreen broad-leaved forest | Castanopsis echinocarpa, Castanopsis mekongensis | 39.3 | 1275 | +++ | |||
| 1400 | 21º35'454''N | 101º32'966''E | Tropical montane evergreen broad-leaved forest | Castanopsis mekongensis, Schima argentea | 64.6 | 1150 | ++ | |||
| Ailao Mountain | 2000 | 24º16'209''N | 101º15'796''E | Subtropical middle montane moist evergreen broad-leaved forest | 18.7 (Zhenyuan County, ele. 1086 m)a | 1237.2 (Zhenyuan County, ele. 1086 m)a | Claoxylon khasianum, Ficus henryi | 55.6 | 1100 | +++ |
| 2200 | 24º16'621''N | 101º15'848''E | Manglietia insignis, Camellia assamica | 58.1 | 1300 | ++ | ||||
| 2400 | 24º17'066''N | 101º15'354''E | Lithocarpus xylocarpus, Eurya obliquifolia | 50.3 | 1850 | + | ||||
| 2600 | 24º17'143''N | 101º15'060''E | Castanopsis rufescens, Camellia forrestii | 78.1 | 1225 | + | ||||
| Lijiang | 3200 | 27º08'363''N | 100º13'757''E | Subalpine coniferous forest | 12.8 (Lijiang County, ele. 2393 m)b | 935.0 (Lijiang County, ele. 2393 m)b | Abies forrestii, Sorbus rufopilosa | 59.4 | 500 | ++ |
| 3400 | 27º09'784''N | 100º13'790''E | Abies georgei, Rhododendron yunnanense | 121.5 | 650 | +++ | ||||
| 3600 | 27º10'244''N | 100º13'977''E | Abies georgei, Abies forrestii, Quercus pannosa | 86.5 | 1375 | + | ||||
| 3800 | 27º11'261''N | 100º13'174''E | Abies georgei | 92.2 | 825 | + | ||||
| a He, Y.L., Zhang, Y.P., 2005. Climate change from 1960 to 2000 in the Lancang River Valley, China. Mt. Res. Dev. 25, 341-348. b Feng, J.M., Wang, R.P., Xu, C.D., Yang, Y.H., Fang, J.Y., 2006. Altitudinal patterns of plant species diversity and community structure on Yulong Mountains, Yunnan, China. J. Mt. Sci. 24, 110–116. |
||||||||||
Xishuangbanna lies on the northern edge of the tropics in southeast Asia and borders Laos and Myanmar to the south and west. This area has a tropical seasonal rain forest below 900 m a.s.l. due to monsoon climate with an alternation between a rainy (May–October) and dry (November–April) season (Cao et al., 1996, 2006). Dominant tree species in the tropical seasonal rain forest study site were Parashorea chinensis, Pittosporopsis kerrii, Garcinia cowa, Castanopsis echidnocarpa, Mezzettiopsis creaghii, and Sloanea tomentosa (Lan et al., 2009). On mountains over 1000 m, tropical montane evergreen broad-leaved forests occur (Zhu et al., 2006).
Ailao Mountain is located in the subtropical zone in central Yunnan Province. Subtropical middle montane moist evergreen broad-leaved forest is distributed between 2000 m–2600 m. The forest is dominated by Castanopsis wattii, Lithocarpus hancei, Lithocarpus xylocarpus and L. truncates.
Lijiang is in northwest Yunnan. Our study site is on Yulong Snow Mountain, which is the southeastern extension of the Tibetan Plateau (Niu et al., 2013). The major forest type between 3100 m–4000 m on this mountain is subalpine coniferous forest dominated by species of Pinaceae, such as Abies forrestii and Abies georgei (Wang et al., 2001).
2.2. Sampling methodsAt each of the four elevations in each vegetation zone, we chose one of the five plots for soil sampling. Twenty soil cores (10 cm × 10 cm × 10 cm) were taken at 2 m intervals along two 20 m-long transects in the plot. Fresh litter on the soil surface was removed before sampling and discarded. Each soil core was sampled in three different layers (depths): 0–2 cm, 2–5 cm and 5–10 cm. This was completed by using two special flat shovels that were exactly 10 cm wide, along with a steel tape (Cao et al., 2000a, b). All samples from each soil core were placed separately in cloth bags and transported to Xishuangbanna Tropical Botanical Garden at Menglun Township, Xishuangbanna Prefecture for germination trials. The soil samples were collected at the end of the rainy season in 2013 for the Xishuangbanna site and in 2014 for the Ailao Mountain and Lijiang sites, when most seeds had dispersed (Zhang and Song, 2015; Yang et al., 2010).
2.3. Seed germinationEach soil sample was sieved with a 2-mm sieve to removed gravels and dead plant material buried in the soil. Soil samples were spread evenly into germination trays of different sizes to a depth of less than 2 cm. Before the soil was put into trays, the bottom of each tray was pierced to prevent the soil from becoming water-saturated (Li et al., 2010). All trays were put in a nontemperature controlled glasshouse to reduce contamination of seeds from the outside. Eight additional trays containing sterilized soil (105 ℃ for 12 h) were used as controls for testing seed contamination.
The germination trays were watered once or twice a day as needed to keep the soil samples moist. Seedlings were counted every 2 days during the first 60 days and then every 5–6 days afterwards. Seedlings were removed once they were identified. Seedlings that were difficult to distinguish were transplanted to separate containers for continued growth until they could be identified. After removing all seedlings within a tray, the soil sample was stirred and kept for germination until no more seedlings emerged for two weeks.
2.4. Data analysisSeed density was calculated as the average number of emerged seedlings per square meter (at a depth of 10 cm) from soil samples. A one-way ANOVA followed by the Tukey–Kramer test (in SPSS 19.0) was used to analyze the differences in seed density among elevations. The level of significance was set at p < 0.05.
Species diversity indices of soil seed banks were measured based on the following formulas:
Shannon–Wiener diversity index: H' = -Σ (PilnPi) (Magurran, 1988)
Simpson diversity index: D = 1 -Σ (Pi)2 (Keylock, 2005) where Pi is the proportion of individuals of the ith species out of the total individuals at each elevation, i.e., Pi = (Ni/N), where N is the total number of individuals recorded in the 20 soil samples at each elevation and Ni is the number of the individuals of the ith species in the 20 soil samples at each elevation.
Non-metric multi-dimensional scaling (NMDS) was used to explore the pattern of species composition of 20 soil samples at each elevation in R 3.2.2 using the package vegan (Legendre and Legendre, 1998).
Similarity between soil seed bank and standing vegetation was calculated using the Sørensen similarity index: S = 2c/(a + b) (Sørensen, 1948), where a is the number of species of soil seed bank, b is the number of species of standing vegetation, and c is the number of species shared by both.
All the information on the soil seed banks for Xishuangbanna site (tropical forest) was analyzed based on the primary data of Zhang and Song (2015).
3. Results 3.1. Seed densityA total of 17, 579 seeds germinated in the soil samples, and seed density varied among the three sites (Table 2). In the tropical forest, the soil seed bank at 800 m had more seeds than that at higher elevations, while the lowest seed density was observed at 1400 m. The highest seed density in soil seed banks of the subtropical forest occurred at 2000 m, although there was no significant difference in seed density between 2200 m, 2400 m and 2600 m (p > 0.05, Table 2). Therefore, both tropical and subtropical sites had the most abundant soil seed banks at the basal elevations (800 m and 2000 m, respectively). The subalpine forest had the highest seed density at 3400 m, but seed densities at the other three elevations did not differ significantly (p > 0.05). Looking at seed density of the basal elevation of the three sites (i.e. 800 m for tropical site, 2000 m for subtropical site and 3200 m for subalpine site), we observed that the soil seed bank at 2000 m (subtropical site) possessed the highest seed density, followed by tropical and subalpine sites (Table 2).
| Site | Elevation (m) | Number of seeds | Seed density (seeds m-2)a | |||
| 0–2 cm | 2–5 cm | 5–10 cm | Total | |||
| Xishuangbanna (Tropical forest) | 800 | 928 | 433 | 747 | 2108 | 10, 540 ± 1578 a |
| 1000 | 308 | 376 | 648 | 1332 | 6660 ± 479 bc | |
| 1200 | 279 | 483 | 1051 | 1813 | 9065 ± 659 ab | |
| 1400 | 170 | 341 | 554 | 1065 | 5325 ± 361 c | |
| Ailao Mountain (Subtropical forest) | 2000 | 1097 | 1080 | 1456 | 3633 | 18, 165 ± 1408 a |
| 2200 | 586 | 496 | 527 | 1609 | 8045 ± 823 b | |
| 2400 | 634 | 618 | 677 | 1929 | 9645 ± 621 b | |
| 2600 | 491 | 510 | 539 | 1540 | 7700 ± 646 b | |
| Lijiang (Subalpine forest) | 3200 | 125 | 132 | 43 | 300 | 1500 ± 333 a |
| 3400 | 660 | 569 | 247 | 1476 | 7380 ± 844 b | |
| 3600 | 102 | 148 | 132 | 382 | 1910 ± 367 a | |
| 3800 | 72 | 105 | 215 | 392 | 1960 ± 194 a | |
| a Mean ± standard error, n = 20; Different letters within a site denote significantly different as determined by TukeyeKramer tests (p < 0.05). | ||||||
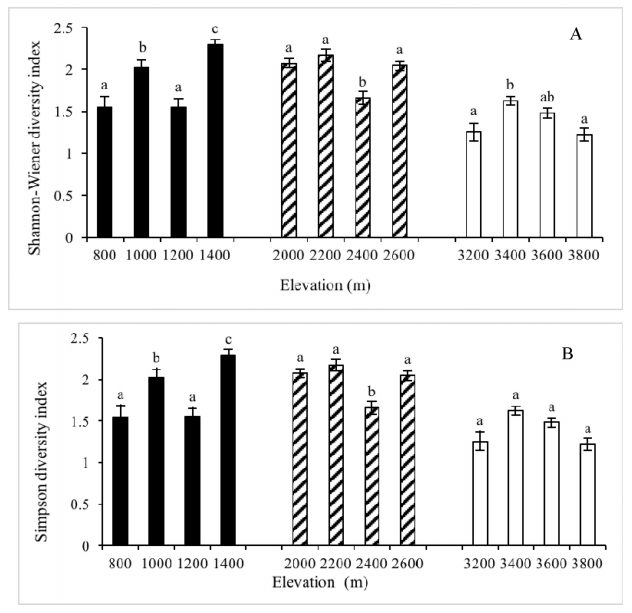
Soil samples from the tropical, subtropical and subalpine sites contained 129, 81 and 50 species (2540 unidentified seedlings excluded), respectively (Appendices 1-3), and number of species tended to decline with an increase in elevation. Among the three sites, soil seed bank at 800 m had the highest number of species, while that at 3800 m had the lowest (Fig. 2). However, the species diversity index was higher in the subtropical than in the tropical and subalpine forests when the three basal elevations (i.e. 800 m, 2000 m, and 3200 m, respectively) in each site were compared (Fig. 3).

|
| Fig. 2 Number of species in soil seed banks across different sites and elevations. |
|
| Fig. 3 Shannon–Wiener diversity index (A) and Simpson diversity index (B) along elevational gradients in tropical, subtropical and subalpine forests. Different letters indicate significant differences among elevations within a climatic zone. |
Woody species (tree + shrub) dominated the soil seed banks in the tropical forest (Table 3), and the highest proportion of tree and shrub seeds occurred in soil at 800 m. In the subtropical forest, shrubs and herbs dominated the soil seed bank, and the highest proportion of tree and shrub species occurred at 2000 m. In the subalpine forest, however, herbs were dominant at all four elevations, and seeds of tree species were nearly absent from the soil seed bank (Table 3).
| Site | Elevation (m) | Life form | |||||
| Tree | Shrub | Herb | Vine | Total | |||
| Xishuangbanna (Tropical forest) | 800 | 22 (31.0%) | 26 (36.6%) | 16 (22.5%) | 7 (9.9%) | 71 (100.0%) | |
| 1000 | 20 (29.4%) | 24 (35.3%) | 19 (27.9%) | 5 (7.4%) | 68 (100.0%) | ||
| 1200 | 13 (25.5%) | 18 (35.3%) | 16 (31.4%) | 4 (7.8%) | 51 (100.0%) | ||
| 1400 | 12 (20.7%) | 21 (36.2%) | 20 (34.5%) | 5 (8.6%) | 58 (100.0%) | ||
| Ailao Mountain (Subtropical forest) | 2000 | 10 (16.9%) | 19 (32.2%) | 25 (42.4%) | 5 (8.5%) | 59 (100.0%) | |
| 2200 | 9 (18.0%) | 18 (36.0%) | 19 (38.0%) | 4 (8.0%) | 50 (100.0%) | ||
| 2400 | 6 (15.8%) | 15 (39.5%) | 15 (39.5%) | 2 (5.3%) | 38 (100.0%) | ||
| 2600 | 7 (17.9%) | 18 (46.2%) | 13 (33.3%) | 1 (2.6%) | 39 (100.0%) | ||
| Lijiang (Subalpine forest) | 3200 | 2 (8.7%) | 21 (91.3%) | 23 (100.0%) | |||
| 3400 | 1 (2.7%) | 5 (13.5%) | 31 (83.8%) | 37 (100.0%) | |||
| 3600 | 3 (13.6%) | 19 (86.4%) | 22 (100.0%) | ||||
| 3800 | 2 (10.0%) | 18 (90.0%) | 20 (100.0%) | ||||
| a Figures in parentheses are percentages for the numbers of species out of the total number of species germinated from the 20 soil samples of each elevation. | |||||||
| Site | Species | Life form | Elevation (m) | |||
| 800 | 1000 | 1200 | 1400 | |||
| Xishuangbanna (Tropical forest) | Neolamarckia cadamba | Tree | 1173 (55.65% | 18 (1.35%) | ||
| Ludwigia hyssopifolia | Herb | 128 (6.07%) | 20 (1.50%) | 1 (0.06%) | ||
| Buddleja asiatica | Shrub | 112 (5.31%) | 12 (0.90%) | 6 (0.56%) | ||
| Digitaria sanguinalis | Herb | 99 (4.70%) | 391 (29.35%) | 938 (51.74%) | 197 (18.50%) | |
| Ficus semicordata | Tree | 56 (2.66%) | 183 (13.74%) | 37 (2.04%) | 118 (11.08%) | |
| Wendlandia uvariifolia | Shrub | 3 (0.14%) | 202 (15.17%) | |||
| Ficus variegata var. chlorocarpa | Tree | 44 (2.09%) | 72 (5.41%) | 1 (0.06%) | 24 (2.25%) | |
| Crassocephalum crepidioides | Herb | 6 (0.28%) | 31 (2.33%) | 7 (0.39%) | 10 (0.94%) | |
| Eurya pittosporifolia | Tree | 15 (1.13%) | 121 (6.67%) | 117 (10.99%) | ||
| Melastoma malabathricum | Shrub | 167 (9.21%) | 128 (12.02%) | |||
| Wendlandia tinctoria | Shrub | 124 (6.84%) | 59 (5.54%) | |||
| Maesa montana | Shrub | 3 (0.23%) | 92 (5.07%) | 25 (2.35%) | ||
| Ailao Mountain (Subtropical forest) | 2000 | 2200 | 2400 | 2600 | ||
| Debregeasia orientalis | Shrub | 1301 (35.81% | 129 (8.02%) | 3 (0.16%) | 3 (0.19%) | |
| Maesa indica | Shrub | 475 (13.07%) | 6 (0.37%) | |||
| Ficus beipeiensis | Tree | 279 (7.68%) | 4 (0.25%) | |||
| Boehmeria clidemioides var. diffusa | Herb | 248 (6.83%) | 3 (0.19%) | |||
| Laggera alata | Herb | 224 (6.17%) | 1 (0.06%) | 1 (0.06%) | ||
| Elatostema laevissimum | Shrub | 1 (0.03%) | 259 (16.10%) | 4 (0.21%) | 44 (2.86%) | |
| Docynia delavayi | Tree | 7 (0.19%) | 178 (11.06%) | 19 (0.98%) | 19 (1.23%) | |
| Oxyspora paniculata | Shrub | 40 (1.10%) | 119 (7.40%) | 66 (3.42%) | 66 (4.29%) | |
| Laportea bulbifera | Herb | 18 (0.50%) | 56 (3.48%) | 35 (1.81%) | 34 (2.21%) | |
| Myrsine semiserrata | Shrub | 18 (0.50%) | 24 (1.49%) | 532 (27.58%) | 226 (14.68%) | |
| Scleria terrestris | Herb | 20 (0.55%) | 29 (1.80%) | 140 (7.26%) | ||
| Agapetes mannii | Shrub | 2 (0.06%) | 44 (2.73%) | 92 (4.77%) | 111 (7.21%) | |
| Carex teinogyna | Herb | 17 (0.47%) | 19 (1.18%) | 85 (4.41%) | 7 (0.45%) | |
| Ilex corallina | Tree | 13 (0.36%) | 47 (2.92%) | 76 (3.94%) | 20 (1.30%) | |
| Rubus sumatranus | Shrub | 6 (0.17%) | 20 (1.24%) | 40 (2.07%) | 85 (5.52%) | |
| Eurya groffii | Tree | 2 (0.06%) | 23 (1.43%) | 39 (2.02%) | 47 (3.05%) | |
| Lijiang (Subalpine forest) | 3200 | 3400 | 3600 | 3800 | ||
| Carex nubigena | Herb | 120 (40.00%) | 17 (1.15%) | 22 (5.76%) | 29 (7.40%) | |
| Philadelphus delavayi | Shrub | 37 (12.33%) | 370 (25.07%) | 15 (3.93%) | ||
| Pilea sinofasciata | Herb | 16 (5.33%) | 340 (23.04%) | 19 (4.97%) | ||
| Stellaria vestita | Herb | 14 (4.67%) | 3 (0.20%) | 17 (4.45%) | 11 (2.81%) | |
| Myriactis wightii | Herb | 12 (4.00%) | 186 (12.60%) | 21 (5.50%) | 23 (5.87%) | |
| Ainsliaea latifolia | Herb | 12 (4.00%) | 111 (7.52%) | 1 (0.26%) | ||
| Clinopodium polycephalum | Herb | 6 (2.00%) | 273 (18.50%) | 1 (0.26%) | ||
| Rubus fockeanus | Herb | 11 (3.67%) | 22 (1.49%) | 83 (21.73%) | ||
| Carex sp1. | Herb | 2 (0.67%) | 9 (0.61%) | 47 (12.30%) | 2 (0.51%) | |
| Ribes glaciale | Shrub | 4 (1.33%) | 4 (0.27%) | 43 (11.26%) | 3 (0.77%) | |
| Juncus effusus | Herb | 35 (9.16%) | ||||
| Astilbe chinensis | Herb | 76 (19.39%) | ||||
| Ribes sp1. | Shrub | 1 (0.07%) | 1 (0.26%) | 71 (18.11%) | ||
| Polygonum runcinatum | Herb | 1 (0.33%) | 33 (8.42%) | |||
| a The number and proportion in bracket of each species in the soil seed bank at each elevation, the bold fonts were the five most abundant species at each elevation. | ||||||
In the Xishuangbanna site, Digitaria sanguinalis obviously was dominant at the four elevations, although this species also commonly occurs in farm fields, roadsides and weedy places in subtropical and tropical areas. In contrast, Neolamarckia cadamba (formerly Anthocephalus chinensis), Buddleja asiatica and Ludwigia hyssopifolia only dominated at 800 m; Ficus variegata var. chlorocarpa, Wendlandia uvariifolia and Crassocephalum crepidioides were dominant only at 1000 m. At higher elevations (1200 m and 1400 m) where tropical montane evergreen broad-leaved forest occurs, Melastoma malabathricum, Wendlandia tinctoria and Eurya pittosporifolia dominated the soil seed banks, although Maesa montana was dominant only at 1200 m.
In the Ailao Mountain site, no species dominated at all four elevations. However, there were some species that were dominant at one elevation, such as Maesa indica, Ficus beipeiensis, Boehmeria clidemioides var. diffusa and Laggera alata at 2000 m; Elatostema laevissimum, Docynia delavayi and Laportea bulbifera at 2200 m; Scleria terrestris, Carex teinogyna; Ilex coralline at 2400 m; and Rubus sumatranus and Eurya groffii at 2600 m.
In the Lijiang site, the soil seed banks at 3200 m and 3400 m shared three dominant species, i.e. Philadelphus delavayi, Pilea sinofasciata and Ainsliaea latifolia. Four and three species were dominant at 3600 m and 3800 m, respectively. However, we did not observe tree species as dominants at the four elevations of the transect.
3.3. NMDS analysisSpecies composition of 20 soil samples at each elevation tended to be more homogeneous than between elevations, as the 20 points representing 20 soil samples from same elevation aggregated in the NMDS plot (Fig. 4).

|
| Fig. 4 Nonmetric multidimensional ordination based on composition of 20 soil samples among elevational gradients in A. Xishuangbanna, B. Ailao Mountain and C. Lijiang. |
In general, the values of Sørensen's index between soil seed bank and standing vegetation at low elevations were lower than those at high elevations in both tropical and subtropical sites. Further, the soil seed bank obviously had more woody species than the standing vegetation at all elevations (Table 5). However, the subalpine site had a completely different pattern, and here no woody species were shared by the standing vegetation and soil seed bank. Contrary to the tropical and subtropical sites, standing vegetation of the subalpine site had more woody species than the soil seed bank at 3200 m, 3400 m and 3600 m (Table 5).
| Site | Elevation (m) | NWSAV | NWSIS | NCS | Sørensen's index |
| Xishuangbanna (Tropical forest) | 800 | 24 | 53 | 0 | 0.00% |
| 1000 | 26 | 48 | 0 | 2.70% | |
| 1200 | 11 | 34 | 2 | 8.89% | |
| 1400 | 7 | 38 | 1 | 4.44% | |
| Ailao Mountain (Subtropical forest) | 2000 | 11 | 30 | 0 | 0.00% |
| 2200 | 14 | 28 | 1 | 4.76% | |
| 2400 | 14 | 22 | 1 | 5.56% | |
| 2600 | 13 | 25 | 2 | 10.53% | |
| Lijiang (Subalpine forest) | 3200 | 6 | 2 | 0 | 0.00% |
| 3400 | 7 | 6 | 0 | 0.00% | |
| 3600 | 5 | 3 | 0 | 0.00% | |
| 3800 | 1 | 2 | 0 | 0.00% | |
| NWSAV: Number of woody species recorded in standing vegetation, NWSIS: Number of woody species in soil seed bank. NCS: Number of common woody species shared by both standing vegetation and soil seed bank. | |||||
Fifteen nonconstituent species germinated from soil samples of Xishuangbanna and four from those of Ailao Mountain, while none was found from those of Lijiang (Table 6). All the nonconstituent species from Xishuangbanna and Ailao Mountain were herbs. D. sanguinalis and C. crepidioides occurred at all elevations, and D. sanguinalis was the most abundant (1625), followed by Ludwigia linifolia (149) and C. crepidioides (54). In Ailao Mountain, Ageratina adenophora, Laggera pterodonta and C. crepidioides occurred at the four elevations, of which A. adenophora was the most abundant species (Table 6).
| Site | Nonconstituent species | Life form | 800 m | 1000 m | 1200 m | 1400 m |
| Xishuangbanna (Tropical forest) | Ludwigia linifolia | Herb | 128 | 20 | 1 | |
| Digitaria sanguinalis | Herb | 99 | 391 | 938 | 197 | |
| Crassocephalum crepidioides | Herb | 6 | 31 | 7 | 10 | |
| Ageratum conyzoides | Herb | 3 | ||||
| Conyza canadensis | Herb | 2 | 4 | |||
| Chromolaena odorata | Herb | 2 | 1 | 1 | ||
| Gnaphalium pensylvanicum | Herb | 1 | 2 | 2 | ||
| Stachytarpheta jamaicensis | Herb | 1 | ||||
| Mirabilis jalapa | Herb | 1 | ||||
| Spermacoce remota | Herb | 3 | 3 | |||
| Hedyotis verticillata | Herb | 3 | ||||
| Hedyotis diffusa | Herb | 2 | ||||
| Hedyotis costata | Herb | 37 | 8 | |||
| Lindernia crustacea | Herb | 13 | ||||
| Lobelia nummularia | Herb | 3 | ||||
| Total number | 243 | 457 | 1002 | 218 | ||
| Percentageb | 11.53% | 34.31% | 55.27% | 20.47% | ||
| Ailao Mountain (Subtropical forest) | Nonconstituent species | 2000 m | 2200 m | 2400 m | 2600 m | |
| Ageratina adenophora | Herb | 48 | 36 | 16 | 30 | |
| Laggera pterodonta | Herb | 35 | 1 | 3 | 5 | |
| Crassocephalum crepidioides | Herb | 14 | 7 | 1 | 5 | |
| Crassocephalum rubens | Herb | 5 | ||||
| Total number | 97 | 44 | 20 | 45 | ||
| Percentageb | 2.67% | 2.73% | 1.04% | 2.92% | ||
| a We did not find any nonconstituent species in the soil samples from Lijiang. b Percentage of the total number of seeds of nonconstituent species from each elevation. |
||||||
Overall, both tropical and subtropical forests had higher seed densities than subalpine forests (Table 2). Seed density at 800 m (10, 540 ± 1577) was much higher than that of the same forest type 10 years ago (5415 ± 3232, Tang et al., 2006). This increase was largely due to an increase in the number of N. cadamba seeds that germinated (1173 seeds germinated in our study and 81 in the study 10 years ago). This species is a common pioneer tree species in tropical Asian forests (Richards, 1996). Meanwhile, the number of species 10 years ago was 87, and it decreased to 71 in our study (Fig. 2).
Previous studies found that the seed density and species diversity of the soil seed bank peaked at an intermediate elevation (Zhang and Fang, 2004b; Li et al., 2012; Erfanzadeh et al., 2013). This seems to be true for the subalpine site in our study, but the tropical site had the highest seed bank species diversity at the highest elevation (1400 m). In addition, species diversity of subtropical soil seed bank did not show significant differences between 2000 m, 2200 m and 2600 m; however, species diversity for the subtropical seed soil bank at 2400 m was significantly lower than at the other three altitudes (Fig. 3).
With regard to the life form spectrum of the dominant species, a transition of tree + shrub → shrub + herb → herb was observed in the tropical, subtropical and subalpine sites (Tables 3 and 4). Some studies proposed that the occurrence of pioneer tree species in the soil seed bank plays an important role in forest dynamics, because the regeneration of forests depends on the alternation of climax species and pioneer species (Swaine and Whitmore, 1988; Whitmore, 1989; Richards, 1996). Thus, the dominance of some tree species such as N. cadamba, Ficus semicordata and Wendlandia spp. in the tropical soil seed bank serves as a species pool for future regeneration. In contrast, the subalpine site was dominated by herbs, and only one deciduous broad-leaved tree species (Padus buergeriana, 2 seeds) germinated from the soil seed bank. These results partially explain why the coniferous forest remains in the meadow stage of succession for a very long time after it is degraded (Liu et al., 2002). On the other hand, this result is different from that obtained for a P. schrenkiana forest in the Tianshan Mountains, northwest China, where seeds of P. schrenkiana occurred in all the soil samples from 13 elevations ranging from 1500 m-2700 m (Li et al., 2012). A study on the soil seed banks in Abies fargesii and Larix chinensis forests in the Qinling Mountains, northern China, also found some seeds of the two tree species (Zhang and Fang, 2004b).
The tropical forest had the highest species richness, followed by the subtropical forest and subalpine forest. Further, species richness of the soil seed banks in tropical and subtropical forests tended to be larger at low than high elevations (Fig. 2). This result is in line with our expectations because tropical and subtropical sites have richer species pools than subalpine sites. However, tropical forests did not show the highest values of species diversity indices (Fig. 3), reflecting the uneven distribution of seed abundance.
4.2. Similarity between soil seed bank and standing vegetationThe similarity of woody species composition between the seed bank and standing vegetation was very low at all elevations and sites (below 11.00% in terms of Sørensen's index) (Table 5), suggesting a minor contribution of the woody species of the standing vegetation to the soil seed banks. This result is consistent with other findings for tropical forests (Tang et al., 1999), subtropical forests (An et al., 1996; Wei et al., 2005), temperate forests (Olano et al., 2002), subalpine and alpine forests (Erfanzadeh et al., 2010; Pei et al., 2012; Zhou et al., 2013) and grasslands (Funes et al., 2001), where the species composition of the soil seed bank differs greatly from that of the standing vegetation.
Some studies have found that species similarity between the soil seed bank and standing vegetation is very low (Hill and Stevens, 1981), especially in the late successional stage of the forest, which has a much lower similarity than early successional stages (Garwood, 1989; Huang et al., 1996; Xiong et al., 1992). The larger number of woody species in the soil seed bank than in the standing vegetation and thus the low similarity between the two can be explained by the fact that (1) the seed bank composition may be derived from a former successional stage (Thompson et al., 1998), (2) seeds from standing vegetation failed to remain viable in the soil seed bank, (3) seeds may germinate immediately once they fall into a moist habitat (Gross-Camp and Kaplin, 2005), and (4) the regeneration of forest depends on the cyclic replacement of different tree species groups (Whitmore, 1982, 1990).
We did not expect to obtain a "0" similarity between the soil seed bank and standing vegetation at the basal elevations of the tropical and subtropical sites. Furthermore, the subalpine site showed the same trends at all elevations ranging from 3200 m throughout 3400 m, 3600 m and 3800 m (Table 5). Does this suggest that none of the seeds of the canopy tree species remain viable in the soils under the forests? This merits long-term monitoring of these soil seed banks.
4.3. Nonconstituent speciesA total of 15 nonconstituent species was recorded in the Xishuangbanna site, and seeds of these species accounted for an average of more than 30% of the total seeds that germinated in the samples, indicating disturbance to some extent. Four of the nonconstituent species were also found along four elevations in the Ailao Mountain site, of which A. adenophora (formerly Eupatorium adenophorum) was the most abundant alien species. This species appears to be very invasive to the native ecosystems in southwest China. Since it became colonized in the 1940s, its dispersal has been closely associated with human activities (Liu et al., 1985). This species has a persistent soil seed bank and can germinate after disturbance occurs in forests (Shen et al., 2006; Song et al., 2017). Lin and Cao, (2009) showed abundant seed storage of this species in the soils in the interior of a subtropical forest in Ailao Mountain because of edge effects, although no individuals of this species were observed in the understory vegetation. Impressively, another herbaceous species, C. crepidioides commonly occurred in both tropical and subtropical soil seed banks under the forests. This is a pantropical weed in fallow fields, on slopes, roadsides, streamsides and thickets in Africa, S and SE Asia, Australia, Central and South America, and the Pacific islands. It was previously recorded mostly below 300–1800 m in Jiangxi, Fujian, Hunan, Hubei, Guangdong, Guangxi, Guizhou, Yunnan, Sichuan, and Xizang provinces of China (Editing Committee of Flora of China, 1999), but we have observed it in the soils of the forest interior even up to 2600 m, indicating the potential colonization of this species at higher habitats in the event of disturbance.
We did not detect nonconstituent species in the forest soils in the subalpine site, but whether this is due to the harsh environment or inaccessibility of these species has not been determined.
5. ConclusionsThe seed density of soil seed banks in tropical and subtropical sites peaked at low elevations (i.e. 800 m and 2000 m, respectively), but at intermediate elevation (3400 m) in the subalpine site. The tropical forest had the highest number of species in the soil seed bank, subtropical forest had a moderate number and subalpine forest had the lowest number. A transition in dominant life form in the soil seed banks was observed: tree + shrub for the tropical site → shrub + herb for the subtropical site → herb for the subalpine site. Tree seeds dominated the tropical soil seed banks, but they were very rare (only 2 seeds) in the subalpine soil seed banks, suggesting a longer regeneration after forest clearance compared with tropical and subtropical forests. Similarity (Sørensen index) of woody species composition between the soil seed bank and standing vegetation was very low at the lowest tropical and subtropical sites and decreased with an increase in elevation. In subalpine forests, there was no common woody species shared by the soil seed bank and standing vegetation. All the nonconstituent species occurring in soil seed banks were herbs, and seed abundance and species number decreased from tropical → subtropical → subalpine forests but not with an increase in elevation in either the tropical or subtropical sites. Nonconstituent species were completely absent from the soil seed bank of the subalpine site.
AcknowledgementsThis study was supported by the National Key Basic Research Program of China (2014CB954100), Yunnan Provincial Foundation of Science and Technology (2014GA003), and the QueenslandChinese Academy of Sciences Biotechnology Fund (GJHZ1130). We are grateful to Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies, Administration Bureau of Ailaoshan Nature Reserves (Zhenyuan) and Lijiang Forest Ecosystem Research Station for the field assistance. We also thank Profs. Peng Hua, Wu Zhikun and Mr. Chen Zhifa of Kunming Institute of Botany, CAS, and also Prof. Tan Yunhong of Xishuangbanna Tropical Botanical Garden, CAS for plant species identification. We extend thanks to Yang Guoshui and Yang Yunzhi for maintaining seed germination trials. We thank Mr. Benjamin Blanchard for careful language editing and suggestions on the early draft of the manuscript. Finally, sincere thanks certainly are due to the anonymous reviewers and editors for their valuable comments and annotations on the manuscript.

|
|
| Species | Life form | 3200 m | 3400 m | 3600 m | 3800 m |
| Ainsliaea latifolia | Herb | 12 (4.00%) | 111 (7.52%) | 1 (0.26%) | |
| Ainsliaea reflexa | Herb | 1 (0.26%) | |||
| Anaphalis sp. | Herb | 1 (0.07%) | |||
| Artemisia sp. | 1 (0.33%) | ||||
| Astilbe chinensis | Herb | 76 (19.39%) | |||
| Cardamine yunnanensis | Herb | 2 (0.14%) | |||
| Carex nubigena | Herb | 120 (40.00%) | 17 (1.15%) | 22 (5.76%) | 29 (7.40%) |
| Carex sp.1 | Herb | 2 (0.67%) | 9 (0.61%) | 47 (12.30%) | 2 (0.51%) |
| Carex sp.2 | Herb | 1 (0.33%) | |||
| Chrysosplenium davidianum | Herb | 7 (0.47%) | 1 (0.26%) | ||
| Circaea alpina | Herb | 10 (3.33%) | 2 (0.14%) | 3 (0.79%) | |
| Clinopodium polycephalum | Herb | 6 (2.00%) | 273 (18.50%) | 1 (0.26%) | |
| Clinopodium sp. | Herb | 8 (0.54%) | |||
| Corydalis petrophila | Herb | 1 (0.33%) | 6 (0.41%) | 1 (0.26%) | |
| Cynoglossum amabile | Herb | 2 (0.14%) | |||
| Epilobium breuifolium | Herb | 2 (0.67%) | 7 (0.47%) | 2 (0.52%) | 1 (0.26%) |
| Epilobium brevifolium | Herb | 2 (0.67%) | |||
| Galium asperuloides | Herb | 1 (0.07%) | |||
| Galium elegans | Herb | 2 (0.14%) | |||
| Geranium sp. | Herb | 4 (1.02%) | |||
| Gnaphalium affine | Herb | 4 (1.33%) | 7 (0.47%) | 3 (0.79%) | 1 (0.26%) |
| Gnaphalium pensylvanicum | Herb | 1 (0.07%) | |||
| Hemiphragma heterophyllum | Herb | 9 (0.61%) | 14 (3.66%) | 1 (0.26%) | |
| Hypericum acmosepalum | Shrub | 1 (0.07%) | |||
| Impatiens delavayi | Herb | 1 (0.33%) | 27 (1.83%) | ||
| Juncus effusus | Herb | 35 (9.16%) | |||
| Laportea interrupta | Herb | 3 (0.20%) | 12 (3.14%) | 1 (0.26%) | |
| Luzula sp. | Herb | 1 (0.07%) | 2 (0.52%) | 13 (3.32%) | |
| Lysimachia violascens var. robusta | Herb | 1 (0.07%) | |||
| Myriactis wightii | Herb | 12 (4.00%) | 186 (12.60%) | 21 (5.50%) | 23 (5.87%) |
| Padus buergeriana | Tree | 2 (0.14%) | |||
| Parasenecio sp. | Herb | 4 (1.02%) | |||
| Parnassia sp. | Herb | 2 (0.52%) | |||
| Philadelphus delavayi | Shrub | 37 (12.33%) | 370 (25.07%) | 15 (3.93%) | |
| Pilea microphylla | Herb | 2 (0.14%) | |||
| Pilea sinofasciata | Herb | 16 (5.33%) | 340 (23.04%) | 19 (4.97%) | |
| Pleurospermum camtschaticum | Herb | 2 (0.51%) | |||
| Pogonatherum paniceum | Herb | 3 (1.00%) | 1 (0.07%) | ||
| Polygonum runcinatum | Herb | 1 (0.33%) | 33 (8.42%) | ||
| Potentilla sp. | Herb | 4 (1.05%) | |||
| Ribes glaciale | Shrub | 4 (1.33%) | 4 (0.27%) | 43 (11.26%) | 3 (0.77%) |
| Ribes sp. | Shrub | 1 (0.07%) | 1 (0.26%) | 71 (18.11%) | |
| Rubia yunnanensis | Herb | 4 (1.33%) | 1 (0.07%) | 4 (1.05%) | |
| Rubus fockeanus | Herb | 11 (3.67%) | 22 (1.49%) | 83 (21.73%) | |
| Sambucus williamsii | Shrub | 3 (0.20%) | |||
| Sedum sp. | Herb | 2 (0.67%) | |||
| Stellaria vestita | Herb | 14 (4.67%) | 3 (0.20%) | 17 (4.45%) | 11 (2.81%) |
| Tripterospermum volubile | Herb | 2 (0.14%) | |||
| Veronica piroliformis | Herb | 9 (3.00%) | 28 (1.90%) | 5 (1.31%) | |
| Viola biflora | Herb | 2 (0.14%) | 3 (0.79%) | ||
| Unidentified | 25 (8.33%) | 11 (0.75%) | 24 (6.28%) | 113 (28.83%) |
This study was supported by the National Key Basic Research Program of China (2014CB954100), Yunnan Provincial Foundation of Science and Technology (2014GA003), and the QueenslandChinese Academy of Sciences Biotechnology Fund (GJHZ1130). We are grateful to Xishuangbanna Station for Tropical Rain Forest Ecosystem Studies, Administration Bureau of Ailaoshan Nature Reserves (Zhenyuan) and Lijiang Forest Ecosystem Research Station for the field assistance. We also thank Profs. Peng Hua, Wu Zhikun and Mr. Chen Zhifa of Kunming Institute of Botany, CAS, and also Prof. Tan Yunhong of Xishuangbanna Tropical Botanical Garden, CAS for plant species identification. We extend thanks to Yang Guoshui and Yang Yunzhi for maintaining seed germination trials. We thank Mr. Benjamin Blanchard for careful language editing and suggestions on the early draft of the manuscript. Finally, sincere thanks certainly are due to the anonymous reviewers and editors for their valuable comments and annotations on the manuscript.
Amiaud B., Touzard B., 2004. The relationships between soil seed bank aboveground vegetation and disturbances in old embanked marshlands of western France. Flora, 199: 25-35. DOI:10.1078/0367-2530-00129 |
An S.Q., Lin X.Y., Hong B.G., 1996. A preliminary study on the soil seed banks of dominant vegetation forms on Baohua Mountain. Acta Phytoecol. Sin, 20: 41-50. |
Baker H.G., 1974. The evolution of weeds. Annu. Ecol. Syst, 5: 1-24. DOI:10.1146/annurev.es.05.110174.000245 |
Bakker J., Bakker E., Rosén E., Verweij G., Bekker R., 1996. Soil seed bank composition along a gradient from dry alvaro grassland to Juniperus shrubland. J. Veg. Sci, 7: 165-176. DOI:10.2307/3236316 |
Cao M., Tang Y., Zhang J.H., Sheng C.Y., 2000a. Storage and dominants in soil seed banks under the tropical forests of Xishuangbana. Acta Bot. Yunnanica, 19: 177-183. |
Cao M., Tang Y., Sheng C.Y., Zhang J.H., 2000b. Viable seeds buried in the tropical forest soils of Xishuangbanna, SW China. Seed Sci. Res, 10: 255-264. DOI:10.1017/S0960258500000283 |
Cao M., Zhang J.H., Feng Z.L., Deng J.W., Deng X.B., 1996. Tree species composition of a seasonal rain forest in Xishuangbanna, Southwest China. Trop. Ecol, 37: 183-192. |
Cao M., Zou X.M., Warren M., Zhu H., 2006. Tropical forests of Xishuangbanna. China. Biotropica, 38: 306-309. DOI:10.1111/btp.2006.38.issue-3 |
Davies A., Waite S., 1998. The persistence of calcareous grassland species in the soil seed bank under developing and established scrub. Plant Ecol, 136: 27-39. DOI:10.1023/A:1009759227900 |
Editing Committee of Flora of China, 1999. Flora China, 77, 304.
|
Erfanzadeh R., Garbutt A., Pétillon J., Maelfait J.P., Hoffmann M., 2010. Factors affecting the success of early salt-marsh colonizers: seed availability rather than site suitability and dispersal traits. Plant Ecol, 206: 335-347. DOI:10.1007/s11258-009-9646-8 |
Erfanzadeh R., Kahnuj S.H.H., Azarnivand H., Pétillon J., 2013. Comparison of soil seed banks of habitats distributed along an altitudinal gradient in northern Iran. Flora, 208: 312-320. DOI:10.1016/j.flora.2013.04.004 |
Esmailzadeh O., Hosseini S.M., Tabari M., Baskin C.C., Asadi H., 2011. Persistent soil seed banks and floristic diversity in Fagus orientalis forest communities in the Hyrcanian vegetation region of Iran. Flora, 206: 365-372. DOI:10.1016/j.flora.2010.04.024 |
Funes G., Basconcelo S., Diaz S., Cabido M., 2001. Edaphic patchiness influences grassland regeneration from the soil seed-bank in mountain grasslands of central Argentina. Austral Ecol, 26: 205-212. DOI:10.1046/j.1442-9993.2001.01102.x |
Funes G., Basconcelo S., Díaz S., Cabido M., 2003. Seed bank dynamics in talltussock grasslands along an altitudinal gradient. J. Veg. Sci, 14: 253-258. DOI:10.1111/j.1654-1103.2003.tb02150.x |
Garwood, N. C., 1989. Tropical soil seed banks: a review. In: Leck, M. A., Parker, V. T., Simpson, R. L. (Eds. ), Ecology of Soil Seed Banks. Acad. Press Inc., San Diego, pp. 149-209.
|
Gross-Camp N., Kaplin B.A., 2005. Chimpanzee (Pan troglodytes) seed dispersal in an afromontane forest: microhabitat influences on the postdispersal fate of large seeds. Biotropica, 37: 641-649. DOI:10.1111/btp.2005.37.issue-4 |
Hill M.O., Stevens P.A., 1981. The density of viable seed in soils of forest plantations in upland Britain. J. Ecol, 69: 693-709. DOI:10.2307/2259692 |
Hopfensperger K.N., 2007. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos, 116: 1438-1448. DOI:10.1111/oik.2007.116.issue-9 |
Huang Z.L., Kong G.H., Wei P., Wang J.H., Huang Y.J., Zhang Y.C., 1996. A study on the soil seed banks at the different succession stages of south subtropical forests. Chin. J. Trop. Subtrop. Bot, 4: 42-49. |
Jalili A., Hamzeh'ee B., Asri Y., Shirvany A., Yazdani S., Khoshnevis M., Zarrinkamar F., Ghahramani M.A., Safavi R., Shaw S., 2003. Soil seed banks in the Arasbaran Protected Area of Iran and their significance for conservation management. Biol. Conserv, 109: 425-431. DOI:10.1016/S0006-3207(02)00170-2 |
Keylock C.J., 2005. Simpson diversity and the ShannoneWiener index as special cases of a generalized entropy. Oikos, 109: 203-207. DOI:10.1111/oik.2005.109.issue-1 |
Lan G.Y., Zhu H., Cao M., Hu Y.H., Wang H., Deng X.B., Zhou S.S., Cui J.Y., Huang J.G., He Y.C., Liu L.Y., Xu H.L., Song J.P., 2009. Spatial dispersion patterns of trees in a tropical rainforest in Xishuangbanna, southwest China. Ecol. Res, 24: 1117-1124. DOI:10.1007/s11284-009-0590-9 |
Legendre, P., Legendre, L., 1998. Numerical Ecology. Elsevier, New York.
|
Li H.D., Pan C.D., Zhang G.L., Wang B., 2012. Distributing patterns of soil seed bank of Picea schrenkiana forests along the altitudinal gradient in the central section of Tianshan Mountains, Xinjiang. Xinjiang Agri. Sci, 49: 1373-1380. |
Li X.S., Liu W.Y., Tang C.Q., 2010. The role of the soil seed and seedling bank in the regeneration of diverse plant communities in the subtropical Ailao Mountains, Southwest China. Ecol. Res, 25: 1171-1182. DOI:10.1007/s11284-010-0742-y |
Li Y.L., Cui J.Y., Zhao X.Y., Zhao H.L., 2004. Floristic composition of vegetation and the soil seed bank in different types of dunes of Kerqin steppe. Arid. Land Res.Manag, 18: 283-293. DOI:10.1080/15324980490451410 |
Lin L.X., Cao M., 2009. Edge effects on soil seed banks and understory vegetation in subtropical and tropical forests in Yunnan, SW China. For. Ecol. Manag, 257: 1344-1352. DOI:10.1016/j.foreco.2008.12.004 |
Lin L.X., Cao M., He Y.T., Baskin J.M., Baskin C.C., 2006. Nonconstituent species in soil seed banks as indicators of anthropogenic disturbance in forest fragments. Can. J. For. Res, 36: 2300-2316. DOI:10.1139/x06-137 |
Liu L.H., Xie S.C., Zhang J.H., 1985. Study on the distribution, harmfulness and control of Eupatorium adenophorum Spreng. Acta Ecol. Sin, 5: 1-6. |
Liu, Q., Wu, Y., Pang, X. Y., 2002. Issues of degradation and evaluation of subalpine coniferous forest ecosystem. In: Liu, Q., Wu, Y., Chen, Q. H. (Eds. ), Ecological Research on Subalpine Coniferous Forests in China. Sichuan Univ. Press, Chengdu, pp. 247-269.
|
Magurran A.E., 1988. Ecological Diversity and Measurement. Princeton: Princeton Univ. Press.
|
Milberg P., 1995. Soil seed bank after eighteen years of succession from grassland to forest. Oikos, 72: 3-13. DOI:10.2307/3546031 |
Niu H.W., He Y.Q., Zhu G.F., Xin H.J., Du J.K., Pu T., Lu X.X., Zhao G.Y., 2013. Environmental implications of the snow chemistry from Mt. Yulong, southeastern Tibetan Plateau. Quat. Int, 313: 168-178. |
Olano J.M., Caballero I., Laskurain N.A., Loidi J., Escudero A., 2002. Seed bank spatial pattern in a temperate secondary forest. J. Veg. Sci, 13: 775-784. DOI:10.1111/j.1654-1103.2002.tb02107.x |
Ortega M., Levassor C., Peco B., 1997. Seasonal dynamics of Mediterranean pasture seed banks along environmental gradients. J. Biogeogr, 24: 177-195. DOI:10.1046/j.1365-2699.1997.00080.x |
Pei Y.H., Yang D.J., Geng Y.F., Shi H.F., 2012. Study on soil seed bank characteristics of vegetation in Shangrila subalpine of northwest Yunnan under different degraded degrees. J. West Chin. For. Sci, 41: 56-61. |
Perera G., 2005. Spatial heterogeneity of the soil seed bank in the tropical semideciduous forest at Wasgomuwa National Park, Sri Lanka. Trop. Ecol, 46: 79-90. |
Richards P.W., 1996. The Tropical Rain Forest, second ed. Cambridge: Camb. Univ. Press.
|
Shen Y.X., Liu W.Y., Baskin J.M., Baskin C.C., Cao M., 2006. Persistent soil seed banks of the globally significant invasive species, Eupatorium adenophorum, in Yunnan Province, south-western China. Seed Sci. Res, 16: 157-162. DOI:10.1079/SSR2006240 |
Song X.Y., Hogan J.A., Brown C., Cao M., Yang J., 2017. Snow damage to the canopy facilitates alien weed invasion in a subtropical montane primary forest in southwestern China. For. Ecol. Manag, 391: 275-281. DOI:10.1016/j.foreco.2017.02.031 |
Sørensen T., 1948. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr, 5: 1-34. |
Swaine M.D., Whitmore T.C., 1988. On the definition of ecological species groups in tropical rain forests. Vegetatio, 75: 81-86. DOI:10.1007/BF00044629 |
Tang Y., Cao M., Fu X., 2006. Soil seedbank in a dipterocarp rain forest in Xishuangbanna, Southwest China. Biotropica, 38: 328-333. DOI:10.1111/btp.2006.38.issue-3 |
Tang Y., Cao M., Zhang H., Sheng C., 1999. The relationship between soil seed banks and aboveground vegetation in Xishuangbanna, Southwest China. Chin. J.Appl. Ecol, 10: 279-282. |
Thompson K., Bakker J.P., Bekker R.M., Hodgson J.G., 1998. Ecological correlates of seed persistence in soil in the northwest European flora. J. Ecol, 86: 163-169. DOI:10.1046/j.1365-2745.1998.00240.x |
Villiers A.J.D., Rooyen M.W.V., Theron G.K., 2003. Similarity between the soil seed bank and the standing vegetation in the Strandveld Succulent Karoo, South Africa. Land Degrad. Devel, 14: 527-540. DOI:10.1002/(ISSN)1099-145X |
Wang B.R., Zhu X., Yang S.H., 2001. Application of RS technique to the study of vegetation mapping in the Yulong snow mountain, Lijiang, Yunnan. Chin. J. Ecol, 20(l): 39-41. |
Wei S.G., Li L., Huang Z.L., Peng S.J., Shi J.H., 2005. Study on the dynamic of seed bank of Dinghushan forest soil. Ecol. Environ, 14: 917-920. |
Welling P., Tolvanen A., Laine K., 2015. The alpine soil seed bank in relation to field seedlings and standing vegetation in subarctic Finland. Arct. Antarct. Alp. Res, 36: 229-238. |
Whitmore, T. C., 1982. On pattern and process in forest. In: Newman, E. I. (Ed. ), The Plant Community as a Working Mechanism. Blackwell Sci. Publ., Oxford, pp. 45-60.
|
Whitmore T.C., 1989. Canopy gaps and the two major groups of forest trees. Ecology, 70: 536-538. DOI:10.2307/1940195 |
Whitmore T.C., 1990. An Introduction to Tropical Rain Forests. Oxford: Clarendon Press.
|
Willems J., Bik L., 1998. Restoration of high species density in calcareous grassland:the role of seed rain and soil seed bank. Appl. Veg. Sci, 1: 91-100. DOI:10.2307/1479088 |
Wu Z.Y., Zhu Y.C., Jiang H.Q., 1987. The Vegetation of Yunnan. Beijing: Science Press.
|
Xiong L.M., Zhong Z.C., Li X.G., 1992. A preliminary study on the soil seed banks of different successional stages of subtropical evergreen broadleaved forest. Chin.J. Plant Ecol, 16: 249-257. |
Yang X.F., Tang Y., Cao M., 2010. Diaspore traits of 145 tree species from a tropical seasonal rainforest in Xishuangbanna, SW China. Acta Bot. Yunn, 32: 367-377. |
Yin H.J., Liu Q., 2004. Soil seed bank of constructive species Picea asperata of subalpine coniferous forest in western Sichuan, China. Chin. J. Appl. Environ.Biol, 10: 581-584. |
Yin H.J., Liu Q., 2005. Seed rain and soil seed banks of Picea asperata of subalpine spruce forests, western Sichuan, China. Acta Phytoecol. Sin, 29: 108-115. |
Zhang L., Fang J.Y., 2004a. Changes in soil seed banks and biodiversity along an altitude gradient in Taibai Mt. Acta Geogr. Sin, 59: 880-888. |
Zhang L., Fang J.Y., 2004b. Reserves and species diversity of soil seed banks in four types of forest on Mt. Taibai, Qinling Mountains. Biodiv. Sci, 512: 131-136. |
Zhang M., Song X.Y., 2015. Response of soil seed banks in tropical forests to an elevational gradient. Chin. J. Ecol, 34: 2390-2400. |
Zhou L., Meng H., Pan C.D., Zhang G.L., Wang B., 2013. Species similarity between soil seed bank and aboveground vegetation in Picea schrenkiana forests in the central part of the Tianshan Mountains, Xinjiang. Xinjiang Agri. Sci, 50: 1235-1245. |
Zhu H., Cao M., Hu H.B., 2006. Geological history, flora and vegetation of Xishuangbanna, southern Yunnan. Biotropica, 38: 310-317. DOI:10.1111/btp.2006.38.issue-3 |



