b. Indian Institute of Science Education and Research, Dr. Homi Bhabha Road, Pashan, Pune, 411008, India
Rattans, members of the family Arecaceae (or Palmae), are a remarkable group of plants known for their lightness, strength, durability and elasticity. There are about 22 genera of rattans in the world comprising more than 650 species (Govaerts et al., 2014). Of these, only about a dozen have high commercial value. Rattans are distributed largely in South and South-East Asia, Africa, China and Australia (Uma Shaanker et al., 2004). Among the 22 genera, Calamus is the largest genus with about 400 species (Govaerts et al., 2014). In India, there are about 61 species of rattans under five genera, Calamus, Daemonorops, Korthalisa, Plectocomia and Zalacca (Uma Shaanker et al., 2004). These are distributed in Peninsular India, North-east India as well as the Andaman and Nicobar Islands (Ravikanth et al., 2002). The rattans comprise more than fifty per cent of the total palm taxa found in India and are distributed from sea level to over an elevation of 3000 m (Basu, 1992). They are found in a wide range of habitats and in a variety of soil types. Studies on rattans from India in general, and the Western Ghats biodiversity hotspot in particular, have been largely focused on taxonomy (Renuka, 1992; Lakshmana, 1995; Sreekumar et al., 2006; Senthilkumar et al., 2015) and population genetics (Ramesha et al., 2007; Lyngdoh et al., 2005; Ravikanth et al., 2010).
Rattans form an important resource after timber in terms of economic importance (Mohan Ram and Tandon, 1997; Senthilkumar et al., 2014). Rattans are a major source of raw material for the furniture and handicraft industry, and are utilized for the manufacture of a wide variety of aesthetic furniture and articles of decoration (Senthilkumar et al., 2014). Rattans also provide a livelihood to a considerable proportion of the rural population engaged in making rattan furniture and handicrafts in many countries like Indonesia, Malaysia, India, China, Myanmar, Thailand and Philippines (Ravikanth et al., 2001).
While there are a number of economically important species endemic to this region, very few studies have looked at the conservation of these taxa (Ravikanth et al., 2002; Singh et al., 2004; Manohara et al., 2007; Bhat et al., 2009, 2010). With the increasing demand for rattan products both locally and internationally, rattan has been heavily extracted from natural populations (Senthilkumar et al., 2014). Being an important non-timber forest product, rattans have a long history of extraction in India, especially from the Western Ghats. Almost all forest ranges in the Western Ghats, which harbour rattans, have been exploited to varying degrees. Consequences of large-scale and intense extraction on the natural stands of rattans have been tremendous. In many areas in the Western Ghats, the heavy extraction of rattans has led to lowered stand density, reduced regeneration, lack of fruit and seed set, which has in recent years led to a reduced supply of rattans (Ravikanth et al., 2001). Large-scale extraction of rattans may not only affect rattan resource availability, but also spell a death knell to the livelihood of number of artisan communities associated with making rattan furniture and handicrafts.
In this regard, conservation of rattan resources is of paramount importance both ecologically and economically. Conservation status has not been assessed for most rattan species of the Western Ghats, making conservation decisions difficult. Hence, an alternate method should be developed and deployed to enable the selection of species or areas that deserve immediate attention. Identifying smaller regions of high diversity and endemism, or "micro-hotspots", within larger hotspots at different scales have been identified for both flora and fauna (Murray-Smith et al., 2006; Raes et al., 2009; Kraft et al., 2010; López-López et al., 2011; Schouten et al., 2010). ''Micro-hotspots'' are endemic-rich areas analogous to biogeographic units (Fenu et al., 2010) and have been used to delineate hotspots within a hotspot. Identifying such "micro-hotspots", especially for rattans, is important for conservation measures to be enacted when resources are scarce (Murray-Smith et al., 2006; Margules and Pressey, 2000; Butchart et al., 2010). Identification of these hot-spots, could help in assigning conservation value based on ecological, biological and threats to each species. Daniels (1992) have developed a conservation value (CV) method for selected birds of the Western Ghats. More recently, a similar approach has been used for butterflies Kunte, 2008. Mohapatra et al. (MS under preparation) have used a slightly different approach for amphibians of the Western Ghats, utilizing available information and considering the biology of the species.
The main aims of this study were to (1) assess the geographical distribution patterns of rattans of the Western Ghats, (2) determine hotspots of rattan diversity in the Western Ghats, (3) evaluate the threat status of rattan species and develop conservation values for each species for in situ conservation, and finally, (4) to assess if protected areas are effective in conserving rattan species. To achieve these goals, we calculated the conservation value for 21 species of rattans of the Western Ghats and identified priority areas for conservation of these rattan species. Based on our results we propose the establishment of extractive reserves to meet the requirements of communities economically dependent on rattan.
Materials and methods Study speciesThe study was carried out for all 21 species of rattans (Genus Calamus) occurring in the Western Ghats. Of these 21, 19 species are endemic to the Western Ghats and two are endemic to the Western Ghats and Sri Lanka. More than half a dozen of these species are commercially exploited (Table 1) for various products ranging from souvenirs to furniture.
| Sr No | Species name | Harve-sted | Single/Multi-stem | Endemic/Non-endemic | Elevation | Regions | Total no. of records | No. of records used for ENM |
| 1 | Calamus brandisii | Yes | Multi | Endemic | 1000–2000 | S | 29 | 24 |
| 2 | Calamus gamblei | Yes | Multi | Endemic | 500–1000 | S, C | 60 | 45 |
| 3 | Calamus karnatakensis | Yes | Multi | Endemic | Up to 1500 | S, C | 9 | 5 |
| 4 | Calamus lakshmanae | Yes | Multi | Endemic | Up to 1000 | C | 13 | 8 |
| 5 | Calamus nagbettai | Yes | Multi | Endemic | Up to 1000 | S, C | 16 | 10 |
| 6 | Calamus thwaitesii | Yes | Multi | WG/SL | 100–900 | S, C, N | 70 | 58 |
| 7 | Calamus travancoricus | Yes | Multi | Endemic | 300–1000 | S, C | 49 | 46 |
| 8 | Calamus delessertianus | Yes | Multi | Endemic | Up to 1000 | S, C | 11 | 7 |
| 9 | Calamus dransfieldii | Yes | Multi | Endemic | 500–600 | S, C | 13 | 8 |
| 10 | Calamus hookerianus | Yes | Multi | Endemic | Up to 1000 | S, C | 36 | 24 |
| 11 | Calamus lacciferus | Yes | Multi | Endemic | Up to 1000 | C | 11 | 6 |
| 12 | Calamus metzianus | Yes | Multi | Endemic | 0–100 | S, C | 16 | 9 |
| 13 | Calamus neelagiricus | Yes | Single | Endemic | 1200 | S | 5 | 4 |
| 14 | Calamus prasinus | Yes | Single | Endemic | 500 | S, C | 20 | 14 |
| 15 | Calamus pseudotenuis | Yes | Multi | WG/SL | 100–1500 | S, C | 65 | 50 |
| 16 | Calamus rheedei | Yes | Multi | Endemic | < 800 | S | 8 | 8 |
| 17 | Calamus vattayila | Yes | Single | Endemic | 500–800 | S, C | 28 | 21 |
| 18 | Calamus shendurunii | Yes | Multi | Endemic | Up to 1000 | S | 3 | 3 |
| 19 | Calamus wightii | Yes | Multi | Endemic | 1300–2000 | S, C | 10 | 8 |
| 20 | Calamus pseudofeanus | Yes | Single | Endemic | < 500 | S | 1 | 1 |
| 21 | Calamus renukae | Yes | Multi | Endemic | 1500 | S | 1 | 1 |
| S = Southern Western Ghats, C = Central Western Ghats, N = Northern Western Ghats. | ||||||||
The Western Ghats, one of four biodiversity hotspots of India (Myers et al., 2000), is a mountain chain running parallel to the west coast of India for about 1600 km between the latitudes 8°N to 21°N (Fig. 1) and covers an area of 160, 000 km2 (Daniels, 1992). The elevation of the Western Ghats ranges from < 100 m amsl on the western side to 2800 m amsl. The high peaks above 2000 m are found in the Nilgiris, Anamalais and Palnis in southern Western Ghats. To the east, the mountains gradually descend to Deccan Plateau at about 600 m. Climate in the Western Ghats varies with elevation and latitude. At the lower reaches of the Ghats, climate is humid and tropical due to proximity to the sea on the Western side with an annual mean temperature of 15 ℃. Mean annual temperature ranges from 20 ℃ in the south to 24 ℃ in the northern regions of the Ghats. The bulk of the rainfall is received in the southwest monsoon between June and October, but the rainfall in the south is often extended due to pre-monsoon and winter showers. The western side (windward side) of the Ghats receives rainfall on an average of 3000 mm–4000 mm with certain regions getting more than 9000 mm per year (e.g., Agumbe, Karnataka). The eastern regions of the Western Ghats, which lie in the rain shadow, receive less rainfall, averaging about 1000 mm. The dry periods in the southern parts of the Western Ghats are shortest, lasting for two to five months while in the northern parts it varies from five to eight months (Daniels, 1992). The Western Ghats forms an important watershed for Peninsular India. Exceptionally rich biodiversity, endemism and historical human interaction in the Western Ghats make this area a high conservation interest.
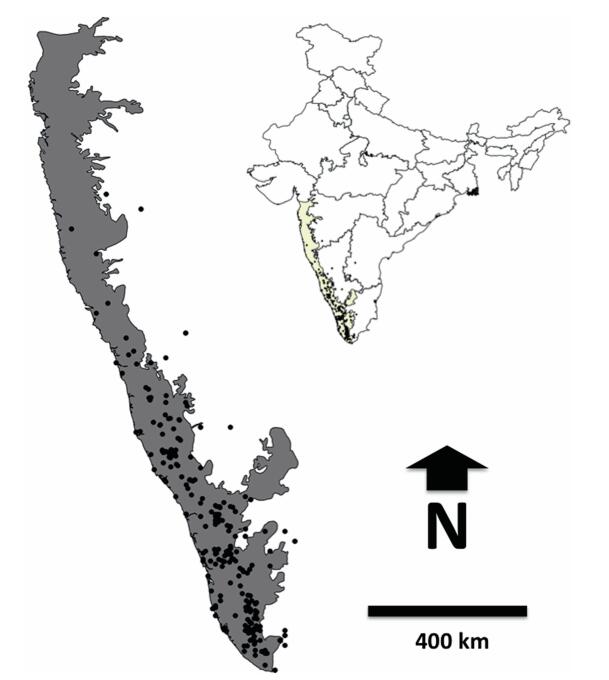
|
| Fig. 1 Distribution of rattans (black dots) in the Western Ghats. Inset map shows the location of the study region. |
The data for 21 endemic species of rattan palms were obtained from primary and secondary sources (Table 1) and developed into a database. Primary occurrence data was collected through field studies. A Garmin GPS was used to record the site of occurrence of each species. Secondary sources included published literature, databases and herbarium records maintained at research organizations such as the Botanical Survey of India (BSI), Kerala Forest Research Institute (KFRI), and Ashoka Trust for Research in Ecology and the Environment (ATREE). Coordinates for each locality were assigned using Google earth and Survey of India toposheets. The location data were geo-tagged to their nearest place when the exact location was not available. The distribution data is available at www.indiabiodiversity.org.
i. Geographic distributionTo understand the geographical distribution patterns of rattans in the Western Ghats, the data were reclassified into every one-degree latitude (8°N–21°N). We have also classified the Western Ghats into three latitudinal divisions: north (between 16°N and 21°N), central (between 12°N and 16°N) and south (between 8°N and 12°N), following Ramesh and Pascal (1996), Subramanian and Shivaramakrishnan (2002), Dahanukar et al. (2004), and Aravind et al. (2005). These three regions fall in a). Kerala and Tamil Nadu (8°N and 12°N), b) Karnataka (12°N and 16°N) and c) Maharashtra (16°N and 21°N) states of India. The Bray–Curtis similarity index (using presence and absence data) was used to assess the similarity between every latitudinal band. We have also assessed the distribution along a 200-m elevational band. Linear regression was performed to assess the changes in species richness from south to north. All analysis was performed in PAST3 (Hammer and Harper, 2007).
ii. Geographic rangeThe distribution range for all 21 species of rattans across the Western Ghats was calculated using EOO (Extent of Occurrence) and AOO (Area of Occupancy) methods. These methods were widely used by IUCN's Red List assessment (IUCN, 2001) for conservation assessment with GeoCAT (Geospatial Conservation Assessment Tool). EOO and AOO were used for assessments of restricted-range species under criterion B of IUCN's Red List procedure as described by Willis et al. (2003). EOO was calculated by constructing the minimum convex hull around known occurrence points of rattans and AOO was calculated as the number of grids occupied by points multiplied by 4 km2 in GEOCAT (Willis et al., 2003; Bachman et al., 2011). A grid size of 2 km × 2 km was used for the present study as recommended by IUCN for conservation assessment (IUCN, 2001). EOO was also calculated using minimum presence area (MPA) after converting the Ecological Niche Modelling (ENM) map to a binary map (see below).
Developing conservation valueDespite the threats faced by rattan species both due to harvesting and land use changes in the Western Ghats, only two species are red-listed by the IUCN. In the absence of the information on red list status of rattan species of the Western Ghats, a different approach was used to assess the status of these species. The approach was based on the distribution of the species, its biology and specific threats for each of the species. The conservation value (CV) for each species was thus developed based on five different approaches.
ⅰ) Extent of Occurrence (EOO): The EOO, calculated using GeoCat, was used in this analysis. The conservation value for each species of rattan based on EOO was developed using the formula:
Conservation value by EOO = 10*[(Max. EOO -Species EOO)/Max. EOO]
The value of EOO ranges from 0 to 1 and in order to get the maximum value of 10 we normalised by multiplying by 10.
ⅱ) Growth form: A value of 5 was assigned for those species with multiple stems and a value of 10 was assigned for species which are single stemmed. This was done because single-stemmed species are more vulnerable to extinction pressures compared to multi-stemmed species, which have coppicing abilities.
ⅲ) Harvesting pressure: Commercially harvested species were assigned a value of 10 and non-harvested species were given 5.
ⅳ) Number of habitat types: The habitat conservation value was calculated using the formula,
Conservation values for habitat H = 10*(N-a)/(N-1)
where, N is total number of habitat and "a" is number of habitat a given species is known to occupy (Daniels et al., 1991). The rattans in the Western Ghats are known to occur in habitats (vegetation types) including evergreen, semi-evergreen, moist deciduous forests and coastal wetlands. In the present study, N = 4 was considered the maximum number of habitat types available for rattans.
ⅴ) Elevation range: Altitudinal conservation value (CV) was calculated using the formula,
CV for elevation A = 10*[(Max. Alt. -Alt. Range)/Max. Alt.]
where, N is maximum elevational range (2800 m asl in this case) and elevation range is the difference between the maximum and the minimum elevation where the species is recorded.
The final Conservation Value for each species was computed by adding all five values (ⅰ–ⅴ). Any given species can score a maximum value of 50.
Hotspots of species richnessThe hotspot of species richness was developed using two methods. In the first method, the species richness was calculated for 0.5° grids (55 km × 55 km) on the rattan species richness vector map. A finer grid of 10 km × 10 km is desired given the high levels of endemism and small ranges. However, we used a half-degree grid due to the absence of good distribution data for rattans of the Western Ghats. For every half-degree grid, total species richness was calculated using the analysis option in DIVA-GIS (ver 7.5) implemented in Windows 7. For the second method, Ecological Niche Modelling (ENM) was used. Two methods were used in order to obtain a proper representation of hotspots. The grid-based approach is biased towards the number of data points and the ENM-based approach cannot be applied for species, which have fewer occurrence points. In order to overcome the limitations of these methods, a combination of both methods was used.
ENM has been used widely to model species ranges with limited data points in biodiversity-rich areas (Cardoso et al., 2011; Pena de Castro et al., 2014; Grossi et al., 2017). Of late, ENM has been used to model species distribution for a number of endemic and economically important plant species in the Western Ghats (Priti et al., 2016; Sen et al., 2016). Recently, IUCN recognised the usefulness of ENM for risk assessment under A3, D2 and E criteria, and Syfert et al. (2014) suggested incorporating ENM into Red List assessments.
For ENM, 19 bioclimatic variables were downloaded from the WordClim database (www.worldclim.org). Apart from these, elevation (https://lta.cr.usgs.gov/SRTM1Arc), population (www.diva-gis.org) and land use (http://glcf.umd.edu/data/lc/) layers were also used. All variables were treated as continuous except for land use, which was treated as categorical (See Supplementary Table 1). QGIS ver, 2.14.2 was used to extract all the bioclimatic variables for each of the 418 locations in the above-mentioned regions for all 21 species. Five species were omitted from the analysis because they had fewer than five occurrence point records (Table 1). All variables were tested for collinearity by examining pairwise Pearson's correlations between them. If a pair of variables had a correlation coefficient more than |r| > 0.8, one of the two variables was eliminated. No edaphic factors were used in the analysis because high resolution layers for soil pH, organic carbon (OC), and other factors were not available for the study region.
Maximum Entropy modelling or MaxEnt (Version 3.3.3k) was used to estimate the probability of presence of species based on presence records and randomly generated background points by finding the maximum entropy distribution (Phillips et al., 2006). The program starts with a uniform distribution and performs a number of iterations, each of which increases the probability of sample locations for the species (Merow et al., 2013). Logistic output was used in Maxent, which is a continuous map with estimated probability of species' presence between 0 and 1. This also allows one to make a distinction between the suitability of different areas. A Jackknife procedure was used to calculate the significance of contribution of each bioclimatic variable to the model. Area under Receiver Operating Characteristic Curve (also known as AUC) was used to evaluate model performance. The model was run with 5000 iterations and 10 replicates for each species with a subsampling procedure. We used 75% of the points for the test and remaining 25% for training the model. Species richness maps were created in QGIS as binary maps of presence ("1") and absence ("0"). Based on the ENM results, we reclassified the suitability maps to binary maps of presence-absence based on minimum training presence (MTP) threshold (Engler et al., 2004; Guisan et al., 2006; Pearson et al., 2007; Jimenez-Alfaro et al., 2012). This method produces minimal omission error (Norris, 2014). Binary maps for all species were stacked to generate a species richness map (De la Estrella et al., 2012). These maps were later clipped onto mask layers of the Western Ghats (the West coast was also included along with the Western Ghats).
Results Geographic distribution patternsThe geographic distribution pattern of endemic rattans indicate that species rich regions are located between 11°N and 13°N latitude (Fig. 2A) and decline significantly from south to north (y = -0.5298x2 + 3.756x + 5.232; R2 = 0.778, P < 0.05). There is a species poor region above 14°N latitude. When data were combined for every 4° bands of latitude, the southern Western Ghats (between 8°N and 12°N) was found to be species rich with 19 species, followed by central Western Ghats (between 12°N and 16°N) with 15 species, while northern Western Ghats was a species poor region (16°N to 21°N). Cluster analysis shows three distinct clusters-latitudinal bands between 8°N and 11°N, 11° N – 14°N and 14°N – 16°N (Fig. 2C).
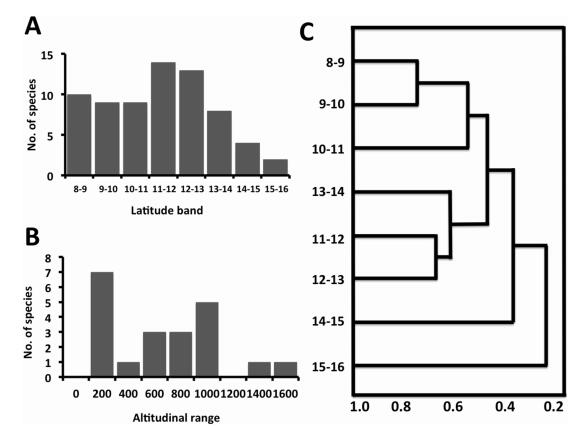
|
| Fig. 2 A) Number of species of rattans at every one-degree latitude band; (B) number of species of rattans at every 200 m elevational range in the Western Ghats and (C) cluster showing the groupings of different latitudinal bands along the Western Ghats. |
Along the altitude range, one-third of the species (seven species) have narrow altitude ranges (200 m). Seven species of rattans have altitude ranges less than 800 m and only three species have ranges more than 1400 m (Fig. 2B; y = -1E-10x3 + 1E-06x2 -0.0054x + 6.108; R2 = 0.601; P < 0.05). There were no rattans exclusive to higher altitudes ( > 2000 m asl) in the Western Ghats. Only Calamus brandisii and Calamus wightii were found to occur up to an altitude of 2000 m asl.
Among the different species of rattans in the Western Ghats, the Extent of Occurrence (EOO) calculated using the Minimum Convex Hull method shows Calamus karnatakensis, Calamus thwaitesii, Calamus metzianus, and Calamus rheedei have high EOO values (Table 2). Five species viz., Calamus nagbettai, Calamus neelagiricus, Calamus shendurunii, Calamus pseudofeanus, and Calamus renukae have EOO values less than 10 km2. C. brandisii and Calamus lakshmanae have EOO values less than 1000 km2. Extent of Occurrence calculated using the Minimum Presence Area (MPA) threshold shows very large areas ( > 40, 000 km2) for all species (Table 2).
| Sr No | Species | MCP-EOO (km2) | AOO (km2) | IUCN statusa | MPA-EOO (km2) | CV | Recommended category | Suitability in PA |
| 1 | Calamus brandisii | 853.19 | 20 | NA | 88, 612.00 | 38.88 | VU | Y (32) |
| 2 | Calamus gamblei | 30, 530.33 | 60 | NA | 92, 047.00 | 38.92 | VU | Y (37) |
| 3 | Calamus karnatakensis | 14, 648.77 | 16 | NA | 47, 608.00 | 33.82 | NT | Y (24) |
| 4 | Calamus lakshmanae | 922.44 | 12 | NA | 1, 71, 158.00 | 39.07 | VU | Y (48) |
| 5 | Calamus nagbettai | 8 | 8 | NA | 42.14 | VU | Y (1) | |
| 6 | Calamus thwaitesii | 23, 632.78 | 56 | NA | 1, 09, 163.00 | 33.25 | NT | Y (45) |
| 7 | Calamus travancoricus | 6431.59 | 36 | NA | 67, 315.00 | 39.62 | VU | Y (24) |
| 8 | Calamus delessertianus | 14, 738.92 | 28 | NA | 1, 61, 182.00 | 38.42 | VU | Y (50) |
| 9 | Calamus dransfieldii | 6811.35 | 12 | NA | 1, 59, 543.00 | 41.74 | VU | Y (48) |
| 10 | Calamus hookerianus | 8740.88 | 48 | NA | 44, 402.00 | 38.45 | VU | Y (37) |
| 11 | Calamus lacciferus | 3872.19 | 16 | NA | 45, 957.00 | 39.13 | VU | Y (19) |
| 12 | Calamus metzianus | 26, 098.16 | 28 | NA | 1, 38, 327.00 | 40.79 | VU | Y (49) |
| 13 | Calamus neelagiricus | 4 | 4 | NA | 47.14 | EN | Y (2) | |
| 14 | Calamus prasinus | 17, 787.02 | 24 | NA | 1, 28, 896.00 | 44.7 | VU | Y (39) |
| 15 | Calamus pseudotenuis | 20, 087.84 | 60 | NA | 1, 92, 732.00 | 36.32 | NT | Y (47) |
| 16 | Calamus rheedei | 37, 173.66 | 16 | NA | 81, 268.00 | 38.38 | VU | Y (30) |
| 17 | Calamus vattayila | 15, 167.83 | 48 | NA | 59, 617.00 | 45.54 | EN | Y (28) |
| 18 | Calamus shendurunii | 4 | 4 | NA | 42.5 | VU | Y (1) | |
| 19 | Calamus wightii | 6482.84 | 20 | NA | 50, 138.00 | 39.62 | VU | Y (46) |
| 20 | Calamus pseudofeanus | 4 | 4 | NA | 47.5 | EN | N (0) | |
| 21 | Calamus renukae | 4 | 4 | NA | 42.5 | VU | N (0) | |
| NA: Not assessed. MCP: Minimum Convex Polygon using GeoCAT analysis. MPA: Minimum Presence Area calculated using ENM method. VU: Vulnerable, EN: Endangered and NT: Near Threatened. a Redlist.org. |
||||||||
The conservation value (CV) for 21 species is given in Table 2. Of these 21 species, two have low CV ( < 35; C. karnatakensis, C. thwaitesii), 10 have moderate CV, five have high CV ( > 35 and < 45) and three have very high CV ( > 45; C. neelagiricus, Calamus vattayila, C. pseudofeanus; Fig. 4; Table 2). Even though Calamus gamblei is more widely distributed than C. karnatakensis, it has received a higher value as it has a narrow elevational range than the latter species. Based on CV, we suggest three species should be treated as Endangered, 15 as Vulnerable and three as Near Threatened (Table 2).
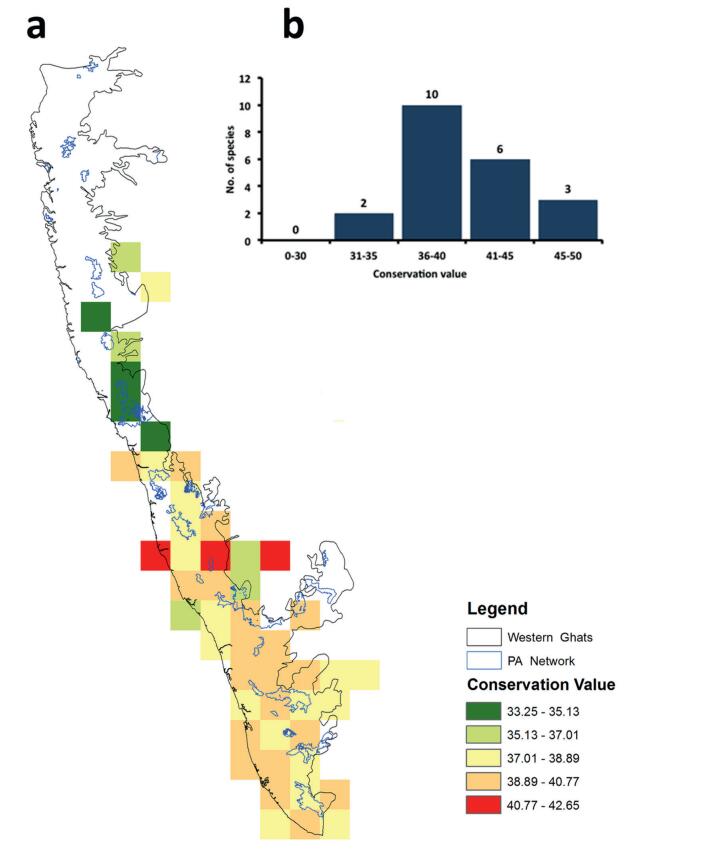
|
| Fig. 4 Distribution of high conservation value (CV) sites in the Western Ghats (a) and frequency distribution of CV for 21 species of Calamus sp. from the Western Ghats (b). |
At half a degree scale, a hotspot of rattan richness in the Western Ghats was found above Palghat Gap, in the Coorg-Wayanad region. There are two "second-hotspot" regions of species richness, one above Coorg-Wayanad region (the first hotspot) and a second in the southern part of the Western Ghats in the Agastyamalai region (Fig. 3a). At a much finer scale, the species richness map from ENM also shows a similar pattern. However, in this map, five species were not included as they had fewer than five records. Thus, the maximum species richness calculated using ENM is 16 compared to 21 when using grid-based analysis (Fig. 3b). It is clear from both the maps that the species rich regions are outside protected areas (PAs). Only Silent Valley National Park and surrounding PAs along with the PAs in Agasthyamalai Biosphere Reserve have moderately high species richness. The grid-based approach identified four micro-hotspots, two in the central Western Ghats and two in southern Western Ghats; and the ENM-based approach identified five regions, three in southern Western Ghats and two in central Western Ghats. These micro-hotspots are at mid-to high elevation in the southern Western Ghats and low-to mid-elevation in the central Western Ghats (Fig. 3).
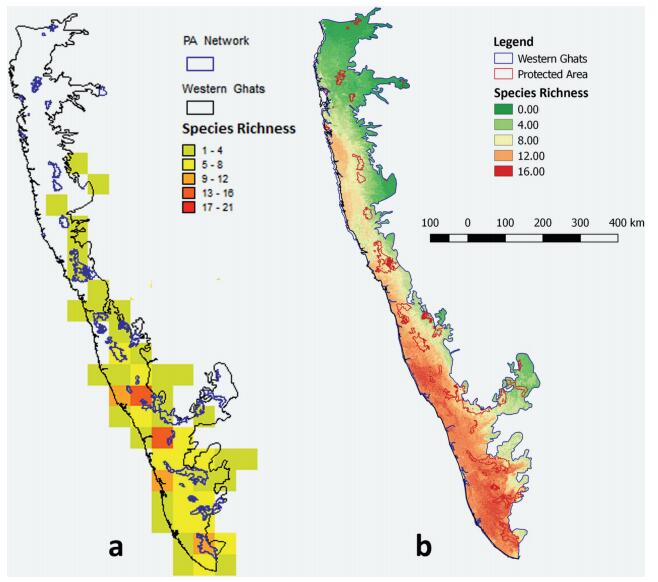
|
| Fig. 3 Species richness map for rattans of the Western Ghats (left grid-based and right ENM-based). The dark polygon shows the protected areas in the study region. |
Only two species, C. nagbettai and C. neelagiricus, have suitable areas in one and two protected areas in the Western Ghats. For the remaining species, there are a large number of PAs with suitable niches (Table 2). The globally threatened, endemic rattan species C. nagbettai has a suitable niche only in Shendurney and Pushpagiri Wildlife Sanctuaries in southern India. These PAs can be selected for future reintroduction and species recovery programmes.
DiscussionIn this paper, we assessed the distribution of all 21 rattan species of the Western Ghats biodiversity hotspot using two approaches. Our results show that of the 21 species, five species have very few distribution records. These five species are rare, have small range sizes or have very low abundance. Many species of rattans have narrow elevational or latitudinal ranges or are restricted to evergreen forests.
Rattans are a very important source of livelihood for the economically and forest-dwelling communities (Uma Shaanker et al., 2004). It is estimated that more than half a million people are directly employed in harvesting and processing rattans in the rural areas of Southeast Asia (Uma Shaanker et al., 2004). In India, the state of Kerala alone has over 300, 000 people involved in cane-based industries. It is estimated that the annual global revenue from the rattan trade exceeds US$6.7 billion (Ravikanth et al., 2001). As the world's demand for rattan and rattan products increases, there is tremendous pressure on natural populations. For instance, almost all the required rattan in the country is supplied by extraction from wild populations. The increasing demand for rattan furniture for both national and international markets has resulted in the loss of several populations (Uma Shaanker et al., 2004). In addition to extraction pressures, rattans are also severely threatened by changes in land use patterns such as conversion of forest lands to agriculture and plantations, as well as other anthropogenic activities such as irrigation and power projects (Ravikanth et al., 2001; Manohara et al., 2007; Senthilkumar et al., 2014). Furthermore, rattans are dioecious and when they are prematurely harvested they rarely flower and fruit, resulting in poor seed set, which in turn adversely affects the regeneration of the species.
In this regard, identifying the species rich regions is important for the conservation of species. This is especially important for those species which are harvested and are under tremendous pressure. The information on the geographic distribution of rattans will provide a geographic perspective of the resources in question and thereby facilitate informed decisions to be made for the conservation and management of these extracted resources. In fact, understanding the geographical variation in species richness and the patterns of species distribution can be valuable for the establishment of conservation sites (Gaston, 2003; Graves and Rahbek, 2005). Specifically, three species, C. neelagiricus, C. vattayila and C. pseudofeanus, have a narrow distribution and need urgent conservation attention.
Geographic distribution patternsOf the 21 endemic rattans, seven species in the Western Ghats have a very narrow elevational range ( < 200 m) and another seven species have an elevational range up to 800 m. The results presented here on the distribution of rattans in the Western Ghats are heavily influenced by the incompleteness of the sampling effort. However, with the existing information, a general conclusion can be drawn on the distribution of rattans in the Western Ghats. The southern Western Ghats (between 8°N and 12°N) and certain parts of the central Western Ghats are relatively better sampled than the northern regions. This is also seen in other taxa such as frogs and aquatic insects (Daniels, 1992; Sarma, 2012). The central Western Ghats is a species rich region (Kodagu district in Karnataka) with 12 of the 21 endemic species of the Ghats (Bhat et al., 2009). Southern Western Ghats, below 12°N is reported to be a species rich region for a number of taxa such as land snails (Aravind et al., 2005), fishes (Dahanukar et al., 2004), Odonates (Subramanian and Sivaramakrishnan, 2002) and endemic plants (Ramesh and Pascal, 1996), but not for rattans.
Five of the 21 species of rattans are known from less than five localities and a couple of other species show patchy distribution. The recent land use pattern might have shaped the patchy distribution of species because the Western Ghats has a long history of human impact (Chandran, 1997). The major change in the landscape has occurred in the evergreen forests of the Western Ghats. Once continuous, the forests of the Western Ghats have been reduced and fragmented to a large extent (Nair and Daniel, 1986; Daniels et al., 1991; Daniels, 1992). Eighteen of the 21 species of endemic rattans of the Western Ghats occur only in evergreen forests. The evergreen forests of the Ghats have undergone drastic reduction in the area in last two centuries. Major land use that has led to reduction in the area is due to the increased cultivation of cash crop plantations such as coffee, tea, as well as the construction of dams, roads, and power lines (Jha et al., 2000). A recent study by Jha et al. (2000) showed that the southern Western Ghats lost up to 25% of evergreen forests between 1975 and 1992 due to various types of activities. In Kodagu, part of the central Western Ghats, the dense forest was reduced from 63% to 45% over two decades (1977–1997) mainly due to conversion to coffee plantations (Ramesh et al., 2009). This change in land use pattern, along with geological and climatic factors, might have shaped current distribution patterns of flora and fauna of the Western Ghats (Ali and Ripley, 1983; Daniels, 1992; Aravind et al., 2005).
Protected areas, hotspots of richness and conservation implicationsCoorg-Wayanad in the central Western Ghats and Agastyamalai region in southern Western Ghats are hotspots of rattan richness. A significant proportion of the suitable areas lie outside the protected areas in central Western Ghats in contrast to southern Western Ghats. The Coorg-Wayanad region contains large areas under coffee and tea plantations. Much of the land conversion in these areas occurred over the last 150 years when agriculture was expanding at a rapid pace (Ravikanth et al., 2000). Rattans are dioecious species and therefore pollination success is hindered by habitat disturbance (Singh et al., 2004). Traditionally, protected areas (PAs) have been considered the most efficient way for conserving biodiversity (Naughton-Treves et al., 2005). However, it has been shown that PAs are insufficient/inefficient in conserving biodiversity, especially in biodiversity rich tropical regions where population is very high (Furze et al., 1996; Fagundes et al., 2016). In the Western Ghats, PAs are well managed and conserved. However, PAs are surrounded by a large number of villages and the boundaries are very porous, which has led to illegal harvesting of rattans from the PAs. Three of the five "micro-hotspots" identified here are in the Kerala part of the Western Ghats. This region also has the highest population density per unit area and much of the natural forests have been converted to rubber and teak plantations at lower elevations or to tea gardens at mid-and high elevations.
The conservation of rattan species is of high priority as they are not only economically important, but many of the species are endemic with a narrow distributional range. Considering the accelerated pace of habitat loss or degradation due to developmental activities in the Western Ghats (Jha et al., 2000), the extinction risk of restricted-range, habitat specialist, non-clump forming and endemic rattans might increase significantly in the near future. In order to overcome this, a sound conservation and management plan needs to be developed. This includes encouraging the establishment of large-scale plantations in private lands, developing agro-forestry systems and domestication as well as large-scale plantations by the state Forest Departments. In these areas, instead of imposing restrictions on the use of these resources, permitting people to manage the resources for their subsistence and livelihoods may be mutually beneficial. Extractive reserves could be established where economically important rattans are planted. These extractive reserves could meet the requirements of communities that depend on these resources. In the Western Ghats region, the State Forest Departments of Kerala, Karnataka, Tamil Nadu and Goa have initiated large-scale plantations of rattans to meet market demand. However, only three or four economically important species are cultivated commercially (Renuka, 1992). Domestication may also contribute significantly to national economies and in maintaining some degree of forest cover in heavily-exploited regions (Mack, 2000; Idohou et al., 2016) and reduce pressure on natural populations. Cultivation may also help to increase recognition of the importance of wild species, and encourage preservation and restoration of wild populations in their natural habitats (Cuni Sanchez et al., 2011). Given the large number of people dependent on rattans for their livelihood, it is imperative to conserve this resource for both ecological as well as economic reasons.
Conservation valueConservation Value has been assigned for a number of taxa, including birds (Daniels et al., 1991), butterflies (Kunte, 2008) and amphibians (Mohapatra et al., MS). Our results are the first attempt to assign CV for a plant species from India. Three species of rattans have very high conservation value (C. neelagiricus, C. vattayila and C. pseudofeanus). None of the rattan species have been added to the IUCN red-list category as they have not been assessed by the IUCN. The CV for these species is 38 and 42 respectively. All three species (C. neelagiricus, C. vattayila and C. pseudofeanus) have very low EOO values (narrow range), and are harvested, single-stemmed species. Not surprisingly, low EOO species generally have higher conservation values. Single-stem species and narrow-range species are highly vulnerable to extinction compared to multi-stem or those species with coppicing abilities. High conservation value species also have narrow elevational range and rainfall regime. Earlier studies on conservation values of birds and butterflies of the Western Ghats have shown that evergreen and high elevation montane forests support a high proportion of endemic and endangered species (Daniels et al., 1991; Kunte, 2008). This is also true for rattans of the Western Ghats. Hence, conservation of these habitats is of utmost importance. The results presented here offer an objective way to assess the status of species in the absence of red listing based on geographic and elevational range, harvest pressures and biology. This method is simple and can be used for any taxa with appropriate modification and/or addition of more variables.
Caveats of the studyThe hotspot map derived from the grid-based and ENM approach shows similar results. The advantage with ENM-based approach is that it normalizes the distribution by extrapolating using environmental layers. However, this model is influenced by a number of data points and its distribution range. The most fundamental constraint of any ENM method is that only those species with adequate numbers of spatially distinct occurrence records can be modelled (Stockwell and Peterson, 2002). Hence, for five species we were unable to model because we could only obtain five distribution records. Though some studies have used three records for modelling (Stockwell and Peterson, 2002), we refrained from modelling these species as the records were closely distributed (clumped distribution). On the other hand, the grid-based approach is appropriate if one is looking at larger areas and more widespread species where a larger grid size is desirable. Hence, the usefulness depends on the number of data points and the grid size (Grossi et al., 2017). For species that have a narrow distribution range, are point endemic and have few distribution records, a smaller grid is preferred. In our study, there are fewer than 1000 records for 21 species ranging from 1 to 200 records per species. This bias in distribution data will influence the selection of grid size and the final results. Given the limitations of the two approaches, the results are consistent between the two models.
ConclusionRattans, one of the most exploited and highly valued non-timber forest products, are a high priority for conservation. We have limited knowledge on patterns of species distribution in the Western Ghats. Despite this limited knowledge, a broad pattern of geographical distribution of rattans of the Western Ghats can be seen. As shown for other mountainous tropical regions of the world, the rattan flora is rich and many species are narrowly endemic. Further studies are required to gain better insight into the patterns, processes and determinants of rattan distribution in the Western Ghats biodiversity hotspot. Further studies might contribute directly to the conservation of this unique palm.
AcknowledgementsAuthors would like to thank Department of Biotechnology, Govt. of India (BT/PR8359/NDB/51/145/2006) and Royal Norwegian Embassy (IND 3025-12/0050) for funding. MJ would like to thank Department of Science and Technology for the INSPIRE Mentorship Grant. Authors are indebted to three anonymous reviewers for the critical comments on the previous version, which helped enormously in improving the MS.
Appendix A. Supplementary dataSupplementary data related to this article can be found at http://dx.doi.org/10.1016/j.pld.2017.08.002.
Ali S., Ripley S., 1983. Handbook of the Birds of India and Pakistan. Oxford University Press Delhi.
|
Aravind N.A., Rajashekhar K.P., Madhyastha N.A., 2005. Species diversity, endemism and distribution of land snails of the Western Ghats, India. Rec. West.Aust. Mus. (Suppl, (Suppl, 68): 31-38. |
Bachman S., Moat J., Hill A.W., de Torre J., Scott B., 2011. Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. Zookeys, 150: 117-126. DOI:10.3897/zookeys.150.2109 |
Basu, S. K., 1992. Rattans (Canes) in India: a Monographic Revision. Rattan Information Centre, Kepong, Kuala Lumpur, Malaysia. http://agris.fao.org/openagris/search.do?recordID=XF2015005857
|
Bhat, P. R., Shenoy, H. S. P., Kaveriappa, K. M., 2009. Studies on the Status of Some Species of Rattans (Calamus spp. ) in the Forests of the Western Ghats of Karnataka, India. Indian for. 135. http://www.cabdirect.org/abstracts/20093215061.html
|
Bhat P.R., Shenoy H.S.P., Kaveriappa K.M., 2010. Status of some species of rattans in the forests of the Western Ghats of Karnataka. India. Afr. J. Plant Sci, 4: 455-463. |
Butchart S.H.M., Walpole M., Collen B., et al, 2010. Global biodiversity: indicators of recent declines. Science, 328: 1164-1168. DOI:10.1126/science.1187512 |
Cardoso P., Borges P.A.V., Triantis K.A., Ferrandez M.A., Martin J.L., 2011. Adapting the IUCN red list criteria for invertebrates. Biol. Conserv, 144: 2432-2440. DOI:10.1016/j.biocon.2011.06.020 |
Chandran M.D.S., 1997. On the ecological history of the Western Ghats. Curr. Sci, 73: 146-155. |
Cuni Sanchez A., Osborne P.E., Haq N., 2011. Climate change and the African baobab (Adansonia digitata L.): the need for better conservation strategies. Afr. J.Ecol, 49: 234-245. DOI:10.1111/aje.2011.49.issue-2 |
Dahanukar N., Raut R., Bhat A., 2004. Distribution endemism and threat status of freshwater fishes in the Western Ghats of India. J. Biogeogr, 31: 123-136. DOI:10.1046/j.0305-0270.2003.01016.x |
Daniels R.J.R., 1992. Geographical distribution patterns of amphibians in the Western Ghats. India. J. Biogeogr, 9: 521-529. |
Daniels R.J.R., Hegde M., Joshi N.V., Gadgil M., 1991. Assigning conservation value:a case study from India. Cons. Biol, 5: 464-475. DOI:10.1111/cbi.1991.5.issue-4 |
De la Estrella M., Mateo R.G., Wieringa J.J., Mackinder B., Muñoz J., 2012. Legume diversity patterns in West Central Africa: Influence of species biology on distribution models. PLoS ONE, 7: e41526. DOI:10.1371/journal.pone.0041526 |
Engler R., Guisan A., Rechsteiner L., 2004. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudoabsence data. J. Appl. Ecol, 41: 263-274. DOI:10.1111/j.0021-8901.2004.00881.x |
Fagundes C.K., Vogt R.C., De Marco Júnior P., 2016. Testing the efficiency of protected areas in the Amazon for conserving freshwater turtles. Divers. Distrib, 22: 123-135. DOI:10.1111/ddi.12396 |
Fenu G., Mattana E., Congiu A., Bacchetta G., 2010. The endemic vascular flora of Supramontes (Sardinia), a priority plant conservation area. Candollea, 65: 347-358. DOI:10.15553/c2010v652a10 |
Furze, B., De Lacy, T., Birckhead, J., 1996. Culture, Conservation and Biodiversity: the Social Dimension of Linking Local Level Development and Conservation through Protected Areas. Wiley, New York. http://www.cabdirect.org/abstracts/19961805865.html
|
Gaston K., 2003. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ.Press.
|
Govaerts, R., Dransfield, J., Zona, S., Hodel, D. R., Henderson, A., 2014. World Checklist of Arecaceae. Facilitated by the Royal Botanic Gardens, Kew. Available from: http://apps.kew.org/wcsp/.
|
Graves G.R., Rahbek C., 2005. Source pool geometry and the assembly of continental avifaunas. Proc. Natl. Acad. Sci. U. S. A, 102: 7871-7876. DOI:10.1073/pnas.0500424102 |
Grossi M.A., Draper D., Apodaca M.J., Vitali M.S., Pataro L., Katinas L., Moreno Saiz J.C., 2017. The road to 2020 targets and the learnings from the emblematic South American plant genus Nassauvia (Asteraceae). Biodivers. Conserv, 26: 329-351. DOI:10.1007/s10531-016-1245-0 |
Guisan A., Broennimann O., Engler R., Vust M., Yoccoz N.G., Lehmann A., Zimmermann N.E., 2006. Using niche-based models to improve the sampling of rare species. Conserv. Biol, 20(2): 501-511. DOI:10.1111/cbi.2006.20.issue-2 |
Hammer O., Harper D.A.T., 2007. Paleontological data analysis. Paleontol. Data Anal, (1999): 1-351. |
Idohou, R., Peterson, T. A., Assogbadjo, A. E., Vihotogbe, R. L., Padonou, E., Kakaï, R. G., 2016. Identification of potential areas for wild palm cultivation in the Republic of Benin through remote sensing and ecological niche modeling. Genet. Resour. Crop. Evol. http://dx.doi.org/10.1007/s10722-016-0443-7.
|
IUCN, 2001. IUCN Red List Categories and Criteria: Version 3. 1. IUCN Species Survival Commission. IUCN, Gland, Switzerland and Cambridge, UK.
|
Jha C.S., Dutt C.B.S., Bawa K.S., 2000. Deforestation and land use changes in Western Ghats, India. Curr. Sci, 79: 231-238. |
Jiménez-Alfaro B., Draper D., Nogués-Bravo D., 2012. Modeling the potential area of occupancy at fine resolution may reduce uncertainty in species range estimates. Biol. Cons, 147: 190-196. DOI:10.1016/j.biocon.2011.12.030 |
Kraft N.J.B., Baldwin B.G., Ackerly D.D., 2010. Range size, taxon age and hotspots of neoendemism in the Californian flora. Divers Distrib, 16: 403-413. DOI:10.1111/j.1472-4642.2010.00640.x |
Kunte K., 2008. The Wildlife (protection) act and conservation prioritization of butterflies of the western Ghats, southwestern India. Curr. Sci, 94: 725-735. |
Lakshmana, A. C., 1995. Rattans of South India. Evergreen Publishers, Bangalore, India, pp. 1-179. http://www.cabdirect.org/abstracts/19950611648.html
|
López-López P., Maiorano L., Falcucci A., Barba E., Boitani L., 2011. Hotspots of species richness, threat and endemism for terrestrial vertebrates in SW Europe. Acta Oecol, 37: 399-412. DOI:10.1016/j.actao.2011.05.004 |
Lyngdoh N., Santosh S.H., Ramesha B.T., Rao M.N., Ravikanth G., Narayani B., Ganeshaiah K.N., Uma Shaanker U., 2005. Rattan species richness and population genetic structure of Calamus flagellum in North-Eastern Himalaya, India. J. Bamboo Rattan, 4: 293-307. DOI:10.1163/156915905774310016 |
Mack R., 2000. Cultivation fosters plant naturalization by reducing environmental stochasticity. Biol. Invasions, 2: 111-122. DOI:10.1023/A:1010088422771 |
Manohara T.N., Ramaswamy S.N., Shivamurthy G.R., 2007. Calamus-dwindling resources?. Curr. Sci, 92: 290-292. |
Margules C.R., Pressey R.L., 2000. Systematic conservation planning. Nature, 405: 243-253. DOI:10.1038/35012251 |
Merow C., Smith M.J., Silander Jr.J.A., 2013. A practical guide to MaxEnt for modelling species' distributions: what it does, and why inputs and settings matter. Ecography, 36: 1058-1069. DOI:10.1111/ecog.2013.36.issue-10 |
Mohan Ram H.Y., Tandon R., 1997. Bamboos and rattans: from riches to rags. Proc.Indian Natl. Sci. Acad, 63: 245-267. |
Mohapatra, B. B., Charles, B., Aravind N. A. Conservation Value for Amphibians of the Western Ghats. (Manuscript Under Preparation).
|
Murray-Smith C., Neil A.B., Oliveira-Filho A.T., Bachman S., Moat J., Lughadha E.M.N., Lucas E., 2006. Plant diversity hotspots in the atlantic coastal forests of Brazil. Con. Biol, 23: 151-163. |
Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent J., 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. DOI:10.1038/35002501 |
Nair N.C., Daniel J.C., 1986. The floristic diversity of the Western Ghats and its conservation: a review. Proc. Indian Acad. Sci. Anim. Sci. Plant Sci., Suppl. 1: 127-163. |
Naughton-Treves L., Holland M.B., Brandon K., 2005. The role of protected areas in conserving biodiversity and sustaining local livelihoods. Annu. Rev. Environ.Resour, 30: 219-252. DOI:10.1146/annurev.energy.30.050504.164507 |
Norris D., 2014. Model thresholds are more important than presence location type:understanding the distribution of lowland tapir (Tapirus terrestris) in a continuous Atlantic forest of southeast Brazil. Trop. Conserv. Sci, 7: 529-547. DOI:10.1177/194008291400700311 |
Pearson R.G., Raxworthy C.J., Nakamura M., Peterson T.A., 2007. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr, 34: 102-117. |
Pena de Castro J.C., Kamino L.H.Y., Rodrigues M., Mariano-Neto E., de Siqueira M.F., 2014. Assessing the conservation status of species with limited available data and disjunct distribution. Biol. Conserv, 170: 130-136. DOI:10.1016/j.biocon.2013.12.015 |
Phillips S.J., Anderson R.P., Schapire R.E., 2006. Maximum entropy modeling of species geographic distributions. Ecol. Modell, 190: 231-259. DOI:10.1016/j.ecolmodel.2005.03.026 |
Priti H., Aravind N.A., Uma Shaanker R., Ravikanth G., 2016. Modeling impacts of future climate on the distribution of Myristicaceae species in the Western Ghats, India. Ecol. Engg, 89: 14-23. DOI:10.1016/j.ecoleng.2016.01.006 |
Raes N., Roos M.C., Slik J.W.F., van Loon E.E., ter Steege H., 2009. Botanical richness and endemicity patterns of Borneo derived from species distribution models. Ecography, 32: 180-192. DOI:10.1111/eco.2009.32.issue-1 |
Ramesh, B. R., Pascal, J. P., 1996. Distribution of endemic arborescent evergreen species in the Western Ghats. In: Karunakaran, C. K. (Ed. ), The Proceedings of the Symposium on Rare, Endangered and Endemic Plants of the Western Ghats, Kerala Forest Department, Special Publication No. Ⅲ, Thiruvananthapuram, pp. 20-29.
|
Ramesh, B. R., Seetharam, M., Guero, M., Michon, R., 2009. Assessment and Conservation of Forest Biodiversity in the Western Ghats of Karnataka, India. 1. General Introduction and Forest Land Cover and Land Use Changes (1977-1997). Institut Francais de Pondichery, Pondy Papers in Ecology no. 6, Head of Ecology Department, Institut Francais de Pondichery.
|
Ramesha B.T., Ravikanth G., Rao M.N., Ganeshaiah K.N., Uma Shaanker R., 2007. Genetic structure of the rattan Calamus thwaitesii in core, buffer and peripheral regions of three protected areas in central Western Ghats, India: do protected areas serve as refugia for genetic resources of economically important plants?. J. Genet, 86: 9-18. |
Ravikanth G., Uma Shaanker R., Ganeshaiah K.N., 2000. Conservation Status of forests in India: a cause for worry? . J. Indian Inst. Sci, 80(6): 591-600. |
Ravikanth, G., Ganeshaiah, K. N., Uma Shaanker, R., 2001. Mapping genetic diversity of rattans in Central Western Ghats: identification of hot-spots of variability for in situ conservation. In: Uma Shaanker, R., Ganeshaiah, K. N., Bawa, K. S. (Eds. ), Forest genetic Resources: Status, Threats and Conservation Strategies. Oxford and IBH Publishing Co. Pvt. Ltd, pp. 69-83.
|
Ravikanth G., Ganeshaiah K.N., Uma Shaanker R., 2002. Identification of hot spots of species richness and genetic variability in rattans: an approach using geographical information systems (GIS) and molecular tools. Plant Genet.Resour. Newsl, 132(17): 21. |
Ravikanth G., Rao M.N., Madhugiri Ajay.N., Uma Shaanker R., Ganeshaiah K.N., 2010. Do endemic rattans have lower genetic variability than their co-generic and con-specific non-endemic rattans?. Genes, Genomes Genomics, 4: 22-27. |
Renuka, C., 1992. Rattans of the Western Ghats: a Taxonomic Manual. Kerala Forest Research Institute, Peechi. http://agris.fao.org/openagris/search.do?recordID=XF2015029410
|
Sarma, R. R., 2012. An Assessment of the Geographical Distribution Pattern in Anurans of the Western Ghats and the Effectiveness of Protected Areas in Conservation of Anurans Using GIS. M. Tech Thesis submitted to Birla Institute of Technology, Mesra.
|
Schouten M.A., Barendregt A., Verweij P.A., Kalkman V.J., Kleukers R.M.J.C., Lenders H.J.R., Siebel H.N., 2010. Defining hotspots of characteristic species for multiple taxonomic groupsin The Netherlands. Biodivers. Conserv, 19: 2517-2536. DOI:10.1007/s10531-010-9857-2 |
Sen S., Gode A., Srirama R., Ravikanth G., Aravind N.A., 2016. Modeling the impact of climate change on wild Piper nigrum (black pepper) in western Ghats, India using ecological niche models. J. Plant Sci, 129: 1033-1040. |
Senthilkumar U., Choudhary R.K., Sanjappa M., Narasimhan D., Uma Shaanker R., Ravikanth G., 2014. Livelihood and revenue: role of rattans among mongoloid tribes and settlers of andaman and Nicobar Islands, India. Ethnobot. Res. Appl, 12: 141-15. DOI:10.17348/era.12.0.141-154 |
Senthilkumar U., Narasimhan D., Sanjappa M., Uma Shaanker R., Ravikanth G., 2015. Species delimitation in congenerics of Genus Daemonorops from India using DNA barcodes. Commun. Plant Sci, 5(1-2): 1-8. |
Singh B.H., Puni L., Jain A., Singh R.S., Rao P.G., 2004. Status, utility, threats and conservation options for rattan resources in Manipur. Curr. Sci, 87: 90-94. |
Sreekumar V.B., Renuka C., Suma T.B., Balasundaran M., 2006. Taxonomic reconsideration of Calamus rivalis Thw. ex Trim. and C. metzianus Schlecht (Arecaceae) through morphometric and molecular analyses. Bot. Stud, 47: 443-452. |
Stockwell D.R.B., Peterson A.T., 2002. Effects of sample size on accuracy of species distribution models. Ecol. Model, 148: 1-13. DOI:10.1016/S0304-3800(01)00388-X |
Subramanian, K. A., Sivaramakrishnan, K. G., 2002. Conservation of Odonate fauna in Western Ghats -A biogeographic perspective. Vistas of Entomological Research for the New Millenium 11-22.
|
Syfert M.M., Joppa L., Smith M.J., Coomes D.A., Bachman S.P., Brummitt N.A., 2014. Using species distribution models to inform IUCN Red List assessments. Biol. Conserv, 177: 174-184. DOI:10.1016/j.biocon.2014.06.012 |
Uma Shaanker, R., Ganeshaiah, K. N., Srinivasan, K., Rao, V. R., Hong, L. T., 2004. Bamboo and Rattans of the Western Ghats: Population Biology, Socioeconomics and Conservation Strategies. ATREE, Bangalore, IPGRI, Malaysia and UAS, Bangalore. http://indianmedicine.eldoc.ub.rug.nl/root/S5/137s/
|
Willis F., Moat J., Paton A., 2003. Defining a role for herbarium data in Red List assessments: a case study of Plectranthus from eastern and southern tropical Africa. Biodivers. Conserv, 12: 1537-1552. DOI:10.1023/A:1023679329093 |



