b. Department of Horticultural Science, Shiraz University, Shiraz, Iran;
c. Department of Horticultural Science, Tarbiat Modares University, Tehran, Iran;
d. Research Institute of Forests and Rangelands, Tehran, Iran;
e. Department of Agronomy and Plant Breeding, Faculty of Agriculture, University of Tehran, Karaj, Iran;
f. Department of Horticulture, Faculty of Agriculture, Shahre Kord University, Shahre Kord, Iran;
g. Department of Physiology & Breeding of Ornamental Plants, Engineering Faculty of Horticulture Sciences, University of Tehran, Karaj, Iran;
h. Department of Plant Sciences, University of California-Davis, Davis, USA
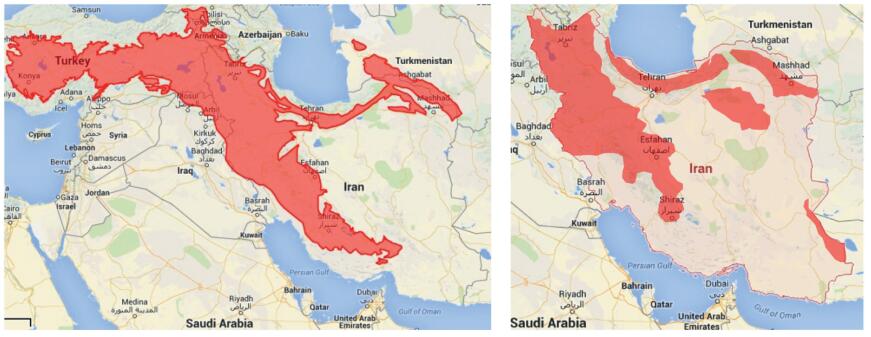
The Middle East enjoys a wealth of biological diversity encompassing a variety of ecosystems and associated habitats, including high-to low-density forests, deserts, plains, rangelands, savannas, oases, and mountains. Three of the world's 34 biodiversity hotspots, the Irano-Anatolian region, the Mediterranean forest region, and the Horn of Africa region, are located in the Middle East and West Asia regions (Hanson et al., 2009). The Irano-Anatolian region hotspot covers an area of about 899, 773 km2, a considerable share of which is found in northern through western Iran, and is a World Heritage Site known for its rich biodiversity with about 6000 plant species. The Irano-Anatolian region also includes parts of Turkey, Iraq, Georgia, Azerbaijan, Armenia and Turkmenistan (Fig. 1, Left).
|
| Fig. 1 Left, Irano-Anatolian hotspot region; Right, an estimation of geographic distribution for Fritillaria spp. throughout Iran. |
Iran is a vast country in Southwest Asia occupying an area of over 1.64 million square km; an area nearly as large as, Italy, France, Spain and the British Isles combined (Firouz et al., 1970). The natural ecosystems of Iran cover about 80% of the country's surface area (Salahi et al., 2008), with 90 million ha of rangeland (nearly 54.8%) (Kharazipour, 2009), 12.4 million ha of forest (nearly 7.5%), and about 33 million ha of desert (about 20%).
Iran is a land of extremes; altitudes range from 5604 m on Mount Damavand's summit (Kharazipour, 2009) to 28 m below sea-level on the shores of the Caspian Sea (Akhani and Ghorbanli, 1993). The cold climates of the Zagros range, running from the northwest to the south, are replaced by the hot desert climates to the center and east. Mean January temperatures range from 20 ℃ along the Persian Gulf in Hormozgan province to about -11 ℃ in Chaharmahal & Bakhtiari (C & B) province. Dry lands of the interior areas change suddenly to the wet and moderate coastal climates of the Caspian coastal areas; the lush Caspian forest may receive an annual rainfall of 1950 mm, whereas lifeless sand-dunes of the 'Lut' remain arid (~30 mm annual rainfall).
Iran supports a great share of plant species and countless natural habitats characterized by many unique plants and centers of local endemism (Khourang et al., 2014). Up to 8200 plant species are recognized throughout the country, of which almost 1900 are endemic (Kharazipour, 2009). The genus Fritillaria, with a variety of species that grow naturally across different areas, is a major component of this botanical richness. However, signs are mounting that the country is truly at a crossroads when it comes to preservation of this national wealth.
Taxonomy and species conservation are often assumed to be interdependent activities; a shortage of taxonomic information and skills causes problems for conservation practitioners (Mace, 2004). From this point of view, dealing with the genus Fritillaria is a tricky effort, as the accepted units of diversity in the genus are hard to define and the exact habitat range of the species is hard to estimate. The existence of intermediate forms and continuous ranges of variation within this group of plants, which are phenomena generally caused by frequent hybridization at the species and subspecies level, accounts for the extreme difficulty in identifying many doubtful species. Furthermore, many of the species are native to remote uninhabited areas, where access to the habitats is extremely difficult. To make this even more complicated, members of the genus in Iran exhibit a wide range of variation in their morphological features (Sharifi-Tehrani and Advay, 2015) and physiological responses to the environment (Khourang et al., 2014). Thus, it is not surprising that among the large number of Fritillaria species known to occur in Iran, the majority of species are little known. Despite the necessity of an accessible comprehensive source of data to set practical conservation strategies, studies on Fritillaria in Iran have been quite sporadic and limited in scope. Following a comparative approach, here, we present a review of detailed basic information necessary for understanding the geographic, taxonomic, cytogenetic and phylogenetic status of Iranian Fritillaria, which seem to be vital to meeting the goal of sustainable conservation of Fritillaria in Iran and neighboring areas.
3. Genus Fritillaria (L.)The name Fritillaria is said to come from either the Latin term fritillus (the chequered Roman dice tower) (Kiani, 2015) or more likely the Latin root frittillo (the chess-board) (Gerard, 1995); both terms, however, denote the checkerboard pattern of the petals in Fritillaria meleagris L., the type species of the genus. Fritillaria is also the scientific name for butterflies in the family Nymphalidae, again referring to their patterned wings (Dunford and Sims, 2008).
Fritillaria (L.) is one of the most complex genera recognized in the family Liliaceae (Li et al., 2009). According to Rix (2001), the genus embraces about 140 species (165 taxa) of geophytic perennials, indigenous to the temperate climatic regions of the Northern Hemisphere (Rønsted et al., 2005) between latitudes 32° and 62°, especially throughout Europe, the Northern part of Africa bordering the Mediterranean Sea, the Mediterranean basin, the Middle East, Northern Asia and as far south as Iran, Afghanistan, the Himalayas, China, Korea and Japan. In North America Fritillaria occurs in Canada and in a narrow coastal strip from the Aleutian Islands to northern Mexico, extending inland to 16 states of the United States, especially California and Oregon (Beetle, 1944; Tekşen and Aytaç, 2011; Tomović et al., 2007). The genus has undergone marked species radiations in the Middle East, Southwest Asia and the eastern Mediterranean regions (Zaharof, 1988), particularly in Turkey and Iran. While Turkey, with 35 species and 6 subspecies (Tekşen and Aytaç, 2011), supports the greatest share of the world's Fritillaria resources, the significance of Iran rather lies in having a greater representation of different subgenera than any other country. Thus, the main center of the genetic diversity of the genus probably is Iran, where subgenera from Caucasus, central Asia and the Mediterranean meet (Rix, 1977). To date, 19 species (including at least 1 variety and 3 subspecies) are reported in Iran, of which at least 10 are endemic to the country (Table 1) (Advay et al., 2015; Bakhshi Khaniki, 1997a, 1997b; Rechinger, 1990; Rix, 1977, 2001).
| Genus Fritillaria | Species |
| Subgenus Petilium | Fritillaria imperialis (also F. imperialis var. lutea, the yellow form of the species), F. raddeanaa |
| Subgenus Thresia | F. persica |
| Subgenus Rhinopetalum | F. gibbosa, F. Ariana |
| Subgenus Fritillaria | |
| Section Fritillaria | |
| Group F. kotschyana | F. kotschyana (ssp. kotschyanaa & ssp. grandifloraa), F. olivieria |
| Group F. crassifolia | F. crassifolia ssp. kurdica, F. poluninii, F. reuteria, F. straussiia |
| Section Olostyleae | |
| Group F. caucasica | F. caucasica, F. uva-vulpis, F. assyriaca, F. zagricaa, F. chloranthaa, F. chlororhabdotaa, F. atrolineataa, F. avromanicaa |
| a Endemic to Iran. | |
Interestingly, the pattern of distribution of Iranian fritillaries largely corresponds to those parts of the country belonging to the Irano-Anatolian biodiversity hotspot (Fig. 1, Right). Fritillares are quite hardy and occur over a wide range of climates and habitats (Beetle, 1944). Most of the Iranian species are well-adapted to the uplands, foothills and associated plains of the Zagros range (Badfar-Chaleshtori et al., 2012), characterized by very rocky and sloping areas, wetlands, riparian zones, and different types of rangelands. The remaining species typically occur either on the northern slopes of the Mountain Elburz (also spelled Alborz) along the Caspian Sea in North Iran (Khaniki and Persson, 1997) or in flat and arid areas of the central parts of the country (Rix and Zarrei, 2007b). Iranian fritillaries mainly inhabit montane to alpine zones (1000–3600 m), but some species e.g. Fritillaria raddeana, at least in some parts, inhabit lowlands too (Khourang et al., 2014). With the exception of Fritillaria imperialis and F. persica, which are more southerly species, from the northern borders of the Zagros chain downwards, the geographical distribution of most of the Iranian species gradually decreases (Sharifi-Tehrani et al., 2015). The Zagros highlands at the border of Kordestan and Kermanshah provinces support the highest level of species richness in Iran (Wallis and Wallis, 2009), while the number of species in the southerly C & B province decreases to only four (Sharifi-Tehrani et al., 2015).
The typical bulb of Fritillaria species consists of a few fleshy, tightly packed scales and a thin, translucent tunic, which usually disappears as the bulb increases in size. Some Fritillaria species have naked bulbs consisting of many scales, which resemble those of Lilium; these species also have numerous bulbils, loosely attached (Rønsted et al., 2005). In the genus Fritillaria, flowers are usually pendant, solitary (sometimes in umbels or many-flowered racemes) and have a campanulate and typical tulip-like perianth (Zych and Stpiczyńska, 2012). Fritillaries generally bear actinomorphic flowers (Khaniki and Persson, 1997), except for members of subgenus Rhinopetalum, in which flowers are usually zygomorphic (Tamura, 1998). Nectaries are often flattish, usually linear, lanceolate or ovate, sometimes circular, and pollen grains are usually faveolate to reticulate (Khaniki, 2003). Species range from < 10 to over 120 cm in height. Although fritillaries can be seen in nearly every color, white-yellow, orange-red and purplish-brown are most common among the Iranian species (Kiani, 2015). F. imperialis is well known for its unpleasant foxy odor (Helsper et al., 2006), emanated by all parts of the plant, which is thought to be a way for repelling herbivores. The majority of the species with green or brown, broadly campanulate, flowers have what is often called a "spermatic scent", associated only with the flowers and presumably related to the attraction of pollinators.
Fritillaries generally produce bisexual flowers (Shimizu et al., 1998); however, a few other mating systems like androdioecy (in F. persica, F. involucrate All., F. messanensis Raf. and F. montana Hoppe ex W.D.J.Koch) and andromonoecy (in F. montana) have also been documented (Peruzzi, 2012). The breeding systems vary from self-compatible to self-incompatible, demonstrating various degrees of out-crossing. Fritillaria species are probably pollinated by insects, especially Hymenoptera (mostly various species of bees and wasps), Diptera, Lepidoptera and Coleoptera (Zych and Stpiczyńska, 2012), and even birds, as in the case of F. imperialis (Burquez, 1989; Peters et al., 1995).
Although variable based on the climatic conditions, species reported as commonly co-occurring with Fritillaria in Iran include Artemisia L. spp., Gagea robusta Zarrei & Wilkin, Tulipa L. spp., Astragalus L. sp., Geranium L. sp., Prunus scoparia (Spach) C.K.Schneid., Rumex acetocella L., Kelussia odoratissima Mozaff., Daphne mucronata Royle, Gundelia tournefortii L., Rosa canina L., Glycyrrhiza glabra L., Anchusa L. sp., Anthemis L. sp., Quercus L. sp., Ranunculus L. sp. and Echinops L. sp. (Babaei, 2014; Bakhshi, 2000; Khourang et al., 2014), many of which are endemic and of ethno-pharmacological value and importance. Furthermore, different species of the genus Fritillaria may occur in same habitat (Khourang et al., 2014).
4. Phylogenetic relationshipThe morphological classification of Fritillaria at the infrageneric level has been reviewed by several authors (Baker, 1874; Bentham and Hooker, 1883; Boissier, 1854; Turrill et al., 1980). According to the last revision by Rix (2001), the genus is subdivided into eight subgenera including Fritillaria [with two sections: Olostylae Boiss. (six series) and Fritillaria (10 series)], Rhinopetalum Fisch., Japonica Rix, Theresia K. Koch, Petilium (L.) Endl., Liliorhiza (Kellogg) Benth. & Hook.f. (three series), Davidii Rix and Korolkowia Rix (Mucciarelli et al., 2016). Although the current classification of the genus is supported by molecular phylogenetic studies (e.g. Rønsted et al., 2005; Day et al., 2014) at the subgeneric level, relationships among species remain incompletely resolved, especially within subgenus Fritillaria.
Up to now, 19 species belonging to four subgenera are recorded in Iran; subgenus Ⅰ) Petilium consists of four morphologically similar species, F. imperialis, F. raddeana, F. chitralensis (auct.) B. Mathew (also treated as F. imperialis var. chitralensis auct.) and F. eduardii A.Regel ex Regel (Wietsma et al., 2015); the first two species have been found in Iran. F. persica is the only species recognized in the monotypic subgenus Ⅱ) Theresia (Türktaş et al., 2012).
Fritillaria gibbosa, F. ariana, F. karelinii, F. stenanthera Rgl. and F. bucharica Rgl. are five morphologically similar species recognized in the subgenus Ⅲ) Rhinopetalum (Rix and Zarrei, 2007b), of which the occurrence of the first two has been confirmed in Iran; historically, a separate genus closely related to Fritillaria, called Rhinopetalum Fisch. ex Alexand. was described in 1830 (Mathew, 2005). Several authors used the genus Rhinopetalum subsequently, but others such as Boissier (1846) and Baker (1874) opted to place these species in a distinct subgenus in the genus Fritillaria. Recent molecular studies of Fritillaria (Rønsted et al., 2005; Day et al., 2014) have confirmed that members of Rhinopetalum are nested in Fritillaria; so the incorporation of Rhinopetalum into Fritillaria is justified.
The largest subgenus of the genus, Ⅳ) Fritillaria, is morphologically classified into six complexes, namely Fritillaria crassifolia, Fritillaria kotschyana, Fritillaria caucasica, Fritillaria graeca, F. meleagris and Fritillaria cirrhosa groups (Rix, 2001), of which members of the first three complexes are reported in Iran. No member of the F. meleagris group has yet been recorded in Iran, but a representative of the group (Fritillaria latifolia) is found in neighboring areas of Turkey and the Caucasus, and is very likely to be found in northwest Iran (Rix, 1977).
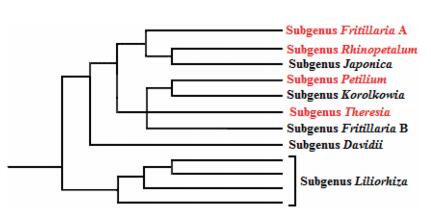
Following the classification proposed by Rix (2001), data in Fig. 2 confirm the monophyly of all subgenera within the genus, except subgenus Fritillaria, which resolves into two phylogenetically distant clades. On the basis of molecular evidence, members of the group Fritillaria A (a large predominantly European, Middle Eastern and North African clade) are distantly related to the group Fritillaria B (a small clade of mainly Chinese and Central Asian species). This result supports recognition of the clade Fritillaria B as a new subgenus, yet to be formally named.
|
| Fig. 2 Subgeneric classification of Fritillaria as adapted from Day et al. (2014); members of the subgenera highlighted in red occur in Iran. |
In Iran, phylogenetic research of Fritillaria is limited to few studies (e.g. Khourang et al., 2014; Sharifi-Tehrani and Advay, 2015). Although the findings of Khourang et al. (2014) reconfirmed most results of the previous phylogenetic studies of Fritillaria (e.g. Rønsted et al., 2005; Day et al., 2014), they suggested a new phylogenetic arrangement within the genus in some cases. In comparison to Day et al. (2014), the most comprehensive phylogenetic study of the genus, Khourang et al. (2014) used nine species (F. imperialis, F. imperialis var. lutea [the yellow form of the species], F. persica, F. crassifolia, F. straussii, F. zagrica, F. kotschyana, F. gibbosa, F. reuteri and F. raddeana), on which no information has been released on molecular phylogeny of F. imperialis var. lutea and F. straussii in the literature. Therefore, based on molecular evidence, the status of these taxa within the genus Fritillaria has remained undefined. Their results confirmed that F. straussii falls into the Middle Eastern group of subgenus Fritillaria (Fritillaria A). Furthermore, they claimed that red-and yellow-colored crown imperials are closely related, and both belong to subgenus Petilium.
5. Karyology and cytogenetic characteristicsThe genus Fritillaria has been used as a cytogenetic model for the study of chromosomal structure and cell division (meiosis & mitosis) processes, due to its exceptionally huge genome size (Darlington and La Cour, 1941); the tetraploid Fritillaria assyriaca is known to be the largest plant genome so far reported with 254.8 pg, nearly 800 times the size of the Arabidopsis thaliana genome (Zonneveld, 2010). Therefore, species of the genus have long held a special place in plant biology (Ambrožová et al., 2010). Chromosome numbers have been reported for over 50 species of Fritillaria; with a few exceptions (Kiran and Gedik, 2014; Matsuura, 1934; Marchant and Macfarlane, 1980; Noda, 1975; La Cour, 1978; Li and Shang, 1989), most of the species within the genus have a basic chromosome number x = 12 (Table 2).
| 2x | 3x | 4x | ||||
| x = 9 | x = 11 | x = 12 | x = 13 | x = 12 | ||
| F. tenella | F. japonica | Most of the examined species, including the Iranian taxab | F. pudica | F. lanceolataa | F. imperialis var. maxima lutea | |
| F. montana | F. amabilis | F. glauca | F. camschatcensisa | F. imperialis var. maxima rubra | ||
| F. ussuriensis | F. ussuriensis | F. assyriacaa | ||||
| F. ruthenica | ||||||
| a Karyo-ecotypes (2x & 3x taxa) reported. b The status of the Iranian F. avromanica is unknown. |
||||||
In addition, Fritillaria is a genus with frequent variations in the chromosome structure, ascribed to polyploidy as well as fusion and fragmentation of some chromosomes. Although tetraploidy in fritillaries has been documented in few cases (Table 2), polyploidy within the genus is almost limited to triploid plants, which are frequently found (Ambrožová et al., 2010). Karyo-ecotypes are reported among Fritillaria species, as some taxa growing in different geographical-environmental conditions show various levels of ploidy, which directly affect their reproductive behavior, ecological tolerance, and distribution range (Marchant and Macfarlane, 1980).
Cytogenetic and karyotypic characteristics are available for all of the Iranian species, except Fritillaria avromanica which has just recently been reported (Khaniki, 2002e, 2002f, 2002g; Advay et al., 2015). All of the Iranian species are diploid (2n = 2x = 24), showing a variety of karyotype formulas; in the majority of the species, the presence of 1–4 pairs of satellites has been confirmed (Advay et al., 2015; Ahmadi-Roshan et al., 2016; Bakhshi, 2000; Bakhshi and Persson, 2002; Bakhshi Khaniki, 1997a, 1997b; Jafari et al., 2014; Khaniki, 2002e, 2002f, 2002g), mostly located on the long arms (Ahmadi-Roshan et al., 2016; Jafari et al., 2014); this may be evaluated as an indicator for chromosomal evolution or possible ways for structural differentiation. Khaniki (2002b) reported the presence of accessory B chromosomes (also known as supernumerary chromosomes) for F. zagrica, whereas Ahmadi-Roshan et al. (2016) and Jafari et al. (2014) found no accessory chromosomes in their assessments. From an evolutionary point of view, this would be considered quite normal, since it seems unlikely that accessories would persist in a species unless there was some significant adaptive advantage.
Different chromosome types have been reported in Fritillaria. In accordance with Peruzzi et al. (2009), Jafari et al. (2014) and Ahmadi-Roshan et al. (2016), four chromosome types have been confirmed to include 'm', 'sm', 'st' and 'T' in nine species (F. imperialis, F. persica, F. raddeana, F. crassifolia ssp. kurdica, F. zagrica, F. kotschyana ssp. kotschyana, F. gibbosa, F. reuteri and F. straussii), with the 'st' and 'sm' types appearing at the highest and the lowest frequencies. The 'T' chromosome type has been reported for the first time in F. imperialis, F. imperialis var. lutea, F. raddeana, F. persica, F. crassifolia, F. zagrica and F. gibbosa (Jafari et al., 2014; Ahmadi-Roshan et al., 2016); the presence of the 'T' type chromosome in the karyotype of species directly correlates with the chromosomal asymmetry inferring the level of the species evolution. According to Stebbins's classification (1971), karyotypes of different species of Iranian Fritillaria can be classified as relatively asymmetric and/or evolved chromosomal structures (Jafari et al., 2014).
Fritillaries are quite different in the geometry of the chromosome structure, which directly affects their cross-compatibility and the fertility of the offspring. Compatible taxa generally show high degrees of chromosomal homology, making them potentially suitable for fertile interbreeding. Accordingly, a set of cross-compatible species, including F. imperialis × F. imperialis var. lutea, F. persica × F. straussii, F. kotschyana × F. crassifolia, F. imperialis × F. crassifolia, F. imperialis × F. gibbosa, F. gibbosa × F. raddeana, F. reuteri × F. zagrica, have been proposed by Ahmadi-Roshan et al. (2016) and Jafari et al. (2014), each of which are expected to make normal chromosome pairing and probably produce the most fertile hybrids when crossed to each other. The specific objectives of Fritillaria cytogenetics may be essentially diverse, but such knowledge is extremely useful, particularly in commercial improvement of cultivars through hybridization and/or identification of doubtful specimens via guestimating potential parents when several taxa inhabit in a given area.
6. Morphology and geographic distributionIn the following sections, detailed morphological characters and distribution ranges of the genus Fritillaria in Iran (including 19 species and 3 subspecies) are discussed. Keys to these taxa are also provided.
1. Flowers in an umbel, surmounted by numerous bracts (subgenus Petilium)
2. Leaves shiny green, inflorescence umbella, perianth segments 40–55 mm, orange to red rarely yellow, nectary ca. 5 mm in diameter – F. imperialis
2. Flowers yellowish–greenish, nectary 2–3 mm in diameter – F. raddeana
1. Flowers in a raceme or solitary, not surmounted by numerous bracts
3. Flowers zygomorphic, the nectary of the upper perianth segment larger than that of the rest, forming a curved protuberance on the back of the segment (subgenus Rhinopetalum)
4. Flowers tessellated, stem papillose throughout – F. gibbosa
4. Flowers not tessellated, stem papillose only at the leaf bases (at the nodes) and below the lowest leaves – F. ariana
3. Flowers actinomorphic, the nectaries all equal, not forming protuberances on the back of the perianth segments
5. Leaves glaucous, stem smooth, inflorescence raceme, 7–20 flowers per stem, perianth segments 15–20 mm, pale yellow, style glabrous, undivided (subgenus Theresia) – F. persica
5. Flowers usually solitary or 2–3 per stem (rarely up to 8–12), style papillose or bifid (subgenus Fritillaria)
6. Perianth broadly campanulate, the nectary at the point of inflection of the segment, ca. 5 mm above its base, style glabrous, usually divided for 1/4–1/2
7. Nectary ovate to lanceolate, less than half as long as the limb of the perianth segment (F. kotschyana group)
8. Perianth not or faintly tessellated, stem usually papillose at the ground level, leaves 7–10 times as long as wide – F. olivieri
8. Perianth heavily tessellated, stem glabrous, leaves 2–4 times as long as wide – F. kotschyana
7. Nectary linear, more than half as long as the limb of the perianth segment (F. crasssifolia group)
9. Stem with 2 leaves at the base of each pedicel – F. reuteri
9. Stem with one leaf at the base of each pedicle, or leaves in a whorl of three below the flower
10. Lower leaves usually trifoliate or opposite, upper usually trifoliate, perianth segments tessellated, without fascia – F. straussii
10. Tepal with warty cells or smooth, perianth segments with/without fascia/tessellation, obtuse or acute.
11. Tepal with warty cells, perianth segments with fascia and tessellated, obtuse – F. crassifolia ssp. kurdica
11. Tepal smooth, perianth segments without fascia and not tessellated, acute – F. poluninii
6. Perianth narrowly campanulate; the nectary at the base or up to 2 mm above the base of the perianth segment (F. caucasica group)
12. Style stout, undivided
13. Leaves usually 5–6 per stem, glaucous, linear, canaliculated; stem often papillose at ground level; style undivided; stigma clavate – F. assyriaca
13. Leaves 3–4 or 4–7 per stem, shiny green or glaucous, lanceolate or oblanceolate
14. Leaves usually 4 per stem, not galucous, lanceolate, flat; stem smooth – F. uva-vulpis
14. Leaves usually 4–7 per stem, galucous, oblanceolate – F. chlororhabdota
12. Style slender, sometimes 3-lobed at apex
15. Leaves 3–4; style 9–17 mm – F. caucasica
15. Leaves 4–10; style 5–10 mm
16. Leaves green, not glaucous; perianth segments usually green – F. chlorantha
16. Leaves usually glaucous
17. Perianth segments striped, without yellow tips – F. avromanica
17. Perianth segments purple with yellow tips, or yellow-greenish to green, nectary green-purplish or black
18. Tepal purple, apex thoroughly bright yellow, nectary green-purplish – F. zagrica
18.
Tepal yellow-greenish to green, nectary black – F. atrolineata
6.1. Subgenus Petilium (L.) BakerSubgenus Petilium comprises a small group of larger and sturdier species, distributed in Turkey, Iraq, Turkmenistan, Iran, Pakistan, Afghanistan, and the western Himalayas (Rønsted et al., 2005). They differ from the other subgenera of Fritillaria in the top half of the robust stem being leafless with a clear tuft of bract leaves above an umbel of 4–8 hanging flowers. The bulbs are much larger than those of most Fritillaria species are, and consist of a few, large, erect, imbricate, fleshy scales (Rønsted et al., 2005). The bulbs typically have a big hole in the top center where last year's stem grew, causing a considerable decrease in the density of the bulb. This subgenus is also characterized by having white to yellowish, broadly triangular-ovate to circular nectaries, which are positioned very close to the base of the perianth segments. The style is 3-fid and the capsules are winged (Rønsted et al., 2005). Two well-known Iranian species, F. imperialis and F. raddeana, are discussed here.
6.1.1. Fritillaria imperialis L.Crown imperial (Fritillaria imperialis L.) is known to be among the most widely grown species of the genus (Fig. 3). The predominant habitats of crown imperials in Iran are high elevations of the alpine Zagros region (Badfar-Chaleshtori et al., 2012) and neighboring areas (Table 3).

|
| Fig. 3 Left, red-and yellow-colored crown imperials naturally co-exist in the same habitats (photo by Hadi Jafari); Right, crown imperials surrounded by giant Rumex acetosella bushes (photo by Abbas Mohamadi). |
| Species | Distribution range | Habitat |
| F. imperialis & F. imperialis var. lutea | Golestan: Khoshyeylaq. West/East Azarbaijan. Kordestan: Marivan; Uramanat; Eight-Frit Mountain. Kermanshah: Sarmil. Lorestan: Dorud; Azna; Aligudarz; Ushtran-Kuh. Hamedan: Malayer. Ilam. C & B: Chelgerd; Fakhr Abad; Sabze Kooh; Ab-kaseh Khoriyeh Mt.; Garganak; Beno-estaki; Qalatak; Maleh Zardeh. Isfahan: Golpaygan (Hende); Alvand-e Khomeyn protected area; Kuh-e Darabshah; Khansar; Fereydunshahr; Fareydan; Booein; Miandasht; Afus; Dehaqan; Semirom; Shahreza; Darreh-Bid. K & B: Pol-e Qarah towards Yasuj; Sisakht; around Pataveh; Yasuj towards Nurabad-e Mamasani. Fars: Sedeh; Tang-e Abdui; east of Kazerun; west of Dasht-e Arjan; around Nurabad-e Mamasani. | Western elevations of the alpine Zagros region and neighboring areas; edge of fields, rocky slopes, stony places, scrub (1000–3000 m). |
| F. raddeana | Golestan: within and close to the Golestan National Park; Almeh valley near Gorgan; Cheshmehy-e Khan. Northern Khorassan: Khombi & Sara Mt. in Garmeh region; Cheshmey-e Eshgh; Dasht intersection; Bahar & Saluk Mt.; Palang & Bozdaghi Mt.; Jowzak and western sides of Aladagh Mt. in Maneh & Samalghan region; Takal-e Qouz Mt. in Raz & Jargalan region; Spidan village; Asadli highlands in Bojnourd region. | Sloping and/or rocky areas, and in dense evergreen forests covered by tangled shrubs and thorny bushes; from near sea level (around the coastal range of the Golestan province) to about 1000 m (throughout the Northern Khorassan province). |
| F. persica | Northwest through southwest Iran especially in East/West Azarbaijan: Urmia; Markazi: Sefidkhani Mountain; Kordestan: Sanandaj; Marivan; Sanandaj to Hamedan. Hamedan: Kabudar Ahang. Lorestan: Khorram Abad; Nurabad; Azna; Ushtran Kuh. Kordestan: Eight-Frit Mountain. Kermanshah: Sarmil; Kuh-e Nova. Ilam: Abdanan. C & B: Along the main road towards Chelgerd; across Koohrang crown imperial plain. K & B. Isfahan: Road between Damaneh-Khansar; Golestan-Kuh. Fars: Between Kazerun-Shiraz; Sepidan as far as Yasuj. | Mountainous oak woodlands; single and/or small colonies can be seen at the borders of roads, plains, hills and valleys, edge of fields, rocky slopes, stony places (1500–2000 m). |
Bulb: up to 10 cm diameter, globose-ovoid; tunica thin and papery, without bulbils or stolons. Stem: 50–100 cm, rarely up to 110 cm, erect, smooth, papillae absent. Leaves: 20–30(–50), glossy, sessile, lanceolate, only adorn the lower part of the stem, arranged in 3–4 whorls of 4–8, the lowest 7–18 × 5–10 cm; bract leaves 6–12 × 0.5–1.5 cm, in a group of 10–20. Flowers: 1–12 (usually 4–7), broadly campanulate, whorled; perianth segments 4–6 cm long, red to orange, rarely yellow, broadly lanceolate, acute, all alike. Nectaries: 5 mm diameter, circular, white, at the base of the perianth segment. Filaments: 25–45 mm, glabrous; anthers 8–12 mm after dehiscence. Style: 30–45 mm, 3-fid to 1–4 mm, papillose towards apex. Capsule: up to 20 mm long, 30 mm diameter, winged. Flowers April to May.
Distribution: Iraq, Turkey, across the plateau of Iran to Afghanistan, Pakistan and the western Himalayan foothills.
Flowers in the crown imperial (in Persian Taaj-e Khosro) are at the top of the stem, topped by a crown of small bract leaves, hence the name. In Iran, crown imperial is also commonly known under the local name of 'Ashk-e Maryam' meaning 'Tears of Mary' (Badfar-Chaleshtori et al., 2012), which refers to the drops of nectar (200–350 μl) that regularly appear at the petal base through the flowering stage, replaceable in less than a day upon extraction. It is also called 'Gel-e Begeriv' (Weepy flower) amongst local 'Lor' people of C & B and Kohgiluyeh & Boyerahmad (K & B) provinces, two provinces with a remarkable share of the distribution.
Although various colors ranging from nearly a true scarlet through orange–yellow are found in crown imperials, they are regularly classified as red-and yellow-colored forms (Alp and Koyuncu, 2009; Kiani, 2015). In comparison to the red-colored form, the yellow-colored (known as F. imperialis var. lutea) individuals are rare in Iran and are considered a critically endangered variety. These are two different forms of the species that naturally co-exist in the same habitats (Khourang et al., 2014). It is worth noting that the yellow form is not a hybrid (Alp and Koyuncu, 2009). Despite the color difference, they show a high level of morphological similarity. The color of the main upright stem is variable based on the flower color; the stem of the red-colored form normally ranges black to dark brownish/purplish, while bright green is the commonest stem color for the yellow-colored individuals (Kiani et al., 2015b).
Crown imperial is mostly an out-crossing species, morphologically best suited to be pollinated by birds; although it may be visited by a variety of insects (Burquez, 1989). Crown imperials usually release a distinctly foxy odor unpleasant to some people, caused by a single sulphurous terpene component identified as 3-methyl-2-butene-1-thiol (Helsper et al., 2006). The odoriferous nature of the bulbs, leaves, and flowers may have evolved as a way to repel herbivores. Like other members of Liliaceae, F. imperialis is susceptible to depredation by the lily beetle Lilioceris Reitter spp. (Salahi Ardakani, 2014).
Crown imperial is among a few species in the genus with the ability to form extraordinarily huge colonies. At least three huge crown imperial colonies are recognized in Iran; 1) Aligoodarz (Dalani) crown imperial plain (~2900 ha), 2) Khansar (Golestan-Kuh) crown imperial plain (~15 ha), and 3) Koohrang (Chelgerd) crown imperial plain (Fig. 12, left), among which the latter, occupying an area of over 3600 ha, is of high national/international importance to tourism.

|
| Fig. 12 Left, Koohrang crown imperial plain (photo by Hossein Hasanzadeh); Right, Eight-Frit Mountain (Photo by Robert B. Wallis). |
Crown imperials, including both red-and yellow-flowered forms, often grow in association with a variety of other species, namely Allium hirtifolium Boiss., Tulipa L. spp., Ixiolirion tataricum (Pall.) Schult. & Schult. f., Asperugo procumbens L., Astragalus brachybotrys Bunge, Astragalus angustiflorus K. Koch., Bromus tectorum L., Geranium persicum Schönb.-Tem., Prunus scoparia, Amygdalus elaeagnifolia Spach, Rumex acetocella, Kelussia odoratissima, Daphne mucronata, Euphorbia microsciadia Boiss., and Tragopogon graminifolius DC. Interestingly, in the Koohrang crown imperial plain, in which huge colonies of the species exist, Rumex acetosella is the primary co-occurring species, with very few others present (Kiani et al., 2015b).
6.1.2. Fritillaria raddeana Rgl.Gorgan lily (Fritillaria raddeana Rgl.) (Fig. 4, Right) is an endemic species native to the semi-humid climate condition and lower elevations of north and northeast Iran. The species is found scattered primarily in Golestan province (Table 3).

|
| Fig. 4 Left, F. persica (photo by Vendelbo, 1980); Right, F. raddeana, (photo by Robert B. Wallis). |
Bulb: up to 8 cm diameter, globose (4–5 × 4–5 cm), fleshy, without bulbils or stolons. Stem: 50–90 cm, smooth. Leaves: numerous, long, not glaucous, lanceolate or elliptic, acute-acuminate, sessile, only adorn the lower part of the stem, arranged in 3–4 loose whorls of 4–8; the lowest up to 7–18 × 5–10 cm, lanceolate, acuminate; bracts leaves, 6–12 × 0.5–2.0 cm, linear-lanceolate, in a group of 8–25, 1–2 per flower. Flowers: 3–10(–20), pendant, whorled, campanulate, pale yellow, green-tinged, rarely pinkish; each flower umbel is topped by an erect crest of bracts; perianth segments 3–3.67 cm, broadly lanceolate, acute, yellowish to greenish; the outer 1.2 cm wide, the inner 1.5 cm wide. Nectaries: 2 mm diameter, broadly ovate to almost circular, placed nearly at the base of the perianth segment and/or 1 mm above the base, slightly impressed at the base, green-yellowish. Filaments: 22 mm, glabrous; anthers 8 mm after dehiscence. Style: 25 mm, 3-fid for 2 mm, papillose towards apex. Capsule: proportionally large, 20–30 × 20–30 mm, 20–30 mm diameter, ovoid, winged. Flowers March to April.
Distribution: Iran, Turkmenistan.
F. raddeana closely resembles the yellow-colored form of crown imperials. F. raddeana is easily distinguished from them by the larger number of flowers in the umbel, the more narrowly campanulate flowers, and much smaller and more angular nectaries (Rix, 1977).
As with crown imperial, F. raddeana is a species with the ability to form huge populations in its normal range. Such colonies are primarily centered in the Khombi and Sara Mountains in Garmeh region (Northern Khorassan province), where at least three plains covered by F. raddeana occupy a total area of over 18 ha (Kiani, 2015).
6.2. Subgenus Theresia (K. Koch) BakerFritillaria persica is the sole member of the monotypic subgenus Theresia. The species has a bulb consisting of only one massive fleshy scale, second in size only to F. imperialis, and numerous flowers on a tall stem. The nectary color largely depends on that of the flower, so that in some populations the nectaries are not very noticeable. Given its striking appearance and geometry, the species can be easily distinguished from other species.
6.2.1. Fritillaria persica L.The distribution of Persian bells (Fritillaria persica L.) (Fig. 4, Left) across Iran is restricted to northwest through southwest areas (Table 3), relatively similar to those of F. imperialis, but at slightly lower elevations (1500–2000 m). In general, F. persica is a species of wider distribution, but of much lower number of plants in comparison to crown imperials (Kiani, 2015).
Bulb: up to 6 cm high, 3–5 cm diameter, solid, fleshy, ovoid or ellipsoid with/or without a tunica formed by the scarious remains of the scales of previous year(s). Stem: 60–120 cm, smooth. Leaves: 9–25, 15 × 3 cm, lanceolate, acute, all alternate. Flowers: 10–50, campanulate, rarely dark purple, usually greenish, gray or yellowish, usually yellowish inside; perianth segments 15–20 × 6–7 mm, oblanceolate, oblong-obovate and obtuse, outer and inner similar. Nectaries: triangular-narrowly ovate, slightly depressed, placed 0.5–1 mm above the base of the perianth segments, surrounding lobs or rims absent, often green-yellowish. Filaments: 5–6 mm, glabrous, at first pressed back against the perianth segment, coming forward at dehiscence; anthers 4 mm. Style: 6–8 mm, entire, slender, glabrous. Capsule: 1–3 cm long, 1.2 cm in diameter, winged. Flowers April to May.
Distribution: Iran, Turkey, Syria, Lebanon, Palestine, Jordan, Cyprus.
F. persica is very variable, especially in flower color, leaf width and in presence or absence of bract leaves in the inflorescence. A variety of flower colors from white to deep purple is known; indeed, pale flowers are in great abundance in Iran (Kiani, 2015). Three forms, F. eggeri, F. libanotica and F. arabica, have been described as separate species, but there is much overlap between them and none seem to merit recognition at even subspecific level (Rix, 1977).
Androdioecy is a rare mating system in plants and F. persica is one of a few plant taxa in which androdioecious reproduction is reported (Mancuso and Peruzzi, 2010). Stem diameter and number of flowers are believed to be higher in the hermaphrodite plants compared with the males (Mancuso and Peruzzi, 2010). Each flowering stem bears numerous basal leaves as well as a terminal pyramidal raceme composed by many small nodding flowers of the same sexual morphology; either male or hermaphrodite. Apparently, flowers open gradually from the bottom of the raceme upwards. Likewise, those species in the Central Asian clade (Fig. 2), have the flowers arranged in a raceme, and open their flowers from the bottom upwards (e.g. F. verticillata, F. olgae).
6.3. Subgenus Fritillaria (L.)Subgenus Fritillaria is the largest subgenus of the genus Fritillaria, comprising more than half of the species, including the type species, F. meleagris. The species in this subgenus are widely distributed from Western Europe and the Mediterranean region to eastern Asia. These are characterized by having the typical Fritillaria bulb; the bulb usually consists of 2 (sometimes 3–4 if one or both of the scales from the previous year have persisted into a second season) subglobose and fleshy scales with/or without a tunica, formed by the scarious remains of the scales of the previous year or years. The stem bears usually 1, sometimes 2–3 flowers, rarely up to 8 flowers as in F. olgae Vved. and F. camschatcensis or up to 12 flowers as in F. pluriflora Torr. ex Benth. (Khaniki and Persson, 1997). Subgenus Fritillaria is normally subdivided into two sections based on the style; species with a clearly 3-fid style are included in section Fritillaria, whereas species with an undivided style or a style that is only shortly trilobulate at the apex are placed in section Olostyleae (Rønsted et al., 2005). Rix (2001) subdivided section Olostyleae into 6 series and section Fritillaria into 10.
6.3.1. F. crassifolia groupThis group consists of a complex of species and subspecies close to Fritillaria crassifolia Boiss. & Reut., with a distribution centered in eastern Anatolia and northern Zagros. The main species in this group is F. crassifolia comprising three dwarf subspecies (Rix, 2000b), of which only ssp. kurdica has been recorded in Iran (Bakhshi Khaniki, 2001a; Wallis and Wallis, 2009). The most widespread member of the complex is F. crassifolia ssp. crassifolia, which occurs in Turkey. Subspecies kurdica is frequent in alpine steppe throughout northwest Iran (Azarbaijan), east Turkey and northeastern Iraq; ssp. hakkarensis is scattered in Turkey and northeastern Iraq. In addition to this typical species, a) F. reuteri, b) F. straussii (Kiani et al., 2015a), and c) F. poluninii (Wallis and Wallis, 2009) are three morphologically distinct species of this group which occur in Iran. They all have usually broadly campanulate flowers pendant at maturity, and rather large linear nectaries, 5–10 × 1.5–2 mm (half or more as long as the perianth segments, placed 3–5 mm above the base), which are usually black-purplish at the base. All members of this complex, except F. poluninii and F. reuteri, have groups of warts or similar processes scattered on the surface of the tepal (Khaniki and Persson, 1997).
Fritillaria crassifolia ssp. kurdica (Boiss. & Noe) Rix (Fig. 5, Left) is a common plant from Zagros Mountains mainly reported along a relatively narrow border strip, bordering Turkey and Iraq in West and Northwest Iran (Rix, 1977).

|
| Fig. 5 Left, F. crassifolia ssp. kurdica; Right, F. poluninii (photos by Robert B. Wallis). |
Bulb: up to 3 cm in diameter, sometimes with bulbils. Stem: up to 10 cm high. Leaves: 4 (5–7), glaucous, lanceolate or linear lanceolate, all alternate; the lowest is wider than the rest, 3–5 cm long, 6–15 mm wide. Flowers: 1–2 (-4), campanulate, pendant at maturity, yellowish or green, stippled and tessellated inside and out, sometimes on a greenish or yellowish background, with a green stripe along the center of each perianth segment; perianth segments, obovate to oblanceolate, obtuse. Nectaries: linear, about 8–10 × 1.5–2 mm, usually with a raised ridges on each side, more blackish at the base. Capsule: 4–4.5 cm long, obvoid, narrowly tapering at base, truncate at apex, not winged. Flowers April to July.
Distribution: Iran, Turkey, Iraq.
The species is rather diminutive, very variable especially in overall size and flower color, ranging from greenish, brownish to yellowish (or combinations thereof), often heavily tessellated, brown or purple inside and outside, often with green fascia. Subspecies kurdica is distinguishable from ssp. crassifolia in relatively very narrower basal leaves that are also higher in number; subspecies crassifolia usually has only 4 leaves, whereas ssp. kurdica bears 5–6 (Rix, 1977). It also differs in its more clearly defined fascia and the raised ridge on the inner side of the perianth segment, which forms the nectary. On the other hand, F. poluninii differs from F. crassifolia ssp. kurdica mainly in narrower perianth segments, smaller flowers and absence of the swollen nectary ridge.
Fritillaria poluninii (Rix) Bakhshi Khaniki & K. Persson (Fig. 5, Right) is the rarest and smallest of four closely related species or subspecies, being recognized by its short stem (Rix, 2006).
Bulb: large for the size of the top growth, up to 2 cm in diameter, without bulbils and stolons. Stem: 4–8 cm, smooth. Leaves: 6–8, usually 6, the lowest narrowly lanceolate 4–7 × 8–10 mm, usually 5–7 times as long as wide, all alternate or subopposite, shiny green. Flowers: usually 1 or 2, rarely 3, nodding or horizontal, broadly campanulate or cup-shaped, pale purple to greyish, not or vaguely tessellated but lined with wavy green or brownish-purple veins, without fascia; tepals smooth, entirely lacking warty cells or other processes, usually spreading and not overlapping, acute, 11.5–14 mm long, the outer 4 mm wide narrowly ovate, the inner slightly wider, obovate. Nectaries: green, linear, 3.5–5 mm long, 1–1.5 mm wide, 3–5 mm above the tepal base. Filaments: ca. 6 mm, sparsely papillose. Style: 4–5 mm, the branches 2–3 mm, reflexed, slender, smooth. Capsule: ca. 35 mm long, cylindrical, tapering towards the base, not winged. Flowers April to May.
Distribution: West Iran and adjacent part of northeastern Iraq in Sulaimaniyah.
Fritillaria poluninii was previously treated as a subspecies of F. crassifolia, but later Khaniki and Persson (1997) raised this to the rank of species, because of several distinctive characteristics. It is differentiated from the other members of the F. crassifolia complex in the narrower leaves, smooth tepal surface without warty cells, much shorter/narrower nectaries, the absence of the swollen nectary ridge and the absence of a dark spot at the base of the nectaries. As opposed to the other subspecies of F. crassifolia that have more oblanceolate tepals, in F. poluninii tepals are usually acute. The flowers are not tessellated (sometimes vaguely tessellated), but lined with green or brownish-purple veins; the veins are distinctly wavy, and can be confused with tessellation. Given these striking features, elevating F. crassifolia ssp. poluninii to a specific level is fully justified.
Frtillaria straussii Bornm (Fig. 6, Left). is a species restricted to northern Zagros Mountains (Rix, 1974).

|
| Fig. 6 Left, F. straussii; Right, F. reuteri (photos by Robert B. Wallis). |
Bulb: up to 2.5 cm in diameter, bulbils or stolons unknown. Stem: up to 30 cm, papillae absent. Leaves: 5–10, not glaucous; the lower 40–80 × 10–35 mm, broadly lanceolate to ovate, opposite or in a whorl of 3; the middle usually opposite; the upper up to 5 cm, linear, in a whorl of three or opposite. Flowers: 1–2 (-4), broadly campanulate; perianth segments greenish when young, often maturing to dark reddish-brown, tessellated all over, fascia obscure, oblanceolate, obtuse, the outer 25–27 × 8–9 mm, the inner 9–12 mm wide, obtuse. Nectaries: 10 × 2 mm, linear, greenish, ca. 5 mm above the base of the segment, distinctly depressed at the base. Filaments: ca. 10 mm, slender, papillose; anthers 3–4 mm after dehiscence. Style: ca. 8 mm, papillose, 3-fid to half or 3/4 of its length; style arms slender, reflexed. Capsule: 3–4.5 × 2.5 cm, obvoid, tapering towards the base, not winged. Flowers May to June.
Distribution: Endemic to Iran.
F. straussii is unique in that the flowers open green, and then mature to dark brown until before fading (Khaniki, 2002d). This is also unlike other species in its combination of a linear nectary and leaves opposite or in whorls (Rix, 1977).
Fritillaria reuteri Boiss (Fig. 6, Right). is restricted to the mountains north and west of Esfahan (east of Shahr-e Kord) (Khaniki, 2002c), where it occurs in a few places, but in great quantities (Rix, 1977).
Bulb: up to 2 cm in diameter, without bulbil or stolons. Stem: 15–25 cm, papillae absent. Leaves: 3–8, all alternate; the lower 6–10 cm, linear lanceolate, at the base of the stem; the upper 3–7 cm, linear, 2 at the base of each pedicle, the intervening stem usually leafless. Flowers: 1–8, usually 2 broadly campanulate, nodding at maturity; perianth segments reddish-purple with a glaucous bloom, always with distinctive yellow tips, and often yellow inside, not tessellated; the outer 16–28 × 5–8 mm, oblanceolate, acute; the inner 8–13 mm wide, often obtuse. Nectaries: ca. 10 mm, linear, green, slightly raised and placed above the tepal base. Filaments: ca. 10 mm, slender, sparsely papillose; anthers 8 mm after dehiscence. Style: 1.3 cm, minutely papillose, 3-fid for 3 mm. Capsule: 35–45 mm long, cylindrical-obvoid, tapering towards the base, not winged. Flowers May to June.
Distribution: Endemic to Iran.
A pure yellow-flowered form has been recorded on several occasions, but otherwise the species is relatively invariable (Rix, 1977).
6.3.2. Fritillaria kotschyana groupIn Fritillaria kotschyana group flowers are nodding and broadly campanulate (Khaniki, 2002b). The nectary is ovate or lanceolate, and always shorter than half the length of the limb of the perianth segment, located at the inflection of the bell (Khaniki and Persson, 1997; Rix, 1977). Two species of this group are restricted to small areas of high elevations; 1) F. olivieri Baker is found in the northern Zagros Range, while 2) F. whittallii Baker is scattered in southwest Anatolia; 3) F. kotschyana is very variable in color (ranging from pure green with little tessellation to brown) and flower size.
Fritillaria kotschyana ssp. kotschyana has been found mainly along upper slopes of the Elburz and Talysh Mountains as well as at the southern end of the Talysh, as far south as Khalkhal, where it inhabits a variety of habitats including sparse meadowland, unstable screes, rock crevices and among roots of small shrubs (Wallis and Wallis, 2009). Khourang et al. (2014) also reported this subspecies in meadows and sloping areas near Mianeh (East Azarbaijan); F. kotschyana ssp. grandiflora is only collected from the northern Talysh; 4) F. hermonis is only found in S. Syria and Lebanon (Lebanon, Chouf and Antilebanon ranges), while 5) F. amana, which was previously treated as a subspecies of F. hermonis, is limited to Turkey and NW Syria (Amanus and Nusairiah Ranges).
F. kotschyana ssp. kotschyana (Fig. 7, Left) is primarily scattered along the Elburz Mountains (Table 4) (Wallis and Wallis, 2009).

|
| Fig. 7 Left, F. kotschyana ssp. kotschyana; Center, F. kotschyana ssp. grandiflora (photos by Marijn Van den Brink); Right, F. olivieri (photo by Robert B. Wallis). |
| Species/Subspecies | Distribution range | Habitat |
| F. crassifolia ssp. kurdica | Azarbaijan: near Baku south to Kordestan near Marivan; Marand, Khoi to Tabriz road; north of Urmia; Kuh-e Sahand; S. foothills of Kuh-e Savalan; W. of Ardabil, pass between Ahar to Tabriz; SW. of Ahar; Bozqush. Kordestan: As far south as Sanandaj extending inland as far as Zanjan; between Marivan and Sanandaj; Dezli | Cliffs and stony banks along rivers, rocky plateau, slopes and ledges or sometimes on screes (but not the loose mobile screes that are the characteristic habitat of ssp. crassifolia); foothills and mountain steppes (1500–3500 m). |
| F. poluninii | West Azarbaijan: Mah Mt.; Qasemlu valley, near Urmia; a pass between Daraki and Nowsud; Hawraman Mt. Kermanshah: Eight-Frit Mountain. Kordestan: Sarv Abad; Seline village; Bandul village; Kale Tang | Limestone screes and grows well in deep soils by late snow patches at high altitudes (2200–2600 m). |
| F. reuteri | C & B: Cheshmeh Kuhrang, Sabze Kuh, Shahr-e Kord valley at foot of Zard Kuh. Esfahan: west of Esfahan, Chehel-Dokhtran Kuh, north-west Daran towards Arak, near Golestan Kuh (south of Khansar). | Boggy banks, wet rocky meadows and mountainous or hilly stony terrains (2500–3000 m) |
| F. straussii | Kermanshah: Kuh-e Sefid; Eight-Frit Mountain. Kordestan: Marivan; Dezli, all the way along the border with Iraq. | Shady places, rangelands of heavy soils and/or loose slopes dominated by Quercus spp. and large Apiaceae (1500–2100 m) |
| F. kotschyana ssp. kotschyana | Tehran: Mt. Alborz; Dizin pass. Guilan: Talysh; Massuleh; Rasht. Mazandaran: Chalus; Gachsar; Kandovan pass; Haraz valley. Golestan. E. Azarbayjan: Mianeh; Bozqush. Ardabil: Khalkhal. Kordestan: Saral | Stony soils often under bushes, rocky slopes and screes (2000–4000 m). |
| F. kotschyana ssp. grandiflora | Along Mt. Alborz scattered in Guilan: Talysh; Massuleh. Golestan: NW. side of the Jahan Nama protected area. | The same as F. kotschyana ssp. kotschyana (about 2200 m). |
| F. olivieri | Hamedan: Mt. Alvand; Ganjnameh; SW. of Hamedan. Kordestan: E. of Sanandaj. Lorestan: Khorramabad; Dorud. E. Azarbayjan: Tabriz | Damp meadows (2000–4000 m). |
| F. caucasica | East Azarbaijan: Tabriz; near Ahar | Subalpine meadows, stony areas of coniferous/oak forests or closed grasslands, grassy slopes, and shady, moist, peaty rock ledges on limestone in drier areas (1700–2900 m). |
| F. assyriaca | Throughout Zagros in W. Azarbaijan: Urmia; Sir kuh. Kermanshah: Eight-Frit Mountain. Kordestan: Sanandaj towards Hamedan. Lorestan: Khorram Abad; Azna; Dorud; Oshtoran Kuh. Fars: Kazerun to Behbahan; valley of Sepidan; Shiraz. Tehran. | Cultivated fields, mountain steppe, rocky slopes and stony hillsides/grounds (1000–2500 m). It is usually found in open habitats and has normal growth either in hot and dry or cool conditions. |
| F. uva-vulpis | Restricted to W. and NW. Iran: W. Azarbaijan: Urmia, 20 km SE. of Mahabad. Kordestan: Eight-Frit Mountain; Mahabad. | Zagrosian Quercus forest zone: crop fields and damp or marshy meadows (1000–2500 m). |
| F. avromanica | Kordestan: Marivan to Paveh (Hawraman area), Seline village; Abdalan Mt., 30 km to Sanandaj; Eight-Frit Mountain. | Rocky terrains and gravelly lands (1300–2100 m). |
| F. atrolineata | One locality in W. Azarbaijan province | Moist areas below cliffs (1500–1800 m). |
| F. chlorantha | Kordestan: Marivan. Hamedan: Alvand. Lorestan: Khorramabad, Gahar, Ushtran Kuh. | Mountain steppe among stones, and rocky and stony hillsides (1800–2000 m). |
| F. zagrica | From south Urmia Lake all the way down along Zagros chains: W. Azarbaijan: Takht-e Soleyman. Markazi: Arak to Golpaygan, 40 km, SE. of Arak; near Arak in Soltanabad village. Hamedan: Alvand Mt., Hamedan to Qazvin, 32 km from Hamedan; Asad Abad pass 23 km W. of Hamedan; Khorram Abad, 40 km S. of Arak. Lorestan: 23 km NE. of Azna. Kordestan: Salavat Abad valley, along the main road towards Sanandaj; Sanandaj to Hamedan, 12 km E. of Sanandaj. Esfahan: Kuh-e Darreh; Golpayegan to Damaneh, Kuh-e Darrebid; Khansar crown imperial plain | Mountain steppe, often by late snow patches (1800–3000 m). |
| F. chlororhabdota | W. Azarbaijan: Urmia-Salmas; Akhordarreh; Sir Kuh. Kordestan: Sanandaj, Salavat Abad valley. Lorestan: Azna; Dorud; Oshtoran Kuh; Khorram Abad | Widespread across Zagros ranges on grassy slopes, sometimes clumps, mountain steppes, stony hills, alpine pastures, rocky and grassy ledges, mainly associated with Euphorbia, Geranium, Thymus, Tulipa, Anthemis, Gagea, Stipa barbata, and grasses (1600–2000). |
| F. gibbosa | Widespread from northeast through northwest, east, center and southwest of Iran, along Zagros chain in W. Azarbaijan: Urmia; Khoi. E. Azarbaijan: Tabriz, 70 km NW Zanjan. Alborz: Karaj elevations. Tehran: Alborz, W side of the road from Tehran-Qom. Qazvin: Between Qazvin-Rasht. Markazi; SE. of Arak; Arak-Golpaygan. Semnan: Miami. Hamedan: NE. of Hamedan, 100 km north of Hamedan; Razan. Kordestan: 60 km east of Bijar. Lorestan: Khorram Abad; SW. of Dorud. C & B: Tangeye Sayyad. Razavi Khorassan: 17 km north of Torbate Heydarieh, on the road to Mashhad; 24 km north of Quchan; Kopet Daq; Between Kashmar and Rivash; Kuh-e Sorkh. Northern Khorassan: Around west of Bojnurd; near Almeh valley. Southern Khorassan. Fars: Niriz; near Marvdasht; Persepolis. Golestan: Gorgan, at the eastern end of the Caspian Sea | Adapted to arid and hot climates; usually associated with Artemisia; open stony or sandy places, steppes, scrubs, rocky screes (1000–2000 m). |
| F. ariana | Confined to east and northeastern Iran in the parts of Razavi Khorassan province, adjacent to the border of Afghanistan: Mash'had; Hari Rud valley, on the Iranian side of frontier; near Sarakhs as far south as Taybad. | Deserted areas, on mobile sand dunes and sandy hills and plains, with a mixture of small annuals, which usually flower and set seed very quickly before the arrival of the dry summer (500–1000 m). |
Bulb: up to 3 cm in diameter, bulbils and stolons sometimes present. Stem: 8–25 cm, papillae absent. Leaves: 4–8, usually 5–6, green glaucous, all alternate; the lowest 7–10 cm, ovate to broadly lanceolate, 2–4 times as long as wide, the uppermost lanceolate, 1–3.5 cm long. Flowers: 1–2, brown-purplish or wholly green, usually on a green background, pendant, broadly campanulate, tessellated, inside and out, usually with green fascia; perianth segments 2.8–4.8 cm, the outer perianth segments 2.5–3.5 × 1.5–2 cm, broadly oblanceolate and obtuse, the inner somewhat wider. Nectaries: ovate to ovate-triangular, ca. 5 mm in diameter, green and then mature to basally dark purplish, at the point of inflection of the bell. Filaments: 7–9 mm, papillose or rarely glabrous; anthers ca. 5 mm after dehiscence. Style: 7–9 mm, 3-fid for 1/4 to 1/2 its length. Capsule: 16–22 mm long, ovoid, tapering towards the base, not winged. Flowers April to July.
Distribution: Endemic to Iran.
Fritilaria kotschyana ssp. grandiflora (Fig. 7, Center) differs from ssp. kotschyana mainly in narrower leaves that resembles those of F. olivieri in shape and size, absence of fascia, narrower and longer nectaries (Khaniki and Persson, 1997), and size difference (Rix, 1977); larger specimens in ssp. kotschyana approach ssp. grandiflora in size.
Bulb: up to 3 cm in diameter, bulbils and stolons unknown. Stem: 30–50 cm. Leaves: 5–10, usually 7–8, lanceolate, all alternate; the lowest 10–23 mm wide, 6–8 times as long as wide. Flowers: 1–3 broadly campanulate, dark purplish or green, obscurely tessellated, without fascia; perianth segments 3.5–5.2 cm, oblong, triangular acute at apex; the outer 1–2 cm, usually oblong sometimes acute; the inner 2–3 cm wide. Nectaries: 7 × 2 mm, ovate-oblong, all over black-purple. Filaments: ca. 10 mm, papillose; anthers 6–7 mm. Style: ca. 10 mm, 3-fid for 1/10 to 1/2. Capsule: not known. Flowers April to May.
Distribution: Endemic to Iran.
Fritillaria olivieri Baker (Fig. 7, Right) is almost restricted to the area around Hamedan, being frequent on Mt. Alvand, where it grows in damp ground by streams (Table 4).
Bulb: up to 3 cm in diameter, solid, button-shaped, with few bulbils. Stem: 15–40 cm, sparsely papillose around ground level. Leaves: 5–10, usually 6–8, all alternate, narrowly lanceolate or oblong, acute, the lowest 7–14 cm long, 5–10 times as long as wide; the uppermost 3–6 cm, linear, usually not glaucous. Flowers: 1(–3), broadly campanulate; perianth segments 25–35 mm, green, pale yellow-green, shaded reddish-brown, untessellated (sometimes lightly tessellated), not tuberculate; the outer ca. 12 mm wide, obovate, obtuse; the inner somewhat wider. Nectaries: ca. 5 mm, dark green, narrowly-acutely lanceolate to linear-lanceolate; 7–9 mm above the base of segment. Filaments: ca. 12 mm, sparsely papillose; anthers ca. 5 mm. Style: 7–9 mm, 3-fid for 1/3 to 1/4, glabrous. Capsule: 4 cm long, obvoid, narrowly tapering towards the base, not winged. Flowers May to June.
Distribution: Endemic to Iran.
6.3.3. Fritillaria caucasica groupFritillaria caucasica is the most diverse group within subgenus Fritillaria in Iran. All Iranian species belonging to F. caucasica group i.e., F. caucasica, F. chlorantha, F. zagrica, F. uva-vulpis, F. assyriaca, F. chlororhabdota, Fritillaria atrolineata and F. avromanica, are distributed along the Zagros chain (Bakhshi Khaniki, 1997a, 1997b; Rechinger, 1990; Rix, 1977). Within this group, the species have comparatively small and narrowly campanulate, narrowly lanceolate flowers, without tessellation. The small nectaries (not or only slightly depressed) are placed 0.5–1 mm above the base of tepals (Khaniki and Persson, 1997). Some species of this group e.g., F. atrolineata, F. armena, F. zagrica and F. chlorantha, have warts or warty cells scattered on the tepal surface.
Caucasian lily (Fritillaria caucasica Adam) (Fig. 8, Left) has only been found in two localities in northwestern Iran. The localities in Iran represent the southernmost extension of its range (Table 4). It differs from other Iranian species in its longer slender style and filaments.

|
| Fig. 8 Left, Caucasian lily (F. caucasica); Center, F. assyriaca; Right, F. cf. uva-vulpis (photos by Robert B. Wallis). |
Bulb: up to 2 cm in diameter, usually without bulbils, stolon unknown. Stem: 10–30 cm, papillae absent. Leaves: 3–4(-6), all alternate, broadly lanceolate to lanceolate, acute, the lowest 3–10 × 0.8–2.0 cm. Flowers: 1(-2), narrowly campanulate, sometimes constricted at mouth, somewhat reflexed at apex; perianth segments 22–30 mm, dark purplish-black, usually with a waxy bloom outside, sometimes paler inside, not tessellated; the outer 5–12 mm wide, usually acute; the inner 6–14 mm wide, acute or obtuse. Nectaries: 4.5–5 × 1 mm, linear-lanceolate to lanceolate, at the base of the segment, dark greenish. Filaments: 10–18 mm, slender, glabrous or papillose; anthers 4–8 mm. Style: 9–17 mm, slender, undivided, glabrous or papillose. Capsule: 4–6 cm long, cylindrical, tapering towards the base, not winged. Flowers April to June.
Distribution: Iran, southern Caucasus, northeast Turkey.
Fritillaria assyriaca Baker (Fig. 8, Center) is one of the most widespread (Table 4) and variable species in the genus and seems to have a quite diverse appearance in Iran (Wallis and Wallis, 2009).
Bulb: up to 3 cm in diameter, often with stolons or bulbils. Stem: 4–20 cm (elongating to 35 cm in fruit), often papillose near the base. Leaves: 4–6(-12), the lowest, 3–9 × 0.3–1.9 cm, sometimes opposite, ovate-lanceolate, the rest shorter, alternate, usually channeled, especially when young, linear, glaucous. Flowers: 1–2(-5), narrowly campanulate; perianth segments very variable in color, greenish, reddish or purplish brown, often with green fascia, yellowish inside, sometimes reflexed towards the tip; the outer 15–25 × 4–5 mm, narrowly oblong; the inner, 5–10 mm wide, usually obtuse. Nectaries: 2–4 × 1 mm, linear-lanceolate, ca. 1 mm above the base of the segment. Filaments: 5–9 mm, swollen, papillose; anthers 4–6 mm. Style: 5–10 mm, usually 7–8 mm long, 1.5–2 mm in diameter, not or slightly lobed at apex. Capsule: ca. 26 mm long, cylindrical, not winged. Flowers March to May.
Distribution: Iran, Iraq, Turkey.
The name 'Fox's grape' was first invented by Rix (2000a) after the folkloric Kurdish name "Tarai Raiwi" for Fritillaria uva-vupis Rix (Fig. 8, Right). F. uva-vupis has been one of the most successful species in cultivation, because they produce many bulbils and grow vigorously (Rix, 2000a).
Bulb: up to 3 cm in diameter, usually with bulbils, without stolons. Stem: 10–20 cm, papillae absent. Leaves: 3–5, usually 4, shiny green, all alternate, the lowest 8–12 × 1–2 cm, narrowly lanceolate, acute, the upper ones smaller. Flowers: 1 (rarely 2), narrowly campanulate, usually rounded, narrowed at the mouth; perianth segments outside dusky purplish-gray, glaucous, with yellow tips outside, inside yellowish, obtuse, the outer 20–28 × 6–7 mm, ovate or ovate-lanceolate, obtuse or acute, the inner 6–12 mm wide, obovate, obtuse. Nectaries: 4 × 1.3 mm, narrowly ovate, 1 mm above the base of the segment, green. Filaments: 6–9 mm, papillose, swollen; anthers 7–9 mm before dehiscence. Style: 5–7 mm, thick, papillose, undivided. Capsule: ca. 35 mm long, obvoid, narrowly tapering towards the base, truncate at apex, not winged. Flowers March to April.
Distribution: Iran, Turkey, Iraq.
F. uva-vulpis is morphologically distinct; it has usually four flat, lanceolate, shiny green leaves spaced evenly up the stem; the radical leaves are narrowly lanceolate. F. assyriaca is widespread and very variable, especially in width of the leaf; it has 4–6 glaucous, narrow and canaliculate leaves, mainly placed on the upper half of the stem; the radical leaves are broadly ovate. F. assyriaca also has narrowly tubular-campanulate flowers with a more greenish tinge and a slightly flaring mouth, while the flowers of F. uva-vulpis are usually more rounded.
Zagrosian lily (Fritillaria zagrica Stapf.) (Fig. 9, Left) is a comparatively dwarf species, native to the Zagros range (Table 4) (Bakhshi Khaniki, 2001b) where it grows as a snowmelt species at very high altitudes (1800–3000 m).

|
| Fig. 9 Left, F. zagrica; Right, F. atrolineata (photos by Robert B. Wallis). |
Bulb: up to 2 cm in diameter, ovoid, sometimes with few bulbils, without stolons. Stem: 4–10 cm, rarely over 10 cm, papillae sometimes present, lowest leaves with a wavy edge. Leaves: 4–7, usually 5–6, smooth, somewhat glaucous, all alternate or the lowest sub-opposite, lanceolate, 3–9 × 0.6–1.6 cm, the lowest narrowly oblanceolate to elliptic-oblong, 5–11 × 1.5–2 cm, usually folded at fruiting time, the uppermost, linear-narrowly lanceolate, 2–5 cm long, acute. Flowers: 1–2(-3), narrowly campanulate, with few warts on the tepal surface; perianth segments 12–18 mm, outside and inside dark purplish-brown with a yellowish tip, and a waxy bloom outside, the outer segments, 12–18 × 4–7 mm, elliptic-lanceolate, acute, the inner 6.5–8 mm wide, lanceolate, obtuse. Nectaries: 3.5–5 × 1–1.5 mm, broadly lanceolate to narrowly elliptic, 1 mm above the base of the segments, green-purplish. Filaments: 8 mm, somewhat swollen, papillose; anthers 3–4 mm. Style: 8–9 mm, slender, papillose or subglobrous. Capsule: cylindrical, 2.5–3.5 × 1.5–5 cm, obvoid, obtuse, tapering towards the base, not winged. Flowers March to May.
Distribution: Endemic to Iran.
Tekşen and Aytaç (2011), in a revision of the genus in Turkey, lumped Fritillaria zagrica with F. pinardii, but they are quite distinct. F. zagrica normally has the little yellow tips to the segments, while F. pinardii sometimes has a yellow distal margin, but if present, this always is a continuous margin on the distal edge. On the other hand, the F. zagrica that Tekşen and Aytaç used as a comparator is not the type specimen, but one from Turkey that is a significant outlier from the normal range of F. zagrica.
Fritillaria atrolineata Bakhshi Khaniki (Fig. 9, Right) has been reported only from one locality in West Azarbaijan province (Table 4) by Bakhshi Khaniki (1997a).
Bulb: 7–11 × 7–10 mm, globose-subglobose, without bulbils or stolons. Stem: 20–25 cm above the bulb, longer in fruit, smooth. Leaves: 4–5, glaucous, the lowest 2–3 usually in a subternate (subverticillate) or subopposite position, the rest alternate, the lowest 9 × 1–1.5 cm, usually 8–9 times as long as wide, oblanceolate, the uppermost (bract leaves) 8.5 × 0.3 cm, linear to narrowly oblanceolate, acute. Flower: 1 (2), narrowly campanulate; perianth segments usually yellowish-green to green, outside more yellowish towards margins, sometimes sparsely brown-dotted, inside tinged or sparsely dotted, pale reddish-brown towards margins, outer segments 20 × 6 mm, oblanceolate, obtuse, inner ones 8 mm wide, oblanceolate, obtuse, fascia obscure. Nectaries: linear, 4–6 × 0.5-l mm, placed 0.5-l mm above the base of tepals, black. Filaments: 7 mm, yellow, densely papillose in upper part. Style: 5–7 mm, stout, greenish-yellow, densely papillose, entire or very slightly 3-lobed, apex of stigma lobes papillose and with a central hole. Capsule: 2.5–3 × 1.5 cm, obovoid, obtuse, tapering towards the base, not winged. Flowers April.
Distribution: Endemic to Iran.
In the type description, Bakhshi Khaniki (1997a) referred to the black nectaries of Fritillaria atrolineata, hence the name. Bakhshi Khaniki classified the species in subgenus Fritillaria (F. caucasica group) based on its comparatively small overall size, geometry, position and color of the nectaries as well as narrowly campanulate flowers.
Fritillaria chlorantha Hausskn. & Bornm (Fig. 10, Left). is a dwarf species confined to Zagros Chains (Table 4) (Rix, 1977).

|
| Fig. 10 Left, F. chlorantha (photo by Robert B. Wallis); Right, F. avromanica (photo by Mahfuz Advay). |
Bulb: up to 2 cm in diameter, bulbils few, without stolons. Stem: 4–10 cm, papillae absent. Leaves: 4–10, usually 5, broad, shiny green, not glaucous, all alternate, the lowest ovate broadly lanceolate, 6–10 × 1.2–5 cm, the upper linear-lanceolate ca. 5 cm long. Flowers: 1–2, narrowly campanulate; perianth segments green outside, yellowish sometimes with purple markings inside and on the inner segments, rarely purple outside, the outer 15–25 × 3–4 mm, the inner somewhat wider. Nectaries: 3 × 1 mm, lanceolate, 1 mm above the base of the segment, green. Filaments: 6–7 mm, swollen, densely papillose; anthers 6–7 mm. Style: 5–10 mm, usually 8 mm, rather stout, 3-fid at apex for 1–2 mm. Capsule: cylindrical, not winged. Flowers April to May.
Fritillaria chlorantha seems to be related to F. atrolineata, primarily on account of the flower color. There are, however, striking differences separating them: F. atrolineata has glaucous, long and narrowly oblanceolate leaves with the lowest ones sometimes two or three in subopposite or subverticillate (subternate) position; a comparatively long stem; small, narrowly campanulate flowers; dark, elongate linear nectaries, and entire styles. The foleolate exine of pollen grains, and slightly trilobulate styles are also typical of the species. By contrast, F. chlorantha has short stems; short, wide, shiny green leaves, all alternate; comparatively big tubular flowers; green, lanceolate nectaries, and styles 3-fid at apex (Bakhshi Khaniki, 1997a; Khaniki, 2002a).
Avroman lily (Fritillaria avromanica M. Advay & M. Teksen) (Fig. 10, Right) has lately been reported only from Hawraman (also spelled Avroman) area in west Iran (Table 4) (Advay et al., 2015). It forms small, few-numbered populations of less than twenty individuals.
Bulb: up to 2.5 cm in diameter, globose, with 1–3 bulblets; tunica yellowish, thin, papery. Stem: erect, 7–20 cm, smooth. Leaves: 3–6(-9), sessile, glaucous, the lowest 3.4–11.0 × 2.0–4.5 cm, subopposite or alternate, ovate, obovate or lanceolate, acute, median leaves 2.5–7.5 × 0.4–3.0 cm, subopposite or alternate, obovate-linear, acute; bract leaves 2–3(-6), 1.5–3.9 × 0.1–0.5 cm, subopposite or alternate, linear, acuminate. Flowers: 1–3, narrowly campanulate; perianth segments purplish-brown with green or yellow stripes, outer 13.6–28.2 × 3.3–6.8 mm, lanceolate, acute, ciliate-tufted at apex, inner 17.9–20.0 × 4.5–6.5 mm, oblanceolate, obtuse, ciliate-tufted at apex. Nectaries: 3.0–3.8 × 1.5 mm, linear, linear-lanceolate, placed 1 mm above the tepal base, green. Filaments: 5.2–7.0 mm, papillose, enlarged to base, yellow; anthers 5.2–8.4 mm, oblong, apiculate, yellow during early anthesis but then blackish, basifixed. Style: 5.7–9.0 mm, 3-lobed, papillose. Capsule: 25–47 × 15–21 mm, cylindrical, truncate at apex, cunate at base, not winged. Flowers February to March.
Distribution: Endemic to Iran.
Advay et al. (2015) argued that Fritillaria avromanica and F. assyriaca are closely related based on floral traits, but these species clearly show very little morphological resemblance since the leaves are a completely different shape. Robert B. Wallis believes F. avromanica is much more similar to F. chlorantha, particularly in its ovate lanceolate lower leaves, and the former may just be a variant of the latter species (Personal communication). However, they are distinct in flower color and to some degree in leaf texture/arrangement. F. chlorantha is relatively invariable, but some collections have perianth segments marked with purple, which may be a sign of hybridization with F. zagrica or F. assyriaca, both of which occur in the same area (Rix, 1977). On the other hand, the patterned coloration of the petals in F. avromanica do not seem to be a sign of variation or random hybridization in F. chlorantha. As in the case of taxonomic status of Rhinopetalum (See Section 4), molecular phylogenetic studies should help shed light on this challenging issue.
The species is mostly accompanied by Bongardia chrysogonum Boiss., Ferula haussknechtii H. Wolff ex Rech.f., Smyrniopsis aucheri Boiss., Muscari neglectum Ten., Gagea graminifolia Vved., Anemone L. spp. and Astragalus L. spp. (Advay et al., 2015).
Fritillaria chlororhabdota Bakhshi Khan. (Fig. 11, Left) was reported in Iran for the first time by Bakhshi Khaniki (1997b), and all information available on this species is restricted to this report (Table 4).

|
| Fig. 11 Left, F. chlororhabdota (photo by Mahfuz Advay); Center, F. gibbosa (Photo by Mehdi Zarrei); Right, F. ariana (Photo by Paul Christian). |
Bulb: 1.5 cm in diameter, ovoid, without bulbils or stolons. Stem: 15–51 cm, 12–3-(–45) cm above ground, smooth. Leaves: 4–7, somewhat glaucous, all alternate, the lowest 5–11 × 1.5–2 cm, narrowly oblanceolate to elliptic-oblong, usually folded at fruiting time; bract leaves 2–5 cm long, linear to narrowly lanceolate, acute. Flowers: 1–2, narrowly campanulate; perianth segments purplish outside, paler inside with median yellowish-green bands extending to apices, sometimes tinged or sparsely green-dotted towards margins, outer segments 12 × 16–46 mm, elliptic-narrowly oblong, obtuse, inner segments 6.5–8 mm wide, lanceolate obtuse. Nectaries: broadly lanceolate to narrowly elliptic, 2.5–4 mm long placed 0.5–1 mm above base of tepals, green. Filaments: 7–8 mm, yellow-brown, stout, glabrous to sparsely papillose at base, densely papillose in upper part; anthers 5–7 mm long before dehiscence, ellipsoid, brown-purplish, basifixed. Style: 6–8 mm, entire, stout, greenish-yellow, densely papillose. Capsule: 2.5–3.5 × 1.5–2 cm, obovoid, obtuse, tapering towards the base, not winged. Flowers April to May.
Distribution: Endemic to Iran.
Among the species belonging to the Caucasica group, Fritillaria chlororhabdota seems to be closest to F. caucasica, by phyllotaxy (position and number of the leaves) as well as shape and its external purplish color of the tepals. However, the species can be distinguished from F. caucasica by several characters, e.g., folded leaves at fruiting time, narrowly campanulate flowers, obtuse tepals with a median green stripe on the inside, wide lanceolate nectaries, shorter filaments, rough pollen exine with big luminae and an entire stout style. In contrast, F. caucasica has flat leaves during the whole of vegetative period, acute tepals (sometimes inner ones obtuse) that are paler purple inside (sometimes greenish) and without a green stripe along the whole of their length, linear-lanceolate nectaries, longer filaments, smooth pollen exine (knobs are absent) with very small luminae (foveolate sculpturing type), and longer tribulate styles.
6.4. Subgenus Rhinopetalum (Fisch. ex Alexand.) BakerThe five members of subgenus Rhinopetalum (F. gibbosa, F. karelinii (Fisch. ex D.Don) Baker, F. stenanthera Rgl., F. bucharica Rgl., F. ariana) all have rather flat, pink and distinctive starry flowers different in shape compared with other subgenera and species of the genus. They are inhabitants of the mountains and steppes of central and western Asia, from the Caspian sea and Iran, to Afghanistan and the dry hills of western Pakistan; two members of this subgenus, F. gibbosa and F. ariana, occur in Iran (Rix and Strange, 2017).
The filament movement is only found in members of Rhinopetalum and Theresia (F. persica); the stamens bend back against the tepals before dehiscence of the anthers, and then bend forward at dehiscence. The nectary structure in members of this subgenus clearly differ from all other species; the nectaries are deeply impressed and the nectary orifice is slit-like which is bordered by two lobes, and appears densely hairy, at least in the lower part (Rix and Zarrei, 2007a, 2007b). As against other subgenera, F. gibbosa and F. ariana are unique in that the flowers are zygomorphic, as the nectary lobes are rather fringed and broad, and the nectary on the uppermost tepal is more depressed than the others. In the other subgenera, the nectaries are less depressed (often flattish), and usually linear to lanceolate or ovate, except in subgenus Petilium having usually circular nectaries. Following a morphological approach, the unique structure of the nectaries and other distinctive floral characters in all species of this subgenus imply that subgenus Rhinopetalum does not seem to be more than distantly related to the genus Fritillaria, and may probably merit formal recognition as a distinct genus (Rhinopetalum Fisch. ex Alexand). We have discussed this uncertainty earlier through Section 4 based on molecular evidence.
6.4.1. Fritillaria gibbosa BoissGibbous lily (Fritillaria gibbosa Boiss.) (Fig. 11, Center) has been claimed (Rix, 1977) to be geographically the most widespread species of the genus in Iran (Table 4). Normally, F. gibbosa is a species adapted to arid climates. Flowering begins very early, from March through May, on arid steppes, often among Artemisia L. spp., Rosa persica Michx. ex Juss., Lactuca orientalis (Boiss.) Boiss. and Poa bulbosa L. In east and southeast Iran where the summers are dry, it is very easy to grow this species.
Bulb: up to 3 cm diameter, without bulbils or stolons. Stem: 6–16 cm, densely papillose, especially below. Leaves: 4–10, twisted, the lower 30–70 × 13–18 mm, lanceolate to ovate, usually opposite, the rest linear, grayish; bract leaves linear, 2 at the base of each pedicle. Flowers: 1–7(-15), flat, horizontal at maturity; perianth segments about 15 × 10 mm, the outer somewhat narrower than the inner, all pinkish spotted and marked towards the apex, dark towards the base. Nectaries: about 4 mm, deeply impressed, the upper larger than the others, with papillose ridge inside. Filaments: 9–10 mm, papillose, especially below, sometimes purple; anthers about 2 mm, spherical after dehiscence. Style: 8–10 mm, entire, slender, glabrous. Capsule: 14 mm long, with 6 about 4 mm teeth. Flowers March to May.
Distribution: Iran, Uzbekistan, Turkmenistan, Armenia, Afghanistan, Pakistan (Baluchistan).
The species is highly variable, especially in the number and color of the flowers (Kiani, 2015). Flower color is usually pink, but ranges from white, orange-yellowish or purplish. The nectaries of F. gibbosa are very complex; the nectary on the topmost outer tepal is the largest (slightly however), and the inner one opposite it, is the smallest. All have two papillose ridges running down their middle, pressed together and concealing the nectar. On the outer part of the ridges, the papillae become flattened yellow lamellae, giving the appearance of an irregular crest. As with F. imperialis, the nectaries may exude drops of nectar in early mornings. The area around the nectaries is yellowish-green, heavily marked with blackish-purple. It differs from F. karelinii in that all the tepals, which are relatively broader, have nectaries, though some are larger than others; in F. karelinii only the uppermost nectary is enlarged and the rest seem to be vestigial, and do not produce nectar (Rix and Zarrei, 2007b).
6.4.2. Fritillaria ariana (Losinsk. & Vved.) RixPink star (Fritillaria ariana (Losinsk. & Vved.) Rix) (Fig. 11, Right) in Iran occurs nowhere in great abundance, but rather in very small populations mainly in northeastern Iran (Table 4) (Rix, 1977). It requires generally the same climatic conditions as were described for F. gibbosa. The geographical distribution of these two species is quite different; F. gibbosa is widespread in center and, northeast through northwest, all the way down along Zagros chain (Rix, 1977), but F. ariana is very rare in Iran (Sharifi-Tehrani et al., 2015) and is categorized as a critically endangered species. On the other hand, F. ariana grows primarily in sandy deserts, while F. gibbosa prefers clay steppes or rocky slopes (Rix and Strange, 2017).
Bulb: up to 3 cm diameter, without bulbils or stolons. Stem: 10–30 cm (-50 cm in fruit), glabrous or papillose only below the lowest leaves and at the nodes. Leaves: 8–12, long, the lowest pair considerably longer up to 120 × 12 mm, linear-lanceolate, opposite, the rest linear, scattered; bract leaves 20–40 × 2 mm, linear, 2 at the base of each pedicle. Flowers: 2–14, usually 6, flat, horizontal at maturity; perianth segments pink-reddish, unspotted or sometimes slightly mottled, marked with brown around the nectaries; the outer 25 × 10 mm, obovate, acute; the inner somewhat wider. Nectaries: about 4 mm long, deeply impressed, the uppermost largest, the outer larger than the inner. Filaments: 6–7 mm, slender, papillose or glabrous only near the base; anthers about 5 mm before, 2 mm after dehiscence. Style: 5–7 mm, entire, slender, glabrous. Capsule: 15 mm, not winged, but toothed at the upper corners. Flowers March to early April.
Distribution: Northeastern Iran, northwestern Afghanistan, southern Turkmenistan.
Fritillaria ariana is very similar to F. gibbosa, but they are separated by several characters; F. ariana is usually a much taller plant with narrower basal leaves, and more numerous flowers nearly always unspotted. The distinction between the broad basal leaves and narrower stem leaves of F. gibbosa, and the narrow basal and stem leaves of F. ariana is usually obvious. The stems of F. gibbosa are usually papillose all over, while those of F. ariana are glabrous or papillose only at the leaf bases (at the nodes) and below the lowest leaves (Rix, 1977; Rix and Zarrei, 2007b; Rix and Strange, 2017).
7. Eight-Frit MountainThe Iraqi border is truly a paradise for those in search of fritillaries; within this region lies a range of mountains which has been christened "Eight-Frit Mountain" (Wallis and Wallis, 2009), as it is home to at least eight different species of the genus, each of which is growing in its own ecological niche. This location is south of Marivan in Kordestan province southwards on the road to Nowsud and on the border with Kermanshah Province. It is very close to the border with Iraq and includes a pass at about 2500 m (Fig. 12, Right).
The place is a habitat of a strange-looking form of F. uva-vulpis growing together with Zagrosia persica (Hausskn.) Speta. and Bellevalia paradoxa (Fisch. & C.A.Mey.) Boiss. There are also populations of F. straussii on the north side of the slope; F. imperialis on the top of the ridge and a very large F. crassifolia ssp. kurdica on the south-facing side of the slope; F. avromanica grows in the spiny bushes on the west-facing slope below the road on the south side of the cleft; F poluninii grows in rock crevices in several parts of the pass; F. assyriaca can be found in several places on the slopes on both sides of the pass but a little lower down; F. persica populations are spread on the slopes while descending from the pass on the road towards Nowsud. Such a rich habitat, where members of the four subgenera known to occur in Iran coexist, is believed to be unique in the world (Wallis and Wallis, 2009).
8. Systematics serves conservationIn general, the large number of species (over 160 taxa), extensive polyploidy and highly polymorphic nature of several species of the genus Fritillaria indicate a genus near its peak of speciation (Beetle, 1944). As stated earlier, the occurrence of four different subgenera of the genus (including 19 species and 3 subspecies) has been confirmed in Iran. This makes the country an important area for diversification and evolution of new species, as species diversity itself could be a driver of further species diversification (Emerson and Kolm, 2005). Increasing species diversity may lead to greater genetic makeup complexity, which has been suggested as an evolutionary force driving speciation. This happens in a variety of ways, among which hybridization has played an especially important role in the evolution of the genus. Frequent hybridization can result in morphologically intermediate specimens and increased confusion in identification of Fritillaria species (Beck, 1947), which in turn increases the difficulty of making decisions related to conservation. Setting conservation priorities in taxonomically complex taxa is an essential but especially complicated process. Incomplete understanding of taxonomy can have disastrous impacts on conservation, since a given threatened species may not be readily distinguishable from a more frequent species.
Although the current classification of the genus proposed by Rix (2001) is supported by molecular phylogenetic studies at the subgeneric level (Rønsted et al., 2005; Day et al., 2014), the whole genus still raises important evolutionary and systematic questions, and many taxa recognized within different species belonging to subgenera Fritillaria, Rhinopetalum and Petilium present taxonomic problems. In the following sections, we provide a brief overview of the possible uncertainties that may easily mislead conservation activities.
8.1. Subgenus FritillariaTaxonomic uncertainties are most frequent in taxa belonging to subgenus Fritillaria. One such problem has been already encountered in the case of F. uva-vulpis and F. assyriaca, which are known as two species of high similarity in general morphological characters. Although the name F. assyriaca has often been used for F. uva-vulpis, detailed examinations reveal their distinctiveness (Rix, 2000b). F. assyriaca is rather more widespread and is a common species in Iran, whereas F. uva-vulpis is considered as an endangered species. To make the case even more complicated, these two are among the most variable species in the genus and have quite diverse morphologies in Iran. In the most confusing case, Wallis and Wallis (2009) recorded a strange-looking F. uva-vulpis in the Eight-Frit Mountain area that is quite different from the description of the species. On the other hand, F. kotschyana subsp. kotschyana has been confused with F. crassifolia, all the subspecies of which are more dwarfed and have linear nectaries (Rix, 1977). In another instance, the name F. kotschyana has been used for dwarf F. latifolia from northern Turkey, a very different species that has never been recorded in Iran. At least two unknown taxa have been recorded in Kordestan, very close to the Iraq border, which seemed to be F. crassifolia, but did not fit any of the known subspecies (Wallis and Wallis, 2009). F. pinardii and F. zagrica are two taxa frequently mixed up in the literature (see Section 6.3.3.3), once again, for extreme resemblance in their morphological characters. For this reason, although, it has been recently a single report of F. pinardii in West Azarbaijan province (Sharifi-Tehrani and Advay, 2015), the inclusion of this species within Iranian flora awaits further studies. In short, there is a variety of similar cases mainly due to crossing compatibility of different taxa and/or morphological similarities (e.g. Beck, 1947; Rix, 1974, 1975; Bakhshi Khaniki, 1997b; Wallis and Wallis, 2009; Tekşen and Aytaç, 2011).
8.2. Subgenus RhinopetalumAs with subgenus Fritillaria, identification errors are frequent and easily occur in subgenus Rhinopetalum mainly due to morphological similarities. For example, the red-listed F. ariana can be easily confused with F. gibbosa, which is widely distributed across Iran (Rix and Zarrei, 2007a). Moreover, F. pterocarpa Stocks, which is native to Baluchistan (Pakistan), is probably identical to F. gibbosa (Rix and Zarrei, 2007a). Although F. karelinii Fisch. ex D.Don has been claimed to occur in Iran on 31st May 1964 by Paul Furse (Rix and Zarrei, 2007b), we strongly believe this is an identification error; when Furse reported this record, the whole genus was poorly known. In addition, F. karelinii normally flowers very early in March; at this late date (31st May), it is very likely to have been in fruit and not in flower, making identification nearly impossible. Furse reported his record in Northern Khorassan (80 km west of Bojnurd, arid plains, yellow fine soil with stones and scattered Artemisia, scrub, 1800 m), where F. gibbosa is recorded 20 km from here in Artemisia steppe at 1300 m. Considering the normal range of F. karelinii, described from steppe in Southern Urals north of the Caspian Sea, as far south as Koppe Dagh, this is likely not F. karelinii.
8.3. Subgenus PetiliumIn a similar case from subgenus Petilium, the abundance of F. imperialis var. imperialis, the most frequent species in Iran, is quite different from its very close relative, F. imperialis var. lutea, which is seriously on the brink of extinction (Khourang et al., 2014). Although the endemic F. raddeana is also reported in Kashmir (Ali, 2007), Pakistan is not within the normal range of the species. This record is doubtful and presumably is correctly assigned to either F. chitralensis, native to the Chitral District of northern Pakistan, or to F. imperialis var. lutea.
8.4. Subgenus TheresiaGeneral morphological characters of F. persica, the sole member of subgenus Theresia, are clear-cut with no indication of introgressions or intermediates in Iran. This is the only species of the genus in Iran with numerous flowers arranged in a raceme, so that it can be easily distinguished from other species. Thus, in contrast to the above-mentioned cases, there is no identification concern about this species in Iran.
9. ConclusionImplementation of conservation frameworks is negatively affected by a lack of basic information on taxonomy and biology of fritillaries in Iran, as taxonomy provides the foundation for conservation practices and sustainable management of the world's remaining resources. Studies carried out on the genus Fritillaria through the last four decades in Iran have been technically sound efforts, but quite sporadic and limited in scope. These studies give a fairly good overview of different aspects of Iranian Fritillaria, yet provide little information relevant to conservation, which remains relatively poorly understood. By describing geographical and ecological features of Iranian Fritillaria along with an intensive look into biodiversity of the genus with respect to its taxonomic status, a much clearer understanding of the essential elements of conservation strategies can be provided for all involved practitioners. Information described in this review may serve as a stepping-stone for setting conservation priorities, hopefully in the near future, in Iran and neighboring centers of biodiversity of the genus Fritillaria.
AcknowledgmentsWe would like to thank Tarbiat Modares University (TMU) and Research Institute of Forests and Rangelands of Iran (RIFRI) for providing financial support and facilities. The assistance of Professor Valiollah Mozaffarian, Mr. Ramezani and Mr. Asghar Shahrokhi, the expert botanists is gratefully acknowledged for their precious contribution and assistance. Thanks are due to Dr. Mehtap Tekşen, Dr. Paul Christian, Mr. Marijn van den Brink, Mr. Monar Peter, Mr. Hossein Hasanzadeh and Mr. Abbas Mohamadi for their contribution. Furthermore, we wish to give a special word of appreciation to Dr. Robert B. Wallis for sharing his pictures, data, and his precious professional comments.
Advay M., Tekşen M., Maroofi H., 2015. Fritillaria avromanica sp. nov. (Liliaeceae)from Iran and notes on F. melananthera in Turkey. Nordic J. Bot, 33: 526-531. DOI:10.1111/njb.2015.v33.i5 |
Ahmadi-Roshan M., Karimzadeh G., Babaei A., Jafari H., 2016. Karyological studies of Fritillaria (Liliaceae) species from Iran. Cytologia, 81: 133-141. DOI:10.1508/cytologia.81.133 |
Akhani, H., Ghorbanli, M., 1993. A Contribution to the Halophytic Vegetation and Flora of Iran. Towards the Rational Use of High Salinity Tolerant Plants. Springer, pp. 35-44. http://link.springer.com/10.1007/978-94-011-1858-3_4
|
Ali S., 2007. A taxanomic study of the genus Fritillaria L. (Liliaceae) from Pakistan and Kashmir. Portugaliae Acta Biol, 22: 221-230. |
Alp Ş., Koyuncu N.A.M., 2009. Established forms of Fritillaria imperialis L. -a naturally growing species in Turkey. Pak. J. Bot, 41: 1573-1576. |
Ambrožová K., Mandáková T., Bureš P., Neumann P., Leitch I.J., Koblížková A., Macas J., Lysak M.A., 2010. Diverse retrotransposon families and an AT-rich satellite DNA revealed in giant genomes of Fritillaria lilies. Ann. Bot, 107: 255-268. |
Babaei, A., 2014. Genetic Fingerprinting of Iranian Tulips (Tulipa spp. ) and Fritillaries(Fritillaria spp. ). 1st National Congress of Flower and Ornamental Plants. National Research Institute of Flower and Ornamental plants, Karaj, Iran.
|
Badfar-Chaleshtori S., Shiran B., Kohgard M., Mommeni H., Hafizi A., Khodambashi M., Mirakhorli N., Sorkheh K., 2012. Assessment of genetic diversity and structure of Imperial Crown (Fritillaria imperialis L.) populations in the Zagros region of Iran using AFLP, ISSR and RAPD markers and implications for its conservation. Biochem. Syst. Ecol, 42: 35-48. DOI:10.1016/j.bse.2011.12.027 |
Baker J.G., 1874. Revision of the genera and species of Tulipeæ. J. Linnean Soc. Lond., Bot, 14: 211-310. DOI:10.1111/j.1095-8339.1874.tb00314.x |
Bakhshi K.G.R., 2000. Giemsa C-banding patterns and chromosome study of Fritillaria subgenus Petilium (Liliaceae) in Iran. Pajouhesh va Sazandegi, 3: 21-25. |
Bakhshi K.G.R., Persson K., 2002. Karyotype and C-banding study in Fritilaria zagrica (Liliaceae). Pajouhesh va Sazandegi, 15: 24-28. |
Bakhshi Khaniki G., 1997a. Fritillaria atrolineata (Liliaceae), a new species from Iran. Edinb. J. Bot, 54: 171-181. DOI:10.1017/S0960428600004017 |
Bakhshi Khaniki G., 1997b. Fritillaria chlororhabdota (Liliaceae), a new species from Iran. Herbertia, 52: 140-152. |
Bakhshi Khaniki G., 2001a. Fritillaria crassifolia ssp. kurdica (Liliaceae):a beautiful bulbous plant in Iran. Herbertia, 56: 77-84. |
Bakhshi Khaniki G., 2001b. Fritillaria zagrica (Liliaceae):an endemic species of fritillary in Iran. Herbertia, 56: 85-90. |
Beetle, D. E., 1944. A monograph of the North American species of Fritillaria. Madroño 133-159. http://www.jstor.org/stable/41426383
|
Beck, C., 1947. Fritillaries (Lecture Given to the Lily Group). Lily Year Book.
|
Bentham, G., Hooker, J., 1883. Fritillaria. In: Genera Plantarum, vol. 3, pp. 817-818.
|
Boissier, E., 1854. Diagnoses Plantarum Orientalium Novarum. B. Herrmann.
|
Burquez A., 1989. Blue tits, Parus caeruleus, as pollinators of the crown imperial, Fritillaria imperialis in Britain. Oikos, 55: 335-340. DOI:10.2307/3565592 |
Darlington C., La Cour L., 1941. The detection of inert genes. J. Hered, 32: 115-121. DOI:10.1093/oxfordjournals.jhered.a105012 |
Day P.D., Berger M., Hill L., Fay M.F., Leitch A.R., Leitch I.J., Kelly L.J., 2014. Evolutionary relationships in the medicinally important genus Fritillaria L. (Liliaceae). Mol. Phylogenet. Evol, 80: 11-19. DOI:10.1016/j.ympev.2014.07.024 |
Dunford, J. C., Sims, K. R., 2008. Greater Fritillaries or Silverspots, Speyeria[=Argynnis] (Lepidoptera: Nymphalidae). In: Encyclopedia of Entomology. Springer, Netherlands, pp. 1718-1722. http://link.springer.com/referenceworkentry/10.1007/978-1-4020-6359-6_1178?no-access=true
|
Emerson B.C., Kolm N., 2005. Species diversity can drive speciation. Nature, 434: 1015-1017. DOI:10.1038/nature03450 |
Firouz E., Hassinger J.D., Ferguson D.A., 1970. The wildlife parks and protected regions of Iran. Biol. Conserv, 3: 37-45. DOI:10.1016/0006-3207(70)90058-3 |
Gerard, J., 1995. The Herball or Generall Historie of Plantes (1597). Bonham & John Norton, London pp[xviii], 1392, 72.
|
Hanson T., Brooks T.M., Da Fonseca G.A., Hoffmann M., Lamoreux J.F., Machlis G., Mittermeier C.G., Mittermeier R.A., Pilgrim J.D., 2009. Warfare in biodiversity hotspots. Conserv. Biol, 23: 578-587. DOI:10.1111/cbi.2009.23.issue-3 |
Helsper J.P.F.G., Bücking M., Muresan S., Blaas J., Wietsma W.A., 2006. Identification of the volatile component(s) causing the characteristic foxy odor in various cultivars of Fritillaria imperialis L. (Liliaceae). J. Agric. Food Chem, 54: 5087-5091. DOI:10.1021/jf0605594 |
Jafari H., Babaei A., Karimzadeh G., Ahmadi-Roshan M., 2014. Cytogenetic study on some Fritillaria species of Iran. Plant Syst. Evol, 300: 1373-1383. DOI:10.1007/s00606-013-0968-6 |
Khaniki G., 2002a. Fritillaria chlorantha (Liliaceae), an endemic species in Iran. Herbertia, 57: 93-96. |
Khaniki G., 2002b. The Fritillaria kotschyana group (Liliaceae) in Iran. Herbertia, 57: 97-107. |
Khaniki G., 2002c. Fritillaria reuteri (Liliaceae), a peculiar species of fritillary in Iran. Herbertia, 57: 109-112. |
Khaniki G., 2002d. Notes on Fritillaria straussii (Liliaceae) in Iran. Herbertia, 57: 125-130. |
Khaniki G.B., 2002e. Chromosome number of all Iranian species of Fritillaria caucasica group (Liliaceae). Nucleus, 45: 103-108. |
Khaniki G.B., 2002f. Chromosome number of Fritillaria subgenera Petilium and Theresia (Liliaceae). Nucleus, 45: 6-11. |
Khaniki G.B., 2002g. Chromosome number of Iranian species of Fritillaria crassifolia group (Liliaceae). Nucleus, 45: 109-113. |
Khaniki G.B., 2003. Fruit and seed morphology in Iranian species of Fritillaria subgenus Fritillaria (Liliaceae). Pak. J. Bot, 35: 313-322. |
Khaniki G.B., Persson K., 1997. Nectary morphology in south west Asian Fritillaria (Liliaceae). Nordic J. Bot, 17: 579-611. DOI:10.1111/j.1756-1051.1997.tb00355.x |
Kharazipour, A. R., 2009. Review of Forests, Wood Products and Wood Biotechnology of Iran and Germany. Universitätsverlag Göttingen.
|
Khourang M., Babaei A., Sefidkon F., Naghavi M.R., Asgari D., Potter D., 2014. Phylogenetic relationship in Fritillaria spp. of Iran inferred from ribosomal ITS and chloroplast trnL-trnF sequence data. Biochem. Syst. Ecol, 57: 451-457. DOI:10.1016/j.bse.2014.10.001 |
Kiani, M., 2015. Morphological & Phytochemical Assessment and Genetic Fingerprinting of Medicinal/Ornamental Fritillaria spp. in Iran. Tarbiat Modares University, Tehran, Iran.
|
Kiani, M., Babaei, A., Sefidkon, F., Naghavi, M., 2015b. Molecular Characterization of Phylogenetic Relationships in Populations of the Medicinale-Ornamental Imperial Crown (Fritillaria imperialis L. ) of Iran Inferred from ITS Sequences.
|
Kiani M., Sefidkon F., Babaei A., Naghavi M.R., 2015a. Phytochemical profiling of medicinal isosteroidal alkaloids of Iranian Fritillaria spp. (Liliaceae). Industrial Crops Prod, 70: 451-458. DOI:10.1016/j.indcrop.2015.03.064 |
Kiran Y., Gedik O., 2014. Karyological study on Turkish endemic Fritillaria baskilensis Behçet. (Liliaceae). Turkish J. Sci. Technol, 9.2: 105-108. |
La Cour L.F., 1978. The constitutive heterochromatin in chromosomes of Fritillaria sp., as revealed by Giemsa banding. Philos. Trans. R. Soc. Lond. B Biol. Sci, 285: 61-71. DOI:10.1098/rstb.1978.0094 |
Li H.-J., Jiang Y., Li P., 2009. Characterizing distribution of steroidal alkaloids in Fritillaria spp. and related compound formulas by liquid chromatographyemass spectrometry combined with hierarchial cluster analysis. J. Chromatogr. A, 1216: 2142-2149. DOI:10.1016/j.chroma.2008.03.093 |
Li R., Shang Z., 1989. The chromosome observation on five species of rare plants of China. J. Wuhan Bot. Res, 7: 217-220. |
Mace G.M., 2004. The role of taxonomy in species conservation. Philos. Trans. R.Soc. B Biol. Sci, 359: 711-719. DOI:10.1098/rstb.2003.1454 |
Mancuso E., Peruzzi L., 2010. Male individuals in cultivated Fritillaria persica L. (Liliaceae):real androdioecy or gender disphasy. Turkish J. Bot, 34: 435-440. |
Marchant C., Macfarlane R., 1980. Chromosome polymorphism in triploid populations of Fritillaria lanceolata Pursh (Liliaceae) in California. Bot. J. Linn. Soc, 81: 135-154. DOI:10.1111/boj.1980.81.issue-2 |
Mathew B., 2005. The Rhinopetalum group. AGS Fritillaria Group J, 16: 1-3. |
Matsuura H., 1934. On karyo-ecotypes of Fritillaria camschatcensis (L.) Ker-Gawler. Hokkaido Imperial Univ, 3(5): 204-219. |
Mucciarelli M., Rosso P., Noble V., Bartolucci F., Peruzzi L., 2016. A morphometric study and taxonomic revision of Fritillaria tubaeformis complex (Liliaceae). Plant Syst. Evol, 302: 1329-1343. DOI:10.1007/s00606-016-1334-2 |
Noda S., 1975. Achiasmate meiosis in the Fritillaria japonica group. Heredity, 34: 373-380. DOI:10.1038/hdy.1975.46 |
Peruzzi L., 2012. Male flowers in Liliaceae are more frequent than previously thought. Bocconea, 24: 301-304. |
Peruzzi L., Leitch I.J., Caparelli K.F., 2009. Chromosome diversity and evolution in Liliaceae. Ann. Bot, 103: 459-475. DOI:10.1093/aob/mcn230 |
Peters W.S., Pirl M., Gottsberger G., Peters D.S., 1995. Pollination of the crown imperial Fritillaria imperialis by great tits Parus major. J. Ornithol, 136: 207-212. DOI:10.1007/BF01651242 |
Rechinger, K. H., 1990. Fritillaria L., Flora Iranica. Akademische Druck-u-Verlagsanstalt, Austria, pp. 61-76.
|
Rix, E., 1974. Notes on Fritillaria (Liliaceae) in the Eastern Mediterranean region, Ⅰ & Ⅱ. Kew Bulletin 633-654. http://www.jstor.org/stable/4102882
|
Rix, E., 1975. Notes on Fritillaria (Liliaceae) in the Eastern Mediterranean region, Ⅲ. Kew Bulletin 153-162. http://europepmc.org/abstract/agr/ind79054341
|
Rix E., 1977. Fritillaria L. (Liliaceae) in Iran. Iran. J. Bot, 1: 75-95. |
Rix M., 2000a. Plate 392. Fritillaria uva-vulpis. Curtis's Bot. Mag, 17: 159-160. DOI:10.1111/curt.2000.17.issue-3 |
Rix M., 2000b. Plate 396. Fritillaria crassifolia subsp. kurdica. Curtis's Bot. Mag, 17: 173-175. DOI:10.1111/curt.2000.17.issue-3 |
Rix M., 2006. 548. Fritillaria poluninii. Curtis's Bot. Mag, 23: 48-50. DOI:10.1111/curt.2006.23.issue-1 |
Rix, M., 2001. Fritillaria: a revised classification: together with an updated list of species. Fritillaria Group of the Alpine Garden Society, pp. 1-14.
|
Rix Strange.K., 2017. 854. Fritillaria ariana. Curtis's Bot. Mag, 34: 2-10. DOI:10.1111/curt.2017.34.issue-1 |
Rix M., Zarrei M., 2007a. 580. Fritillaria karelinii. Curtis's Bot. Mag, 24: 46-49. DOI:10.1111/curt.2007.24.issue-1 |
Rix M., Zarrei M., 2007b. 581. Fritillaria gibbosa. Curtis's Bot. Mag, 24: 50-53. DOI:10.1111/curt.2007.24.issue-1 |
Rønsted N., Law S., Thornton H., Fay M.F., Chase M.W., 2005. Molecular phylogenetic evidence for the monophyly of Fritillaria and Lilium (Liliaceae; Liliales)and the infrageneric classification of Fritillaria. Mol. Phylogenet. Evol, 35: 509-527. DOI:10.1016/j.ympev.2004.12.023 |
Salahi, A., Asareh, M. H., Kharazipour, A., Müller, C., Schöpper, C., 2008. Iran and its Natural Resources Features. Review of Forests, Wood Products and Wood Biotechnology of Iran and Germany, Part Ⅱ. Universitätsverlag Göttingen.
|
Salahi Ardakani A., 2014. Intensive damage of Lilioceris chodjaii on Fritillaria imperialis in Kohgiluyeh va Boyerahmad province, Iran. Adv. Environ. Biol, 8: 478-482. |
Sharifi-Tehrani M., Advay M., 2015. Assessment of relationships between Iranian Fritillaria (Liliaceae) species using chloroplast trnH-psbA sequences and morphological characters. J. Genet. Resour, 1: 89-100. |
Sharifi-Tehrani M., Advay M., Shabani L., 2015. Fritillaria (Liliaceae) in Iran:distribution and nomenclature. Taxonomy Biosyst, 22: 49-70. |
Shimizu T., Hatanaka Y., Zentoh H., Yashima T., Kinoshita E., Watano Y., Shimizu T., 1998. The role of sexual and clonal reproduction in maintaining population in Fritillaria camtschatcensis (L.) Ker-Gawl. (Liliaceae). Ecol. Res, 13: 27-39. DOI:10.1046/j.1440-1703.1998.00245.x |
Stebbins, G. L., 1971. Chromosomal Evolution in Higher Plants. Chromosomal Evolution in Higher Plants. Edward Arnold Publisher, London, p. 216.
|
Tamura, M., 1998. Liliaceae. Flowering Plants. Monocotyledons. Springer, pp. 343-353.
|
Tekşen M., Aytaç Z., 2011. The revision of the genus Fritillaria L. (Liliaceae) in the Mediterranean region (Turkey). Turkish J. Bot, 35: 447-478. |
Tomović G., Vukojićić S., Niketic M., Zlatkovic B., Stevanović V., 2007. Fritillaria (Liliaceae) in Serbia:distribution, habitats and some taxonomic notes. Phytol.Balc, 13: 359-370. |
Türktaş M., Türktaş M., Aslay M., Ertuǧrul F., 2012. Molecular characterization of phylogenetic relationships in Fritillaria species inferred from chloroplast trnLtrnF sequences. Turkish J. Biol, 36: 552-560. |
Turrill, W.B., Sealy, J.R., Ross-Craig, S., 1980. Studies in the Genus Fritillaria (Liliaceae). Bentham-Moxon Trustees.
|
Wallis B., Wallis R., 2. Fritillaries in Iran. Plantsman, 8: 184-188. |
Wietsma W.A., Deinum D., Teunissen H.A., van den Berg R.G., 2015. Phylogenetic relationships within Fritillaria section Petilium based on AFLP fingerprints. Plant Syst. Evol, 301: 1043-1054. DOI:10.1007/s00606-014-1135-4 |
Zaharof E., 1988. A phenetic study of Fritillaria (Liliaceae) in Greece. Plant Syst. Evol, 161: 23-34. DOI:10.1007/BF00936009 |
Zonneveld B.J.M., 2010. New record holders for maximum genome size in eudicots and monocots. J. Bot, 2010: 1-4. |
Zych M., Stpiczyńska M., 2012. Neither protogynous nor obligatory out-crossed:pollination biology and breeding system of the European Red List Fritillaria meleagris L. (Liliaceae). Plant Biol, 14: 285-294. DOI:10.1111/plb.2012.14.issue-2 |



