b. University of Chinese Academy of Sciences, Beijing 100049, China
Cushion-forming plants are widely distributed in global alpine regions and have long fascinated botanists around the world. According to an updated catalogue of cushion plant species by Aubert et al. (2014), 1309 cushion-forming species belonging to 272 genera and 63 families of angiosperms have been recorded worldwide. Temperate Asia contains the greatest abundance of cushion species (37.1%), followed by South America (26.7%), Europe (14.3%), Australasia (10.4%) and tropical Asia (9.3%); North America, Africa and Antarctic share a combined 12% of cushion species (Aubert et al., 2014). Regionally, New Guinea, New Zealand, the Andes and Patagonia, the Himalayas, the Hengduan Mountains, the European Alps and the Pyrenees show the highest concentration of cushion species (Boucher et al., 2016). These findings support a previous study that suggests the Qinghai-Xizang Plateau, hosting 85 cushion species belonging 15 genera and 8 families, is a center of diversification for cushion plants (Li et al., 1987). Overall, the repeated development of cushion morphology across numerous angiosperm clades in disparate regions of the world suggests a remarkable convergence in the evolution of these plant species. This convergence may have happened relatively recently in the history of angiosperms since the alpine and arctic environments where cushion plants mainly occur are relatively young (Fine and Ree, 2006).
Studies of plant ecology and ecophysiology have shown that cushion species form efficient heat and water traps, and cycle nutrients within their compact structure by accumulating litter (reviewed by Körner, 2003). Recent studies in community ecology have indicated that cushion species may act as keystone species in communities in alpine and arctic environments, by facilitating the establishment of plant species. Furthermore, arthropods and insects associate with particular fungal and bacterial communities in the roots and rhizomes of cushion species (Roy et al., 2013), thereby increasing diversity in alpine environments (Callaway and Walker, 1997; Cavieres et al., 2002, 2007, 2008, 2014; Badano and Cavieres, 2006a, b; Badano et al., 2006; Molina-Montenegro et al., 2006; Cavieres and Badano, 2009; Yang et al., 2010, 2017; Reid and Lortie, 2012; Lortie and Reid, 2012; Chen et al., 2014, 2015).
Because of their ability to modify microenvironments and their key role in alpine ecosystems, cushion species are considered foundation species in the process of community succession in alpine ecosystems (Molenda et al., 2012; Reid and Lortie, 2012; Schöb et al., 2012) and an insurance policy for diversity in alpine communities (Cavieres et al., 2016). The stability of cushion populations in the natural ecosystem is of great importance for their ecosystem functions. Previous studies have examined how the compact structures of cushion species (Körner, 2003; Aubert et al., 2014) and their long life-spans (Benedict, 1989; Molau, 1997; Morris and Doak, 1998; Forbis and Doak, 2004) have enabled them to persist in hostile environments. However, the reproductive strategies of cushion species, which are key processes for occupying hostile habitats and maintaining communities in these environments, have been largely ignored. The few studies that have examined reproductive strategies have focused on a relatively small number of species, and have been unable to draw major conclusions. In this paper, we first review recent advances in understanding reproduction and/or community recruitment in alpine cushion species. We then present a preliminary study addressing sexual reproduction of a typical cushion species, i.e. Arenaria polytrichoides (Caryophyllaceae), and its relationship with one abiotic factor (i.e. elevation) in the Hengduan Mountains. Cushion species inhabiting high elevations of the Hengduan Mountains have been shown to play an important role in enhancing alpine community diversity (Yang et al., 2010, 2017; Chen et al., 2014, 2015). However, to our knowledge, no information related to reproduction and community recruitment of cushion species has been reported before in this area. Finally, we highlight limitations in the current research and propose directions for future study.
Material and methods Publication collection and reviewTo collect publications referring to reproduction and community/seedling recruitment of alpine cushion species, we searched Web of Science (1980–2017) using the following search items: "cushion OR cushion species OR cushion-forming" AND "reproduction OR pollination OR community recruitment OR growth rate" AND "plant" AND "mountain OR alpine". We also searched these items, but in Chinese, using the CNKI (China National Knowledge Infrastructure) database for papers, theses or dissertations which were on alpine cushion species.
For publications that met the above criteria, we read the titles and abstracts and retained those that: (1) were conducted in the field in mountain regions; (2) mainly investigated or partly referred to the growth rate, reproductive strategies (including pollination, breeding system) or community/seedling recruitment of cushion species. Additionally, for the retaining publications, we checked their reference lists and if there were additional references relevant to our study, we included them to our reference list.
As a result, we collected 34 publications on reproduction (including pollination and breeding systems), growth rate and/or community/seedling recruitments of alpine cushion species. These studies referred to 18 different species, including two subspecies and one variant, among which Silene (Caryophyllaceae) and Eritrichium (Boraginaceae) are the mostly studied genera, with 14 (41.18%) publications studying on Silene species (including two subspecies and one variant) and eight (23.53%) publications on Eritrichium species (only one species, i.e. Eritrichium numum); the remaining 13 cushion species comprised 35.29% of the total publications (Fig. 1). Most studies were conducted in the northern hemisphere (North America, Canada and Europe); only a few studies were conducted in the southern hemisphere (Chile, New Zealand, Australia). Although Asia owns the largest number of cushion species (Aubert et al., 2014), no studies about cushion reproduction were conducted in this area.
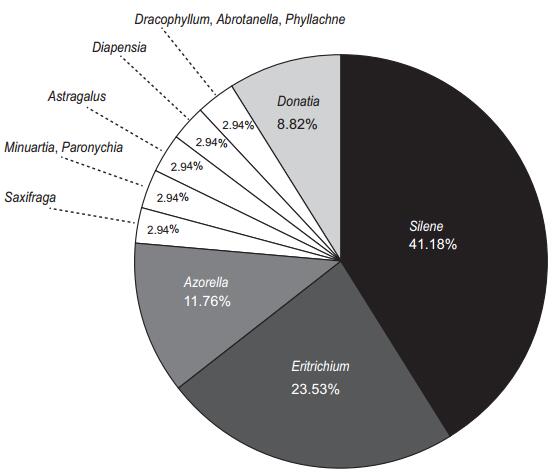
|
| Fig. 1 Proportion of publications collected and cushion species investigated (genus level). |
After reading the publications, we summarized the key results and conclusions about growth rate, the strategies of reproduction and community recruitment and discussed the potential generality of these findings. Finally, based on this review and the following case study, we outlined limitations and proposed directions for the future work.
Case study in the Hengduan Mountains . Study sites and speciesThis case study was performed on the Baima snow mountain at Pujin pasture in early June (i.e. flowering time) and late September (i.e. fruiting time) 2015. Three A. polytrichoides populations (Fig. 2) were selected along elevational gradient, the first located at elevation of 4380 m (hereafter 'low site/population'; 28°26'51.11″N, 099°59'51.98″E), the second located at 4520 m (hereafter 'middle site/population'; 28°28'07.93″N, 099°00'01.23″E) and the third located at 4780 m (hereafter 'high site/population'; 28°28'55.82″N, 099°00'12.11″E). A. polytrichoides is a long-lived perennial herb that forms hemispherical cushions. Vegetative growth of this plant starts as soon as the snow cover disappears, and flowering occurs from late May to early July (personal observations). According to Peng et al. (2012), most alpine plants in the Hengduan Mountains are hermaphroditic; but A. polytrichoides is a gynodioecious species, with females and hermaphrodites coexisting in the same population (Fig. 2). To understand the evolutionary adaptations which allow cushion species populations to be maintained in severe ecosystems, it is important to examine the reproductive strategies of such a sexual system.
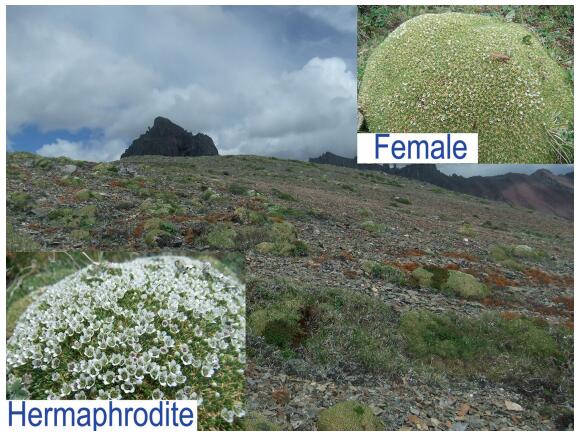
|
| Fig. 2 Alpine Arenaria polytrichoides population in which female (top right) and hermaphroditic (left bottom) individuals co-exist. |
This region is characterized by a monsoon climate, with annual precipitation between 300 and 1300 mm, most of which is concentrated during the summer (Zhang et al., 1997). The annual mean temperature varies from below 0 ℃ to over 20 ℃, depending on geographical location; and the minimum and maximum values generally occur in January and July, respectively (Zhang et al., 1997).
. Flower, fruit and seed productionTo determine the female frequency in each population, we randomly selected 204, 225, 232 cushion individuals and checked their gender morphs. The female frequency was calculated as: number of female individuals/number of all individuals × 100%.
The number of flowers per area was counted in three randomly placed 2 cm × 2 cm quadrates on 10 female and 10 hermaphroditic cushion individuals in each population. The same protocol was used for determining the number of fruits per area. Given the difficulty in distinguishing females from hermaphrodites at the fruiting stage, we tagged females and hermaphrodites using iron labels with different colors during flowering time. For flower and seed number counts, three values from each cushion individual were averaged and taken as replicates in later analyses.
. Pollen productionTo compare pollen production among three different populations, we measured pollen production of a single flower. For this, two to three flowers from 10 hermaphroditic individuals were randomly collected prior to anthesis. The petals were carefully removed, and the anthers were stored in 1.5 ml ethanol in an Eppendorf tube. Pollen production was determined according to Chen et al. (2016).
. Data analysisFor each population, we compared flower production between different genders using paired-sample t tests. For seed production, since we collected seeds from different numbers of individuals of each gender morph, independent-sample t tests were chosen to conduct comparisons. The differences in flowers, seeds and pollen production among three different populations (elevations) were tested with One-Way ANOVA, within which elevation was taken as the independent factor and the reproductive measurements were taken as dependent factors. All the analyses were conducted using SPSS 16.0.
Results and discussion Growth rate, life-span and seedling recruitmentTo understand community recruitment in alpine cushion species, relative growth rates and life-spans must be involved. All species studied show slow growth rates, varying from 0 (or even negative growth) to ca. 7 cm year-1 in diameter, depending on habitat, species and life-history stage (Benedict, 1989; Gibson, 1990; Morris and Doak, 1998; Kleier and Rundel, 2004; le Roux and McGeoch, 2004; Méndez, 2011; Kleier et al., 2015). For example, the growth rate of Silene acaulis increases slowly from the seedling stage, reaching a maximum of 2–3 cm year-1, until surface diameters measure between 20 and 30 cm, before decreasing in the later stages of its life history (Benedict, 1989). One possible explanation for this is that at the seedling stage individual plants have relatively small photosynthetic areas and consequently allocate available resources and energy mainly to the taproots below ground for the absorption of soil water and nutrients. At the adult stage (larger than 20–30 cm in diameter), the demands of water and nutrients, in addition to the distance over which these substances must be transported from the central taproot to peripheral growing shoot, both increase, thus resulting in a decrease in the supply of these substances and further leading to a decrease in growth rate (Benedict, 1989). Slow growth rates are common among alpine plants (Agakhanyantz and Lopatin, 1978) due to the high environmental stress of alpine ecosystems, e.g. low temperature and poor soil nutrients. Because of the slow growth rate, some cushion species (e.g. Diapensia lapponica) may need 20 years of vegetative growth to reach reproductive stage (Molau, 1997). However, once these plants begin to reproduce, they are able to reproduce for the remainder of their life history (Molau, 1997; Morris and Doak, 1998).
If cushion species grow so slowly, one interesting question is how they persist in alpine ecosystems. The answer seems simple: although they grow slowly, they have very long life-spans (Benedict, 1989; Molau, 1997; Morris and Doak, 1998; Forbis and Doak, 2004). Many cushion species can live for centuries (Mccarthy, 1992; Molau, 1997; Morris and Doak, 1998; Forbis and Doak, 2004; le Roux and McGeoch, 2004), and may persist for additional centuries. That means once the communities are established, they will not decline for a long period. Additionally, cushion species can produce a large number of progeny (seeds) throughout their life history. Production of progeny should promote substantial seedling recruitment, which is central to population maintenance for nonclonal plant species (Forbis and Doak, 2004). Although, for some species (e.g. Minuartia obtusiloba and Paronychia pulvinata), the contribution of seedling establishment to population maintenance may be low. For example, when the longevity of cushion life-spans is taken into account, cushion populations are able to maintain their stability for a long time (Forbis and Doak, 2004). Indeed, it is a common strategy for alpine plants to have long life-spans to adapt to harsh and unpredictable environmental conditions (Morris and Doak, 1998; Körner, 2003). Even so, few other life-forms (herbaceous) in alpine ecosystems are known to have life-spans as long as cushion species (Ehrlén and Lehtil, 2002).
Sexual reproductionTheoretical and empirical studies have predicted that alpine plants benefit from asexual reproduction (Bliss, 1971; Klimes et al., 1997; Milla et al., 2009) due to severe environmental conditions (e.g. low diversity and activities of pollinators). However, existing studies reveal that population recruitment in cushion species like S. acaulis and Eritrichium nanum are entirely dependent on sexual reproduction. S. acaulis is a polymorphic species that exhibits great variation in its breeding system (Hermanutz and Innes, 1994; Maurice et al., 1998). S. acaulis has been treated as gynodioecious (Shykoff, 1988, 1992; Hermanutz and Innes, 1994; Delph et al., 1999; Delph and Carroll, 2001), trioecious (Philipp et al., 1990; Alatalo and Molau, 2001) and subdioecious (Maurice et al., 1998). Plant sexual reproductive strategies commonly reflect the adaptive strategies of sexual dimorphisms under specific environmental conditions (Case and Ashman, 2005; Ashman, 2006). Different sexual morphs achieve reproductive success through different means, leading to remarkable differences in their reproductive efforts (Case and Ashman, 2005). Females, usually achieve reproductive success through seed production (Charlesworth and Charlesworth, 1978; Ashman, 1994; Sakai et al., 1997; Williams et al., 2000); while hermaphrodites achieve reproductive success through both seeds and pollen production (Charlesworth and Morgan, 1991). Accordingly, hermaphrodites should have an advantage over females because they transfer both male and female genes to future generations. It has been suggested that females are at a disadvantage in gynodioecious populations and must gain extra advantages over hermaphrodites in order to persist (Charlesworth and Charlesworth, 1978; Arnan et al., 2014). For example, Shykoff (1988) found that seeds produced by female cushion individuals might contain more nutrients such as nitrogen because females do not need to allocate resources to pollen production (pollen grains contain a relatively high concentration of nitrogen, Carroll and Delph, 1996). Hence, seeds and/or seedlings from S. acaulis females may have higher survival rates than those of hermaphrodites because they are better provisioned (Shykoff, 1988). In addition, females have been confirmed to have additional reproductive advantages, such as seed, capsule/ovule and fruit production (Shykoff, 1988; Hermanutz and Innes, 1994; Delph and Carroll, 2001; Lortie and Reid, 2012), receptivity/pollen germinability of/on stigmas (Shykoff, 1992; Hermanutz and Innes, 1994) and seedling survival (Shykoff, 1988). Female fitness is higher than hermaphrodite fitness when examined in relation to these traits.
The results of our case study support previous studies (e.g. Charlesworth and Charlesworth, 1978; Shykoff, 1988, 1992; Lortie and Reid, 2012; Arnan et al., 2014), with female cushion individuals of A. polytrichoides producing fewer flowers (d.f. = 9, P < 0.001; Figs. 2 and 3a) but more fruits and seeds (P < 0.001; Fig. 3b and c) than hermaphroditic individuals no matter what environmental conditions (altitudes) they experience. Increased seed numbers should promote population expansion via seedling establishment. These results indicate that for S. acaulis, females achieve more fitness via seed production (female function) than hermaphrodites, while hermaphrodites may mainly achieve fitness through pollen exportation (male function). Thus, like many other non-cushion species (e.g. Ashman, 1994; Delph and Carroll, 2001; Chen et al., 2016, 2017), female cushions promote their persistence in populations by enhancing female functions (as mentioned above).
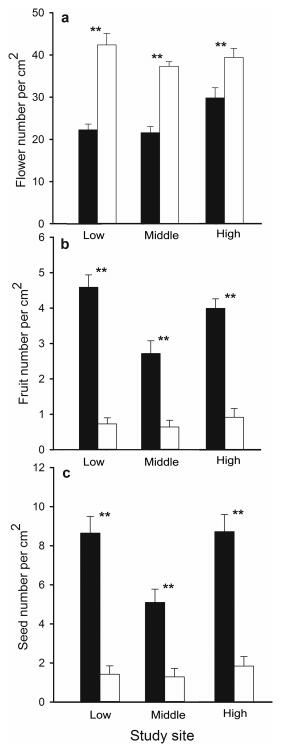
|
| Fig. 3 Reproductive measurements between female (black bars) and hermaphroditic (empty bars) cushions at three study sites. Mean ± s.e.; for flower production, n = 10 for all three sites; for fruit and seed production, low: n = 63; middle: n = 54; high: n = 59; **P < 0.01. |
Like many other species, reproductive function in S. acaulis is usually determined by ecological factors. For example, it has been shown that the frequency of S. acaulis females in population varies dramatically among different environmental conditions (Hermanutz and Innes, 1994), and increases with increasing elevation (Alatalo and Molau, 1995). Higher female frequencies should be favored in stressful environmental conditions (Delph, 1990; Delph and Carroll, 2001) where hermaphrodites may reduce their fruit or seed set and allocate the saved resources to vegetative growth and maintenance, thereby enhancing their flower and pollen production while reducing their relative seed fitness (Lloyd, 1976), further allowing a higher frequency of females (Delph and Carroll, 2001). In addition, reproductive traits such as flower, ovule and seed number, as well as fruit set, were also influenced by the environmental conditions that populations experience (Delph and Carroll, 2001); however, the magnitude and mode of the influence may be species specific and/or variable depending on the environment. In addition to abiotic factors, some biotic factors also influence the reproductive success of S. acaulis. For example, predation by seed-sucking bugs (Nysius groenlandicus) may lead to loss of seed mass, while simultaneously increasing the seed germination rate and speed, and thus might benefit the establishment of seedlings (Lundbye et al., 2012). However, it seems difficult to provide an explanation for how ecological factors influence the sexual reproduction of cushion species, especially sexual dimorphic species.
Our results show that the frequency of female cushions increased at higher elevations, which are in agreement with previous predictions and empirical studies (e.g. Shykoff, 1992; Alatalo and Molau, 1995). Specifically, female cushions comprise 43% of populations at a low elevation, whereas they are 55% and 61% of populations at middle and high elevations, respectively. However, in contrast with previous hypotheses that in populations with higher female frequency, the selective pressure should favor mutations that increase pollen production by hermaphrodites at the cost of a decrease in their seed production (Lloyd, 1976; Delph and Carroll, 2001), our results showed that hermaphroditic A. polytrichoides cushions produce similar flowers (d.f. = 2, F = 1.20, P = 0.316; Fig. 4a), fruits (d.f. = 2, F = 0.45, P = 0.64; Fig. 4b), seeds (d.f. = 2, F = 0.97, P = 0.38; Fig. 4c) and pollen grains (d.f. = 2, F = 0.764, P = 0.473) among three populations, even though they experience different environmental stresses. Similar results have been reported in other gynodioecious non-cushion species in the same region, e.g. Cyananthus macrocalyx (Chen et al., 2016) and Cyananthus delavayi (Chen et al., 2017). We still lack a strong explanation for this finding. Pollen production of hermaphrodites in the study region may be independent of environmental stress and female frequency.
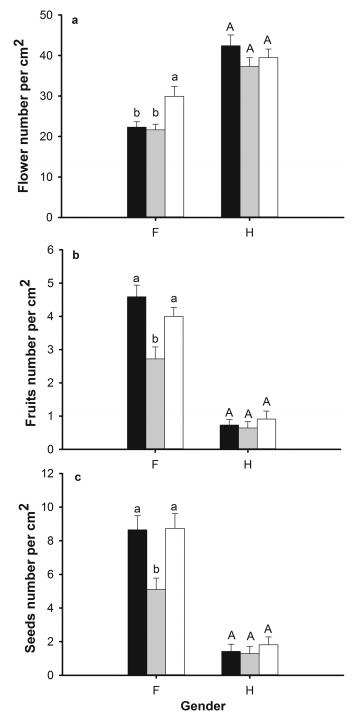
|
| Fig. 4 Reproductive measurements of female (F) and hermaphroditic (H) cushions among three different sites (Low: black bars; Middle: grey bars; High: empty bars). mean ± s.e.; for flower production, n = 10 for all three sites; for fruit and seed production, low: n = 63; middle: n = 54; high: n = 59; for each gender group, superscripts with different letters indicate significantly different means. |
Overall, both previous studies and our case study indicate that females of cushion species enhance female functions and produce more seeds to promote their persistence in ecosystems. More seeds should lead to advantages during population expansion via seedling establishment. Additionally, females are more resistant to harsh environments, which may indicate they easily adapt to higher elevations and occupy new habitats therein. In contrast, hermaphrodites of cushion species achieve fitness by allocating resources to male functions, including advertising to pollinators (large flower number) and pollen production. In our case study, A. polytrichoides hermaphrodites showed conservatism in reproductive outputs. Both female (seeds) and male (pollen grains) functions seem to be independent of environmental stress. These strategies are in direct contrast with A. polytrichoides females.
In contrast with S. acaulis and A. polytrichoides, E. nanum has a simple breeding system, i.e. androgynous (Zoller et al., 2002; Wirth et al., 2010a, b). Although E. nanum is self-compatible, it is predominantly outcrossed and pollinated by various generalist pollinators, mostly Diptera (Zoller et al., 2002). For species with simple breeding systems, ecologists have paid more attention to the effects that ecological factors (e.g. elevation) have on their reproductive success. It has been suggested that the selfing rate of plant species should increase at higher elevations (García–Camacho and Totland, 2009; Körner and Paulsen, 2009) because pollinator abundance and activity decreases at higher elevation (Arroyo et al., 1982; Medan et al., 2002). However, studies of E. nanum found decreased selfing rates at higher elevations (Zoller et al., 2002; Wirth et al., 2010b). Similar results were reported for Saxifraga oppositifolia, a cushion species in eastern Switzerland (Gugerli, 1998). It has been shown that E. nanum produces a large number of flowers to enhance attraction of pollinators and increase pollination efficiency. Moreover, Wirth et al. (2010b) attributed the higher selfing rate at lower elevations to the unfavorable weather conditions (low temperature) at the beginning of the growing season of E. nanum, which limited pollinator abundance and activities and further led to intensive selfing. In addition, seeds from outcrossing should allow these plants to avoid inbreeding depression and enhance the fecundity of progeny (Sakai et al., 1997; Ramsey et al., 2006; Dufay et al., 2010), which in turn should favor seedling survival and establishment under stressful alpine conditions. With elevation increasing, the seed production in E. nanum populations also increased (Zoller et al., 2005), but seed mass decreased (Wirth et al., 2010a). The increased seed production, attributed to the increase of population density but not the productivity of individuals, has been suggested to favor successful recruitment and appears to be decisive for E. nanum (Zoller et al., 2005). Similarly, individuals of the cushion Donatia novae-zelandiae produced more flowers and seeds at higher elevations than individuals at lower elevations, possibly because this cushion form increases heat stress at low elevations to the point that fitness is reduced (Cranston et al., 2015). Recent studies have suggested that biotic factors such as neighborhood facilitation may play a role in population density, but this strategy is only apparent at larger spatial scales; at small scales, neither competition nor facilitation has been found (Sieber et al., 2011; Wirth et al., 2011). Moreover, E. nanum seed set was higher when growing at low conspecific density than when growing at high density. In addition, seeds from cushions growing at low conspecific density were more highly outbred than seeds from cushions at high density (Wirth et al., 2011). This indicates the population density indeed, to some extent, determines reproductive success and may further influence population recruitment.
ConclusionAlthough the reproductive and maintenance strategies of alpine cushions have only been studied in a very small proportion of cushions, some common features can be elucidated: (1) species sharing the cushion morphology usually grow very slowly but have high adult survival rates and very long life-spans, which is sufficient for the stability and maintenance of cushion populations; (2) for cushion species with polymorphic breeding systems, female individuals can gain extra fitness through enhancing female functions (e.g. high seed production and progeny quality), thus promoting their maintenance in populations; while hermaphrodites can achieve fitness through both female and male functions, which may vary with changes of environmental conditions (plasticity); (3) cushion species with simple breeding systems outcross even under more stressful conditions, probably because progeny from outcrossing have higher fecundity than from selfing.
Directions for future studiesWe have to acknowledge that there still are many gaps in our understanding of reproduction in cushion species. Firstly, present studies are mainly focused on Northern American and European alpine regions; while reproductive or maintenance strategies of cushion species is still very poorly understood in regions with different climatic conditions, in particular temperate Asia, including Qinghai-Xizang Plateau, Hengduan Mountains, which is a hotspot for diversification of cushion species. Secondly, compared to the large number of cushion species in the world, studies on reproduction of cushion species are still very scarce, in particular for those with sexual polymorphisms. Thirdly, the global climate change is widely accepted and has proved to dramatically influence alpine ecosystems (Körner, 2003; Mackay, 2008), however, how cushion reproduction responds to the global climate change remains unknown (but see Cranston et al., 2015).
AcknowledgementsWe thank Yang B for his assistance in references collection and Zhang YZ for his assistance in the field work; Ma XG gave some useful suggestions on this manuscript. This work was supported by grants from the National Key Research and Development Program of China (grant no. 2017YFC0505200), the Major Program of National Natural Science Foundation of China (grant no. 31590823) to H Sun, the Natural Science Foundation of China (Grant no. 31500185 to JG Chen, 31470321 to Y Yang).
Agakhanyantz O.E., Lopatin I.K., 1978. Main characteristics of the ecosystems of the Pamirs, USSR. Arct. Alp. Res, 10(2): 397-407. DOI:10.2307/1550770 |
Alatalo J.M., Molau U., 1995. Effect of altitude on the sex ratio in populations of Silene acaulis (Caryophyllaceae). Nord. J. Bot, 15(3): 251-256. DOI:10.1111/j.1756-1051.1995.tb00150.x |
Alatalo J.M., Molau U., 2001. Pollen viability and limitation of seed production in a population of the circumpolar cushion plant, Silene acaulis (Caryophyllaceae). Nord. J. Bot, 21(4): 365-372. DOI:10.1111/j.1756-1051.2001.tb00780.x |
Arnan X., Escola A., Rodrigo A., et al, 2014. Female reproductive success in gynodioecious Thymus vulgaris:pollen versus nutrient limitation and pollinator foraging behaviour. Bot. J. Linn. Soc, 175(3): 395-408. DOI:10.1111/boj.2014.175.issue-3 |
Arroyo M.T.K., Primack R., Armesto J., 1982. ommunity studies in pollination ecology in the high temperate Andes of central Chile. 1. Pollination mechanisms and altitudinal variation. Am. J. Bot, 69(1): 82-97. DOI:10.2307/2442833 |
Ashman T.L., 1994. Reproductive allocation in hermaphrodite and female plants of Sidalcea oreganas sp. spicata (Malvaceae) using 4 currencies. Am. J. Bot, 81(4): 433-438. DOI:10.2307/2445492 |
Ashman, T. L., 2006. The evolution of separate sexes: a focus on the ecological context. In: Harder, L. D., Barrett, S. C. H. (Eds. ), Ecology and Evolution of Flowers. Oxford University Press, Oxford, pp. 204-219.
|
Aubert S., Boucher F., Lavergne S., et al, 2014. 1914-2014:a revised worldwide catalogue of cushion plants 100 years after Hauri and Schroter. Alp. Bot, 124(1): 59-70. DOI:10.1007/s00035-014-0127-x |
Badano E.I., Cavieres L.A., 2006a. Ecosystem engineering across ecosystems:do engineer species sharing common features have generalized or idiosyncratic effects on species diversity? J. Biogeogr, 33(2): 304-313. DOI:10.1111/jbi.2006.33.issue-2 |
Badano E.I., Cavieres L.A., 2006b. Impacts of ecosystem engineers on community attributes:effects of cushion plants at different elevations of the Chilean Andes. Divers. Distrib, 12(4): 388-396. DOI:10.1111/ddi.2006.12.issue-4 |
Badano E.I., Jones C.G., Cavieres L.A., et al, 2006. Assessing impacts of ecosystem engineers on community organization:a general approach illustrated by effects of a high-Andean cushion plant. Oikos, 115(2): 369-385. DOI:10.1111/oik.2006.115.issue-2 |
Benedict J.B., 1989. Use of Silene acaulis for dating:the relationship of cushion diameter to age. Arct. Alp. Res, 21(1): 91-96. DOI:10.2307/1551520 |
Bliss L.C., 1971. Arctic and alpine plant life cycles. Annu. Rev. Ecol. Syst, 2(2): 405-438. |
Boucher F.C., Lavergne S., Basile M., et al, 2016. Evolution and biogeography of the cushion life form in angiosperms. PPEES, 20: 22-31. |
Callaway R.M., Walker L.R., 1997. Competition and facilitation:a synthetic approach to interactions in plant communities. Ecology, 78(7): 1958-1965. DOI:10.1890/0012-9658(1997)078[1958:CAFASA]2.0.CO;2 |
Carroll S.B., Delph L.F., 1996. The effects of gender and plant architecture on allocation to flowers in dioecious Silene latifolia (Caryophyllaceae). Int. J. Plant. Sci, 157(4): 493-500. DOI:10.1086/297367 |
Case, A. L., Ashman, T. L., 2005. Sex-specific physiology and its implications for the cost of reproduction. In: Reekie, E., Bazzaz, F. A. (Eds. ), Reproductive Allocation in Plants. Elsevier Academic Press, UK, pp. 126-154.
|
Cavieres L.A., Arroyo M.T.K., Molina-Montenegro M., et al, 2002. Nurse effect of Bolax gummigera (Apiaceae) cushion plants in the alpine vegetation of the Chilean Patagonian Andes. J. Veg. Sci, 13(4): 547-554. |
Cavieres L.A., Badano E.I., 2009. Do facilitative interactions increase species richness at the entire community level? J. Ecol, 97(6): 1181-1191. DOI:10.1111/jec.2009.97.issue-6 |
Cavieres L.A., Badano E.I., Sierra-Almeida A., et al, 2007. Microclimatic modifications of cushion plants and their consequences for seedling survival of native and non-native herbaceous species in the high Andes of central Chile. Arct.Antarct. Alp. Res, 39(2): 229-236. DOI:10.1657/1523-0430(2007)39[229:MMOCPA]2.0.CO;2 |
Cavieres L.A., Brooker R.W., Butterfield B.J., et al, 2014. Facilitative plant interactions and climate simultaneously drive alpine plant diversity. Ecol. Lett, 17(2): 193-202. DOI:10.1111/ele.12217 |
Cavieres L.A., Hernandez-Fuentes C., Sierra-Almeida A., et al, 2016. Facilitation among plants as an insurance policy for diversity in alpine communities. Funct.Ecol, 30(1): 52-59. DOI:10.1111/1365-2435.12545 |
Cavieres L.A., Quiroz C.L., Molina-Montenegro M.A., 2008. Facilitation of the nonnative Taraxacum officinale by native nurse cushion species in the high Andes of central Chile:are there differences between nurses? Funct. Ecol, 22(1): 148-156. |
Charlesworth B., Charlesworth D., 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat, 112(988): 975-997. DOI:10.1086/283342 |
Charlesworth D., Morgan M.T., 1991. Allocation of resources to sex functions in flowering plants. Trans. R. Soc. B-Biol. Sci, 332(1262): 91-102. DOI:10.1098/rstb.1991.0036 |
Chen J.G., Niu Y., Yang Y., et al, 2016. Sexual allocation in the gynodioecious species Cyananthus macrocalyx (Campanulaceae) at high elevations in the SinoHimalaya Mountains. Alp. Bot, 126(1): 49-57. DOI:10.1007/s00035-015-0154-2 |
Chen J.G., Niu Y., Li Z.M., et al, 2017. Sex allocation in gynodioecious Cyananthus delavayi differs between gender morphs and soil quality. Plant Reprod, 30: 107-117. DOI:10.1007/s00497-017-0303-4 |
Chen J.G., Schöb C., Zhou Z., et al, 2015. Cushion plants can have a positive effect on diversity at high elevations in the Himalayan Hengduan Mountains. J. Veg.Sci, 26(4): 768-777. DOI:10.1111/jvs.12275 |
Chen J.G., Yang Y., Stöcklin J., et al, 2014. Soil nutrient availability determines the facilitative effects of cushion plants on other plants species at high elevations in the South-Eastern Himalayas. Plant Ecol. Divers, 8(2): 199-210. |
Cranston B.H., Monks A., Whigham P.A., et al, 2015. Variation and response to experimental warming in a New Zealand cushion plant species. Austral Ecol, 40(6): 642-650. DOI:10.1111/aec.2015.40.issue-6 |
Delph L.F., 1990. Sex ratio variation in the gynodioecious shrub Hebe strictissima(Scrophulariaceae). Evolution, 44(1): 134-142. DOI:10.1111/evo.1990.44.issue-1 |
Delph L.F., Bailey M.F., Marr D.L., 1999. Seed provisioning in gynodioecious Silene acaulis (Caryophyllaceae). Am. J. Bot, 86(1): 140-144. DOI:10.2307/2656963 |
Delph L.F., Carroll S.B., 2001. Factors affecting relative seed fitness and female frequency in a gynodioecious species, Silene acaulis. Evol. Ecol. Res, 3(4): 487-505. |
Dufay M., Lahiani E., Brachi B., 2010. Gender variation and inbreeding depression in gynodioecious-gynomonoecious Silene nutans (Caryophyllaceae). Int. J. Plant Sci, 171(1): 53-62. DOI:10.1086/647916 |
Ehrlén J., Lehtilä K., 2002. How perennial are perennial plants?. Oikos, 98(98): 308-322. |
Fine P.V.A., Ree R.H., 2006. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat, 168(6): 796-804. |
Forbis T.A., Doak D.F., 2004. Seedling establishment and life history trade-offs in alpine plants. Am. J. Bot, 91(7): 1147-1153. DOI:10.3732/ajb.91.7.1147 |
García-Camacho R. Totland Ø., Totland Ø., 2009. Pollen limitation in the alpine:a metaanalysis. Arct. Antarct. Alp. Res, 41(103): 103-111. |
Gibson N., 1990. The environments and primary production of cushion species at Mt Field and Mt Wellington, Tasmania. Aust. J. Bot, 38(3): 229-243. DOI:10.1071/BT9900229 |
Gugerli F., 1998. Effect of elevation on sexual reproduction in alpine populations of Saxifraga oppositifolia (Saxifragaceae). Oecologia, 114(1): 60-66. DOI:10.1007/s004420050420 |
Hermanutz L.A., Innes D.J., 1994. Gender variation in Silene acaulis (Caryophyllaceae). Plant Syst. Evol, 191(1-2): 69-81. DOI:10.1007/BF00985343 |
Kleier C., Rundel P.W., 2004. Microsite requirements, population structure and growth of the cushion plant Azorella compacta in the tropical Chilean Andes. Austral Ecol, 29(4): 461-470. DOI:10.1111/aec.2004.29.issue-4 |
Kleier C., Trenary T., Graham E.A., et al, 2015. Size class structure, growth rates, and orientation of the central Andean cushion Azorella compacta. PeerJ, 3: e843. DOI:10.7717/peerj.843 |
Klimes, L., Klimesova, J., Hendriks, R., et al., 1997. Clonal plant architecture: a comparative analysis of form and function. In: de Kroon, J. V. G. E. H. (Ed. ), The Ecology and Evolution of Clonal Plants. Backhuys Publishers, Leiden, The Netherlands, pp. 1-29.
|
Körner, C., 2003. Alpine Plant Life, second ed. Springer, Berlin, DE.
|
Körner, C., Paulsen, J., 2009. Exploring and explaining mountain biodiversity. In: Spehn, E. M., Korner, C. (Eds. ), Data Mining for Global Trends in Mountain Biodiversity. CRC Press, Boca Raton, Florida, USA, pp. 1-10.
|
le Roux P.C., McGeoch M.A., 2004. The use of size as an estimator of age in the subantarctic cushion plant, Azorella selago (Apiaceae). Arct. Antarct. Alp. Res, 36(4): 509-517. DOI:10.1657/1523-0430(2004)36[509:TUOSAA]2.0.CO;2 |
Li B.S., Wang J.T., Li S.Y., 1987. The floristic features and geographic distribution of the cushion plant in Xizang. Mt. Res, 5(1): 14-20. |
Lloyd D.G., 1976. The transmission of genes via pollen and ovules in gynodioecious angiosperms. Theor. Pop. Biol, 9(3): 299-316. DOI:10.1016/0040-5809(76)90050-2 |
Lortie C.J., Reid A.M., 2012. Reciprocal gender effects of a keystone alpine plant species on other plants, pollinators, and arthropods. Botany-Botanique, 90(4): 273-282. DOI:10.1139/b11-112 |
Lundbye H., Johansson D.K., Andersen M.R., et al, 2012. The effect of a seedsucking bug on seed germination of an arctic cushion plant. Ecoscience, 19(3): 209-212. DOI:10.2980/19-3-3510 |
Mackay A., 2008. Climate change 2007:impacts, adaptation and vulnerability.Contribution of working group Ⅱ to the fourth assessment report of the intergovernmental panel on climate change. J. Environ. Qual, 37(6): 2407. |
Maurice S., Desfeux C., Mignot A., et al, 1998. Is Silene acaulis (Caryophyllaceae) a trioecious species? Reproductive biology of two subspecies. Can. J. Bot, 76(3): 478-485. |
Mccarthy D.P., 1992. Dating with cushion plants e establishment of a Silene acaulis growth curve in the Canadian Rockies. Arct. Alp. Res, 24(1): 50-55. DOI:10.2307/1551319 |
Medan D., Montaldo N.H., Devoto M., et al, 2002. Plant-pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arct. Antarct. Alp. Res, 34(3): 233-241. DOI:10.2307/1552480 |
Méndez E., 2011. Growth and covering of Azorella monantha Clos (Apiaceae) in the high central Andes of Mendoza Argentina. Rev. Fac.Cien. Agrar, 43(1): 219-229. |
Milla R., Gimenez-Benavides L., Escudero A., et al, 2009. Intra-and interspecific performance in growth and reproduction increase with altitude:a case study with two Saxifraga species from Northern Spain. Funct. Ecol, 23(1): 111-118. DOI:10.1111/fec.2009.23.issue-1 |
Molau U., 1997. Age-related growth and reproduction in Diapensia lapponica, an arctic-alpine cushion plant. Nord. J. Bot, 17(3): 225-234. DOI:10.1111/j.1756-1051.1997.tb00314.x |
Molenda O., Reid A., Lortie C.J., 2012. The alpine cushion plant Silene acaulis as foundation species:a bug's-eye view to facilitation and microclimate. PLoS One, 7: e37223. DOI:10.1371/journal.pone.0037223 |
Molina-Montenegro M.A., Badano E.I., Cavieres L.A., 2006. Cushion plants as microclimatic shelters for two ladybird beetles species in alpine zone of central Chile. Arct. Antarct. Alp. Res, 38(2): 224-227. DOI:10.1657/1523-0430(2006)38[224:CPAMSF]2.0.CO;2 |
Morris W., Doak D., 1998. Life history of the long-lived gynodioecious cushion plant Silene acaulis (Caryophyllaceae), inferred from size-based population projection matrices. Am. J. Bot, 85(6): 784-793. DOI:10.2307/2446413 |
Peng D.L., Zhang Z.Q., Xu B., et al, 2012. Patterns of flower morphology and sexual systems in the subnival belt of the Hengduan Mountains, SW China. Alp. Bot, 122(2): 65-73. DOI:10.1007/s00035-012-0104-1 |
Philipp M., Böcher J., Mattson O., et al, 1990. A quantitative approach to the sexual reproductive biology and population structure in some arctic flowering plants:Dryas integrifolia, Silene acaulis and Ranunculus nivalis. Medd. Grønl. Biosci, 34: 3-60. |
Ramsey M., Vaughton G., Peakall R., 2006. Does inbreeding avoidance maintain gender dimorphism in Wurmbea dioica (Colchicaceae)? J. Evol. Biol, 19(5): 1497-1506. DOI:10.1111/jeb.2006.19.issue-5 |
Reid A.M., Lortie C.J., 2012. Cushion plants are foundation species with positive effects extending to higher trophic levels. Ecosphere, 3(11): 96. |
Roy J., Albert C.H., Ibanez S., et al, 2013. Microbes on the cliff:alpine cushion plants structure bacterial and fungal communities. Front. Microbiol, 4(64): 1-14. |
Sakai A.K., Weller S.G., Chen M.L., et al, 1997. Evolution of gynodioecy and maintenance of females:the role of inbreeding depression, outcrossing rates, and resource allocation in Schiedea adamantis (Caryophyllaceae). Evolution, 51(3): 724-736. DOI:10.1111/evo.1997.51.issue-3 |
Schöb C. Butter field B.J., Butter field B.J., Pugnaire F.I., 2012. Foundation species influence traitbased community assembly. New Phytol, 196(3): 824-834. DOI:10.1111/nph.2012.196.issue-3 |
Sieber Y., Holderegger R., Waser N.M., et al, 2011. Do alpine plants facilitate each other's pollination? Experiments at a small spatial scale. Acta Oecol. Int. J. Ecol, 37(4): 369-374. DOI:10.1016/j.actao.2011.04.005 |
Shykoff J.A., 1988. Maintenance of gynodioecy in Silene acaulis (Caryophyllaceae):stage-specific fecundity and viability selection. Am. J. Bot, 75(6): 844-850. DOI:10.2307/2444003 |
Shykoff J.A., 1992. Sex polymorphism in Silene acaulis (Caryophyllaceae) and the possible role of sexual selection in maintaining females. Am. J. Bot, 79(2): 138-143. DOI:10.2307/2445100 |
Williams C.F., Kuchenreuther M.A., Drew A., 2000. Floral dimorphism, pollination, and self-fertilization in gynodioecious Geranium richardsonii (Geraniaceae). Am.J. Bot, 87(5): 661-669. DOI:10.2307/2656852 |
Wirth L.R., Graf R., Gugerli F., et al, 2010a. Between-year variation in seed weights across altitudes in the high-alpine plant Eritrichium nanum. Plant Ecol, 207(2): 227-231. DOI:10.1007/s11258-009-9667-3 |
Wirth L.R., Graf R., Gugerli F., et al, 2010b. Lower selfing rate at higher altitudes in the alpine plant Eritrichium nanum (Boraginaceae). Am. J. Bot, 97(5): 899-901. DOI:10.3732/ajb.0900297 |
Wirth L.R., Waser N.M., Graf R., et al, 2011. Effects of floral neighborhood on seed set and degree of outbreeding in a high-alpine cushion plant. Oecologia, 167(2): 427-434. DOI:10.1007/s00442-011-1985-1 |
Yang, Y., Chen, J. G., Schöb, C., et al., 2017. Size-mediated interaction between a cushion species and other non-cushion species at high elevations of the Hengduan Mountains, SW China. Front. Plant Sci. http://dx.doi.org/10.3389/fpls.2017.00465online.
|
Yang Y., Niu Y., Cavieres L.A., et al, 2010. Positive associations between the cushion plant Arenaria polytrichoides (Caryophyllaceae) and other alpine plant species increase with altitude in the Sino-Himalayas. J. Veg. Sci, 21(6): 1048-1057. DOI:10.1111/j.1654-1103.2010.01215.x |
Zhang R.Z., Zheng D., Yang Q.Y., et al, 1997. Physical Geography of Hengduan Mountains. Beijing, China: Science Press,.
|
Zoller H., Lenzin H., Erhardt A., 2002. Pollination and breeding system of Eritrichium nanum (Boraginaceae). Plant Syst. Evol, 233(1): 1-14. |
Zoller H., Lenzin H., Rusterholz H.P., et al, 2005. Increasing population density and seed production with altitude in Eritrichium nanum (Boraginaceae) e an arctic alpine obligatory seeder. Arct. Antarct. Alp. Res, 37(1): 41-48. DOI:10.1657/1523-0430(2005)037[0041:IPDASP]2.0.CO;2 |



