b. Shangri-La Alpine Botanical Garden, Shangri-la 674400, China;
c. Yunnan Academy of Biodiversity, Southwest Forestry University, Kunming 650224, China
Plant abundance is thought to be closely related to climatic conditions, species interactions and human disturbance (Liu et al., 2011; Soliveres and Maestre, 2014; Hawkins et al., 2015; Junker et al., 2015). Many studies have shown that temperature and rainfall are vital to the survival of species (Junker et al., 2015). Biotic species interactions, like facilitation and competitive exclusion, are also known to have a great effect on plant community structure (Maestre et al., 2005; Soliveres and Maestre, 2014), and further influence the abundance of each species. In addition, disturbance, especially human caused, is an increasingly important factor for determining species survival (Hawkins et al., 2015).
The rapid development of traditional Chinese medicine pharmacies has increased the demand for Fritillaria cirrhosa D. Don, a well-known and valuable traditional Chinese herb, augmenting its over-exploitation and leading to its decline in the wild. Based on our market survey, from 2013 to 2016, 1 kg of high-quality F. cirrhosa commanded about 3000 yuan (RMB). The high value placed on F. cirrhosa bulbs has spurred excessive collecting and exacerbated its scarcity.
Over-exploitation may lead to changes in various traits. For example, over-collection of plants with shallow stems will increase the mean underground bulb depth of the remaining plants. Plants farther from roads are more difficult to collect. Therefore, we hypothesized that collection pressure would weaken with increasing distance from roads, and we predicted that consequently the abundance of over-collected plants like F. cirrhosa would increase. Here, we integrate information about F. cirrhosa from 78 plots at 14 sites in the Hengduan Mountains area to assess how climatic conditions, biological interactions and human-caused disturbance affect F. cirrhosa abundance and other traits. We therefore examined: 1) whether the abundance of F. cirrhosa is related to the distance from roads, which is directly linked to human-caused disturbance, 2) whether traits such as diameter/height of the fruit/bulb reflect the same response to human disturbance, and 3) how important climatic factors, interactions with other species, and disturbance are to F. cirrhosa abundance. We also tried to speculate on the underlying reasons for abundance patterns of F. cirrhosa.
2. Materials and methods 2.1. Study materialFritillaria is a genus of about 130 species in the Liliaceae distributed throughout the north temperate hemisphere (Chen et al., 2000). In Chinese medical books, the bulbs of Frittilaria cirrhosa, Fritillaria unibracteata, Fritillaria przewalskii, Fritillaria unibracteata and Fritillaria delavayi are treated under the medical name "Fritillariae Cirrhosae Bulbus" (Chinese-Pharmacopoeia-Commission, 2005). Their distribution center is in the Hengduan Mountains area of western Sichuan, southeastern Tibet and northwestern Yunnan (Li, 1987). Fritillaria spp. grow in alpine shrub or alpine meadow communities between 3200 and 4600 m (Chen et al., 2000). The average annual temperature in the distribution area, as in Litang County, is only 3.0 ℃, whereas the highest temperature recorded is 25.6 ℃, and the lowest temperature is -25.8 ℃. Average annual precipitation is 784.9 mm (Chen et al., 2003). The dominant flora in the distribution area of F. cirrhosa is north temperate (Li and Li, 1993). North temperate genera, such as Rhododendron, Spiraea, Sibiraea, Saussurea, Potentilla, Salix, and Anaphalis play a vital role in the communities in which Fritillaria occur.
We sampled F. cirrhosa in its distribution center using 2 m × 2 m quadrats to determine its status in nature (Fig. 1). The quadrats were chosen randomly at different distances from the main road (national road, province road, or county road). Because of its rarity in the wild, the number of quadrats at each site was not equal (Table 1).

|
| Fig. 1 Study sites of Fritillaria cirrhosa (the map is available from http://srtm.csi.cgiar.org/). Sites: 1. Dongda Mountain, 2. Yela Mountain, 3. Zongla Mountain, 4. Aila Mountain, 5. Que'er Mountain Ⅰ, 6. Que'er Mountain Ⅱ, 7. Site between Luhuo County and Maerkang County, 8. Baozuo Xiang, 9. Yakexia Mountain, 10. Zhegu Mountain, 11. Mengbi Mountain, 12. Zheduo Mountain, 13. Jianziwan Mountain, 14. Baima Mountain. |
| No. | Sites | Latitude (°N) |
Longitude (°E) |
Elevation (m) |
Quadrats number | Province |
| 1 | Dongda Mountain | 29.73 | 98.07 | 4885 | 5 | Tibet |
| 2 | Yela Mountain | 30.15 | 97.29 | 4529 | 10 | Tibet |
| 3 | Zongla Mountain | 31.29 | 97.58 | 4052 | 6 | Tibet |
| 4 | Aila Mountain | 31.64 | 98.45 | 4329 | 10 | Tibet |
| 5 | Que'er Mountain Ⅰ | 31.92 | 98.95 | 4460 | 4 | Sichuan |
| 6 | Que'er Mountain Ⅱ | 31.91 | 98.98 | 4281 | 4 | Sichuan |
| 7 | Luhuo-Maerkang County | 31.77 | 100.75 | 4081 | 4 | Sichuan |
| 8 | Baozuo Xiang | 33.06 | 103.35 | 3768 | 8 | Sichuan |
| 9 | Yakexia Mountain | 32.23 | 102.59 | 4056 | 2 | Sichuan |
| 10 | Zhegu Mountain | 31.86 | 102.68 | 4081 | 3 | Sichuan |
| 11 | Mengbi Mountain | 31.7 | 102.32 | 3970 | 5 | Sichuan |
| 12 | Zheduo Mountain | 30.05 | 101.83 | 3911 | 9 | Sichuan |
| 13 | Jianziwan Mountain | 30.01 | 100.86 | 4328 | 3 | Sichuan |
| 14 | Baima Mountain | 28.38 | 99 | 4459 | 5 | Yunnan |
Vascular plant species were recorded in each quadrat of each site. At the same time, the number of plants of F. cirrhosa, the latitude and longitude of the site, the elevation of each quadrat, and the coverage of each species were recorded. The climate of each quadrat, based on 19 parameters, was extracted from the WorldClim version 1.4 database (www.worldclim.org; Hijmans et al., 2005). The distance from the main road of each quadrat was documented in Google Maps using the coordinates of the sample site. The diameter and height of bulbs and fruits, as well as the underground bulb depth were also measured and recorded for further analysis.
2.3. AnalysesTo determine whether proximity to the road affects the abundance of F. cirrhosa, the number of plants of F. cirrhosa and the underground bulb depth in each quadrat were related to the distance from the main road. The slope difference between sites was determined using the command 'diffslope' in R software (version 3.3.1, http://www.r-project.org). The relationships between underground bulb depth and the height/diameter of the bulbs/fruits of each quadrat were analyzed to test if the habitat was degraded throughout the whole distribution area. The relationship between underground bulb depth and height/diameter of the bulbs/fruits at each quadrat was determined and the slope difference among them was tested to examine if the height/diameter of bulbs/fruits showed the same trends under the pressure of heavy collecting.
To assess the correlations between the number of individuals of F. cirrhosa and all measured variables, a variation partitioning method (Borcard et al., 1992) was applied to distinguish the pure and joint effects of environmental factors, the effects caused by other factors, and human disturbance on the number of individuals of F. cirrhosa in the distribution center. The variation partitioning and regression analyses were performed in the R software (version 3.3.1, http://www.r-project.org).
3. ResultsThe relationship between F. cirrhosa number and distance from the road for the Dongdala, Zongla Mountain, and Yela Mountain sites in Tibet had the largest slopes. Abundance of bulbs at Zongla Mountain, increased significantly with increasing distance from the main road, while showing only a marginally significant increase at other sites (Fig. 2, Table 2).
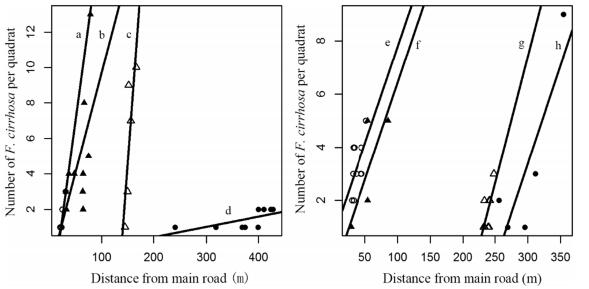
|
| Fig. 2 Relationship between distance from main road and number of individuals of Fritillaria cirrhosa in each quadrat (2 m × 2 m). a: Zongla Mountain, Tibet (open circles), b: Yela Mountain, Tibet (solid triangles), c: Dongda Mountain, Tibet (open triangles), d: Zheduo Mountain, Sichuan (solid circles), e: Aila Mountain, Tibet (open circles), f: Mengbi Mountain, Sichuan (solid triangles), g: Baozuo Xiang, Sichuan (open triangles), h: Baima Mountain, Yunnan (solid circles). The quadrats number less than 4 was not included. |
| Sites | d.f. | F | Slope | Adjusted R2 | P-value | Difference |
| Dongda Mountain | 1, 3 | 6.676 | 0.3987 | 0.5866 | 0.08151 | A |
| Zongla Mountain | 1, 4 | 22.7 | 0.2186 | 0.8127 | 0.008876 | B |
| Yela Mountain | 1, 8 | 4.178 | 0.1128 | 0.261 | 0.0752 | BC |
| Baozuo Xiang | 1, 6 | 5.179 | 0.0952 | 0.3738 | 0.06317 | C |
| Baima Mountain | 1, 3 | 8.353 | 0.0742 | 0.6477 | 0.06301 | CD |
| Mengbi Mountain | 1, 3 | 7.028 | 0.0727 | 0.6011 | 0.07693 | De |
| Aila Mountain | 1, 8 | 3.78 | 0.0724 | 0.236 | 0.08776 | eF |
| Zheduo Mountain | 1, 7 | 5.41 | 0.0058 | 0.3554 | 0.05293 | G |
The underground bulb depth significantly decreased with increasing distance from the main road; Bulb depth was deeper for plants near the road, while it was shallower farther from the road (Fig. 3).
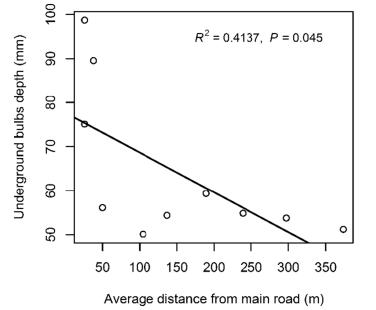
|
| Fig. 3 Relationship between average distance from main road and average underground bulb depth of each site in Hengduan Mountains. |
The size (height/diameter) of the bulbs and fruits showed a significant or marginally significant decrease with underground bulb depth (Fig. 4, Table 3). The slope between the diameter of the bulb and the underground bulb depth was significantly smaller than the slope between the height of the bulb and the underground bulb depth, while the slope between the diameter of the fruits and the underground bulbs depth was significantly greater than the slope between the height of the fruits and underground bulb depth (Fig. 4, Table 3).
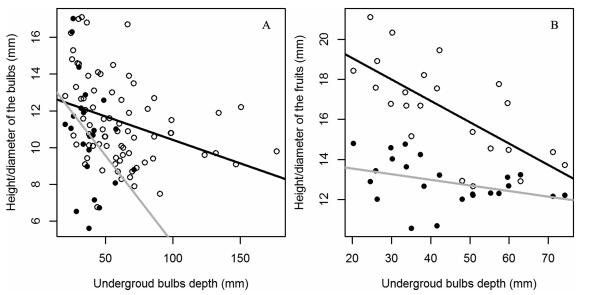
|
| Fig. 4 Relationship between underground bulb depth and height/diameter of the bulbs/fruits in Hengduan Mountains. a, the line of bulb height along underground bulb depth; b, the line of the bulb diameter along underground bulb depth; c, the line of fruit height along underground bulb depth; d, the line of the fruit diameter along underground bulb depth (Table 3). |
| Items | d.f. | F | Slope | Adjusted R2 | P-value | Difference |
| Height of bulbs | 1, 73 | 9.622 | -0.0255 | 0.1044 | 0.002733 | A |
| Diameter of bulbs | 1, 21 | 3.971 | -0.0950 | 0.119 | 0.05946 | B |
| Height of fruits | 1, 21 | 19.23 | -0.1067 | 0.4531 | 0.0002588 | A |
| Diameter of fruits | 1, 21 | 3.607 | -0.0282 | 0.106 | 0.07135 | B |
Overall, community species, climatic conditions and spatial position can explain 58%, 25% and 31% of the variance in the abundance of F. cirrhosa, respectively, for the samples examined in the Hengduan Mountains area (Fig. 5). Among the dominant species, Juniperus pingii and Caragana jubata had the most vital influence on the abundance of F. cirrhosa. Other shrubs, like Potentilla fruticosa, Lonicera hispida, Salix oritrepha, Potentilla stenophylla, Anaphalis muliensis, Spiraea alpina, Codonopsis alpina and Rhododendron nivale also showed a significant influence on the abundance of F. cirrhosa (Fig. 5A). Among the 19 climate factors, annual precipitation had the greatest impact on the abundance of F. cirrhosa; temperature seasonality and minimum temperature of the coldest month also showed significant impacts on the abundance of F. cirrhosa (Fig. 5B). Elevation and longitude were significantly related to the abundance of F. cirrhosa (Fig. 5C).
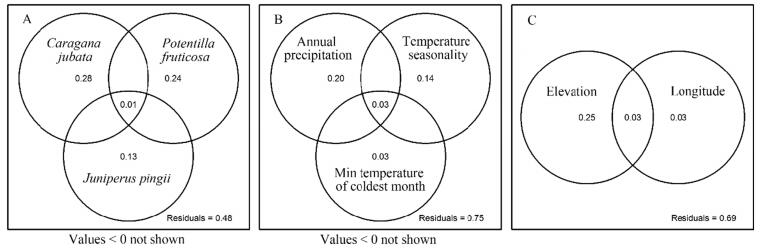
|
| Fig. 5 Percentage of variance in Fritillaria cirrhosa abundance explained by presence of other species (A), environmental conditions (B), and spatial elements (C). |
Our results indicate that climatic conditions, biotic settings and disturbance are important factors in the abundance of F. cirrhosa.
4.1. Biotic settingsIn plant communities where F. cirrhosa is distributed, we found that the predominant species are always shrubs with dense branches, thorns or slim stems. Our results showed that the ambient species, especially predominant shrubs, had an important effect on the abundance of F. cirrhosa, likely indicating that the shrubs protect F. cirrhosa from frequent human disturbance.
Seed germination is very high in culture dishes under natural conditions, and they grow well in the Greenhouse at Shangri-La Alpine Botany Garden without the protection of shrubs. Therefore, the predominant shrub species in the wild probable provide a habitat refuge from human disturbance for F. cirrhosa. Juniperus spp. have spiny-tipped leaves which deter collectors and their strong roots make it difficult to dig the bulbs out. The strong thorns of Caragana jubata, as at Yela Mountain, are effective in deterring collectors and livestock. Some species of Juniperus and Rhododendron form dense communities, as at Dongdala and Zheduo Mountain, preventing feet from stepping on them and protecting the species living among their branches. The slim, hard stems of Salix and Potentilla are similar to the stems of Fritillaria, making Fritillaria difficult to be detected. Shrubs may therefore provide refuge for Fritillaria and other plants compared to open areas.
4.2. Climatic conditionsClimatic conditions are important factors in determining the abundance of plant species (Mohamed et al., 2014). Temperature and precipitation are recognized to be the most important factors in explaining plant distribution in mountains (Guisan and Zimmermann, 2000; Korner, 2003). A previous study indicated that temperature was a key ecological factor for F. unibracteata in the wild (Xu et al., 2013). In this study, we found that among various climate conditions, annual precipitation, temperature seasonality and minimum temperature of the coldest month have the largest effect on the number of individuals of F. cirrhosa (Fig. 5B).
Annual precipitation always has a great impact on the distribution of organisms, and can be used to predict patterns of plant density (Richerson and Lum, 1980; Carpenter, 2005; Bhandari et al., 2015). Carpenter (2005) found that woody plant diversity was positively related to annual precipitation during a study of environmental factors affecting plant species density in eastern Nepal. A study conducted on the northern Tibetan Plateau found that species richness responds positively to precipitation at the community level (Wu et al., 2014). Our results also provide evidence that higher levels of annual precipitation result in greater abundance of F. cirrhosa. In addition, the seeds of F. cirrhosa need an after-ripening period (Pan, 2004). During this process, humid conditions are required for proper development of the embryo. Precipitation can therefore be inferred to be a vital factor in the seed after-ripening process.
Our results also showed that seasonal temperatures and the minimum temperature of the coldest month have a great impact on the abundance of F. cirrhosa, which conformed with previous studies (Pither, 2003; Khanum et al., 2013; Kreyling et al., 2015; Yu et al., 2016). Yu et al. (2016) reported climatic variation to be a substantial and important factor for wide-ranging alpine and subalpine plants. Pither (2003) reported that extreme climate (represented by the lowest temperature of the coldest month or quarter) determined the distribution of plants. Over a large area and wide range of elevations, seasonality indicates periodic departures from optimal climatic conditions for living organisms. The climatic variation may train the resistance of the plant, and the minimum temperature of the coldest month may be related to the vernalization of alpine plants and reduce diseases and pests in alpine regions. Therefore, climatic variation and the minimum temperature of the coldest month are thought to be important determinants for the distribution of F. cirrhosa.
4.3. DisturbanceHuman disturbance has an influence on the distribution of organisms, especially resource plants such as medicinal and ornamental plants. As a medicinal plant, the Fritillaria Cirrhosa Bulb is commonly used in traditional Chinese medicine as a cough remedy. According to our market research, the price of high-quality Fritillaria Cirrhosa Bulb is more than 3000 RMB (Yuan) per kilo. Because natural resources are being diminished, the price may possibly increase in the future. In our field work in 2015, seeds were being sold at the price of 1–2 yuan per seed. Because of the higher price, F. cirrhosa has been increasingly over-collected in the wild and its habitat has been seriously disturbed.
Elevation, like temperature, represents an environmental gradient. In this study, it can also serve as a proxy for disturbance, because higher sites are visited by fewer people. We found that elevation has a great effect on the abundance of F. cirrhosa (Fig. 5C). This also shows varying human influence along an elevation gradient.
We found that F. cirrhosa was less abundant nearer to the road (Figs. 2 and 3), indicating that sites which are easier to access are under greater collection pressure. This can also be inferred by the underground bulb depth, as bulbs are deeper when they are nearer to the road. Our findings also showed that habit disturbance along the road was different in Tibet than Sichuan and Yunnan (Table 2). These findings also reflect the fact that there was a significant increase in F. cirrhosa abundance at Dongdala, Yela and Zonlga mountain areas, all of which are farther from cities than the other sites and accessed by fewer people.
In these Tibetan areas, the disturbed areas are mainly near the road; places far from the road were disturbed less, so the slope of regression was high. The sites in Sichuan and Yunnan were more disturbed and F. cirrhosa was less abundant, even far from the road, so the slope of the regression was low.
Bulb diameter and fruit height showed a significantly different slope with the underground bulb depth compared to bulb height and fruit diameter, respectively (Fig. 4). The longer the fruits, the easier the plants can be found because of the horizontal view when collectors look for them. The larger the diameter of the bulbs, the easier they can be collected because of the vertical view when collectors dig them. Because certain traits are easier to detect by people, these traits may be preferentially selected in response to heavy collecting.
Other factors, such as soil conditions and plant-animal interactions, can also influence the F. cirrhosa abundance. However, human disturbance may have an increasingly important effect on F. cirrhosa habitat, and may explain the large variance in F. cirrhosa numbers, especially if we assume that elevation and distance from the road are valid proxies for human disturbance.
4.4. ConservationOur results indicate that plants of F. cirrhosa with bigger fruits and bulbs are easier to collect. Thus, human collection drives reduction in fruit size, which may influence the reproductive fitness of the plants. This is a latent risk in addition to the direct risk posed by collection of bigger bulbs, which leaves only small bulbs in natural habitats.
5. ConclusionSerious over-collecting, climatic conditions and biotic settings are important factors determining the abundance of F. cirrhosa. The most important among the three factors is disturbance by human collecting. The fruits of F. cirrhosa become thick and short, while bulbs become thin and tall under human heavy collecting. Biotic factors, such as shrubs with thorns or dense shrub communities, have a positive influence on the survival of F. cirrhosa.
AcknowledgmentsThis work was supported by Yunnan Environmental Protection Special Fund 2013, Grant No. 214203, the National Natural Science Foundation of China (NSFC) Grant No. 31560063 and Key Disciplines (Ecology) Project of Yunnan Education Department.
Bhandari J., Pan X., Zhang L., et al, 2015. Diversity and productivity of semi-arid grassland of Inner Mongolia:influence of plant functional type and precipitation. Pak. J. Agric. Sci, 52: 259-264. |
Borcard D., Legendre P., Drapeau P., 1992. Partialling out the spatial component of ecological variation. Ecology, 73: 1045-1055. DOI:10.2307/1940179 |
Carpenter C., 2005. The environmental control of plant species density on a Himalayan elevation gradient. J. Biogeogr, 32: 999-1018. DOI:10.1111/jbi.2005.32.issue-6 |
Chen S.L., Jia M.R., Wu Y., et al, 2003. Study on the plant community of Fritillaria cirrhosa. China J. Chin. Mater. Med, 28: 18-22. |
Chen, X. Q., Mordak, H. V., 2000. Fritillaria. In: Wu, Z. Y., Raven, P. H., Hong, D. Y. (Eds. ), Flora of China, vol. 24. Science Press, Beijing, pp. 127-133. Missouri Botanical Garden Press, St Louis.
|
Chinese-Pharmacopoeia-Commission, 2005. Chinese Pharmacopoeia. Beijing: Chemical Industry Press.
|
Guisan A., Zimmermann N.E., 2000. Predictive habitat distribution models in ecology. Ecol. Model, 135: 147-186. DOI:10.1016/S0304-3800(00)00354-9 |
Hawkins C.P., Mykra H., Oksanen J., et al, 2015. Environmental disturbance can increase beta diversity of stream macroinvertebrate assemblages. Glob. Ecol.Biogeogr, 24: 483-494. DOI:10.1111/geb.2015.24.issue-4 |
Hijmans R.J., Cameron S.E., Parra J.L., et al, 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol, 25: 1965-1978. DOI:10.1002/(ISSN)1097-0088 |
Junker R.R., Bluethgen N., Keller A., 2015. Functional and phylogenetic diversity of plant communities differently affect the structure of flowerevisitor interactions and reveal convergences in floral traits. Evol. Ecol, 29: 437-450. DOI:10.1007/s10682-014-9747-2 |
Khanum R., Mumtaz A.S., Kumar S., 2013. Predicting impacts of climate change on medicinal asclepiads of Pakistan using Maxent modeling. Acta Oecol, 49: 23-31. DOI:10.1016/j.actao.2013.02.007 |
Korner, C., 2003. Alpine Plant Life, second ed. Springer, Berlin.
|
Kreyling J., Schmid S., Aas G., 2015. Cold tolerance of tree species is related to the climate of their native ranges. J. Biogeogr, 42: 156-166. DOI:10.1111/jbi.2014.42.issue-1 |
Li B.Y., 1987. On the boundaries of the Hengduan Mountains. Mountain Res, 5: 74-82. |
Li X.W., Li J., 1993. A preliminary floristic study on the seed plants from the region of Hengduan Mountain. Acta Bot. Yunnanica, 15: 217-231. |
Liu Y., Reich P.B., Li G., et al, 2011. Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology, 92: 1201-1207. DOI:10.1890/10-2060.1 |
Maestre F.T., Valladares F., Reynolds J.F., 2005. Is the change of planteplant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol, 93: 748-757. DOI:10.1111/j.1365-2745.2005.01017.x |
Mohamed A., Reich R.M., Khosla R., et al, 2014. Influence of climatic conditions, topography and soil attributes on the spatial distribution of site productivity index of the species rich forests of Jalisco, Mexico. J. For. Res, 25: 87-95. DOI:10.1007/s11676-014-0434-5 |
Pan X., 2004. Lilium, Fritillaria cirrhosa, Fritillaria ussuriensis, Fritillaria pallidiflora. Beijing: Scientific and Technical Documents Publishing House.
|
Pither J., 2003. Climate tolerance and interspecific variation in geographic range size. J. Ecol, 270: 475-481. |
Richerson P.J., Lum K., 1980. Patterns of plant-species diversity in Californiarelation to weather and topography. Am. Nat, 116: 504-536. DOI:10.1086/283645 |
Soliveres S., Maestre F.T., 2014. Planteplant interactions, environmental gradients and plant diversity:a global synthesis of community-level studies. Perspect.Plant Ecol. Evol. Syst, 16: 154-163. DOI:10.1016/j.ppees.2014.04.001 |
Wu J., Shen Z., Shi P., et al, 2014. Effects of grazing exclusion on plant functional group diversity alpine grasslands along a precipitation gradient on the Northern Tibetan Plateau. Arct. Antarct. Alp. Res, 46: 419-429. DOI:10.1657/1938-4246-46.2.419 |
Xu B., Wang J., Shi F., et al, 2013. Adaptation of biomass allocation patterns of wild Fritillaria unibracteata to alpine environment in the eastern Qinghai-Xizang Plateau. Chin. J. Plant Ecol, 37: 187-196. DOI:10.3724/SP.J.1258.2013.00019 |
Yu F., Groen T.A., Wang T., et al, 2016. Climatic niche breadth can explain variation in geographical range size of alpine and subalpine plants. Int. J. Geogr. Inform.Sci, 31: 190-212. |



