b. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, 650201 Kunming, Yunnan, China
The organization of male and female reproductive functions exhibits great flexibility in flowering plants, forming various sexual systems (Barrett, 2002). These systems can be categorized into two general classes, monomorphic and dimorphic, depending on whether all individuals in a population adopt the same or different gender strategies (Geber et al., 1999). Sexually dimorphic systems often include unisexual individuals that lack either male or female function in reproduction, e.g., gynodioecy and androdioecy, respectively. To overcome this disadvantage in reproduction and maintain such sexual systems, it is essential that unisexual individuals have higher fitness than hermaphrodites. This can be achieved by producing a larger number of offspring (resource compensation) or breeding offspring with greater viability (heterozygote advantage, Geber et al., 1999).
Androdioecy is a very rare sexual system in nature which is characterized by male individuals coexisting with hermaphrodites in the same populations (Richards, 1997). Both theoretical and empirical studies have suggested that to invade and maintain an outcrossing population with hermaphrodites, male individuals need to disperse more pollen and sire more than twice the number of progeny as hermaphrodites (reviewed in Pannell, 2002a). Datisca glomerata (Datiscaceae) is one of the very few cases of androdioecy. Male individuals of D. glomerata produce more than three times as much pollen as hermaphrodites, and exhibit outcrossing advantage (Fritsch and Rieseberg, 1992; Rieseberg et al., 1993). Indeed, compared to female function (seed production), it is difficult for unisexual individuals to achieve a greater reproductive advantage than hermaphrodites through male function (pollen export, Pannell, 2002a, b). This may explain why androdioecy is the rarest sexual system in nature and only occurs in a few plant taxa.
Despite this, the frequency of androdioecy is prone to be overestimated because the sex of plants is often described based on morphological criteria, which may not reflect their actual function in reproduction. For example, some morphologically "androdioecious" plants are actually functionally dioecious, because morphologically hermaphroditic flowers only function as females in many cases (Mayer and Charlesworth, 1991). In addition, androdioecy may be confused with the phenomenon of gender diphasy (or "gender switching"). For example, some perennial plants can change their sex expression in different years, effectively "choosing" to be either a functional male or hermaphrodite in a specific flowering season. If investigations are limited to a single season, these plants may easily be regarded as androdioecious (Manicacci and Després, 2001). Panax trifolium was regarded as androdioecious initially, but verified to change sex after long-term study (Schlessman, 1991). In these circumstances, if the lifetime contribution of male and female reproductive function for each individual is considered, they are likely to adopt the same gender strategy, which should therefore not be regarded as a dimorphic sexual system (Zhang et al., 2014).
Gender-diphasic species "choose" to express a specific sex in a certain period, and the sex expression of these organisms is not only genetically controlled but also influenced by changes in the environment (Vega-Frutis et al., 2014). This phenomenon can be considered an extreme situation within a broader framework of size-dependent sex allocation (Zhang et al., 2014). As a strategy, interannual sex change may optimize resource allocation between two sexual functions depending on changing allocation-gain curves (Lloyd, 1979; Lloyd and Bawa, 1984; Zhang, 2006). The "size advantage hypothesis" is widely accepted to explain sex change in many animals. It predicts that the efficiency of fitness achievement through either male or female function is size-dependent (reviewed in Vega-Frutis et al., 2014). Specifically, this hypothesis suggests that selection should favour a sex change strategy if individuals have higher fitness as a given sex (male, in most circumstances) when they are young or small, and as the other sex (female, in most circumstances) when they are older or larger (Ghiselin, 1969).
For animal-pollinated plants, the resource requirements for female function are larger than those for male function, therefore only older or lager individuals can accumulate sufficient resources to achieve female fitness (Charnov, 1982). It is advantageous for young or small individuals to gain reproductive fitness through male function (e.g., produce flowers with more pollen or relatively more male flowers) and older or larger individuals gain fitness through female function (produce more ovules or relatively more female flowers, Lloyd and Bawa, 1984). In cases where plants produce only one flower, the adjustment of sex allocation cannot be achieved by regulating the number of flowers with different sexual function, and plants exhibit a specific sex in a given season. Observed through a plant's lifetime, these plants will exhibit an interannual change of sex expression. Although this hypothesis is reasonable, the phenomenon has been well studied in only a few plant taxa.
To obtain evidence of size-dependent gender diphasy, investigations are required that record the sex and size change for each of the sampled individuals for several years, which is often difficult to conduct in the field. Taking advantage of an ideal study system, however, these investigations can be done in a single season. Lloydia oxycarpa Franch. (Liliaceae) is a perennial alpine herb distributed in SW China. Most of these plants produce a single flower each year, with a few individuals having two or more flowers. Based on preliminary observations, we found a considerable number of plants only produce male flowers, which coexist with hermaphroditic individuals in the same population, appearing androdioecious. In addition, the natural fruit set of this alpine plant has been observed to be high, likely because of spontaneous selfpollination. This may provide an opportunity to predict the sex status of plants in the previous year using the presence or absence of the persistent capsular fruit (matured in last year). Here we investigate natural populations of L. oxycarpa in order to address four questions: 1) What is the sexual system of this species? 2) What is the relative reproductive function of male and hermaphroditic flowers? 3) Does L. oxycarpa change sex between years? 4) Is sex expression of this plant associated with individual size?
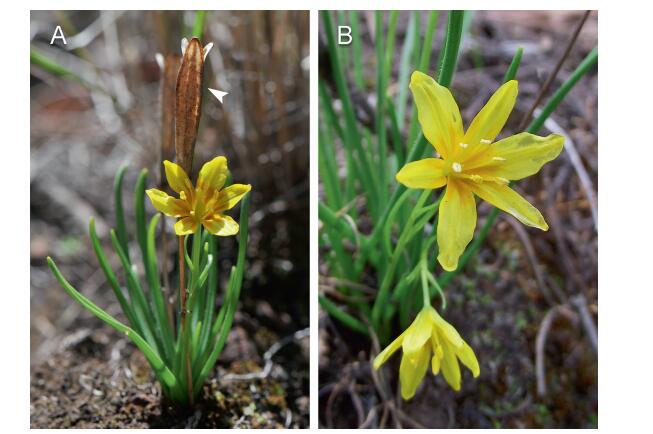
2. Materials and methods 2.1. Species and locationL. oxycarpa is a bulbiferous perennial herb that is distributed in the subalpine region in SW China. Each plant produces several linear leaves and a single scape that bears one, rarely two or more, flowers. L. oxycarpa has two flower morphs: hermaphroditic (Fig. 1A) or male (female sterile, Fig. 1B). Male flowers have normal anthers and a much smaller pistil than hermaphroditic flowers, which cannot set seed after hand pollination. The hermaphroditic flowers have both normal anthers and pistils. These flowers are yellow and visited by insects. L. oxycarpa flowers from May to July, and seeds mature in late August. We carried out the field experiments in Lijiang Alpine Botanical Garden (27°00'17″N, 100°11'05″E, 3246 m a.s.l.), NW Yunnan, China, 2013. This is a natural botanical garden that protects the natural vegetation of this area, where L. oxycarpa is found on the edge of pine forest.
|
| Fig. 1 Hermaphroditic (A) and male (B) flowers of Lloydia oxycarpa in its natural habitat. Please note that the pistil was almost absent in the male flower. The arrow (A) shows a persistent capsular fruit that matured the previous year. |
To investigate the breeding system of L. oxycarpa in natural conditions, we manipulated pollination in a series of experiments. 1) To determine whether L. oxycarpa exhibits apomixes, we emasculated six flowers and netted the flowers with nylon nets (10 × 10 cm-2). Flowers were sampled randomly from separate individuals. 2) To examine whether spontaneous self-pollination occurs in L. oxycarapa, 38 flowers were netted (NE). 3) To quantify the contribution of cross-pollination in natural conditions, 31 flowers were emasculated and exposed to pollinators (EM). 4) To investigate pollen limitation in L. oxycarpa (which occurs when this manipulation results in higher fruit set or seed production than under natural conditions), we pollinated 35 flowers by hand (SP). Pollen used in pollination experiments were collected from three individuals located at least 10 m away from the target individual. 5) To examine potential inbreeding depression, we pollinated 32 flowers by hand and subsequently isolated the flowers with a nylon net (SE) for comparison with the results of the cross-pollination experiment (CR, see below). 6) Fifty-one flowers were chosen as natural controls (NC).
For all experiments mentioned above, receiver flowers were hermaphroditic, and the emasculation manipulation was conducted at the beginning of anthesis, using fine scissors. We netted the whole plant rather than individual flowers, avoiding any artificial influence on pollination through physical contact between flower and net. Fruits were harvested in August when mature. Fruit set was calculated as the number of flowers that set fruit (containing at least one seed) divided by the total number of flowers sampled. For those fruits that contained seeds, we also counted the seed number per fruit. As some fruits were partly consumed by herbivores, the exact seed number could not be obtained. Therefore, the sample sizes for seed number per fruit are slightly smaller than the total number of fruits harvested.
We used one-way ANOVA (followed by Tukey's test) to analyse the difference in seed number per fruit among NE, EM and NC groups, and used independent sample t-tests to analyse the difference between SP and NC, and between SF and CF. Differences in fruit set between groups were analysed using Chi-square tests.
2.3. Floral functions of male and hermaphroditic flowersTo investigate the potential difference in reproduction function between male and hermaphroditic flowers, several floral traits and actual reproductive output were compared between the two floral morphs.
2.3.1. Pollen productionThe sex of flower buds was checked just before anthesis, and then collected and preserved in alcohol for pollen counting. The number of pollen grains in each flower (32 male and 27 hermaphroditic) was estimated using the method detailed by Dafni (1992).
2.3.2. Floral longevityFloral status was monitored from anthesis to withering in 12 male and 14 hermaphroditic flowers. The status was recorded as either open or not open, at ca. 11:00 am every day from June 1 to June 7. Floral longevity was then calculated as the total lifespan (number of days) from anthesis to withering.
2.3.3. Attractiveness to visitorsTo examine the potential difference in the attractiveness to visitors, we observed floral visitation for the two sexes every day from May 28 to June 16, 2013, from 10:00 to 18:00. To reduce the effect of extraneous factors on visitation, male and hermaphroditic individuals with similar floral display were observed simultaneously. For each visit, we recorded the visitor species, number of visits and their behaviour (nectar feeding, pollen feeding or pollen collection). Observations were carried out over a 30-min period; visitations were calculated as the number of visits per flower per hour (VFH). A Paired sample t-test was used to analyse the difference in visitation rate between sexes.
2.3.4. Siring successTo examine the potential difference in siring ability between two floral morphs, 32 and 31 hermaphroditic flowers were emasculated at the beginning of anthesis and received pollen from male and hermaphroditic donors, separately. Pollen was collected from individuals 10 m away from the receiver plant in the same population, and three donors were used in pollination for a single receiver. Fruit set and seed number per fruit were calculated when mature. Sex differences in the terms mentioned above were analysed by independent sample t-tests. Seed production of both these two pollination treatments was used as the results of crosspollination to compare with that of the self-pollination.
2.4. Sex expression patternSex expression was determined in 140 individuals in 2013. Flowers with normal pistils and stamens were identified as hermaphrodites, whereas those with sterile pistils were identified as males. Based on this survey, we divided these individuals into three categories: male individuals (M, only produce male flowers), hermaphroditic individuals (H, only produce hermaphroditic flowers), and a few individuals that produce both male and hermaphroditic flowers (H + M).
Spontaneous self-pollination contributes to high fruit set in hermaphrodites (see Results). The presence of persistent capsular fruits in these plants allowed us to speculate the plant's sex expression from the previous year (Fig. 1A). Specifically, the presence of persistent capsular fruit indicated the sexual expression was H or H + M (i.e. the plants produced hermaphroditic flowers) the previous year. Using this method, we surveyed the sex expression of 140 plants in 2013, and speculated their sex for the previous year (2012). We checked whether sex changed between seasons for an additional 117 individuals. Please note that when the persistent capsular fruit is absent we cannot determine if a plant was M the previous year, as it may not have flowered.
2.5. Plant size and sex expressionTo examine the potential relationship between plant size and sex expression, plant height (mm), bulb diameter (mm), dry weight (mg), corolla diameter (mm) and flower production (the proportion of individuals that produce more than one flower) were measured in 30 M and 30 H plants. Independent t tests were used to compare the difference in these parameters between sexes except for flower production, which was analysed using a Chi-square test. Furthermore, we used binary logistic regression to analyse the relationship between plant size (predictor: plant height, bulb diameter and dry weight) and sex expression (response variable, whether it produces hermaphroditic flowers).
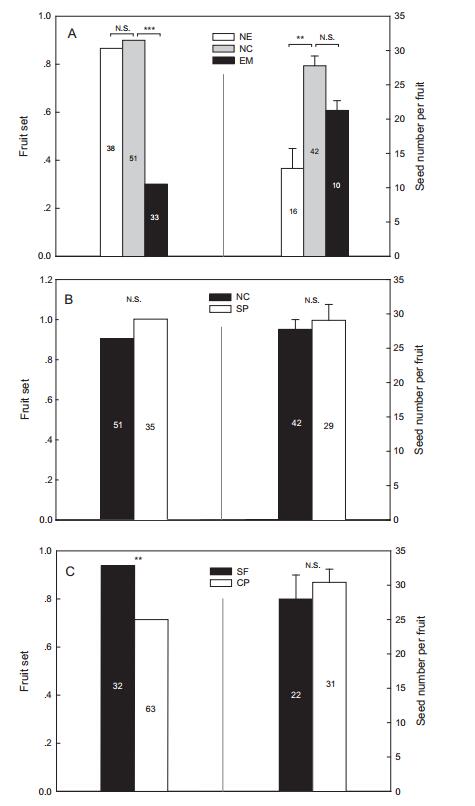
3. Results 3.1. Breeding systemEmasculated and netted flowers produced no fruit and their ovaries did not expand, indicating there is no apomixis in L. oxycarpa. There were significant differences in fruit set and seed number per fruit (F = 12.915, df = 2, P < 0.001) among the three groups (i.e. NE, EM and NC). Specifically, netted flowers produced lower seed number per fruit (Fig. 2A), but they had high fruit set (87%), which was not significantly different from the natural control (90%, χ2 = 0.246, P = 0.433), indicating the presence of spontaneous self-pollination. Emasculated flowers that were exposed to pollinators had significantly lower fruit set than the natural control (χ2 = 32.34, P < 0.001), though the seed number per fruit did not decrease (Fig. 2A), indicating that the opportunity of crosspollination is limited in natural conditions. Compared to natural controls, supplementary pollination did not increase fruit set or seed production significantly (Fig. 2B), indicating that there was no pollen limitation. In addition, cross-pollinated individuals failed to produce higher seed numbers per fruit, and they even had lower fruit set (χ2 = 6.362, P = 0.015) than in self-pollinated individuals (Fig. 2C), indicating that there is no obvious inbreeding depression at least in the life stages monitored.
|
| Fig. 2 Fruit set (left) and seed number per fruit (right) of manipulated pollination experiments, examining the breeding system of Lloydia oxycarpa. A: Comparison between netted (NE, testing the possibility of spontaneous self-pollination), emasculated (EM, testing seed production by outcross pollination in natural conditions) flowers and natural control (NC). B: Comparison between supplementary pollination (SP) and natural control (NC), testing the occurrence of pollen limitation. C: Comparison between self-(SF) and cross-pollination (CP), examining potential inbreeding depression. Numbers on the columns are sample sizes. **P < 0.01; ***P < 0.001. NS, not significant. |
Hermaphroditic flowers were larger and had significantly higher number of pollen grains than the male flowers (Fig. 3A and B). Floral longevity was slightly longer in male flowers (4.64 ± 0.14 days, mean ± SE) than in hermaphrodites (4.25 ± 0.12 days, Fig. 3C, t = -2.093, df = 24, P = 0.047). Our observations revealed that most visitors of L. oxycarpa were members of Syrphidae, including Scaeva pyrastri and Scaeva selenitica; both are pollen feeders. We did not find a significant difference in floral attractiveness between male flowers and hermaphroditic flowers (Fig. 3D).

|
| Fig. 3 A comparison between male (M) and hermaphroditic (H) flowers floral traits. Floral diameter (A), number of pollen grains per flower (B), floral longevity (C) and visitation rate (D). Numbers in the brackets are sample sizes. *P < 0.05, ***P < 0.001. NS, not significant. |
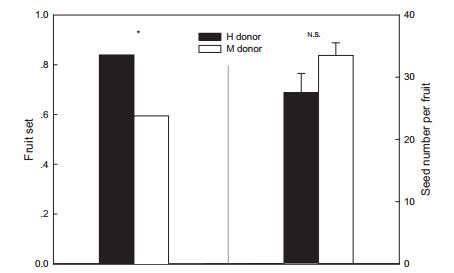
Flowers that were pollinated by hermaphroditic donors had higher fruit set than flowers pollinated by male donors, whereas both of these groups produced a similar number of seeds (Fig. 4), indicating that males are not better than hermaphrodites in male function.
|
| Fig. 4 Fruit set (left) and seed number per fruit (right) of flowers that received pollen from male ("M") and hermaphroditic ("H") donors, respectively. Numbers on the columns are sample sizes. *P < 0.05. NS, not significant. |
For 140 plants surveyed in 2013, there were 71 H (50.71%), 66 M (47.14%) and 3 H + M (2.14%). Thirty-three individuals did not have a persistent capsular fruit, indicating that they produced no flower or only male flowers the previous year. Of the remaining 117 plants that produced at least one hermaphroditic flower the previous year (2012), 40 (34.2%) remained H, 42 (35.9%) changed to M; 4 (3.4%) changed to H + M and 31 (26.5%) did not flower the following year.
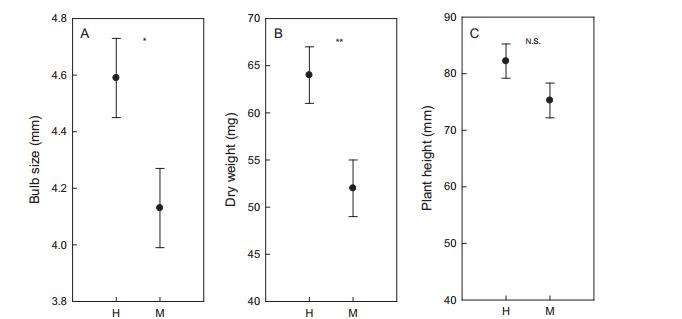
3.4. Relationship between sex and sizeMost hermaphrodites (83.8%) and males (98.5%) produced only one flower. While, a significantly higher ratio of hermaphrodites produced two or more flowers compared with males (χ2 = 8.951, P = 0.003). In addition, bulb size and dry weight were significantly larger in hermaphrodites than males (Fig. 5A and B). But there was no significant difference between the two "morphs" in terms of plant height (Fig. 5C). Binary logistic regression indicated that both bulb size and plant dry weight can predict sexual expression well (Table 1), but dry weight was a slightly better predictor than bulb size (χ2 = 3.371, P = 0.07).
|
| Fig. 5 Bulb size (A), dry weight (B) and plant height (C) between individuals that produce male (M) and hermaphroditic (H) flowers. Sample sizes of all these groups are 30. *P < 0.05, **P < 0.01. NS, not significant. |
| Predictor | χ2 | df | P | -2log likelihood |
| Bulb size | 5.46 | 1 | 0.019 | 77.72 |
| Dry weight | 8.83 | 1 | 0.003 | 74.35 |
| Height | 2.63 | 1 | 0.105 | 80.55 |
A considerable number of "male individuals" (up to 47.2%) were observed to coexist with hermaphrodites in the same population of L. oxycarpa, however, here we conclude that the sexual system of L. oxycarpa is not androdioecy.
To coexist with hermaphrodites, which possesses both the male and female function in reproduction, males need to have much higher fitness than hermaphrodites (Pannell, 2002a). For example, males may produce a larger number of pollen grains, which have a greater opportunity to sire more offspring, or they may have a higher outcrossing rate and show a bigger heterogeneous advantage (Pannell, 2002a). In L. oxycarpa, however, we failed to find any male advantage except for slightly longer floral longevity. Fewer male individuals produced more than one flower compared with hermaphrodites. In addition, male flowers were smaller than hermaphrodite flowers and produced a smaller number of pollen grains per flower. Furthermore, male flowers did not attract more pollinators than hermaphrodites. When receiver flowers were pollinated by different pollen donors (M and H), we failed to find any advantage in siring success of males. These results are in line with the study on Fritillaria montana (Peruzzi et al., 2012) and Lilium apertum (Zhang et al., 2014) in the same family.
There was no pollen limitation in L. oxycarpa and the natural fruit set was high (largely due to spontaneous self-pollination), which may make most of the ovules unavailable for males to fertilize. If this selfing occurs at the end of the anthesis (as a delayed selfing mechanism, Richards, 1997), males may still have the opportunity to sire more offspring. However, this possibility was not supported by the finding that fruit set from outcrossing flowers (EM group) was relatively low, and seed production mainly resulted from self-pollination. Furthermore, we did not detect any inbreeding depression in terms of fruit set or seed number per fruit. Therefore, even if males have a higher outcrossing rate, the outcrossing advantage of male individuals cannot be achieved, at least at these life stages. In addition, phylogenetic evidence has shown that true androdioecy is often derived from a dioecious ancestor (Pannell, 2002b). Presently, no dioecious member has been recorded in either Lloydia or Liliaceae (Renner, 2014). Taken together, these results indicate that males are unlikely to be maintained in the population we studied. Importantly, we found clear evidence that L. oxycarpa changes sex between years, which is not a characteristic of androdioecy.
4.2. Size-dependent sex expressionTaking advantage of the spontaneous self-pollination that results in high fruit set, we found that plants changed their sex between years. Specifically, 35.9% individuals that produced hermaphroditic flowers in 2012 produced only male flowers in 2013. Similar results have been found in a congener, Lloydia serotina (Jones and Gliddon, 1999; Manicacci and Després, 2001). This indicates that it is more reasonable to regard the "male individual" as a transient rather than a life-time gender strategy. Although we cannot completely rule out the possibility that some individuals may maintain male function through their life, unisexual individuals can hardly be maintained in populations, as discussed above. Thus, we suggest L. oxycarpa is a gender diphysic plant rather than androdioecious.
The ability of individuals to acquire resources is largely sizedependent and plants may increase reproductive allocation by increasing individual size. Consequently, what we observed in nature is not a balance between two sexual functions (as expected by the classical sex allocation theory), but a size-dependent sexual allocation (Lloyd and Bawa, 1984; Zhang, 2006). We showed a clear relationship between size and sex expression in this study. Specifically, hermaphrodites had heavier weight and larger bulb size, and they produced higher number of pollen grains, larger flower size and greater number of flowers. In general, larger individuals tend to produce hermaphroditic flowers, whereas smaller individuals tend to produce male flowers or no flowers. This size-dependent sex expression pattern has also been reported in other members of Liliaceae (Peruzzi et al., 2012; Zhang et al., 2014; Gong et al., 2015). Although this phenomenon has only been well studied in a few taxa, we speculate it may occur in many other members of perennial taxa that have bulbs, tubes or other resource storage organs. An interesting example is the genus Fritillaria, a plant used in traditional Chinese medicine that received considerable attention for its growth habit. Flora of China (Chinese edition) records that in the first flowering year, Fritillaria species produce flowers but fail to set fruit, and they can only set fruit at the second flowering year (Chen, 1980). We believe that this may reflect a size/age-dependent annual change of sex from male to hermaphrodite in this taxon.
Alternatively, it is possible that the female-biased sexual allocation may be related to the direct effect of individual size rather than its ability to access resources. For example, taller individuals may allocate more resources to female function merely because it enhances female fitness in terms of seed dispersal (de Jong and Klinkhamer, 2005). However, this may not be the case in our study given that there was no significant difference in plant height between the two sex morphs. In addition, dry weight and bulb size were better predictors than plant height, implying that sex expression is related to resource budget rather than the direct effect of plant size.
In conclusion, we found considerable numbers of males coexist with hermaphrodites in a population of L. oxycarpa. However, this plant is not androdioecious because male individuals are unlikely to be maintained in conditions with no obvious fitness advantage. Furthermore, sex changes were observed between years. The sex expression of these plants is clearly related to individual size, with larger and small individuals producing hermaphroditic and male flowers, respectively. Based on these results, we conclude that L. oxycarpa represents a case of size-dependent gender diphasy.
AcknowledgementsWe thank Mr. Chen L.-Y., Dr. Wu Z.-K., Mr. Zhou Y.-Q., Ms. Zhang Y., Mr. Zhang Y.-Z. and Sun W.-G. for their help in field and lab experiments, Dr. Zhang Y.-H., Dr. Zhang Z.-Q., Dr. Chen J.-G. and Dr. Song B. for their advices in data analysis. This study was supported by The National Key Research and Development Program of China (grant no. 2017YFC0505200), NSFC (grant 30360049 to Z.-M. L. and 31200183 to Y.N.) and major Program of NSFC (grant 31590823 to H.S.).
Barrett S.C.H., 2002. The evolution of plant sexual diversity. Nat. Rev. Genet., 3: 274-284. DOI:10.1038/nrg776 |
Charnov E.L., 1982. The Theory of Sex Allocation. Princeton: Princeton University Press.
|
Chen, X. Q., 1980. Fritillria L. In: Wang, F. Z., Tang, J. (Eds. ), Flora Republicae Popularis Sinicae, Liliaceae, vol. 14. Science Press, Beijing, pp. 97-116.
|
Dafni A., 1992. Pollination Ecology:A Practical Approach. Oxford: Oxford University Press.
|
de Jong T., Klinkhamer P., 2005. Evolutionary Ecology of Plant Reproductive Strategies. Cambridge: Cambridge University Press.
|
Fritsch P., Rieseberg L.H., 1992. High outcrossing rates maintain male and hermaphrodite individuals in populations of the flowering plant Datisca glomerata. Nature, 359: 633-636. DOI:10.1038/359633a0 |
Geber M., Dawson T.E., Delph L., 1999. Gender and Sexual Dimorphism in Flowering Plants. Berlin, Heidelberg: Springer-Verlag.
|
Ghiselin M.T., 1969. The evolution of hermaphroditism among animals. Q. Rev. Biol., 44: 189-208. |
Gong Q.-B., Li Z.-M., Peng D.-L., Niu Y., Sun H., Zhang Z.-Q., 2015. Male flowers and relationship between plant size and sex expression in herbaria of Nomocharis species (Liliaceae). Plant Divers. Resour., 37: 11-20. |
Jones B., Gliddon C., 1999. Reproductive biology and genetic structure in Lloydia serotina. Plant Ecol., 141: 151-161. DOI:10.1023/A:1009805401483 |
Lloyd D.G., 1979. Parental strategies of angiosperms. New Zeal. J. Bot., 17: 595-606. DOI:10.1080/0028825X.1979.10432573 |
Lloyd D.G., Bawa K.S., 1984. Modification of the gender of seed plants in varying conditions. Evol. Biol., 17: 255-338. |
Manicacci D., Després L., 2001. Male and hermaphrodite flowers in the alpine lily Lloydia serotina. Can. J. Bot., 79: 1107-1114. |
Mayer S., Charlesworth D., 1991. Cryptic dioecy in flowering plants. Trends Ecol.Evol., 6: 320-325. DOI:10.1016/0169-5347(91)90039-Z |
Pannell J.R., 2002a. The evolution and maintenance of androdioecy. Annu. Rev. Ecol.Syst., 33: 397-425. DOI:10.1146/annurev.ecolsys.33.010802.150419 |
Pannell J.R., 2002b. What is functional androdioecy?. Funct. Ecol., 16: 862-865. DOI:10.1046/j.1365-2435.2002.06893.x |
Peruzzi L., Mancuso E., Gargano D., 2012. Males are cheaper, or the extreme consequence of size/age-dependent sex allocation:sexist gender diphasy in Fritillaria montana (Liliaceae). Bot. J. Linn. Soc., 168: 323-333. DOI:10.1111/j.1095-8339.2011.01204.x |
Renner S.S., 2014. The relative and absolute frequencies of angiosperm sexual systems:dioecy, monoecy, gynodioecy, and an updated online database. Am. J.Bot., 101: 1588-1596. DOI:10.3732/ajb.1400196 |
Richards A.J., 1997. Plant Breeding Systems. London: Garland Science.
|
Rieseberg L.H., Philbrick C.T., Pack P.E., Hanson M.A., Fritsch P., 1993. Inbreeding depression in androdioecious populations of Datisca glomerata (Datiscaceae). Am. J. Bot., 80: 757-762. DOI:10.2307/2445595 |
Schlessman M.A., 1991. Size, gender, and sex change in dwarf ginseng, Panax trifolium (Araliaceae). Oecologia, 87: 588-595. DOI:10.1007/BF00320425 |
Vega-Frutis R., Macías-Ordoñez R., Guevara R., Fromhage L., 2014. Sex change in plants and animals:a unified perspective. J. Evol. Biol., 27: 667-675. DOI:10.1111/jeb.2014.27.issue-4 |
Zhang, D. -Y., 2006. Evolutionarily stable reproductive investment and sex allocation in plants. In: Harder, L. D., Barrett, S. C. H. (Eds. ), Ecology and Evolution of Flowers. Oxford University Press, Oxford, pp. 41-60.
|
Zhang Z.-Q., Zhu X.-F., Sun H., Yang Y.-P., Barrett S.C.H., 2014. Size-dependent gender modification in Lilium apertum (Liliaceae):does this species exhibit gender diphasy?. Ann.Bot., 114: 441-453. DOI:10.1093/aob/mcu140 |



