b. School of Life Science, Yunnan Normal University, Kunming 650500, Yunnan, China;
c. Germplasm Bank of Wild Species in Southwest China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, Yunnan, China;
d. University of Chinese Academy of Sciences, Beijing 100049, China
Seed germination timing is crucial for seedling survival in plants. Seed dormancy is the main mechanism controlling the timing of germination (Baskin and Baskin, 2014), allowing seeds to delay germination until the conditions are optimal for growth and survival of the seedlings (Baskin and Baskin, 2004). Thus, seed dormancy is generally considered as an adaptation to harsh environments, such as in alpine areas (Jaganathan et al., 2015). In alpine plants, dormancy is a very common phenomenon, and more than 70% of plants produce seeds that exhibit dormancy, mainly physiological dormancy (PD) (Schwienbacher et al., 2011; Baskin and Baskin, 2014). Light is an important environmental factor stimulating germination in many seeds (Baskin and Baskin, 2014), therefore, in addition to dormancy, the light requirement for germination also plays a pivotal role in controlling the timing of germination from the soil seed bank (Densmore, 1997).
For non-dormant seeds, temperature is one of the main environmental factors that regulates seed physiology, having major effects on the rate of germination and final germination percentage (Batlla and Benech-Arnold, 2015; Dürr et al., 2015). The germination response to accumulated temperatures can be modeled by a thermal time (θ) approach. In this model, cardinal temperatures of each species can be calculated. When seeds are subjected to temperatures (T) above a base temperature for germination (Tb), at which the germination rate is zero, and below an optimum temperature (To), above which the germination rate does not increase anymore, the rate of germination (1/t) usually increases linearly with temperature, at least within the suboptimal range (Tb-To)(Garciahuidobro et al., 1982). Germination times for seeds can be modeled using this thermal time approach (Garciahuidobro et al., 1982; Hardegree, 2006), in which a threshold cumulative quantity of thermal time (θ, ℃days or hours) is required above the base temperature to initiate germination.
The rhubarb genus (Rheum, Polygonaceae) is composed of about 60 species, most of which are endemic to the Qinghai-Tibetan Plateau and adjacent regions (Sun et al., 2012). Rheum nobile Hook.f. et Thomson typically occurs on dryish alpine scree, and occasionally on alpine scrub, growing at altitudes of 4000-6000 m a.s.l. in the Himalaya-Hengduan Mountains (HHM) (Chowdhery and Agrawala, 2009). Rheum alexandrae Batalin is mainly found in alpine wetlands, including marsh, swampy meadows, and lake shores, growing at altitudes of 3000-4800 m a.s.l. in HHM (Xu et al., 2014). Morphological traits of the rhubarb genus are highly diverse (Sun et al., 2012), but only Rh. nobile and Rh. alexandrae have large translucent cream-colored bracts wrapping flowers/fruits, and thus have attracted much attention from evolutionary biologists. These developed bracts represent an adaptive strategy to protect the interior reproductive organs from hostile alpine environments (e.g., low temperatures, ultraviolet radiation and wetting) (Omori and Ohba, 1996; Tsukaya, 2002; Song et al., 2013b, 2015). However, thus far, the seed germination strategy these two species use to adapt to the hostile alpine environments remains unknown. In addition, it is not known whether these species, which occur in contrasting habitats, have different seed dormancy types or germination responses to temperature and light conditions after dormancy release.
In this study, we investigated seed dormancy and germination in Rh. nobile and Rh. alexandrae. We specifically addressed the following questions: (1) What is the dormancy type of fresh seeds in Rh. nobile and Rh. alexandrae? (2) How do temperature and light conditions affect germination for these species after dormancy release? (3) What are the cardinal temperatures and thermal times for germination for each species?
2. Materials and methods 2.1. Seed collectionMature samaras (seeds) of each species were collected from two natural populations (Table 1) in early October 2014, in the alpine subnival belt of the Hengduan Mountains, SW China. Rh. nobile and Rh. alexandrae are giant perennial monocarpic herbs, with only a few flowering individuals ( < 10 individuals) in each population, therefore seeds were only collected from five individuals. Non-seed structures and empty seeds were removed by aspirator and hand in the laboratory. Except for a set of fresh seeds used directly in experiments, seeds were air-dried (dry after-ripening, DAR) in a paper bag at room temperature for six months until the start of the experiments.
| Species | Population code | Region | Locality | Location | Altitude (m a.s.l.) | Substrate |
| Rh. nobile | RnobPY | Yunan Province | PuYong Pass | 99°55'E, 28°24'N | 4670 | Alpine scree |
| Rh. nobile | RnobHLH | Yunan Province | HuLuHai Lake | 99°57'E, 28°31'N | 4650 | Alpine scree |
| Rh. alexandrae | RaleJZW | Sichuan Province | JianZiWan Mountain | 100°50'E, 29°58'N | 4530 | Alpine wetland |
| Rh. alexandrae | RaleYJG | Sichuan Province | YaJiaGeng Mountain | 102°00'E, 29°53'N | 3930 | Alpine wetland |
Experiments on fresh seeds from RnobPY and RaleJZW populations were initiated one week after collection on 1% water agar substrate, in plastic Petri dishes (90 mm) in the light under two alternating temperatures: 15/5 and 25/15 ℃. The daily photoperiod was 12 h light with 22.2 μmol m-2 s-1 illumination from cool white fluorescent light coinciding with the higher temperature and 12 h dark (hereafter referred to as in the light). When seeds were airdried for three months, the weight of 100 seeds was measured. After approx. six months, seeds were moved to germination conditions (15/5 and 25/15 ℃) again. To determine the level of physiological dormancy (if it presents) after dry ripening, DAR seeds were also incubated on 1% water agar substrate with 100 mg L-1 of GA3 at aforementioned temperatures.
2.2.2. Effect of temperature and light on germinationAfter approx. six months, DAR seeds from the two species (two populations each) were incubated in the light at seven constant temperatures (5, 10, 15, 20, 25, 30 and 35 ℃) and two alternating temperatures (15/5 and 25/15 ℃) starting in April 2015. To determine the effect of darkness on germination, Petri dishes with seeds were wrapped in two layers of aluminum foil, and then incubated at the aforementioned temperatures (except 30 and 35 ℃).
Due to limited well-developed seed availability (larvae damage), three replicates of a few (20) seeds each were used in each condition. The Petri dishes were put into transparent plastic bags to prevent desiccation. Seeds incubated in the light were counted daily and germinated seeds were discarded, while dark-incubated seeds were counted only once at the end of the test to avoid any exposure to light. The criterion for germination was visible radicle protrusion (>2 mm). All experiments were terminated after up to eight consecutive weeks. The viability of ungerminated seeds was checked by a cut-test. Seeds with a plump, firm, and white embryo were considered viable.
2.3. Data analysisThe final germination percentage was calculated as follows: GP = ∑Gi/N, where i is the day of germination, counted from the day of sowing, Gi is the number of seeds germinated on day i, and N is the total number of filled seeds.
All data were analyzed using the procedures in PASW Statistics 18, and all data were expressed as mean ± SE. A one-way ANOVA (P < 0.05) was carried out on the germination percentage to determine whether DAR and DAR + GA3 significantly increased seed germination, compared with fresh seeds. A two-way ANOVA (P < 0.05) was carried out on the germination percentage to assess the effects of the fixed factors (temperature and light) and their interactions on final germination percentage. Tukey's HSD was used to compare means of germination percentage between treatments when significant differences were found. An independent-sample t-test was carried out on the germination rate to determine whether alternating temperatures significantly accelerated germination, compared with constant temperatures (i.e., 10 vs. 15/5 ℃, 20 vs. 25/15 ℃).
A thermal-time model was applied to estimate the cardinal temperatures (Hardegree, 2006). Germination time courses for all three replicates at a given temperature were combined and fitted using the Weibull Function (Brown and Mayer, 1988) in OriginPro 8.0. The day to 50% germination (t50) was obtained by fitting cumulative germination progress curves. Germination rate (GR, 1/t50) was plotted as a function of temperature and regressed using a linear model, to estimate the base temperature below which germination rate was equal to zero (Tb). The slope of the linear regression line corresponded to the reciprocal of the thermal-time requirement at suboptimal temperatures (θ50).
3. Results 3.1. Weight of the 100 seedsThe 100 seed weights were (n = 10): 2.09 ± 0.017 g (RnobPY), 2.022 ± 0.028 g (RnobHLH) for Rh. nobile; 0.776 ± 0.008 g (RaleJZW), 0.895 ± 0.029 g (RaleYJG) for Rh. alexandrae.
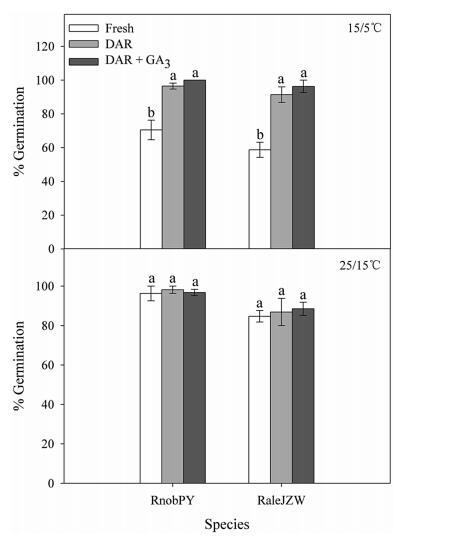
3.2. Effects of DAR on seed germinationA one-way ANOVA showed that GP in DAR seeds was significantly higher than in fresh seeds at 15/5 ℃ (RnobPY and RaleJZW; Fig. 1). However, GA3 did not significantly increase GP in DAR seeds in either species (Fig. 1).
|
| Fig. 1 Final germination percentages at two alternating temperatures for fresh, DAR and DAR + GA3 seeds of Rh. nobile and Rh. alexandrae. Error bars indicate SE for three replicates of 20 seeds, and bars with different letters indicate significant differences at P < 0.05. |
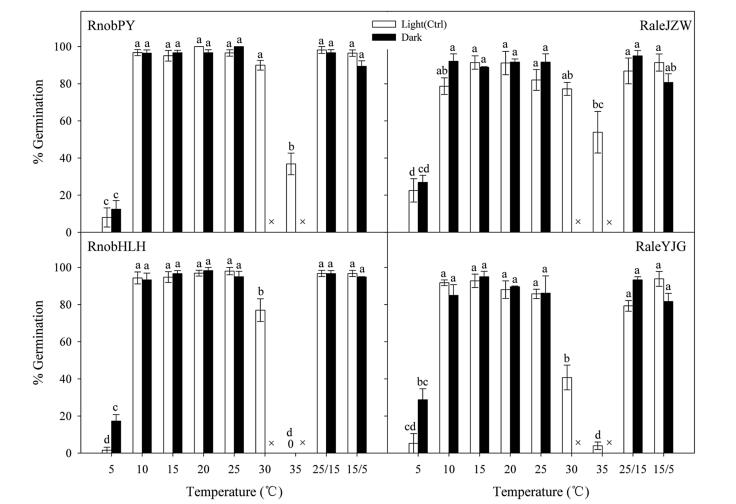
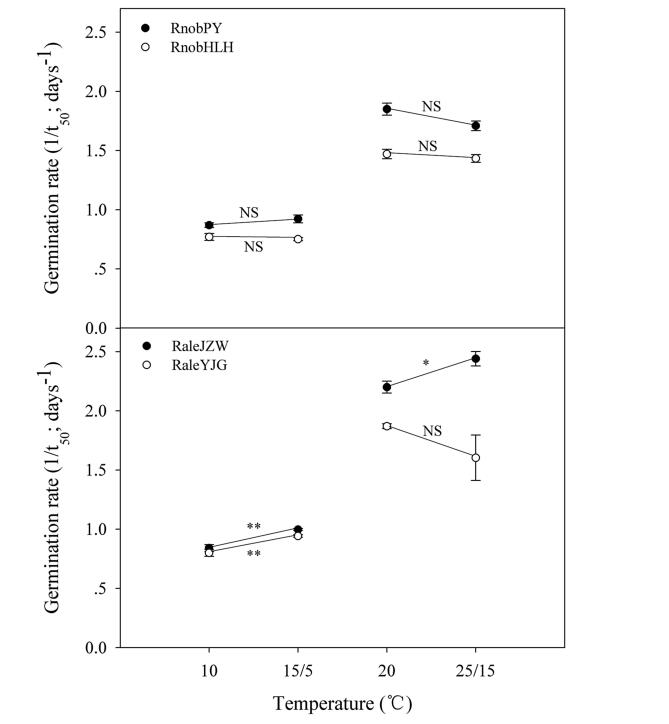
For both Rh. nobile and Rh. alexandrae, the two-way ANOVA showed that temperature significantly affects germination, whereas light does not (Table 2; Fig. 2). Germination increased initially and then decreased with increasing temperature, regardless of light condition. Both species germinated to a higher percentage (>80%) at 10 ℃ to 25 ℃ (Fig. 2). For all of the seed lots, final germination at 5 and 35 ℃ were significantly lower than at all other temperature treatments, and no germination occurred at 35 ℃ in the population of RnobHLH. RnobHLH and RaleYJG GPs at 30 ℃ were also significantly lower than at other temperatures (10-25 ℃). Incubation at two alternating temperatures (25/15 and 15/5 ℃) did not significantly increase the GP of the two species in the light or in the dark (Fig. 2). However, in two Rh. alexandrae populations, alternating temperatures increased the seed germination rate (1/t50) compared with the corresponding constant temperatures in the light, especially at 15/5 ℃ (versus 10 ℃; Fig. 3).
| Source of variation | RnobPY | RnobHLH | RaleJZW | RaleYJG | |||||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | ||||
| Light | 1 | 0.058 | 0.812 | 1 | 1.893 | 0.178 | 1 | 1.378 | 0.249 | 1 | 1.789 | 0.190 | |||
| Temperature | 8 | 217.539 | 0.000 | 8 | 370.058 | 0.000 | 8 | 34.865 | 0.000 | 8 | 85.434 | 0.000 | |||
| Light × temperature | 6 | 0.943 | 0.478 | 6 | 3.145 | 0.015 | 6 | 1.250 | 0.308 | 6 | 3.538 | 0.008 | |||
|
| Fig. 2 Effect of light and temperature on seed germination percentages of Rh. nobile and Rh. alexandrae. Error bars indicate SE for three replicates of 20 seeds. ×, no test in this treatment; 0, no germination at this temperature. Different letters indicate significant differences at P < 0.05. |
|
| Fig. 3 Effect of alternating and corresponding constant temperatures on seed germination rate (1/t50) of Rh. nobile and Rh. alexandrae under each treatment. Error bars indicate SE for three replicates of 20 seeds. "*" and "**" indicate significant difference at 0.05 and 0.01 levels, respectively, and "N.S." indicates no significant difference at 0.05 level between alternating and corresponding constant temperatures (10 vs. 15/5 ℃, 20 vs. 25/15 ℃). |
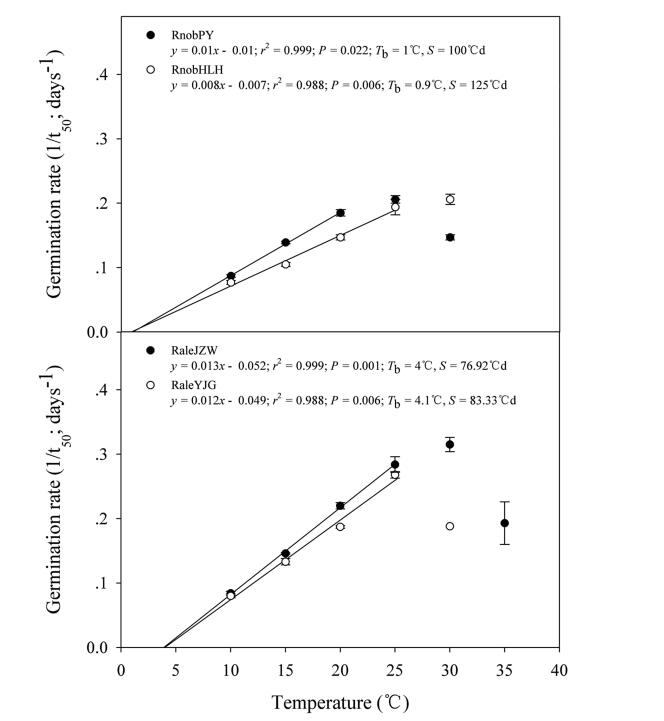
Generally, the germination rate (1/t50) of all four populations increased and then decreased as temperature increased (Fig. 4). It was not possible to calculate 1/t50 for 5 ℃ due to the low final germination ( < 50%) at this temperature (Fig. 2). The germination rates for the range of the tested constant temperatures allowed a suboptimal range to be identified from 10 to 25 ℃ (20 ℃ in RnobPY), with 30 and 35 ℃ being already in the supra-optimal range (Fig. 4). Seed germination in response to temperature was well described by the thermal-time model at suboptimal temperatures. For two Rh. nobile populations, the estimated Tb values were 1 and 0.9 ℃, respectively; for two Rh. alexandrae populations they were 4 and 4.1 ℃, respectively. Thermal time (θ50) for two Rh. nobile populations were 100 and 125 ℃d, respectively; for two Rh. alexandrae populations, thermal times were 76.92 and 83.33 ℃d, respectively. In addition, considering that 30 ℃ was already in the supraoptimal range, the optimal temperature for germination can be estimated to range between 25 and 30 ℃, except for RnobPY, where the optimal temperature was estimated to range between 20 and 25 ℃ (Fig. 4).
|
| Fig. 4 Seed germination rates of Rh. nobile and Rh. alexandrae calculated on the basis of the reciprocal of the times to reach 50% germination. Points correspond to the actual data and solid lines indicate the fitted lines from the linear regressions (RnobPY 5-20 ℃; RnobHLH, RaleJZW and RaleYJG 5-25 ℃). Error bars indicate SE for three replicates of 20 seeds. |
This study explored the characteristics of seed dormancy and germination of two endemic Rheum species which occur at high altitudes of the Himalaya-Hengduan Mountains (HHM). Overall, for both Rheum species, we found that fresh seeds are dormant, and seeds can germinate at a wide range of temperatures after dormancy release, regardless of light condition. Such seed dormancy mechanisms, and light and temperature requirements for germination may delay germination until spring in the field, when both temperature and soil water content are favorable, representing an advantageous ecological adaptation towards the unpredictable alpine environments.
4.1. Type of dormancyIn RnobPY and RaleJZW, fresh seeds showed higher germination percentages at a higher alternative temperature (25/15 ℃) than at a lower temperature (15/5 ℃) (Fig. 1). DAR final germination increased significantly at the lower temperature (15/5 ℃), but was not affected at the higher temperature (25/15 ℃); GA3 did not increase final germination at either temperatures (Fig. 1). DAR was sufficient to break seed dormancy, which suggests that the seeds of the two Rheum species have non-deep PD. Further, the expanded temperature range at which the seeds of the two Rheum species were able to germinate (widened from higher to lower) indicates that the seeds have type 2 non-deep PD (Baskin and Baskin, 2004).
4.2. Effect of temperature on germinationTypically, in alpine climates, germination is limited to spring, which maximizes the length of the growing season before the onset of winter cold (Baskin and Baskin, 2014). Seeds of the two Rheum species were able to germinate over a wide range of temperatures (both constant and alternating) (Fig. 2). The thermal-time model provided new insights into the effects of suboptimal temperatures on seed germination. The base temperatures for germination (Tb) in non-dormant seeds (i.e., after-ripening) of the two populations in each species were found to be very low (Fig. 4), namely 1 ℃ (RnobPY), 0.9 ℃ (RnobHLH), 4 ℃ (RaleJZW) and 4.1 ℃ (RaleYJG). To our knowledge, this is the first report of Tb values for any members of the Polygonaceae. The two Rheum species had similar Tb values ( < 5 ℃) as two Labiatae species in the alpine region of the Hengduan Mountains (Peng et al., 2017). Lower Tb allows seeds to accumulate heat when ambient temperatures are low (below 5 ℃). Such thermal characteristics of both species would probably allow early germination after snowmelt in spring, which would ensure a sufficiently long growing period allowing seedling recruitment in the alpine region.
Final germination of the two Rheum species did not exhibit a positive response to temperature fluctuation in the light or in the dark (Fig. 2). However, germination rates of the seeds of Rh. alexandrae for both populations in light were elevated by alternating temperatures, especially at 15/5 ℃ (Fig. 3), which corresponds to the daily mean high and low temperature, respectively, at the study sites during the spring germination period. These results are consistent with previous studies that have suggested that seed germination of many species living in marshlands requires alternating temperatures (Thompson and Grime, 1983; Liu et al., 2013). The two Rh. alexandrae populations are found in alpine marsh habitats, where the water level is subject to seasonal fluctuation with a minimum in winter and a maximum in summer. The onset of snowmelt in spring is accompanied by a rise in water level after dry, cold winter soil becomes warmer (with larger temperature fluctuations) and waterlogged. Moreover, Song et al. (2013a) found that Rh. alexandrae seedling survival was reduced significantly after being submerged in water. In this situation, rapid germination of Rh. alexandrae seeds in response to large temperature fluctuations in spring would promote seedling establishment by avoiding flooding.
4.3. Effect of light on seed germinationAlthough the two Rheum species have clearly contrasting habitats in terms of the soil water conditions (i.e., Rh. nobile in dryish habitat, Rh. alexandrae in swampy habitat), our results show that light had no significant effect on germination for either species (Table 2; Fig. 2). Notably, seed germination for both populations of Rh. alexandrae was not inhibited by dark. These results do not agree with Grime et al. (1981), who suggested that many wet-habitat species require light for germination compared with "dryish" habitat species. The similar light requirements for Rh. nobile and Rh. alexandrae may be explained by their close phylogenetic relationship (Sun et al., 2012) or morphological and life history similarities. In addition, Grime et al. (1981) found that if seed mass is over 0.1 mg, the light requirement for germination may not be absolute. Rh. nobile and Rh. alexandrae seed mass both exceed 0.1 mg, therefore the similar light requirement for germination may also be due to their large seeds. The indifference of the two Rheum species seeds to germination in light or dark, combined with the lack of an alternating temperature requirement (Fig. 3), highlights their capacity to germinate when deeply buried in the gravelly soils of the alpine habitat, confirming the lack of a permanent soil seed bank.
5. ConclusionSpecies occurring in specific habitats often produce seeds with specialized adaptations (Kudo et al., 2010; Hu et al., 2015). Rh. nobile and Rh. alexandrae are similar in morphology and life history, but they are distributed in contrasting habitats (i.e., dryish and wet habitats), in term of soil water condition. It has been suggested that in these two Rheum species seed germination and seedling establishment respond to habitat-specific soil water conditions (Song et al., 2013a). In this study, we found that the two Rheum species shared a similar seed dormancy type (type 2 nondeep PD) and germination patterns (e.g., Tb, light and temperature requirements), indicating that they have the same germination strategy adapted to the same alpine environments. But these findings (except alternating temperature) do not explain why the two Rheum species are distributed in contrasting habitats. Thus, soil water conditions, rather than temperature and light conditions, appear to be the crucial factor in determining germination and seedling establishment. In turn, this may explain how these species have survived in dryish and wet habitats, respectively.
AcknowledgmentsThe first author thanks Y.G. Guo, J. Yang and Dr. X.Y. Yang of the Germplasm Bank of Wild Species in Southwest China for facilities used during the research. The authors are grateful to Dr. J.W. Zhang, L.E. Yang and M.S. Song for their assistance in seed collection and laboratory work. The authors thank two anonymous reviewers for comments. This study was supported by National Key Research and Development Program of China (2017YFC0505201), NSFC (grant 31670206 to Z.-M. L. and 31570228 to B. S.), major Program of NSFC (grant 31590823 to H. S.), the Orientation Training Programme for Postdoctoral Fellows from Yunnan Province to D.-L. P., and the Young Academic and Technical Leader Raising Foundation of Yunnan Province to B. S.
Baskin C.C., Baskin J.M., 2014. Seeds:Ecology, Biogeography, and Evolution of Dormancy and Germination. San Diego: Elsevier Academic Press Inc.
|
Baskin J.M., Baskin C.C., 2004. A classification system for seed dormancy. Seed Sci.Res., 14: 1-16. |
Batlla D., Benech-Arnold R.L., 2015. A framework for the interpretation of temperature effects on dormancy and germination in seed populations showing dormancy. Seed Sci. Res., 25: 147-158. DOI:10.1017/S0960258514000452 |
Brown R.F., Mayer D.G., 1988. Representing cumulative germination.2. The use of the Weibull function and other empirically derived curves. Ann. Bot., 61: 127-138. DOI:10.1093/oxfordjournals.aob.a087535 |
Chowdhery H., Agrawala D., 2009. Rheum nobile Hook. f. & Thoms. (Polygonaceae) -a rare and highly specialized plant of Himalayan region. Indian J. For., 32: 145-148. |
Dürr C., Dickie J.B., Yang X.Y., et al, 2015. Ranges of critical temperature and water potential values for the germination of species worldwide:contribution to a seed trait database. Agric. For. Meteorol., 200: 222-232. DOI:10.1016/j.agrformet.2014.09.024 |
Densmore R.V., 1997. Effect of day length on germination of seeds collected in Alaska. Am. J. Bot., 84: 274-278. DOI:10.2307/2446088 |
Garciahuidobro J., Monteith J.L., Squire G.R., 1982. Time, temperature and germination of Pearl Millet (Pennisetum typhoides S. & H.) Ⅰ. Constant temperature. J. Exp. Bot., 33: 288-296. DOI:10.1093/jxb/33.2.288 |
Grime J.P., Mason G., Curtis A.V., et al, 1981. A comparative-study of germination characteristics in a local flora. J. Ecol., 69: 1017-1059. DOI:10.2307/2259651 |
Hardegree S.P., 2006. Predicting germination response to temperature.Ⅰ. Cardinal-temperature models and subpopulation-specific regression. Ann. Bot., 97: 1115-1125. DOI:10.1093/aob/mcl071 |
Hu X.W., Fan Y., Baskin C.C., et al, 2015. Comparison of the effects of temperature and water potential on seed germination of Fabaceae species from desert and subalpine grassland. Am. J. Bot., 102: 649-660. DOI:10.3732/ajb.1400507 |
Jaganathan G.K., Dalrymple S.E., Liu B., 2015. Towards an understanding of factors controlling seed bank composition and longevity in the alpine environment. Bot. Rev., 81: 70-103. DOI:10.1007/s12229-014-9150-2 |
Kudo G., Kimura M., Kasagi T., et al, 2010. Habitat-specific responses of alpine plants to climatic amelioration:comparison of fellfield to snowbed communities. Arct. Antarct. Alp. Res., 42: 438-448. DOI:10.1657/1938-4246-42.4.438 |
Liu K., Baskin J.M., Baskin C.C., et al, 2013. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PLoS One, 8: e69364. DOI:10.1371/journal.pone.0069364 |
Omori Y., Ohba H., 1996. Pollen development of Rheum nobile Hook f Thomson (Polygonaceae) with reference to its sterility induced by bract removal. Bot. J. Linn. Soc., 122: 269-278. |
Peng, D. L., Hu, X. J., Yang, J., et al., 2017. Seed dormancy, germination and soil seed bank of Lamiophlomis rotata and Marmoritis complanatum (Labiatae), two endemic species from HimalayaeHengduan Mountains. Plant Biosyst. doi: 10.1080/11263504.2017.1311959.
|
Schwienbacher E., Navarro-Cano J.A., Neuner G., et al, 2011. Seed dormancy in alpine species. Flora, 206: 845-856. DOI:10.1016/j.flora.2011.05.001 |
Song B., Stocklin J., Gao Y.Q., et al, 2013a. Habitat-specific responses of seed germination and seedling establishment to soil water condition in two Rheum species in the high Sino-Himalayas. Ecol. Res., 28: 643-651. DOI:10.1007/s11284-013-1057-6 |
Song B., Zhang Z.Q., Stocklin J., et al, 2013b. Multifunctional bracts enhance plant fitness during flowering and seed development in Rheum nobile (Polygonaceae), a giant herb endemic to the high Himalayas.. Oecologia, 172: 359-370. DOI:10.1007/s00442-012-2518-2 |
Song B., Stoecklin J., Peng D.L., et al, 2015. The bracts of the alpine 'glasshouse' plant Rheum alexandrae (Polygonaceae) enhance reproductive fitness of its pollinating seed-consuming mutualist. Bot. J. Linn. Soc., 179: 349-359. DOI:10.1111/boj.12312 |
Sun Y.S., Wang A.L., Wan D.S., et al, 2012. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Mol. Phylogenet. Evol., 63: 150-158. DOI:10.1016/j.ympev.2012.01.002 |
Thompson K., Grime J.P., 1983. A comparative study of germination responses to diurnally fluctuating temperatures. J. Appl. Ecol., 20: 141-156. DOI:10.2307/2403382 |
Tsukaya H., 2002. Optical and anatomical characteristics of bracts from the Chinese "glasshouse" plant, Rheum alexandrae Batalin (Polygonaceae), in Yunnan, China. J. Plant Res., 115: 59-63. DOI:10.1007/s102650200009 |
Xu B., Li Z.M., Sun H., 2014. Seed Plants of the Alpine Subnival Belt from the Hengduan Mountains, SW China. Beijing: Science Press.
|



