b. Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Kunming, China
The association between figs (Ficus, Moraceae) and their pollinating fig wasps (Hymenoptera, Agaonidae, Chalcidoidea) is generally thought to be an ideal system for investigating coevolution and the maintenance of mutualisms. Both figs and their pollinating fig wasps are completely dependent on each other for survival and reproduction, as figs can only be pollinating by pollinating fig wasps, and pollinating fig wasps can only reproduce within figs. Although previously studies reported that each fig species had its own speciesspecific pollinating fig wasp species (Ramirez, 1970; Wiebes, 1979; Bronstein, 1987; Herre et al., 1996; Anstett et al., 1997), recent work has found examples of multiple pollinating fig wasp species cooccurring on the same fig species (Kerdelhué et al., 1999; Molbo et al., 2003, 2004). These findings suggest that the phenomenon of pollinator host-switching may be more complex than previously thought in fig-wasp mutualism (Machado et al., 2005).
Some Agaonids are passive pollinators, transporting pollen on their body surface, while others actively collect and store pollen and then distribute it on the styles of fig flowers while they are ovipositing. Active pollination is much more efficient, allowing their host figs to produce far fewer male flowers because less pollen is required. In about one-third of fig species (Kjellberg et al., 2001), pollination is passive. The mode of pollination occurring in a species of Ficus can be consistently predicted from the anther-to-ovule ratio (Kjellberg et al., 2001). On a representative number of fig species, these authors showed that an anther-to-ovule ratio of less than 0.16 indicates active pollination, while a ratio over 0.20 is characteristic of passively pollinated species.
Figs also host numerous non-pollinating fig wasp species that depend on figs for their development and reproduction without providing any benefit to their figs (Bronstein, 1991; Compton and Hawkins, 1992; Boucek, 1993; West and Herre, 1994; West et al., 1996; Cook and Rasplus, 2003; Pereira and Prado, 2005). They do not belong to the pollinating lineage Agaonidae (Rasplus et al., 1998). Most non-agaonid fig wasps oviposit through the fig wall from the exterior of the fig. However, some species enter figs and oviposit in the female flowers, just as the pollinating fig wasps do.
Biological data on these internally-ovipositing non-agaonid fig wasps is limited. Their hosts are often largely unknown for Asian species. Some internally ovipositing non-agaonid fig wasps Diaziella (Pteromalidae, Sycoecinae) and Lipothymus species (Pteromalidae, Otitesellinae) are known to be able to act as pollinators. They develop in figs that produce abundant pollen and consequently their Waterstoniella and Eupristina agaonids are passive pollinators (Jousselin et al., 2001; Zhang et al., 2008). In contrast, there is no evidence of a mutualism between an internally ovipositing non-agaonid fig wasp and its actively-pollinated host fig, Ficus glaberrima (Zhang et al., 2009). Here, we examine the anatomy of the Eupristina pollinators of eight closely related fig tree species, and relate this to mode of pollination and the possibility of non-agaonids being recruited for pollination.
2. Materials and methods 2.1. Study site and study speciesFigs were collected from eight species of (subgenus Urostigma, subsection Conosycea), in the vicinity of the Xishuangbanna Tropical Botanical Garden (XTBG), in South-West China (101° 15' E, 21° 55' N), at the northern margin of tropical South-east Asia. The genus Eupristina belongs to Agaonidae. Two internally ovipositing non-agaonid fig wasp species (Diaziella yangi and Diaziella bizarrea) belong to Pteromalidae, Sycoecinae. Another internally ovipositing non-agaonid fig wasp species (Lipothymus sp.) belongs to Pteromalidae, Otitesellinae. The species of fig wasps and their biological characteristics are given in Table 1.
| Ficus | Species | Diagnosis | Coexisting role |
| Ficus curtipes | Eupristina sp. 1 | Female: body black color with clear wings; male: wingless | Gall maker |
| F. curtipes | Diaziella yangi | Female: the large dark patch on the forewings; male: winged | Inquiline |
| F. curtipes | Lipothymus sp. | Female: body metallic color with clear wings; Male: wingless | Inquiline |
| F. glaberrima | Eupristina sp. 2 | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. glaberrima | D. bizarrea | Female: body metallic color with clear wings; Male: winged | Inquiline |
| F. altissima | E. altissima | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. benjamina | E. oningsbergeri | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. stricta | E. cyclostigma | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. microcarpa | E. verticillata | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. macellandi | Eupristina sp. 3 | Female: body black color with clear wings; Male: wingless | Gall maker |
| F. drupacea pubescens | Eupristina sp. 4 | Female: body black color with clear wings; Male: wingless | Gall maker |
We first assessed whether internally ovipositing non-agaonid wasps were regularly found in Ficus species pollinated by the agaonid genus Eupristina. For eight Ficus species pollinated by fig wasps of this genus, figs were sampled. Figs were collected when fig wasps are emerging and pollen is mature. They were kept in fine-mesh bags and fig wasps were allowed to emerge. Wasps were the preserved in 70% alcohol for identification. In addition, all female and males flowers of figs were counted.
2.3. Direct evidence of mode of pollinationThe mode of pollination was confirmed by using our own observations and published data. Receptive and mature figs were collected. Then we bisected the fig lengthways, from the stalk to the ostiole, to reveal the lumen. Observation of pollen-loading and pollen-deposition behavior was performed under microscope. In order to further detect the pollination mode of Eupristina species, SEM photos of the pollen pockets and coxal combs were took. Pollen pockets and coxal combs are two traits associated with the mode of pollination in agaonid fig wasps. Coxal combs are considered as the most reliable trait for inferring mode of pollination, as many species of agaonids still possess pollen pockets but do not actively collect and deposit pollen (Kjellberg et al., 2001).
3. Results 3.1. Frequencies of internally ovipositing non-agaonid fig wasps in fig crops pollinated by EupristinaAmong the eight species of Ficus collected at fig maturity. Interestingly, two Ficus species host one or two species of nonagaonid wasps that enter the fig to oviposit in addition to agaonid wasp belonging to the genus Eupristina. Ficus species collected and their associated wasps are given in Table 2. Diaziella, Lipothymus and Eupristina emerged simultaneously from figs. Each species of Diaziella and Lipothymus was specific to its associated fig.
| Ficus species | Number of trees | Internally-ovipositing fig wasps | |
| Agaonids | Others | ||
| Ficus curtipes | 4 | Eupristina sp. 1 | Diaziella yangi Lipothymus sp. |
| F. glaberrima | 3 | Eupristina sp. 2 | Diaziella bizarrea |
| F. altissima | 3 | Eupristina altissima | |
| F. benjamina | 2 | Eupristina koningsbergeri | |
| F. stricta | 2 | Eupristina cyclostigma | |
| F. microcarpa | 2 | Eupristina verticillata | |
| F. macellandi | 2 | Eupristina sp. 3 | |
| F. drupacea pubescens | 1 | Eupristina sp. 4 | |
The anther-to-ovule ratio of Ficus curtipes was high (0.84 ± 0.10 (mean ± SD, n = 67)), suggesting that this Ficus species is passively pollinated. This was consistent with the anatomy of adult female Eupristina sp. (Fig. 1a), the pollinator Eupristina sp. females of F. curtipes possessed obviously non-functional pollen pockets and had no the coxal combs. The females become completely dusted with pollen when they emergence from figs. When the fig wasps enter a receptive fig, pollen is progressively dispersed within the cavity. The fig females did not show pollination behavior.
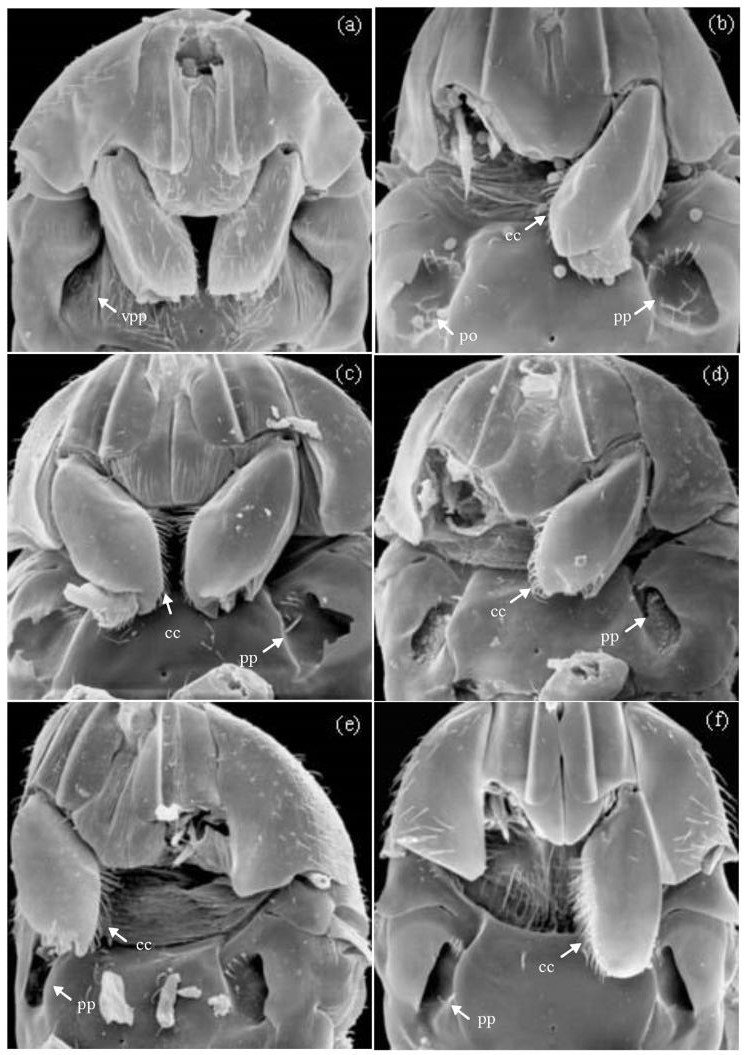
|
| Fig. 1 Ventral views of female agaonid mesosoma: (a): Eupristina sp.1 (host Ficus curtipes), (b) Eupristina sp.2 (host Ficus glaberrima), (c) Eupristina koningsbergeri (host Ficus benjamina), (d) Eupristina cyclostigma (host Ficus stricta), (e) Eupristina sp.3 (host Ficus macellandi), (f) Eupristina sp. 4 (host Ficus drupacea pubescens). The head is pointing upward. Species pollinating actively are shown in bef. Species pollinating passively is shown in a. Abbreviations: vpp, vestigial pollen pocket; pp, pollen pocket; po, pollen seen through pollen-pocket opening; cc, coxal comb. |
The anther-to-ovule of other seven Ficus species was low, and the data on mode of pollination and on the fig traits were presented in Table 3, implying that these Ficus species are actively pollinated. These were consistent with the anatomy of their associated female agaonids (Fig. 1), which have well defined thoracic pollen pockets (to carry pollen) and coxal combs on their fore coxae (to manipulate pollen). Active pollination of these agaonids was confirmed by observation of pollen-loading and pollen-deposition behavior in mature and receptive figs respectively.
| Ficus species | Sample size (figs) | Anthers/ovules (mean ± SD) | Pollinating fig wasps | Pollen pocket | Coxal comb | Pollination behavior (Sources) |
| Ficus curtipes | 67 | 0.84 ± 0.10 | Eupristina sp.1 | vestigial | No | Passive (observed) |
| F. glaberrima | 42 | 0.11 ± 0.02 | Eupristina sp. 2 | Yes | Yes | Active (observed) |
| F. altissima | 30 | 0.09 ± 0.02 | Eupristina altissima | Yes | Yes | Active (observed) |
| F. benjamina | 26 | 0.10 ± 0.02 | Eupristina koningsbergeri | Yes | Yes | Active (observed) |
| F. stricta | 29 | 0.15 ± 0.02 | Eupristina cyclostigma | Yes | Yes | Active (observed) |
| F. microcarpa | 26 | 0.08 ± 0.03 | Eupristina verticillata | Yes | Yes | Active (observed) |
| F. macellandi | 34 | 0.14 ± 0.04 | Eupristina sp. 3 | Yes | Yes | Active (observed) |
| F. drupacea pubescens | Not recorded | 0.09 (K) | Eupristina sp. 4 | Yes | Yes | Active (observed) |
| Sources: data from (K): Kjellberg et al., 2001. | ||||||
Jousselin et al. (2001) reported seven Ficus species pollinated by wasps of the genus Waterstoniella in Brunei Darussalam, on the island of Borneo, their result showed that six species of Ficus that were passively pollinated by the agaonid genus Waterstoniella hosted internally ovipositing non-agaonid wasps (the genera Diaziella (Otitesellinae) and Lipothymus (Sycoecinae)). In contrast, the colonize rate of internally ovipositing non-agaonid wasps in Ficus species pollinated by the genus Eupristina was low. Our study shows that two species of Ficus pollinated by the genus Eupristina host one or two specific species of internally ovipositing nonagaonid wasps of the genera Lipothymus or Diaziella in Xishuangbanna, China.
Based on our observations, only about one species (F. curtipes) is passively pollinated, the other (F. glaberrima, Ficus altissima, Ficus benjamina, Ficus stricta, Ficus macrocarpa, Ficus macellandi, Ficus drupacea pubescens) is actively pollinated. Action pollinating is probably generally beneficial for both wasp and fig. Indeed, previous report showed that active pollination benefited the fig by allowing a strong reduction in pollen production (Kjellberg et al., 2001). It was also generally thought that ovule fertilization benefited the fig wasp, either because it limited fig abortion (Janzen, 1979) or because it ensured better survivorship of their larvae (Jousselin et al., 2003; Tarachai et al., 2008). In the genus Eupristina, most species pollinate actively and only one species pollinate passively. This pattern suggests that rare passively pollinating species in mainly actively pollinating species represent relatively recent cases of loss of active pollination. This conclusion is consistent with previous report (Kjellberg et al., 2001).
Some non-agaonid fig wasps (Diaziella and Lipothymus species) that enter figs to oviposit are capable of pollinating them, and thereby establishing a mutualism with their host plants (Jousselin et al., 2001; Zhang et al., 2008). In all reported cases the host plants are nonetheless also associated with a 'typical' agaonid pollinator, and so are not dependent upon these substitute pollinators. Furthermore, the agaonids are passive pollinators and their host figs produce large amounts of pollen that becomes scattered within the figs when adult females are preparing to leave their natal figs. Pollinators of such figs do not require any specific adaptations to achieve effective pollination, so long as foundresses enter figs during their receptive period, which they are forced to do because this is the only time that the ostiolar bracts loosen to form a passage. In contrast, active pollinators, such as the Eupristina species associated with F. glaberrima, have complex morphological and behavioral adaptations that ensure that the relatively small amounts of pollen available to them can be collected and transferred. There is no evidence of mutualism between D. bizarrea and its actively-pollinated host fig, F. glaberrima (Personal communication). The contrast between the result and those of Jousselin et al. (2001) and Zhang et al. (2008) confirms that the ability of nonagaonids to act as substitute pollinators is dependent of the mode of pollination of their associated agaonid fig wasps.
When the associated agaonid fig wasp pollinates actively, only a very small quantity of pollen is produced by the fig (Galil and Meiri, 1981; Kjellberg et al., 2001). The internally ovipositing nonagaonids do not present any pollen collecting behavior, the low level of seed production is supposed to be due to incidentally passive pollen transfer on the body of these fig wasps (Newton and Lomo, 1979; Compton et al., 1991). In contrast, in passively pollinated figs, abundant pollen is produced in cavity of fig, which ensures the passive coating of fig wasps with pollen as they emerge, the internally ovipositing non-agaonids become efficient pollinators (Jousselin et al., 2001; Zhang et al., 2008).
Our study suggests that the mode of interaction between internally ovipositing non-agaonids and figs relies on the mode of pollination of the associated agaonid wasp. Studies of the molecular phylogenies of Ficus, pollinating and externally ovipositing nonpollinating fig wasps have already given valuable insights into the putative factors that are responsible for the origin and evolution of the fig-wasp mutualism (Machado et al., 1996; Rasplus et al., 1998; Marussich and Machado, 2007). Similarly, internally ovipositing non-agaonid wasps and pollinating and externally ovipositing nonpollinating fig wasps will be very interesting models for comparative analysis. The combination of mode of pollination will open a whole fig-wasp world of exciting possibilities. Further studies of internally ovipositing non-agaonid wasps present not only a tremendous advance in general fig knowledge, but an exceptionally good set of opportunities for future work.
AcknowledgementsWe thank Li Zong-Bo for assistance. We are grateful to Prof. Stephen G. Compton for his useful comments and help in improving the English text. This study was funded by the National Natural Science Foundation of China (30571507, 30670358).
Anstett M.C., Hossaert-McKey M., Kjellberg F., 1997. Figs and fig pollinators: evolutionary conflicts in a coevolved mutualism. Trends Ecol. Evol, 12: 94-99. DOI:10.1016/S0169-5347(96)10064-1 |
Boucek Z., 1993. The genera of Chalcidoid wasps from Ficus fruit in the New World. J. Nat. Hist, 27: 173-217. DOI:10.1080/00222939300770071 |
Bronstein J.L., 1991. The nonpollinating wasp fauna of Ficus pertusa: exploitation of a mutualism?. Oikos, 61: 175-186. DOI:10.2307/3545335 |
Bronstein J.L., 1987. Maintenance of species-specificity in a neotropical fig pollinator wasp mutualism. Oikos, 48: 39-46. DOI:10.2307/3565686 |
Compton S.G., Holton K.C., Rashbrook V.K., van Noort S., Vincent S., Ware A.B., 1991. Studies of Ceratosolen galili, a non pollinating agaonid fig wasp (Hymenoptera, Agaonidae). Biotropica, 23: 188-194. DOI:10.2307/2388305 |
Compton S.G., Hawkins B.A., 1992. Determinants of species richness in southern African fig wasp assemblages. Oecologia, 91: 68-74. DOI:10.1007/BF00317243 |
Cook J.M., Rasplus J.Y., 2003. Mutualists with attitude: coevolving fig wasps and figs. Trends Ecol. Evol, 18: 241-248. DOI:10.1016/S0169-5347(03)00062-4 |
Galil J., Meiri L., 1981. Number and structure of anthers in fig syconia in relation to behaviour of the pollen vectors. New Phytol, 88: 83-87. |
Herre E.A., Machado C.A., Bermingham E., Nason J.D., Windsor D.M., McCafferty S., van Houten W., Bachmann K., 1996. Molecular phylogenies of figs and their pollinator wasps. J. Biogeogr, 23: 521-530. DOI:10.1111/jbi.1996.23.issue-4 |
Janzen D.H., 1979. How to be a fig. Annu. Rev. Ecol. Syst, 10: 13-51. DOI:10.1146/annurev.es.10.110179.000305 |
Jousselin E., Hossaert-McKey M., Herre E.A., Kjellberg F., 2003. Why do fig wasps actively pollinate monoecious figs?. Oecologia, 134: 381-387. DOI:10.1007/s00442-002-1116-0 |
Jousselin E., Rasplus J.Y., Kjellberg F., 2001. Shift to mutualism in parasitic lineages of the fig/fig wasp interaction. Oikos, 94: 287-294. DOI:10.1034/j.1600-0706.2001.940209.x |
Kerdelhu#233; C. , Le Clainche I., Rasplus J.Y., 1999. Molecular phylogeny of the Ceratosolen species pollinating Ficus of the subgenus Sycomorus sensu stricto: biogeographical history and origins of the species-specificity breakdown cases. Mol. Phylogeny Evol, 11: 401-414. DOI:10.1006/mpev.1998.0590 |
Kjellberg F., Jousselin E., Bronstein J.L., Patel A., Yokoyama J., Rasplus J.Y., 2001. Pollination mode in fig wasps: the predictive power of correlated traits. Proc. R. Soc. Lond. B, 268: 1113-1121. DOI:10.1098/rspb.2001.1633 |
Machado C.A., Herre E.A., McCafferty S., Bermingham E., 1996. Molecular phylogenies of fig pollinating and non-pollinating wasps and the implications for the origin and evolution of the fig-fig wasp mutualism. J. Biogeogr, 23: 531-542. DOI:10.1111/jbi.1996.23.issue-4 |
Machado C.A., Robbins N., Gilbert M.P.T., Herre E.A., 2005. Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proc. Natl. Acad. Sci. U. S. A, 102: 6558-6565. DOI:10.1073/pnas.0501840102 |
Marussich W., Machado C.A., 2007. Host-specificity and coevolution among pollinating and non-pollinating New World fig wasps. Mol. Ecol, 16: 1925-1946. DOI:10.1111/j.1365-294X.2007.03278.x |
Molbo D., Machado C.A., Sevenster J.G., Keller L., Herre E.A., 2003. Cryptic species of fig-pollinating wasps: implications for the evolution of the fig-wasp mutualism, sex allocation, and precision of adaptation. Proc. Natl. Acad. Sci. U. S. A, 100: 5867-5872. DOI:10.1073/pnas.0930903100 |
Molbo D., Machado C.A., Herre E.A., Keller L., 2004. Inbreeding and population structure in two pairs of cryptic fig wasp species. Mol. Ecol, 13: 1613-1623. DOI:10.1111/mec.2004.13.issue-6 |
Newton L.E., Lomo A., 1979. The pollination of Ficus vogelii in Ghana. Bot. J. Linn. Soc, 78: 21-30. DOI:10.1111/j.1095-8339.1979.tb02183.x |
Pereira R.A.S., Prado A.P., 2005. Recognition of competitive asymmetries reduces the severity of fighting in male Idarnes fig wasps. Anim. Behav, 70: 249-256. DOI:10.1016/j.anbehav.2004.09.029 |
Ramirez W.B., 1970. Host specificity of fig wasps (Agaonidae). Evolution, 24: 680-691. DOI:10.1111/evo.1970.24.issue-4 |
Rasplus J.Y., Kerdelhué C., Clainche ILe, Mondor G., 1998. Molecular phylogeny of fig wasps (Hymenoptera). Agaonidae are not monophyletic. Compte Rendu de l'Académie des Sciences de Paris, 321: 517-527. |
Tarachai Y., Compton S.G., Trisonthi C., 2008. The benefits of pollination for a fig wasp. Symbiosis, 45: 29-32. |
West S.A., Herre E.A., 1994. The ecology of the New World fig-parasitizing wasps Idarnes and implications for the evolution of the fig-pollinator mutualism. Proc. R. Soc. Lond., 258(B): 67-72. |
West S.A., Herre E.A., Windsor D.M., Green P.R.S., 1996. The ecology and evolution of the New World non-pollinating fig wasp communities. J. Biogeogr, 23: 447-458. DOI:10.1111/jbi.1996.23.issue-4 |
Wiebes J.T., 1979. Co-evolution of figs and their insect pollinators. Annu. Rev. Ecol. Syst, 10: 1-12. DOI:10.1146/annurev.es.10.110179.000245 |
Zhang F.P., Peng Y.Q., Guan J.M., Yang D.R., 2008. A species of fig tree and three unrelated fig wasp pollinators. Evol. Ecol. Res, 10: 611-620. |
Zhang F.P., Peng Y.Q., Compton S.G., Zhao Y., Yang D.R., 2009. Host pollination mode and mutualist pollinator presence: net effect of internally ovipositing parasite in the fig-wasp mutualism. Naturwissenschaften, 96: 543-549. DOI:10.1007/s00114-008-0502-9 |



