b. Biological Sciences and Technology College, Baotou Teachers' College, Baotou 014030, China
Mattirolomyces terfezioides (Mattir.) E. Fisch., the type species of Mattirolomyces E. Fisch. (Pezizaceae, Pezizales), is one of the truffle species repeatedly documented in taxonomic and phylogenetic literatures (Kagan-Zur et al., 2014). It was originally described from Northern Italy by Mattirolo (1887) in the genus Choiromyces Vittad. Fischer (1938) erected a monotypic genus Mattirolomyces using Choiromyces terfezioides Mattir. as the type. Trappe (1971) transferred Mattirolomyces to Terfezia (Tul. and C. Tul.) Tul. and C. Tul. based on the overlapping characters between some species of Terfezia and Mattirolomyces. Molecular phylogenetic analyses, however, supported Mattirolomyces to be a separate genus from Terfezia within the same family, Pezizaceae (Hansen et al., 2001; Læssøe and Hansen, 2007; Percudani et al., 1999), thus making the name M. terfezioides has been fixed since then. Unlike Terfezia species (desert truffle), which are mostly found in arid to semi-arid sandy environments in Mediterranean region and form mycorrhizae with herbaceous species of Cistaceae (Díez et al., 2002), M. terfezioides is often found under artificially planted trees [e.g. Robinia pseudoacacia L., Diospyros kaki Thunb. and Prunus avium (L.) L.] in southern and central Europe and its mycorrhizal status is not clearly answered up to now.
Among the five known species of Mattirolomyces, M. terfezioides and Mattirolomyces spinosus (Harkn.) Kovács et al. have been recorded from China. M. spinosus is only listed by Tai (1979), whereas M. terfezioides is one of the mostly documented true truffles in the country (Alsheikh, 1994; Liu, 1991; Liu and Guo, 1984; Liu and Tao, 1989; Liu et al., 2002; Zhang, 1990) and enumerated as one of the Chinese edible fungi (Dai et al., 2010). These records, however, has never been tested with DNA sequence data. Alsheikh (1994) observed a specimen collected from Beijing (HMAS 32656). The identity of this specimen, however, was left as an open issue by Kovács and Trappe (2014) when they said "it (Mattirolomyces) includes five species … from four continents (or five, if we consider the Beijing urban collection of M. terfezioides as well…". Moreover, Kovács and Trappe (2014) found that most of the Chinese desert truffles are misidentified. In such scenario, as well as based on a assumption that truffles normally have a less efficient dispersal ability (Trappe and Claridge, 2005) that will result in relatively narrow distribution (Bonito et al., 2010), it is natural to question if the Chinese specimens labelled as M. terfezioides could be conspecific with authentic (European) M. terfezioides. Aiming to answer this question, we re-examined five historical specimens (possibly) related with M. terfezioides in HMAS and amplified the ITS and LSU regions for them. The results are reported herein.
2. Materials and methods 2.1. MaterialsFive specimens under Terfezia (where M. terfezioides has long been placed) deposited in HMAS were studied. Three of them were labelled as Terfezia terfezioides, one as Terfezia leonis and one as Terfezia sp. This sampling includes a specimen collected from Shanxi Province in October, 1983 (HMAS 76805). Since many specimens have been transferred from the Mycological Herbarium of Shanxi University to HMAS and this specimen meets the date and locality of the specimen cited by Liu and Guo (1984), we believe this specimen presents the voucher that Liu and Guo (1984) used to the report Terfezia eonis [later corrected to T. terfezioides by Liu and Tao (1989) and Liu (1991)] in China. HMAS 32656, HMAS 60273, HMAS 76805 and HMAS 88581 were described as T. terfezioides by Zhang (1990). The specimen (HMAS 32656) labelled as T. leonis was cited by Alsheikh (1994) under M. terfezioides. This is the only specimen under the name T. leonis collected before 1963, and we believe this is the voucher of T. leonis in Teng (1963). The specimen labelled as Terfezia sp. (HMAS 83766) was cited as a desert truffle record in China by Kovács and Trappe (2014).
2.2. Morphological observationMacroscopic observations are based on dried specimens. Microscopy mainly followed Kovács et al. (2011). Dried ascomata were sectioned with a stainless razor blade. Slides were made by mounting the tissue in 5% or 10% KOH. Micro-morphological features observed included shape and size of ascus, number of ascospore in mature asci, size, shape and surface ornamentation of mature ascospores. Slides were observed under a Leica DM2500 stereoscope and photographed with a Leica DFC450C camera installed in it. Thirty spores that came from different asci or dissociate outside the asci were measured from mature ascoma. Reactions were tested using Melzer's reagent and Cotton Blue.
2.3. DNA extraction, PCR and phylogenetic analysesTotal DNA was extracted from dried gleba with a modified CTAB protocol (Doyle and Doyle, 1987). Since the most samples are rather old (up to 54 years old), an extra purification step was performed for the extracted DNA using GeneClean® II Kit (MP Biomedicals), according to the manufacturer's instructions. The primer pairs ITS5þ ITS4 (or ITS1þ ITS4) and LR0R þ LR5 were used to amply the ITS region and part of the 28S respectively (White et al., 1990; R. Vilgalys lab, http://www.biology.duke.edu/fungi/mycolab/primers.htm). PCR amplification was performed with Takara® DNA polymerase (Dalian, China) using the following protocol (25 ml reaction mixture): 2.5 μl buffer, 2.5 μl 0.1% BSA, 2 μl 2.5 mM dNTPs, 0.5 μl 10 μM of forward and reverse primers, 0.2 μl 5 U/μl Taq polymerase, 5 μl total DNA solution, and 12 ml ddH2O. The following PCR programs were used: 5 min at 94 ℃, 38 cycles of 1 min at 94 ℃, 1 min at 58 ℃ and 1 min 30 s at 72 ℃, and a final extension of 72 ℃ for 10 min. For two samples with problem to get the whole ITS region, HMAS 76805 and HMAS 88581, internal primers ITS2 and 5.8SR were used with ITS1 and ITS4 respectively to amplify the ITS-1 and ITS-2 regions separately. Cycling parameters for the two short regions were set as: an initial denaturalization step for 5 min at 94 ℃, 38 cycles consisting of 30 s at 94 ℃, 30 s at 60 ℃, and 50 s at 72 ℃, and a final extension at 72 ℃ for 7 min. The PCR products, pre-judged by gel electrophoresis were purified and sequenced at Sangon Biotech Corporation, Shanghai, China. Sequences were deposited in GenBank with accession numbers in Table 1.
| Species | Voucher | Locality | Collector and date | GenBank No. | |
| ITS | LSU | ||||
| Elderia avenivaga | OSC 111751 | Australia | R. Helms, 1891 | GQ231733 | GQ231734 |
| Elderia avenivaga | OSC 111641 | Australia | D. Albrecht, 2000 | GQ231736 | GQ231737 |
| Mattirolomyces austroafricanus | OSC 58845 | South Africa | E. L. Stephens | GQ231752 | GQ231753 |
| Mattirolomyces mexicanus | OSC 131669 | Mexico | J. Muñoz, 1980.07.08 | HQ660378 | HQ660379 |
| M. mulpu | OSC 131319 | Australia | E. Mantatjara, 1983.05.26 | GQ231739 | GQ231740 |
| M. spinosus | Ellis & Everhart 1782 | USA | E. Forges, 1886.11 | HQ660381 | HQ660382 |
| M. spinosus | CUP 56967 | Pakistan | S. Ahmed, 1949.08 | HQ660384 | HQ660385 |
| M. terfezioides (labelled as T. leonis) | HMAS 32656 | China: Beijing | D.L. Guo & H.Z. Li, 1961.09.20 | KT963175 | KT963180 |
| M. terfezioides | HMAS 60273 | China: Hebei Province | Z.J. He & Z.J. Han, 1986 | KT963177 | KT963179 |
| M. terfezioides | HMAS 76805 | China: Shanxi Province | S.X. Guo, 1983.10.17 | KT963176 | - |
| M. terfezioides (labelled as Terfezia sp.) | HMAS 88581 | China: Shanxi Province | S.X. Guo, 1984.05 | KT963178 | - |
| Choiromyces sp. (labelled as M. terfezioides) | HMAS 83766 | China: Heilongjiang Province | J.X. Zhuang, 2001 | KU531609 | KT531618 |
| M. terfezioides | Trappe 4548 | France | L. Riousset, 1974.11.02 | GQ231754 | - |
| M. terfezioides | MA 8212 | Spain | 1984.08.30 | GQ422438 | - |
| M. terfezioides | Bratek 1131 | Hungary | Z. Bratek, 1996.11.13 | AJ272445 | - |
| M. terfezioides | Bratek 1873 | Hungary | Z. Bratek, 1998.10.15 | AJ305045 | - |
| M. terfezioides | Bratek 2197 | Hungary | Z. Bratek, 1991.09.10 | AJ272443 | - |
| M. terfezioides | KMG 10125_4 | Hungary | G.M. Kova'cs, 1999.08.30 | AJ305169 | - |
| M. terfezioides | Rob 01 | Hungary | J. Díez | AF276680 | - |
| M. terfezioides | Rib 02 | Hungary | J. Díez | AF276681 | - |
| M. terfezioides < / td > | environmental sample | Hungary | - | AJ875015 | - |
| M. terfezioides | environmental sample | Hungary | - | AJ875016 | - |
| M. terfezioides | KMG 10124 | Italy | G.M. Kova'cs, 1995.12.02 | AJ305170 | - |
| M. terfezioides | 17086 | Italy | A. Montecchi, 1989.10.10 | JF908728 | - |
| M. terfezioides | KFRI 2829 | South Korea | - | KT025693 | - |
DNA sequences were assembled in Sequencher 4.1.4 (Gene Codes Corp., Ann Arbor, MI). The obtained sequences were firstly submitted to the Nucleotide Basic Local Alignment Search Tool (BLAST) to find sequences with high homology. For M. terfezioides samples, 17 ITS sequences and six LSU sequences with 97-100% similarity were retrieved from GenBank. Duplicate sequences with identical characters were removed if they have the same biogeographic origin or of the same material type (azenic culture, environmental samples or ascomata). The 13 sequences left, the four Mattirolomyces ITS sequences obtained in this study, and five ITS sequences of Mattirolomyces austroafricanus, Mattirolomyces mexicanus, Mattirolomyces mulpu and M. spinosus published by Kovács et al. (2011)were used to conduct the phylogenetic analyses. Elderia arenivaga, which is shown to be the closest relative of Mattirolomyces by Trappe et al. (2010) and Kovács et al. (2011) was used as outgroup.
Alignments were made using the online version of the multiple sequence alignment program MAFFT v7 (Katoh and Toh, 2008), applying the L-INS-I strategy and manually adjusted in BioEdit Version 5.0.9 (Hall, 1999). Maximum Likelihood (ML) and Bayesian Inference (BI) analyses were performed to find the placement of the Chinese samples in the ITS phylogeny of Mattirolomyces. ML analysis was conducted in RAxML v7.2.6 (Stamatakis, 2006) and BI in MrBayes v3.2.1 (Ronquist et al., 2012). ML analyses applied the Rapid Bootstrapping algorithm with 1000 replicates, followed by a ML tree search. In the BI analysis, the GTR + I + G model was used and all parameter values, except branch lengths and tree topologies, were set unlinked. The BI analyses were conducted using two runs with four chains each for 1 × 107 generations sampling every 100th tree. Amajority rule consensus treewas built after discarding trees from a 25% burning. Trees generated by the two analyses were viewed and then exported as PDF in FigTree v1.3.1.
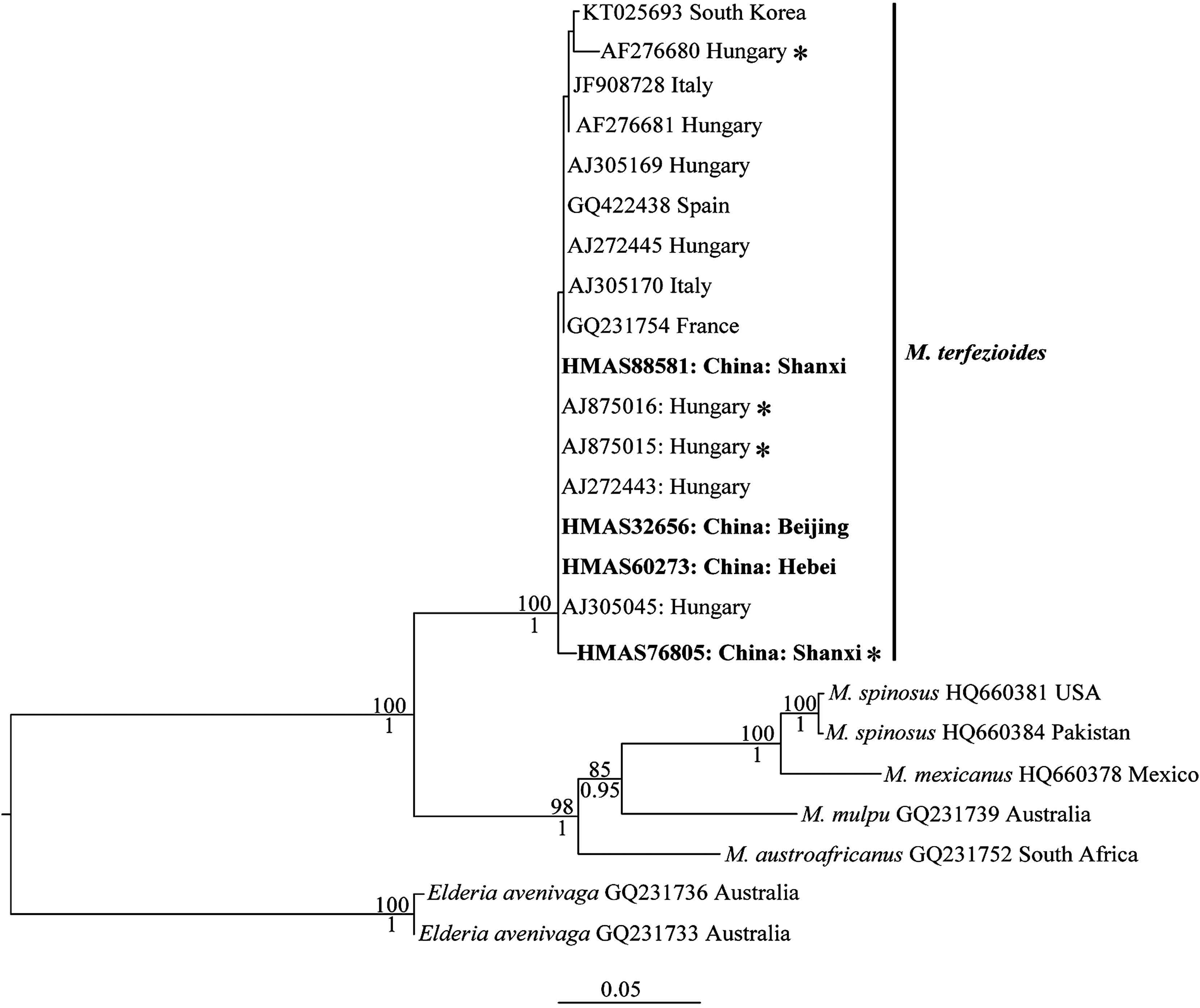
3. Results 3.1. Sequences comparison and molecular phylogenetic analysesWe produced five ITS and three LSU sequences from the five specimens sampled. By BLAST, we found that the ITS and LSU sequence of the specimen HMAS 83766 (KU531609 and KU531618) has 99% similarity with ITS sequence of Choiromyces sp. (KP019343) and ten LSU sequences of Choiromyces sp. (represented by KP019354, KP019355, KP019356). For the other four samples, we got 17 hits of ITS sequences with 97-100% similarity and six hits of LSU sequences with 98-99% similarity. All the retrieved ITS sequences are labelled as M. terfezioides and the six LSU sequences belong to Mattirolomyces. Compared with the retrieved ITS sequences, two Chinese samples (HMAS 32656 and HMAS 60273, with complete ITS sequences) have identical ITS sequences with three Hungary samples (Bratek2197 and two environmental samples with GenBank numbers AJ875015 and AJ875016). We only successfully amplified the ITS-1 and part of the 5.8S regions for HMAS 76805 and HMAS 88581. The two short sequences have one specific change compared with the other sequences of M. terfezioides. The two LSU sequences of the Chinese samples (KT963179 from HMAS 60273 and KT963180 from HMAS 32656) have one specific change compared with the only available LSU sequence of M. terfezioides from a European sample (Trappe4548). One Chinese sample (HMAS 76805) and three Hungarian samples were collected under or from the root of black locust (R. pseudoacacia) (Fig. 1).
|
| Fig. 1 Maximum Likelihood (ML) phylogram of Mattirolomyces and based on the ITS region, rooted with Elderia avenivaga. ML Bootstrap proportions higher than 70% and posterior probabilities from the Bayesian Inference analysis higher than 0.95 are indicated above and below the branches respectively. Samples are provided with GenBank accessions. Sequences generated in this study are in bold. Samples marked with "*" are collected under or from the roots of Robinia pseudoacacia. |
In the ITS phylogeny, our four Chinese samples formed a highly supported clade with 11 European samples and one South Korea sample (BI-PP = 1.00, ML-BP = 100%). These European samples are from four countries. There is neither clear genetic nor geographic structure within the clade of M. terfezioides. Similar to the results of Kovács et al. (2011), the M. terfezioides clade is the earliest divergent clade within Mattirolomyces.
3.2. Morphological observationM. terfezioides (Mattir.) E. Fisch., In Fischer In Engler A. & Prantl K. Nat. Pfl. Ed.: 39 (1938).
≡ Choiromyces terfezioides Mattir., Mém. R. Accad. Sci. Torino, Ser. 2 37: 10 (1887).
≡ Terfezia terfezioides (Mattir.) Trappe, Trans. Br. mycol. Soc. 57(1): 91 (1971).
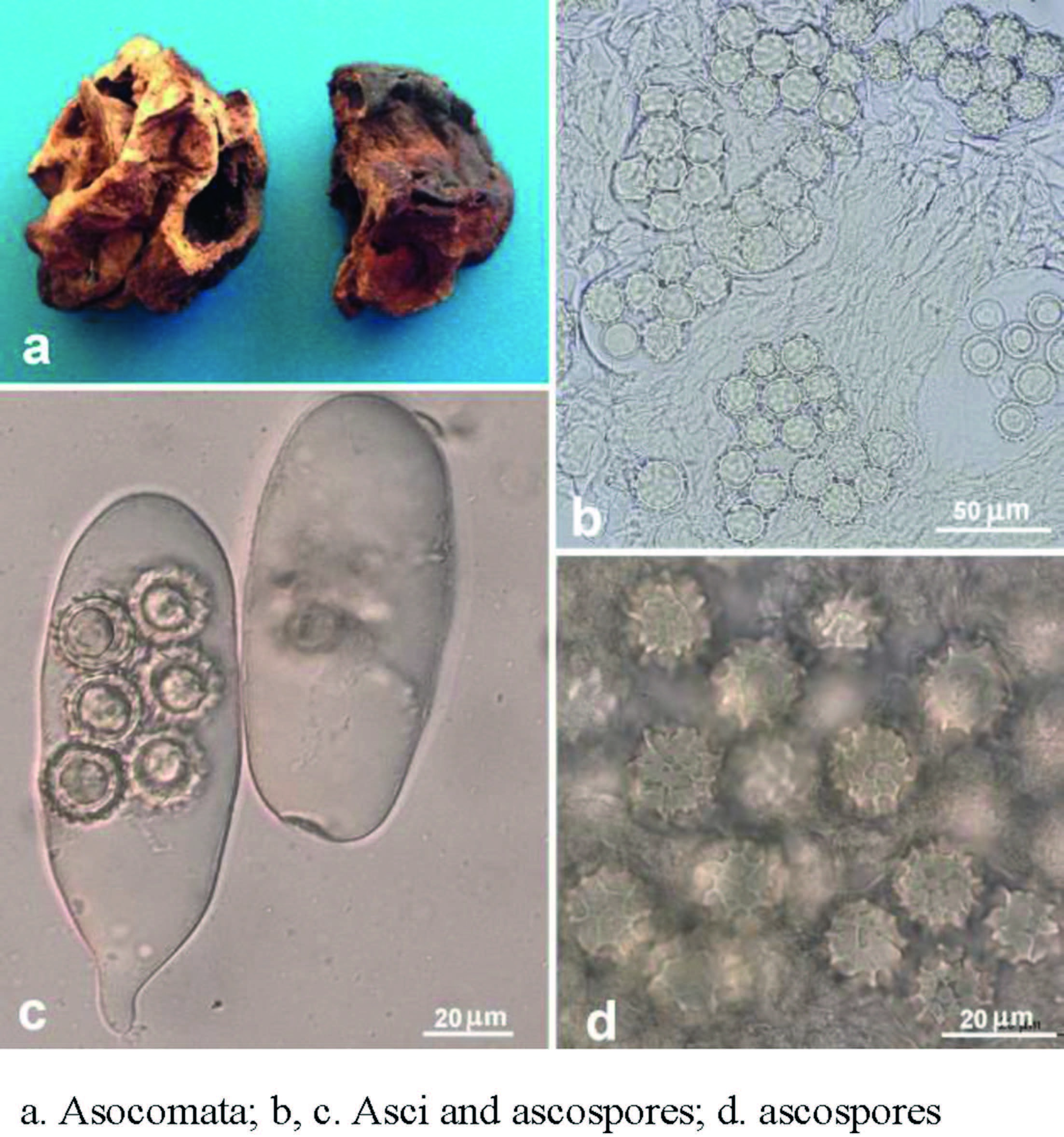
Ascomata (dry specimens, Fig. 2a) hypogeous or subepigeous, 1.5-3 (-8) cm in diam., subglobose to irregular massy, whitishyellow to yellow brown, fragile, surface smooth to scabrous, lobed, furrowed or wrinkled. Gleba subsolid, spongy with minute pockets, yellow to yellowish brown, some ascomata with narrow, white to pale yellow veins. Taste and odor sweet when fresh based on record.
|
| Fig. 2 Mattirolomyces terfezioides. |
Paraphyses absent. Peridium 160-310 mm thick, no clear differentiation from the gleba, composed of inflated hyphae and irregular, hyaline or pale yellowish cells 9-19 mm broad, often collapsing in maturity. Gleba composed of interwoven septate hyphae 2-8 mm broad, with some free hyphal ends. Asci (Fig. 2b and c) randomly arranged in gleba, 10 or (2-) 8-spored, hyaline, globose to ellipsoid, pockety, saccate, cylindrical or clavate, (40-) 55-110 (-130) × (20-) 35-60 (-70) µm, sessile or occasionally substipitate with a short stalk, disintegrating with age, thin-walled, readily separable from glebal hyphae, in youth sometimes the spores clustered in the tip of the ascus, later migrating to the middle, biseriate or irregularly arranged, nonamyloid. Ascospores (Fig. 2b-d) hyaline to pale yellow, globose, (11) 13-19 (-21) mm in diam. excluding the ornamentation (120 spores from four specimens measured); ornamentation of blunt spines connected in an irregular alveolate reticulum 1-4 mm high, mostly have a de Bary bubble and uniguttulate; walls 1-1.5 mm thick, dark yellow to yellowish brown in Melzer's, light blue in Cotton Blue.
Specimens examined: CHINA. Beijing, Luodaozhuang, 1961.9.20, leg. D.L. Guo and H.Z. Li, HMAS 32656; Hebei Province, Wanxian, 1986, leg. Z.J. He and Z.J. Han, HMAS 60273; Shanxi Province, Taiyuan, 1983.10.17, leg. S.X. Guo, HMAS 76805 (MHSU 1457); Shanxi Province, Taiyuan, 1984.5, leg. S.X. Guo, HMAS 88581 (MHSU 1458).
4. DiscussionThe typical characters of M. terfezioides include the whitish to yellowish brown ascomata with subsolid whitish to yellowish gleba with minute pockets asci and globose ascospores with blunt spines connected in an irregular alveolate reticulum 1-4 (-5) mm high. The four Chinese specimens that were confirmed to be conspecific with European M. terfezioides by ITS and LSU data match the morphological descriptions of T. terfezioides given by Babos (1981), Király and Bratek (1992), Ławrynowicz et al. (1997), and Alsheikh (1994). Among the other four know species of Mattirolomyces, M. spinosus is highly similar to M. terfezioides (Alsheikh, 1994) and distinguishing the two species has to relay on DNA sequences (Kovács et al., 2011).
Up to now, there are two molecular evidences convincing the presence of M. terfezioides in Asia: our data in this study and the GenBank sequences KT025693 from South Korean sample. Alsheikh (1994) cited a specimen from Pakistan under M. terfezioides from Pakistan, but this specimen was found to be M. spinosus by Kovács et al. (2011) with ITS and LSU sequences. The confirmed conspecificity of the Chinese specimens with European material might be due to shared host. Among our specimens, HMAS 76805 was collected under R. pseudoacacia. M. terfezioides has been reported to be associated with R. pseudoacacia or grow in (mixed) R. pseudoacacia forest many times [Bratek et al., 1996; Díez et al., 2002; Montecchi and Lazzari, 1993, and literatures cited by Kovács et al. (2003)]. In our ITS dataset, four samples of M. terfezioides are related with R. pseudoacacia (Fig. 1). Although Kovács et al. (2003) did not confirm the M. terfezioides-R. pseudoacacia interaction to be real mycorrhiza, they did find that the root cells of R. pseudoacacia could be colonized by the hyphae of M. terfezioides or the septate hyphal coils are similar to the endogenous structure formed by M. terfezioides (Kovács and Bagi, 2001, Kovács et al., 2007). Given the frequent co-occurrence of M. terfezioides with R. pseudoacacia, even if they do not form real well-defined mycorrhizae, their internal interaction cannot be excluded. The co-occurrence of M. terfezioides with R. pseudoacacia in Northern China will add new evidences in understanding the ecological habit and distribution of this edible truffle.
AcknowledgementsWe thank the curator of HMAS to arrange the loan of the specimens studied. We are grateful for Dr. X. H. Wang (Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China), who helped to revise the first draft and gave some valuable suggestions. This study was financed by the Joint Funds of the National Science Foundation of China and Yunnan Province Government (No. U1202262), the National Natural Science Foundation of China (No. 30470011, 31270075), the Local Project Y234011261 (Alxa League, Inner Mongolia) and Y21C211211 (Kunming, Yunnan Province), Key Laboratory of The Research Group of Systematics & Resources of Higher & Marco-Fungi, Kunming Institute of Botany, Chinese Academy of Sciences (No. 0806361121).
Alsheikh, A. M. , 1994. Taxonomic and Mycorrhizal Ecology of the Desert Truffles in the Genus Terfezia (Ph. D. Dissertation). Oregon State University, Corvallis.
|
Babos M, 1981. Distribution of Choiromyces venosus and Terfezia terfezioides in Hungary. Mikol. Köozl, 20(1-2): 47-56. |
Bonito G.M, Gryganskyi A, Trappe J.M, Vilgalys R, 2010. A global meta-analysis of Tuber ITS Rdna sequences: species diversity, host associations and longdistance dispersal. Mol. Ecol, 19: 4994-5008. DOI:10.1111/j.1365-294X.2010.04855.x |
Bratek Z, Jakucs E, Bóoka K, Szedlay G, 1996. Mycorrhizae between black locust (Robinia pseudoacacia) and Terfezia terfezioides. Mycorrhiza, (6): 271-274. |
Dai Y.C, Zhou L.W, Yang Z.L, et al, 2010. A revised checklist of edible fungi in China. Mycosystema, 29(1): 1-21. |
Díez J, Manjóon J.L, Martin F, 2002. Molecular phylogeny of the mycorrhizal desert truffles (Terfezia and Tirmania), host specificity and edaphic tolerance. Mycologia, 94: 247-259. DOI:10.1080/15572536.2003.11833230 |
Doyle J.J, Doyle J.L, 1987. A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem. Bull, 19: 11-15. |
Fischer, E. , 1938. Klasse Ascomycetes, Reihe Euascales. Unterreihe Ⅷ. Tuberineae. In: Engler, A. , Harms, H. (Eds. ), Natürlichen Pflanzenfamilien V Wilhelm Engelmann Verlag, Leipzig, p. 39.
|
Hall T.A, 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser, 41: 95-98. |
Hansen K, Læssøe T, Pfister D.H, 2001. Phylogenetics of the Pezizaceae, with an emphasis on Peziza. Mycologia, 93(5): 958-990. DOI:10.2307/3761760 |
Kagan-Zur V, Roth-Bejerano Sitrit S, Morte A, 2014. Desert truffles phylogeny, psysiology, distribution and domestication. Soil Biol, 38. |
Katoh K, Toh H, 2008. Recent developments in the MAFFT multiple sequence alignment program. Briefings Bioinf, 9: 286-298. DOI:10.1093/bib/bbn013 |
Kiráaly I, Bratek Z, 1992. Terfezia terfezioides, a common truffle in Hungary. Micol. Veg. Med, 7: 57-64. |
Kovács G.M, Bagi I, 2001. Mycorrhizal status of a mixed deciduous forest from the Great Hungarian Plain with special emphasis on the potential mycorrhizal partners of Terfezia terfezioides (Matt.). Trappe. Phyton, 41: 161-168. |
Kovács G.M, Trappe J.M, 2014. Nomenclatural history and genealogies of desert truffles. Desert truffles. Soil Biol, 38: 21-36. DOI:10.1007/978-3-642-40096-4 |
Kovács G.M, Váagvöolgyi C, Oberwinkler F, 2003. In vitro interaction of the truffle Terfezia terfezioides with Robinia pseudoacacia and Helianthemum ovatum. Folia Microbiol, 48: 369-378. DOI:10.1007/BF02931369 |
Kovács G.M, Jakucs E, Bagi I, 2007. Identification of host plants and description of sclerotia of the truffle Mattirolomyces terfezioides. Mycol. Prog, 6: 19-26. DOI:10.1007/s11557-006-0520-y |
Kovács G.M, Trappe J.M, Alsheikh A.M, Hansen K, Healy R.A, Váagi Páal, 2011. Terfezia disappears from the American truffle mycota as two new genera and Mattirolomyces species emerge. Mycologia, 103(4): 831-840. DOI:10.3852/10-273 |
Liu, B. , 1991. The Edible Macrofungi in Shanxi. United Press of Shanxi University, Taiyuan.
|
Liu B, Guo S.X, 1984. Terfezia leonis from Chinese Shanxi. Edible Fungi Chin, 22(2): 1. |
Liu B, Tao K, 1989. Hypogeous fungus research history and Chinese known species. Edible Fungi China, 11(1): 2. |
Liu B, Liu H.Y, Liu Z.H, 2002. Hypogeous fungus from China. Edible Fungi China, 21(1): 3-4. |
Læssøe T, Hansen K, 2007. Truffle trouble: what happened to the Tuberales? Mycol. Res, 111: 1075-1099. |
Mattirolo, O. , 1887. Illustrazione di tre nuove specie di Tuberacee Italiane. Mem. della R. Accad. Sci. Torino Second. Ser. 38, 377-393.
|
Montecchi, A. , Lazzari, G. , 1993. Atlante fotogróafico di funghi ipogei, vol. 490. AMB Fondazione Centro Studi Micologici, Vicenza, Italy.
|
Percudani R, Trevisi A, Zambonelli A, Ottonello S, 1999. Molecular phylogeny of truffles (Pezizales: Terfeziaceae, Tuberaceae) derived from nuclear rDNA sequence analysis. Mol. Phylogenet. Evol, 13: 169-180. DOI:10.1006/mpev.1999.0638 |
Ronquist F, Teslenko M, van der Mark P, Ayres D.L, Darling A, Hohna S, Larget B, Liu L, Suchard M.A, Heulsenbeck J.P, 2012. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Biol, 61(3): 539-542. |
Stamatakis A, 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688-2690. DOI:10.1093/bioinformatics/btl446 |
Tai, F. L. , 1979. Sylloge fungorum sinicorum. Science Press, Peking.
|
Teng, S. Q. , 1963. Fungi of China. Science Press, Beijing.
|
Trappe J.M, 1971. A synopsis of the Carbomycetaceae and Terfeziaceae (Tuberales). Trans. Br. Mycol. Soc, 57: 85-92. DOI:10.1016/S0007-1536(71)80083-9 |
Trappe, J. M. , Claridge, A. W. , 2005. Hypogeous fungi: evolution of reproductive and dispersal strategies through interactions with animals and mycorrhizal plants. In: Dighton, J. , Oudemans, P. , White, J. (Eds. ), The Fungal Community. CRC Press, Boca Raton, Florida, USA, pp. 599-623.
|
Trappe J.M, Kovács G, Claridge A.W, 2010. Comparative taxonomy of desert truffles of the Australian outback and the African Kalahari. Mycol. Prog, 9: 131-143. DOI:10.1007/s11557-009-0612-6 |
White, T. J. , Bruns, T. , Lee, S. S. , Taylor, J. , 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A. , Gelfand, D. H. , Sninsky, J. J. , White, T. J. (Eds. ), PCR Protocols, a Guide to Methods and Appliations. Academic Press Inc, New York.
|
Zhang, B. C. , 1990. Study on Systematic Position of Tuber at Tuberales and Chinese Tuber Species (Ph. D. thesis). Institute of Microbiology, CAS, Beijing.
|
Ławrynowicz M, MarkoviĆ M, MilenkoviĆ M, IvanČeviĆ B, 1997. Terfezia terfezioidesd-a new hypogeous fungus for Balkan Peninsula. Acta Mycol, 32: 233-238. |



