b. Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, Yunnan, China;
c. University of Chinese Academy of Sciences, Beijing 100049, China
Floral odors are found in the vast majority of flowering plants and they play an important role in plant-pollinator interactions, being the chemical cues for locating food and mating sites for the insects (Azuma et al., 1997; Dobson and Bergström, 2000; Jürgens et al., 2006; Gong et al., 2015). Because floral scents attract pollinators, to a large extent they determine a plant's reproductive success (Galen, 1999; Thien et al., 2000; Azuma et al., 2001).
Many plant species have low fruit/seed set due to lack or low visitation rate of pollinators (Sih and Baltus, 1987; Kunin, 1993; Rathcke and Jules, 1993; Hirayama et al., 2005). Many threatened plant species especially suffer from reproductive failure, and knowledge of their reproductive biology is essential to understand the cause of this failure (Duan and Liu, 2007).
The family Magnoliaceae is an ancient and important group in the evolution of flowering plants (Seymour et al., 2010). One of the largest genera in the family is the genus Magnolia sensu lato. Magnoliaceae plants, in particular the genus Magnolia, are earlydivergent flowering plants, bearing a number of large odoriferous flowers with chamber blossoms (Azuma et al., 1997; Gottsberger et al., 2012). Pollination in the family is primarily by nocturnal beetles, although other insects may also be involved (Thien, 1974; Bernhardt, 2000; Matsuki et al., 2008). Beetles are rewarded with pollen and other floral structures that they eat (Seymour et al., 2010).
Manglietia ventii(Magnoliaceae) was firstly described by Tiep (1980) based on specimens collected from Pingbian County in Yunnan Province, China. It has also been known as Manglietia hebecarpa (Law, 1996), Magnolia hebecarpa (Kumar, 2006), M. ventii (Law, 2006; Xia et al., 2008) and Magnolia ventii (Kumar, 2006; Wang et al., 2016a). M. ventii, an endemic species to Yunnan, China, has been approved as one of the 62 PSESP (Plant Species with Extremely Small Populations) for rescue conservation before 2020 by the Yunnan government (Ma et al., 2013). It has also been proposed as a second-ranked plant for national protection in China (Anonymous, 1999) and designated as an endangered tree in the Red List issued by Ministry of Environmental Protection of China and Chinese Academy of Science (http://www.zhb.gov.cn/gkml/hbb/bgg/201309/t20130912_260061.htm).
To date, reports about the distribution of M. ventii have been sporadic and include Flora Reipublicae Popularis Sinicae (Law, 1996), Flora Yunnanica (Law, 2006) and Flora of China (Xia et al., 2008), Seed Plants of Honghe Region in SE Yunnan, China (Shui, 2003) and National Protected Wild Plants in Yunnan Province China (Li, 2004). Recent studies provided more detail on species distribution and population sizes (Chu et al., 2011; Rivers et al., 2016; Wang et al., 2016a). The habitats have been badly degraded and fragmented due to heavy habitat destruction in the past, resulting in most (sub) populations being isolated from each other (Wang et al., 2016b). Our observations during the years 2013-2015 revealed abundant flowering but very low fruit and seed set, and very few seedlings, indicating problems with reproduction. Thus far, the causes of low fecundity in M. ventii are not known, largely because our knowledge of the species pollination ecology and breeding system is incomplete or lacking. Therefore, to conserve this critically endangered species, we must insure its successful reproduction and recruitment. To this end, our research had the following aims: (1) to provide a description of the floral period, (2) to identify visiting insects during different flowering stages, (3) to analyze floral scent compounds and their relationship with pollinator visitation, and (4) to verify the breeding system of M. ventii. Based on these results, a number of conservation recommendations were made.
1. Material and methods 1.1. Study sites and plant choiceThis study was conducted at two sites: Huangtian and Shuijing of Maandi village, Jinping County, Honghe Hani and Yi Autonomous prefecture, South Yunnan (Fig. 1). This region has a subtropical plateau monsoon climate and an altitude range of 76-3074 m. The mean annual temperature range is 15-22 ℃ and the mean annual precipitation is 800-1600 mm. The region is abundant in flora and fauna, and is one of the areas with the highest number of plant species in Yunnan. The total population size of M. ventii in all known existing locations (including three counties: Jinping, Hekou, and Pingbian) has been estimated around 580-630 individuals (Wang et al., 2016a). We located 330 individuals during our latest field surveys in the three counties, and grouped them into five categories, based on diameter at breast height (DBH), with the percentages are as follows: 15.71% (DBH < 2.5 cm, n=52), 14.20% (DBH 2.5-7.5 cm, n=47), 34.14% (DBH 7.5-22.5 cm, n=113), 34.44% (DBH 22.5-67.5 cm, n=114) and 1.51% (DBH≥67.5 cm, n=5) (unpublished data).

|
| Fig. 1 A map showing study sites and individuals in the Jinping Couty. The red asterisks indicate the trees subjected to observations of floral period and visitor, tests of breeding system and collections of floral scents. The red circles indicate the individuals used only for floral scents collection. |
M. ventii trees generally flower from the end of April to mid-May, with peak blooming in early-May, and our observations were conducted from late April to mid-May of 2013, 2014, and 2015. Based on the observation of movements of the petals, stigmas and anthers for many flowers (n=30, annually), floral period of a flower was consecutively recorded by a camera in the same position for each flower.
Flowers of M. ventii appear facing the ground like a bell (this flower orientation probably evolved to prevent washing away of pollen by rain during flowering) with 10 or 11 tepals in four whorls. Three to 10 flowers per tree and a total of three trees were selected for examining floral traits, including the color of the tepals in various whorls using the color chart of the Royal Horticultural Society of London and the Flower Council of Holland.
One tree in Huangtian (31 flowers) and two trees in Shuijing (30 and 30 flowers) were selected for observations. The behavior and number of floral visitors were observed and recorded over ten consecutive days for a total of 91 flowers on the three trees. Observations were conducted concentrating on the first day of anthesis for each designated flower for 5.5 h from 18:00 to 23:30. In the pre-staminate stage the flower visitors were collected by putting a plastic self-sealing bag over a flower. Before it re-opened the next day, the flower, which locked almost all floral visitors in its chamber, was cut off and brought to the laboratory for identification of the floral visitors. All insects were picked out of the flower by carefully taking apart the tepals and stamens, and each insect was checked for whether pollen was on its body surface. The insects of each type were counted, classified to family or genus, and three representative samples for each type of floral visitor were collected and fixed by insect pins for later identification to the species level.
1.3. Floral scentsOdor production was studied during the flowering period under natural conditions. Thirteen flowers were selected in the middle or lower canopy of six trees indicated in Fig. 1. From these 13 flowers, samples of floral scents were taken from one (6 flowers) or two floral stages (7 flowers) of the floral cycle of each flower (18:00-23:30 on the first day for the pistillate stage, and 16:00-23:00 on the next day for the staminate stage).
Based on our observations in 2013, the flowering period for M. ventii lasts about six days, but odor production occurs mainly during the first two days. Therefore, floral scents were collected using the dynamic headspace adsorption method over the period 18:00-23:30 on the first day and 16:00-23:00 on the second day, coinciding with the time of highest floral odor emission and the time for attracting floral visitors. Flowers were cut from different individuals and the flower stalks were wrapped in wet cotton. The wrapped flower was placed in a culture dish. The dish was enclosed in a Tedlar bag (Dupont, USA). The scent was drawn from the bag into a tube containing the adsorbent Porapak Q (150 mg, mesh 60/ 80, Waters Associates, Inc.) using a pump with an inlet flow rate of 300 mL min-1 for 4 h. To identify background contamination, the scent of stems with unopened floral buds was collected as a control. Floral scent collection was undertaken according to the method described in Chen et al. (2015). Trapped volatile organic compounds (VOCs) were eluted with 300 ìL dichloromethane (99.5%, chromatographically pure) and concentrated to one-fifth of the original volume by a gentle stream of nitrogen (200 mL min-1). Before gas chromatography analysis, 7200 ng n-nonane was added as an internal standard to all samples. Extracts were stored at-20 ℃ in a refrigerator for subsequent analysis.
Samples from flowers and controls were analyzed using an Agilent Technologies HP 6890 gas chromatograph (GC), equipped with an HP-5MS column (5% phenylmethyl polysiloxane; 60 m long, 0.32 mm inner diameter, 0.25 mm film thickness), and linked to an HP 5973 mass spectrometer (MS). Helium was used as a carrier gas at a flow rate of 1 mL min-1. Split inlet and FID were held at 250 ℃. Column temperature was programmed to rise from 40 ℃ (5-min hold) to 250 ℃ (20-min hold) at 3 ℃ min-1. The mass spectra were taken at 70 eV (in EI mode) with a scanning speed of one per scan from m/z 35 to 500. Compounds were tentatively identified by comparing mass spectra and relative retention times with those of standard compounds purchased from SigmaeAldrich, USA and with the Wiley 7n.L mass spectral library. Their relative proportions (%) were determined by peak area measurements.
To characterize the floral scent variation among populations, a non-metric multi-dimensional scaling (NMDS) ordination based on Bray-Curtis similarities, calculated on the relative amounts of volatile compounds (in % of the total blend) was performed on all samples, i.e. samples taken from 6 flowers at the first floral stage, and samples taken from 7 flowers at two floral stages. To visualize the contribution of three main scent compounds to the variation in floral scent among populations, we used principal components analysis (PCA) performed on a variance-covariance matrix of the floral scents (relative amount, in %). The 'Biplot' option shows a projection of the original axes (variables) onto the scattergram and it is a visualization of the PCA loading, which implies the contributions of the three compounds among populations within a species. Both NMDS and PCA were performed in PAST version 2.08 (Hammer et al., 2001).
1.4. Breeding systemPollination treatments were performed on three randomly chosen individual trees at Huangtian and Shuijing during the flowering period from late April to mid-May of 2015 (Fig. 1). Five pollination treatments were applied to each of 30 flowers per tree: (1) open pollination: floral buds were labeled and left untouched under natural conditions; (2) pollinator exclusion: floral buds were labeled and bagged without any manipulations to test for autogamy; (3) apomixis: the flowers were emasculated before pollen had landed on any of the stigmas, labeled and bagged to test for asexual reproduction; (4) geitonogamy: hand self-pollinations (using pollen from the same tree) were performed on emasculated flowers; (5) xenogamy: hand cross-pollinations (using pollen from different trees) were performed on emasculated flowers. The treated flower organs were bagged with water-proof paper bags. The bags were removed six days after bagging (on the last day of the single flower period).
Labeled fruits were harvested one month after the pollination treatment, in early September of each year and viable seeds were distinguished from aborted ones by the color of the aril (blood red vs. white or green, respectively) (Zhao and Sun, 2009). The effect of pollination treatments was analyzed for two traits: fruit set [(number of fruits/number of tested flowers) × 100] and seed set [(number of viable seeds/number of initial ovules) × 100] by One Way ANOVA using SPSS 16.0. All data were examined for normality and homogeneity of variance. Tukey post-hoc test was performed to compare the group means.
2. Results 2.1. Determination of the floral periodflowers of m. ventii appear facing the ground like a bell (this flower type probably prevents the pollen from being washed away by rainfall) with 10 or 11 tepals in four whorls. the color of flower buds is red-purple (74a-d) or green (143b-d); the color of the abaxial outer tepal is red-purple (74a-d) or yellow-green (145a-d); the tepal of the second and third whorl (as counted from the outside inwards) is yellow-white (158c-d), and the base of the petal is flushed purple-violet (80a-b, 81c-d) or yellow-green (145a-d). the abaxial outer stamen is greyed-purple (187b-d) or red-purple (74a-d) (supplementary fig. 1).
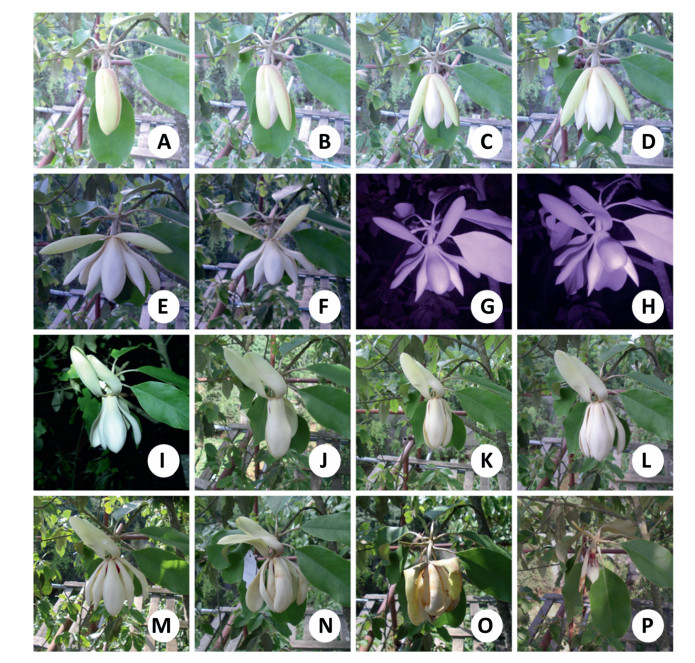
The flowers, which open and close in a two-night rhythm, were hermaphroditic, nocturnal, protogynous and the anthesis of a flower lasted for about 6 days. The floral period can be divided into five distinct stages over the first two days of the flower period: prepistillate, pistillate, pre-staminate, staminate and post-staminate (Supplementary Table 1). The pre-pistillate stage, in which the outer tepals appear slightly loose as a hint of blooming, commenced at about noon on the right opening day and ended at about sunset before flower opening (at ca. 18:00) (Fig. 2A). Upon opening, soon after the first of the outer tepals was held erect away from the bud (at 19:00) (Fig. 2B), the rest of tepals quickly opened at almost the same time, at about 19:20, when the pistillate stage commenced (Fig. 2C). During the pistillate stage, the flowers were functionally female: the pistil was open and the receptive stigmas produced nectar-like exudates (Fig. 2C-I). The pistillate stage lasted until 23:30 when the tepals, except for the three outermost tepals, completely closed and formed a floral chamber (Fig. 2I), immediately entering the pre-staminate stage (Fig. 2J). This stage, which was characterized by the receptivity of the stigma being gradually lost and stamens having indehiscent anthers, ended at about the second sunset before flower re-opening (at ca. 16:00). At this point, the flower reopened, the receptivity of stigma was lost completely and the anthers began to dehisce, which indicated the start of the staminate stage (Fig. 2K-M). This latter stage lasted 2-4 days until the pollen, along with the stamen, was shed indicating the poststaminate stage (Fig. 2N-P).
|
| Fig. 2 Five stages of M. ventii floral period: the pre-pistillate (A, B), pistillate (CeI), pre-staminate (J), staminate (KeM), and post-staminate (NeP). |
| Compound | Retention time (min) | Pistillate stage (%) | Staminate stage (%) |
| Limonene | 13.37 | 15.41±8.76 | 21.98±6.28 |
| β-Pinene | 11.01 | 14.10±3.61 | 14.38±4.09 |
| α-Pinene | 9.25 | 12.17±4.10 | 13.49±4.51 |
| 1, 8-Cineole | 13.41 | 8.72±8.51 | 8.88±7.35 |
| Methyl 2-methylbutyrate | 4.40 | 8.24±12.31 | 6.56±9.72 |
| p-Cymene | 13.15 | 5.82±6.34 | 7.38±7.30 |
| Methyl 3-methyl-2-butenoate | 6.15 | 5.19±2.81 | 3.73±2.09 |
| 2-Methoxy-2-methyl-3-buten | 3.96 | 5.19±5.16 | 3.08±5.06 |
| 3-Methyl-2-buten-1-ol | 4.37 | 5.40±5.37 | 1.19±1.17 |
| Myrcene | 11.75 | 2.77±2.36 | 3.52±2.67 |
| trans-β-Ocimene | 14.25 | 2.35±2.05 | 2.64±2.36 |
| Sabinene | 10.88 | 1.64±1.51 | 1.74±2.01 |
| Camphene | 9.83 | 1.34±0.77 | 1.59±0.49 |
| Linalool | 16.41 | 0.85±0.81 | 1.10±0.89 |
| cis-β-Ocimene | 13.82 | 0.61±0.95 | 0.45±0.38 |
| Note: The floral scents were sampled at 18:00 for the pistillate stage and 16:00 for the staminate stage. | |||
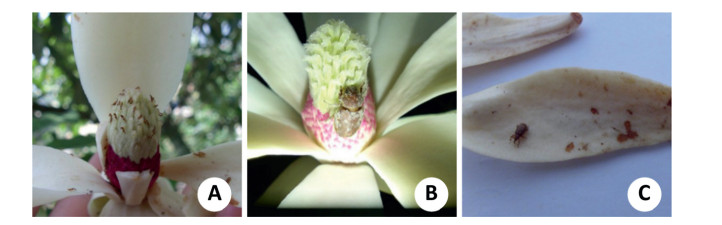
Several species of insects were attracted to and visited the flowers of M. ventii during our observations, including rove beetles (Aleochara sp., Aleocharinae, Staphylinidae, Staphylinidea, Coleoptera) (Fig. 3A), thrips (Thrips sp., Thripidae, Terebrantia, Thysanoptera) (Fig. 3A), weevils (Sitophilus sp., Curculionidae, Curculionoidea, Coleoptera) (Fig. 3C) and beetles (Anomala sp., Rutelidae, Scarabaeoidea, Coleoptera) (Fig. 3B). At approximately the beginning of the pistillate stage of a single flower, the floral visitors began to fly around the flowering tree and then visited the flowers. The period of most movement activity of visitors correlated with scent emissions (personal observation of the first author). Once visitors had entered a single flower in the pistillate stage, they did not actively fly from one flower to another until the flower reopened in the staminate stage (via the intermediate closing stage of the pre-staminate stage). Visitors stayed overnight in a single flower and went through both the pistillate and prestaminate stages of the flower. Before leaving the flower in staminate stage and switching to another pistillate stage flower, the pollinators carried pollen on their bodies. During their most active period (in the pistillate stage of a flower), the greater number of visitors assembled in the flowers and were observed to mate, to feed on stigmas and petal exudates, and to gnaw on the tissue of the inner petals. Visitors inside the first-night pistillate flowers were trapped when the petals closed tightly. The trapped visitors continued feeding on exudates (as long as they were available), feeding on petal tissue, and mating. The next evening, upon flower re-opening (in the staminate stage), the visitors were finally released. Before entering another newly-opened pistillate-stage flower, they unceasingly ate pollen of the flower in staminate stage.
|
| Fig. 3 Flower-visiting insects at different stages of anthesis. (A) Aggregation of both rove beetles (Aleochara sp.) and thrips (Thrips sp.) on the surface of gynoecia during the pistillate stage. (B) A beetle (Anomala sp.) with pollen on its body feeding on stigmas during the pistillate stage. (C) A weevil (Sitophilus sp.) crawling on an inner petal, which was gnawed on during the pre-staminate stage. |
We collected a total of 6549 visitors from the 91 sampled flowers. Rove beetles were the dominant flower visitors (63.2%), followed by thrips (35.4%), weevils (0.3%) and beetles (0.1%). The average number of visitors (±SE) in each flower was 45.46±36.45 rove beetles, 25.47±21.79 thrips, 0.19±0.58 weevils and 0.04±0.21 beetles. The above floral visitors were observed both in the pistillate and staminate stages and all of them landed on the pistillate-stage flowers and crawled on the stamens and gynoecia. However, we observed that only the bodies of weevils and beetles carried the sticky pollen grains during the pistillate stage in which the floral visitors could really play a role in pollination. Therefore, weevils and beetles appear to be the most effective pollinators of M. ventii. In contrast, rove beetles and thrips, although they represented the highest proportion of all the floral visitors, cannot be considered effective pollinators.
2.3. Floral scentsAn aromatic scent (personal sense of smell) was perceptible when flower buds were held close to the nose during the late phase of the pre-pistillate stage. In other words, the scent emissions of a flower first occurred just before the flowers open when the outer tepals separate slightly and the inner yellowish-white tepal tips became visible. The floral scent intensified strongly with the onset of anthesis, both in the pistillate and staminate stages. Floral scent analyses were conducted during the two developmental stages (Table 1).
The total amount of scent trapped using dynamic headspace adsorption varied between the two key flowering stages and also among replicates within specific stages. In total, 52 different compounds were detected in samples (n=20), but only the primary 15 appeared in at least half of the samples and contributed≥1% to the total scent of the samples. These compounds are listed in Table 1. According to the relative percentage contents of volatile compounds, the dominant compounds are Limonene, β-Pinene, α-Pinene, 1, 8-Cineole, Methyl-2-methylbutyrate, p-Cymene, Methyl-3-methyl-2-butenoate and 2-Methoxy-2-methyl-3-buten.
The non-metric multi-dimensional scaling (NMDS) ordination based on floral scent data from 20 samples of M. ventii produced high linear fit (R2=0.713) along the x-axis (NMDS1) (Fig. 4A). The NMDS biplot indicates that different M. ventii (sub) populations formed discrete clusters and illustrates intraspecific differentiation in floral scents among populations. Intraspecific contribution of each floral scent based on relative amount among five M. ventii (sub) populations was further evaluated through principal components analysis (PCA). Methyl 2-methylbutyrate mainly contributed to (sub) population Huangtian (HT); Limonene primarily contributed to (sub) populations Huangtian Longshu (HTL), Huangtian Guaijiao (HTG) and Shuijing (SJ); 1, 8-Cineole mainly contributed to another four (sub) populations Shuitou (ST), Shuijing (SJ), Huangtian (HT) and Huangtian Guaijiao (HTG) (Fig. 4B).

|
| Fig. 4 Floral scent differentiation based on 15 main identified floral scent compounds from 20 samples of M. ventii between five locations. (A) Non-metric multi-dimensional scaling (NMDS); (B) Principal component analysis (PCA). The first and second PC explained 55.7 and 15.3% of variation. The abbreviations correspond to the distribution sites, defined as follows: HT, Huangtian; HTG, Huangtian Guaijiao; HTL, Huangtian Longshu; SJ, Shuijing; ST, Shuitou. |
Pollination treatments had a highly significant effect on fruit set in all five treatments (F4, 10=31.6, P < 0.0001, ANOVA). The average fruit set that resulted from open pollination, pollinator exclusion, apomixes, geitonogamy (hand self-pollination), and xenogamy (hand cross-pollination) was 8.89±5.09%, 0.00±0.00%, 0.00±0.00%, 40.00±28.87%, and 100.00±0.00%, respectively. Effects of open pollination and geitonogamy did not differ (P=0.140, Tukey's post-hoc test), while xenogamy resulted in significantly higher fruit set than either open pollination or geitonogamy (P=0.001 and 0.012, both Tukey's post-hoc test). Pollination treatment had a strong effect on seed set (F2, 170=950.1, P < 0.0001, ANOVA) with seed set per fruit for open pollination, geitonogamy, and xenogamy being significantly different (P < 0.0001, Tukey's post-hoc test). The average values for the latter were 3.08±1.64%, 25.43±27.13%, and 99.04±2.27%, respectively.
3. DiscussionM. ventii is similar to most other members of the Magnoliaceae in being bisexual, and having protogynous and nocturnal flowers that emit a strong fragrance over two consecutive evenings. There is a closing period (the pre-staminate stage) during the process of anthesis of a single flower, and we characterize the key flowering process as an "open-close-reopen" flowering rhythm. During the first night of anthesis, the flowers are functionally female in the pistillate stage, during which stigmas are receptive. In the meantime, floral scent is emitted to attract pollinators, at about sunset, and lasts about 5.5 h until all the tepals but the outermost three are completely closed. This is the time when the stigmas are receptive and the scents are emitted. During the second night of anthesis, the flowers enter the staminate stage with dehiscent stamens and the stigmas having already lost receptivity. Both Magnolia ovata and Magnolia sprengeri exhibit a highly specialized pollination system involving floral thermogenesis over the two consecutive opening evenings. Heat production of M. sprengeri flowers occurs in its gynoecia during anthesis, and its flower may act as shelter for pollinators during cold nights (Wang et al., 2014). In this study, the pollinators were not able to fly from a flower in the pistillate stage to another flower on the same night, being kept in the flower until the flower reopened in the staminate stage. After entering the flower in the pistillate stage, all visitors stayed in the flower until the flower reopened in the staminate stage. By that time they were apt to carry the pollen on their bodies and to visit another pistillate stage flower in the evening. During this period, an increasingly large number of visitors assembled on the stigma area in the flower (Fig. 3A), and the flower provided a chamber for visitors to mate, to feed on stigmas and petal exudates, and to gnaw on the tissue of the inner petals, especially during the pistillate stage. Before leaving the flower in the staminate stage, the visitors predominantly ate pollen.
Many pollinators are floral scent-dependent. Floral scents have been well documented to play an important role in plant-pollinator interactions through various effects on visiting insects, including feeding cues, nectar guides (Endress, 2010; Wang et al., 2014), and sex pheromones (Schatz, 1990). Floral scents are complexes of chemical compounds (Suinyuy et al., 2013), and different visiting insects prefer certain odors (Kumano-Nomura and Yamaoka, 2009). In this study, we also found some variation in the content level of dominant scent compound composition of floral scents both at pistillate and staminate stages. The process of odor release between the pistillate and staminate stage is slightly different from that described in previous studies performed on Magnolia tamaulipana (Dieringer et al., 1999), M. ovata (Gottsberger et al., 2012), and M. sprengeri (Wang et al., 2014). For ensuring successful pollination, the flower of M. ventii mainly adopt a 'push-pull' pollination strategy (Terry et al., 2007) which could be portrayed as 'pulling' pollinators via one odor cue in the first night, and, via the other odor cue which has a slightly different combination in content level of each compound, 'pushing' pollinators toward another new blooming flower in the second night. It could be inferred that the different combination of these compounds involved in attracting or rejecting pollinators appear to have some sort of 'cocktail effect' (Chen et al., 2015).
Both apomixis and pollinator exclusion treatments resulted in no fruits produced, while the open pollination treatment, where flowers were untreated and accessible to natural pollinators, produced a small amount of fruits, each fruit containing only a very few seeds. In contrast, hand-pollination (especially cross-pollination with pollen from a different individual) resulted in greatly improved seed production. Thus, M. ventii is pollinator-dependent, and low seed set in natural populations is a result of insufficient pollen deposition.
Outcrossing plant species rely on vectors transferring pollen to their flowers' stigmas for fertilization, and both adequate fertile pollen and carrying pollen pollinators are essential for seed production. However, small and fragmented plant populations are often less attractive to pollinators due to small population size and plant density and may be visited less frequently (Rathcke and Jules, 1993; Kunin, 1997; Roll et al., 1997) leading to disruption of plant-pollinator interactions and reduced reproduction (Ægisdóttir et al., 2007; Chacoff et al., 2008; Colling et al., 2004; Kearns et al., 1998; Moody-Weis and Heywood, 2001).
Small population sizes have many negative consequences both demographic and genetic (Ellstrand and Elam, 1993; Severns, 2003). Inbreeding depression is a major concern in the management and conservation of endangered species (Hedrick and Kalinowski, 2000), and negative effects from small population sizes on seed production have been found in many species (Ke'ry et al., 2001; Morgan, 1999).
M. ventii has been recently designated as endangered and approved as one of the PSESP for rescue conservation by the Yunnan provincial government (Ma et al., 2013). We believe that the improvement of following aspects may be beneficial to the regeneration of M. ventii: (1) Habitat protection. Gottsberger and Amaral (1984) reported that many dynastid beetles (tribe Cyclocephalini), the larvae of which develop in the soil affiliated to the habitat of the targeted plant species and feed on litter and roots (García et al., 2009; Villegas et al., 2008), have been observed emerging from the soil, e.g., below Philodendron selloum plants, and then fly directly to inflorescences. Those previous studies reveal that litter removal and micro habitat destruction will affect the number of pollinators and thereby reduce the fitness of plants. In this study, the low fruit and seed set in open pollination treatment indicates insufficient number of M. ventiipollinators. Many individual trees of M. ventii are located at the border of croplands and villages where soil conditions are unfavorable for the development of dynastid beetle larvae. To insure the regeneration of M. ventii, soil disturbances removing litter (tillage or crop cultivation) in the vicinity to M. ventii trees must be prevented. The local government should find a way to encourage local farmers to retreat from the edge of current M. ventii distribution, or at least restrain from expanding their farmlands into it. Establishing one or several (micro) nature reserves in the least degraded habitats occupied by M. ventii is absolutely vital for the species survival.
(2) Ex situ-seed collecting must be initiated from every reproducing adult in order to capture the maximum level of remaining genetic diversity (Volis, 2016). This is really important because xenogamy was found to result in 100% of the fruit and seed set. The collected seeds must be used to raise saplings for the augmentation of existing populations.
(3) Population reinforcements. There are two main factors limiting reproduction in M. ventii: the overlap of the flowering period with the local rainy season, which hinders the diffusion of floral scent; and the high dependence on pollinator activity (including their recognition of floral odor). The negative impact of these factors is exacerbated when adult individuals are scattered and far apart. Therefore, a reinforcement of the remaining wild populations with genetically variable saplings, planted at the distance from each other within a migrating distance of the effective pollinators, may substantially improve reproduction and regeneration in M. ventii.
AcknowledgementsFunding (No. U1302262) to W.B. Sun from the NSFC-Yunnan joint fund on key projects and Survey and Germplasm Conservation of PSESP in Southwest China (2017-2020, 2017FY100100) are particularly acknowledged. This study was also partly supported by the Young Academic and Technical Leader Raising Foundation of Yunnan Province (2015HB091) and the Science and Technology Research Program of Kunming Institute of Botany, the Chinese Academy of Science (KIB2016005) to G. Chen. We gratefully thank Drs Z.L. Dao & C.Y. Han, J.Y Liu, Z.Y. Yu, Z.L. Liang, X.W. Li, W.Q. Yao, S.X. Zhu, J. Bai and J. Qian for their help during fieldwork; the local forestry authorities of Pingbian, Hekou and Jinping for assistance in field work. We also thank Z. Yu for floral scents analysis; Dr Y.P. Ma for giving valuable advice for data processing; Drs T. and J. Marczewski for reviewing the text; Dr W.C. Gong for drafting Fig. 4, and Y. Qin for photographing insects and for their identification. We are also grateful to Prof. Sergei Volis and four anonymous reviewers for their valuable comments on the manuscript.
Appendix A. Supplementary dataSupplementary data related to this article can be found at http://dx.doi.org/10.1016/j.pld.2017.01.001.
Ægisdóttir H.H., Jespersen D., Kuss P., et al, 2007. No inbreeding depression in an out crossing Alpine species: the breeding system of Campanula thyrsoides. Flora, 202: 218-225. DOI:10.1016/j.flora.2006.06.003 |
Anonymous, 1999. List of national key protected wild plants (first group). In: The Order of National Forestry Bureau and Agricultural Ministry of China, Beijing, vol. 4, pp. 2-13.
|
Azuma H., Toyota M., Asakawa Y., 1997. Chemical divergence in floral scents of Magnolia and allied genera (Magnolliaceae). Plant Species Biol, 12: 69-83. DOI:10.1111/psb.1997.12.issue-2-3 |
Azuma H., Toyota M., Asakawa Y., 2001. Intraspecific variation of floral scent chemistry in Magnolia kobus DC. (Magnoliaceae). J. Plant Res, 114: 411-422. DOI:10.1007/PL00014006 |
Bernhardt, P. , 2000. Convergent evolution and adaptive radiation of beetlepollinated angiosperms. Plant Syst. Evol. 222, 293-320. http://dx.doi.org/10.1007/BF00984108.
|
Chacoff N.P., Garcia D., Obeso J.R., 2008. Effects of pollen quality and quantity on pollen limitation in Crataegus monogyna (Rosaceae) in NW Spain. Flora, 203: 499-507. DOI:10.1016/j.flora.2007.08.005 |
Chen G., Jürgens A., Shao L.D., et al, 2015. Semen-like floral scents and pollination biology of a sapromyophilou plant Stemona japonica (Stemonaceae). J. Chem. Ecol, 41: 244-252. DOI:10.1007/s10886-015-0563-0 |
Chu Y.X., Ouyang Z.Q., Zhang R.G., et al, 2011. Distribution and protection measures of Manglietia hebecarpa resources in Dawei Mountain area of Yunnan. For. Inventory Plan, 36(4): 63-65, 70. |
Colling G., Reckinger C., Matthies D., 2004. Effects of pollen quantity and quality on reproduction and offspring vigor in the rare plant Scorzonera humilis (Asteraceae). Am. J. Bot, 91: 1774-1782. DOI:10.3732/ajb.91.11.1774 |
Dieringer G., Cabrera R.L., Lara M., et al, 1999. Beetle pollination and floral thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int. J. Plant Sci, 160(1): 64-71. DOI:10.1086/314099 |
Dobson H.E.M., Bergström G., 2000. The ecology and evolution of pollen odors. Plant Syst. Evol, 222: 63-87. DOI:10.1007/BF00984096 |
Duan Y.W., Liu J.Q., 2007. Pollinator shift and reproductive performance of the Qinghai-Tibetan Plateau endemic and endangered Swertia przewalskii (Gentianaceae). Biodivers. Conserv, 16: 1839-1850. DOI:10.1007/s10531-006-9076-z |
Ellstrand N.C., Elam D.R., 1993. Population genetic consequences of small population size: implications for plant conservation. Annu. Rev. Ecol. Syst, 24: 217-242. DOI:10.1146/annurev.es.24.110193.001245 |
Endress P.K., 2010. The evolution of floral biology in basal angiosperms. Philos. Trans. R. Soc. B Biol. Sci, 365: 411-421. DOI:10.1098/rstb.2009.0228 |
Galen C., 1999. Flowers and enemies: predation by nectar thieving ants in relation to variation in floral form of an alpine wildflower, Polemonium viscosum. Oikos, 85: 426-434. DOI:10.2307/3546692 |
García G.L., Ortega-Arenas L., Hernández H.G., et al, 2009. Description of Melo-lonthidae (Coleoptera) third instar larvae associated to Agave tequilana var. Azul and their population fluctuation in Jalisco Mexico. Neotrop. Entomol, 38: 769-780. |
Gong W.C., Chen G., Vereecken N.J., et al, 2015. Floral scent composition predicts bee pollination system in five butterfly bush (Buddleja, Scrophulariaceae) species. Plant Biol, 17: 245-255. DOI:10.1111/plb.12176 |
Gottsberger G., Amaral Jr., A ., 1984. Pollination strategies in Brazilian Philodendron species. Ber. Dtsch. Bot. Ges. Bd, 97(S): 391-410. |
Gottsberger G., Silberbauer-Gottsberger I., Seymour R.S., et al, 2012. Pollination ecology of Magnolia ovata may explain the overall large flower size of the genus. Flora, 207: 107-118. DOI:10.1016/j.flora.2011.11.003 |
Hammer Ø, Harper D.A.T., Ryan P.D., 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron, 4: 83-93. |
Hedrick P.W., Kalinowski S.T., 2000. Inbreeding depression in conservation biology. Annu. Rev. Ecol. Syst, 31: 139-162. DOI:10.1146/annurev.ecolsys.31.1.139 |
Hirayama K., Ishida K., Tomaru N., 2005. Effects of pollen shortage and selfpollination on seed production of an endangered tree, Magnolia stellata. Ann. Bot, 95: 1009-1015. DOI:10.1093/aob/mci107 |
Jürgens A., Dötterl S., Meve U., 2006. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae). New Phytol, 172: 452-468. DOI:10.1111/nph.2006.172.issue-3 |
Kearns C.A., Inouye D.W., Waser N.M., 1998. Endangered mutualisms: the conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst, 29: 83-122. DOI:10.1146/annurev.ecolsys.29.1.83 |
Ke'ry M., Matthies D., Fischer M., 2001. Interactions between the rare plant Gentiana cruciata and the specialized herbivore Maculinea rebeli. J. Ecol, 89: 418-427. DOI:10.1046/j.1365-2745.2001.00550.x |
Kumano-Nomura Y., Yamaoka R., 2009. Beetle visitations, and associations with quantitative variation of attractants in floral odors of Homalomena propinqua (Araceae). J. Plant Res, 122: 183-192. DOI:10.1007/s10265-008-0204-6 |
Kumar V.S., 2006. New combinations and new names in Asian Magnoliaceae. Kew Bull, 61: 183-186. |
Kunin W.E., 1993. Sex and the single mustard: population density and pollinator behavior effects on seed set. Ecology, 74: 2145-2160. DOI:10.2307/1940859 |
Kunin W.E., 1997. Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. J. Ecol, 85: 225-234. DOI:10.2307/2960653 |
Law, Y. W. , 1996. Magnoliaceae. In: Flora Reipublicae Popularis Sinicae. Science Press, Beijing, China, pp. 94-96.
|
Law, Y. W. , 2006. Magnoliaceae. In: Flora Yunnanica. Science Press, Beijing, China, pp. 7-9.
|
Li, Y. Y. , 2004. National Protected Wild Plants in Yunnan Province China. Yunnan Science and Technology Press, Kunming, pp. 233-234.
|
Ma Y.P., Chen G., Grumbine R.E., et al, 2013. Conserving plant species with extremely small populations (PSESP) in China. Biodivers. Conserv, 22: 803-809. DOI:10.1007/s10531-013-0434-3 |
Matsuki, Y. , Tateno, R. , Shibata, M. , et al. , 2008. Pollination efficiencies of flowervisiting insects as determined by direct genetic analysis of pollen origin. Am. J. Bot. 95, 925-930. http://dx.doi.org/10.3732/ajb.0800036.
|
Moody-Weis J.M., Heywood J.S., 2001. Pollination limitation to reproductive success in the Missouri evening primrose, Oenothera macrocarpa (Onagraceae). Am. J. Bot, 88: 1615-1622. DOI:10.2307/3558406 |
Morgan J.W., 1999. Effects of population size on seed production and germinability in an endangered, fragmented grassland plant. Conserv. Biol, 13: 266-273. DOI:10.1046/j.1523-1739.1999.013002266.x |
Rathcke B.J., Jules E.S., 1993. Habitat fragmentation and plant-pollinator interactions. Curr. Sci, 65: 273-277. |
Rivers, M. , Beech, E. , Murphy, L. , et al. , 2016. The Red List of Magnoliaceae-revised and Extended. BGCI, Richmond, UK.
|
Roll J., Mitchell R.J., Cabin R.J., et al, 1997. Reproductive success increases with local density of conspecifics in a desert mustard (Lesquerella fendleri). Conserv. Biol, 11: 738-746. DOI:10.1046/j.1523-1739.1997.96013.x |
Schatz, G. E. , 1990. Some aspects of pollination biology in Central American forests. In: Bawa, K. S. , Hadley, M. (Eds. ), Reproductive Ecology of Tropical Forest Plants. Parthenon, Paris, pp. 69-84.
|
Severns P., 2003. Inbreeding and small population size reduce seed set in a threatened and fragmented plant species, Lupinus sulphureus ssp. kincaidii (Fabaceae). Biol. Conserv, 110: 221-229. DOI:10.1016/S0006-3207(02)00191-X |
Seymour R.S., Silberbauer-Gottsberger I., Gottsberger G., 2010. Respiration and temperature patterns in thermogenic flowers of Magnolia ovata under natural conditions in Brazil. Funct. Plant Biol, 37: 870-878. DOI:10.1071/FP10039 |
Shui, Y. M. , 2003. Seed Plant of Honghe Region in SE Yunnan, China. Yunnan Science and Technology Press, Kunming, p. 54.
|
Sih A., Baltus M.-S., 1987. Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology, 68: 1679-1690. DOI:10.2307/1939860 |
Suinyuy T.N., Donaldson J.S., Johnson S.D., 2013. Patterns of odour emission, thermogenesis and pollinator activity in cones of an African cycad: what mechanisms apply? Ann. Bot, 112: 891-902. DOI:10.1093/aob/mct159 |
Terry I., Walter G.H., Moore C., et al, 2007. Odor-mediated push-pull pollination in cycad. Scienc, 318: 70. DOI:10.1126/science.1145147 |
Thien, L.B., 1974. Floral biology of Magnolia. Am. J. Bot. 61, 1037-1045. http://dx.doi.org/10.2307/2441921.
|
Thien L.B., Azuma H., Kawano S., 2000. New perspectives on the pollination biology of basal angiosperms. Int. J. Plant Sci, 6 Suppl.: S225-S235. |
Tiep N.V., 1980. Beitrage zur Sippenstruktur der Gattung Manglietia Bl. (Magnoliaceae. Feddes Repert, 91(9-10): 497-576. |
Villegas N.P., Gaigl A., Vallejo L.F., 2008. The white grub complex (Coleoptera: Melolonthidae) associated with onion and pasture in Risaralda, Colombia. Rev. Colomb. Entomol, 34: 83-89. |
Volis S., 2016. How to conserve threatened Chinese plant species with extremely small populations?. Plant Divers, 1: 53-62. |
Wang B., Han C.Y., Li C.R., et al, 2016a. Population status, distribution and conservation of Magnolia ventii (Magnoliaceae), a critically endangered species endemic to Yunnan province in China. Magnolia, J. Mag. Soc. Int, 51(1): 14-22. |
Wang B., Ma Y.P., Chen G., et al, 2016b. Rescuing Magnolia sinica (Magnoliaceae), a critically endangered species endemic to Yunnan, China. Oryx, 50(3): 446-449. DOI:10.1017/S0030605315000435 |
Wang, R. H. , Xu, S. , Liu, X. Y. , et al. , 2014. Thermogenesis, flowering and the association with variation in floral odour attractants inMagnolia sprengeri (Magnoliaceae). PLoS One 9 (6), e99356. http://dx.doi.org/10.1371/journal.pone.0099356.
|
Xia, N. H. , Liu, Y. H. , Nooteboom, H. P. , 2008. Magnoliaceae. In: Flora of China. Science Press, Beijing, China & Missouri Botanical Garden Press, St. Louis, USA, pp. 48-91.
|
Zhao, X. F. , Sun, W. B. , 2009. Abnormalities in sexual development and pollinator limitation in Michelia coriacea(Magnoliaceae), a critically endangered endemic to Southeast Yunnan, China. Flora 204, 463-470.
|



